1. Introduction
Autism spectrum disorder (ASD) is a heterogenous group of neurodevelopmental disorders manifested by the presence of qualitative and quantitative deficits in the creation and maintenance of interpersonal relationships, social communication, repetitive and stereotyped behaviors and activities [
1]. In the latest editions of the ICD-11 (2019) and DSM-5 (2013) classifications, nosological units formerly functioning as separate within one diagnosis - ASD - have been unified.
Data from the Centers of Disease Control and Prevention (CDC, 2023) reported that in 2020, 1 in 36 children aged 8 years was diagnosed with ASD [
2]. This represents a sharp increase in the number of diagnoses since 1985, when the ratio was 1:2500 children. Later data from 1995 indicate the occurrence of ASD at a frequency of 1:500, while in 2005 it was 1:150 [
2]. It is suggested that the increase in the frequency of ASD diagnoses may be due to unspecified environmental factors or to a change in the diagnostic paradigm and a gradual widening of the existing criteria. The latter reason is probably also responsible for difference in the incidence of ASD between sexes, where the ratio of boys to girls ranges was 43.0:11.4 [
2]. A separate issue to remember is the hypothesis of common occurrence of the autistic traits in general population, which postulates a departure from the dichotomous division into
children without diagnosis and neuroatypical people. According to this concept, the severity of autistic traits in the population would be continuous, and the clinically relevant level would be determined by the individual needs of each person, with an ambiguous division into "autistic" and "non-autistic" people.
The pathophysiology of autistic traits, despite many studies and hypotheses, still remains unclear [
1]. Nevertheless, literature sources agree that they are primarily genetically determined, and their clinical presentation is subject to significant shaping byfactors of these environments [
3]. One of the hypotheses suggests the occurrence of intestinal microbiota disorders in children diagnosed with autism spectrum disorders, both in terms of quantitative and qualitative abnormalities (e.g. increased colony size, e.g.
Streptococcus, Clostridium and
Candida fungi with a reduced amount, e.g.
Pseudomonas or
Citrobacter). However, the direction of this type of dependence is not clear. Some authors suggest that dysbiosis may be the basis of some of the disorders observed in children with ASD.
Meanwhile, from the available literature in the case of healthy siblings, the microbiota profile was intermediate between the subjects and the population not affected by the disorder [
4]. This, in turn, may suggest an inverse relationship, in which ASD and the food selectivity observed in their course lead to microbiome abnormalities. The difference in the siblings' microbiome would then result from the family's diet adapted to the food selectivity of the child with autism, enriched with deviations in the form of, for example, snacks.
Such reports and studies direct the interest of scientists towards the impact of a proper diet on the clinical picture of ASD. One of the areas in this type of analysis is the use of elimination diets. In 2009 Mulloy et al. [
5] conducted a systematic review on the effect of gluten- and casein-eliminating diets on ASD symptoms. It showed that majority of studies that presented positive effect of nutritional interventions on ASD symptoms were of poor methodological quality. The rest of the studies analyzed showed negative or mixed effects. None of the papers gave conclusive results [
5,
6]. Despite the lack of convincing evidence, it is currently estimated that up to 26% of children with ASD are on a casein and gluten-free diet regardless of the existence of objective indications for its implementation [
4], which can also significantly affect the composition of the intestinal microflora. Notwithstanding in the case of patients with food intolerances, the use of elimination diets may bring some clinical effects. However, they are not related to the ASD itself, but rather to the coexisting alexithymia in this group of people which according to the literature may affect around 55% of ASD population.
The concept of alexithymia refers to problems with recognizing and distinguishing between emotions and sensations coming from the body, difficulties in expressing emotions, as well as a deficit of imagination. People with alexithymia may have difficulties in identifying and understanding the source of unpleasant gastric symptoms [
7], which are very common in people with ASD, although not secondary to that diagnosis, and depending on the source, occur in 9 to 90% of neuroatypical children. This relationship may be confirmed by a strong correlation between the severity of autism symptoms and the severity of gastrointestinal symptoms [
8]. In addition, the discomfort resulting from the ailments may generate behavioral disorders and difficulties in functioning, which may be wrongly perceived as elements of the clinical picture of ASD.
One of the key symptoms of ASD are repetitive and stereotyped patterns of interests and activities (RRB) which include adherence to rituals, the need for consistency, and poor tolerance for new and unfamiliar activities and sensations. DSM-5 also lists sensory integration disorders within the RRB domain. Originally described by Jean Ayres, they consist of the inability to organize the stimuli reaching the nervous system from the outside and integrate them with past experiences. As a consequence, hypersensitivity to noise, unpleasant tastes, smells and tactile stimuli occurs [
9].
Hypersensitivity to specific food characteristics, such as taste, color, temperature or texture, leads to food selectivity [
3,
10,
11]. Disorders of chewing food associated with unpleasant sensations during biting and with impaired muscle tone lead to impaired coordination of chewing and swallowing. As a consequence, there are serious dietary restrictions [
12]. . It has been suggested that food selectivity is more common in children with autism spectrum disorders and may be associated with nutritional deficiencies [
13,
14]. Food selectivity may also occur in typically developing children, but in the case of patients with ASD, the repertoire of food intake is more limited [
13,
15]. Refusing foods with specific characteristics or reluctance to try new foods may occur with varying frequency in many patients and may interfere with the child's daily functioning and integration in the peer group [
16].
The results of studies by several authors indicate that children with ASD have significantly noticeable food selectivity compared to children without ASD, which may result in nutrient deficiency [
17,
18,
19]. Considering how strongly dietary issues are related to the clinical picture and the functioning of people with ASD, it was of the interest how the food preferences of children on the spectrum are shaped. Previous studies of food selectivity in neuroatypical population suggest the need to investigate factors related to food selectivity, such as sensory integration disorders, behavioral disorders, or family food preferences.
2. Materials and Methods
The presented study was conducted using an authors’ own questionnaire addressed to parents/guardians of children. Demographic questions regarded the age and sex of the child, the place of residence and the fact if the examined person had a diagnosis of ASD. The main part of the questionnaire consisted of 37 items dividing foods into categories according to their crunchiness, appearance, taste, smell, temperature, color, moisture, consistency, degree of mixing of ingredients, consumption of vegetables, fruits, vitamins and supplements and willingness to try new dishes. The aim of the study was to determine how willingly a children eat food according to a specific characteristic of it on a 5-point scale: does not eat at all, eats reluctantly, has no influence, eats willingly, eats very willingly in order to check differences in the desire to eat food. The last part of the questionnaire would be a non-obligatory open question about the child's unusual behaviors related to nutrition.
Between May 2021 to December 2021, both electronic and paper-based methods were used to gather questionnaires for our study. The electronic questionnaire was primarily shared on social media, where we aimed at collecting the sample of parents and caregivers of children and adolescents without diagnosis of ASD.
In addition to our online efforts, we collected paper-based surveys in person at two primary locations. These included the John Paul II Paediatric Center in Sosnowiec and the Center for Therapy and Diagnostics of Children with Autism in Biała Podlaska. At these sites, we distributed the surveys to parents of children with ASD diagnoses, made by psychiatric specialists based on the DSM-V and ICD-10 criteria, during follow-up visits and handed out questionnaires directly to parents.
Inclusion criteria to our study group included ASD diagnosis made by psychiatry specialists based on DSM-V and ICD-10 criteria. Exclusion criteria included history of metabolic disorders and diseases requiring introduction of diets (f.e. food intolerances). In control group inclusion criteria comprised lack of the ASD diagnosis. Exclusion criteria included ASD diagnosis made by a psychiatry specialist, ASD diagnosis among 1st degree family and history of metabolic disorders and diseases requiring introduction of diets (f.e. food intolerances).
By strategically targeting various Facebook groups to form a control group and leveraging in-person data collection at key sites, we were able to assemble a comprehensive and diverse set of data for our study.
The data was collected into Excel and then subjected to statistical analysis in StatSoft Statistica software v. 13. Confidence level =0.05 was assumed. In order to carry out the statistical analysis, questions were also divided into two groups:
1. Sensory preferences – including questions about the color, structure, temperature or consistency of food (15 questions).
2. Stereotypical preferences – including questions about organization of ingredients on the plate (e.g. ingredients mixed/separated on the plate) or type of food (8 questions).
For further analysis, the collected values within the Likert scale were transformed assuming that the value of 3 points describes the "neutral" attitude to a given type of food, and the values obtained by the respondents were converted into a deviation from the value "0", obtaining the so-called "relative deviation" in the range of preferences (having values -2 to 2) indicating the direction of preference (did children of the respondents prefer to eat such foods or feel aversion to them), and then obtaining their absolute value using the modulus from the obtained number (values 0-2), which made it possible to compare the "strength" of preferences between groups.
The study included 219 people aged 3 to 18 years, of whom 115 had a diagnosis of autism spectrum disorder (52.3%) and 92 had no diagnosis. 12 children undergoing diagnosis were excluded from the analyses due to the high confounding potential therefore, 207 people were subjected to statistical analysis. The age range we established in our study, setting the lower limit at 3 years, is informed by the common diagnostic practices surrounding Autism Spectrum Disorder (ASD). It is frequently observed that diagnoses are made between the ages of 3 and 18. The early symptoms before the age of 3 years often go unrecognized by parents and specialists. It's important to note that these early symptoms under the age of 3 should be viewed as potential risk indicators for ASD, rather than definitive evidence of the disorder. Thus, we have set our lower age limit at 3 years to align our study with the age at which these early signs become more readily identified and result in a formal diagnosis.
Due to the assumed criteria, 21 individuals without an autism diagnosis and 11 individuals with an autism diagnosis were excluded because their ages did not fall within the specified range. Additionally, 12 individuals who self-reported not having received a complete psychiatric diagnosis yet were also excluded. These individuals demonstrated certain symptoms, yet the absence of a definitive diagnosis risked introducing inconsistencies into our study. Therefore, they were not included in our analysis. In summary, a total of 44 individuals were excluded from the study due to the rigorous application of inclusion and exclusion criteria.
Children with a diagnosis formed the study group, while children who did not have ASD were the control group. In order to better assess the effect of age on food preferences, the analyzed group was also divided into age subgroups according to the most commonly used method of periodization of human development:
Middle childhood (preschool age) – 4-6 years of age (this group also includes people in 3 years of age
Late childhood (school age) – 7 – 12 years of age
Early adolescence – 13-16 years of age
Late adolescence 17-18 years of age
However, due to the low size of group 4, it was decided to merge it with group 3 into one "adolescence".
Table 1 presents a distribution of the diagnosis in the created groups.
3. Results
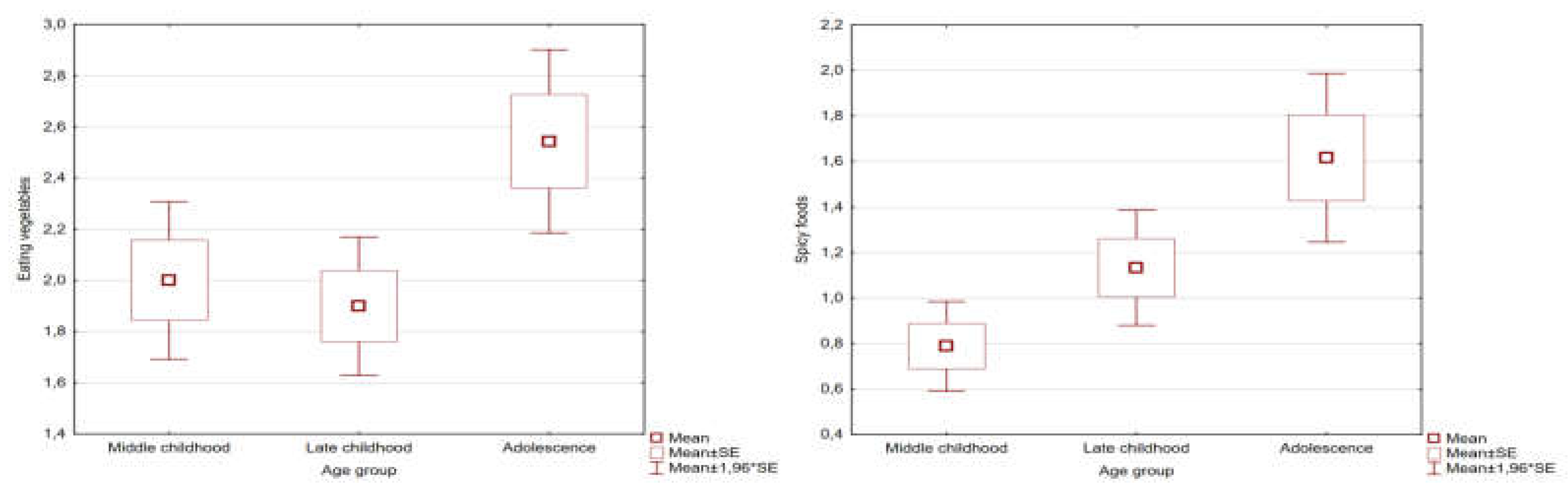
The mean age of the study group was 8.6 (95%Cl: 7.9-9.3) years, while for the control group it was a 9.42 (95%Cl: 8.5-10.3). The age difference between the groups was not statistically significant, and the age of the respondents alone had no significant effect on the mean relative and absolute deviations of food preference parameters in the Spearman rho analysis. Furthermore, in the analysis broken down by age subgroups using the Kruskal-Wallis ANOVA test, no statistically significant differences were observed in the raw values of the analyzed parameters for all variables except spicy foods (p = 0.02) and the desire to eat vegetables (p = 0.02). These differences were presented on
Figure 1. Diagnosis and gender did not affect the obtained results.
Females accounted for 37.19% (n=77) of the group. In the study group this percentage was 25.3% (n=29) and in the control group 52.17% (n=48), the difference was statistically significant in the chi 2 test with p<0.05. In contrast to age, the gender of the respondents had a statistically significant effect on the parameters of food preferences in the entire population, however, this result lost its statistical significance when analyzed separately within the study and control groups.
Table 2 shows descriptive statistics for raw values of statistically significant variables: mean values, standard deviations, minimum and maximum values, Mann-Whitney U test results. In the analysis based only on the values translated from the Likert scale, it was shown that there were differences in preferences between children diagnosed with ASD and without diagnosis in the case of 9 variables. It has been observed that children with ASD show a clear preference to avoid meals that are sticky or acidic and avoid trying things they do not know (trying novelties). Moreover, after averaging the preference values across all variables, it was shown that, overall, people diagnosed with ASD in all categories scored lower than
children without diagnosis subjects – 2.19 (95%CI:2.08-2.30) vs. 2.45 (95%CI: 2.34-2.56) with p=0.001 in the Mann-Whitney U test.
The average number of "food selectivity traits" in the group of children with and without autism diagnosis was also analyzed, where answers "does not eat" and "eat reluctantly" to questions about food preferences were treated as a "food selective trait". In each of the subjects, the above-mentioned answers were added together and the average number of traits per child was calculated. The average "food selectivity traits" in those diagnosed with ASD was 9.1 and in those without a diagnosis 6.9. Autistic people exhibited 24% more of these traits.
Mean relative and absolute deviations from the neutral approach to all analyzed food groups and in terms of sensory and stereotyped preferences in groups of people with ASD and
children without diagnosis were also analysed. A comparison of deviations in sensory preferences and stereotyped preferences in both groups was also performed with the Wilcoxon test for dependent variables. The results of the analyses are presented in
Table 3.
The effect of food colour on the willingness to eat a given food was also analyzed, with no significant differences in the absolute degree of preferences between the study and control groups. Interestingly, in both groups, a significant, negative effect of the determined colour of food on the child's preferences was observed (average absolute value >1 points for all colours) regardless of belonging to the study and control groups. In terms of food consistency, type that showed a difference in absolute deviation were sticky foods, which were significantly less likely to be consumed by people in the study group (-1.09 [95%CI: -1.26 - -0.94] vs. -0.60 [95%CI: -0.77 - -0.42]; p<0.05).
Subsequently a logistic regression analysis was carried out, modelling the predictive effectiveness of the mean relative deviation parameters in the range of preferences for the respondent's belonging to the control group. The created model obtained statistical significance with p=0.007 and the obtained parameters were OR=0.09 (95%CI: ±0.001) for sensory selectivity and OR=0.56 (95%CI: ±0.16) for stereotypical selectivity. Next, the predictive capacity of absolute values was analysed, obtaining similar results. Finally, an analysis was carried out for relative and absolute deviation values for average values of variables concerning preferences as to colour, taste and structure of food, respectively, obtaining a model devoid of statistical significance. The results of the questionnaire are included in
Table 4.
4. Discussion
Authors should discuss the results and how they can be interpreted from the perspective of previous studies and of the working hypotheses. The findings and their implications should be discussed in the broadest context possible. Future research directions may also be highlighted.
In the presented study, no statistically significant difference in age was observed between the study and control groups, and the age of the respondents itself did not significantly affect their food preferences, as a rule, it coincides with the results of other studies [
20,
21]. Statistically significant differences in the gender distribution of respondents were observed between groups. This was the expected result given the sex ratio in the group of people diagnosed with ASD. However, the observed disproportion did not significantly affect the results obtained due to the lack of relationship between the sex of the subjects and food selectivity shown by other researchers [
20].
Analysis of raw parameters representing respondents' answers to specific questions indicates that, on average, children with ASD show more food selectivity traits compared to children without a diagnosis. Although the severity of food selectivity in neuroatypical children was greater than in
children without diagnosis, it should be emphasized that children without ASD diagnosis also showed specific food preferences especially in terms of the specific taste of the dishes. The obtained results coincide with studies by Tomova et al.[
22] and Vernocchi et al.[
23], who also described the frequent occurrence of food selectivity in children with autism spectrum disorder but also the presence of food selectivity in some
children without diagnosis [
23,
24].
The obtained data also suggest that they are more likely to refuse foods with mixed ingredients and dishes in which individual ingredients have come into contact on the plate. The obtained results coincide with the available literature, which also indicate a preference for dishes with a uniform structure and the separation of individual elements of the dish on the plate [
13,
21]. The effect of a specific appearance of a meal on the willingness to eat it, suggested by some authors [
20], was not present in the studied group. However, the fact of contact between the products on the plate was a significant food preference in the group of neuroatypical children.
In terms of the taste of the dish, it is worth noting that in the presented study, children with ASD presented significant aversion to the sour food but for the remaining flavours there was no statistically significant difference. This is especially interesting in context of data available in literature indicating that patients aged 10-19 years with ASD are less accurate in identifying sour and bitter tastes [
22]. In terms of specific types of foods, the presented study indicates that children with ASD showed statistically significantly greater aversion to sticky foods compared to
children without diagnosis. Such difficulties may be a manifestation of sensory integration disorders as an axial symptom of ASD. These results may be indirectly consistent with studies showing that children with ASD prefer crispy products [
14,
25,
26]. If we assume that stickiness is the inverse of crunchiness, then the research has a common denominator. Neuroatypical people were also less likely to reach for fruit than
people without diagnosis. The study by Cornish et al. [
18] indicates the reluctance of children with ASD to eat fruits and vegetables. Perhaps this is due to the fact that people with ASD have trouble understanding their parents' point of view on the importance of eating fruits and vegetables that are perceived as healthy.
Neuroatypical children presented a significantly greater severity of food restriction that
children without diagnosis group, and, interestingly, they showed more deviations towards "preferences" than "aversions" to the specific foods. Valenzuela-Zamora et al. [
26] describe the phenomenon of a strong aversion to specific foods due to their texture, temperature, taste, colour and smell with a coexisting preference for specific foods with different characteristics. Among children with ASD, a significantly higher severity of sensory food preferences were observed [
3,
8]. In addition, people with ASD are less likely to change their habits [
27], and may be less likely to give up a "safe" eating regimen including products that have been introduced earlier. Food selectivity may be observed in the population of children up to age of six regardless of diagnosis, however, in
children without diagnosis there is a reduction in selectivity after the of 6, while in neuroatypical children selectivity may persist throughout life. The expansion of the nutritional repertoire in children without diagnosis may be explained by the social function of food consumption [
28].
Literature reports indicate the occurrence of food selectivity in children with ASD. In a series of studies, the most frequently appearing element that is subject to selectivity is the texture of dishes. Not without significance are also the appearance, taste, smell and temperature of food. It is also indicated that there is a reluctance to try new dishes and to take medication. Available literature data and results obtained in the presented study indicate that the symptom of food selectivity is a heterogeneous phenomenon covering several diagnostic domains. This interpretation of selectivity seems to explain the variability in its prevalence and the nature of the population of children with ASD. The results of the presented study indicate that patients observe both symptoms of sensory aversion to certain food structures as well as symptoms of stereotypical attachment to certain feeding patterns or organization of dishes on the plate.
Also, statistical analysis indicates that although both the sensory and stereotyped domain clearly distinguish itself in the group of people with ASD, the food parameters associated with stereotypes are significantly more intense than sensory parameters. Logistic regression also indicates that stereotyped symptoms in terms of selectivity discriminated better against people without diagnosis than neuroatypical people, and their presence were significantly linked to the risk of ASD diagnosis. Perhaps this is due to the fact that stereotypical habits in children with ASD may seem more unusual to the parent and thus easier to notice, which may affect the frequency of their reporting.
Although parents of children with ASD are more likely to report food selectivity in their children compared to parents of children without a diagnosis, available literature points out that there is a relationship between an individual's eating habits and the eating habits of his entire family [
29]. In this context, an interesting issue may be the occurrence of broad autism phenotype (BAP) or simply subclinical features of ASD in the family of children studied. As these are genetic disorders, it can be assumed that they will be passed from generation to generation within one family [
30] determining the dietary choices of its members. In subsequent generations, the eating habits of a given family may be shaped on the basis of food choices of older family members with subclinical autistic features, which in turn may deepen the food selectivity of the child on the spectrum. However, this type of phenomenon requires further research to understand its fastic significance and assess its scale.
Food selectivity leading to excessive consumption of certain types of foods and limiting the consumption of others is not only a problem in terms of finding acceptable meals, but can also lead to a number of health complications. The most visible health risks are gastrointestinal ailments. According to researchers, they may concern up to 88.9% of children and adolescents with ASD, with girls more often affected [
20]. In other works, the frequency of gastrointestinal symptoms such as constipation, diarrhoea, abdominal pain, bloating or gastroesophageal reflux is estimated to 23-70% of neuroatypical population [
31].
Difficulties in estimating the real scale of the phenomenon may result from the fact that, especially in children with ASD with limited verbal communication, some of the symptoms may be overlooked [
32]. Gastrointestinal symptoms in children with ASD are present in all age groups and, as with food selectivity, their degree is not correlated with the severity of ASD symptoms [
33]. The most common gastrointestinal symptom in children with ASD is constipation, which can be explained by the aversion to fiber-rich foods such as vegetables also demonstrated in our study[
26]. What's more, an additional factor hindering the maintenance of a proper diet may be the co-occurrence of several diseases, e.g. inflammatory bowel disease (IBD), type 1 diabetes, neurological diseases: CNS anomalies, skull structure or muscular dystrophy with ASD[
34]. The microbiota of children with ASD, just as their eating habits, is different from
children without diagnosis. The core microbiota, i.e. the number and type of bacteria that are common to different individuals [
23]) is similar in children with and without ASD, but it has been found that
Dichelobacter, Nitriliruptor and Constrictibacter may be probable markers of ASD.
Evidence suggests that food selectivity may be associated with changes in the gut microbiota in children with ASD. The intestinal microbiota in children with ASD presenting food selectivity has a more heterogeneous composition compared to children with ASD who did not present food selectivity or presented it to a lesser extent. Food selectivity makes it possible to distinguish the fecal microbiota of "picky eaters" from "non-nutritious eaters" among children with ASD. Representatives of
Enterobacteriacaea,
Escherichia/Shigella and
Salmonella from
Proteobacteria, as well as
Clostridium XlVa,
Anaerofilum from
Clostridia,
Firmicutes , turned out to be characteristic of "picky eaters". These types of bacteria may also be associated with a higher incidence of gastrointestinal symptoms in children with ASD.
Prevotella, Bacteroides
, Parabacteroides and
Bacteroidetes characterize "indiscriminate eaters". Therefore modulating the eating habits of children with ASD may affect the composition of the gut microbiota, which is particularly important because changes in the gut microbiota are associated with behavioral symptoms of ASD by acting on the microbiota-gut-brain axis [
35].
As the role of the gut microbiota in gastrointestinal diseases is increasingly recognized, ways in which it can be modulated have been investigated. For microbiome disorders occurring in ASD, the combination of diet, prebiotics, and probiotics may provide a potential therapeutic solution to restore and maintain a normal microbiome. Various studies using animal models with ASD, as well as clinical trials in children with ASD, have investigated the use of probiotics as a potential treatment for ASD. Probiotic interventions were variedin preclinical and clinical studies, and most supplemented probiotics consisted of Lactobacillus species. In some clinical trials, probiotics have shown potential benefits in alleviating gastrointestinal disorders and in reducing the severity of ASD symptoms by correcting dysbiosis (36). It should be noted, however, that these effects were not due to the effect of the described interventions on the axial symptoms of ASD, but rather by improving the functioning of people with ASD by reducing the number of gastrointestinal symptoms felt.
Studies show that food selectivity can lead to a deficiency of vitamins K, B6, C, iron, copper, docosahexaenoic acid and docosapentanoic acid[
11], which suggests the potential benefits of supplementation of these ingredients. In addition, the increasingly discussed impact of the microbiome on ASD also encourages parents to try various dietary interventions. Some studies indicate that up to 25% of people with ASD use dietary interventions, but this percentage may vary depending on the country in which the study was conducted. The results regarding the effectiveness of dietary interventions such as restrictive diets (most often gluten-free and casein-free) and dietary supplements such as vitamins, minerals, amino acids, omega-3s and herbal compounds in ASD are still controversial [38]. This meta-analysis showed that in people with ASD, dietary supplementation with omega-3 acids and vitamin supplementation was more effective than placebo in improving specific symptoms, e.g. omega-3 supplementation worked better than placebo, improving language function and social functioning, while vitamin supplementation reduced stereotypes and had a positive effect on behavioral symptoms. The cited meta-analysis also draws attention to the low size of the effect of the analyzed studies compared to placebo. Thus, they suggest that dietary supplements may exert a nonspecific and minor effect on ASD [
37].
5. Conclusions
The results of the presented study indicate a higher incidence of food selectivity and its greater severity in children with ASD than in children without diagnosis. It should be pointed out that children without diagnosis also present food selectivity to some extent. Especially in the context of taste aversions, which were more unambiguous in the group of people without diagnosis. In addition, food preferences in children with ASD can be varied. The results obtained in the present study indicate that the symptoms of food selectivity constitute a heterogeneous phenomenon covering several diagnostic domains. This interpretation seems to explain well the variability in prevalence and the nature of selectivity in the population in children with ASD. Although it is partially linked to the sensory domain, food selectivity in neuroatypical population seem to be predominantly stereotypical in its nature.
Author Contributions
Conceptualization, Anna.Byrska., Idalia.Błażejczyk. and Krzysztof.M.Wilczyński; methodology, Idalia.Błażejczyk. and Krzysztof.M.Wilczyński; software, Maria.Potaczek. and Anna.Faruga; validation, Anna.Byrska. and Anna.Faruga..; formal analysis, Maria.Potaczek., Anna.Faruga. and Krzysztof.M.Wilczyński; investigation, Idalia.Błażejczyk.; resources, Anna.Byrska.; data curation, Maria.Potaczek.; writing—original draft preparation, Maria.Potaczek, Anna.Byrska. and Idalia.Błażejczyk.; writing—review and editing, Krzysztof.Wilczyński.; visualization, Anna.Faruga.; supervision, Małgorzata Janas-Kozik.; project administration, Krzysztof.M.Wilczyński.; All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and was part of the study approved by the Institutional Ethics Committee of Medical University of Silesia (KNW/0022/KB1/123/18/19).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Acknowledgments
We want to thank the Center for Therapy and Diagnostics of Children with Autism in Biała Podlaska for the opportunity for collecting the surveys among their patients.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lord, C., Elsabbagh. Autism spectrum disorder. Lancet 2018, 392, 508–520. [Google Scholar] [CrossRef] [PubMed]
- Maenner MJ, Warren Z, Williams AR; et al. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years — Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2020. MMWR Surveill Summ 2023, 72, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Gerhant, A., Olajossy. Neuroanatomical, genetic and neurochemical aspects of childhood autism. Pol. Psychiatry 2013, 47. [Google Scholar]
- Ciéslińska, A., Kostyra. Treating autism spectrum disorder with gluten-free and casein-free diet: the underlying microbiota-gut-brain axis mechanisms. HSOA J. Clin. Immunol. Immunother. 2017, 3. [Google Scholar]
- Mulloy, A., Lang. Gluten-free and casein-free diets in the treatment of autism spectrum disorders: A systematic review. Res. Autism Spectr. Disord. 2010, 4, 328–339. [Google Scholar] [CrossRef]
- Abdellatif, B., McVeigh. The promising role of probiotics in managing the altered gut in autism spectrum disorders. Int. J. Mol. Sci. 2020, 21, 4159. [Google Scholar] [CrossRef]
- Kinnaird, E., Stewart. Investigating alexithymia in autism: A systematic review and meta-analysis. Eur. Psychiatry 2019, 55, 80–89. [Google Scholar] [CrossRef]
- Vuong, H. E., & Hsiao. Emerging roles for the gut microbiome in autism spectrum disorder. Biol. Psychiatry 2017, 81, 411–423. [Google Scholar] [CrossRef]
- Bandini, L. G., Anderson. Food selectivity in children with autism spectrum disorders and typically developing children. J. Pediatr. 2010, 157, 259–264. [Google Scholar] [CrossRef]
- Cermak, S. A., Curtin. Food selectivity and sensory sensitivity in children with autism spectrum disorders. J. Am. Diet. Assoc. 2010, 110, 238–246. [Google Scholar] [CrossRef]
- Marí-Bauset, S., Zazpe. Food selectivity in autism spectrum disorders: a systematic review. J. Child Neurol. 2014, 29, 1554–1561. [Google Scholar] [CrossRef] [PubMed]
- Masgutova, S., & Masgutov. Reflex integration disorder as a ne w treatment paradigm for children with autism. A Collective Work; SMEI: Florida, FL, USA, 2015; pp. 171–180. [Google Scholar]
- Page, S. D., Souders. Correlates of Feeding Difficulties Among Children with Autism Spectrum Disorder: A Systematic Review. J. Autism Dev. Disord. 2022, 52, 255–274. [Google Scholar] [CrossRef]
- Nadon, G., Feldman. Association of sensory processing and eating problems in children with autism spectrum disorders. Autism Res. Treat. 2011, 2011. [Google Scholar] [CrossRef]
- Skawina, B. "Autism and Asperger's syndrome, symptoms, causes, diagnosis and modern therapeutic methods". Nová Sociálna Edukácia Človeka V Medzinárodná Interdiscip. Ved. Konf. Prešov 2016, 7. [Google Scholar]
- Siracusano, M., Riccioni. Vitamin D deficiency and autism spectrum disorder. Curr. Pharm. Des. 2020, 26, 2460–2474. [Google Scholar] [CrossRef]
- Robea, M. A., Luca. Relationship between vitamin deficiencies and co-occurring symptoms in autism spectrum disorder. Medicina 2020, 56, 245. [Google Scholar] [CrossRef]
- Cornish, E. A balanced approach towards healthy eating in autism. Top. Clin. Nutr. 1999, 14, 85. [Google Scholar] [CrossRef]
- Babinska, K., Celusakova. Gastrointestinal symptoms and feeding problems and their associations with dietary interventions, food supplement use, and behavioral characteristics in a sample of children and adolescents with autism spectrum disorders. Int. J. Environ. Res. Public Health 2020, 17, 6372. [Google Scholar] [CrossRef]
- Schreck, K. A., & Williams. Food preferences and factors influencing food selectivity for children with autism spectrum disorders. Res. Dev. Disabil. 2006, 27, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Bennetto L, Kuschner ES, Hyman SL. Olfaction and taste processing in autism. Biol. Psychiatr. 2007, 62, 1015–21. [Google Scholar] [CrossRef]
- Tomova, A., Sołtys. The influence of food intake specificity in children with autism on gut microbiota. Int. J. Mol. Sci. 2020, 21, 2797. [Google Scholar] [CrossRef] [PubMed]
- Vernocchi, P., Ristori. Gut microbiota ecology and inferred functions in children with ASD compared to neurotypical subjects. Front. Microbiol. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Huxham, L., Marais. Idiosyncratic food preferences of children with autism spectrum disorder in England. South Afr. J. Clin. Nutr. 2021, 34, 90–96. [Google Scholar] [CrossRef]
- Gray, H. L., & Chiang. Brief Report: Mealtime Behaviors of Chinese American Children with Autism Spectrum Disorder. J. Autism Dev. Disord. 2017, 47, 892–897. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela-Zamora, A. F., Ramírez-Valenzuela. Food selectivity and its implications associated with gastrointestinal disorders in children with autism spectrum disorders. Nutrients 2022, 14, 2660. [Google Scholar] [CrossRef]
- Chistol, L. T., Bandini. Sensory sensitivity and food selectivity in children with autism spectrum disorder. J. Autism Dev. Disord. 2018, 48, 583–591. [Google Scholar] [CrossRef]
- Poljac, E., Hoofs. Understanding Behavioural Rigidity in Autism Spectrum Conditions: The Role of Intentional Control. J. Autism Dev. Disord. 2017, 47, 714–727. [Google Scholar] [CrossRef]
- Tanoue, K., Takamasu. Food repertoire history in children with autism spectrum disorder in Japan. Pediatr. Int. Off. J. Jpn. Pediatr. Soc. 2017, 59, 342–346. [Google Scholar] [CrossRef]
- Curtin, C., Hubbard. Food selectivity, mealtime behavior problems, spousal stress, and family food choices in children with and without autism spectrum disorder. J. Autism Dev. Disord. 2015, 45, 3308–3315. [Google Scholar] [CrossRef]
- Hirokawa, K., Kimura. Associations Between Broader Autism Phenotype and Dietary Intake: A Cross-Sectional Study (Japan Environment & Children's Study). J. Autism Dev. Disord. 2020, 50, 2698–2709. [Google Scholar]
- Ristori, M. V., Quagliariello. Autism, Gastrointestinal Symptoms and Modulation of Gut Microbiota by Nutritional Interventions. Nutrients 2019, 11, 2812. [Google Scholar] [CrossRef] [PubMed]
- Holingue, C., Poku. Gastrointestinal concerns in children with autism spectrum disorder: A qualitative study of family experiences. Autism Int. J. Res. Pract. 2022, 26, 1698–1711. [Google Scholar] [CrossRef] [PubMed]
- Adams, J. B., Johansen. Gastrointestinal flora and gastrointestinal status in children with autism--comparisons to typical children and correlation with autism severity. BMC Gastro\Enterol. 2011, 11, 22. [Google Scholar] [CrossRef]
- Kohane, I. S., McMurry. The co-morbidity burden of children and young adults with autism spectrum disorders. PloS One 2012, 7, e33224. [Google Scholar] [CrossRef] [PubMed]
- Fattorusso, A., Di Genova. Autism Spectrum Disorders and the Gut Microbiota. Nutrients 2019, 11, 521. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P. A., Hyman. Dietary Supplementation in Children with Autism Spectrum Disorders: Common, Insufficient, and Excessive. J. Acad. Nutr. Diet. 2015, 115, 1237–1248. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).