1. Introduction
Application of tissue-engineered conduits in peripheral nerve regeneration has been extensively studied and successfully implemented in clinical practice [
1]. Although the peripheral nervous system (PNS) possesses inherent regenerative capabilities, simple transverse or small-scale injuries can be effectively treated with epineural and/or fascicular sutures [
2]. However, larger gaps in nerve segments (>3 mm) present a challenge, as axonal reconnection cannot occur. Autologous nerve grafting (typically from the sural nerve of the patient) has become the “gold standard” for treating long-gap nerve defects. Autologous cells provide a natural nerve structure that serves as a template for regeneration. Additionally, extracellular matrix proteins (ECM), Schwann cells, and growth factors create an ideal microenvironment for peripheral nerve regeneration [
3]. However, autologous grafts also have inherent disadvantages including limited donors, loss of donor function, neuroma formation, nerve distortion or dislocation, and nerve diameter mismatch. Tissue reperfusion following autologous nerve transplantation causes apoptosis and necrosis. These limitations of autografts have prompted the development of alternative therapeutic options to improve patient outcomes [
4].
In this context, tissue-engineered peripheral nerve guidance conduits (NGCs) have emerged as a viable solution to overcome the limitations of autologous tissues [
5]. Typically, NGCs are cylindrical tubular structures composed of degradable or nondegradable synthetic or natural materials that are implanted at the site of a nerve defect to facilitate specific regeneration of proximal axons toward their distal target organ [
6]. Numerous artificial peripheral nerve repair grafts based on commercial products have been successfully implemented in clinical practice, including the Avance Nerve Graft (AxoGen, Inc.), NeuraWrapTM (Integra Life Sciences), and NeuroFlex (Collagen Matrix). With development of neural tissue engineering and regenerative medicine, several ideal design requirements have emerged as guiding principles for continued nerve graft development [
5]. NGCs in clinical application should have the following properties: (1) excellent bio-activity that can guide axons to grow from the proximal end into the distal stump, avoiding formation of neuroma [
7]; (2) mechanical performance to avoid wall breakage during suture and sufficient flexibility to avoid compression of neural tissue [
8]; (3) biocompatibility and nontoxicity to cells and tissue, with almost no immune response [
8]; (4) a controllable degradation rate that matches the regeneration phase time of peripheral nerves, avoiding a second operation to remove undegraded implant materials and compression of the regenerated peripheral nerve by undegraded NGCs [
9]; (5) a suitable porous structure that can limit invasion of fibrous scarring into the NGC, hinder axon growth, and facilitate communication of biomolecule signals inside and outside the conduits and between cells and axons [
10].
A porous structure with suitable pore size and connectivity has been shown to be a determining factor for completion of the biological functions of NGCs, promoting early adhesion, spreading, proliferation, and differentiation of Schwann cells to form cord-like structures (Bungner bands), promote vascularisation, and reduce formation of fibrous scars [
11]. The concept of a nonpermeable silicone “nerve regeneration chamber” presented by Lundborg
et al. [
12] in the 1990s must be innovated, although its closed space can enrich bioactive factors (such as PDGF, FGF, and TGF-β secreted by Schwann cells or macrophages) and effectively prevent invasion of fibrous scars.
In vivo studies [
13,
14] have shown that the peripheral nerve repair effects of hollow impermeable conduits are outmatched by those of porous, permeable conduits. The 3D topology of the NGC directly affects the behaviour of cells during nerve regeneration. The porosity and permeability of NGCs play important roles in the flow of oxygen, nutrients, and bioactive molecules between the internal and external environments [
15]. Thus, porous structures with specific biological functions are critical for positive results in peripheral nerve repair. However, porous structures also have drawbacks; an excessively large pore size causes fibroblast deposition and hinders axon growth [
2]. A lack of porosity affects the exchange efficiency of the internal and external walls of the conduits [
11]. What type of porous structure should ideal NGCs have? What effect does the pore structure have on the physiological process of peripheral nerve regeneration? These questions do not seem to have clear answers in previous research. Transformation of peripheral nerve tissue repair materials in clinical applications can be promoted through summary and analysis of the effects of porous structures on the regeneration and repair of NGCs.
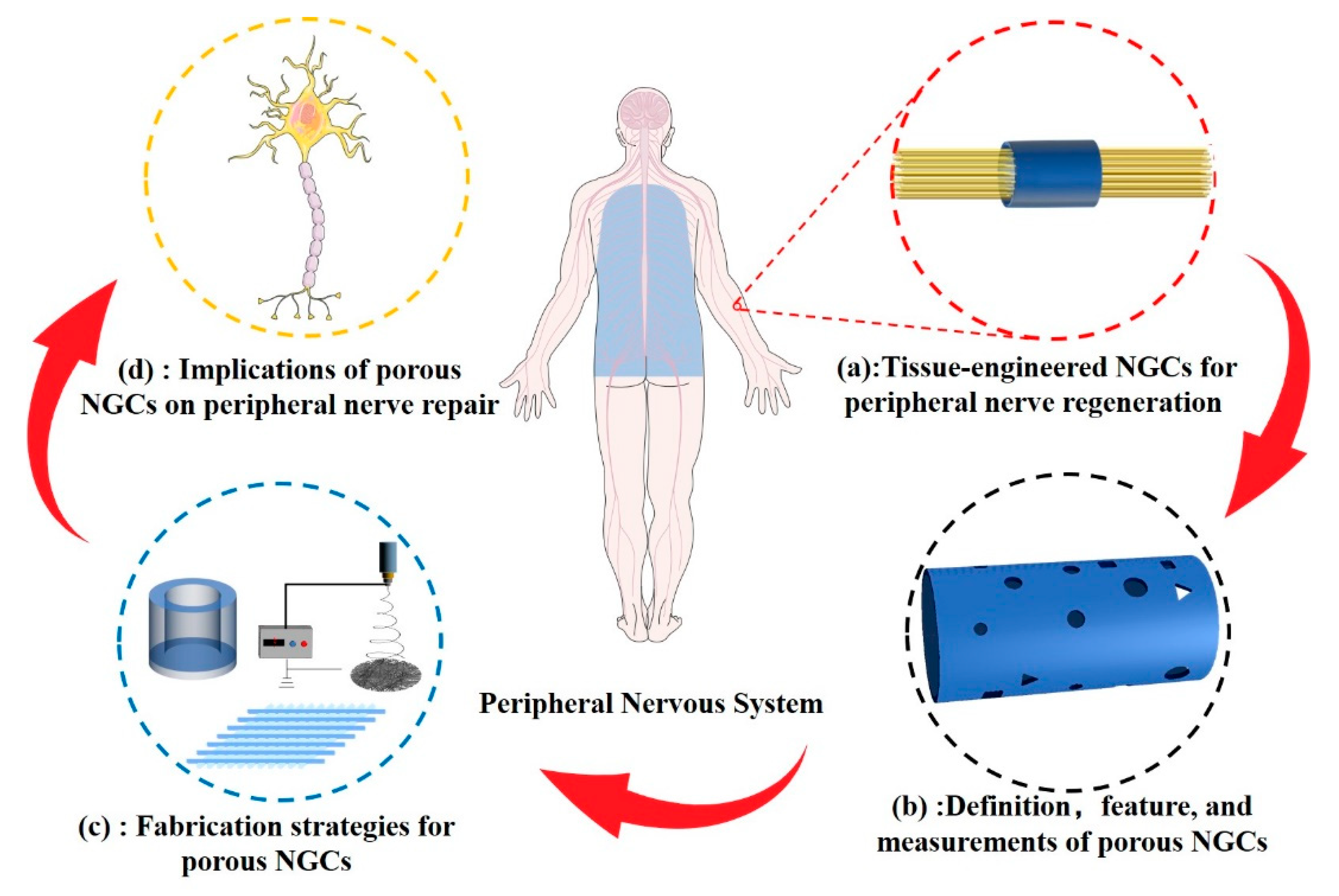
As shown in
Figure 1, this review reports the latest progress and applications of porous structures in peripheral nerve tissue-engineered conduits. Classification of NGCs according to pore structure characteristics and current methods of porosity measurement are summarised. We describe the techniques and principles used in constructing porous structures in NCGs. The physical properties of the porous structure including biodegradability, mechanical performance, and permeability of NGCs, and the biological behaviour of cells related to peripheral nerve regeneration are discussed.
2. Definition and measurement of pore structure
The porous structure of an NGC refers to the three-dimensional morphology of closed or penetrating voids in the biomaterials [
16]. The pore structure is characterised by size, connectivity, uniformity, and three-dimensional morphology, which affect the biocompatibility, permeability, density, and mechanical performance of the NGC [
11]. Generally, biomaterial pores can be delineated according to their size as macropores (100–500 μm), micropores (< 100 μm), and nanopores (< 1000 nm) [
17]. The pore size of NGCs determines which molecules can be exchanged between the regenerated peripheral nerve and the surrounding microenvironment through the conduit walls. Depending on whether the cells can freely infiltrate the inside of the conduits, NGCs can be categorised as semi-permeable (< 10 μm) or fully permeable (> 50 μm) membranes [
18]. Spherical, tubular, and irregularly shaped porous NGCs have been fabricated; however, with development of new technologies, more complex structures with higher resolution will be developed to meet the needs of peripheral nerve regeneration [
19]. Based on these interconnections, pore structures can be divided into isolated and open structures. Almost all studies have considered open and penetrating pore structures; isolated and closed pore structures fail to achieve material exchange and reduce the mechanical performance of NGCs.
Pore size, porosity, permeability, and connectivity are important in characterising the porous structure of NGCs, particularly in development of innovative 3D topographies as they play a decisive role in nerve regeneration. Conduits constructed from spun fibres are usually characterised by the fibre diameter and thickness. The morphology of porous NGCs can be observed through field-emission scanning electron microscopy (SEM) and transmission electron microscopy (TEM); the pore size distribution can be statistically analysed using image analysis software [
16]. This method has been widely used to characterise porous biomaterials. Although it allows for intuitive observation of the 2D morphology of NGCs, it falls short of revealing the complete 3D morphology, and its objectivity is subject to the observation site.
Porosity is defined as the void volume as a percentage of the total NGC volume[
20]. The liquid displacement method in an ethanol medium has been widely used in recent studies. The main steps are described as follows [
21]. The conduits are immersed in a known volume (V
1) of anhydrous ethanol. Ethanol is forced into the pores of the conduit through a series of vacuum-release cycles, and the volume of the solution is recorded as V
2. The conduits are removed, and the volume is recorded as V
3. The NGC porosity is calculated using the following equation: Porosity rate (%) = (V
1 - V
3)/(V
2 - V
3)×100%. This method is simple and convenient, and is not limited by the experimental equipment; however, the accuracy varies due to the volatility of ethanol, and closed pores cannot be measured. The gas adsorption method is an alternative for measuring the porosity and pore distribution of a conduit and is based on the adsorption of gas molecules on a solid surface [
22]. The principle of this method is exposure of a solid material to a gas environment at pressure, calculating the porosity and pore size distribution of the solid material by measuring the change in the gas adsorption [
23]. Both approaches have advantages and disadvantages. The liquid displacement method can measure larger pores but is insensitive to pores below the surface tension. The gas adsorption method is sensitive in measuring micropores but ignores the number of macropores and macroscale pores.
Permeability is primarily determined by the size and connectivity of the pore structure [
11]. NGCs with larger pores and high connectivity allow molecules and cells to in-fold and out-fold the tube walls more freely. Waste products, such as cellular phospholipid debris due to Wallerian degeneration after peripheral nerve injury, should be released from conduits. Macrophages, vascular endothelial cells, fibroblasts, and Schwann cells gather at the site of peripheral nerve injury and participate in regeneration of peripheral nerves by releasing bioactive signals via autocrine or paracrine pathways. Thus, the efficiency and mode of substance exchange inside and outside NGCs are important standards for evaluating the conduit repair function. Solutes with different molecular weights including glucose (Mw 180 Da), lysozyme (Mw 14600 Da), and BSA (Mw 62000 Da) were used to simulate the osmotic flow dynamics of signal molecules with different molecular weights in the tube wall [
24].
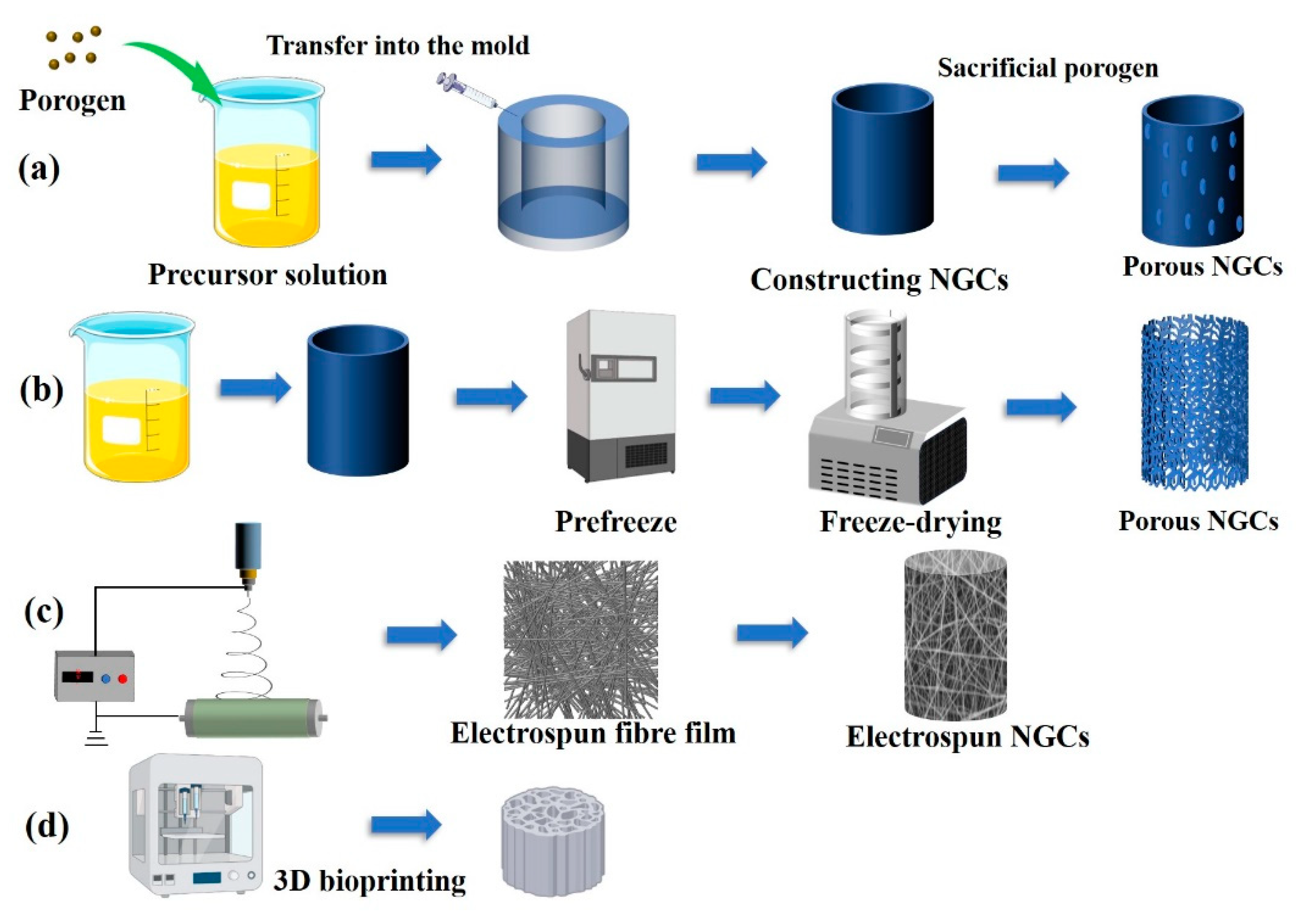
3. Fabrication strategies
For tissue-engineering regenerative materials such as bone tissue repair scaffolds, skin regeneration accessories, and corneal tissue-engineering materials [
25], preparation methods have been developed for construction of porous biomaterials. The fabrication strategy for constructing porous peripheral nerve regeneration materials does not differ from existing preparation technologies for other porous biomaterials. Generally, these methods are either subtractive or additive to the main material of the nerve repair conduits [
26]. Although the preparation strategy for the porous structure of peripheral nerve repair materials is not unique, the specific preparation process has differences owing to the biological functions. For example, a porous structure is constructed in a bone repair scaffold; the main consideration is that a suitable pore structure can provide a point for early attachment of cells and further promote functionalisation of pre-osteoblasts. The porous structure of the graft plays a key role in the biological responses of Schwann cells, macrophages, fibroblasts, and vascular endothelial cells related to peripheral nerve regeneration. The porous structure and induced permeability of conduits are currently mainstream research directions. A summary of porous fabrication methods and the resulting pore features are presented in
Table 1 and shown in
Figure 2.
3.1. Templating
Templating is a traditional and frequently used method for constructing the porous structure of biomaterials, and allows fabrication of pores ranging from nanometres to micrometres in size [
27]. It is a subtractive method that fabricates porous structures by adding a porogen into a polymer or metal precursor and removing the sacrificial porogen by mechanical stimuli or dissolution such that only the host materials remain. The porosity, size, connectivity, and shape of a porous material can be controlled through careful selection of the porogen morphology [
28]. Salt particles and spheres are the most commonly used porogens in biomedical materials. The advantages of the salt template method are presented as follows: (1) biological stability and no chemical reaction with other materials; (2) ease of removal, complete removal by soaking in the aqueous phase; (3) biocompatibility and no residual biotoxic substances. Li
et al. [
29] developed a porous structure on a gastrodin/polyurethane film through salt leaching to promote peripheral nerve regeneration. Their results showed that the interconnected porous structure endowed films with a high surface area; a 10–60 μm pore size could improve cell adhesion and axonal regeneration.
Spheres are another commonly used porogen for constructing 3D porous structures of biomaterials [
30]. Compared to the salt leaching method, the shape and size of the spheres can be more strictly controlled; thus, the pore size and 3D morphology of the porous structure of biomaterials can be more precisely regulated. Additionally, a spherical porogen has a higher specific surface area, greater possibility of contact between spheres, and better connectivity of the prepared porous structure. Draghi
et al. [
31] compared the effects of gelatine microspheres, paraffin microspheres, and NaCl crystals as porogens on the porosity, permeability, morphology, and biocompatibility of scaffolds. They demonstrated that a porous structure fabricated with gelatine and paraffin spheres had higher connectivity and an open-pore structure with lower liquid flow resistance through the scaffold.
3.2. Freeze-drying
Freeze-drying or freeze-casting is a common method for preparing porous 3D NGC structures [
32]. Chitosan, fibroin, gelatine, and other water-soluble, degradable biopolymer materials have shown great advantages in conduit preparation. Water is the most commonly used solution; in some studies, a mixture of ethanol and water has been used. Vacuum freeze-drying technology is based on vacuum sublimation of ice, freezing water into a solid (phase transition) by cooling and sublimating water molecules in vacuum conditions to obtain porous materials [
33]. This method is more physical, meaning that the 3D structure of the pores mainly depends on the concentration of the polymer solution and the method of freezing into a solid.
Typically, freeze-drying includes the following steps [
34]: (1) freezing; (2) sublimation drying; (3) secondary drying. Freezing is a decisive step in constructing porous structures for biomaterials in which the pore size and morphology are tailored by the freezing temperature and method. In the frozen and formed precursor solution, free water that is not bound to the polymer forms ice crystals and sublimates directly from the solid state to the gas state, leaving space to form pore structures. Haugh
et al. [
33] constructed different pore sizes in collagen/glycosaminoglycan (CG) scaffolds by varying the freezing temperature (-10 ℃ to -70 ℃). The results showed that the pore size decreased with decreasing freezing temperature [
35]. Jiang
et al. [
36] regulated the pore structure of a scaffold by adding different amounts of DMSO to a gelatine solution to control ice crystal formation during the freezing stage. Their results suggested that the formation of ice crystals during freezing could be controlled by adding DMSO, glycerol, or methanol to control the pore size of the scaffold. The size and morphology of the pore structure can also be affected by the concentration of the precursor polymer solution and the freezing method. A higher concentration of the polymer solution induces a lower free water content in the frozen solid, and thus a smaller pore size after sublimation [
37]. Because the shape of the pores is consistent with the morphology of ice crystals, the morphology of the pores can be tailored by changing the freezing method. Wu
et al. [
38] used rapid unidirectional freezing of liquid nitrogen to control the directionality of ice crystal formation to fabricate a gelatine scaffold with a unidirectional pore structure. The porosity of the scaffold was reduced by increasing the gelatine solution concentration.
The freeze-drying method maintains the physical 3D morphology of the nerve graft because the pore structure is formed through sublimation of solid ice crystals in the frozen state. Thus, unpredictable deformation of the NGC is avoided [
39]. Moreover, the low temperature (usually -50 ℃) and vacuum environment can protect the bioactive substances (NGF, BDNF, and Neurotrophin-3 frequently used in peripheral nerve repair) loaded on artificial nerve repair conduits. In this study, freeze-drying was used to prepare conduits with high porosity. A chitosan conduit with a porosity of 94.24% was prepared using the freeze-drying method by Li
et al. [
21]. Ma
et al. [
40] constructed porous multichannel conductive conduits for peripheral nerve regeneration. Scanning electron microscopy revealed that the fibroin nerve conduit had excellent porosity and pore connectivity.
3.3. Electrospinning fibre technology
Electrospinning is a common method for preparing microscale and nanoscale fibres. The method includes four main elements: a high voltage, a propulsion pump, a syringe, and a receiving device. The principle of electrospinning is based on a high-voltage device applying a voltage of 10–20 kV to the polymer solution. The accumulation of charge on the surface of the polymer solution of the syringe needle gradually forms a hanging drop (Taylor cone), followed by formation of a charged polymer jet. The polymer fibre falls into the receiving device through solvent evaporation or curing [
41]. Electrospun fibre NGCs have unique advantages in peripheral nerve regeneration due to their native extracellular matrix (ECM) mimicking and porous structure that can improve cell-substrate interactions [
42]. This technology can produce random or directional fibre films; the cross-space between the fibres forms a porous structure. The diameter of the fibres can be adjusted by controlling the solution concentration, process, and environmental parameters. The size and permeability of the pore structure can be tailored by adjusting the thickness and diameter of the fibre membranes [
43]. The porous structure of the electrospun fibre conduit can increase the diffusion of biomolecules and metabolic substances and effectively prevent fibrous scar deposition through pore size adjustment. More important, directional fibres can provide direct physical cues for directional regeneration of axons from the proximal to the distal end [
44].
Aligned fibre nerve conduits have recently been shown to provide mechanical cues for Schwann cell proliferation and axonal outgrowth. An aligned methacrylated silk fibroin electrospun fibre nerve conduit was reported by Chen
et al. [
45]. Mechanical cues can enhance enriched myelination of Schwann cells through nuclear translocation of Yes-associated protein 1 (YAP) to secrete neurotrophins to support axonal growth. Core-shell electrospun fibres have also been fabricated to endow them with hydrophilicity, early cell adhesion, strong toughness, and degradation properties. As electrospun fibre conduits can provide topological and biological cues to facilitate rapid and directed nerve regeneration, Deng
et al. [
46] prepared a nanofibre nerve repair graft with an acellular matrix shell and a PCL core. They demonstrated that electrospun fibres with a porous biomimetic extracellular matrix topology could effectively promote nerve fibre maturation and functionalisation. Electrospinning has a high specific surface area. Thus, nerve repair drugs can be loaded; the pore structure can promote diffusion of nerve repair drugs or growth factors. Puhl et al. reported an aligned fibre nerve conduit using a pseudouridine-modified neurotrophin-3 (NT-3) mRNA delivery system [
47]. The results showed that electrospun fibre conduits could provide ideal structural support and sustained drug release properties for peripheral nerve regeneration.
3.4. Three-dimensional bioprinting
Three-dimensional bioprinting is an additive technology that has become a reliable method for constructing biomaterials with complex and precise geometries from 3D model data, especially NGCs with porous structures [
48]. The most attractive aspect of 3D printing technology is that it can be used to prepare personalised grafts for patients through bioinfluence and biofabrication technology [
49]. Under computer control, printed conduits are stacked layer by layer, quickly producing a precision structure. The porous structure of NGCs is formed by the spacing between the deposited or solidified material [
50]. Although 3D printing technology has been widely used for degradable and non-degradable materials, polymer-degradable materials have been widely used in 3D printing in tissue engineering due to the demand for nerve graft repair materials [
51]. There are different 3D printing methods, such as stereolithography (SLA) and fused deposition modelling (FDM). A detailed and comprehensive description of these printing methods is provided in a review by Ngo
et al. [
19].
Compared with traditional pore structure construction methods such as templating and freeze-drying, 3D printing can effectively overcome the limitations of low surface porosity to produce a highly regular and consistent 3D porous structure. According to current research, porous structures prepared using 3D printing technology are macroporous, owing to limited accuracy. However, this method can overcome the problem of reduced mechanical performance of NGCs fabricated using traditional templating, freeze-drying, and electrospinning methods [
52]. Qian
et al. [
53] fabricated multi-layered conduits with macroporous structures by integrating 3D printing and layer-by-layer casting. The results indicated that the 3D porous conduit could improve peripheral nerve regeneration through macroporous structures for exchange of nutrients and oxygen, with ideal flexibility and rigidity. Construction of biomimetic repair materials similar to natural peripheral nerve tissue in terms of composition and 3D structure has always been the goal of peripheral nerve tissue-engineering research. Biomimetic NGCs were fabricated by Vijayavenkataraman
et al. [
54] using 3D printing technology. A regular square porous structure with a pore diameter of 125 ± 15 μm was fabricated; the NGCs achieved PC12 cell spreading.
4. Implications of porous NGCs on peripheral nerve regeneration
Peripheral nerve regeneration is a complex physiological process involving Schwann cells, macrophages, fibroblasts, PDGF, FGF, TGF-β and other cells, and mutual synergistic participation of bioactive factors [
62]. The size, permeability, morphology, and interconnection of the pore structure matching the pathophysiological needs of peripheral nerve repair guarantee the synergistic action of these factors [
63]. For peripheral nerve repair grafts, pores are typically intended to permit cell migration, maintain an adequate influx of nutrients and oxygen, and eliminate metabolic waste. Thus, the permeability of the pore structure is directly related to its positive role in promoting peripheral nerve regeneration [
64]. Pore size and morphology also affect early cellular behaviours such as adhesion, spreading, and migration [
65]. Porosity also affects the surface-to-volume ratio, biodegradation rate, stiffness, and mechanical properties of NGCs.
4.1. Porous structure impact on physical properties of NGCs
In clinical applications, ideal NGCs are not evaluated based on a single property; their biodegradation rate, mechanical performance, and permeability should be comprehensively considered. NGC functions are largely responsible for the prognostic efficacy of peripheral nerve regeneration therapies. The 3D porous structure has a direct effect on the properties of NGCs, although the material itself is decisive. It is necessary to fully consider and characterise the effects of porous structures on conduit properties.
4.1.1. Degradability
Degradation of NGCs after implantation
in vivo should be coordinated with the process of peripheral nerve regeneration to avoid a lack of structural support for axon regeneration caused by rapid degradation and compression of the newly regenerated nerve caused by slow degradation [
6]. At present, the materials for constructing NGCs are mainly chitosan, gelatine, collagen, PLA (
polylactic acid), PLGA (polylactic-co-glycolic acid), and high-molecular polymers. After implantation, the polymer NGCs are excreted via chemical degradation and
in vivo biodegradation. Chemical degradation refers to the hydrolysis of polymer materials to form small molecular monomers that leave the material body, resulting in a loss of material quality [
66]. Biodegradation, also known as bioerosion, refers to the inflammatory response caused by foreign body stimulation after implantation of polymer materials in the body [
67]. The organism secretes cellular mediators, enzymes, and free radicals to accelerate the hydrolysis of materials, some of which directly cleave their chemical bonds. The pore structure of the NGC can affect the degradation rate of the material through its relationship with the two degradation modes.
Pore size and porosity affect the surface-to-volume ratio of NGCs, as greater porosity and smaller pores increase the specific surface area of the materials. Thus, NGCs with high porosity, small pores, and good connectivity have a larger contact area with biological enzymes and a faster degradation rate [
68]. Chemical degradation of polymer materials is mainly related to the degradation activity of the backbone; however, the pore structure can change the degradation of NGCs by affecting the accumulation of local monomers and PH [
69]. Wu
et al. [
70] studied the in vitro chemical degradation performance of PLGA 3D porous scaffolds based on their porosity and pore size. The results showed that scaffolds with lower porosity or larger pores had thicker pore walls and smaller surface areas, which inhibited diffusion of acidic degradation products, leading to stronger acid-catalysed hydrolysis and a faster chemical degradation rate. Song
et al. [
71] investigated the degradation properties of porous polyester materials using a mathematical modelling strategy. The results indicated that materials with high porosity delayed the degradation process by slowing the autocatalytic reaction. Odelius
et al. [
72] studied the effects of pore size and porosity on the degradability of PLA scaffolds and degradation product monomers. Their results suggested that larger pore structures can accelerate degradation by autocatalysis.
4.1.2. Mechanical performance
The mechanical properties of NGCs include axial tensile strength, suture traction strength, radial compressive strength, and bending resistance to evaluate their operability during surgery and the expected protection of newly regenerated axons [
73]. NGCs with excellent mechanical performance can effectively avoid suture breakage during operation. More important, strong tensile mechanical and anti-distortion properties can reduce the risk of conduit fracture after implantation. When conduits are squeezed by the surrounding tissue, the original round tubular structure is maintained, avoiding compression of the regenerated nerve tissue and blocking of nerve regeneration.
The porosity, pore size, and pore distribution of NGCs affect their mechanical performance. The porous structure reduces the tensile and compressive strengths as the apparent density of the material and its integrity are destroyed [
74]. The Young's modulus of elastomer NGCs, used to evaluate the deformability of elastomers, also changes owing to the pore structure [
75]. Wang
et al. [
76] used the salt-template method to prepare porous polyurethane scaffolds with different porosities to investigate the relationship between material porosity and mechanical properties. Their results showed that the mechanical properties of scaffolds were initially positively correlated with the concentration of materials; the mechanical performance of materials with higher porosity decreased at a certain concentration. Chao
et al. [
77] studied the permeability and mechanical properties of porous scaffolds fabricated using additive manufacturing. A compressive mechanical property test showed that when the porosity was 70%, the elastic modulus and compressive strength of the porous structure tended to decrease with an increase in average pore size. However, when the porosity was 80%, the elastic modulus of the porous structure decreased first and then increased with an increase in average pore size. The compressive strength decreased with an increase in average pore size.
4.1.3. Permeability
Permeability is the most important NGC function provided by the pore structure. Signalling molecule exchange and cell infiltration of conduit walls are key mediators of NGC success. The permeability of the NGC is determined by the size and connectivity of its pore structure [
78]. Conduits with semi-permeable, fully permeable, and asymmetric structures have been prepared; however, the optimal solution remains controversial [
2]. The scar barrier caused by fibroblasts invading the NGC is the difference between fully permeable structures, which allow cells, nutrients, and molecular signals to enter the nerve conduit, and semi-permeable structures that do not allow cells to enter the conduit [
79]. Vleggeert Lankamp
et al. [
80] compared the peripheral nerve repair ability of semi-permeable (pore size: 1–10 μm) and fully-permeable (pore size: 10–230 μm) conduits. The results showed that the nerve electrophysiology, muscle morphology, and nerve fibre diameter of the semi-permeable nerve conduit group were better than those of the fully permeable and non-porous structure groups. However, with the discovery that fibroblasts can promote axonal regeneration and Schwann cell proliferation and migration, communication between cells and axons through paracrine signalling in semi-permeable structures cannot meet the needs of nerve regeneration because peripheral nerve regeneration involves direct contact between cells and newly regenerated axons to regulate the regeneration process [
81].
Table 2. summarizes the classification of NGCs according to their passability and their respective characteristics.
Asymmetric high-outflow conduits with large external and small internal pores have become a research focus for promoting the outflow rate of waste products and preventing invasion of fibroblasts into NGCs. Zhang et al. developed asymmetric NGCs with internal nanoporous structures [
82]. With the nanoporous structure inside the tube wall and the asymmetric structure of the hybrid hydrogel on the outer wall, such conduits can spatially limit the distribution of compliance factors, improving the efficacy of peripheral nerve repair. Chang
et al. [
24] prepared asymmetric PLGA conduits with high outflow using the immerse-precipitation method and implanted them into a 10-mm sciatic nerve defect in rats as an animal model. The silicone conduits of the non-porous structure as the control group showed that asymmetric NGCs significantly promoted the efficiency of metabolic waste removal and inhibited invasion of exogenous cells. Microporous PLA conduits with an asymmetric structure were implanted into a rabbit model of a 20-mm sciatic nerve defect in Hus
et al. [
18]. Eighteen months later, the electrophysiological function recovered to 82%, and the extension of the affected limb recovered to 81%. Tissue sections showed that the newly regenerated nerve fibres formed a normal sciatic nerve bundle.
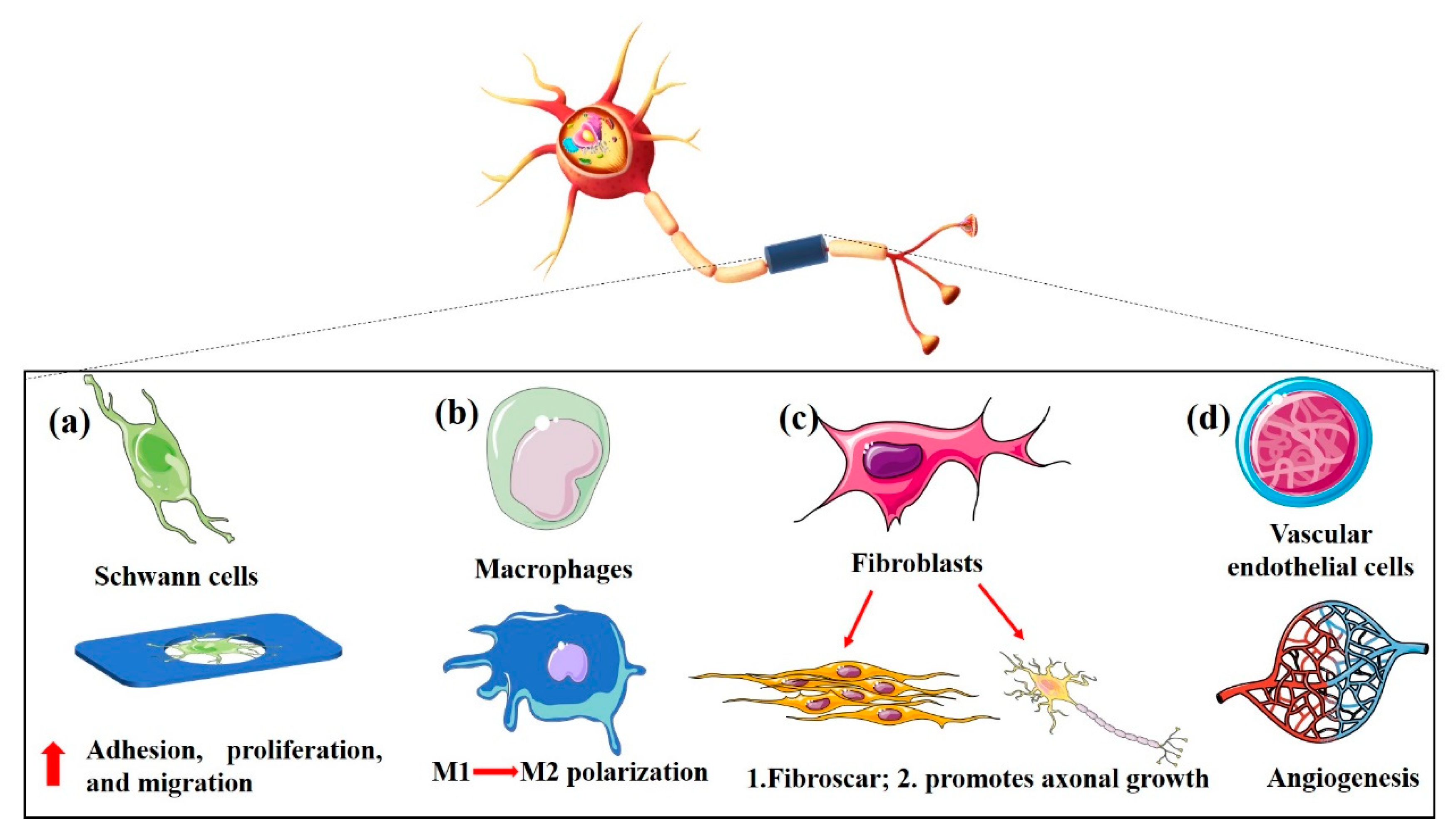
4.2. Porous structure determines bio-function of NGCs
After peripheral nerve injury, both the distal and proximal ends of the nerve degenerate. Neurones undergo rapid apoptosis if the cell body of the neuron or the axon near the cell body is seriously injured [
83]. Peripheral nerve injury can only occur far from the nerve body or with minor injury, and mainly involves three major processes: Wallerian degeneration, axon regeneration, and terminal nerve reinnervation [
84]. Failure of any of these processes may lead to irreversible neurological dysfunction in patients with peripheral nerve injury. Wallerian degeneration occurs seven days after traumatic peripheral nerve injury, creating a favourable microenvironment for axonal regeneration. Wallerian degeneration is the injury and rupture of the axons of peripheral nerve fibres, blocking the nutritional protection of the axoplasmic flow and the degeneration of the axons and myelin at the distal end of the fibres [
85]. The myelin sheath distal to the nerve stumps is swollen and ruptures with neuronal fibres; macrophages remove the debris and release growth factors and other substances to stimulate Schwann cell proliferation. Schwann cells migrate to the injured sites and secrete growth factors and adhesion molecules to create a regenerative environment [
86]. The proximal end of the injured axon generates a tooth germ that grows at a rate of approximately 1 mm/day along cord-like structures formed by Schwann cells (Bungner bands) to reach the target organ and form new synapses. The axon is remyelinated and the regeneration process is complete [
87].
This is only the general physiological process of peripheral nerve regeneration; a more detailed mechanism of regeneration remains to be determined in neuroscience and clinical specialties. The current research directions for peripheral nerve tissue engineering are: (1) improving the proliferation and migration of Schwann cells [
88]; (2) regulating the macrophage phenotype [
89] (3) inhibiting scar blocking induced by fibroblasts [
63]; (4) promoting angiogenesis of newly regenerated nerve tissue [
90]. The pore size, morphology, and porosity can affect the surface roughness, stiffness, and permeability of NGCs, affecting the biological functions of Schwann cells, fibroblasts, macrophages, and vascular endothelial cells (
Figure 3).
4.2.1. Schwann cells
Schwann cells, also known as nerve membrane or sheath cells, are unique glial cells found in the peripheral nervous system. Schwann cells have a variety of important functions [
91]: (1) supporting and protecting axons, maintaining a good axon microenvironment; (2) forming a myelin sheath that plays an insulating role on myelinated fibres and accelerates conduction of nerve axons; (3) having a nutrient-metabolising effect on nerve axons. After peripheral nerve injury, Schwann cells change from a quiescent state with a stable myelin structure to a proliferative and vegetative state, creating a suitable environment for nerve regeneration [
92]. Phenotypic changes of Schwann cells are crucial for Schwann cells to promote nerve regeneration. After Wallerian degeneration at the distal end of the injured nerve, Schwann cells divide, proliferate, participate in the phagocytosis of degenerated axons and myelin debris, and form Bungner bands to guide the growth of regenerated axons. The expression and secretion of nerve growth factors, cytokines, and other active substances can induce and regulate axon regeneration and myelin sheath formation, which are conducive to axon maturation and reinnervation [
93].
An appropriate pore structure size can provide attachment points for early adhesion of Schwann cells by changing the surface roughness of the NGC. This is conducive to early functionalisation of cells, such as spreading, and promotes proliferation and migration, ultimately promoting formation of Bungner bands to guide axon regeneration. Zhang
et al. [
94] compared Schwann cell proliferation in 3D-printed cold glue/starch composite scaffolds with gaps of 2 mm, 2.5 mm, and 3 mm. After 5 d of culture, proliferation of Schwann cells in the 3-mm group was more significant than that in the other groups. For improvement of the pore size, porosity and mechanical properties of traditional electrospun scaffolds, an antheraea pernyi silk fibroin scaffold was prepared by Zhou
et al. [
95]. Adjusting the pore size and the distance between the fibre collector and syringe, the viability, penetration, and migration of Schwann cells varied according to the pore size.
4.2.2. Macrophages
The traditional view is that the macrophages involved in peripheral nerve injury and regeneration are mainly involved in Wallerian degeneration, engulfment, and clearance of deforming axons and myelin debris [
96]. In recent years, with development and application of cellular and molecular biology techniques, a new understanding of the role of macrophages in peripheral nerve regeneration has emerged. After peripheral nerve injury, macrophages have active phagocytic function and also directly or indirectly participate in peripheral nerve regeneration through their cellular activities and secretion of a variety of growth factors, cytokines, and proteases [
93]. The role of macrophages in peripheral nerve injury and regeneration has been studied.
In the process of peripheral nerve regeneration, macrophages exhibit a “classically activated” pro-inflammatory phenotype and a “selectively activated” anti-inflammatory phenotype [
97]. Previous studies [
98] have shown a correlation between graft surface topography and macrophage polarisation. Jiang
et al. [
36] investigated the effect of pore size and stiffness of scaffolds on macrophage polarisation. Macrophages in scaffolds with small pores and softness tended to be activated toward a proinflammatory phenotype, whereas cells in scaffolds with large pores and rigidity tended to be activated toward an anti-inflammatory phenotype. Yu
et al. [
99] investigated the effects of zein microspheres on induction of immune responses, microsphere size, pore structure, and drug loading during sciatic nerve regeneration. Their results showed that modulation of pore structure could effectively regulate zein-induced immune responses, inhibit neutrophil recruitment, and promote macrophage polarisation to the M2 phenotype. High-porosity conduits induce more M2 macrophages to accelerate nerve regeneration.
4.2.3. Fibroblasts
In the peripheral nervous system, fibroblasts are the main cellular components of the endoneurium, epineurium and perineurium [
100]. In the traditional view, scars produced by fibroblasts are detrimental to peripheral nerve regeneration. Fibroblasts invade the inner part of the NGC to form scar tissue that blocks axonal growth into the target organ. The foreign body reaction caused by graft implantation leads to a long-term inflammatory reaction that forms a fibrous wrapping on the outer surface of the NGC and causes adhesion of the newly regenerated nerve to the surrounding tissue [
101]. However, an increasing number of studies have shown that fibroblasts and their secretions play important roles in peripheral nerve regeneration. He
et al. [
102] reported that peripheral nerve fibroblasts greatly promoted outgrowth of motor neuron neurites compared with cardiac fibroblasts. Zhao
et al. [
81] demonstrated that oligosaccharides, chitosan degradation products, could promote peripheral axon regeneration through the miR-132-5p/CAMKK1 axis pathway through the induced fibroblast exosome TFAP2C. Thus, the traditional concept of blocking fibroblasts from entering the NGC must be reconsidered, and a more advanced pore structure should be explored in future research.
4.2.4. Vascular endothelial cells
When a peripheral nerve is injured, the local microenvironment around the injured nerve and the nutrients needed for nerve regeneration are important for repair and reconstruction; these have an important relationship with the local blood supply [
103]. Early vascularisation of nerve grafts can provide sufficient nutrition for transplanted cells to promote axonal growth and nerve regeneration [
104]. Formation of fibrous capsules due to foreign body reactions can be reduced. In addition, vascularisation of nerve grafts can gather macrophages from the blood to quickly remove degeneration products of axons and the myelin sheath after peripheral nerve injury, providing a good channel for new axons to grow to the distal end [
105].
The pore structural characteristics of biomaterials such as pore size, porosity, and connectivity significantly affect cell invasion and angiogenesis. Macroporous structures contribute to cell permeability, oxygen and nutrient transport, and formation of mature vascularised tissues [
106]. Some studies have suggested that the effect of pore structure on vascularisation is mainly due to regulation of the immune environment of the material [
107]. Studies have shown that collagen–chitosan scaffolds with a larger pore size (360 μm) promoted transformation of macrophages from M1 to M2 and constructed a suitable immune microenvironment for vascularisation, inducing angiogenesis. Porosity is an important factor in determining blood vessel ingrowth. The porosity of biomaterials is significantly positively correlated with the invasion depth of endothelial cells and diameter of new blood vessels [
108]. Shen
et al. [
58] used electrospinning to construct a pre-vascularised nerve conduit with high porosity and connectivity. In a 10-mm rat sciatic nerve defect model, pre-vascularised 3D porous NGCs greatly enhanced intra-nerve angiogenesis and promoted peripheral nerve regeneration.
5. Conclusions
Significant development of medical tissue engineering peripheral NGCs has enabled their successful use in clinical practice, which will be more extensive and routine in the future. Many studies have explored the 3D topological morphology of nerve grafting, from relatively closed nerve regeneration chambers to construction of irregular pore structures, to current pore structure instruments with controlled morphology and asymmetric pore structures. A unified standard for the porous structure of nerve conduits is still lacking, and the ideal porous structure for promoting peripheral nerve regeneration remains controversial. According to recent research results, pore structure characteristics such as porosity, pore size, and connectivity have a comprehensive effect on the nerve regeneration graft, including the degradation rate, mechanical performance, permeability, and other physical properties. The most important factor is that the porous structure can change the roughness and hardness of the NGC to have a comprehensive effect on the physiological process of peripheral nerve regeneration. In future studies, additional pore structure construction methods and pore structures that are more consistent with the physiological characteristics of peripheral nerve regeneration will be studied and developed. Considering the practicability and foundation of porous structures as conduits to promote peripheral nerve regeneration, pore morphology standards will be formulated for future medical nerve repair grafts.
Author Contributions
Conceptualization, P.-X.Z. and T.W.; methodology, T.W.; software, F.S.Z and M.Z.; validation, T.W., H.-R.J. and Y.-C.Z.; formal analysis, X.-M.Z., Q.C.L; investigation, Y.-L.W.; re-sources, P.-X.Z.; data curation, T.W.; writing—original draft preparation, T.W.; writing—review and editing, P.-X.Z. and X.-M.Z.; visualization, M.Z.; supervision, P.-X.Z.; project administration, P.-X.Z.; funding acquisition, P.-X.Z. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Not applicable.
Acknowledgments
This study was financially supported by the National Natural Science Foundation of China (Project No.22278003).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bell, J.H.A.; Haycock, J.W. Next Generation Nerve Guides: Materials, Fabrication, Growth Factors, and Cell Delivery. Tissue Eng Part B-Re 2012, 18, 116–128. [Google Scholar] [CrossRef]
- Lackington, W.A.; Ryan, A.J.; O'Brien, F.J. Advances in Nerve Guidance Conduit-Based Therapeutics for Peripheral Nerve Repair. Acs Biomater Sci Eng 2017, 3, 1221–1235. [Google Scholar] [CrossRef]
- Vijayavenkataraman, S. Nerve guide conduits for peripheral nerve injury repair: A review on design, materials and fabrication methods. Acta Biomater 2020, 106, 54–69. [Google Scholar] [CrossRef]
- Yan, Y.X.; Yao, R.T.; Zhao, J.Y.; Chen, K.L.; Duan, L.R.; Wang, T.; Zhang, S.J.; Guan, J.P.; Zheng, Z.Z.; Wang, X.Q.; et al. Implantable nerve guidance conduits: Material combinations, multi-functional strategies and advanced engineering innovations. Bioact Mater 2022, 11, 57–76. [Google Scholar] [CrossRef]
- Wieringa, P.A.; de Pinho, A.R.G.; Micera, S.; van Wezel, R.J.A.; Moroni, L. Biomimetic Architectures for Peripheral Nerve Repair: A Review of Biofabrication Strategies. Adv Healthc Mater 2018, 7. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, J.H.; Wang, Y.; Shang, L.R.; Chai, R.J.; Zhao, Y.J. Natural Polymer-Derived Bioscaffolds for Peripheral Nerve Regeneration. Adv Funct Mater 2022, 32. [Google Scholar] [CrossRef]
- Daly, W.; Yao, L.; Zeugolis, D.; Windebank, A.; Pandit, A. A biomaterials approach to peripheral nerve regeneration: bridging the peripheral nerve gap and enhancing functional recovery. J R Soc Interface 2012, 9, 202–221. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Tsai, T.Y.; Young, T.H.; Yang, H.J.; Ji, Y.R. An electroactive alginate hydrogel nanocomposite reinforced by functionalized graphite nanofilaments for neural tissue engineering. Carbohyd Polym 2019, 224. [Google Scholar] [CrossRef]
- Dong, Q.; Yang, X.D.; Liang, X.; Liu, J.; Wang, B.Y.; Zhao, Y.T.; Huselstein, C.; Feng, X.L.; Tong, Z.; Chen, Y. Composite Hydrogel Conduit Incorporated with Platelet-Rich Plasma Improved the Regenerative Microenvironment for Peripheral Nerve Repair. Acs Appl Mater Inter 2023, 15, 24120–24133. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J.; Zheng, Z.; Yan, J.; Zhang, L.; Li, Y.; Zhang, J.; Li, G.; Wang, X.; Kaplan, D. Porous nerve guidance conduits reinforced with braided composite structures of silk/magnesium filaments for peripheral nerve repair. Acta Biomater 2021, 134, 116–130. [Google Scholar] [CrossRef]
- Apablaza, J.A.; Lezcano, M.F.; Marquez, A.L.; Sanchez, K.G.; Oporto, G.H.; Dias, F.J. Main Morphological Characteristics of Tubular Polymeric Scaffolds to Promote Peripheral Nerve Regeneration-A Scoping Review. Polymers-Basel 2021, 13. [Google Scholar] [CrossRef]
- Williams, L.R.; Longo, F.M.; Powell, H.C.; Lundborg, G.; Varon, S. Spatial-Temporal Progress of Peripheral-Nerve Regeneration within a Silicone Chamber - Parameters for a Bioassay. J Comp Neurol 1983, 218, 460–470. [Google Scholar] [CrossRef]
- Uz, M.; Sharma, A.D.; Adhikari, P.; Sakaguchi, D.S.; Mallapragada, S.K. Development of multifunctional films for peripheral nerve regeneration. Acta Biomater 2017, 56, 141–152. [Google Scholar] [CrossRef]
- Vijayavenkataraman, S.; Kannan, S.; Cao, T.; Fuh, J.Y.H.; Sriram, G.; Lu, W.F. 3D-Printed PCL/PPy Conductive Scaffolds as Three-Dimensional Porous Nerve Guide Conduits (NGCs) for Peripheral Nerve Injury Repair. Front Bioeng Biotech 2019, 7. [Google Scholar] [CrossRef]
- Oh, S.H.; Kim, J.H.; Song, K.S.; Jeon, B.H.; Yoon, J.H.; Seo, T.B.; Narngung, U.; Lee, I.W.; Lee, J.H. Peripheral nerve regeneration within an asymmetrically porous PLGA/Pluronic F127 nerve guide conduit. Biomaterials 2008, 29, 1601–1609. [Google Scholar] [CrossRef]
- Hsieh, W.C.; Chang, C.P.; Lin, S.M. Morphology and characterization of 3D micro-porous structured chitosan scaffolds for tissue engineering. Colloid Surface B 2007, 57, 250–255. [Google Scholar] [CrossRef]
- Sayed, E.; Haj-Ahmad, R.; Ruparelia, K.; Arshad, M.S.; Chang, M.W.; Ahmad, Z. Porous Inorganic Drug Delivery Systems-a Review. Aaps Pharmscitech 2017, 18, 1507–1525. [Google Scholar] [CrossRef]
- Hsu, S.H.; Chan, S.H.; Chiang, C.M.; Chen, C.C.C.; Jiang, C.F. Peripheral nerve regeneration using a microporous polylactic acid asymmetric conduit in a rabbit long-gap sciatic nerve transection model. Biomaterials 2011, 32, 3764–3775. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos Part B-Eng 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Saltzman, W.M.; Langer, R. Transport Rates of Proteins in Porous Materials with Known Microgeometry. Biophys J 1989, 55, 163–171. [Google Scholar] [CrossRef]
- Li, G.C.; Xue, C.B.; Wang, H.K.; Yang, X.M.; Zhao, Y.X.; Zhang, L.Z.; Yang, Y.M. Spatially featured porous chitosan conduits with micropatterned inner wall and seamless sidewall for bridging peripheral nerve regeneration. Carbohyd Polym 2018, 194, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Wan, T.; Jiao, Z.X.; Guo, M.; Wang, Z.L.; Wan, Y.Z.; Lin, K.L.; Liu, Q.Y.; Zhang, P.B. Gaseous sulfur trioxide induced controllable sulfonation promoting biomineralization and osseointegration of polyetheretherketone implants. Bioact Mater 2020, 5, 1004–1017. [Google Scholar] [CrossRef] [PubMed]
- Menezes, I.R.S.; Sakai, T.; Kaneko, K. Evaluation of graphene oxide nanoporosity by multiprobe gas adsorption analysis. J Mater Sci 2023, 58, 4439–4449. [Google Scholar] [CrossRef]
- Chang, C.J.; Hsu, S.H. The effect of high outflow permeability in asymmetric poly(DL-lactic acid-co-glycolic acid) conduits for peripheral nerve regeneration. Biomaterials 2006, 27, 1035–1042. [Google Scholar] [CrossRef]
- Hernandez, J.L.; Woodrow, K.A. Medical Applications of Porous Biomaterials: Features of Porosity and Tissue-Specific Implications for Biocompatibility. Adv Healthc Mater 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.C.; Jamshidi, P.; Eisenstein, N.M.; Webber, M.A.; Burton, H.; Moakes, R.J.A.; Addison, O.; Attallah, M.; Shepherd, D.E.T.; Grover, L.M. Surface Finish has a Critical Influence on Biofilm Formation and Mammalian Cell Attachment to Additively Manufactured Prosthetics. Acs Biomater Sci Eng 2017, 3, 1616–1626. [Google Scholar] [CrossRef]
- Galperin, A.; Long, T.J.; Garty, S.; Ratner, B.D. Synthesis and fabrication of a degradable poly(N-isopropyl acrylamide) scaffold for tissue engineering applications. J Biomed Mater Res A 2013, 101, 775–786. [Google Scholar] [CrossRef]
- Huczko, A. Template-based synthesis of nanomaterials. Appl Phys a-Mater 2000, 70, 365–376. [Google Scholar] [CrossRef]
- Li, Q.; Li, L.M.; Yu, M.L.; Zheng, M.; Li, Y.; Yang, J.; Dai, M.; Zhong, L.M.; Sun, L.; Lu, D. Elastomeric polyurethane porous film functionalized with gastrodin for peripheral nerve regeneration. J Biomed Mater Res A 2020, 108, 1713–1725. [Google Scholar] [CrossRef]
- Stein, A. Sphere templating methods for periodic porous solids. Micropor Mesopor Mat 2001, 44, 227–239. [Google Scholar] [CrossRef]
- Draghi, L.; Resta, S.; Pirozzolo, M.G.; Tanzi, M.C. Microspheres leaching for scaffold porosity control. J Mater Sci-Mater M 2005, 16, 1093–1097. [Google Scholar] [CrossRef] [PubMed]
- Grenier, J.; Duval, H.; Barou, F.; Lv, P.; David, B.; Letourneur, D. Mechanisms of pore formation in hydrogel scaffolds textured by freeze-drying. Acta Biomater 2019, 94, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Haugh, M.G.; Murphy, C.M.; O'Brien, F.J. Novel Freeze-Drying Methods to Produce a Range of Collagen-Glycosaminoglycan Scaffolds with Tailored Mean Pore Sizes. Tissue Eng Part C-Me 2010, 16, 887–894. [Google Scholar] [CrossRef]
- Mackenzie, A.P. A Review of the Freeze-Drying Process. Cryobiology 1988, 25, 574–574. [Google Scholar] [CrossRef]
- de Waard, H.; De Beer, T.; Hinrichs, W.L.J.; Vervaet, C.; Remon, J.P.; Frijlink, H.W. Controlled Crystallization of the Lipophilic Drug Fenofibrate During Freeze-Drying: Elucidation of the Mechanism by In-Line Raman Spectroscopy. Aaps J 2010, 12, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.M.; Lyu, C.; Zhao, P.; Li, W.J.; Kong, W.Y.; Huang, C.Y.; Genin, G.M.; Du, Y.N. Cryoprotectant enables structural control of porous scaffolds for exploration of cellular mechano-responsiveness in 3D. Nat Commun 2019, 10. [Google Scholar] [CrossRef]
- Fissore, D.; Pisano, R.; Barresi, A.A. Process analytical technology for monitoring pharmaceuticals freeze-drying - A comprehensive review. Dry Technol 2018, 36, 1839–1865. [Google Scholar] [CrossRef]
- Wu, X.; Liu, Y.; Li, X.; Wen, P.; Zhang, Y.; Long, Y.; Wang, X.; Guo, Y.; Xing, F.; Gao, J. Preparation of aligned porous gelatin scaffolds by unidirectional freeze-drying method. Acta Biomater 2010, 6, 1167–1177. [Google Scholar] [CrossRef]
- Hou, Q.P.; Grijpma, D.W.; Feijen, J. Preparation of interconnected highly porous polymeric structures by a replication and freeze-drying process. J Biomed Mater Res B 2003, 67b, 732–740. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, H.; Wang, Q.Q.; Cao, X.D.; Gao, H.C. Piezoelectric conduit combined with multi-channel conductive scaffold for peripheral nerve regeneration. Chem Eng J 2023, 452. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, J.D.; Cheng, H.; Li, G.Q.; Cho, H.J.; Jiang, M.J.; Gao, Q.; Zhang, X.W. Developments of Advanced Electrospinning Techniques: A Critical Review. Adv Mater Technol-Us 2021, 6. [Google Scholar] [CrossRef]
- Sun, R.Y.; Wang, B.L.; Zhang, L.F.; Lang, Y.A.; Chang, M.W. Engineering Three-Dimensional Bendable Helix Conduits for Peripheral Nerve Regeneration via Hybrid Electrotechnologies. Acs Mater Lett 2022, 4, 2210–2218. [Google Scholar] [CrossRef]
- Zhan, L.; Deng, J.X.; Ke, Q.F.; Li, X.; Ouyang, Y.M.; Huang, C.; Liu, X.Q.; Qian, Y. Grooved Fibers: Preparation Principles Through Electrospinning and Potential Applications. Adv Fiber Mater 2022, 4, 203–213. [Google Scholar] [CrossRef]
- Wei, Z.D.; Jin, F.; Li, T.; Qian, L.L.; Zheng, W.Y.; Wang, T.; Feng, Z.Q. Physical Cue-Based Strategies on Peripheral Nerve Regeneration. Adv Funct Mater 2022. [Google Scholar] [CrossRef]
- Chen, X.L.; Tang, X.X.; Wang, Y.L.; Gu, X.Y.; Huang, T.T.; Yang, Y.M.; Ling, J. Silk-inspired fiber implant with multi-cues enhanced bionic microenvironment for promoting peripheral nerve repair. Biomater Adv 2022, 135. [Google Scholar] [CrossRef] [PubMed]
- Deng, R.L.; Luo, Z.L.; Rao, Z.L.; Lin, Z.D.; Chen, S.H.; Zhou, J.; Zhu, Q.T.; Liu, X.L.; Bai, Y.; Quan, D.P. Decellularized Extracellular Matrix Containing Electrospun Fibers for Nerve Regeneration: A Comparison Between Core-Shell Structured and Preblended Composites. Adv Fiber Mater 2022, 4, 503–519. [Google Scholar] [CrossRef]
- Puhl, D.L.; Funnell, J.L.; Fink, T.D.; Swaminathan, A.; Oudega, M.; Zha, R.H.; Gilbert, R.J. Electrospun fiber-mediated delivery of neurotrophin-3 mRNA for neural tissue engineering applications. Acta Biomater 2023, 155, 370–385. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, D.; Faisal, N.; Sharma, A.; Ansu, A.K.; Goyal, A.; Saxena, K.K.; Prakash, C.; Kumar, D. Application of 3D printing technology for medical implants: a state-of-the-art review. Adv Mater Process Te 2023. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Yue, K.; Aleman, J.; Mollazadeh-Moghaddam, K.; Bakht, S.M.; Yang, J.Z.; Jia, W.T.; Dell'Erba, V.; Assawes, P.; Shin, S.R.; et al. 3D Bioprinting for Tissue and Organ Fabrication. Ann Biomed Eng 2017, 45, 148–163. [Google Scholar] [CrossRef]
- Heinrich, M.A.; Liu, W.J.; Jimenez, A.; Yang, J.Z.; Akpek, A.; Liu, X.; Pi, Q.M.; Mu, X.; Hu, N.; Schiffelers, R.M.; et al. 3D Bioprinting: from Benches to Translational Applications. Small 2019, 15. [Google Scholar] [CrossRef]
- Petcu, E.B.; Midha, R.; McColl, E.; Popa-Wagner, A.; Chirila, T.V.; Dalton, P.D. 3D printing strategies for peripheral nerve regeneration. Biofabrication 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Guzzi, E.A.; Tibbitt, M.W. Additive Manufacturing of Precision Biomaterials. Adv Mater 2020, 32. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Zhao, X.T.; Han, Q.X.; Chen, W.; Li, H.; Yuan, W.E. An integrated multi-layer 3D-fabrication of PDA/RGD coated graphene loaded PCL nanoscaffold for peripheral nerve restoration. Nat Commun 2018, 9. [Google Scholar] [CrossRef]
- Vijayavenkataraman, S.; Thaharah, S.; Zhang, S.; Lu, W.F.; Fuh, J.Y.H. Electrohydrodynamic jet 3D-printed PCL/PAA conductive scaffolds with tunable biodegradability as nerve guide conduits (NGCs) for peripheral nerve injury repair. Mater Design 2019, 162, 171–184. [Google Scholar] [CrossRef]
- Jeon, J.; Lee, M.S.; Lim, J.; Park, S.; Kim, S.M.; Kim, D.I.; Tae, G.; Yang, H.S. Micro-grooved nerve guidance conduits combined with microfiber for rat sciatic nerve regeneration. J Ind Eng Chem 2020, 90, 214–223. [Google Scholar] [CrossRef]
- Fadia, N.B.; Bliley, J.M.; DiBernardo, G.A.; Crammond, D.J.; Schilling, B.K.; Sivak, W.N.; Spiess, A.M.; Washington, K.M.; Waldner, M.; Liao, H.T.; et al. Long-gap peripheral nerve repair through sustained release of a neurotrophic factor in nonhuman primates (vol 12, eabc4054, 2020). Sci Transl Med 2020, 12. [Google Scholar] [CrossRef]
- Choi, J.; Kim, J.H.; Jang, J.W.; Kim, H.J.; Choi, S.N.; Kwon, S.W. Decellularized sciatic nerve matrix as a biodegradable conduit for peripheral nerve regeneration. Neural Regen Res 2018, 13, 1796–1803. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.J.; Wang, J.Y.; Liu, X.Z.; Sun, Y.; Yin, A.L.; Chai, Y.M.; Zhang, K.H.; Wang, C.Y.; Zheng, X.Y. In Situ Prevascularization Strategy with Three-Dimensional Porous Conduits for Neural Tissue Engineering. Acs Appl Mater Inter 2021, 13, 50785–50801. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.L.; Zhu, L.; Shi, X.W.; Xia, B.; Liu, Z.Y.; Zhu, S.; Yang, Y.F.; Ma, T.; Cheng, P.Z.; Luo, K.; et al. A compound scaffold with uniform longitudinally oriented guidance cues and a porous sheath promotes peripheral nerve regeneration in vivo. Acta Biomater 2018, 68, 223–236. [Google Scholar] [CrossRef]
- Zheng, T.T.; Wu, L.L.; Xu, J.W.; Sun, S.L.; Guan, W.C.; Han, Q.; Zhang, L.Z.; Gu, X.S.; Yang, Y.M.; Li, G.C. YR/DFO@DCNT functionalized anisotropic micro/nano composite topography scaffolds for accelerating long-distance peripheral nerve regeneration. Compos Part B-Eng 2022, 246. [Google Scholar] [CrossRef]
- Namhongsa, M.; Daranarong, D.; Sriyai, M.; Molloy, R.; Ross, S.; Ross, G.M.; Tuantranont, A.; Tocharus, J.; Sivasinprasasn, S.; Topham, P.D.; et al. Surface-Modified Polypyrrole-Coated PLCL and PLGA Nerve Guide Conduits Fabricated by 3D Printing and Electrospinning. Biomacromolecules 2022. [Google Scholar] [CrossRef]
- Qian, Y.; Lin, H.; Yan, Z.W.; Shi, J.L.; Fan, C.Y. Functional nanomaterials in peripheral nerve regeneration: Scaffold design, chemical principles and microenvironmental remodeling. Mater Today 2021, 51, 165–187. [Google Scholar] [CrossRef]
- Zheng, F.R.; Li, R.; He, Q.D.; Koral, K.; Tao, J.Y.; Fan, L.H.; Xiang, R.Z.; Ma, J.Y.; Wang, N.; Yin, Y.X.; et al. The electrostimulation and scar inhibition effect of chitosan/oxidized hydroxyethyl cellulose/reduced graphene oxide/asiaticoside liposome based hydrogel on peripheral nerve regeneration in vitro. Mat Sci Eng C-Mater 2020, 109. [Google Scholar] [CrossRef] [PubMed]
- Stocco, E.; Barbon, S.; Emmi, A.; Tiengo, C.; Macchi, V.; De Caro, R.; Porzionato, A. Bridging Gaps in Peripheral Nerves: From Current Strategies to Future Perspectives in Conduit Design. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Maksoud, F.; De la Paz, M.F.V.; Hann, A.J.; Thanarak, J.; Reilly, G.C.; Claeyssens, F.; Green, N.H.; Zhang, Y.S. Porous biomaterials for tissue engineering: a review. J Mater Chem B 2022, 10, 8111–8165. [Google Scholar] [CrossRef]
- Wang, L.; Wang, C.Y.; Wu, S.; Fan, Y.B.; Li, X.M. Influence of the mechanical properties of biomaterials on degradability, cell behaviors and signaling pathways: current progress and challenges. Biomater Sci-Uk 2020, 8, 2714–2733. [Google Scholar] [CrossRef]
- Huang, J.Y.; Fan, C.M.; Chen, Y.W.; Ye, J.C.; Yang, Y.W.; Tang, C.Q.; Zhang, H.; Fei, Y.; An, C.R.; Xie, Y.H.; et al. Single-cell RNA-seq reveals functionally distinct biomaterial degradation-related macrophage populations. Biomaterials 2021, 277. [Google Scholar] [CrossRef]
- Lu, X.Z.; Lai, C.P.; Chan, L.C. Novel design of a coral-like open-cell porous degradable magnesium implant for orthopaedic application. Mater Design 2020, 188. [Google Scholar] [CrossRef]
- Wan, Y.; Zhang, J.; Luo, Y.; Zhou, T.; Wu, H. Preparation and degradation of chitosan-poly(p-dioxanone)/silk fibroin porous conduits. Polym Degrad Stabil 2015, 119, 46–55. [Google Scholar] [CrossRef]
- Wu, L.B.; Ding, J.D. Effects of porosity and pore size on in vitro degradation of three-dimensional porous poly(D,L-lactide-co-glycolide) scaffolds for tissue engineering. J Biomed Mater Res A 2005, 75a, 767–777. [Google Scholar] [CrossRef]
- Song, C.B.; Zhang, J.P.; Cen, L.; Xi, Z.H.; Zhao, L.; Yuan, W.K. Modeling Strategies for the Degradation Behavior of Porous Polyester Materials Based on Their Key Structural Features. Ind Eng Chem Res 2020, 59, 14806–14816. [Google Scholar] [CrossRef]
- Odelius, K.; Hoglund, A.; Kumar, S.; Hakkarainen, M.; Ghosh, A.K.; Bhatnagar, N.; Albertsson, A.C. Porosity and Pore Size Regulate the Degradation Product Profile of Polylactide. Biomacromolecules 2011, 12, 1250–1258. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y.; Zhang, B.; Li, L.; Yin, J.; Fu, J.Z. Additive-lathe 3D bioprinting of bilayered nerve conduits incorporated with supportive cells. Bioact Mater 2021, 6, 219–229. [Google Scholar] [CrossRef]
- Farokhi, M.; Mottaghitalab, F.; Shokrgozar, M.A.; Kaplan, D.L.; Kim, H.W.; Kundu, S.C. Prospects of peripheral nerve tissue engineering using nerve guide conduits based on silk fibroin protein and other biopolymers. Int Mater Rev 2017, 62, 367–391. [Google Scholar] [CrossRef]
- Yucel, D.; Kose, G.T.; Hasirci, V. Polyester based nerve guidance conduit design. Biomaterials 2010, 31, 1596–1603. [Google Scholar] [CrossRef]
- Wang, Y.F.; Barrera, C.M.; Dauer, E.A.; Gu, W.Y.; Andreopoulos, F.; Huang, C.Y.C. Systematic characterization of porosity and mass transport and mechanical properties of porous polyurethane scaffolds. J Mech Behav Biomed 2017, 65, 657–664. [Google Scholar] [CrossRef]
- Chao, L.; Jiao, C.; Liang, H.X.; Xie, D.Q.; Shen, L.D.; Liu, Z.D. Analysis of Mechanical Properties and Permeability of Trabecular-Like Porous Scaffold by Additive Manufacturing. Front Bioeng Biotech 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Jeon, E.Y.; Nam, J.J.; Park, J.H.; Choi, I.C.; Kim, S.H.; Chung, J.J.; Lee, K.; Park, J.W.; Jung, Y. Development of a regenerative porous PLCL nerve guidance conduit with swellable hydrogel-based microgrooved surface pattern via 3D printing. Acta Biomater 2022, 141, 219–232. [Google Scholar] [CrossRef]
- Bian, Y.Z.; Wang, Y.; Aibaidoula, G.; Chen, G.Q.; Wu, Q. Evaluation of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) conduits for peripheral nerve regeneration. Biomaterials 2009, 30, 217–225. [Google Scholar] [CrossRef]
- Vleggeert-Lankamp, C.L.A.M.; de Ruiter, G.C.W.; Wolfs, J.F.C.; Pego, A.P.; van den Berg, R.J.; Feirabend, H.K.P.; Malessy, M.J.A.; Lakke, E.A.J.F. Pores in synthetic nerve conduits are beneficial to regeneration. J Biomed Mater Res A 2007, 80a, 965–982. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Liu, J.N.; Liu, S.; Yang, P.P.; Liang, Y.Y.; Ma, J.Y.; Mao, S.S.; Sun, C.; Yang, Y.M. Fibroblast exosomal TFAP2C induced by chitosan oligosaccharides promotes peripheral axon regeneration via the miR-132-5p/CAMKK1 axis. Bioact Mater 2023, 26, 249–263. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, H.; Wang, H.; Zhao, Y.J.; Chai, R.J. Natural proteins-derived asymmetric porous conduit for peripheral nerve regeneration. Appl Mater Today 2022, 27. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Gong, J.X.; Zhang, J.Y.; Zhu, Z.Y.; Qian, Y.; Lu, K.J.; Zhou, S.Y.; Gu, T.Y.; Wang, H.M.; He, Y.; et al. Three Potential Elements of Developing Nerve Guidance Conduit for Peripheral Nerve Regeneration. Adv Funct Mater 2023. [Google Scholar] [CrossRef]
- Babu, S.; Krishnan, M.; Panneerselvam, A.; Chinnaiyan, M. A comprehensive review on therapeutic application of mesenchymal stem cells in neuroregeneration. Life Sci 2023, 327, 121785. [Google Scholar] [CrossRef]
- Liu, J.M.; Li, L.X.; Zou, Y.; Fu, L.Y.; Ma, X.R.; Zhang, H.W.; Xu, Y.Z.; Xu, J.W.; Zhang, J.Q.; Li, M.; et al. Role of microtubule dynamics in Wallerian degeneration and nerve regeneration after peripheral nerve injury. Neural Regen Res 2022, 17, 673. [Google Scholar] [CrossRef]
- Huang, Z.; Powell, R.; Phillips, J.B.; Haastert-Talini, K. Perspective on Schwann Cells Derived from Induced Pluripotent Stem Cells in Peripheral Nerve Tissue Engineering. Cells-Basel 2020, 9. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.N.; Xiao, C.S.; Liu, B. Engineered hydrogels for peripheral nerve repair. Mater Today Bio 2023, 20. [Google Scholar] [CrossRef] [PubMed]
- Nocera, G.; Jacob, C. Mechanisms of Schwann cell plasticity involved in peripheral nerve repair after injury. Cell Mol Life Sci 2020, 77, 3977–3989. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, H.; Zhang, Y.; Liu, Z.; He, D.; Xu, W.; Li, S.; Zhang, C.; Zhang, Z. Li-Mg-Si bioceramics provide a dynamic immuno-modulatory and repair-supportive microenvironment for peripheral nerve regeneration. Bioact Mater 2023, 28, 227–242. [Google Scholar] [CrossRef]
- Ma, T.; Hao, Y.M.; Li, S.Y.; Xia, B.; Gao, X.; Zheng, Y.; Mei, L.W.; Wei, Y.T.; Yang, C.B.; Lu, L.; et al. Sequential oxygen supply system promotes peripheral nerve regeneration by enhancing Schwann cells survival and angiogenesis. Biomaterials 2022, 289. [Google Scholar] [CrossRef]
- Borger, A.; Stadlmayr, S.; Haertinger, M.; Semmler, L.; Supper, P.; Millesi, F.; Radtke, C. How miRNAs Regulate Schwann Cells during Peripheral Nerve Regeneration-A Systemic Review. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef]
- Jones, S.; Eisenberg, H.M.; Jia, X.F. Advances and Future Applications of Augmented Peripheral Nerve Regeneration. Int J Mol Sci 2016, 17. [Google Scholar] [CrossRef]
- Hercher, D.; Nguyen, M.Q.; Dworak, H. Extracellular vesicles and their role in peripheral nerve regeneration. Exp Neurol 2022, 350. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zheng, T.T.; Wu, L.L.; Han, Q.; Chen, S.Y.; Kong, Y.; Li, G.C.; Ma, L.; Wu, H.; Zhao, Y.H.; et al. Fabrication and characterization of 3D-printed gellan gum/starch composite scaffold for Schwann cells growth. Nanotechnol Rev 2021, 10, 50–61. [Google Scholar] [CrossRef]
- Zou, S.Z.; Wang, X.R.; Fan, S.N.; Yao, X.; Zhang, Y.P.; Shao, H.L. Electrospun regenerated Antheraea pernyi silk fibroin scaffolds with improved pore size, mechanical properties and cytocompatibility using mesh collectors. J Mater Chem B 2021, 9, 5514–5527. [Google Scholar] [CrossRef] [PubMed]
- Li, L.X.; Xu, Y.Z.; Wang, X.H.; Liu, J.M.; Hu, X.F.; Tan, D.D.; Li, Z.L.; Guo, J.S. Ascorbic acid accelerates Wallerian degeneration after peripheral nerve injury. Neural Regen Res 2021, 16, 1078–1085. [Google Scholar] [CrossRef] [PubMed]
- Wofford, K.L.; Shultz, R.B.; Burrell, J.C.; Cullen, D.K. Neuroimmune interactions and immunoengineering strategies in peripheral nerve repair. Prog Neurobiol 2022, 208. [Google Scholar] [CrossRef]
- Cerri, F.; Salvatore, L.; Memon, D.; Boneschi, F.M.; Madaghiele, M.; Brambilla, P.; Del Carro, U.; Taveggia, C.; Riva, N.; Trimarco, A.; et al. Peripheral nerve morphogenesis induced by scaffold micropatterning. Biomaterials 2014, 35, 4035–4045. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.W.; Lin, Y.F.; Wang, G.W.; Song, J.L.; Hayat, U.; Liu, C.; Raza, A.L.; Huang, X.Y.; Lin, H.D.; Wang, J.Y. Zein-induced immune response and modulation by size, pore structure and drug-loading: Application for sciatic nerve regeneration. Acta Biomater 2022, 140, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.Y.; Gu, X.K.; Zhang, R.R.; Qian, T.M.; Li, S.Y.; Yi, S. Biological characteristics of dynamic expression of nerve regeneration related growth factors in dorsal root ganglia after peripheral nerve injury. Neural Regen Res 2020, 15, 1502–1509. [Google Scholar] [CrossRef]
- Yang, H.C.; Li, Q.; Li, L.M.; Chen, S.C.; Zhao, Y.; Hu, Y.R.; Wang, L.; Lan, X.Q.; Zhong, L.M.; Lu, D. Gastrodin modified polyurethane conduit promotes nerve repair via optimizing Schwann cells function. Bioact Mater 2022, 8, 355–367. [Google Scholar] [CrossRef] [PubMed]
- He, Q.R.; Cong, M.; Yu, F.H.; Ji, Y.H.; Yu, S.; Shi, H.Y.; Ding, F. Peripheral nerve fibroblasts secrete neurotrophic factors to promote axon growth of motoneurons. Neural Regen Res 2022, 17, 1833–1840. [Google Scholar] [CrossRef] [PubMed]
- Saio, S.; Konishi, K.; Hohjoh, H.; Tamura, Y.; Masutani, T.; Iddamalgoda, A.; Ichihashi, M.; Hasegawa, H.; Mizutani, K. Extracellular Environment-Controlled Angiogenesis, and Potential Application for Peripheral Nerve Regeneration. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef]
- Yamada, Y.; Nihara, J.; Trakanant, S.; Kudo, T.; Seo, K.; Iida, I.; Izumi, K.; Kurose, M.; Shimomura, Y.; Terunuma, M.; et al. Perivascular Hedgehog responsive cells play a critical role in peripheral nerve regeneration via controlling angiogenesis. Neurosci Res 2021, 173, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Ramasamy, T.S.; Lin, S.C.; Chen, S.H.; Lu, J.; Liu, Y.H.; Lu, F.I.; Hsueh, Y.Y.; Lin, S.P.; Wu, C.C. Autologous Platelet-Rich Growth Factor Reduces M1 Macrophages and Modulates Inflammatory Microenvironments to Promote Sciatic Nerve Regeneration. Biomedicines 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Zhang, T.; Lin, Y.; Cai, X. Vascularization in Craniofacial Bone Tissue Engineering. J Dent Res 2018, 97, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; He, X.T.; Wang, J.; Wu, R.X.; Xu, X.Y.; Hong, Y.L.; Tian, B.M.; Chen, F.M. S Pore size-mediated macrophage M1-to-M2 transition influences new vessel formation within the compartment of a scaffold. Appl Mater Today 2020, 18. [Google Scholar] [CrossRef]
- Wang, W.L.Y.; Kent, R.N.; Huang, S.I.A.; Jarman, E.H.; Shikanov, E.H.; Davidson, C.D.; Hiraki, H.L.; Lin, D.E.; Wall, M.A.; Matera, D.L.; et al. Direct comparison of angiogenesis in natural and synthetic biomaterials reveals that matrix porosity regulates endothelial cell invasion speed and sprout diameter. Acta Biomater 2021, 135, 260–273. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).