1. Introduction
ICH accounts for 15 to 20% in all stroke types and is commonly accompanied with high morbidity and mortality [
1,
2,
3]. While the acute hematoma compression leads to immediate mechanical brain damage, adverse neurological outcomes are strongly related to the occurrence of SBI [
4,
5]. SBI of ICH is believed to result from the
potentiation of various parallel cascades, including toxicity of extravasated blood components [
6,
7], oxidative stress and inflammation [
8,
9,
10], which ultimately leads to blood-brain-barrier (BBB) disruption and brain edema with massive brain cell death [
11,
12,
13]. Unfortunately, currently agents afforded limited neuroprotection against inflammation induced SBI following ICH.
The orally available compound Fingolimod (FTY720) is therapy for multiple sclerosis approved by Food and Drug Administration (FDA) in 2010[
14,
15]. Modulation of S1PR by FTY720 in both the immune system and CNS may offer a combination of anti-inflammatory and neuroprotective effects in patients with cerebral hemorrhage [
16,
17,
18]. However, due to its low solubility and instability in an aqueous medium, the currently available FTY720 medicine for oral administration must be administered daily to achieve active steady-state levels[
19]. In addition, alternative approaches of FTY720 for renal transplantation [
20] and different types of cancers [
21,
22] reinforced its instability problems, toxicity potential in the maintenance of therapeutic doses.
Nanomedicine, application of nanotechnology in medical field, is of vital importance in medicine delivery [
23,
24]. It is well established that nano-based drug delivery facilitates effective delivery and systemic toxicity reduction [
25,
26]. In recent decades, the polymeric micelles (PMs), self-assembled from amphiphilic block copolymers with hydrophobic and hydrophilic blocks, have gained significance in the field of drug delivery [
27,
28,
29]. Poly (etheylene glycol) (PEG), an FDA-approved polymer with good biocompatibility and good tolerance, with no cytotoxicity, is one of the most commonly investigated biocompatible polymers [
30,
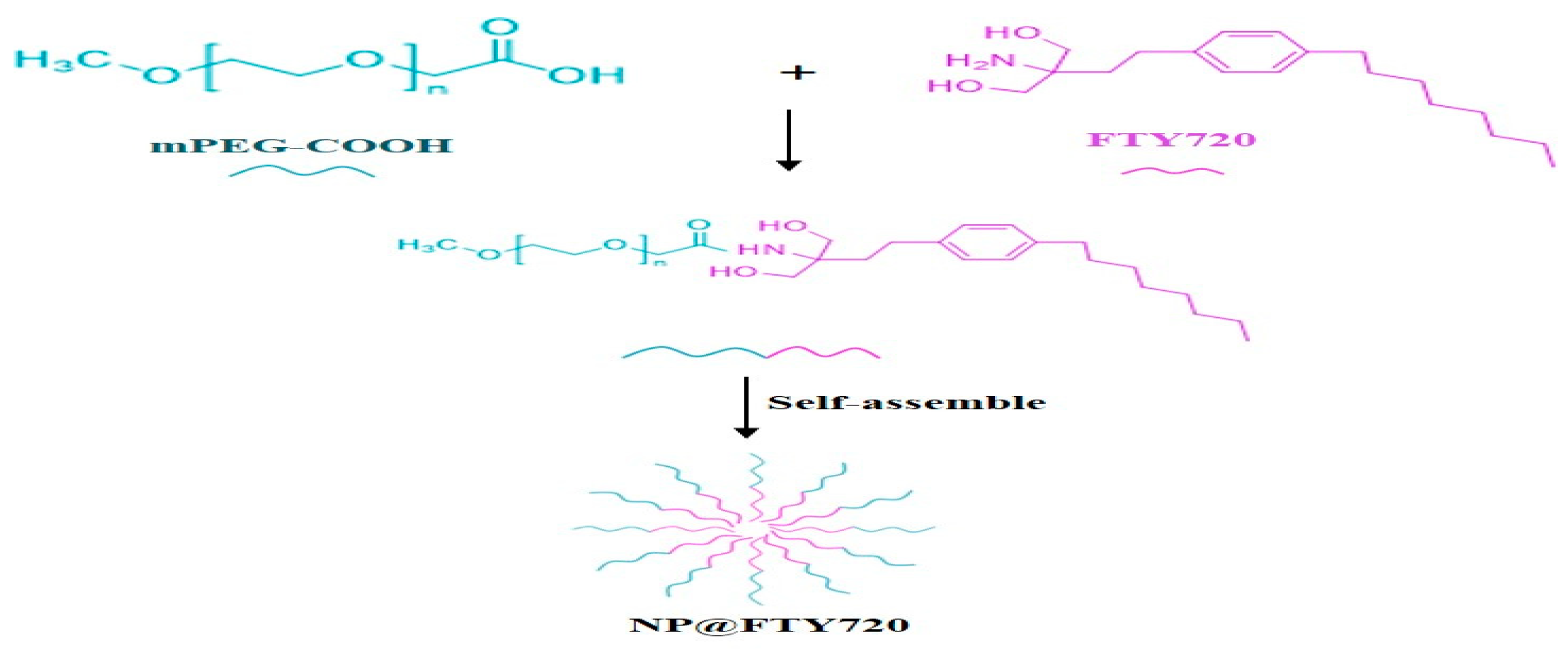
31]. Since FTY720 displays amino groups, an amphiphilic block copolymer composed of mPEG and FTY720 was constructed in our study (Figure1). The terminated carboxy group of mPEG coupled with FTY720 through amide bond linker. The NP@FTY720 were characterized in terms of physicochemical, morphological properties, drug release profiles and cytotoxicity. Near-infrared fluorescence imaging was also performed after administration of NP@FTY720 intravenously to assess the biodistribution
in vivo and
in vitro. Furthermore, we explored the neuroprotective effect of NP@FTY720 in a mouse model of ICH. The results demonstrated that the prepared micelles encapsulated FTY720 into the hydrophobic core in blood circulation. Once entering cells, the amide bond break under the conditions of tissue proteases, and then FTY720 was released. It led to a striking reduction of neuronal damage and BBB injury after ICH. Overall, we identified that the nanotechnology allied to NP@FTY720 might represent a potential strategy for ICH treatment.
2. Materials and Methods
2.1. Materials
FTY720 was purchased from Aikon Biopharmaceutical R&D Co. Ltd (Jiangsu, China). mPEG-COOH was provided by Yu CAI Medical Technology Co Ltd (Chongqing, China). Ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC) and N-hydroxysuccinimide (NHS) were purchased from Sinopharm Chemical Reagent Co Ltd. (Shanghai, China). Dulbecco’s Modified Eagle Medium (DMEM/F12), penicillin/streptomycin solution, fetal bovine serum (FBS), and trypsin-ethylene diamine tetraacetic acid (Trypsin-EDTA, 0.05%) were supplied by Gibco BRL Co Ltd (Gaithersburg, USA). 3-[4,5-Dimethylthiazol-2-yl]-2, 5-diphenylte-trazolium bromide (MTT), dimethyl sulfoxide (DMSO), and phosphate-buffered saline (PBS) were purchased from Biosharp Co Ltd (Anhui, China). Cathepsin D enzyme was purchased from Sigma-Aldrich (St. Louis, USA).
2.2. Synthesis and Characterizations of Self-Assembling FTY720 Nanoparticle (NP@FTY720)
NP@FTY720 was synthesized as following: First, mPEG-COOH was pre-dissolved in deionized H2O and then activated using EDC/NHS solution (EDC: NHS=2:1) for 0.5h. Subsequently, FTY720 solution was added into above reaction system and stirred for 40h to obtain white products. To remove unreacted FTY720, EDC and NHS, the white products were disposed by dialysis bag for 4 times to ultimately gain NP@FTY720. The morphology and size of NP@FTY720 were examined by a transmission electron microscopy (TEM, Tecnai G2 20S-Twin). Chemical bonds of nanoparticle composition were detected by a Fourier transform infrared spectrometer (FTIR, Nicolet 6700, Thermo Electron Scientific Instruments). The 1H NMR spectrum was analyzed by a Bruker Avance 400 NMR spectrometer (Bruker Corporation, Switzerland).
2.3. Measurement of FTY720 Release In Vitro
In vitro drug release of FTY720-loaded nanoparticles was examined through the dialysis method[
32]. Different pH values were chosen to simulate the physiological condition of the blood (pH 7.4) and intracellular lysosomers (5.0), respectively. The release of FTY720 concentration was measured by ultraviolet spectrophotometer at 265nm.
2.4. Cell Culture
Human neuroblastoma (SH-SY5Y) (ATCC®, CRL-2266™) cells were cultured in DMEM/F12 and 10% FBS in an incubator (Thermo Scientific, USA) under an atmosphere of 5% CO2 at 37℃.
2.5. Cytotoxicity Experiments In Vitro
Vitro cytotoxicity was examined by MTT assay. SH-SY5Y cells were seeded in 96-well plates for 24 h and incubated with gradient concentration of FTY720 and NP@FTY720 for another 24h, respectively. MTT solution was added to each well and then incubated for 4h. The cellular viability was investigated via absorbance.
2.6. Animals and Groups
Male adult C57BL/6 mice, 8-10 week-old (25g to 28g), were used in all animal experiments. The animal study protocol was approved by the Institutional Animal Care and Use Committee (IACUC)The Second Xiangya Hospital, Central South University, China (No 2021709). Mice were randomly divided into three groups: ICH group (normal saline + ICH), free FTY720 + ICH (FTY720 group), the NP@FTY720 group + ICH (NP@FTY720 group). The mice were intravenously injected via tail vein with normal saline, FTY720, NP@FTY720 (0.5mg/kg, dose equivalent to FTY720) respectively, once a day after ICH until sacrifice.
2.7. ICH Model
ICH surgery was induced by stereotactic-guided injection of collagen type IV into the left basal ganglia as previously described. Briefly, mice were anesthetized and placed on a stereotaxic frame. A 1-mm cranial burr hole was drilled in the skull, and the Hamilton syringe was inserted into the right basal ganglia following the stereotactic guide (coordinates: 0.2 mm anterior, 2.5 mm lateral, and 3.5 mm deep relative to the bregma). collagen type IV(0.0375U in 0.5μL) was infused using a microinfusion pump (Stoelting, Harvard Apparatus, Holliston, MA) at a rate of 2 μL/min.
2.8. Biodistribution Assay
DiR-loaded nanoparticles, a near-infrared fluorophore dye, were prepared. Mice with a model of ICH were intravenously injected with NP@FTY720/DIR (0.5mg/kg). After 24h, the mice were observed using vivo imaging system of IVIS Lumina III (Perkin Elmer, USA). Ultimately, the mice were sacrificed and organs (brain, heart, brain, liver, spleen, and kidney) were obtained for vivo imaging.
2.9. Neurobehavioral Assessment
The neurological deficit scores were investigated by modified neurological severity score (mNSS, n = 8) 1, 3 and 7 days after ICH, which includes motor, sensory, balance, and reflex tests, together with the corner-turning test. The score ranges from 0 to 18 points, and 1 point is given for failing to perform one task.
2.10. Hematoxylin and Eosin (H&E) Staining
The brain, heart, liver, spleen, lung and kidney of mice in different groups were obtained and fixed with 4% paraformaldehyde for 24 h. Paraffin-embedded tissue was sectioned, and deparaffinized and stained with hematoxylin and eosin (HE).
2.11. Nissl Staining
The brain was removed and placed in 4% PFA for 24 h at 4 °C. Paraffin-embedded brain was sectioned and then treated with FD Cresyl Violet SolutionTM for 2 min, followed by dehydration and sealing with neutral resin.
2.12. Brain Water Content Measurement
The brain-water content was assessed using the wet-dry method. Brains were divided into five parts: ipsilateral and contralateral cortex, ipsilateral and contralateral basal ganglia, and cerebellum. Each brain section was measured immediately on an analytical microbalance to obtain the wet weight (WW) and then dried for 24 h at 100 °C to obtain the dry weight (DW). Brain water content was calculated through the following formula: brain water content (%) = [(WW − DW)/WW] × 100%.
2.13. Evans Blue Analysis
EB dye extravasation was used to evaluate BBB permeability[
33]. At 4h before sacrifice, 2% EB dye in saline was injected intravenously as a BBB permeability tracer.Mice were anesthetized and then transcardially perfused with saline. The brain was then removed and cut into five 1-mm-thick coronal slices and photographed to visualize EB leakage.
2.14. Statistical Analysis
Statistical analysis was performed by SPSS 21.0 software. The quantitative data was presented as mean ± SD. Statistical differences were analyzed with one-way ANOVA as the data followed normal distribution, otherwise with Kruskal-Wall H. P <0.05 was considered as significance.
3. Results and Discussion
3.1. Preparation and Characterization of NP@FTY720
NP@FTY720 preparation is presented in
Figure 1.
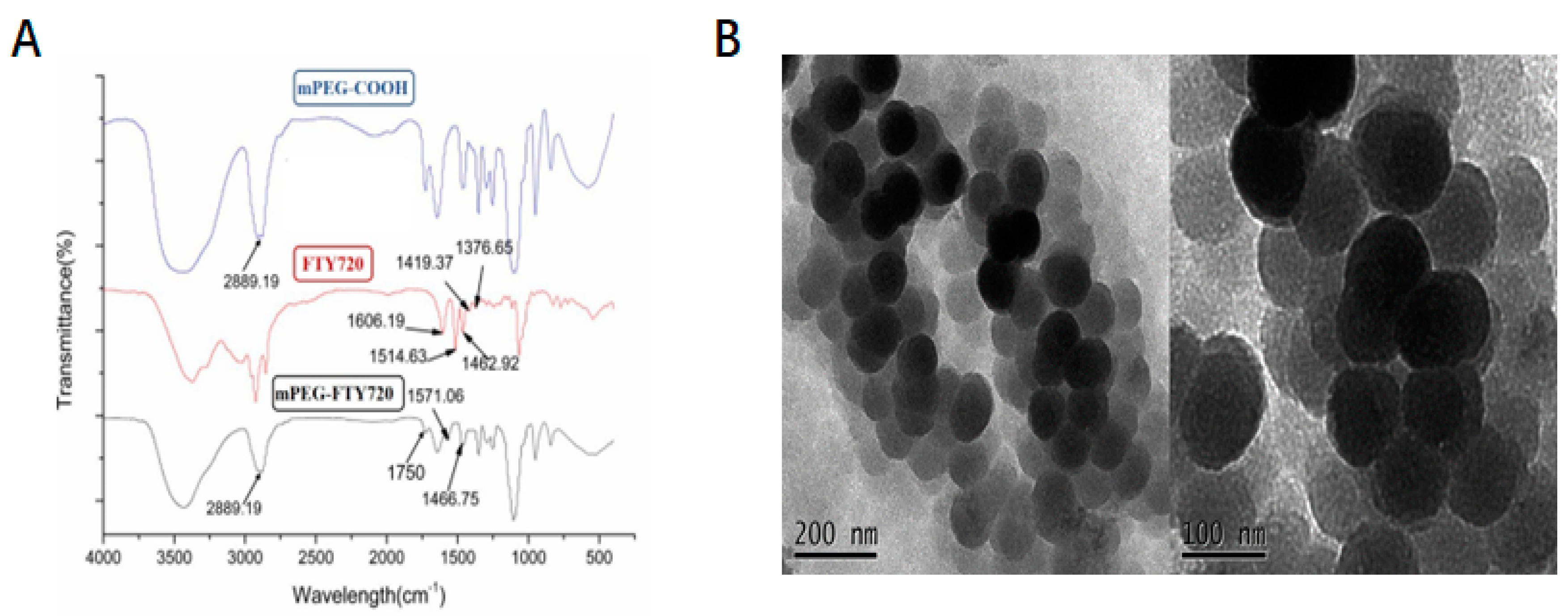
The FTIR spectra of compounds are provided to confirm their structures. After modification, characteristic peak of FTY720 was revealed at 1420-1620 cm
-1 and that of mPEG at 2750-3000 cm
-1. There was a new amide peak at 1750 cm
-1 indicating that
dicarboxy-terminated polyethylene glycol mPEG was successfully
covalently grafted to FTY720 via amide crosslinking (Figure 2A). The morphological structures of
NP@FTY720 were analyzed by transmission electron microscopy (TEM) images. As shown in
Figure 2B,
NP@FTY720 exhibited a regular spherical morphology with the average diameter of about 113nm. The TEM data validated the creation of
polymeric micelles.
3.2. Measurement of FTY720 Release In Vitro
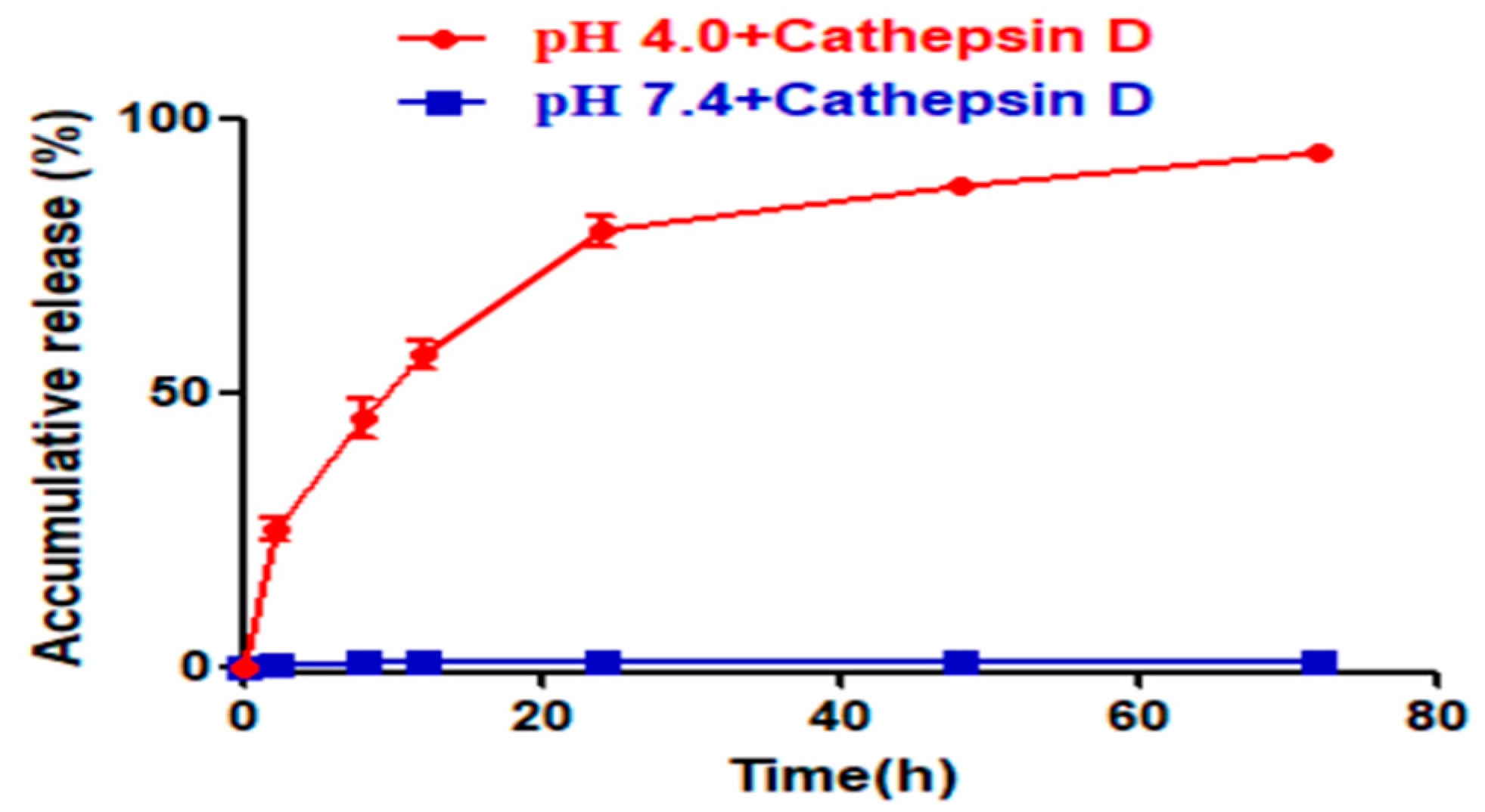
At pH = 7.4, scarcely any FTY720 was released from NP@FTY720 over 48h indicating that drug carriers exist in a form of stable micelle structure. When pH was decreased to 4.0, very fast and high cumulative FTY720 release could be observed, with more than 77.68% drug released within 20min. It may be inferred that under the acidic environment,
carboxy-amino groups, the ionic interaction between the encapsulated FTY720 and
mPEG in amphiphile micelle cores was significantly weakened, which finally led to copolymer micelle disassembly and fast FTY720 release (
Figure 3). The drug release study demonstrated its significant pH-responsive release behavior, which emphasizes that
NP@FTY720 could keep a prominent stability in the course of systematic circulation, while cell internalization facilitates effective release of loaded drug.
3.3. Cytotoxicity of NP@FTY720
Cytotoxicity of
NP@FTY720 was estimated in SHSY-5Ycells by MTT assay after being incubated with FTY720 and
NP@FTY720 at different concentrations for 24h. The data showed that concentrations above 2.5 μg/mL of both free FTY720 and
NP@FTY720 were cytotoxic to SHSY-5Ycells cells. Free and encapsulated FTY720 induced a significant decrease in cell viability in a concentration-dependent manner. However, Free FTY720 presented significantly higher cytotoxicity compared with
NP@FTY720. Thus, the block copolymer was proved to decrease cytotoxicity of FTY720, especially at high doses (
Figure 4), which serves as a promising candidate for drug delivery.
3.4. Biosafety Assessment of NP@FTY720 In Vivo
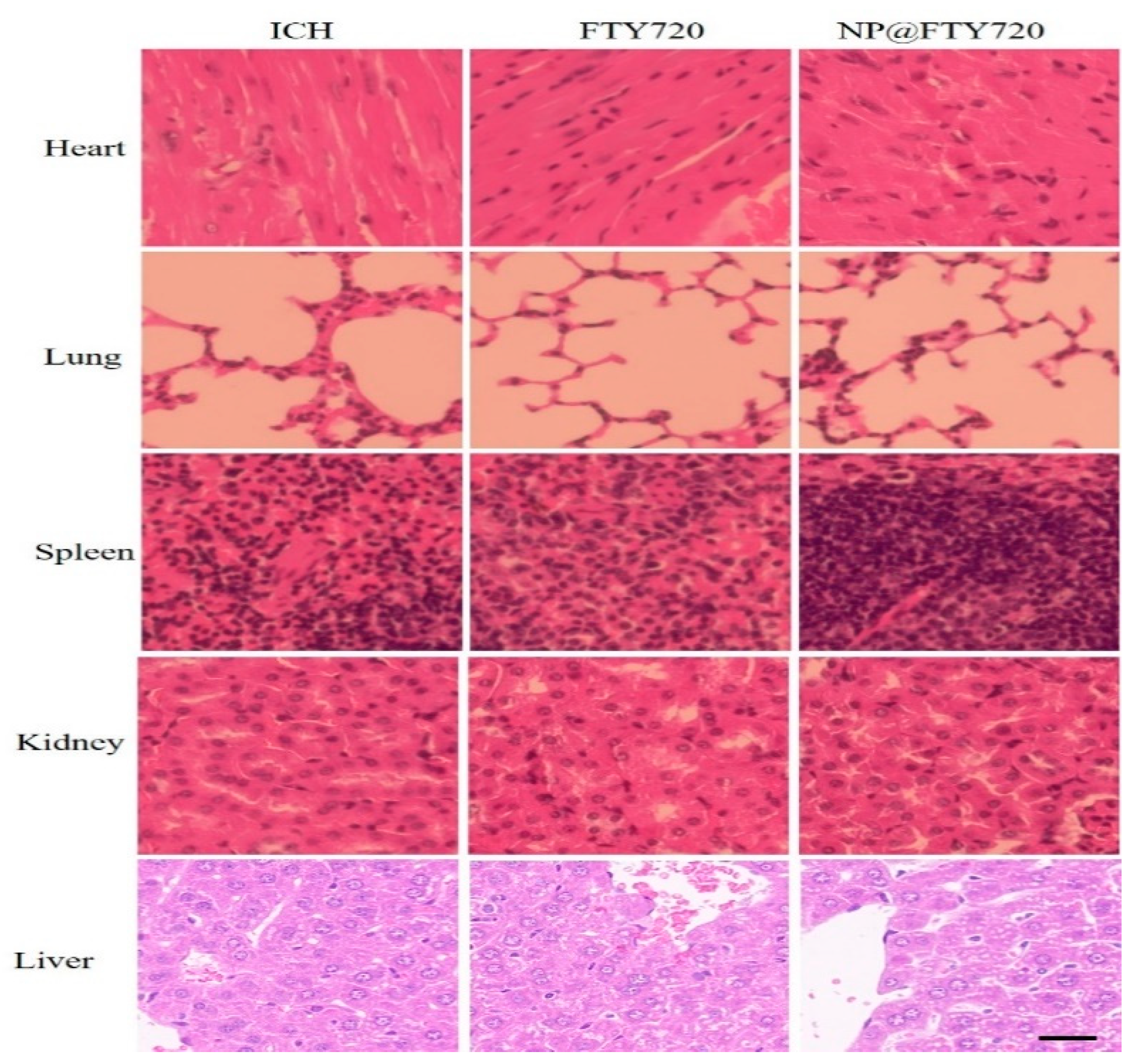
The possible side effects produced by the intravenous injection of
NP@FTY720 were evaluated by histological analyses. Representative histological images using hematoxylin-eosin staining of samples of heart, lung, liver, spleen and kidney from saline and
NP@FTY720-treated animals at day 7 following treatment are shown in
Figure 5. The results indicated that no significant abnormalities were observed in vital organs, highlighting the excellent biosafety profile for
NP@FTY720 in our study.
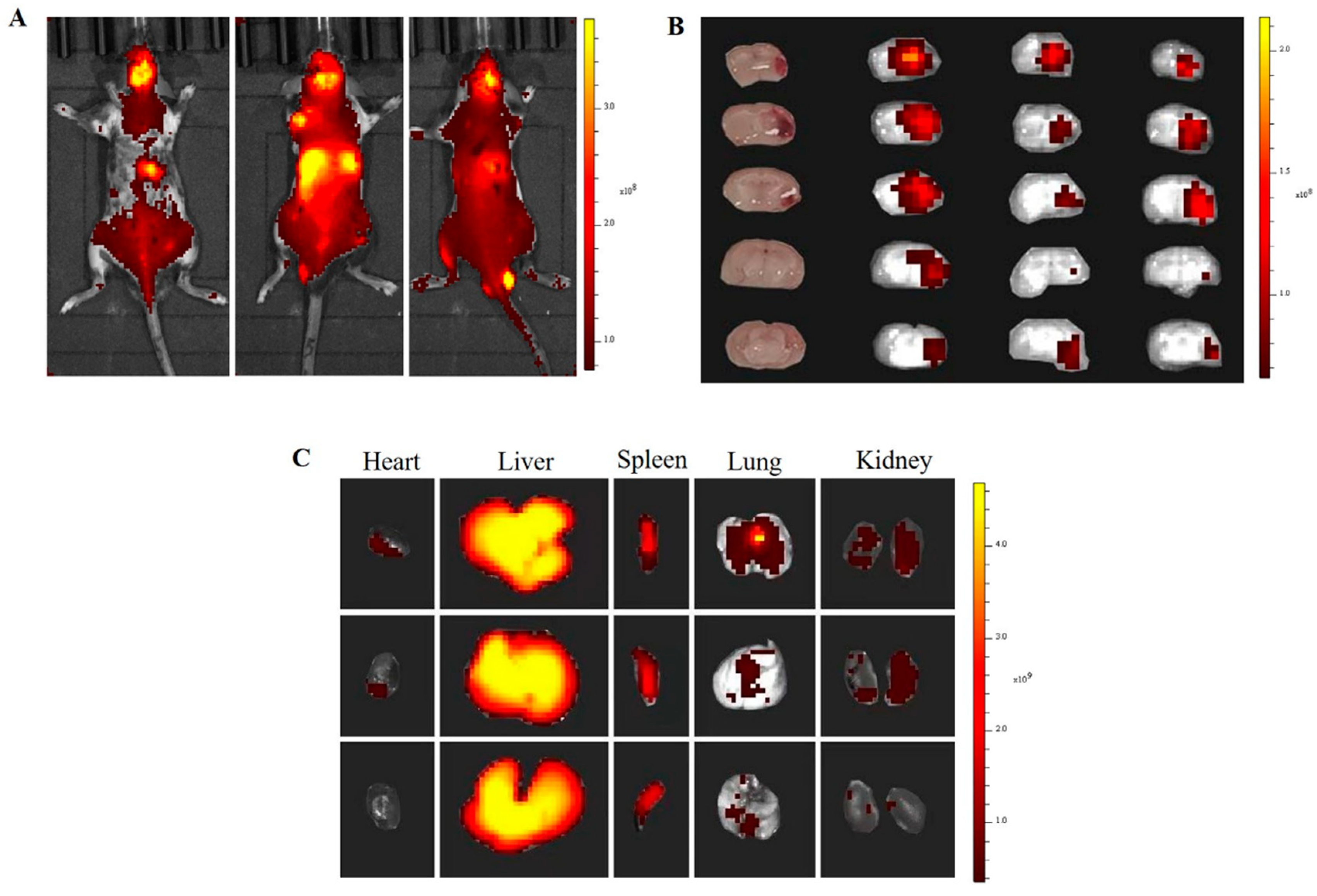
3.5. In Vivo and Ex Vivo Biodistribution
In vivo biodistribution assay was performed using a near-infrared fluorophore dye (DiR)-loaded nanoparticle with an
in vivo fluorescence imaging system (IVIS spectrum). As shown in
Figure 6A,
NP@FTY720 was distributed throughout the body through blood circulation and was enriched in brain tissue of ICH models after 24h (
Figure 6A)
. Mice were sacrificed after 24h and major organs (brain, heart, lung, liver, spleen and kidney) were collected for ex vivo imaging. The cryosection of brain tissues were undertaken to detect brain distribution of NP@FTY720. The data showed that (DiR)-labeled
NP@FTY720 mainly accumulated in hematoma and perihematomal area
ex vivo (
Figure 6B). The distribution of
NP@FTY720 in other main organs was also assayed, with high accumulation in liver and spleen (
Figure 6C). These results revealed that the block copolymer can penetrate the BBB rapidly and deliver drugs to the targeted brain tissues.
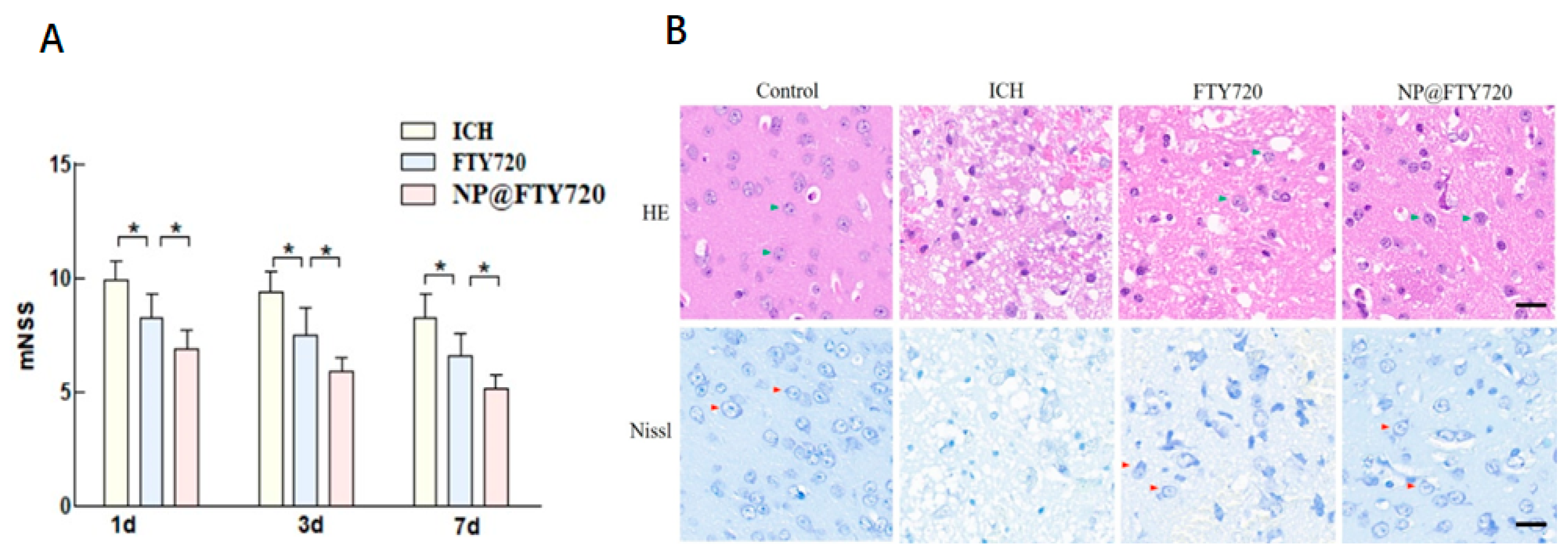
3.6. NP@FTY720 Alleviated ICH-Induced Neurological Deficits and Ameliorated ICH-Induced Neuronal Damage
To comprehensively determine the impact of NP@FTY720 on ICH in mice, neurological function was evaluated by a modified Neurological Severity Scores (mNSS) at 1d, 3d and 7d after ICH. Treatment of FTY720 and
NP@FTY720 after ICH significantly attenuated the severity of behavioural symptoms starting from day 1 after ICH, as evidenced by substantial decreased mNSS. Additionally, the overall behavioral performance showed that compared with FTY720 treatment, the NP@FTY720 held the better effect (
Figure 7A).
3.7. NP@FTY720 Alleviated ICH-Induced Brain Injury
As shown in
Figure 7B (upper panel), HE staining showed that in sham group, neurons in perihematomal tissue were neatly arranged with clearly visible nuclei and dense cytoplasm. In ICH group, the number of neurons significantly decreased. Moreover, cells exhibited a disorderly arrangement and nuclear pyknosis. The damage was substantially reduced in ICH mice treated with FTY720 or
NP@FTY720, and the
NP@FTY720 treatment had greater improvement in neuronal damage after ICH induction. Neuronal cells showed greater benefit from treatment with
NP@FTY720 than FTY720. Additionally, Nissl staining was then performed to evaluate ICH-induced neuronal damage extent after ICH. Compared to the sham group, the number of Nissl's bodies was dropped significantly, and neuronal cell exhibited small, darkly stained, shrunken nuclei in ICH group. In contrast, FTY720 and
NP@FTY720 group revealed increased number of Nissl bodies with improvement of morphology, while
NP@FTY720 exhibited a more profound protective effect on neuronal cells than FTY720 (
Figure 7B, lower panel).
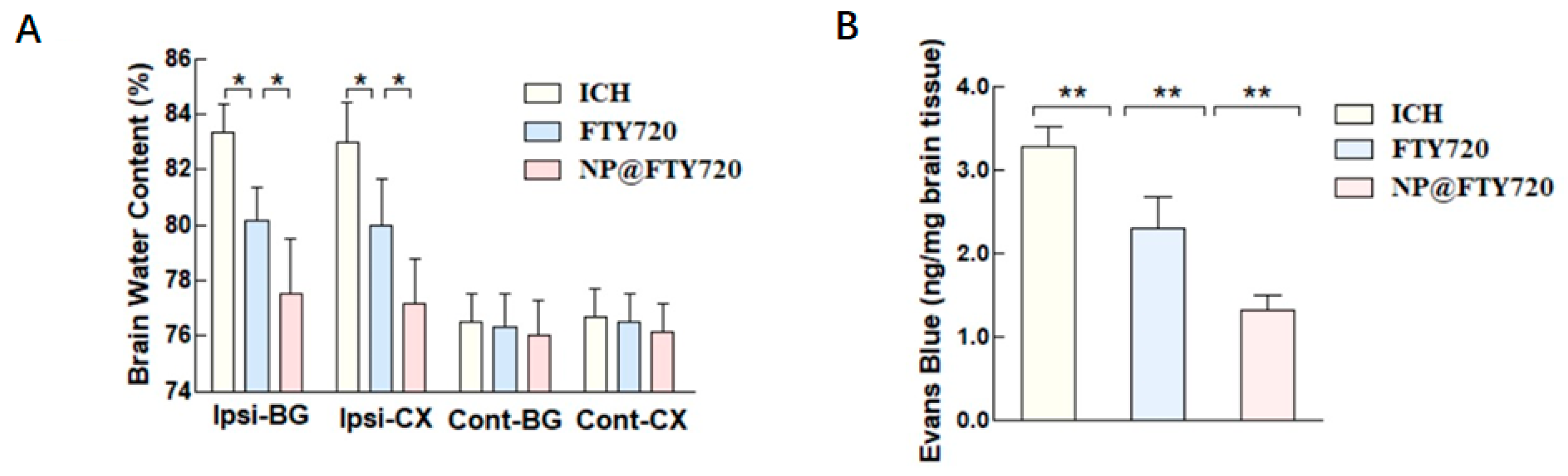
3.8. NP@FTY720 Reduced Brain Edema and Ameliorated BBB Disruption
Brain water content was examined to explore the effect of NP@FTY720 treatment on ICH-induced brain edema. No significant differences were observed in the contralateral cortex, contralateral basal ganglia, or cerebellum in these three groups at 72 h post-ICH. However, the ICH group showed significant increase in water content in the ipsilateral cortex and ipsilateral basal ganglia, compared to the sham group, while both FTY720 and
NP@FTY720 treatment significantly reduced the brain water content. Moreover, the
NP@FTY720 group exhibited significantly milder brain edema compared with FTY720 group (
Figure 8A). We further evaluated Evans blue (EB) leakage in each group at 72 h post-ICH, which is an important hallmark of BBB disruption. Compared with the sham group, ICH mice displayed increased EB extravasation in the hemorrhagic hemisphere, which were markedly decreased by both FTY720 and
NP@FTY720 respectively. EB leakage was significantly reduced in
NP@FTY720 group compared with FTY720 group (
Figure 8B). These results indicated that
NP@FTY720 alleviated brain edema and BBB dysfunction.
4. Conclusion
Based on the chemical structure of FTY, we successfully fabricated a diblock copolymer, which was self-assembled by covalently grafting dicarboxy-terminated polyethylene glycol on the surfaces of FTY720 with an amino group. The NP@FTY720 presented a relatively uniform spherical shape morphology with a mean diameter of about 120 nm. Subsequently, the PMs were stable at neutral pH but rapidly release the loaded FTY720 once encountering an acidic environment. NP@FTY720 penetrated through BBB and efficiently accumulated in peri-hematomal area. We further revealed that NP@FTY720 significantly enhanced protective effects against ICH-induced neuronal death and BBB damage while showing no toxicity to SH-SY5Y cells in vitro and mice in vivo. This observation opens up a novel promising avenue for ICH therapy.
Author Contributions
Conceptualization, YBB and ZFF; methodology, GXY and ZFF.; validation, GXY, ZFF and HST; formal analysis, ZFF; investigation, ZFF,GXY, HST, HYJ, and YBB; project administration, ZFF,GXY, HST, HYJ, and YBB;data curation, ZFF and GXY ; writing—original draft preparation, ZFF GXY, HST, HYJ; writing—review and editing, YBB; funding acquisition, ZFF and YBB. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Science and health Union Foundation of Hunan Province (No. 2022JJ70147) and Natural Science Foundation of Hunan Province (No. 2022JJ30859).
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Animal Care and Use Committee (IACUC), The Second Xiangya Hospital, Central South University, China (No. 2021709).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data can be made available upon request from the corresponding authors.
Acknowledgments
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Banzrai, C.; Bosookhuu, O.; Yadamsuren, E.; Dambasuren, B.; Turbat, S.; Erdenedalai, T.; Myadagsuren, M.; Munkhtur, U.; Baatar, K.; Boldbayar, P.; et al. Incidence and outcomes for stroke in Ulaanbaatar, Mongolia, during 2019-21: a prospective population-based study. The Lancet. Global health 2023, 11, e942–e952. [Google Scholar] [CrossRef]
- Hu, R.; Zhang, C.; Xia, J.; Ge, H.; Zhong, J.; Fang, X.; Zou, Y.; Lan, C.; Li, L.; Feng, H. Long-term Outcomes and Risk Factors Related to Hydrocephalus After Intracerebral Hemorrhage. Translational stroke research 2021, 12, 31–38. [Google Scholar] [CrossRef]
- Global, regional, and national burden of stroke, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet. Neurology 2019, 18, 439–458. [CrossRef]
- Lok, J.; Leung, W.; Murphy, S.; Butler, W.; Noviski, N.; Lo, E.H. Intracranial hemorrhage: mechanisms of secondary brain injury. Acta neurochirurgica. Supplement 2011, 111, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Cordonnier, C.; Demchuk, A.; Ziai, W.; Anderson, C.S. Intracerebral haemorrhage: current approaches to acute management. Lancet (London, England) 2018, 392, 1257–1268. [Google Scholar] [CrossRef]
- Babu, R.; Bagley, J.H.; Di, C.; Friedman, A.H.; Adamson, C. Thrombin and hemin as central factors in the mechanisms of intracerebral hemorrhage-induced secondary brain injury and as potential targets for intervention. Neurosurgical focus 2012, 32, E8. [Google Scholar] [CrossRef] [PubMed]
- Wagner, K.R.; Sharp, F.R.; Ardizzone, T.D.; Lu, A.; Clark, J.F. Heme and iron metabolism: role in cerebral hemorrhage. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 2003, 23, 629–652. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Y.; Wang, J.; Anne Stetler, R.; Yang, Q.W. Inflammation in intracerebral hemorrhage: from mechanisms to clinical translation. Progress in neurobiology 2014, 115, 25–44. [Google Scholar] [CrossRef]
- Wang, J.; Doré, S. Inflammation after intracerebral hemorrhage. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 2007, 27, 894–908. [Google Scholar] [CrossRef]
- Shao, L.; Chen, S.; Ma, L. Secondary Brain Injury by Oxidative Stress After Cerebral Hemorrhage: Recent Advances. Frontiers in cellular neuroscience 2022, 16, 853589. [Google Scholar] [CrossRef]
- Felberg, R.A.; Grotta, J.C.; Shirzadi, A.L.; Strong, R.; Narayana, P.; Hill-Felberg, S.J.; Aronowski, J. Cell death in experimental intracerebral hemorrhage: the "black hole" model of hemorrhagic damage. Annals of neurology 2002, 51, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Xu, Y.; Guo, P.; Chen, Y.J.; Zhou, J.; Xia, M.; Tan, B.; Liu, X.; Feng, H.; Chen, Y. CCL5/CCR5-mediated peripheral inflammation exacerbates blood‒brain barrier disruption after intracerebral hemorrhage in mice. Journal of translational medicine 2023, 21, 196. [Google Scholar] [CrossRef]
- Keep, R.F.; Xiang, J.; Ennis, S.R.; Andjelkovic, A.; Hua, Y.; Xi, G.; Hoff, J.T. Blood-brain barrier function in intracerebral hemorrhage. Acta neurochirurgica. Supplement 2008, 105, 73–77. [Google Scholar] [CrossRef]
- Chiba, K.; Adachi, K. Discovery of fingolimod, the sphingosine 1-phosphate receptor modulator and its application for the therapy of multiple sclerosis. Future medicinal chemistry 2012, 4, 771–781. [Google Scholar] [CrossRef]
- Ali, R.; Nicholas, R.S.; Muraro, P.A. Drugs in development for relapsing multiple sclerosis. Drugs 2013, 73, 625–650. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, H. FTY720 in CNS injuries: Molecular mechanisms and therapeutic potential. Brain research bulletin 2020, 164, 75–82. [Google Scholar] [CrossRef]
- Fu, Y.; Hao, J.; Zhang, N.; Ren, L.; Sun, N.; Li, Y.J.; Yan, Y.; Huang, D.; Yu, C.; Shi, F.D. Fingolimod for the treatment of intracerebral hemorrhage: a 2-arm proof-of-concept study. JAMA neurology 2014, 71, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Diaz Diaz, A.C.; Shearer, J.A.; Malone, K.; Waeber, C. Acute Treatment With Fingolimod Does Not Confer Long-Term Benefit in a Mouse Model of Intracerebral Haemorrhage. Frontiers in pharmacology 2020, 11, 613103. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Wang, J.; Zhao, Y.; Wu, Y.; Kwak, K.J.; Chen, C.S.; Byrd, J.C.; Lee, R.J.; Phelps, M.A.; Lee, L.J.; et al. A novel liposomal formulation of FTY720 (fingolimod) for promising enhanced targeted delivery. Nanomedicine : nanotechnology, biology, and medicine 2014, 10, 393–400. [Google Scholar] [CrossRef]
- Budde, K.; Schütz, M.; Glander, P.; Peters, H.; Waiser, J.; Liefeldt, L.; Neumayer, H.H.; Böhler, T. FTY720 (fingolimod) in renal transplantation. Clinical transplantation 2006, 20 Suppl 17, 17–24. [Google Scholar] [CrossRef]
- Garner, E.F.; Williams, A.P.; Stafman, L.L.; Aye, J.M.; Mroczek-Musulman, E.; Moore, B.P.; Stewart, J.E.; Friedman, G.K.; Beierle, E.A. FTY720 Decreases Tumorigenesis in Group 3 Medulloblastoma Patient-Derived Xenografts. Scientific reports 2018, 8, 6913. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Wang, J.; Zheng, T.; Liang, Y.; Yin, D.; Song, R.; Pei, T.; Pan, S.; Jiang, H.; Liu, L. FTY720 inhibits proliferation and epithelial-mesenchymal transition in cholangiocarcinoma by inactivating STAT3 signaling. BMC cancer 2014, 14, 783. [Google Scholar] [CrossRef]
- Zhu, H.; Zheng, J.; Oh, X.Y.; Chan, C.Y.; Low, B.Q.L.; Tor, J.Q.; Jiang, W.; Ye, E.; Loh, X.J.; Li, Z. Nanoarchitecture-Integrated Hydrogel Systems toward Therapeutic Applications. ACS nano 2023, 17, 7953–7978. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wu, M.; Wei, Q.; Xu, D.; He, X.; Wang, J.; Wu, J.; Chen, L. Conjugated Polymer Nanoparticles for Tumor Theranostics. Biomacromolecules 2023, 24, 1943–1979. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Centurion, F.; Misra, A.; Patel, S.; Gu, Z. Molecularly targeted nanomedicine enabled by inorganic nanoparticles for atherosclerosis diagnosis and treatment. Advanced drug delivery reviews 2023, 194, 114709. [Google Scholar] [CrossRef]
- Wong, X.Y.; Sena-Torralba, A.; Álvarez-Diduk, R.; Muthoosamy, K.; Merkoçi, A. Nanomaterials for Nanotheranostics: Tuning Their Properties According to Disease Needs. ACS nano 2020, 14, 2585–2627. [Google Scholar] [CrossRef]
- Liang, Y.; Sullivan, H.L.; Carrow, K.; Mesfin, J.M.; Korpanty, J.; Worthington, K.; Luo, C.; Christman, K.L.; Gianneschi, N.C. Inflammation-Responsive Micellar Nanoparticles from Degradable Polyphosphoramidates for Targeted Delivery to Myocardial Infarction. Journal of the American Chemical Society 2023, 145, 11185–11194. [Google Scholar] [CrossRef]
- Ghezzi, M.; Pescina, S.; Padula, C.; Santi, P.; Del Favero, E.; Cantù, L.; Nicoli, S. Polymeric micelles in drug delivery: An insight of the techniques for their characterization and assessment in biorelevant conditions. Journal of controlled release : official journal of the Controlled Release Society 2021, 332, 312–336. [Google Scholar] [CrossRef]
- Perumal, S.; Atchudan, R.; Lee, W. A Review of Polymeric Micelles and Their Applications. Polymers 2022, 14. [Google Scholar] [CrossRef]
- Hoang Thi, T.T.; Pilkington, E.H.; Nguyen, D.H.; Lee, J.S.; Park, K.D.; Truong, N.P. The Importance of Poly(ethylene glycol) Alternatives for Overcoming PEG Immunogenicity in Drug Delivery and Bioconjugation. Polymers 2020, 12. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, T.; Liu, Q.; He, J. PEG-Derivatized Dual-Functional Nanomicelles for Improved Cancer Therapy. Frontiers in pharmacology 2019, 10, 808. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Luo, T.; Sheng, R.; Li, H.; Sun, J.; Cao, A. Amphiphilic Diblock Terpolymer PMAgala-b-P(MAA-co-MAChol)s with Attached Galactose and Cholesterol Grafts and Their Intracellular pH-Responsive Doxorubicin Delivery. Biomacromolecules 2016, 17, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Huang, B.; Khatibi, N.; Rolland, W., 2nd; Suzuki, H.; Zhang, J.H.; Tang, J. PDGFR-α inhibition preserves blood-brain barrier after intracerebral hemorrhage. Annals of neurology 2011, 70, 920–931. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).