1. Introduction
Bee products contain biologically active principles with an antioxidant, antimicrobial, antiinflammatory, antiviral and antitumor role [
1,
2,
3]. The biological activities of active products are highly variable and depend on many factors, such as floral sources, apiary location, beekeepers' experience, environmental conditions or bee breed [
1]. The variable and complex composition of bee products and the multitude of laboratory methods used to study their antioxidant activities are responsible for the wide range of results reported [
4].
Royal jelly is a bee product resulting from the secretion of the hypopharyngeal and mandibular glands, yel-lowish-white in color, with a sour taste and a viscous-gelatinous consistency, its smell being slightly similar to phenol [
5,
6,
7,
8,
9,
10]. It is produced by nurse bees, aged between 5 and 15 days [
9], being used within the bee family to feed queens and larvae. Its secretion is correlated with the abundance of nectar and pollen collection [
11]. Royal jelly has an acidic pH with values between 3.6-4.2, being mainly composed of water (60%-70%), but also contains proteins (12%), lipids (5%-6%), sugar (12%-15%), small amounts of vitamins and mineral salts, but has a considerable number of amino acids [
5].
Apilarnil is a product obtained from drone larvae harvested at the age of 7 days together with the contents of the cell in which they develop. After harvesting and mixing, the apilarnil is viscous, with fine granulation, white color with shades of gray to brown, characteristic smell of food for brood [
12]. The high content of biologically active substances of apilarnil determines a lot of pharmacological characteristics, especially the antioxidant, immunotropic, adaptogenic and anabolic actions [
13]. The studies carried out by Bognanov, 2015 [
14] showed that drone larvae contain estradiol, prolactin, progesterone and testosterone, showing both estrogenic and androgenic effects.
Propolis is produced by honey bees from resinous substances harvested from various plants, such as leaves, flowers and buds, to which are added glandular secretions of worker bees, residues from the digestion of wax and pollen [
5,
15]. The raw material for its production is harvested mainly from plants such as willow, conifers, beech, poplar, oak, alder, ash, chestnut and others [
16,
17,
18,
19,
20]. Each year, about 100-300 g of propolis can be obtained from the cleaning of the interior of the hives and the frames during the active season, from a family of bees [
11]. It is composed of approximately 50% resins, 30% wax, 10% various essential oils, 5% pollen and 5% various organic compounds, including polyphenols, ethanol, amino acids, vitamin A, minerals, vitamin E complex and vitamin B [
5,
21,
22]. Propolis is used as an antimicrobial agent against bacteria, viruses and other pathogen-ic microorganisms [
23,
24,
25,
26,
27] and has more than 70 pharmaceutical properties. Research was carried out on the activity of propolis and it was found that it can be used as an additive with an antimicrobial, immuno-modulatory, antioxidant, antiinfective, anti-inflammatory, antiparasitic role [
28,
29,
30]. Propolis can be incorporated into food, beverages and cosmetic products, being commercially available in different forms, capsules, creams, throat lozenges, mouthwash solutions [
31].
The main objectives of this study were to evaluate the chemical composition of some bee products collected by us directly from the apiary (royal jelly, apilarnil and propolis) compared to the same raw products purchased commercially, with commercial lyophilized products (royal jelly and apilarnil) and as tincture (propolis). At the same time, we evaluated the antioxidant and antiinflammatory activity of these bee products.
2. Materials and Methods
2.1. Samples of Bee Products
We analyzed the royal jelly, apilarnil and propolis products (
Table 1) obtained in 2022. The analyzed propolis came from the own apiary and from the market, the form of presentation being raw propolis and propolis tincture prepared by macerating 20 g of propolis (from the own apiary) in 100 ml of alcohol. Royal jelly was tested in freezedried and pure form with commercial and freshly harvested origin. Apilarnil was subjected to research in the same forms as royal jelly but the lyophilized apilarnil was obtained by lyophilizing commercial pure apilarnil using the Unicryo MC4L -60˚C lyophilizer (Uniequip Germany). The apiary chosen for collecting samples in fresh form is located in the town of Caransebeș, Banat region, Romania (45024`48.6``N22012`53.7``E). All samples were stored in glass containers, in a refrigerator, at a temperature of 0±5⁰C. Analyzes were performed at the Interdisciplinary Research Platform (PCI) belonging to the University of Life Sciences “King Mihai I” from Timisoara.
2.2. Determination of Humidity
It was made according to the SR-784-3 standard by the oven drying method. From each sample, 2 determinations were made in which 3 g/sample were weighed for all bee products except for commercial royal jelly (lyophilized) for which 1.5 g/sample were examined and dried in an oven (BINDER GmbH, Tuttingen, Germany) at a temperature of 103±2⁰C until constant mass. After 4 hours, the samples were removed from the oven, cooled and weighed, and later the result was calculated according to the following formula [
32,
33]:
where:
G1—the weight of petri dish and sample before drying (g);
G2—the weight of petri dish and sample after drying (g);
G—the weight of Petri dish (g).
2.3. Determination of Dry Matter
The determination was made after obtaining the moisture percentages according to the formula:
2.4. Determination of Acidity
To determine the acidity, the standardized method SR 784-3 was used, so in each sample of bee products (1 g/sample) we added 5 ml of water and 2 drops of phenolphthalein, and then they were introduced into the DLAB shaker, SK-L330 -PRO (China) for 30 minutes. After homogenization, the samples were filtered through filter paper and titrated with sodium hydroxide solution 0.1 n until a persistent pink color, for 30 seconds [
33,
34]. The temperature of the room where the acidity of the samples was determined was between 23⁰-24⁰C, and to calculate and express the results we used the formula:
where:
V—represents the volume of sodium hydroxide solution used in the titration (mL);
0.1—represents the normality of sodium hydroxide solution used for titration.
2.5. Determination of pH
The inoLab pH 720 pH meter (Xylem Analytics, Weilheim, Germany) was used to determine the pH, and the amount of bee products used was 1 g/sample. They were dissolved in 30 ml of water and homogenized with the DLAB shaker (SK-L330-PRO, China) for 30 minutes [
34,
35]. The room temperature at which the pH of the propolis, royal jelly and apilarnil samples was determined was between 23⁰-24⁰C, and the pH working range was -2.000 ± 19.999, with accuracies of ± 0.005.
2.6. Determination of Impurities
It was carried out according to the SR-784-3:2009 standard, so that for the samples of propolis, royal jelly and apilarnil, 1 g/sample was weighed, dissolved in 10 ml of water and homogenized using the DLAB shaker (SK-L330 -PRO, China), for 30 minutes. Afterwards the filter papers were prepared and weighed, and after weighing the solutions were filtered. The samples obtained after filtration were placed in the oven at a temperature of 103⁰C for 10 minutes to dry the filter paper, then they were weighed. The content of impurities was expressed in percentages and was determined according to the formula:
where:
I—represents the quantity of impurities (%);
m₁—represents the mass of the sample taken for analysis (g);
m₂—represents the mass of residue left on the filter paper after drying (g).
2.7. Determination of Sugars
1.5 g of each sample of bee products were weighed, and with the help of a volumetric flask it was quantitatively brought to 100 ml with water, then it was stirred well for 30 minutes with the help of the DLAB shaker (SK-L330-PRO, China). From the solution obtained, the working solution was prepared by pipetting 10 ml of the previously prepared solution into graduated flasks, and then it was completed with water up to the 50 ml mark of each flask. After these stages, 10 ml of copper sulfate solution was introduced into 150 ml containers and proportional in number to the number of samples, together with 10 ml of alkaline Seignette salt solution and 10 ml of water. It was homogenized with the DLAB shaker (SK-L330-PRO, China). The containers were placed on the flame of a Bunsen bulb and the boiling temperature was expected to be reached, when 10 ml of the working solution was introduced with a pipette and the boiling process was expected to resume for 5 minutes, a measured time. After this time, the containers were immediately cooled in water, 15 ml of sodium chloride solution was added to each and the homogenization process was resumed by stirring in the DLAB device (SK-L330-PRO, China). To neutralize the hydrochloric acid, 1.5 g of sodium bicarbonate was added, and the color of the solution changed from blue-green to intense blue, and further it was titrated with iodine solution (0.05 n) under manual homogenization until the liquid became clear, and the green color showed no tendency to return to blue, thus suggesting that the titration at that point was complete, and we could note the amount of iodine solution used.
For the determination of excess iodine, 0.25 ml of starch solution was added and then titrated drop by drop with sodium thiosulphate (0.05 n) until the dark blue color suddenly changed to light blue. The amount of iodine used for oxidation was determined by subtracting the amount of iodine solution used in the titration from the amount of the thiosulphate solution used in the back titration.
The results were expressed as a percentage using the formula:
where:
C invert sugar — represents the amount of inverted sugar
m – the amount of inverted sugar
m1 – the amount of bee products used
5 – the ratio between the volume of the solution in the 100 ml volumetric flask and the volume of the solution used in the titration
2,5 – the ratio of the volume of the solution in the 50 ml volumetric flask to the volume of the diluted solution used for analysis
The amount of inverted sugar (m) in milligrams was determined using
Table 2, where it corresponds to the volume of iodine used to oxidize the cuprous cation.
2.8. Determination of Protein
The protein content was determined for royal jelly and apilarnil samples (0.5 g in 25 mL distilled water). The obtained solutions were filtered with filter paper in glass tubes. The protein content was assayed according to the Lowry method, using the Folin-Ciocalteu’s phenol reagent and bovine serum albumin (BSA) as standard [
36,
37]. The colour reaction was carried out using the clear solutions of royal jelly and apilarnil obtained previously, to which was added alkaline solution (containing NaOH, Na2CO3, sodium potassium tartrate and CuSO4) and Folin Ciocalteu reagent. The extinction was measured by using a T 60U Spectrometer (PG Instruments Ltd) at 660 nm against the blank solution. The protein content was calculated by using the equation of the calibration line and was calculated according to the formula:
where:
p—represents the quantity of protein (mg protein/ml)
Emed—represents the average extinction of duplicate samples;
Eblank —represents the extinction of blank
10 - the correction factor for expressing the protein content per one ml sample
50 - the correction factor for expressing the protein content per one g sample
2.9. Determination of Ash
It was determined according to the SR 784-3:2009 standard. The empty crucibles required for each sample were kept at a temperature of 525˚C for two hours in the calcination furnace (190945, Nabertherm Lilienthal, Germany), and later they were cooled and submitted to weighing. In the next step, 1 g samples of each bee product were weighed, placed in the cooled crucibles, and later the crucibles were reintroduced into the calcination furnace (190945, Nabertherm Lilienthal, Germany) and were calcined up to a temperature of 525˚C. After the completion of calcination, the crucibles were cooled and weighed individually.
The ash content is expressed as a percentage and determined according to the formula:
where:
Cash = total ash content
m – the mass of the crucible with ash after the calcination process, in grams;
m1 – the mass of the empty crucible, in grams;
m2– the mass of the crucible with the bee products, before the calcination process, in grams.
2.10. Determination of Mineral Substance Content (Ash)
In the calcined samples to determine the ash, 10 ml of hydrochloric acid was added to each sample, after which the samples thus obtained were placed in glass test tubes to determine the content of microelements and macroelements. 18 graduated flasks of 50 ml were prepared (double determinations were made for each beekeeping product), the samples were filtered and filled with double-distilled water up to the 50 ml mark of each graduated flask. Centipur Merk multielement standard solution was used for calibration, and the determinations were made by the atomic absorption spectrometry technique, with the help of a VARIAN 240 FS spectrophotometer (U.S.A). The working conditions of the device were: air:acetylene ratio of 13.50:2, and the absorption rate of the nebulizer: 5ml/min. Each item was read according to
Table 3.
2.11. Determination of Total Amino Acids
For the determination of total amino acids, 18 containers with lids were prepared, which correspond to the 2 determinations of each bee product. 1 g/sample was weighed into each plastic container, over which 24 ml of 70% alcohol was added, thus resulting in a dilution of 1:250 for all bee products. They were homogenized with the DLAB shaker (SK-L330-PRO, China) for 30 minutes, and then they were filtered with filter paper and 1 ml of the resulting extract was pipetted into a glass tube for each of the 18 assays. 0.5 ml of Phosphate buffer solution (ph – 8.04) 1/15 mol/L + 0.5 ml Ninhydrin (2% + 0.8 mg/ml SnCI2*2H2O) was added to each test tube, after they stirred with the help of the DLAB shaker (SK-L330-PRO, China) for 30 minutes, being later placed in the oven (BINDER GmbH, Tuttingen, Germany), at a temperature of 103±2⁰C, for 10 minutes. After removing the test tubes from the oven, each one was filled with distilled water up to 10 ml and homogenized manually. Finally, they were left for 15 minutes at rest, after which the absorbance was read at 570 nm using the UV-VIS spectrometer (Analytical Jena Specord 205, Jena Germany). All determinations were made in triplicate, and the values were expressed based on the calibration curve.
2.12. Determination of Total Phenolic Content (TPC)
From the bee products: „LMS”; „LML”; „LMC” and „AP”, 1 g/sample was weighed in a plastic container with a lid, over which 10 ml of 70% alcohol was added, thus resulting in a dilution of 1:10. Also from the bee products: „PS”; „PC”; „PT”; „AS” and „ALC” dilutions of 1:250 were used to be able to take UV-VIS spectrometer readings (Analytical Jena Specord 205, Jena, Germany). The samples were homogenized with the DLAB shaker (SK-L330-PRO, China) for 30 minutes, and then they were filtered with filter paper and 0.5 ml of each filtered sample was pipetted into a glass test tube. The titration process followed with 1.25 ml Folin-Ciocalteu reagent (Sigma-Aldrich Chemic GmbH, München, Germania), diluted 1:10 with distilled water and placed in each test tube from those previously prepared. The 18 prepared samples (2 of each bee product) were incubated for 5 minutes at room temperature, 1 ml of Na₂CO₃ (60g/L aqueous solution) was added to each and then placed in the incubator (Memmert GmbH, Schwabach, Germany) at 50⁰C for 30 minutes. After this period, the absorbance was read at 750 nm with a UV-VIS spectrometer (Analytical Jena Specord 205, Jena, Germany), using the ethanol solution as a control, and the calibration curve of the device was obtained by using gallic acid (concentration range: 2.5- 250 μg/mL). All determinations were made in triplicate, and the results obtained were expressed in mg GAE/g dry substance (d.m.).
2.13. Determination of Flavonoid Content (FC)
For the determination of flavonoids, 18 containers with lids were prepared (corresponding to the 2 determinations of each bee product) in which the following dilutions were made: 1:10 for the bee products „LMS”; „.LML”; „LMC” and „AP”, where 1 g/sample was weighed in a plastic container with a lid, over which 10 ml of 70% alcohol was added, and for the bee products „P21”; „PC”; „PT”; „AS” and „ALC” dilutions of 1:250 were used, i.e. 24 ml of 70% alcohol was added to 1 g/sample, in order to be able to take readings with a UV-VIS spectrometer (Analytical Jena Specord 205, Jena, Germany). They were homogenized with the DLAB shaker (SK-L330-PRO, China) for 30 minutes, and then filtered with filter paper and 1 ml of the resulting extract was pipetted together with: 4 ml H₂O, 0.3 ml NaNO₂5%, 0.3 ml Al(NO3)3 10% and 2 ml NaOH1M in glass tubes, after which they were homogenized and 70% alcohol was added up to 10 ml, and finally the samples were left for 15 minutes at rest. The absorbance was read at 510 nm using the UV-VIS spectrometer (Analytical Jena Specord 205, Jena, Germany). 70% ethyl alcohol was used as a control sample, and quercitin solution in the concentration range 5-100 ug/ml was used for the calibration curve, and the results were expressed in mg QUE/1g. All determinations were made in triplicate.
2.14. Determination of the Antioxidant Capacity by DPPH (2,2-diphenyl-1-picrylhydrazyl) Assay
DPPH (2,2-diphenyl-1-pyrylhydrazyl) test for antioxidant capacity is a commonly used classical method for different types of extracts. The analysis was performed according to the method described by Duca et al. (2019) [
38] with minor modifications. Thus from each sample 5 alcoholic extracts of different concentrations (0.5; 1; 2.5; 5 and 10 mg/mL) were prepared. To make the extracts, 70% ethyl alcohol was used. Thus in the present study, 0.5 mL of each alcoholic extract prepared was tak[
1]en from each extract and 2.5 mL of 0.3 mM DPPH solution in ethanol (Calbiochem®, EMD Millipore Corp., Billerica, MA, USA, lot: D00174004). The samples were left for 30 min in the dark at room temperature and then the absorbance was read at 518 nm using a UV-VIS spectrophotometer (Analytic Jena Specord 205). In parallel, a control sample was also made in which the extract was replaced with 70% ethyl alcohol. Ascorbic acid 0.16 mg/mL in 70% (v/v) ethanol was used as a positive control. Ascorbic acid was purchased from Lach-Ner Company (Czech Republic). Radical scavenging activity (RSA) was calculated with the formula: RSA (%) = (A control - A sample)/(A control) × 100), where RSA = radical scavenging activity of the extract (%), A control = absorbance of the control sample; A sample = absorbance of the sample measured at 518 nm. The antioxidant capacity of the extracts was expressed as IC50 value and compared with that of ascorbic acid.
2.15. Determination of the Antimicrobial Activity
The antimicrobial activity of the samples was determined by broth microdilution against Gram-positive, Gram-negative and fungal ATTC strains.
The ATTC strains used in the present study were obtained from the Laboratory of Microbiology culture collection in the Interdisciplinary Research Platform within the University of Life Sciences "King Mihai I of Romania" Timisoara. The tested strains were: Streptococcus pyogenes (ATCC 19615), Staphylococcus aureus (ATCC 25923), Shigella flexneri (ATCC 12022), Pseudomonas aeruginosa (ATCC 27853), Escherichia coli (ATCC 25922), Salmonella typhimurium (ATCC 14028), Haemophilus influenzae tip B (ATCC 10211), Bacillus cereus (ATTC 10876), C. perfringens (ATTC 13124), C. albicans (ATCC 10231), and C. parapsilopsis (ATCC 22019).
2.5.1. Bacterial Culture
Our previous study describes the methods used Obistioiu et all., 2021 [
39]. The extracts were used directly by adding 2.5, 5, 7.5, 10, 25, 50, 75 or 100 µL over the bacterial suspension. Pure uninhibited strain in BHI was used as a positive control and the value was subsequently used to calculate the bacterial growth and inhibition rates.
The MIC was determined by measurement of OD using the spectrophotometric method, according to ISO 20776-1:2019. The MIC is the lowest compound concentration that yields no visible microorganisms growth.
BGR (bacterial growth rate) and BIR (bacterial inhibition rate) were calculated as indicators for interpreting the results using the following formulas:
where: OD sample—optical density at 540 nm as a mean value of triplicate readings;
OD negative control—optical density at 540 nm as a mean value of triplicate readings for the selected bacteria in BHI.
2.5.2. Fungal Culture
The analysis was done according to our previous research [
40], with small modifications regarding the quantity tested. The extracts were used directly by adding 2.5, 5, 7.5, 10, 25, 50, 75 or 100 µL over the bacterial suspension. The plates were incubated for 48 h at 37 °C. After incubation, the OD was measured at 540 nm. All samples were read in triplicate.
The following formulas were used to calculate MGR (mycelial growth rate) and MIR (mycelial inhibition rate):
where: OD sample—optical density at 540 nm as a mean value of triplicate readings;
OD negative control—optical density at 540 nm as a mean value of triplicate readings for the selected fungi in BHI.
2.16. Determination of Antiinflammatory Activity
Heat-induced haemolysis Assay
Heat-induced haemolysis assay was performed according to Okoli et al., 2008 [
41], with some modifications exposed by Gunathilake et al., 2018 [
42]. Briefly, the method consists of two-step: the preparation of cell suspensions and hemolysis induced by exposure of the samples to a temperature of 54°C.
Preparation of red cell suspension
The erythrocyte suspension was prepared by using heparinized human blood, which was centrifuged at 3000 rpm for 10 minutes. The supernatant was removed, and the erythrocyte mass was washed with an equal volume of sterile isotonic sodium chloride solution (0,9%). After three centrifugation and washing step, the blood volume was measured and reconstituted as 40% suspension with isotonic PBS solution (pH - 7.4).
Heat-induced haemolysis
The reaction mixture consisted of different concentrations of the samples (25%, 75% and 100%) suspended in 5 ml of PBS isotonic solution (RemedLab, Bucharest, Romania) and 100 µl red blood cell suspension. The mixtures were incubated in a water bath at 54°C for 20 minutes and then were centrifuged at 2500 rpm for 3 min. The absorbance of the supernatant was read at 540 nm using a UV-VIS spectrophotometer (Specord 205; Analytik Jena AG, Jena, Germany).
The positive control sample consisted of PBS and 100µl erythrocyte suspension. The negative control sample consisted of dexamethasone (0,1 mg/ml) diluted in 5 ml of PBS and 100µl erythrocyte suspension.
To calculate the haemolysis inhibition percentage it was used the formula:
Where:
A1 represents the absorbance of the tested sample
A2 represents the absorbance of the positive control
The effect on protein denaturation
The protein denaturation assay was performed according to the method described by Gunathilake et al., 2018 [
42], with slight modifications. Briefly, 1 ml of 1% egg albumin (Oxford Lab Fine Chem, Maharashtra, India) and 4 ml of PBS (pH 6,4) (RemedLab, Bucharest, Romania) containing different concentrations of tested samples (25%, 50%, 75%, 100%) were incubated for 15 min at a temperature of 37°C. Then the mixture was heated at 70 °C for 5 min. After cooling, the absorbance of the mixture was read at 660 nm using a UV-VIS spectrophotometer (Specord 205; Analytik Jena AG, Jena, Germany).
The control solution was a mixture between albumin and PBS without any essential oil.
The value of the percentage inhibition of protein denaturation was calculated using the following formula:
Where:
A1 represents the absorbance of the tested sample
A2 represents the absorbance of the positive control
2.17. Statistical Analysis
The results presented in this study were determined using the IBM SPSS 22 statistical program. In the case of statistical differences (p<0.05) between the bee products analyzed, they were processed using the Anova program with Tukey's test.
3. Results and Discussion
3.1. Chemical Composition
3.1.1. Chemical Composition of Royal Jelly
Royal jelly is a valuable source of nutrients and bioactive components whose composition depends on a multitude of factors such as the beekeeping season, climatic conditions, the ecosystem in which bees live, honey sources to which bees have access, pollution sources within flight of bees but also the genetics of the colony. In the hive, it intervenes in the phenotypic development of worker bee larvae when they benefit from abundant feeding over a longer period of time, turning them into queens, but it also plays an important role in the social behavior of the bee colony by stimulating memory and learning. Consumed by humans, it is a functional food with high biological and therapeutic value [
43].
The samples of fresh royal jelly analyzed by us had a water content of 64.84-66.78%, proteins 36.02-42.73mg/ml and ash 0.92-1.48%, the minimum values being observed in the case of commercial royal jelly and the maximum of the one we harvested from the beehive (
Table 1). In the case of protein, the content differences were statistically significant (p<0.05) between the two sources of origin. Impurities had close values between the two lots analyzed, being in the range of 9.28-10.59%. The acidity of the analyzed samples had values between 29.41 mg/100 g (the royal jelly from the analyzed apiary) and 33.46 mg/100 g (commercial royal jelly) and the pH was in the range of 3.12-3.46 (
Table 4). The highest proportion of macroelements was occupied by potassium with 889.11-2019.78 mg, followed by phosphorus 990.25-1891.5 mg, magnesium 312.71-437.01 mg, calcium 199.38-403.84 mg and sodium 176.69-193.72 mg (
Table 4). We observed statistically significant differences in calcium content (p<0.05) between the analyzed samples, probably due to the different geographical location of the apiaries and the food sources to which the bees had access. The maximum values of the macroelements were recorded in the case of commercial royal jelly, with the exception of phosphorus, at which the maximum threshold was found in the case of royal jelly from the own apiary. In the case of microelements, zinc (16.63-24.12 mg) and iron (12.96-24.63 mg) occupy the largest share in the composition of fresh royal jelly, followed by copper (5.23-7.70 mg), manganese (3.75-6.71 mg) and chromium (0.79-5.89 mg), the maximum values being recorded by the commercial one.
In the case of lyophilized royal jelly, the water content was 3.32-3.33%, the proteins were represented in the amount of 89.05-100.46 mg/g and the ash 2.90-3.0%. The weight of macro and microelements was close to that recorded in fresh royal jelly (
Table 4). In the case of heavy metals, nickel and lead were not detected in the analyzed samples and cadmium was detected in the range of 0.58-1.58 ppm, exceeding the allowed values being observed in the case of commercial royal jelly, both fresh and freeze-dried (table 4).
Studies by Kunugi and Mohammed, 2019, [
44] showed that the major component of fresh royal jelly is water 60-70%, while proteins represent 50% of the dry matter. Sugars have an important weight of 7.5-15% of which 90% are represented by fructose and glucose and lipids are present in royal jelly in a proportion of 7-18%. Sidor et al., 2021, reported for fresh royal jelly from Poland a water content of 65.4-69%, protein 10.43-18% and pH 3.97-3.98. Balkanska and Kashanov, 2011, [
45] highlighted that Bulgarian lyophilized royal jelly has in its composition water 3.49-4.76%, dry matter 95.24-96.51%, proteins 34.09-41.80%, lipids 3.09-8.56%, sugars 24.27-32.67% and acidity of 10.67-12.88 mg/100 g, values that confirm the results obtained by Sabatini et al., 2009 [
46].
The macroelement content of royal jelly studied by Sidor et al., 2021, [
13] showed, as in our case, the presence as the main element of phosphorus 338.4-412.1 mg, followed by potassium 321.1-357.4 mg, magnesium 44-50.4 mg, calcium 22.8-24 mg and sodium 10.3-13.8 mg. Microelements had values of 2.07-2.58 mg in the case of zinc, 0.31-0.39 mg copper, 0.03-0.15 mg and 0.01-0.08 mg manganese lower compared to those observed by us.
3.1.2. Chemical Composition of Apilarnil
Apilarnil is a product of yellowish color, milky consistency and sour taste, obtained after triturating 7-day-old drone larvae collected together with the cell contents [
47]. The composition of the apiarnil is variable and depends on the age of the larvae, the food sources to which the bees have access, the beekeeping season.
The fresh apilarnil, analyzed by us, had a water content of 68.34-78.59%, acidity 12-27.72 mg/g and pH 4.15-5.75 (
Table 5). Commercial samples had a percentage of impurities of 5.45-5.53 and those collected from the apiary 15.18-15.36, with statistically significant differences (p<0.05) between the two sources of origin. Large variations in the protein content were observed in the case of fresh commercial apilarnil samples, respectively 20.80-45.19 mg/g. The ash of the analyzed samples was 0.85-1.46%, the maximum values being observed in the case of commercial samples. The main macroelement we found was phosphorus 344.25-2125.75 mg, followed by potassium 235.68-1339.61 mg, calcium 159.7-408.75 mg, magnesium 106.25-633.61 mg and sodium 106.57-181.47 mg. The best represented microelement was iron 5.62-19 mg, followed by zinc 4.09-13.22 mg, chromium 2.69-6.83 mg, manganese 3.57-5.84 mg and copper 2.46-5.24 mg.
The lyophilized apilarnil had a water content of 6.30-7.24%, proteins were represented in the amount of 112.55-121.94 mg/g and ash 1.88-1.90%. The weight of macro and microelements was close to that observed in fresh apilarnil (
Table 5).
Among the analyzed contaminants, nickel and lead were not detected in any sample of fresh or lyophilized apilarnil and cadmium was identified in the range of 1.15-2.48 ppm, the higher values were in the case of commercial samples (
Table 5).
The results obtained by us fall within the data presented in the specialized literature, the differences may be the result of geographical and meteorological conditions, the beekeeping season in which the harvesting was done, food sources, etc. The research carried out by Mărgăoan et al., 2017 [
47] highlighted the following chemical composition for apilarnil: water 73.25%, total protein 9.47%, lipids 8.38%, while the royal jelly analyzed had a water content of 66.03%, total protein 11.14% and lipids 3.96 %.
Kim et al., 2018, [
48] reported the nutritional profile of fresh apilarnil from Apis mellifera pupae drones aged 21-24 days as humidity 74.23 g/100g, protein 11.05/100g, fat 8.19 g/100 g, ash 0.85 g/100 g, and for the dry one obtained from drone pupae aged 16-20 days [
49] humidity 1.69 g/100 g, protein 48.52 g/100 g, fat 23.41 g/100 g and ash 4.05 g/100 g. The protein level of bee brood (pupae and larvae) evaluated by Choi et al., 2009, [
50] was 46.4-46.73 g/100 g and the fat level 18.84-20.75 g/100 g.
3.1.3. Chemical Composition of Propolis
Propolis is the result of harvesting and processing by bees of resinous substances from some plants, to which they add glandular secretions, wax, residues from the digestion of pollen. In the hive, bees use propolis for sealing and sanitizing the nest and secondarily for polishing the walls of the hive and the cells, covering the killed pests. Even though the chemical composition varies a lot being correlated with the geographical area and the plants used by the bees to produce it, propolis has similar activities, such as antibacterial, antifungal, antiparasitic, antiviral, anti-inflammatory and antioxidant [
51,
52,
53]. Raw propolis usually contains 50% vegetable resins, 30% wax, 10% essential and aromatic oils, 5% pollen and 5% other organic substances [
16].
The raw propolis samples analyzed by us had a humidity content of 0.34-0.67%, the maximum value was recorded in commercial samples with statistically significant differences (p<0.05) compared to the propolis from our own apiary (
Table 6). We observed that the acidity of the commercial samples was higher 5.94-5.96 mg/g compared to the samples collected from the own apiary (3.95-3.98 mg/g) with statistically significant differences (p<0.05), a similar situation was also observed in the case of impurities (
Table 6). The evaluation of macroelements revealed the presence of calcium as the main element 542.49-849.27 mg, with significant differences between the sources of origin (p<0.05), followed by magnesium 133.13-149.05 mg, phosphorus 99.0-175.25 mg, potassium 78.62-163.62 mg and sodium 97.89-115.03 mg. The best represented microelement was iron 41.01-68.14 mg, followed by zinc 11.23-50.41 mg, manganese 4.45-6.23 mg and copper 2.06-4.19 mg, with statistically insignificant differences between the sources of propolis (
Table 6). Chromium was not detected in raw propolis samples. Among the contaminants, nickel was identified in commercial propolis samples in the range of 1.09-2.33 ppm, cadmium was 0.17-0.70 ppm and lead was not detected in any sample under study.
The propolis tincture studied had a humidity of 96.67-97.01%, an acidity of 15.84-15.90 mg/g, a pH of 2.92-2.97 and a percentage of impurities of 0.07-0.10 (
Table 6). Calcium was the best represented macroelement 423.99-450.74 mg, followed by sodium 81.12-88.13 mg, magnesium 54.99-61.52 mg, potassium 15.68-20.02 mg and phosphorus 5.75-10 mg. Zinc was the major microelement of the propolis tincture analyzed by us, with a content of 2.43-5.85 mg, followed by manganese 2.61-2.86 mg, copper 2.11-2.32 mg and iron 1.69-2.36 mg. Among the analyzed heavy metals, cadmium was identified in a concentration of 0.49-074 ppm and nickel and lead were below the detection limit.
The studies carried out so far have shown that there are correlations between the chemical composition of propolis and the plants used by bees for its production [
54]. The type of soil and its parameters, the botanical origin of the samples, respectively the geographical area and the climatic conditions can determine differences in the mineral profile of propolis from different areas [
55]. The mineral content of the propolis samples evaluated by us have values close to those reported by Cvek et al., 2008 [
56], in the case of propolis from Croatia, and Tosic et al., 2017, [
55] for propolis from Serbia, the order of macrominerals being Ca›K›Mg›P›Na and in the case of microelements, the largest share is occupied by iron, followed by zinc. The authors reported for the 25 propolis samples analyzed the following values: calcium 627-1168 mg/kg, potassium 324-1157 mg/kg, magnesium 157 mg/kg, phosphorus 134-422 mg/kg, sodium 63.5-256 mg/kg, 116-284 mg/kg, zinc 19.2-241 mg/kg, manganese 6.1-14.36 mg/kg and copper 2.22-8.70 mg/kg [
55].
3.2. Antioxidant Activity by DPPH Method
The radical reduction activity by the DPPH method of the ethanolic extracts from the 9 analyzed samples was determined for 5 concentrations (10 mg/mL, 5 mg/mL, 2.5 mg/mL, 1 mg/mL and 0.5 mg/mL) (
Table 7). In parallel, the antioxidant activity of 5 ascorbic acid solutions in different concentrations (0.06 – 0.16 mg/mL) was evaluated as a positive control, resulting in an inhibition of 94.54% for the highest tested concentration (0.16 mg/mL). IC50 (concentration of each extract causing 50% DPPH inhibition) was subsequently calculated and expressed in mg/mL (
Table 7,
Figure 1).
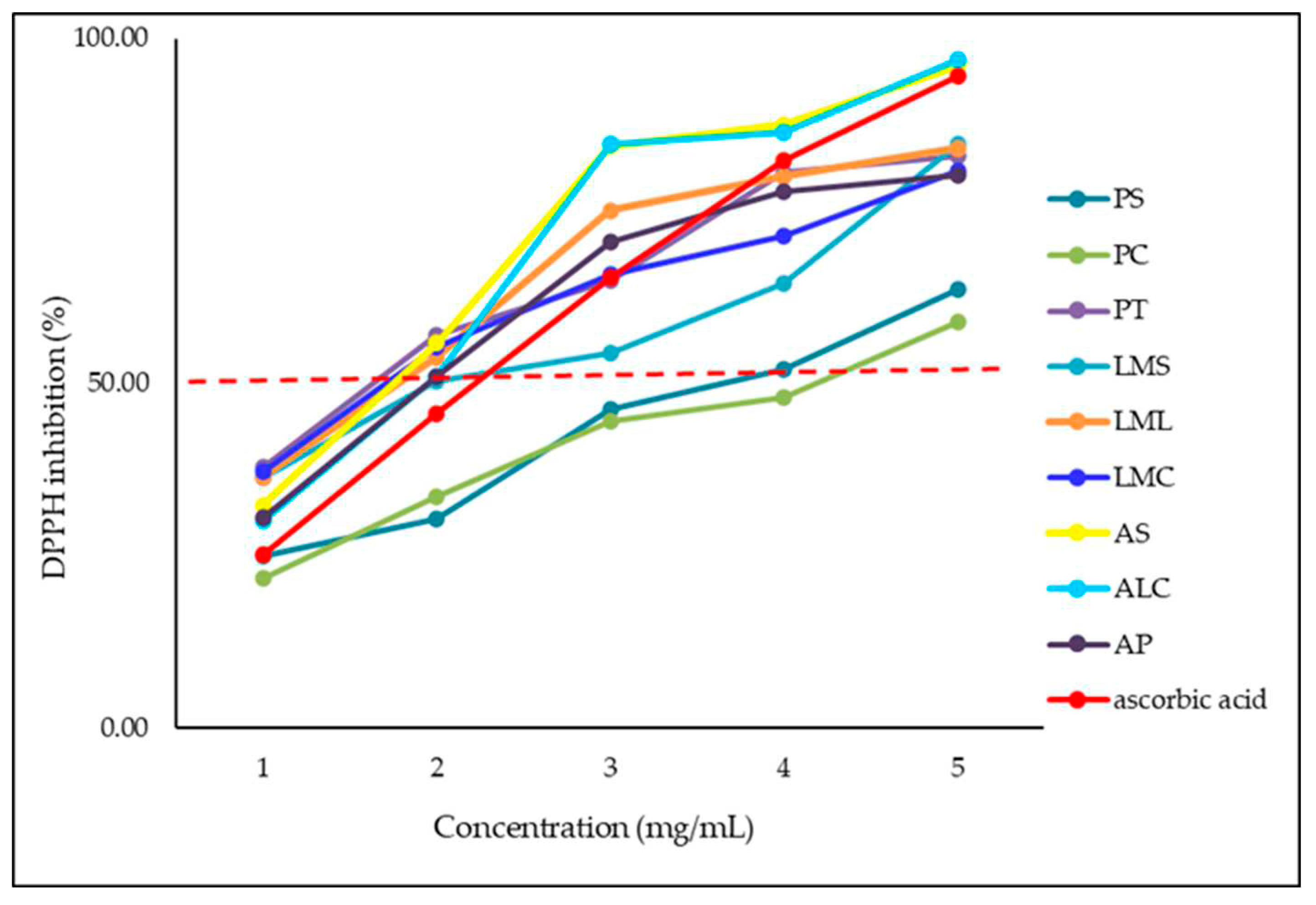
As can be seen from the values presented in
Table 7, the maximum radical scavenging activity was recorded for the highest concentration (10 mg/mL) for all samples. In the case of AS and ALC samples, higher values were obtained than in the case of the control sample, ascorbic acid, analyzed in the concentration range 0.06 – 0.16 mg/mL.
For the 5 mg/mL concentration, with the exception of the PS, PC and LMS samples, for the other samples similar values were obtained to those recorded for ascorbic acid.
The DPPH inhibition percentage still remained high for the next two lower concentrations (2.5 mg/mL and 1 mg/mL, respectively) for all samples except PS and PC. However, the recorded values are comparable to those recorded in the case of ascorbic acid. At the lowest tested concentration (0.3 mg/mL) the antioxidant activity showed a significant decrease for all samples, the values being comparable to those recorded for ascorbic acid, for some samples even higher.
The DPPH inhibition percentage for at 10 mg/mL concentration was >90% for AS and ALC, >80% for PT, LMS, LML, CML and AP and >50% for PS and PC. At 5 mg/mL, > 80% for PT, CML, AS, and ALC, > 70% for CML and AP, > 50% for PS and LMS, and < 50% for PC. At a concentration of 2.5 mg/mL, > 80% was recorded for AS and ALC, > 50% for PT, LMS, LML and AP, and PS and PC recorded DPPH values < 50%. At 1 mg/mL for all samples except PS and PC values > 50% were recorded. At the lowest concentration values > 30% were recorded except for PS and PC samples.
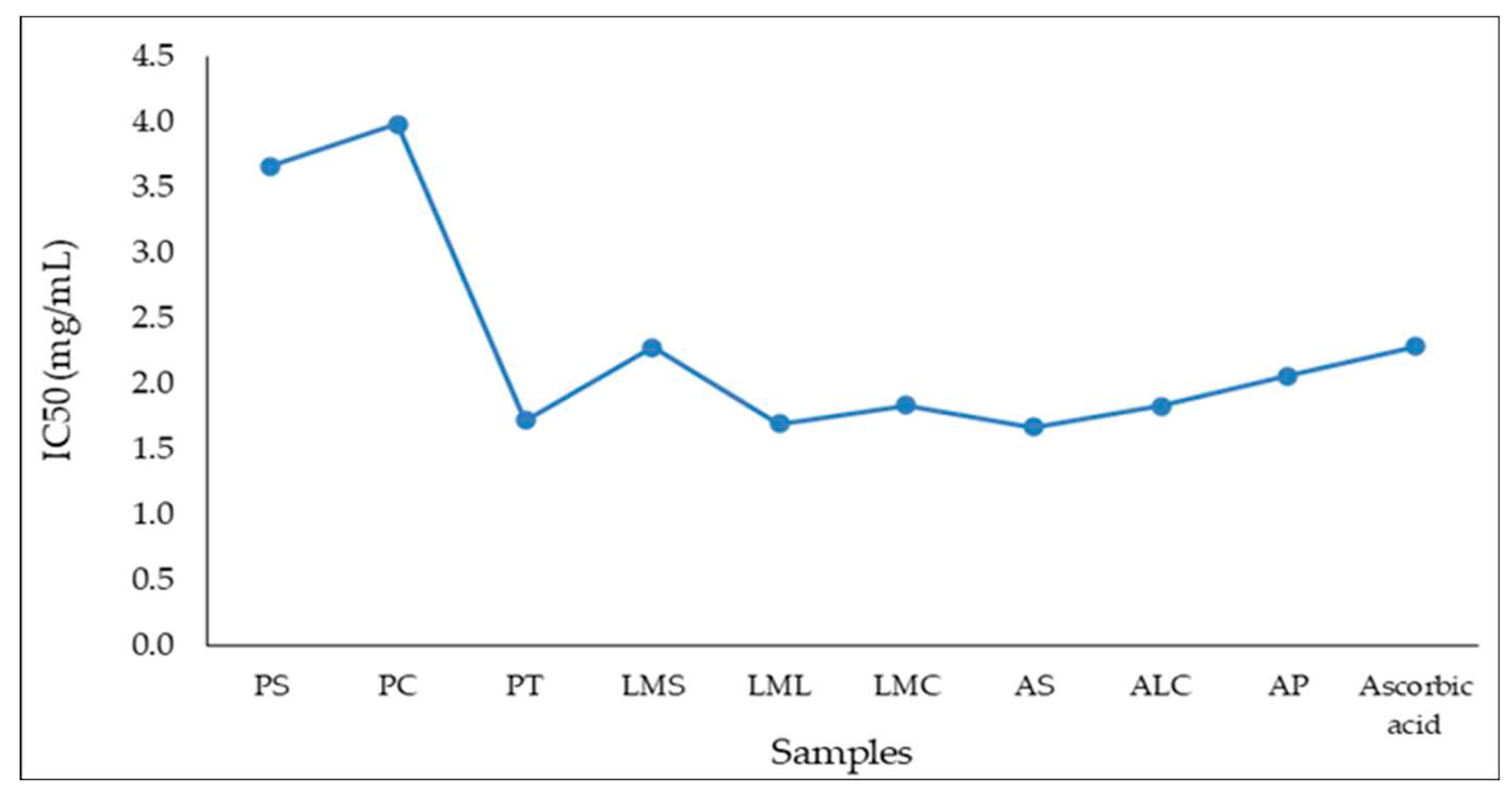
Table 8 shows the values obtained for IC50 compared to the value obtained for the control sample, ascorbic acid.
IC50 values (
Table 8) ranged from 1,669 mg/mL for the AS sample (the highest antioxidant capacity) to 3,979 mg/mL (the lowest antioxidant capacity) in the case of the PC sample. The IC50 variation of the analyzed samples is presented in
Figure 1.
The concentration dependence of the radical reduction activity of the nine analyzed samples is described in
Figure 2.
IC50 values between 0.3 mg/mL and 5.6 mg/mL were identified by Mărghitaș et al. (2009) [
57] for 13 ethanolic extracts of propolis originating from beehives in Transylvania. Chinese propolis was analyzed by Sun et al. (2015), [
58] and its antioxidant activity and reported IC50 values for different propolis extracts between 0.633 mg/mL and 13.798 mg/mL. Guzman-Gutierrez et al. (2018) [
59] in the research conducted on extracts made from Mexican propolis and ethyl acetate reported a strong DPPH scavenging activity (IC50= 16.55 ± 0.87 μg/mL). A strong antioxidant activity was mentioned by Belfar et al. (2015) [
60] for 4 methanolic extracts of propolis from Algeria with IC50 values between 0.007 and 0.066 mg/mL, but lower compared to the control ascorbic acid value (0.184 mg/mL).
Brazilian propolis tincture extracts were reported to exhibit antioxidant activity, and the DPPH scavenging activity differed depending on the dose between 23.7% and 43.5%, with ascorbic acid being used as a positive control [
61], the values being similar to those obtained by us in the case of concentrations of 0.5 (mg/mL). A study on propolis extracts from 14 countries in the world carried out by Kumazawa et al. (2004) [
62] evaluated their antioxidant activity and reported a large variation in DPPH radical scavenging activity (from 10% to 90% ), values similar to those obtained by us for the 3 propolis samples, and according to the study the strongest results were obtained by propolis from countries such as: Australia, China, Hungary, and New Zealand.
In the case of propolis originating in India, IC50 determinations for 10 ethanolic extracts varied between 0.33348 mg/mL and 0.60088 mg/mL, and for ascorbic acid it was 0.28492 mg/mL [
63] . Wang et al. (2016) [
64] reported IC50 values for Korean propolis from 0.043 to 0.269 mg/mL for 20 investigated samples.
3.2. Antimicrobial Activity
The antimicrobial activity of the samples taken in the analysis began by evaluating the antibacterial activity against the S. pyogenes strain (
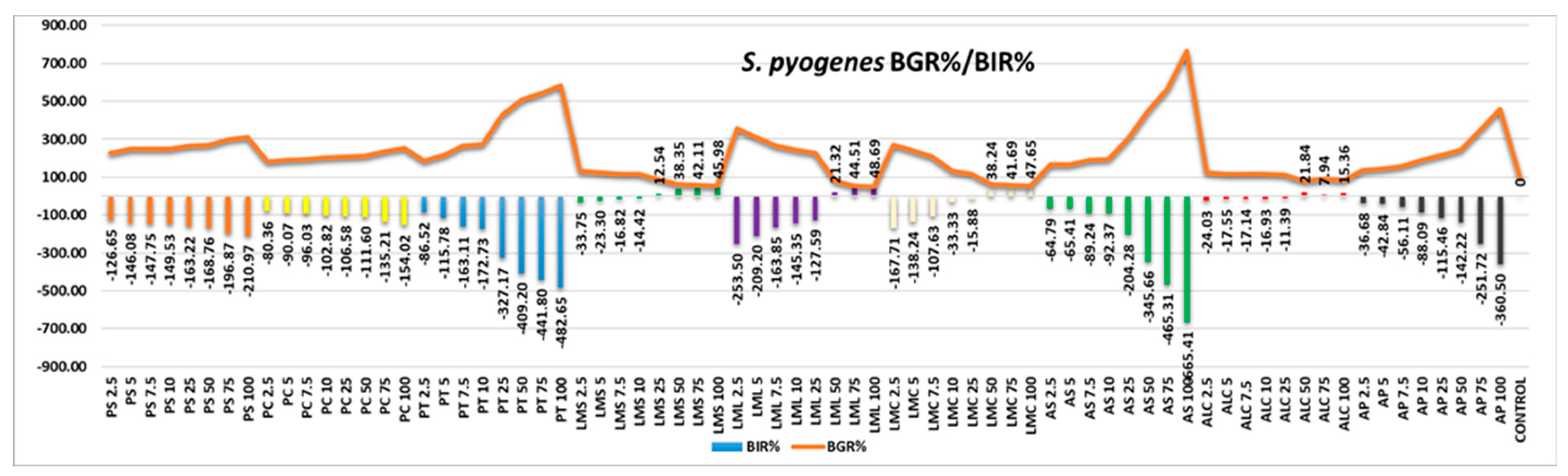
Figure 3). The results are pre-sented in graphical form, the values presented being those of bacterial growth rate (BGR%) as well as bacterial inhibition rate (BIR%). The results show a negative picture from the inhibition point of view. The only positive results obtained are those obtained in samples LMS, MLL, MCL and ALC. In all the samples showing positive results, these occurred only at high quantities tested above 25%. All other samples tested showed an-tibacterial efficacy in negative correlation with the increase in quantity. All BIR % val-ues obtained are negative and increased with the increase in the tested quantity. The results obtained show a low efficacy against S. pyogenes in the case of the 4 less efficient LMS, LML, LMC and ALC samples. All the other samples proved a strain boosting ef-fect, an effect proved by the increase in the optical density.
Concerning the antimicrobial activity against
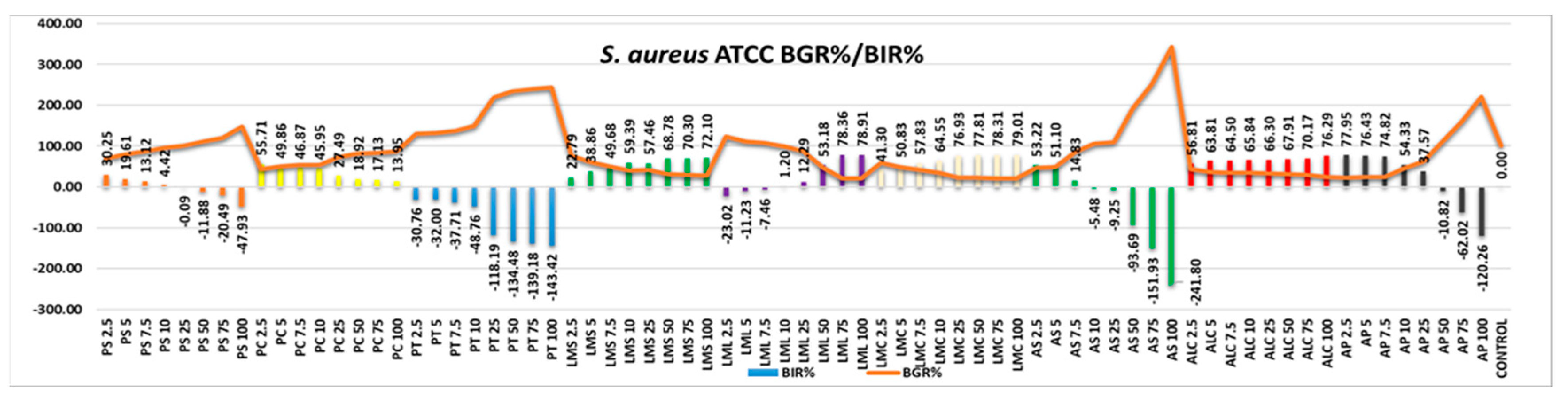
S. aureus five of the tested samples showed no effect in regard to inhibition (
Figure 4). PS, PC, PT, AS and AP showed a strain boosting effect, the trend being a negative one, in negative correlation with the increase in quantity. The least effective of the samples tested proved to be a AS, with a negative value regarding BIR% of -241.80%, followed by PT, AP and PS. PC also showed a strain boosting effect with the value of inhibition decreasing alongside the increase in quantity, but the values obtained are still higher than the value obtained for the control therefore remaining to be considered as effective. PS an AP prove inhibitory values that are positive only concerning the smaller quantity tested, values that reached only the 25µL quantity tested. LML developed antibacterial efficiency but only starting from 10%. LMS LMC and ALC where the samples identified with good antibacterial efficiency. In their cases BIR% reached levels varying from 22.79% to 79.01%.
Regarding the antibacterial efficiency of the tested samples against
S. flexneri, the percentage of effectiveness is similar to the ones obtained in case of
S. aureus as presented in
Figure 5 PS demonstrates negative inhibition values, but with a positive trend the values increasing with increasing quantity, demonstrating that the tested quantities have not yet reached the value required to cause a greater than control inhibition of bacterial growth. PT shows from the beginning an activity to stimulate bacterial growth, the trend being a negative one, the inhibition rate decreasing with increasing quantity, reaching values of -100.69%. PC, LMS, LMC, LML and ALC have demonstrated antibacterial efficacy with positive trends correlated with increasing quantity, the most effective being shown to be LM, followed by ALC and CML. AS and AP show negative inhibition trends with efficacy only in the first 4 and 5 quantities tested, respectively.
P. aeruginosa proved to be one of the strains most resistant to the action of the tested samples, antibacterial activity being identified only in the case of samples: LMS, LMC, LML and ALC (
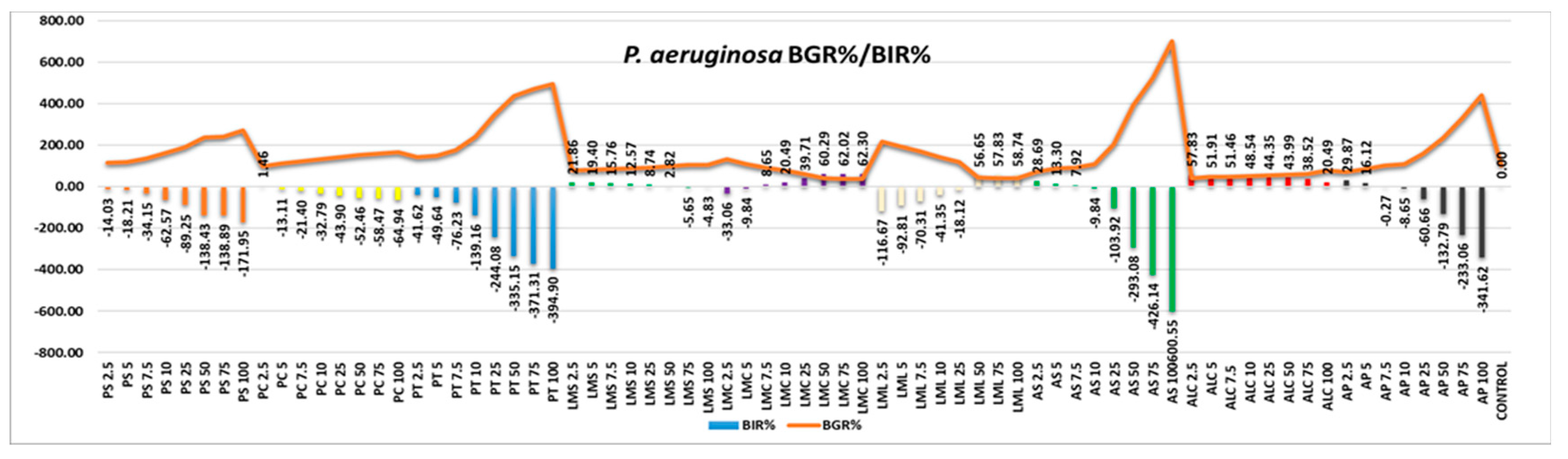
Figure 6). Even in the case of these samples, positive trends correlated with the increase in quantity showed only LML and AML, the other 2 samples having inhibition values that decrease with increasing quantity of the tested samples. All other samples had a growth-stimulating effect on the bacterial strain, with inhibition values up to -600.55 percent in the AS sample and -394.90 percent in the PT sample. This demonstrates 500 percent higher bacterial growth in the AS sample and 294 percent higher in the PT sample compared to the control.
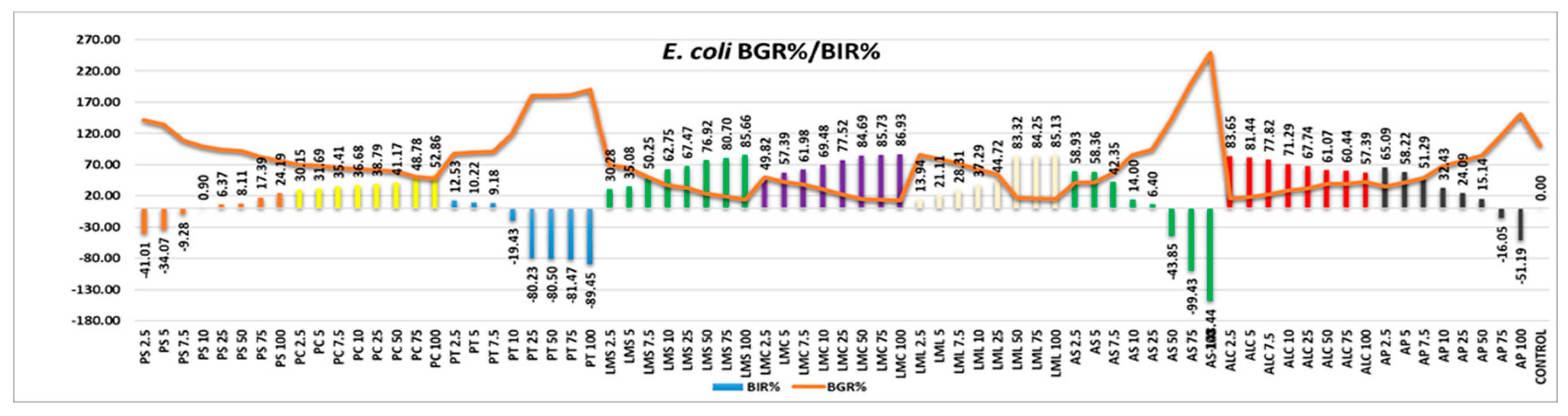
Analyzing the results obtained from the point of view of the efficacy of the samples tested against the E
. coli strain, the picture presented is one with a higher efficacy than average (
Figure 6). Most of the samples tested showed efficacy with positive trend in direct correlation with increased quantity. Thus, the efficacy of the samples classified ascending was PS, PC, LML, LMS and LMC. PT, AS, and AP showed a negative trend, with inhibition values decreasing with increasing quantity, proving a stimulating effect on the bacterial strain. ALC has also demonstrated a strain-boosting effect, but the values obtained are clearly superior to those compared to those of control and can be classified as effective.
Figure 8 presents the graphical representation of the results obtained in case of antibacterial efficiency analysis of samples tested against
S. typhimurium. The general picture is one with an efficacy above average, a negative trend correlated with the increase in quantity being found only in the case of PS, PT, AS and AP samples. In the case of the PS sample, the values obtained, although decreasing, are positive from 50.17% and decreasing to 12.96% in the case of the maximum tested quantity. All other samples tested demonstrate very good efficacy, with maximum values recorded at the last tested quantity ranging from 60 % to 89,37 %.
Concerning the antibacterial efficacy of the tested samples,
Figure 9 presents the graphical representation of the BGR% and BIR% values. PS, PT, AS and AP presented negative correlation with the increase in quantity of the inhibition values. The other samples demonstrated good efficiency in the following decreasing order ALC>LMC>LMS>LML>PC.
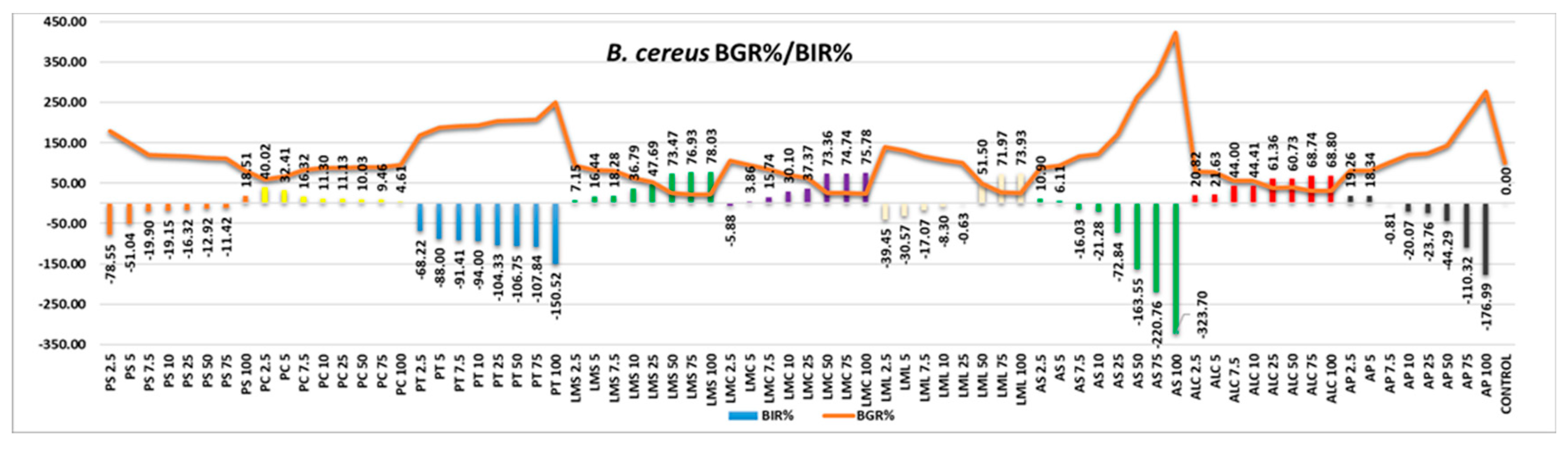
B. cereus presented components of the bacterial wall that formed a synergy with components from the samples tested (
Figure 10), the effect being a strain boosting effect in most of the extracts tested. Negative correlation with the increase in quantity proved PC, PT, AS and AP. PS demonstrated MIC only at 100µL tested, LMC at 7.5 and LML only at 75µL. LMS and ALC were the only samples that proved efficacy, in positive correlation with the increase in the quantity tested, with values of BIR% ranging from 68.80% to 78.03%.
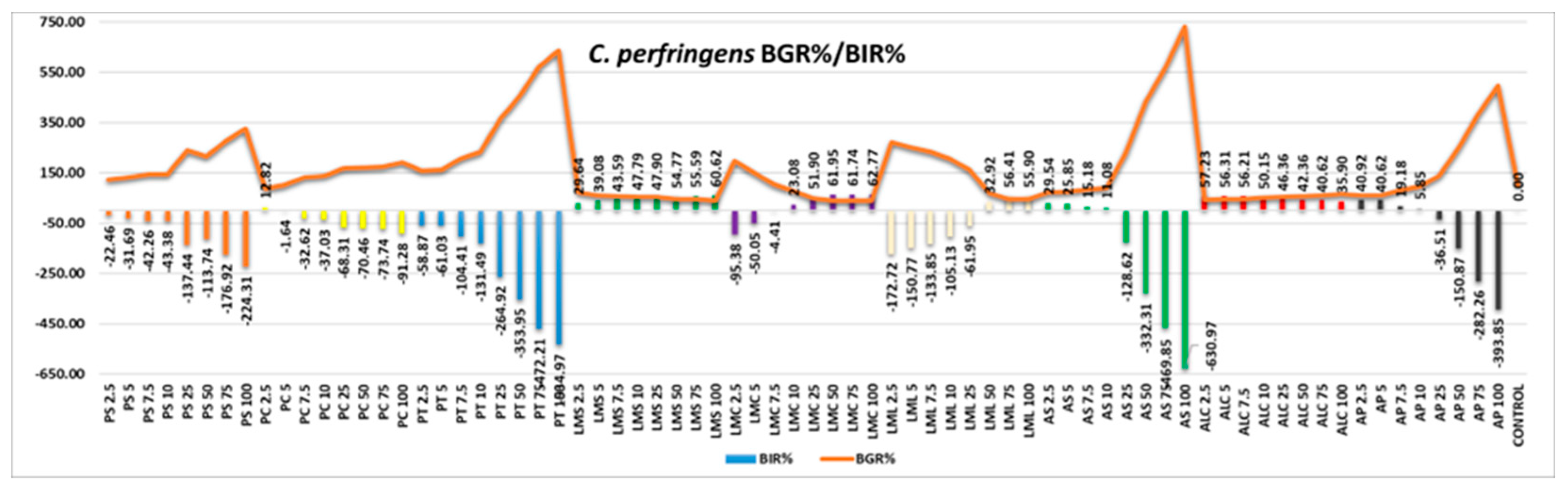
Figure 11 represents the antimicrobial activity of the samples tested against
Cl. perfingens. The overall picture is one of low efficacy, most of the samples proving a strain-boosting effect: PS, PC, PT, AS, ALC (in which, even if the trend is negative, the values are positive, reaching MIC at the first quantity tested) and AP. LMC and LML showed negative values at the first quantities tested therefore LMS being the only one with a positive effect.
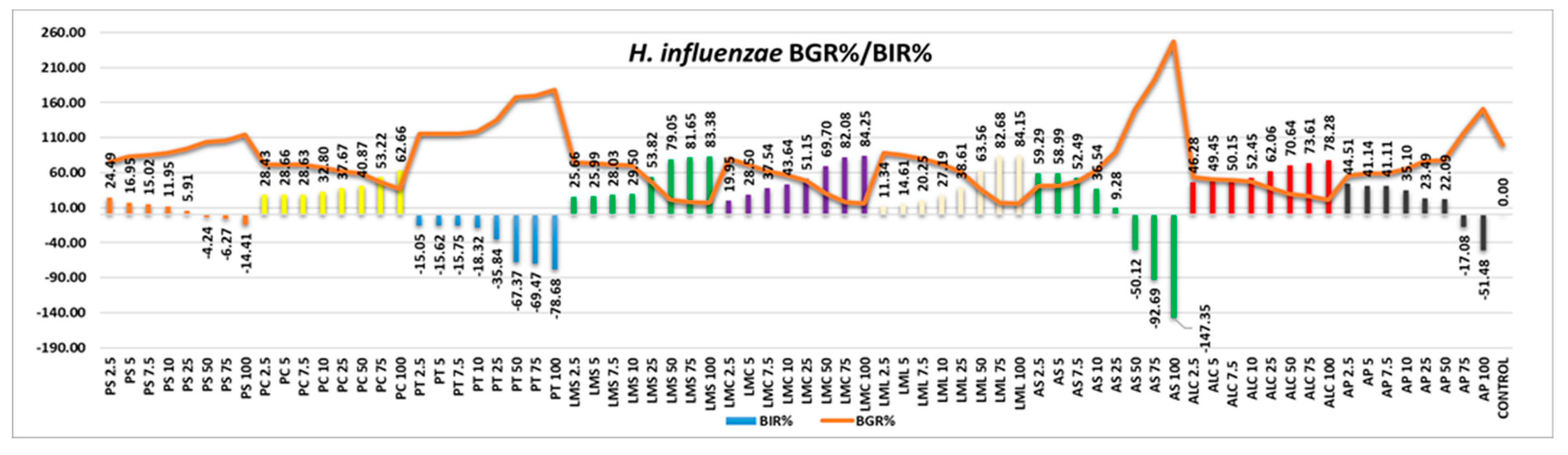
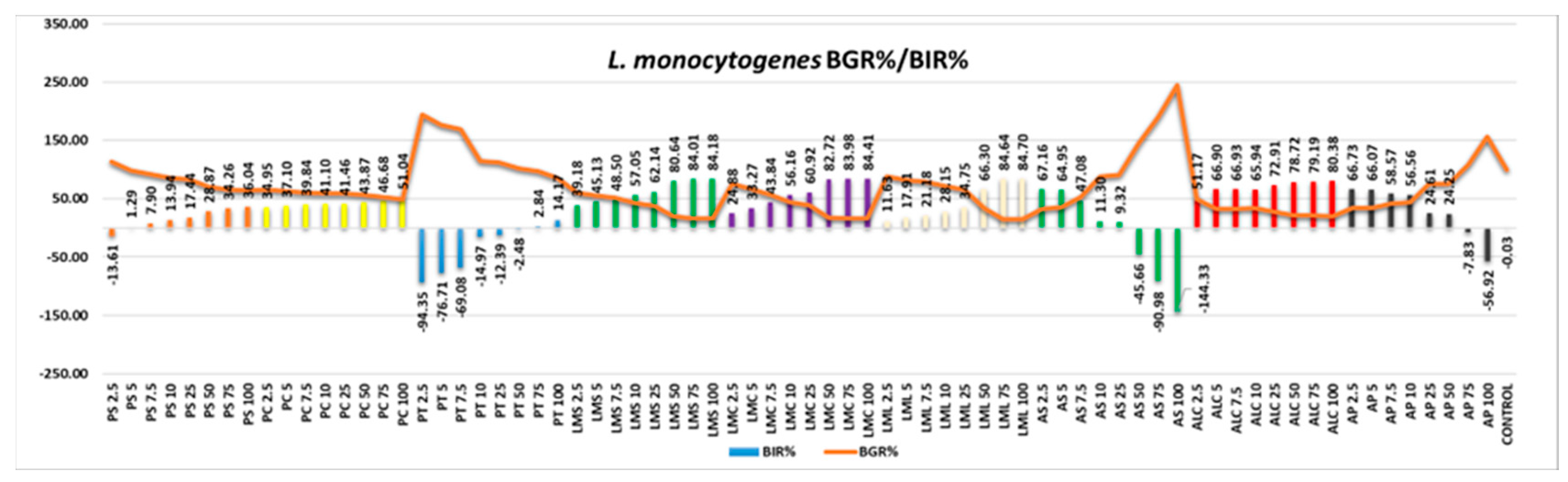
L. monocytogenes responded well to the activity of the tested samples (
Figure 12), AS and AP being the only two with effect only at the first quantities tested, the values becoming negative alongside the increase in the quantity tested. All the other samples were effective, the efficacy being in a positive correlation with the increase in quantity.
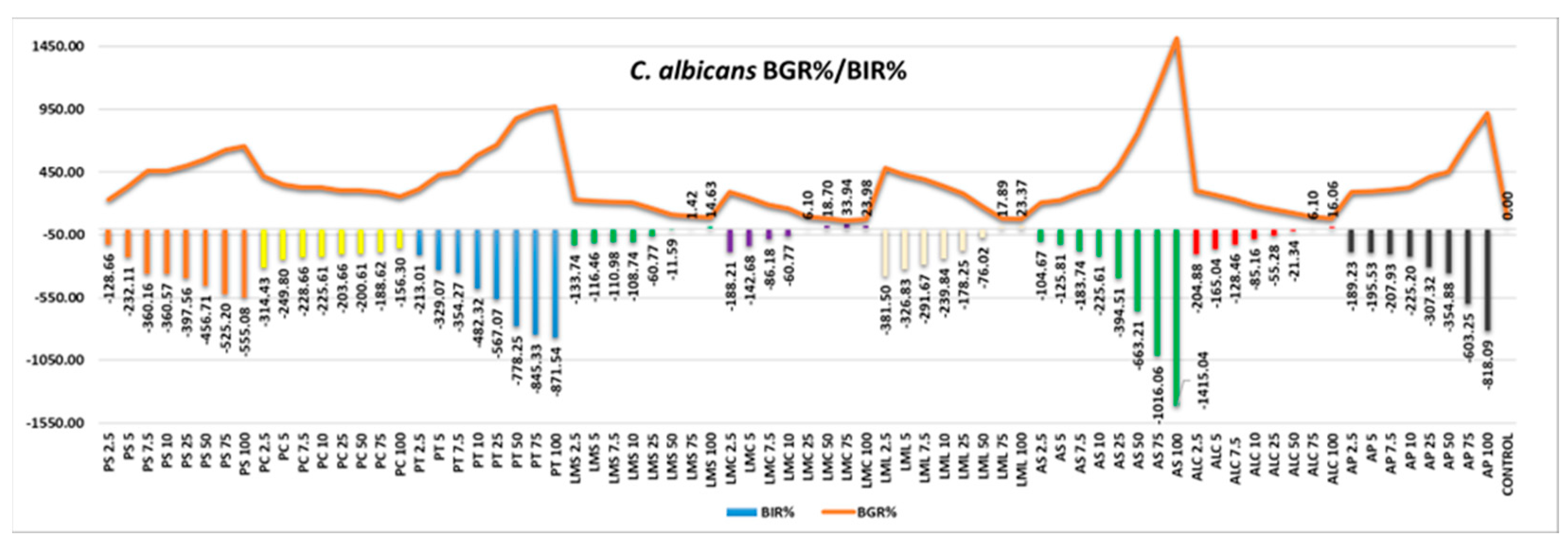
Regarding the antifungal effect, C. albicans proved to be resistant to all the samples tested, only LMS, LMC, LML and ALC developing small percentages of inhibition and only at high quantities tested, as presented in
Figure 13 and
Table 9.
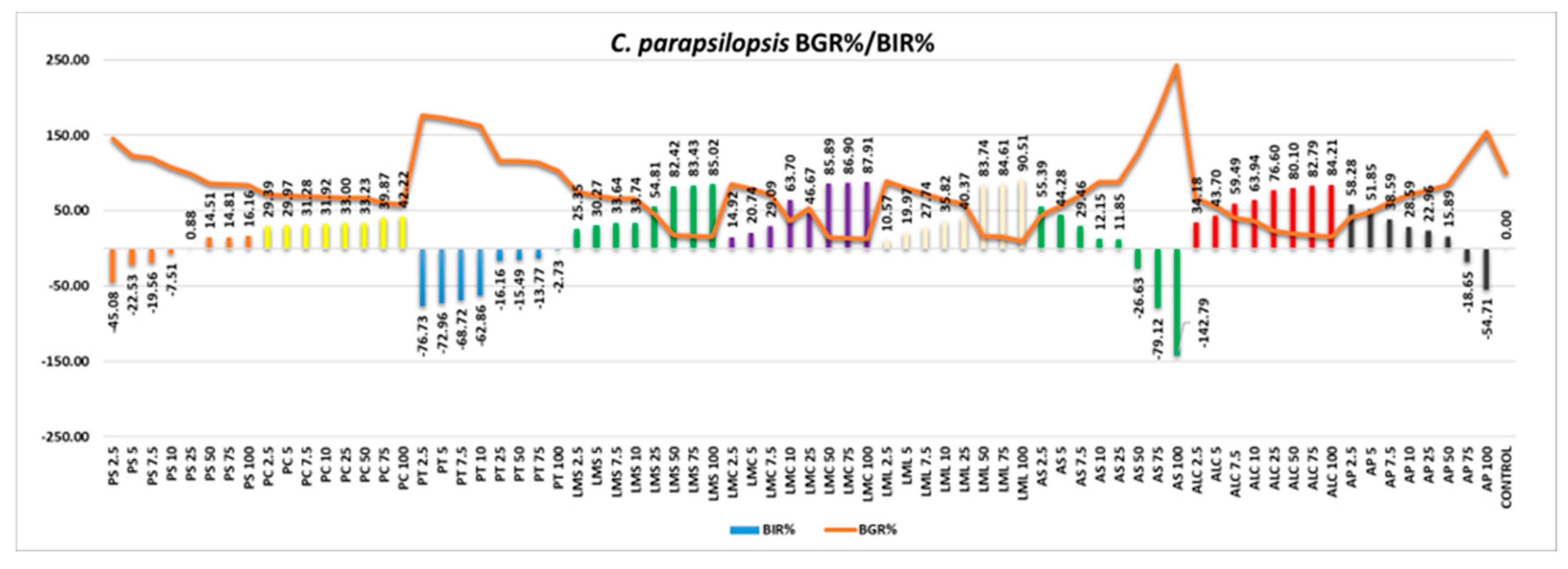
C. parapsilopsis appeared more susceptible to the antifungal effect of the samples (
Figure 14). AS and AP were the only samples with a negative inhibitory trend, all the other samples tested proving good efficacy, with BIR% values reaching maximum values ranging from 16.16% to 90.51%. All the MIC values recorded are presented in
Table 9.
The studies carried out by János Dégi et al., 2022, [
40] confirm that propolis has a diverse chemical composition and good therapeutic properties against bacterial infections, in vitro investigations for the antimicrobial activity of propolis ethanol extract, against isolated clinical strains of
Staphylococcus aureus from clinical samples of superficial dermatitis in dogs by the microdilution broth technique to assess the susceptibility profile of the bacteria. The minimum inhibitory concentration (MIC) of the ethanol extracts of Romanian propolis had a very well developed antibacterial activity, and these results demonstrate the fact that propolis can be used for in vivo experiments as a good therapeutic agent against
Staphylococcus aureus [
40].
In the study carried out by L.Drago, et al. (2013) [
65] the antibacterial and antifungal properties of propolis were highlighted, it being demonstrated that the minimum inhibitory concentrations and the minimum bactericidal concentrations were determined on 320 strains, including:
Staphylococcus aureus , Streptococcus pneumoniae, group A beta-hemolytic streptococci, Moraxella catarrhalis, Haemophilus influenzae, Klebsiella pneumoniae, Escherichia coli, Proteus mirabilis, Pseudomonas aeruginosa and
Candida albicans. Propolis presented in this study similar to those achieved by a good antimicrobial activity against most bacteria,
especially S. pneumoniae, H. influenzae and M. catarrhalis, but not against group A beta-hemolytic streptococci, and the curves of killing over time demonstrated bacteriostatic rather than bactericidal activity of propolis, the latter being evident only at high concentrations [
65].
3.3. Antiinflammatory Activity
The results obtained by heat-induced haemolysis assay are presented in the
Table 9.
It was observed that PS, PC and LML had a protective effect against haemolysis starting from 0,25 mg/ml; the percentage of haemolysis inhibition increased values with the concentration of samples. Of the three, at a concentration of 25%, PC has the highest value of hemolysis inhibition percentage, respectively, of 47,22 %, reaching 58,83% at the highest concentration tested. Similarly, LML showed a high value of the hemolysis inhibition percentage at a concentration of 25%, respectively of 41,56%, reaching a value slightly over 50% at the maximum tested concentration. The percentage of inhibition of haemolysis value for PC at a concentration of 25% is only 0,75%, reaching 18,71% at a concentration of 100%. The samples demonstrated the antiinflammatory activity by membrane lysis assay in the following order: PS> LML > PC. However, all these samples showed that a concentration of 100% determined the inhibition percentage values close to those obtained by using 0.1 mg/ml dexamethasone.
Regarding the other samples, only LMC and AP demonstrated the protective effect against membrane lysis at the highest tested concentration for the rest of the samples.
The effect on protein denaturation
The results for the effect on protein denaturation are presented in the
Table 10.
From all the studied samples, PS and PC demonstrated that the percentage inhibition of protein denaturation values follows an ascending curve with minimum values at a concentration of 25% and maximum values at a concentration of 100%.
For the other samples, the percentage inhibition of protein denaturation followed a descending slope with maximum values at a minimum tested concentration of the samples. However, even at the maximum concentration tested (100%), the inhibition of protein denaturation values were more than 50% for LML, LMC and AP.
A study carried out by Mendez et al. (2023) [
66] highlighted the fact that seasonal extracts of Sonoran propolis have antiinflammatory activity depending on the time of collection and the dose, but also the fact that they were directly influenced by variations in its chemical composition. Antiinflammatory tests showed that Sonoran propolis seasonal extracts significantly reduced NO levels to basal levels on cells and inhibited heat-induced protein denaturation, thus protecting the HRB membrane, thus being considered mechanisms of Sonoran propolis with antiinflammatory effect, and some of its constituents could be used for the development of new antiinflammatory drugs.
Other research carried out by Park Eun Hee et al. (1996) [
67] demonstrated similar effects to our study using Korean propolis, which was extracted with ethanol and used as a test material by oral administration (100 mg/kg), and the results demonstrated the significant inhibition of the increase in vascular permeability and of acetic acid-induced writhing in mice, thus Korean propolis was shown to have strong antiinflammatory activity. According to the research carried out by Borrelli in 2002 [
68] several studies demonstrated that propolis would have an action as a strong antiinflammatory agent against acute and chronic inflammation, and two phenolic compounds: galangin and CAPE, major components of bee propolis, inhibited the development of inflammation induced by various pathogens.
Figure 1.
IC50 variation for the 9 analyzed samples vs ascorbic acid.
Figure 1.
IC50 variation for the 9 analyzed samples vs ascorbic acid.
Figure 2.
Concentration dependency of radical scavenging activity of the samples.
Figure 2.
Concentration dependency of radical scavenging activity of the samples.
Figure 3.
Antibacterial activity against the S. pyogenes strain.
Figure 3.
Antibacterial activity against the S. pyogenes strain.
Figure 4.
Antibacterial activity against the S. aureus strain.
Figure 4.
Antibacterial activity against the S. aureus strain.
Figure 5.
Antibacterial activity against the S. flexneri strain.
Figure 5.
Antibacterial activity against the S. flexneri strain.
Figure 6.
Antibacterial activity against the S. aeruginoasa strain.
Figure 6.
Antibacterial activity against the S. aeruginoasa strain.
Figure 7.
Antibacterial activity against the E. coli strain.
Figure 7.
Antibacterial activity against the E. coli strain.
Figure 8.
Antibacterial activity against the S. typhimurium strain.
Figure 8.
Antibacterial activity against the S. typhimurium strain.
Figure 9.
Graphical representation of the BGR% and BIR% values.
Figure 9.
Graphical representation of the BGR% and BIR% values.
Figure 10.
B.cereus - Graphical representation of the BGR% and BIR% values.
Figure 10.
B.cereus - Graphical representation of the BGR% and BIR% values.
Figure 11.
C. perfringens - Graphical representation of the BGR% and BIR% values.
Figure 11.
C. perfringens - Graphical representation of the BGR% and BIR% values.
Figure 12.
L.monocytogenes - Graphical representation of the BGR% and BIR% values.
Figure 12.
L.monocytogenes - Graphical representation of the BGR% and BIR% values.
Figure 13.
C.albicans - Graphical representation of the BGR% and BIR% values.
Figure 13.
C.albicans - Graphical representation of the BGR% and BIR% values.
Figure 14.
C.parapsilopsis - Graphical representation of the BGR% and BIR% values.
Figure 14.
C.parapsilopsis - Graphical representation of the BGR% and BIR% values.
Table 1.
Bee product samples analyzed.
Table 2.
Expression of the amount of inverted sugar according to the volume of iodine used for oxidation (According to SR 784-3 / July 2009).
Table 2.
Expression of the amount of inverted sugar according to the volume of iodine used for oxidation (According to SR 784-3 / July 2009).
| Iodine solution 0.05 n ml |
Inverted sugar mg |
1.0
1.2
1.4
1.8
2.0
2.2 |
1.7
2.1
2.4
3.1
3.5
3.9 |
Table 3.
Parameters used when reading mineral elements.
Table 3.
Parameters used when reading mineral elements.
| Symbol Metal |
Wavelenght
ʎ (nm) |
Lamp current
(mA) |
Slit width
(nm) |
Pb
Ca
Ni
Mg
Fe
Cu
Na
Cr
Zn
K
Mn
Cd |
217.0
422.7
232.0
285.2
248.3
324.8
589.0
357.9
213.9
766.5
279.5
228.8 |
10
10
4
4
5
4
3
8
5
4
5
4 |
1.0
0.5
0.2
0.5
0.2
0.5
0.8
0.2
1.0
0.2
0.2
0.5 |
Table 4.
Chemical composition of fresh and lyophilized royal jelly.
Table 4.
Chemical composition of fresh and lyophilized royal jelly.
| Royal jelly |
Fresh |
Lyophilized |
| S |
C |
C+S |
| Mean ±SD |
Min-Max values |
Mean ±SD |
Min-Max values |
Mean ±SD |
Mean ±SD |
Min-Max values |
| Humidity (%) |
65.56±1.064a
|
64.81-66.78 |
64.75±0.079a
|
64.69-64.84 |
65.16±0.572A
|
3.33±0.010B
|
3.32-3.33 |
| DM (%) |
34.17±0.987a
|
33.22-35.19 |
35.22±0.078a
|
35.16-35.31 |
34.70±0.742A
|
94.64±2.000B
|
92.66-96.66 |
| Acidity |
29.45±0.045a
|
29.41-29.50 |
31.77±1.485a
|
30.66-33.46 |
30.61±1.640A
|
33.03±1.704A
|
32.00-35.00 |
| pH |
3.41±0.042a
|
3.38-3.46 |
3.14±0.020b
|
3.12-3.16 |
3.28±0.190A
|
3.15±0.050A
|
3.10-3.20 |
| Impurities (%) |
9.96±0.656a
|
9.28-10.59 |
9.32±0.809a
|
8.71-10.24 |
9.64±0.452A
|
16.79±0.738B
|
16.16-17.60 |
| Protein |
41.72±1.005a
|
40.72-42.73 |
36.13±0.110b
|
36.02-36.24 |
38.93±3.952A
|
94.76±5.705B
|
89.05-100.46 |
| Ash (%) |
1.46±0.015a
|
1.45-1.48 |
0.93±0.015b
|
0.92-0.95 |
1.20±0.374A
|
2.95±0.050B
|
2.90-3.00 |
| Macroelements |
| Na |
182.67±8.457a
|
176.69-188.65 |
187.53±8.761a
|
181.33-193.72 |
185.10±3.429A
|
179.11±4.992A
|
175.58-182.64 |
| Ca |
200.79±1.994a
|
199.38-202.20 |
382.12±30.716b
|
360.40-403.84 |
291.46±128.21A
|
1218.42±1101.62A
|
439.45-1997.38 |
| K |
1182.42±414.80a
|
889.11-1475.73 |
1659.43±509.61a
|
1299.08-2019.78 |
1420.93±337.29A
|
2553.17±878.77A
|
1931.78-3174.55 |
| Mg |
365.77±75.038a
|
312.71-418.83 |
423.32±19.367a
|
409.62-437.01 |
394.54±40.686A
|
782.40±109.17B
|
705.20-859.60 |
| P |
1648.38±343.83a
|
1405.25-1891.50 |
1125.75±191.62a
|
990.25-1261.25 |
1387.07±369.55A
|
2799.00±890.95A
|
2169.00-3429.00 |
| Microelements |
| Fe |
17.87±7.219a
|
12.76-22.97 |
19.43±7.353a
|
14.23-24.63 |
18.65±1.110A
|
36.34±6.243A
|
31.92-40.75 |
| Mn |
4.20±0.636a
|
3.75-4.65 |
5.32±1.965a
|
3.93-6.71 |
4.76±0.791A
|
4.91±0.155A
|
4.80-5.02 |
| Cu |
6.26±1.449a
|
5.23-7.28 |
7.12±0.820a
|
6.54-7.70 |
6.69±0.615A
|
15.78±0.721B
|
15.27-16.29 |
| Zn |
19.71±5.769a
|
16.63-23.79 |
21.95±3.075a
|
19.77-24.12 |
20.83±1.583A
|
51.07±4.617B
|
47.80-54.33 |
| Cr |
1.03±0.332a
|
0.79-1.26 |
2.95±4.164a
|
0.00-5.89 |
3.46±3.436A
|
2.68±0.784A
|
2.12-3.23 |
| Contaminants |
| Ni |
Undetectable |
undetectable |
undetectable |
undetectable |
undetectable |
undetectable |
undetectable |
| Cd |
0.70±0.169a
|
0.58-0.82 |
1.51±0.106b
|
1.43-1.58 |
1.11±0.572A
|
1.29±0.247A
|
1.11-1.46 |
| Pb |
Undetectable |
undetectable |
undetectable |
undetectable |
undetectable |
undetectable |
undetectable |
Table 5.
Chemical composition of fresh and lyophilized apilarnil.
Table 5.
Chemical composition of fresh and lyophilized apilarnil.
| Apilarnil |
Fresh |
Lyophilized |
| S |
C |
C+S |
| Mean ±SD |
Min-Max values |
Mean ±SD |
Min-Max values |
Mean ±SD |
Mean ±SD |
Min-Max values |
| Humidity (%) |
68.54±0.295a
|
68.34-68.88 |
78.58±0.005b
|
78.58-78.59 |
73.56±7.099A
|
6.78±0.470B
|
6.30-7.24 |
| DM (%) |
31.36±0.274a
|
31.12-31.66 |
21.43±0.017b
|
21.42-21.45 |
26.40±7.021A
|
93.19±0.475B
|
92.76-93.70 |
| Acidity |
26.62±0976a
|
25.85-27.72 |
12.62±0.69b
|
12.00-13.36 |
19.62±9.899A
|
78.25±0.160B
|
78.12-78.43 |
| pH |
5.74±0.010a
|
5.73-5.75 |
4.17±0.152b
|
4.15-4.18 |
4.96±1.110A
|
5.55±0.050A
|
5.50-5.60 |
Impurities
(%) |
15.28±0.091a
|
15.18-15.36 |
5.47±0.026b
|
5.45-5.53 |
10.38±6.936A
|
5.65±0.735A
|
4.92-6.39 |
| Protein (mg/g) |
44.86±0.335a
|
44.52-45.19 |
22.04±1.235a
|
20.80-45.19 |
33.45±16.136A
|
117.25±4.695B
|
112.55-121.94 |
| Ash (%) |
0.88±0.025a
|
0.85-0.90 |
1.45±0.010b
|
1.44-1.46 |
1.17±0.403 A
|
1.89±0.010 B
|
1.88-1.90 |
| Macroelements |
| Na |
144.02±52.962a
|
106.57-181.47 |
159.53±8.209a
|
153.72-165.33 |
151.78±10.967A
|
184.00±8.174A
|
178.22-189.78 |
| Ca |
284.23±176.104a
|
159.70-408.75 |
309.11±36.465a
|
283.32-334.89 |
296.67±17.585A
|
413.81±40.722A
|
385.01-442.60 |
| K |
787.65±780.596a
|
235.68-1339.61 |
578.89±338.69a
|
339.39-818.38 |
683.27±147.615A
|
2252.91±1262.09A
|
1360.47-3145.34 |
| Mg |
369.93±372.89a
|
106.25-633.61 |
206.06±11.377a
|
198.01-214.10 |
287.99±115.880A
|
459.60±187.333A
|
327.13-592.06 |
| P |
1233.00±1256.88a
|
344.25-2121.75 |
375.78±85.878a
|
315.05-436.50 |
804.39±606.146A
|
1343.25±691.903A
|
854.00-1832.50 |
| Microelements |
| Fe |
12.31±9.461a
|
5.62-19.00 |
7.86±2.064a
|
6.40-9.32 |
10.09±3.146A
|
32.58±19.219A
|
18.99-46.17 |
| Mn |
4.71±1.605a
|
3.57-5.84 |
4.79±0.919a
|
4.14-5.44 |
4.75±0.056A
|
4.22±0.403A
|
3.93-4.50 |
| Cu |
3.71±1.767a
|
2.46-4.96 |
4.54±0.989a
|
3.84-5.24 |
4.13±0.586A
|
7.40±3.478A
|
4.94-9.86 |
| Zn |
8.66±6.455a
|
4.09-13.22 |
9.34±2.743a
|
7.40-11.28 |
9.00±0.487A
|
30.10±23.129A
|
13.74-46.45 |
| Cr |
3.29±0.848a
|
2.69-3.89 |
6.80±0.049b
|
6.76-6.83 |
5.04±2.474A
|
6.40±0.127A
|
6.31-6.49 |
| Contaminants |
| Ni |
Undetectable |
undetectable |
undetectable |
undetectable |
Undetectable |
undetectable |
undetectable |
| Cd |
1.34±0.268a
|
1.15-1.53 |
2.22±0.374a
|
1.95-2.48 |
1.78±0.615A
|
1.68±0.516A
|
1.31-2.04 |
| Pb |
Undetectable |
undetectable |
undetectable |
undetectable |
Undetectable |
undetectable |
undetectable |
Table 6.
Chemical composition of fresh propolis and tincture.
Table 6.
Chemical composition of fresh propolis and tincture.
| Propolis |
Fresh |
Tincture |
| C |
S |
C+S |
| Mean ±SD |
Min-Max values |
Mean ±SD |
Min-Max values |
Mean ±SD |
Mean ±SD |
Min-Max values |
| Humidity (%) |
0.66±0.010a
|
0.65-0.67 |
0.35±0.010b
|
0.34-0.36 |
0.51±0.219A
|
96.79±0.188B
|
96.67-97.01 |
| DM (%) |
99.15±0.175a
|
99.00-99.34 |
98.47±1.051a
|
97.66-99.66 |
98.81±0.480A
|
3.14±0.173B
|
2.99-3.33 |
| Acidity |
5.95±0.010a
|
5.94-5.96 |
3.97±0.015b
|
3.95-3.98 |
4.96±1.400A
|
15.87±0.031B
|
15.84-15.90 |
| pH |
3.55±0.136a
|
3.44-3.70 |
3.92±0.020b
|
3.90-3.94 |
3.74±0.261A
|
2.95±0.025B
|
2.92-2.97 |
| Impurities (%) |
12.40±0.791a
|
11.83-13.30 |
5.74±0.393b
|
5.50-6.19 |
9.07±4.709A
|
0.09±0.015B
|
0.07-0.10 |
| Ash (%) |
0.94±0.010a
|
0.93-0.95 |
0.98±0.025a
|
0.95-1.00 |
0.96±0.028A
|
0.93±0.015A
|
0.92-0.95 |
| Macroelements |
| Na |
103.29±7.636a
|
97.89-108.69 |
110.33±6.648a
|
105.63-115.03 |
106.81±4.978A
|
84.63±4.956A
|
81.12-88.13 |
| Ca |
840.40±12.537a
|
831.54-849.27 |
587.05±63.01b
|
542.49-631.60 |
713.72±179.15A
|
437.36±18.915A
|
423.99-450.74 |
| K |
97.34±26.467a
|
78.62-116.05 |
128.79±44.491A
|
156.88-163.62 |
160.25±4.765a
|
17.85±3.068A
|
15.68-20.02 |
| Mg |
144.24±15.711a
|
133.13-155.35 |
148.99±0.084a
|
148.93-149.05 |
146.62±3.358A
|
58.26±4.617B
|
54.99-61.52 |
| P |
132.00±46.669a
|
99.00-165.00 |
152.63±31.99a
|
130.00-175.25 |
142.32±14.587A
|
7.88±3.005B
|
5.75-10.00 |
| Microelements |
| Fe |
54.58±19.183a
|
41.01-68.14 |
41.97±0.883a
|
41.34-42.59 |
48.27±8.909A
|
2.03±0.473B
|
1.69-2.36 |
| Mn |
4.91±0.650a
|
4.45-5.37 |
5.67±0.926a
|
5.01-6.32 |
5.29±0.530A
|
2.74±0.176B
|
2.61-2.86 |
| Cu |
3.13±1.506a
|
2.06-4.19 |
2.32±0.120a
|
2.23-2.40 |
2.72±0.572A
|
2.22±0.148A
|
2.11-2.32 |
| Zn |
15.85±6.533a
|
11.23-20.47 |
42.73±10.868a
|
35.04-50.41 |
29.29±19.007A
|
4.14±2.418A
|
2.43-5.85 |
| Cr |
Undetectable |
undetectable |
undetectable |
undetectable |
undetectable |
0.49±0.692 |
0.00-0.98 |
| Contaminants |
| Ni |
1.71±0.876a
|
1.09-2.33 |
undetectable |
undetectable |
Undetectable |
undetectable |
undetectable |
| Cd |
0.55±0.212a
|
0.40-0.70 |
0.24±0.091a
|
0.17-0.30 |
0.39±0.226A
|
0.62±0.176A
|
0.49-0.74 |
| Pb |
Undetectable |
undetectable |
undetectable |
undetectable |
Undetectable |
undetectable |
undetectable |
Table 7.
The DPPH radical scavenging activity (% inhibition) of ethanolic extracts vs. ascorbic acid.
Table 7.
The DPPH radical scavenging activity (% inhibition) of ethanolic extracts vs. ascorbic acid.
| Concen-tration (mg/mL) |
Samples |
Ascorbic acid |
| PS |
PC |
PT |
LMS |
LML |
LMC |
AS |
ALC |
AP |
Concen-tration (mg//mL) |
% inhi-bition |
| % Inhibition |
|
|
| 10 |
63.65 |
58.91 |
82.93 |
84.57 |
84.09 |
80.77 |
96.00 |
97.00 |
80.08 |
0.16 |
94.54 |
| 5 |
52.07 |
47.89 |
80.52 |
64.45 |
80.02 |
71.33 |
87.51 |
86.40 |
77.68 |
0.14 |
82.32 |
| 2.5 |
46.23 |
44.50 |
64.85 |
54.44 |
75.06 |
65.76 |
84.36 |
84.70 |
70.38 |
0.10 |
65.24 |
| 1 |
30.34 |
33.55 |
56.86 |
50.25 |
53.76 |
55.31 |
55.77 |
50.90 |
50.89 |
0.08 |
45.48 |
| 0.5 |
24.89 |
21.76 |
37.77 |
36.34 |
36.39 |
37.15 |
32.30 |
30.09 |
30.40 |
0.06 |
25.22 |
Table 8.
The IC50 value of samples vs ascorbic acid.
Table 8.
The IC50 value of samples vs ascorbic acid.
| Samples |
PS |
PC |
PT |
LMS |
LML |
LMC |
AS |
ALC |
AP |
Ascorbic acid |
| IC50 ± SEM (mg/mL) |
3.661 ± 0.002 |
3.979 ± 0.002 |
1.720 ± 0.001 |
2.276 ± 0.002 |
1.696 ± 0.001 |
1.832 ± 0.001 |
1.669 ± 0.001 |
1.830 ± 0.001 |
2.058 ± 0.001 |
2.284 0.001 |
| R2
|
0.9788 |
0.9752 |
0.9488 |
0.9497 |
0.9067 |
0.9556 |
0.9027 |
0.904 |
0.9017 |
0.9913 |
| Hill Slope |
9.925 |
8.864 |
11.398 |
11.066 |
12.166 |
10.326 |
15.914 |
16.932 |
12.615 |
17.548 |
Table 9.
MIC values recorded against the ATCC strains.
Table 9.
MIC values recorded against the ATCC strains.
| |
S. pyogenes |
S. aureus |
S. flexneri |
P. aeruginosa |
E. coli |
S. typhimurium |
H. influenzae |
B. cereus |
Cl perfringens |
L. monocytogenes |
C.parapsilopsis |
C. albicans |
| PS |
undetectable |
10 |
2.5 |
undetectable |
10 |
2.5 |
2.5 |
undetectable |
undetectable |
5 |
25 |
undetectable |
| PC |
undetectable |
undetectable |
undetectable |
undetectable |
undetectable |
undetectable |
undetectable |
undetectable |
undetectable |
undetectable |
undetectable |
undetectable |
| PT |
undetectable |
undetectable |
2.5 |
undetectable |
2.5 |
2.5 |
undetectable |
undetectable |
undetectable |
75 |
undetectable |
undetectable |
| LMS |
25 |
2.5 |
2.5 |
2.5 |
2.5 |
2.5 |
2.5 |
2.5 |
2.5 |
2.5 |
2.5 |
75 |
| LML |
50 |
10 |
2.5 |
7.5 |
2.5 |
2.5 |
2.5 |
5 |
10 |
2.5 |
2.5 |
25 |
| LMC |
50 |
2.5 |
2.5 |
50 |
2.5 |
2.5 |
2.5 |
50 |
50 |
2.5 |
2.5 |
75 |
| AS |
undetectable |
2.5 |
2.5 |
2.5 |
2.5 |
2.5 |
2.5 |
2.5 |
2.5 |
2.5 |
2.5 |
undetectable |
| ALC |
50 |
undetectable |
undetectable |
undetectable |
undetectable |
undetectable |
undetectable |
undetectable |
undetectable |
undetectable |
undetectable |
100 |
| AP |
undetectable |
2.5 |
2.5 |
2.5 |
2.5 |
2.5 |
2.5 |
2.5 |
2.5 |
2.5 |
2.5 |
undetectable |
Table 10.
The percentage of inhibition of haemolysis.
Table 10.
The percentage of inhibition of haemolysis.
| Samples abr. |
The concentrations of the samples |
| 25% |
50% |
75% |
100% |
25% |
50% |
75% |
100% |
| % Inhibition of haemolysis values |
% Inhibition of protein denaturation |
| PS |
47,22 |
52,78 |
57,28 |
58,83 |
19,81 |
82,77 |
83,23 |
83,32 |
| PC |
0,75 |
3,41 |
44,02 |
51,46 |
28,40 |
34,30 |
42,29 |
50,61 |
| PT |
-19,13 |
-5,99 |
0,84 |
18,71 |
10,98 |
8,64 |
0,77 |
-7,73 |
| LMS |
-18,17 |
-11,55 |
-8,73 |
-4,11 |
34,43 |
18,75 |
8,22 |
7,40 |
| LML |
41,56 |
47,2 |
55 |
59,58 |
81,17 |
71,18 |
65,82 |
65,03 |
| LMC |
-26,36 |
-13,36 |
-5,48 |
14,37 |
58,42 |
53,48 |
51,48 |
51,42 |
| AS |
-74,69 |
-73,92 |
-61,8 |
-42,14 |
49,03 |
38,13 |
34,69 |
9,81 |
| ALC |
-49,51 |
-5,78 |
-4 |
-2,85 |
45,63 |
30,35 |
14,07 |
13,99 |
| AP |
-34,41 |
-32,56 |
-15,59 |
44,72 |
58,83 |
58,05 |
51,15 |
50,33 |
| CTR -dexamethasone 0,1 mg/ml |
61,28 |
67,18% |