1. Introduction
One of the Rubiaceae family,
Mitragyna speciosa Korth (Kratom), is a native tree that popularly cultivated in Thailand, Indonesia, and Malaysia, and is also known as Thom, Ketum, Biak, and Thang [
1]. Kraatom leaves have long been utilized in Thai folk medicine to treat pain and generate euphoric effects through chewing, drinking, and smoking [
2,
3]. The leaves of
M. speciosa are recognized to be high in alkaloids, flavonoids, and phenolic compounds [
2]. Mitragynine, an interesting compound in the alkaloid class, can be extracted from
M. speciosa leaves and has an antinociceptive effect, similar to 7-hydroxymitragynine [
3]. In addition, mitragynine has been found to have other clinical effects such as antidiabetic, antidiarrheal, antidepressant, anti-inflammatory, antinociceptive, antitussive, antipyretic, anxiolytic, appetite-suppressing, blood pressure-lowering, and euphoric [
2].
In recent decades, the extraction of
M. speciosa mitragynine has been carried out in various ways, including ultrasound (US) [
4,
5,
6], maceration [
4,
7], and accelerated solvent extraction (ASE) [
8]. Conventional extractions, such as soaking, maceration, and Soxhlet extraction, have more or fewer deficiencies and limitations, including long processing time, low extraction yields, and poor extraction efficiency [
4,
9]. To overcome these limitations of conventional extraction methods, new and promising extraction techniques have been introduced. The innovative techniques, including microwave-assisted extraction (MAE), ultrasonic-assisted extraction (UAE), and supercritical carbon dioxide extraction (SFE-CO
2), were chosen to maximize the content of mitragynine, and it was found that the best yield of mitragynine was obtained when UAE was used [
4]. Furthermore, an optimized condition of the UAE was modeled at 25 °C, 15 minutes of sonication time, and a 10 mL/g of solvent to solid ratio [
5].
Pulse electric field (PEF) is one of the advanced non-thermal technologies that has been successfully introduced to extract various plant and natural food pigments [
10]. PEF provides substantial advantages over other non-thermal approaches, such as a quick processing time (nanoseconds to milliseconds), higher efficiency, and lower energy consumption [
11,
12,
13]. PEF can be used alone or in conjunction with other technologies to minimize extraction temperature and time. For example, PEF can be combined with MAE to extract pectin from jackfruit waste [
14] and US can be combined with PEF to improve the olive oil yield [
15]. Furthermore, PEF can be applied for pasteurization, as well as the enhancement of drying and freezing processes [
16].
In this investigation, we extracted mitragynine from M. speciosa leaves using a newly built pulsed electric field-ultrasound (PEF-US) system. PEF-US extraction efficiency was equivalent to other modes, including PEF, US, US + PEF, and simple maceration. We believe this is the first time PEF + US has been used to treat mitragynine. In addition, energy consumption was examined. SEM and FTIR were used to examine changes in the structural and functional features of plant materials after employing four distinct modes of the prototype.
2. Materials and Methods
2.1. Plant material and preparation
Dried leaves of M. speciosa were purchased from a local farm in Ratchaburi, Thailand. The leaves were pulverized in a multipurpose grinder (Thaigrinder model WF-04, Nonthaburi, Thailand) into a fine powder form (1.0 mm) and used for the experiments.
The powder of 10% (w/v) was used in all extraction experiments; specifically, 100 g of powder was mixed with 1000 mL of extraction solvent [
4]. The extraction solvent consisted of 1% of acetic acid in 80% ethanol. After extraction, the mixture of solvent and
M. speciosa powder was immediately filtered and adjusted to reach 1000 mL with 1% of acetic acid in 80% ethanol.
2.2. Pulsed electric field and ultrasonic (PEF-US) apparatus
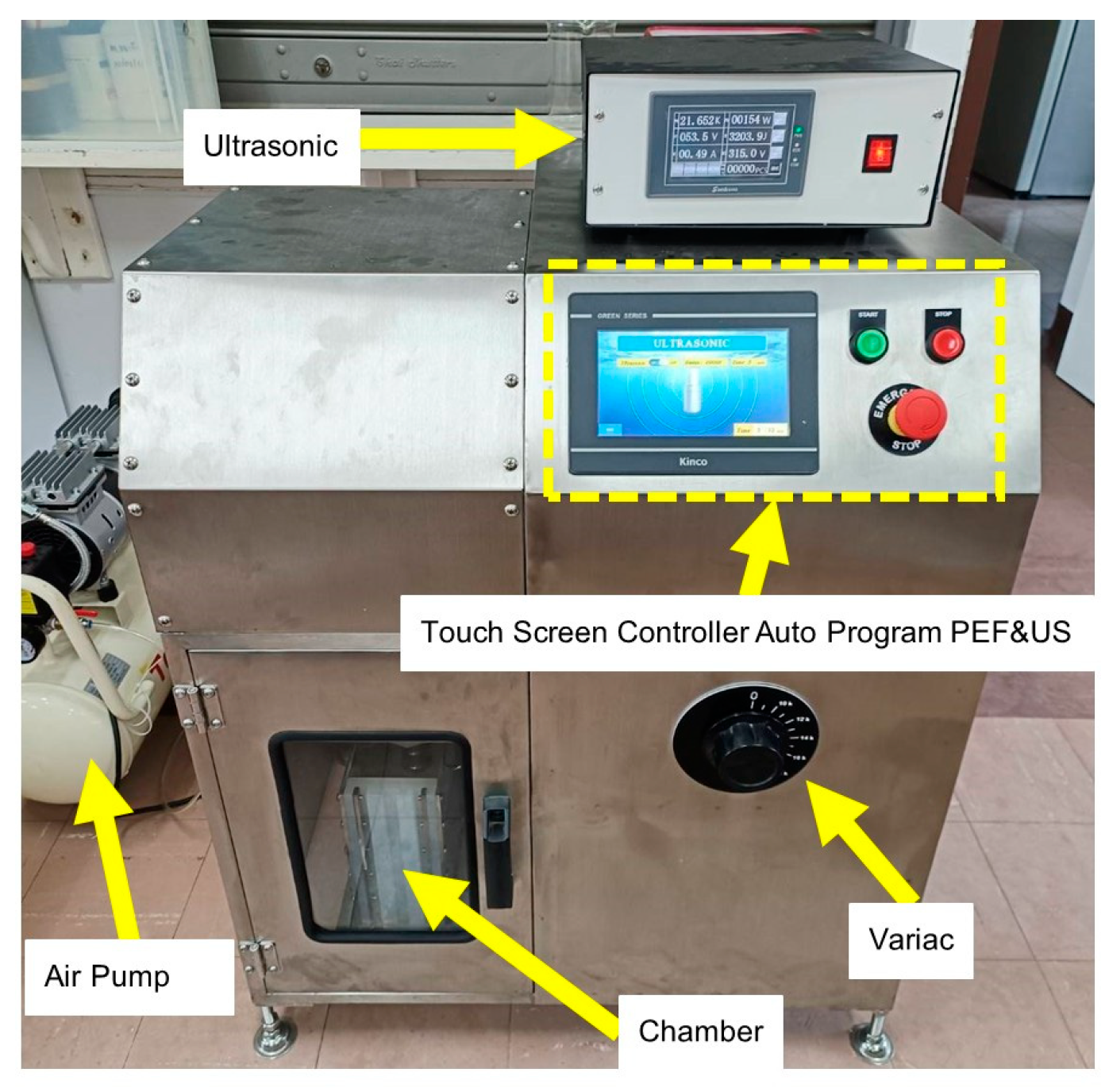
The prototype of PEF-US was designed and built at the Research Center of Methodology in Producing Active Ingredients from Cannabis and Herbs by Bioreactor (RPAH), Payap University, Chiang Mai, Thailand (
Figure 1). The PEF-US equipment layout consisted of a high-voltage generator with a 20 kV of a maximum voltage, a pulse generator, an ultrasonic horn, a chamber, and an air pump. This device can be operated in four modes: PEF, US, US+PEF, and PEF+US.
The voltage, frequency, pulse duration, and ultrasonic conditions were all controlled via the digital touchscreen. A polypropylene enclosure and two stainless steel electrodes make up the treatment chambers. Inside the treatment chamber, cat- and an-ion electrodes were arranged in parallel, separated by a 100 mm interval. The chamber capacity was 5 L. The US was operated with maximum power of 300 W at the frequency of 20 kHz. The tip of the US horn placed 50% of the way into liquid.
2.3. Kratom extraction procedures
2.3.1. PEF extraction
The PEF extraction was performed after the sample was added to the treatment chamber. PEF treatment of 1.3 kV/cm was applied to the mixture at a 1 of Hz frequency and with 100 pulses [
17]. The experiment was performed at room temperature (30°C). The temperature of sample was measured after extraction and was 30±1°C on average for PEF extraction. The sample was collected after 20 min and kept at -20 °C until used within 24 h.
2.3.2. US extraction
After transferring the sample into the treatment chamber, the US mode was operated following the modified protocol [
5] by fixing the operation at 20 min. The initial temperature was 30°C, while the final temperature was 33±2°C on average for US extraction.
2.3.3. PEF+US extraction
In the first step, PEF was operated following the condition at 2.3.1, then the auto control operation was switched to the US condition. The final temperature of PEF+US extraction was 33±2°C.
2.3.4. US+PEF extraction
Initially, the US followed with PEF mode was set. The condition was operated following the procedure of 2.3.1 and 2.3.2. The final temperature of US+PEF extraction was 31±2°C.
2.3.5. Maceration extraction
The mixture of sample and extraction solvent was stood at room temperature (30±2°C) for 24 h. This sample was a control sample.
2.4. Liquid chromatography analysis of kratom extracts
Mitragynine content was determined using a UHPLC model QSight LX50 and an LC-MS/MS model QSight 110 (PerkinElmer, MA, USA). Before injection, the samples were filtered via a 0.22 m nylon filter. The gradient program for mobile phase A (water containing 0.1% Formic Acid) and mobile phase B (methanol) was adjusted at 0–1-minute, 25% B; 1–3-minute, 30% B; and 3–4-minute, 25% B. A 10 L needle and a 20 L loop were connected to the solvent delivery module. The MS operation was set at 350 psi nebulizer pressure, 5000 V electrospray voltage, and 340 °C source temperature. The drying gas was set to 120 degrees Celsius, while the HSID was set at 320 °C. In positive mode, the mass scan mode was done from 100 to 1000 m/z. A Quasar SPP C18 column (100 2.1 mm, 2.6 m-PerkinElmer, Buckinghamshire, UK) was used to separate mitragynine at 40 °C. At 1.22 min, a mitragynine standard (1.0-5.0 ppm) (Sigma-Aldrich, St Louis, MO, USA) was eluted. The aforementioned literatures were used to identify MS/MS data [
6,
7,
8,
18].
2.5. Extraction efficiency
The extraction efficiency was calculated in comparison with the maceration technique using the equation:
where:
M0 = mitragynine content of control (mg/L)
Mt = mitragynine content of sample using PEF-US apparatus mode (mg/L)
2.6. Energy comsumption dertermination
The amount of electricity consumed under US extraction was measured by an electricity meter (OKELE, Wenzhou, China) and calculated according to the equation (2).
where E is the energy measured in J or kWh,
P is the power (W),
t is the time (h).
The energy consumption under PEF was calculated using equations (3) and (4) [
19].
where E
J is Joule energy (J),
N is the number of applied pulses,
V(t) is the voltage across the treatment cell (kV/cm),
tp is the pulse duration (s),
R is the resistance (Ω),
d is the separation between the two electrodes (cm),
σ is the conductivity of the liquid suspension (mS/cm),
A is the cross-sectional area of the exposed electrode surface (cm2).
2.7. Scanning Electron Microscopy
The morphology of kratom powder was observed using scanning electron microscopy (SEM; PentaFETTM precision, X-act, Oxford Instruments, Abingdon, UK). The powder was placed on SEM stubs using double-sided tape and examined at a magnification of 4000x, with an accelerating voltage of 10 kV.
2.8. Fourier Trasnformed Infrared Spectroscopy
Fourier Transform Infrared Spectroscopy (FTIR) was performed to detect the functional groups in the extract using an Infrared Spectrophotometer (Jasco FTIR Analyzer, FT/IR-4700, Jasco, Japan). The spectrum was analyzed in the range of 500 to 3500 cm-1, with a resolution of 4 cm-1.
2.9. Statistical Analysis
All experiments were done at least three times. The mean and standard deviation (S.D.) of the experimental values were provided. SPSS (SPSS Inc., Chicago, IL, USA) was used to assess the differences across extraction modalities using a one-way ANOVA test. The threshold for a meaningful difference was fixed at p<0.05.
3. Results and Discussion
3.1. Mitragynine content
The mitragynine content (y = 131,610x + 49,454; R
2 = 0.9963) of the different extraction modes of the developed PEF-US apparatus was assessed and expressed as mg/L (
Table 1). The mitragynine content ranged from 73.13 ± 0.40 to 106.63 ± 0.85 mg/L and decreased in this order: PEF + US > US + PEF > PEF > US > maceration. The PEF + US mode exhibited a higher mitragynine content (106.63 ± 0.85 mg/L) than the other modes. The four different modes significantly increased the mitragynine content by 45.81 ± 0.59%, 33.00 ± 1.85%, 23.06 ± 1.87%, and 13.77 ± 0.47%, respectively, compared to the control. From these results, it is evident that the mitragynine content of advanced methods (PEF, US, US + PEF, and PEF + US) is superior to conventional methods. However, the combination of PEF and US techniques could increase the mitragynine content by 8.08 - 18.48% for PEF alone and 16.91 - 28.16% for US alone. These findings supported the findings of Parniakov et al. [
20] and [
21], who demonstrated that combining PEF or US-pretreated microalgae suspensions in a binary mixture of water and organic solvent improved the pigment yields and extraction efficiency of microalgae Nannochloropsis spp. Furthermore, the combination of US and PEF might boost virgin olive oil extraction yield from 16.3% to 18.1% [
15].
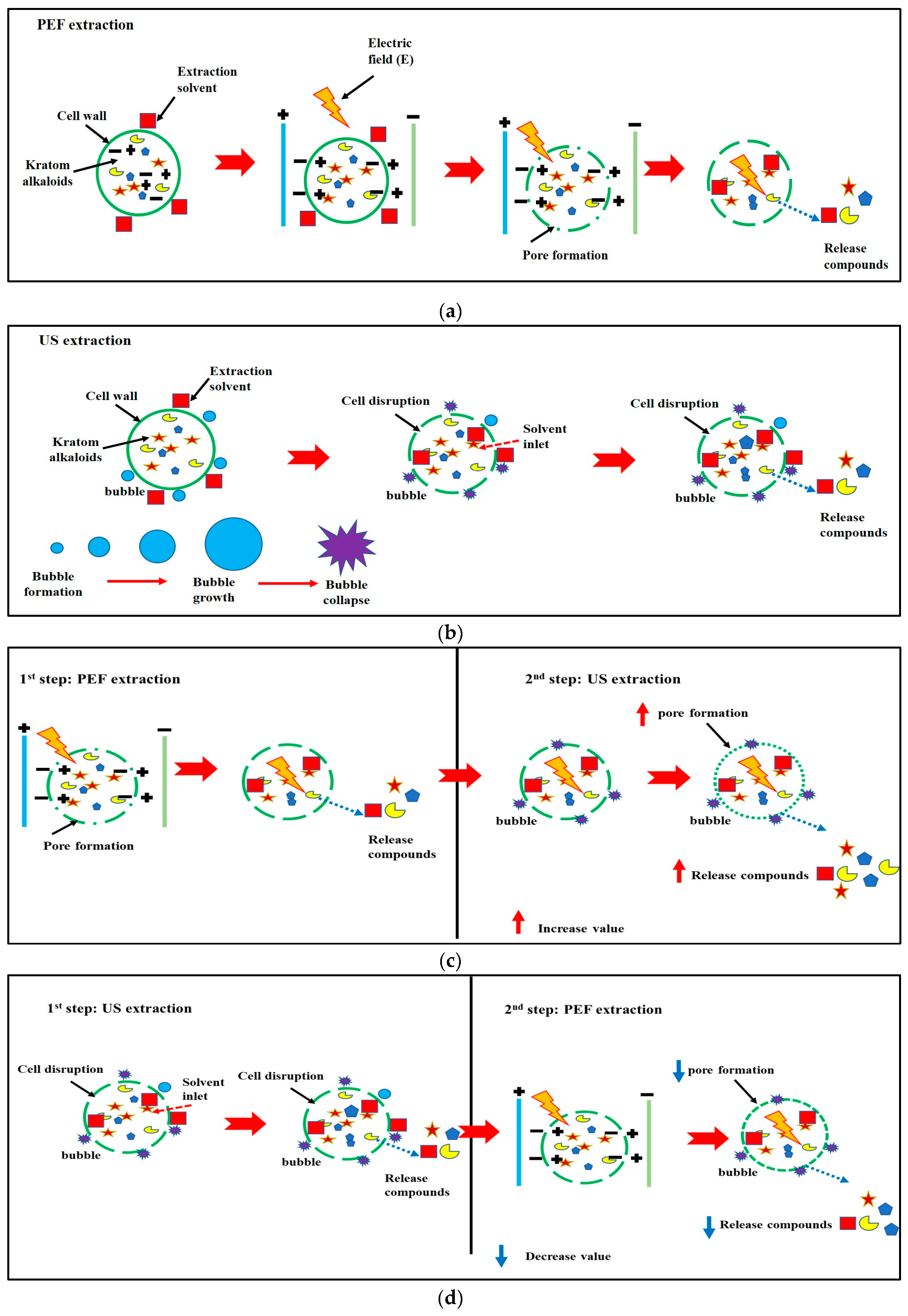
The efficiency of PEF in assisting with the extraction processes can be explained by the electroporation of the cell membranes (electrically induced pore formation of the membranes) [
22], as shown in
Figure 2a. When an electric field is applied, cell membranes act like capacitors with a low dielectric constant. As a result, the membrane becomes thinner due to electrostatic attraction between opposite charges that results from the growing accumulation of charges across the membrane. Trans-membrane pores form when the membrane breaks down and the critical breakdown voltage is attained by an additional increase in the external field strength [
22]. According to Kumari et al. [
22] PEF-assisted extraction entails the use of brief pulses of moderate electric power (about 0.5–20 kV/cm). These PEF intensities are thought to be an efficient pretreatment technique for improving extraction yields [
23].
During the rarefaction phase, ultrasound waves with frequency larger than 20 kHz created negative pressure, resulting in the creation of cavitation bubbles from the solvent's gas nuclei [
22]. These bubbles grew over a number of cycles until they became unstable and finally violently collapsed/exposed, which was called the acoustic cavitation phenomenon (
Figure 2b). It also generates powerful micro-streaming currents that can damage the cell wall, resulting in greater diffusion and a faster mass transfer rate, which leads to better biological chemical release [
22].
Due to the application of 1.3 kV/cm of PEF intensities before US extraction, the PEF procedure acted as a pretreatment method. Therefore, the application of US could increase the extraction efficiency by increasing pore formation and enhancing solvent diffusion (
Figure 2c). While the use of ultrasound followed by the PEF technique (
Figure 2d) exhibited a mitragynine content of 97.27 ± 1.33 mg/L, which decreased efficiency by 12% in comparison with the PEF + US method, this phenomenon might be caused by irreversible electroporation, which causes mechanical breakdown of the cell membrane and renders cells unviable [
20,
22].
3.2. Energy consumption
The total energy required to extract mitragynine from kratom leaves was 4.94 ± 0.31 kJ/kg for PEF alone, 1.03 ± 0.01 kJ/kg for US alone, 3.72 ± 0.13 kJ/kg for PEF + US, and 3.64 ± 0.02 kJ/kg for US + PEF (
Table 1). Thus, the combination of PEF and US can benefit mitragynine extraction by reducing energy consumption. Therefore, it might be potential to apply in industrial extraction as a green technology. Moreover, the energy consumption under this prototype was equivalent to the extraction of aromatic plants [
24] and rosemary [
25] by microwave extraction at 4.2 kJ/kg.
3.3. Change of surface structure
SEM analysis was used to confirm the deformation of
M. speciosa surface after the application of different extraction techniques of the prototype (
Figure 3). Conventional extraction showed closed cells and a rough surface (
Figure 3a). After being subjected to different extraction modes, the physical modification of
M. speciosa cell wall could be noticed. The use of PEF formed a porous layer on the surface of M. speciosa (
Figure 3b), which was caused by the electroporation of PEF, which strengthened the electric field (cations and anions) on the plant cell wall [
26]. While the use of US changed rough the to be surface (
Figure 3c). Thus, a porous and smooth surface could be found when PEF was used before US extraction (
Figure 3d). The SEM micrographs of the sample after US+PEF (
Figure 3e) were not considerably different from those of US samples (
Figure 3c), but only minor damage was observed on the external surface. This indicates that US and PEF treatments affect the structure of the cell due to the high, localized pressures induced by cavitation of US and the electroporation of PEF. Therefore, the mitragynine content was related to the physical modification of
M. speciosa surface. According to Xing et al. [
27], the considerable level of cell wall breakdown increased the release of target chemicals from the plant matrix and improved extraction efficiency.
3.4. Fourier infrared spectroscopy analysis
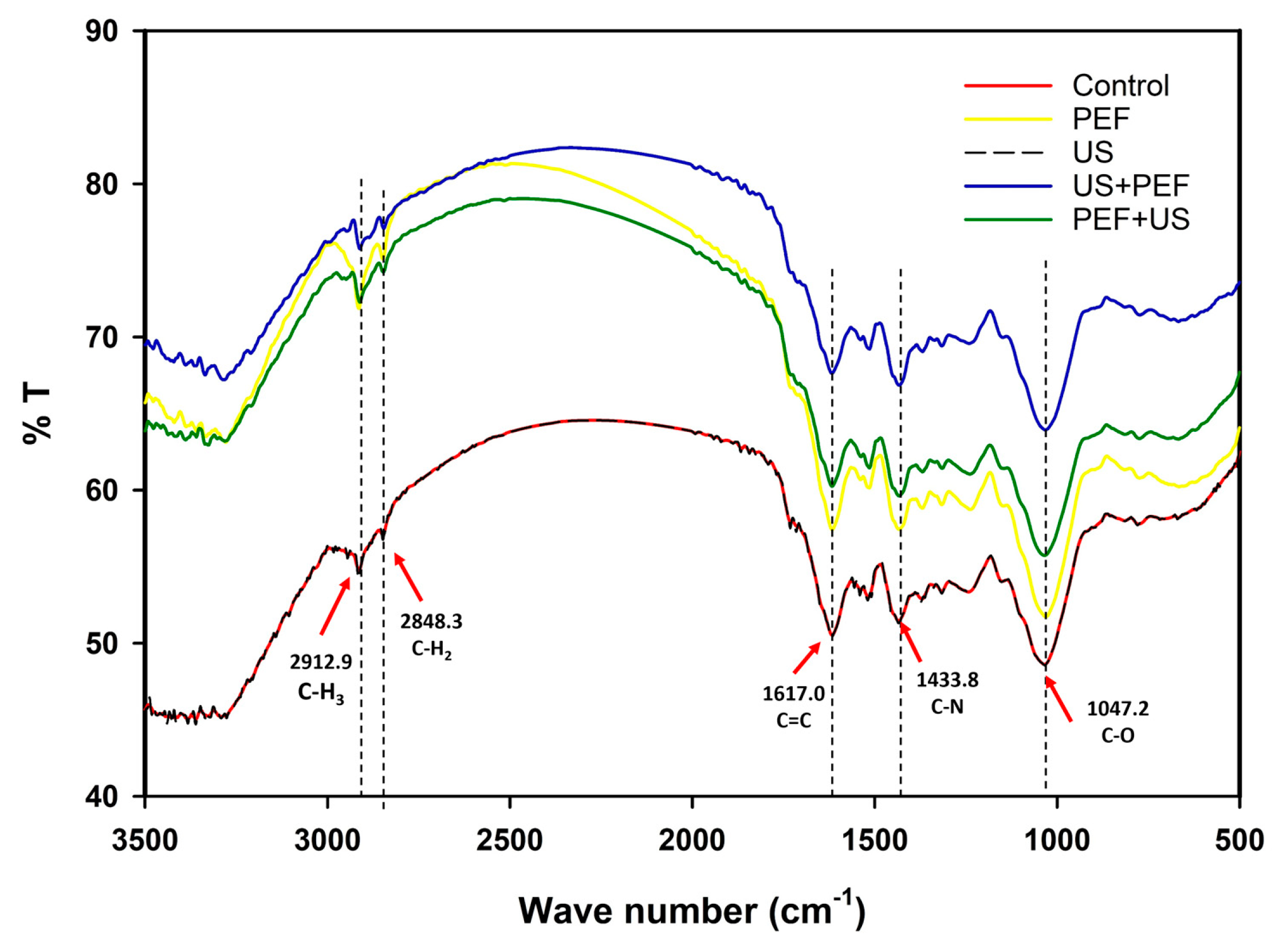
Figure 4 illustrates the FTIR functional group of M. speciosa powder extracted with various conditions of the prototype in the range of 500 to 3500 cm
-1. Peaks at 2912.9 and 2848.3 cm
-1 were attributed to C-H stretching vibrations, which are present due to the polyphenolic compound and TPC concentration. At 1617.0 cm
-1, the absorption bands correspond to the C=C in aromatic groups. The ring C-C stretching vibrations occur in the bands 1625–1430 cm
−1. The distinctive band at 1047.2 cm
-1 can be associated with C-O deformation of phenolic compounds [
28]. The weak C-H, C=C, C-C, and C-O were attributed to the higher amount of active molecules being disintegrated into extraction solvents. The use of the combined US and PEF methods This phenomenon was consistent with the mitragynine content, as presented in
Table 1.
3.5. LC-MS/MS profiles
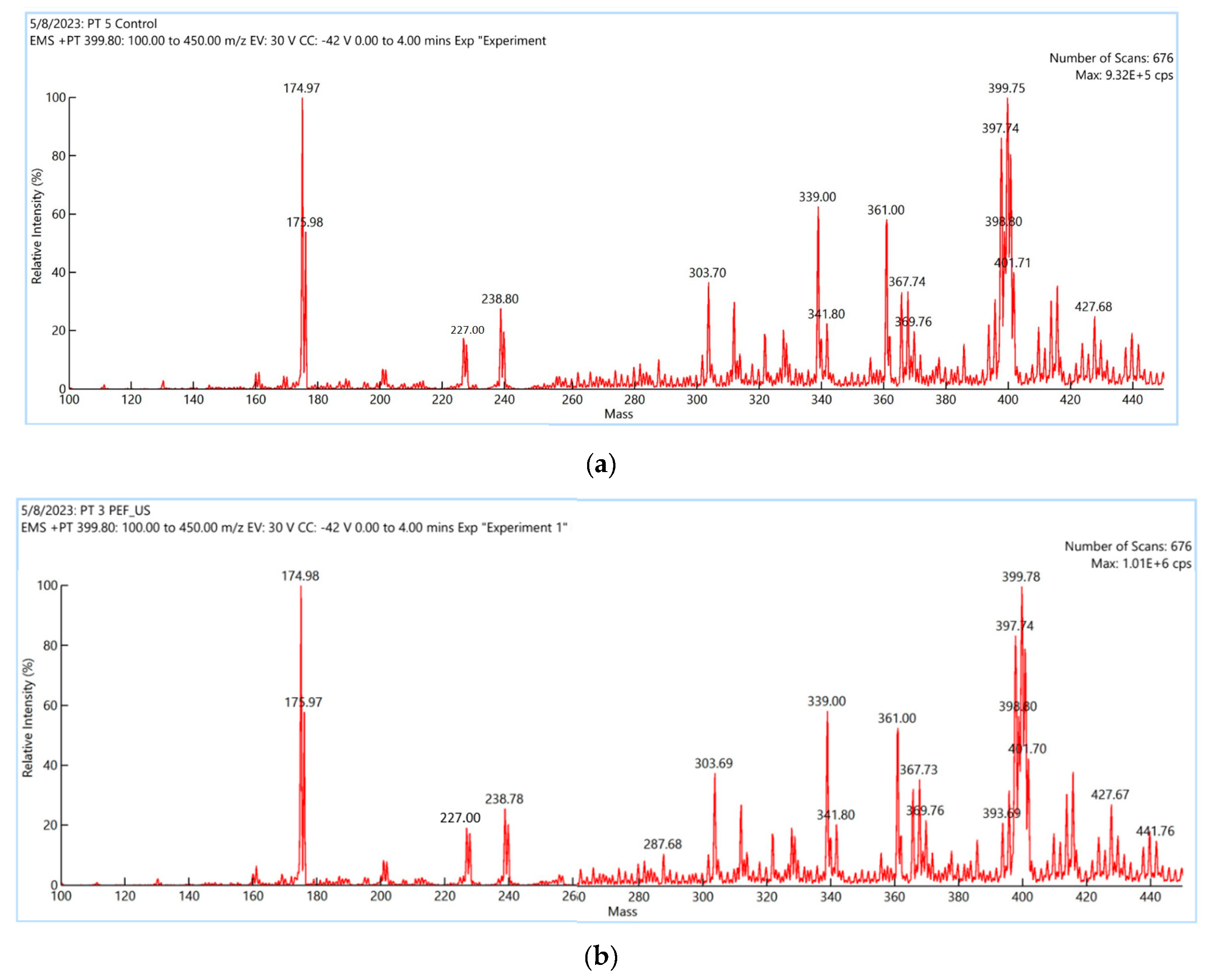
Mass spectrum of
M. speciosa leaves extracted by maceration and PEF + US was showed in
Figure 5. A similar pattern was followed for the control and PEF + US extraction.
Table 2 summarizes the identification of the mitragynine confirmed with the published literatures [
6,
7,
8,
18]. The precursor ion and product ion of mitragynine were m/z 399.75→ 174.97 for maceration extraction and 399.78→ 174.98 for PEF + US PEF + US extracts. The fragmentation pattern was helpful tool to resolve unknow alkaloids in the
M. speciosa extracts.
4. Conclusions
The purpose of this research was to establish whether the combination of PEF and US equipment could be used to produce plant extracts. During this investigation, four different mechanisms of mitragynine synthesis from dried M. speciosa leaves were employed. Regarding the evaluation of extractability, the PEF + US device has proved to be very efficient in extracting mitragynine from the dried leaves of kratom, significantly increasing the efficiency of mitragynine extracted compared to the control. Together with a higher mitragynine content, less energies were required to extract if PEF and US were input in a contact way. The physical modification in the kratom leaves obtained by the different techniques were evaluated using SEM and FTIR analysis. However, developing low-cost, environmentally friendly extraction methods that yield a viable extract rich in bioactive chemicals remains a difficult task. Therefore, further work needs to be done to focus on the impact of PEF and US parameters on cell wall material of kratom leaves to better improve the alkaloid compounds, especially mitragynine content.
5. Patents
The prototype of pulsed electric field and ultrasonic (PEF-US) apparatus is the subject of the Petty Patent No. 2303000855, with the title Extraction processing of mitragynine from kratom leaves using the combination of pulse electric field and ultrasonic technique (in Thai), released by Department of Intellectual Property, Thailand on 27.03.2023.
Author Contributions
Conceptualization, S.S. and A.K.; methodology, S.S. and A.K.; validation, A.K. and T.P.; investigation, P.J., R.J., N.P. and R.H.; resources, P.K.; data curation, A.K., R.J., N.P. and R.H.; writing—original draft preparation, R.J., A.K. and N.P.; writing—review and editing, A.K., P.J., N.P., S.S. and T.P.; visualization, A.K., S.S. and T.P.; supervision, S.S. and T.P.; project administration, S.S. and T.P.; funding acquisition, P.K and T.P.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This study was supported by APA industries Co., Ltd. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Trakulsrichai, S.; Sathirakul, K.; Auparakkitanon, S.; Krongvorakul, J.; Sueajai, J.; Noumjad, N.; Sukasem, C.; Wananukul, W. Pharmacokinetics of mitragynine in man. Drug Des. Devel. Ther. 2015, 9, 2421–2429. [Google Scholar] [CrossRef] [PubMed]
- Eastlack, S.C.; Cornett, E.M.; Kaye, A.D. Kratom—pharmacology, clinical implications, and outlook: A comprehensive review. Pain Ther. 2020, 9, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Fluyau, D.; Revadigar, N. Biochemical Benefits, Diagnosis, and Clinical Risks Evaluation of Kratom. Front. Psychiatry 2017, 8, 1–8. [Google Scholar] [CrossRef]
- Orio, L.; Alexandru, L.; Cravotto, G.; Mantegna, S.; Barge, A. UAE, MAE, SFE-CO2 and classical methods for the extraction of Mitragyna speciosa leaves. Ultrason. Sonochem. 2012, 19, 591–595. [Google Scholar] [CrossRef]
- Zakaria, F.; Tan, J.-K.; Mohd Faudzi, S.M.; Abdul Rahman, M.B.; Ashari, S.E. Ultrasound-assisted extraction conditions optimisation using response surface methodology from Mitragyna speciosa (Korth.) Havil leaves. Ultrason. Sonochem. 2021, 81, 105851. [Google Scholar] [CrossRef]
- Karunakaran, T.; Goh, Y.S.; Santhanam, R.; Murugaiyah, V.; Abu Bakar, M.H.; Ramanathan, S. RP-HPLC-DAD analysis of mitragynine content in Mitragyna speciosa Korth. (Ketum) leaf extracts prepared using ultrasound assisted extraction technique and their cytotoxicity. Separations 2022, 9, 345. [Google Scholar] [CrossRef]
- Limcharoen, T.; Pouyfung, P.; Ngamdokmai, N.; Prasopthum, A.; Ahmad, A.R.; Wisdawati, W.; Prugsakij, W.; Warinhomhoun, S. Inhibition of alpha-glucosidase and pancreatic lipase properties of Mitragyna speciosa (Korth.) Havil. (Kratom) leaves. Nutrients 2022, 14, 3909. [Google Scholar] [CrossRef] [PubMed]
- Goh, Y.S.; Karunakaran, T.; Murugaiyah, V.; Santhanam, R.; Abu Bakar, M.H.; Ramanathan, S. Accelerated solvent extractions (ASE) of Mitragyna speciosa Korth. (Kratom) leaves: evaluation of its cytotoxicity and antinociceptive activity. Molecules 2021, 26, 3704. [Google Scholar] [CrossRef]
- Soquetta, M.B.; Terra, L.d.M.; Bastos, C.P. Green technologies for the extraction of bioactive compounds in fruits and vegetables. CyTA - J. Food 2018, 16, 400–412. [Google Scholar] [CrossRef]
- Bocker, R.; Silva, E.K. Pulsed electric field assisted extraction of natural food pigments and colorings from plant matrices. Food Chemistry: X 2022, 15, 100398. [Google Scholar] [CrossRef]
- Toepfl, S.; Mathys, A.; Heinz, V.; Knorr, D. Potential of high hydrostatic pressure and pulsed electric fields for energy efficient and environmentally friendly food processing. Food Rev. Int. 2006, 22, 405–423. [Google Scholar] [CrossRef]
- Toepfl, S.; Heinz, V.; Knorr, D. High intensity pulsed electric fields applied for food preservation. Chem. Eng. Process.: Process Intensif. 2007, 46, 537–546. [Google Scholar] [CrossRef]
- Ranjha, M.M.A.N.; Kanwal, R.; Shafique, B.; Arshad, R.N.; Irfan, S.; Kieliszek, M.; Kowalczewski, P.Ł.; Irfan, M.; Khalid, M.Z.; Roobab, U.; et al. A Critical Review on pulsed electric field: A novel technology for the extraction of phytoconstituents. Molecules 2021, 26, 4893. [Google Scholar] [CrossRef] [PubMed]
- Lal, A.M.N.; Prince, M.V.; Kothakota, A.; Pandiselvam, R.; Thirumdas, R.; Mahanti, N.K.; Sreeja, R. Pulsed electric field combined with microwave-assisted extraction of pectin polysaccharide from jackfruit waste. Innovative Food Science & Emerging Technologies 2021, 74, 102844. [Google Scholar] [CrossRef]
- Grillo, G.; Boffa, L.; Calcio Gaudino, E.; Binello, A.; Rego, D.; Pereira, M.; Martínez, M.; Cravotto, G. Combined Ultrasound and Pulsed Electric Fields in Continuous-Flow Industrial Olive-Oil Production. Foods 2022, 11, 3419. [Google Scholar] [CrossRef]
- Nowosad, K.; Sujka, M.; Pankiewicz, U.; Kowalski, R. The application of PEF technology in food processing and human nutrition. J. Food Sci. Technol. 2021, 58, 397–411. [Google Scholar] [CrossRef]
- Shiekh, K.A.; Olatunde, O.O.; Zhang, B.; Huda, N.; Benjakul, S. Pulsed electric field assisted process for extraction of bioactive compounds from custard apple (Annona squamosa) leaves. Food Chem. 2021, 359, 129976. [Google Scholar] [CrossRef]
- Avula, B.; Sagi, S.; Wang, Y.-H.; Wang, M.; Ali, Z.; Smillie, T.J.; Zweigenbaum, J.; Khan, I.A. Identification and characterization of indole and oxindole alkaloids from leaves of Mitragyna speciosa Korth using liquid chromatography–accurate QToF mass spectrometry. J. AOAC Int. 2015, 98, 13–21. [Google Scholar] [CrossRef]
- Qin, S.; Timoshkin, I.V.; Maclean, M.; Wilson, M.P.; MacGregor, S.J.; Given, M.J.; Anderson, J.G.; Wang, T. Pulsed electric field treatment of microalgae: Inactivation tendencies and energy consumption. IEEE Transactions on Plasma Science 2014, 42, 3191–3196. [Google Scholar] [CrossRef]
- Parniakov, O.; Barba, F.J.; Grimi, N.; Marchal, L.; Jubeau, S.; Lebovka, N.; Vorobiev, E. Pulsed electric field assisted extraction of nutritionally valuable compounds from microalgae Nannochloropsis spp. using the binary mixture of organic solvents and water. Innov. Food Sci. Emer. Technol. 2015, 27, 79–85. [Google Scholar] [CrossRef]
- Parniakov, O.; Apicella, E.; Koubaa, M.; Barba, F.J.; Grimi, N.; Lebovka, N.; Pataro, G.; Ferrari, G.; Vorobiev, E. Ultrasound-assisted green solvent extraction of high-added value compounds from microalgae Nannochloropsis spp. Bioresour. Technol. 2015, 198, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Kumari, B.; Tiwari, B.K.; Hossain, M.B.; Brunton, N.P.; Rai, D.K. Recent advances on application of ultrasound and pulsed electric field technologies in the extraction of bioactives from agro-industrial by-products. Food Bioproc. Tech. 2018, 11, 223–241. [Google Scholar] [CrossRef]
- Balasa, A.; Janositz, A.; Knorr, D. Electric field stress on plant systems. Encyclopedia Biotechnol. Agric. Food 2011, 2, 208–211. [Google Scholar] [CrossRef]
- Lucchesi, M.E.; Chemat, F.; Smadja, J. Solvent-free microwave extraction of essential oil from aromatic herbs: comparison with conventional hydro-distillation. Journal of Chromatography a 2004, 1043, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Bousbia, N.; Vian, M.A.; Ferhat, M.A.; Petitcolas, E.; Meklati, B.Y.; Chemat, F. Comparison of two isolation methods for essential oil from rosemary leaves: Hydrodistillation and microwave hydrodiffusion and gravity. Food chemistry 2009, 114, 355–362. [Google Scholar] [CrossRef]
- Supasin, S.; Kantala, C.; Intra, P.; Rattanadecho, P. Postharvest preservation of Thai mango var. Chok-Anan by the combination of pulsed electric field and chemical pickling. Horticulturae 2022, 8, 584. [Google Scholar] [CrossRef]
- Xing, C.; Cui, W.-Q.; Zhang, Y.; Zou, X.-S.; Hao, J.-Y.; Zheng, S.-D.; Wang, T.-T.; Wang, X.-Z.; Wu, T.; Liu, Y.-Y.; et al. Ultrasound-assisted deep eutectic solvents extraction of glabridin and isoliquiritigenin from Glycyrrhiza glabra: Optimization, extraction mechanism and in vitro bioactivities. Ultrasonics Sonochemistry 2022, 83, 105946. [Google Scholar] [CrossRef]
- Manik, U.P.; Nande, A.; Raut, S.; Dhoble, S.J. Green synthesis of silver nanoparticles using plant leaf extraction of Artocarpus heterophylus and Azadirachta indica. Results in Materials 2020, 6, 100086. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).