You are currently viewing a beta version of our website. If you spot anything unusual, kindly let us know.
Preprint
Article
INFLUENCE OF GRAPHENE OXIDE ON THE TEXTURE AND CHEMISTRY OF N, S-DOPED HIGHLY POROUS CARBON AND IMPLICATIONS FOR ELECTROCATALYTIC AND ENERGY STORAGE APPLICATION POTENTIAL
Altmetrics
Downloads
89
Views
17
Comments
0
A peer-reviewed article of this preprint also exists.
This version is not peer-reviewed
Abstract
High surface area carbon was obtained from -carrageenan – urea cryogels doped with 1.25 – 5 wt% graphene oxide (GO). Incorporation of GO resulted in multifaceted modification in the textural and chemical properties of the carbon cryogel. The thermal decomposition of GO facilitated the formation of micropores resulted in a substantial increase in the apparent surface area (up to 1780 m2/g). The annealing at 1000 °C removed part of the heteroatoms but left behind a high number of defects. While the volatile thermal degradation products of GO affected the porous texture and the surface chemistry in a complex way, its reduced residue resulted in a gradually increasing electrical conductivity. The influence of GO was studied in oxygen reduction reaction (ORR) and in Li-ion storage application. GO affected the electron transfer mechanism in the ORR tests and challenged the stability of the electrodes. Even if the electrode has been stabilized under oxygen-free conditions, later may undergo destabilization processes during the ORR. The 5 wt% GO doped sample was the most sensitive under oxidative conditions, but finally it exhibited the highest capacitance. In the Li-ion battery tests the coulombic efficiency of all the samples was consistently above 98 %, indicating the great potential of such carbons for efficient Li-ion insertion and reinsertion during the charge – discharge process, thereby providing a promising alternative for graphite-based anodes. The cell from the 1.25% sample showed an initial discharge capacity of 313 mAh/g, 95.1 % capacity retention and 99.3 % coulombic efficiency after 50 charge – discharge cycles.

Keywords:
Subject: Chemistry and Materials Science - Materials Science and Technology
1. Introduction
The fascinating microstructural and surface properties of biomass-based carbon materials justify the extensive interest in their application in various fuel cells [1,2,3], supercapacitors [4,5] and alkaline ion batteries [6,7,8]. Compared to terrestrial biomass the potential of marine-based sources, despite their abundance, is far less explored. Crustaceans rich in N-containing chitin, or edible red seaweed with reasonable sulfated polysaccharide content also may serve as precursors for N or S containing porous carbon materials [9,10,11].
Porous carbons with high surface area, rich porosity and tuned surface chemistry are essential to boost their electrochemical properties. The high surface area, which goes hand in hand with microporous texture and facilitates the exposure of active sites, might, however, limit the diffusion-controlled processes. In contrast, macroporous materials exhibit better transport kinetics, but their correspondingly lower surface area hampers the number of the exposed active sites, and therefore results in poorer surface area related performance. Well-tailored porous carbons with interconnected pore morphology make it possible to fabricate high-performance electrodes in fuel cells, as battery anodes [12] and high-rate electrochemical capacitive energy storage devices [13]. In fuel cells the ultra-micro and micropores are pseudo active sites for adsorption and cleavage of O2 molecules, while the meso- and macropores promote the oxygen reduction reaction (ORR) by facilitating the mass transfer [14,15,16]. Ultra-micropores of similar size to O2 molecules act as pseudo catalytic centers. They stimulate the O2 adsorption and consequently promote O = O bond splitting, fostering the ORR through the 4 e- reduction mechanism [17]. The enhanced interface between the electrolyte and the electrodes shortens the ion transport pathways, fosters electrolyte penetration, and facilitates mass transport and ion diffusion in Li-ion batteries as well as in supercapacitors [18,19,20,21]. Excessive widening of the pores decreases the coulombic efficiency and thus the reversible capacity of batteries; however, pores that are too narrow restrict the access of the bulky solvated Li-ions. Fine tuning of the carbon microstructure is therefore necessary for an effective balance between rate performance and storage capacity [22]. The bulky Li-ions solvated in a tetrahedral arrangement within the electrolyte [23] become intercalated and stored in the nanopores of the electrode in de-solvated form. The maximum Li-ion storage capacity can be achieved when the actual size of the Li-ion fits perfectly into the pore size. The benefits of carbon aerogels as anode materials in lithium-ion batteries can be manifested in three aspects: i) the carbon skeleton is a good electronic conductor that can ensure efficient electron transport; ii) the 3D interconnected and open porous framework facilitates the electrolyte penetration and provide fast Li ion diffusion channels; iii) the open interconnected pores offer enough space to accommodate the volume changes (expansion and contraction during repeated charge – discharge processes) of the electroactive materials, thus helping to maintain the structural and thereby electrochemical stability during cell cycling [24,25,26].
Besides the pore structure the other key factor is the surface chemistry of the carbons. The fundamentals of both texture and surface chemistry design and the corresponding challenges of nanostructured mesoporous carbon materials including carbon gels and their application were summarized by Enterría et al [27]. While the parameters of the sol-gel synthesis technique can be used to tune the adsorption and diffusion characteristics of carbon aerogels, non-metallic heteroatoms (e.g., B, N, O, P, S) generate additional active sites and defects, increase the activity and/or selectivity of the electrochemical processes, and construct more channels for electron and ion transfer [28,29,30].
Although the role of the various N species in the electrocatalytic oxygen reduction process is not fully elucidated, the graphitic and/or pyridinic nitrogen atoms seem to be the most efficient ones in the ORR [31,32,33,34]. Pyridinic nitrogens present in the carbon structure are Lewis bases, while graphitic or quaternary N may act as n-type dopants in amorphous carbon structures. The higher electronegativity of N (3.04 vs. 2.55 of C) facilitates the electron transfer and increases the charge density on the neighboring carbon atom, thus fostering the electrical conductivity, polarity, and surface wettability. In consequence, N doping enhances the high-rate capability in Li-ion battery anode applications and the pseudo-capacitance in supercapacitor applications [35,36,37,38].
The large covalent radius of the S atoms alters the electronic and metallic properties of the carbon matrix [39]. Sulfoxide, sulfones, and sulfonic acids located in the mesopores foster the access of the electrolyte delivering dissolved oxygen into the pore system [40]. The thiophenic sulfur compounds appear to be particularly active in enhancing the physisorption of the oxygen from electrolytes in small carbon pores [40]. The S atom incorporated in aromatic rings causes a slightly positive charge on the neighboring C atoms [40,41] and brings additional hydrophobicity to the surface, thus promoting the adsorption of molecular oxygen [34].
DFT calculations revealed that dual S and N doping results in a large number of active sites through the redistribution of spin and charge densities, thus synergistically improving the ORR performance [41]. Atomic level distribution of (hetero)atoms in the porous carbon matrix significantly improves the rate of the ORR, which is otherwise too slow for practical applications [42,43]. They also affect the performance of supercapacitors [44]. They reduce or may even prevent the need for Pt loading, which is an economic obstacle in commercializing fuel cells. N, P, and S atoms in biomass-based carbon electrodes act as extra active Li-ion storage sites [45,46]. The kinetics of the electrochemical reaction in heteroatom doped carbons are much faster, resulting in higher capacity and better rate performance. The C-S defect sites induce torsion in the graphitic layer structure and the extended interlayer spacing facilitates the insertion of lithium ions during the charge – discharge cycling of the cells [47].
Nanocarbons (carbon nanotubes, carbide and carbonitride graphene) themselves may form electrically conductive interconnected aerogel matrix through van der Waals interactions [48]. Graphene, due to its outstanding properties (high electrical and thermal conductivity, good mechanical strength) plays a particularly important role in the development of a new generation of batteries. Their surface area, mechanical flexibility, and broad electrochemical window [49] make graphene-based electrodes promising for fuel cells [50], batteries [51,52,53], supercapacitors [54,55,56,57], etc. Graphene oxide (GO) has been used as a platform [58] for the facile synthesis of N-doped graphene-like catalysts using e.g., urea [59], or polypyrrole [60] as doping agents. The thermal/annealing treatment during the synthesis converts the GO to reduced GO (rGO) and at least partly restore the desired sheet-like structure and physico-chemical properties of the electrocatalytically more attracting graphene.
Although thermal insulation and electric conductivity are uniquely combined in carbon aerogels, incorporation of carbon nanoparticles can considerably improve their electric properties. These particles can be also used as templating agents [61] and/or conductive additives [62]. Incorporation of 0.23 – 0.46 wt% well dispersed GO into the resorcinol – formaldehyde (RF) polymer xerogel precursor resulted in a carbon xerogel of high porosity and of excellent electrical conductivity: when used as electrode in aqueous supercapacitors at high current density, the capacitance and the power were enhanced by 25 and 100 %, respectively, compared of the undoped carbon xerogel [63]. It was also concluded that the electrical conductivity seems to play a more important role than the specific surface area in the electrode performance of mesoporous carbon xerogels. The optimum GO loading was below 2 wt% as higher loadings resulted in lower energy and power densities in spite of the increased electric conductivity [64]. In Li-ion batteries, graphene-doped carbon/carbon aerogel electrodes can accommodate lithium more easily than the common graphite anode [49]: in addition to the intercalation mechanism, they can exhibit fast lithium adsorption [23,65,66], defect trapping [67], build-up of faradaic capacitance [68], etc.
In spite of the intensive studies on the performance of electrodes fabricated from carbons doped with two heteroatoms or carbon nanoparticles, their simultaneous application is rare [69,70] and none of them addressed the influence of the nanoparticle concentration. Recently our group studied the oxygen reduction activity of a N, S co-doped metal free carbon synthesized from a ι-carreageenan – urea polymer cryogel precursor [71]. In this work we use the same matrix to synthesize GO-doped carbons with interconnected porous structure and high surface area to study the influence of the added GO on the morphology and chemistry of the carbon cryogel obtained. In RF based carbon xerogels developed for electric application the optimal GO content was found below 2 wt % [64]. Li et al [69] incorporated 4 wt% SWCNT, while the other group [70] added 13 wt% GO in the polymer precursor. Earlier we found that in responsive 3D poly(N-isopropylacrylamide) networks the percolation threshold was around 5 wt% related to the polymer matrix [72]. Based on these references the GO content was varied between 1.25 and 5 wt%. The texture of the carbon cryogels was characterized by electron microscopic imaging, N2 and CO2 adsorption measurements, Raman spectroscopy and powder X-ray diffraction (XRD). X-ray photoelectron spectroscopy (XPS), SEM supported electron dispersive spectroscopy (EDS), ultimate elemental analysis and FTIR were used to study the surface and bulk chemistry. The samples were probed as electrodes i) in a fuel cell under conditions, similar to alkaline anion exchange membrane fuel cells and ii) in Li-ion batteries in order to reveal the effect of the rGO in these applications.
2. Materials and methods
2.1. Synthesis
The rGO containing N and S double-doped carbon aerogels were synthesized according to the method of Li et al [11], using ι-carrageenan, urea (both from Sigma Aldrich) and aqueous GO suspension. The aqueous GO suspension (0.96 wt%) was prepared from natural graphite using an improved Hummers’ method [73,74]. Hydrogels were obtained by mixing 2 g urea and 2 g ι-carrageenan with 100 mL aqueous GO suspension (containing 50, 100 and 200 mg GO, respectively) at 80 °C. Thus, polymer gels with 1.25, 2.5, and 5 % GO were obtained. A GO-free gel was also prepared for comparison. The cooled hydrogels were freeze dried and pyrolyzed in a rotary quartz reactor at 700 °C (20 °C/min) in dry N2 flow (25 L/h) for 1 h. After washing with aq. 1.0 M HCl, the samples were annealed at 1000 ˚C in Ar flow for 1 h [71]. The resulting carbon aerogels are labeled as CA, CAGO50, CAGO100 and CAGO200.
2.2. Characterization methods
Scanning electron micrographs were taken by a Zeiss Sigma 300 FESEM field emission scanning electron microscope (Carl Zeiss QEC GmbH, Germany). Low temperature (-196.15 ˚C) nitrogen adsorption measurements were performed after 24 h degassing at 110 ˚C on a NOVA 2000e (Quantachrome, USA) automatic volumetric instrument. The apparent surface area SBET was determined using the Brunauer-Emmett-Teller (BET) model [75]. A pore volume V0.98 was estimated from the amount of vapor adsorbed at p/p0 = 0.98, assuming that the adsorbed gas fills the pores as liquid. The Dubinin-Radushkevich (DR) plot [76] was used to calculate the micropore volume Vmicro. The pore size distribution was computed by the Quenched Solid Density Functional Theory (QSDFT) for slit/cylindrical pore geometry [77]. Carbon dioxide adsorption was measured at 0 °C up to atmospheric pressure with an AUTOSORB-1 (Quantachrome, USA) computer-controlled analyzer. Evaluation of the primary adsorption data was performed with the Quantachrome ASiQwin software (version 3.0). Raman spectra were obtained using a LabRAM (Horiba Jobin Yvon) instrument. The laser source was a λ = 532 nm Nd-YAG (laser power at the focal point 15 mW). A 0.6 OD filter was used to reduce the power of the beam. Parameter optimization and data analysis were performed by a LabSpec 5 software. Powder X-ray diffractograms (XRD) were obtained in the range 2𝜃 = 10˚ - 130˚ with an X’Pert Pro MPD (PANalytical Bv., Almelo, The Netherlands) X-ray diffractometer using an X’celerator type detector and monochromatic Cu Kα radiation with a Ni filter foil (λ = 1.5406 Å).
The ultimate elemental analysis was performed in triplicates on an Elementar Vario Micro (CHNS) instrument. The lack of metal traces was confirmed with SEM/EDS (JEOL JSM 6380LA, Tokyo, Japan). X-ray photoelectron spectra were recorded on a Kratos XSAM 800 spectrometer operating in fixed analyzer transmission mode, using Mg Kα1,2 (1253.6 eV) excitation. The pressure of the analysis chamber was lower than 1⋅10-7 Pa. Survey spectra were recorded in the 150–1300 eV range in 0.5 eV steps. The photoelectron lines of C1s, O1s, N1s and S2p were measured in 0.1 eV steps with 1 s dwell time. The spectra were referenced to the energy of the C1s line of the sp2 type graphitic carbon, set at 284.3 ± 0.1 eV binding energy (BE). Peak decomposition was performed after Shirley-type background removal using Gaussian–Lorentzian peak shape with 70:30 ratio. Details of the applied fitting procedure are described elsewhere [78]. Quantitative analysis, based on integrated peak intensity, was performed by the XPS MultiQuant program [79], applying the conventional infinitely thick layer model using the experimentally determined photoionization cross-section data of Evans et al. [80] and the asymmetry parameters of Reilman et al. [81]. Attenuated total reflectance Fourier transform infrared spectroscopy (FTIR-ATR) were recorded on powdered carbons in the 4000 - 400 cm−1 wavelength range at a resolution of 4 cm-1 by 32 scans using a Tensor 27 (Bruker Optik GmbH, Leipzig, Germany) spectrophotometer equipped with a Platinum ATR unit A225. The crystal was made of diamond having refractive index of 2.4. For the background signal, the measured medium was air. Since absorption by the powders was very strong, a moderate polynomial baseline correction and smoothing were required.
2.3. Electrochemical performance tests
The electrical conductivity was estimated by a laboratory made instrument. The powdered samples were gradually compressed (0.5-5 MPa) in a rigid polytetrafluoroethylene (PTFE) tube (1 cm2 internal cross section) with two copper bolts (one on each side of the tube). The conductivity was estimated from the pressure dependence of the resistance [82]. The electrocatalytic ORR tests were performed using a glassy carbon (GC) rotating disc electrode (RDE, Pine Research Instrumentation, Durham, NC, USA). The ink for the working electrodes was prepared by dispersing 2 mg powdered annealed carbon (CA, CAGO50, CAGO100, or CAGO200) in a mixture of 1.6 mL MilliQ water, 0.4 mL isopropyl alcohol and 8 µL 5% Nafion® solution. After 30 min sonication, the ink was pipetted on to the dry mirror-polished GC and dried at room temperature. The loading varied between 50 and 400 μg/cm2. Measurements were implemented in 0.1 M KOH electrolyte using three-electrode systems with a hydrogen electrode as a reference and Pt wire as a counter electrode in a three-compartment PTFE cell [71]. All potentials are given vs. the reversible hydrogen electrode (RHE).
The working electrodes for the Li-ion storage studies were prepared by mixing the carbon samples and polyvinylidene fluoride binder (PVDF 99.9 %, Solvay) in a weight ratio of 95:5 in a ball mill for 1 hour. After adding an adequate amount of N-methyl-2-pyrrolidone (NMP) the slurry was cast on to a copper foil using an automatic film applicator (BYK Gardner GmbH, Germany). 14 mm diameter circular discs were cut from the dried film (70 °C in a vacuum oven, overnight). CR2032 coin-type cells were assembled in an argon-filled glove box using the as-prepared coated disc and Li metal disc as working and counter electrode, respectively, Whatman glass fiber as the separator, and 1.0 M lithium hexafluorophosphate (LiPF6) in ethylene carbonate and diethyl carbonate (EC: DEC 1:1 vol %) as electrolyte and employing a manual crimping machine (MTI MSK 110). The electrochemical performance of the half-cell was performed using an electrochemical workstation (Biologic VMP 300) at room temperature. The galvanostatic charge – discharge cycling of the half-cell was done in a potential window of 0-3 V (vs. Li+/Li).
3. Results and Discussion
3.1. Development of the morphology during the annealing
The FESEM images demonstrate the complexity of the pore structure of the annealed carbon gels (Figure 1). The early inclusion of GO already in the precursor suspension influences the morphology developing during gelation and cryogenic drying and is reflected also in the final annealed state (Figure 1c). It is apparent that the reduced GO formed during the heat treatments is evenly distributed in the carbon matrix. The typical nanopores within the CA matrix are of 2-3 nm (Figure S1). The yield of the synthesis steps is reported in Table S1.
The low temperature (-196 ˚C) N2 adsorption isotherms of the annealed carbon samples having various GO content are shown in Figure 2a. All the isotherms are of composite Type IV + Type II with an H4 hysteresis loop displaying a sharp step at p/p0 = 0.45 [83]. The shape and type of the hysteresis loops suggest an interconnected pore network of micro-, meso- and macropores. Numerical data deduced from the measured isotherms are presented in Table 1. Since the macropores are not totally filled with condensed nitrogen, the liquid equivalent volume V0.98 was determined at p/p0 = 0.98.
The effect of the GO is most spectacular in the micropore region of the isotherm. Incorporation of GO increases not only the carbon content of the polymer matrix, but also the volatile products that are released during the thermal treatments. The 1.25 - 2.5 % GO (samples CAGO50 and CAGO100) increased the pore volumes and the apparent surface area almost proportionally, while addition of 5 % GO (CAGO200) resulted in a drop both in surface area and pore volume. The ultra-micropore region was revealed by CO2 adsorption measurements performed at 0 °C. The combined pore size distribution curves in Figure 2b indicate the presence of ultra-micropores as well as significant micro- and narrow mesoporosity in the range 1.7 – 6 nm. The increasing amount of oxidative volatile gases evolving during the thermal decomposition of the GO may be responsible for the decreasing trend in ultra-microporosity.
The Raman spectra in Figure S2 exhibit the iconic D (~1350 cm-1; defects, edges, and disordered carbon sites) and G (~1580 cm-1; vibration of sp2-hybridized graphitic carbon) band regions typical of carbon materials [84]. The first and second order regions of the spectra were deconvoluted for further analysis (Figure S2b, Tables S2 and S3 [85,86,87,88]). The D band (around 1350 cm-1) indicates the aromatic clusters with edges/borders of mainly amorphous carbon (sp3 -bonded) structures [89]. The blue shift of the G band may be caused by an internal stress developing when the incorporated GO and/or newly developing clusters collide during the annealing treatment at 1000 °C [89,90]. The D' band, associated with disordered graphenic lattices (around 1620 cm-1) appeared only in the GO-free CA sample. On the other hand, the second order region was detected only in the GO doped samples. The trend in the ID/IG ratio along with the relative sharpening of the G band and the reduced intensity D” in the GO doped samples confirms an ordering effect of the added GO in the carbon matrix [86].
3.2. Surface chemistry of the samples
The effect of GO on the surface composition was studied by XPS (Table 2). The successful removal of the metal impurities was confirmed by SEM supported ED spectroscopy (Figure S3). As expected, the O content of the incorporated GO affects not only the porous texture but also the surface chemistry of the annealed samples. The complexity of the thermal treatments explains the change in the overall C or O content and the trend of the heteroatom/carbon ratios.
The composite photoelectron lines (C1s, O1s, N1s and S2p) were decomposed into different chemical states. The self-oxidation alters the surface composition as well as the chemical environment. Due to the complicated structure of the samples, however, the chemical states cannot be resolved exactly. The suggested states refer to a broad range of chemical bonds. The shape of the C1 (sp2 C) component is asymmetric, as in pure and low heteroatom-containing graphite and graphene. The tail of the asymmetric peak overlaps with the peaks of the C–O(N) components; using this line-shape also gives satisfactory oxygen + nitrogen balance (the ratio of the measured and calculated concentrations) for all samples.
Figure 3 presents a typical decomposition scheme of the C1s, O1s, N1s and S2p spectral regions, while Table 3 and Table 4 show the quantitative results of the decomposition, together with the binding energy ranges of the components and their assignations to various chemical states.
The decomposition of the C1s signal revealed three different carbon species. The most abundant is the sp2 form (C1). The presence of the π-π* shake-up satellite also confirms the assignation of this component. The concentration of C1 is slightly higher in the GO-doped samples. The other components can be assigned to carbon atoms connected to one (C2) or two (C3) heteroatoms.
The O1 and O2 oxygen components are found in all samples; they can be assigned to S–O and to various C–O bonds, respectively. The O3 component, present in the high GO-containing samples, can be connected to either highly oxidized carbon (anhydride, carbonate). Owing to their low concentration, the corresponding carbon species cannot be resolved in the spectra. Nitrogen is also present in three chemical states. N1 is the simple C–N bond, while N2 is bonded to carbon with further heteroatoms. The quantities of these nitrogens are commensurable. The low concentration, high binding energy N3 component is a kind of quaternary ammonium. Due to the synthesis procedure, it is assumed that the nitrogen atoms are homogeneously distributed in the matrix and also decorate the walls of the micropores and ultra-micropores [91]. The sulfur content, present as C–S sulfide (S1) and in oxidized forms (S2; sulfone, sulfate), is the least affected by the treatments, but it should be noted that all the sulfur in the CAGO50 sample is sulfidic, while in the others the contribution of the S2 forms reaches 20-30 %.
The FTIR measurements give complementary information about the chemical structures of the samples with diverse GO content (Figure S4, Table 4). From the rich spectra three main regions were considered to monitor the chemical changes in the bulk [92,93]. All spectra display a strong adsorption band at 1554 cm-1, assigned to the –C=C– skeletal vibration of aromatic regions. The 1749 cm-1 band of the GO-loaded samples implies the presence of carbonyl groups (–C=O), stemming from aldehydes, ketones, esters and carboxylic acids. The band at 1372 cm-1, which is absent in the starting material, is ascribed to the –OH in plane bending of phenols. If we compare the amount of the latter two signals to the corresponding carbon skeleton peak at 1554 cm-1, we can establish that the ratio of oxygen-containing groups increases with increasing GO content.
In summary, addition of GO to the carrageenan – urea carbon xerogel results in multifaceted modification of the textural and chemical properties of the samples. The thermal decomposition of GO enhances self-activation and facilitates the formation of micropores which, for the CAGO50 and CAGO100 samples, also result in a considerable increase in the apparent surface area. With 5 wt% GO probably too much oxygen was introduced to the matrix which destroyed the finer porosity. The annealing at 1000 °C certainly removed part of the heteroatoms but left behind a high number of defects. It is expected that these new active sites (N-S-C) “reconstructed” from edged thiophene S, graphitic N, and pentagon defects and the 3D porous architecture result in an outstanding electrocatalytic activity [11]. There is a significant difference between the bulk and surface compositions: the population of the heteroatoms is much richer in the bulk. They are present in the expected chemical forms in the surface of all the samples.
3.3. Effect of GO on ORR performance
Likewise other groups we also found that incorporation of GO improved the electric conductivity of the cryogels [64,94]. In our samples the improvement was proportional to the amount of GO added, in spite of the lower apparent surface area of CAGO200 (Figure S5).
The ORR performance of the GO doped samples was investigated under conditions similar to alkaline anion exchange membrane fuel cells. The linear sweep voltammograms (LSVs) of the four samples are plotted in Figure 4. The high potential regions reveal not only the slight differences among the various samples but also their alteration during the consecutive cycles. CAGO200 was found to be particularly unstable. The addition of GO gradually shifted the onset potential. CAGO50 exhibited the highest half-wave potential, which gradually decreased back to the value observed with the GO-free CA carbon. In Table 5 we compare the onset and half-wave potentials (E1/2) of these curves with the results of other groups.
The Koutecky-Levich (KL) equation [103] was used to estimate the correlation between the current density j and the rotation rate ω
where jk is the kinetic current density, jlim is the limiting diffusion current density, n is the number of electrons transferred in ORR per oxygen molecule, F is the Faraday constant, D is the diffusion coefficient of oxygen in the electrolyte, ν is the kinematic viscosity of the electrolyte, C is the concentration of oxygen in the electrolyte. KL plots of CAGO50 (Figure 5) display a slope closest to the theoretical curve corresponding to the 4 e- mechanism, i.e., incorporation of 1.25% GO improved the performance, whereas further added GO has the opposite impact. At higher GO loadings the lines become steeper, nearer to the theoretical 2 e- slope. Based on these findings from our samples CAGO50 seems to have the optimum pore texture and chemistry for ORR under the investigated conditions. Although the electrical conductivity increased linearly with increasing GO loading, no similar change in ORR by adding GO is apparent. This agrees well with the work by Ramos-Fernández et al. who also observed that increasing GO content improved the electric conductivity of carbon xerogels. They also observed that enhanced porosity may spoil the electrochemical performance by disrupting the GO network throughout the carbon matrix [64].
The (in)stability of the electrodes was further explored with CV responses measured after a series of treatments simulating application conditions. Based on the KL evaluation the GO-free CA and the CAGO50 and CAGO200, showing the lowest and highest slopes were selected for stability studies. The fresh electrodes made from the carbons, after running a CV cycle (curve 1) were exposed to further 50 cycles in argon treated oxygen-free KOH electrolyte and then another 50 cycles in oxygen-saturated KOH, finally an ORR test was run (sweep rate: 5 mV/s, rotation rates: 400, 625, 900, 1250 rpm). The voltammograms, curves 2, 3 and 4, obtained after each set of treatment are compared in Figure 6. (The effect of the electrode loading is discussed in the Supplement.) The slightly distorted rectangular shapes may stem from the pseudo-faradaic reactions of the corresponding functional groups [104]. The width of the hysteresis is not correlated with the BET surface area (Table 1), but the presence of the GO apparently reduces the gravimetric capacitance. The coincidence of curves 1 and 2 in CA carbon (Figure 6) reveal that this sample is stable under oxygen-free conditions. After an additional 50 cycles in oxygen-saturated electrolyte (curve 3) the widening of the CV curve implies that the electrocatalytically active surface area increases in the presence of oxygen. The consecutive four ORR cycles further modify the electrode surface (curve 4). The performance of the CAGO50 carbon is very similar, however, it seems somehow even less stable under oxygenic conditions. The CAGO200 electrode was instable already in oxygen-free conditions (curves 1 and 2). The change in the surface was even more expressed after being cycled in oxygen-saturated electrolyte, which apparently stabilized the surface: no further changes were seen after these ORR cycles (curve 4). These observations indicate that the electron transfer mechanism deduced from the KL plot can only be considered as an estimation. The kinetics of the reduction reaction in the presence of the heteroatoms is indeed more sophisticated, and further influenced by the rGO. The chemical species locally evolving in the ORR may initiate various processes including restructuring, activation, degradation, etc. of the electrode surface. The plots in Figure 6 clearly show that even if the electrode has been stabilized under oxygen-free conditions, later may undergo destabilization processes during the ORR. Nevertheless, the widening of the CV curves mark an increase in the electrocatalytically active surface area. The deviation of the corresponding curves confirms that addition of GO to the precursor affect the stability of the electrodes. As expected from the KL plot, the CAGO200 sample is more sensitive to the oxidative conditions, but after stabilization this sample exhibited the highest capacitance. The trend of the gravimetric capacitance in this figure is different from the trend expected from the apparent surface area (1070, 1479 and 933 m2/g for CA, CAGO50 and CAGO200, respectively) implying that the accessibility of the nitrogen molecules is different from that of the species involved in the electrocatalytic processes [105].
3.4. Effect of GO on Li-ion battery application
In order to reveal the eff+ect of GO on the performance of the carbon xerogel as anode material in Li-ion batteries galvanostatic charge – discharge cycling tests were performed. In the half-cell setup the CA, CAGO50, CAGO100, or CAGO200 sample was the working electrode and the Li metal disc served as the counter and reference electrode, i.e., no further conductive agent was used. In the galvanostatic test a constant current I is applied to the cell and the potential is evaluated as a function of time t. The specific capacity Cs during complete charge – discharge is given by
Cs = I⋅t / m
The nature of the charge – discharge plots largely depends on the surface chemistry (microstructure, porosity, heteroatom content, etc.) and the adsorption capacity of the carbon host. Figure 7 shows the cell voltage vs. specific capacity charge – discharge cycling plots of the samples under investigation. The cyclability of the materials is presented here as the total charge – discharge capacity (or capacity retention) as a function of the number of cycles (Figure 8).
Figure 7 shows the cell voltage vs. specific capacity charge – discharge cycling plots of the samples under investigation. While highly ordered graphitized carbon anodes show a steep charge – discharge curve [106], sloping shapes similar to Figure 8 are distinctive for amorphous biomass-derived carbon anodes in Li-ion battery applications [25,107,108]. The broad peaks in the XRD patterns (Figure S7) reveal that the carbon cryogels studied in this work are typically amorphous, therefore charge – discharge curves with this shape expected, i.e., the CA, CAGO50, CAGO100, and CAGO200 have the potential to store the Li-ions gradually and consistently over their operating potential. A comparable charge – discharge plateau was measured also on RF polymer based carbon xerogels [109]. The latter microporous carbon xerogels were successfully tested as anode material in lithium-ion cells exhibiting a specific capacity 288 mAh/g and retaining 97% of the initial capacity after 100 charge–discharge cycles.
The cyclability of the carbons is presented here as the total charge – discharge capacity (or capacity retention) as a function of the number of cycles (Figure 9). The undoped carbon, CA shows an initial discharge capacity 332 mAh/g at a constant current density 100 mA/g. The cell shows good capacity retention and high coulombic efficiency (92.9% and 99.7%, respectively) even after 50 charge – discharge cycles.
For comparison, 218 mAh/g at a current density 0.372 A/g was reported by Wang et al. [6]. Among the GO doped carbons CAGO50 exhibits the best performance in terms of cycling stability. The cell shows an initial discharge capacity of 313 mAh/g and a highly reversible capacity of 297 mAh/g after 50 cycles, corresponding to 95.1 % capacity retention. This good stability agrees well with the impedance analysis results: this carbon has the lowest impedance. The enhanced performance of CAGO50 compared to pristine CA can be explained in terms of the surface area and porosity [110]. The higher surface area of CAGO50 improves the electrolyte accessibility and ion diffusion in the pores of the electrode, which in turn results in higher coulombic efficiency and cell stability [22,110]. The charge – discharge plateau recorded in the 1st and 50th cycle is almost identical confirming the resilient stability of the electrode [111]. Following this, CAGO200 showed an initial discharge capacity of 240 mAh/g and a capacity retention of 93.6% after 50 cycles (Figure 8).
The overall electrochemical performance of CAGO200 in the Li-ion battery tests was better than undoped CA and slightly lower than CAGO50. CAGO100 presented the poorest electrochemical performance in spite of having the largest surface area. This apparent surface area, however, is also combined with a larger pore volume in the wider pore size region, which seems to decrease the initial coulombic efficiency and reduces the reversible capacity of the battery [22]. The initial discharge capacity of CAGO100 is 255 mAh/g, but the cell poorly maintained its stability. The low retention capacity (67.2% after 50 cycles) might be due to a kinetic limitation in the porous electrode [110]. The compatibility of the pore and the Li+ ion sizes is also responsible for the charge capacity. CAGO100 and CAGO200 showed the highest and lowest pore volume, respectively, both in the micro- and mesopore regions. On the one hand wider pores may not be completely filled by the Li+ ions, and on the other, low porosity limits the entrance of Li+ containing electrolyte into the pores. CAGO50, which seems to have the optimum pore size compatible with Li+ and is capable to effectively accommodate the Li+ ions, exhibits the highest overall capacity among the GO doped samples.
As shown in Figure 8, the coulombic efficiency of all the samples was consistently maintained above 98.5 % indicating the efficient Li-ion insertion and reinsertion potential of these carbons during the charge – discharge process [111]. CA and CAGO100 displayed lower coulombic efficiency than CAGO50 and CAGO200 (>99.3 %) indicating that addition of GO is clearly beneficial for more stable electrochemical performance in Li ion batteries, but the GO doping level must be carefully tuned.
It may be concluded that the carbons investigated here can be utilized as potential anodes in Li-ion batteries. According to our results the pore structure and the chemical composition corresponding to CAGO50 proved to be the best to achieve an acceptably high reversible discharge capacity and electrode stability under prolonged cycling. The electrochemical performance of these carbon electrodes proved to be comparable to the results previously reported on carbon electrode anode in Li-ion batteries [112,113,114,115,116].
Conclusions
Addition of GO successfully increases the surface area and the porosity of the carbon material up to 2.5% (up to SBET 1780 m2/g and V0.98 1.7 cm3/g), but 5% is destroyed the finer pores. The self-activating effect of the GO influences the surface chemistry in a complex way, but sufficient O, N, and S heteroatoms were preserved in various chemical forms. The highest concentration of sp2 carbons was detected on the surface of sample CAGO50 (1.25 wt% GO). Although the volatile thermal degradation products of GO affected the porous texture and the surface chemistry in a subtle way, the residual rGO results in monotonically increasing electrical conductivity. GO affected the electron transfer mechanism and challenged the stability of the electrodes in ORR tests. TheCAGO50 sample displayed the advantageous 4e- mechanism. The CAGO200 (5 wt% GO) sample was the most sensitive under oxidative conditions, but after conditioning it exhibited the highest capacitance in ORR. In the Li-ion battery tests the coulombic efficiency of all the samples was consistently above 98%. The cell made from CAGO50 showed the lowest impedance, an initial discharge capacity of 313 mAh/g, 95.1 % capacity retention and 99.3 % coulombic efficiency after 50 charge – discharge cycles. Despite having the largest surface area sample CAGO100 performed more poorly than CAGO50 in both application tests. This is a clear indication that concerted fine-tuning of the pore morphology and surface chemistry is required for both the advanced electrocatalytic and electrochemical performance. The as-prepared GO-doped carbon samples with their ultra-micropores and high surface area performed well and can be a promising electrode material for both applications.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Acknowledgments
We extend our warm thanks to A. Farkas and J. Madarász for their invaluable assistance in Raman spectroscopy and XRD, as well as to G. Bosznai for his technical assistance. We are thankful to B. Pinke and A. Bulátkó for their help with SEM-EDS measurements. Financial support from the Hungarian Scientific Research Funds OTKA K128410 and K 143571 is acknowledged. The research is part of project no. BME-NVA-02, implemented with the support of the Ministry of Innovation and Technology of Hungary from the National Research, Development and Innovation Fund, and financed under the TKP2021 funding scheme. SKSA is grateful to the Stipendium Hungaricum scholarship program of the Hungarian Government. SSL and RK thank the financial support of project no. RRF-2.3.1-21-2022-00009, titled National Laboratory for Renewable Energy, implemented with support from the Recovery and Resilience Facility of the European Union within the framework of Programme Széchenyi Plan Plus.
References
- P. Chen, L.K. Wang, G. Wang, M.R. Gao, J. Ge, W.J. Yuan, Y.H. Shen, A.J. Xie, S.H. Yu. Nitrogen-doped nanoporous carbon nanosheets derived from plant biomass: an efficient catalyst for oxygen reduction reaction. Energy Environ. Sci., 2014, 7 (12), 4095–4103. [CrossRef]
- J. Zhao, Y. Liu, X. Quan, S. Chen, H. Yu, H. Zhao. Nitrogen-doped carbon with high degree of graphitization derived from biomass as high-performance electrocatalyst for oxygen reduction reaction. Appl. Surf. Sci. 2017, 396, 986–993. [CrossRef]
- Q. Zhao, Q. Ma, F. Pan, Z. Wang, B. Yang, J. Zhang, J. Zhang. Facile synthesis of nitrogen-doped carbon nanosheets as metal-free catalysts with excellent oxygen reduction performance in alkaline and acidic media. J Solid State Electrochem., 2016, 20, 1469-1479. [CrossRef]
- P. Cheng, T. Li, H. Yu, L. Zhi, Z. Liu, Z. Lei. Biomass-derived carbon fiber aerogel as a binder-free electrode for high-rate supercapacitors. J. Mater. Chem. C, 2016, 120 (4), 2079–2086. [CrossRef]
- ZT You, L Zhao, KH Zhao, HX Liao, SJ Wen, YH Xiao, BC Cheng, SJ Lei. Highly tunable three-dimensional porous carbon produced from tea seed meal crop by-products for high performance supercapacitors. Appl. Surf. Sci. 2023, 607, 155080.
- L. Wang, Z. Schnepp, M.M. Titirici. Rice husk-derived carbon anodes for lithium-ion batteries. J. Mater. Chem. A, 2013, 1 (17), 5269-5273. [CrossRef]
- T. Liu, X. Li. Biomass-derived nanostructured porous carbons for sodium ion batteries: a review. Mater. Technol., 2018, 34 (4), 232–245. [CrossRef]
- H. Li, Z. Cheng, Q. Zhang, A. Natan, Y. Yang, D. Cao, H. Zhu. Bacterial-derived, compressible, and hierarchical porous carbon for high-performance potassium-ion batteries. Nano Lett., 2018, 18 (11), 7407–7413. [CrossRef]
- Z. Sebestyén, E. Jakab, A. Domán, P. Bokrossy, I. Bertóti, J. Madarász, K. László. Thermal degradation of crab shell biomass, a nitrogen-containing carbon precursor. J. Therm. Anal. Calorim., 2020, 142, 301-308. [CrossRef]
- K.M. Zia, S. Tabasum, M. Nasif, N. Sultan, N. Aslam, A. Noreen, M. Zuber. A review on synthesis, properties and applications of natural polymer based carrageenan blends and composites. Int. J. Biol. Macromol., 2017, 96, 282-301. [CrossRef]
- D. Li, Y. Jia, G. Chang, J. Chen, H. Liu, J. Wang, Y. Hu, Y. Xia, D. Yang, X. Yao. A defect-driven metal-free electrocatalyst for oxygen reduction in acidic electrolyte. Chem, 2018, 4, 2345–2356. [CrossRef]
- J. Hou, C. Cao, F. Idrees, X. Ma. Hierarchical porous nitrogen-doped carbon nanosheets derived from silk for ultrahigh-capacity battery anodes and supercapacitors. ACS Nano, 2015, 9 (3), 2556–2564. [CrossRef]
- D.W. Wang, F. Li, M. Liu, G.Q. Lu, H.M. Cheng. 3D aperiodic hierarchical porous graphitic carbon material for high-rate electrochemical capacitive energy storage. Angew. Chem. Int. Ed., 2007, 47 (2), 373–376. [CrossRef]
- Gabe, R. Ruiz-Rosas, C. González-Gaitán, E. Morallón, D. Cazorla-Amorós. Modeling of oxygen reduction reaction in porous carbon materials in alkaline medium. Effect of microporosity. J. Power Sources, 2019, 412, 451-464. [CrossRef]
- Y. Liu, K. Li, B. Ge, L. Pu, Z. Liu. Influence of micropore and mesoporous in activated carbon air-cathode catalysts on oxygen reduction reaction in microbial fuel cells. Electrochim. Acta, 2016, 214, 110-118. [CrossRef]
- J. Cao, C. Zhu, Y. Aoki, H. Habazaki. Starch-derived hierarchical porous carbon with controlled porosity for high performance supercapacitors. ACS Sustainable Chem. Eng., 2018, 6 (6), 7292-7303. [CrossRef]
- T.J. Bandosz. Revealing the impact of small pores in oxygen reduction on carbon electrocatalysts: A journey through recent findings. Carbon, 2022, 188, 289-304. [CrossRef]
- M. Huang, S.J. Yoo, J.S. Lee, T.H. Yoon. Electrochemical properties of an activated carbon xerogel monolith from resorcinol-formaldehyde for supercapacitor electrode applications. RSC Adv., 2021, 11, 33192-33201. [CrossRef]
- X. Feng, Y. Bai, M. Liu, Y. Li, H. Yang, X. Wang, C. Wu. Untangling the respective effects of heteroatom-doped carbon materials in batteries, supercapacitors and the ORR to design high performance materials. Energy Environ. Sci., 2021, 14, 2036-2089. [CrossRef]
- Y. Zhai, Y. Dou, D. Zhao, P.F. Fulvio, R.T. Mayes, S. Dai. Carbon materials for chemical capacitive energy storage. Adv. Mater., 2011, 23, (42), 4828-4850. [CrossRef]
- W. Tang, Y. Zhang, Y. Zhong, T. Shen, X. Wang, X. Xia, J. Tu. Natural biomass-derived carbons for electrochemical energy storage. Mater. Res. Bull., 2017, 88, 234–241. [CrossRef]
- Z. Zhu, Z. Xu. The rational design of biomass-derived carbon materials towards next-generation energy storage: A review. Renewable and Sustainable Energy Reviews, 2020, 134, 110308. [CrossRef]
- J.R. Dahn, T. Zheng, Y.H. Liu, J.S. Xue. Mechanisms for lithium insertion in carbonaceous materials. Science, 1995, 270, 590−593. [CrossRef]
- S. Sun, Q. Yan, M. Wu, X. Zhao. Carbon aerogel based materials for secondary batteries. Sustain. Mater. Technol., 2021, 30, e00342. [CrossRef]
- D. Li, Y. Wang, Y. Sun, Y. Lu, S. Chen, B. Wang, H. Zhang, Y. Xia, D. Yang. Turning gelidium amansii residue into nitrogen-doped carbon nanofiber aerogel for enhanced multiple energy storage. Carbon, 2018, 137, 31–40. [CrossRef]
- Q.Q. Xiong, Z.G. Ji. Controllable growth of MoS2/C flower-like microspheres with enhanced electrochemical performance for lithium ion batteries. J Alloys Compd., 2016, 673, 215–219. [CrossRef]
- M. Enterría, J.L. Figueiredo. Nanostructured mesoporous carbons: Tuning texture and surface chemistry. Carbon, 2016, 108, 79-102. [CrossRef]
- J. Zhang, J. Zhang, F. He, Y. Chen, J. Zhu, D. Wang, M. Shichun, H.Y. Yang. Defect and doping co-engineered non-metal nanocarbon ORR electrocatalyst. Nano-Micro Lett., 2021, 13, 65. [CrossRef]
- X Wang, LW Yang, R Li, YX Chen, ZG Wu, BH Zhong, XD Guo. Heteroatom-doped Ginkgo Folium porous carbon modified separator for high-capacity and long-cycle lithium-sulfur batteries. Appl. Surf. Sci. 2022, 602, 154342.
- B Yan, L. Feng, JJ Zheng, Q. Zhang, YZ Dong, YC Ding, WS Yang, JQ Han, SH Jiang, SJ He. Nitrogen-doped carbon layer on cellulose derived free-standing carbon paper for high-rate supercapacitors. Appl. Surf. Sci. 2023, 608, 155144.
- Z. Duan, G. Henkelman. Identification of active sites of pure and nitrogen-doped carbon materials for oxygen reduction reaction using constant-potential calculations. J. Phys. Chem. C., 2020, 124 (22), 12016-12023. [CrossRef]
- B. Nagy, S. Villar-Rodil, J.M.D. Tascón, I. Bakos, K. László. Nitrogen doped mesoporous carbon aerogels and implications for electrocatalytic oxygen reduction reactions. Microporous and Mesoporous Mater., 2016, 230, 135-144. [CrossRef]
- D. Guo, R. Shibuya, C. Akiba, S. Saji, T. Kondo, J. Nakamura. Active sites of nitrogen-doped carbon materials for oxygen reduction reaction clarified using model catalysts. Science, 2016, 351 (6271), 361–365. [CrossRef]
- Dewang Kong, Wenjing Yuan, Cun Li, Jiming Song, Anjian Xie, Yuhua Shen: Synergistic effect of nitrogen-doped hierarchical porous carbon/graphene with enhanced catalytic performance for oxygen reduction reaction. Appl. Surf. Sci., 2017, 393, 144-150. https://doi.org/10.1016/j.apsusc.2016.10.019.
- K. Lee, L. Shabnam, S.N. Faisal, V.C. Hoang, V.G. Gomes. Aerogel from fruit biowaste produces ultracapacitors with high energy density and stability. J. Energy Storage, 2020, 27, 101152. [CrossRef]
- Gopalakrishnan, S. Badhulika. Effect of self-doped heteroatoms on the performance of biomass-derived carbon for supercapacitor applications. J. Power Sources, 2020, 480, 228830. [CrossRef]
- Donglin He , Wang Zhao, Ping Li, Zhiwei Liu, Haoyang Wu, Luan Liu, Kun Han, Lang Liu, Qi Wan, Faheem K. Butt, Xuanhui Qu: Bifunctional biomass-derived 3D nitrogen-doped porous carbon for oxygen reduction reaction and solid-state supercapacitor. Appl. Surf. Sci., 2019, 465, 303-312.
- HQ Gao, D Zhang, HT Zhou, JC Wu, GJ Xu, ZL Huang, MH Liu, JH Yang, D Chen. Boosting gravimetric and volumetric energy density of supercapacitors by 3D pomegranate-like porous carbon structure design. Appl. Surf. Sci. 2020, 534, 147613.
- S. Ito, T. Murata, M. Hasegawa, Y. Bito, Y. Toyoguchi. Study on CxN and CxS with disordered carbon structure as the anode materials for secondary lithium batteries. J. Power Sources, 1997, 68 (2), 245-248. [CrossRef]
- M. Seredych, K. László, E. Rodríguez-Castellón, T.J. Bandosz. S-doped carbon aerogels/GO composites as oxygen reduction catalysts. J. Energy Chem., 2016, 25 (2), 236-245. [CrossRef]
- J. Liang, Y. Jiao, M. Jaroniec, S.Z. Qiao. Sulfur and nitrogen dual-doped mesoporous graphene electrocatalyst for oxygen reduction with synergistically enhanced performance. Angew. Chem. Int. Ed., 2012, 51 (46), 11496-11500. [CrossRef]
- M. Jiang, X. Yu, H. Yang, S. Chen. Optimization strategies of preparation of biomass-derived carbon electrocatalyst for boosting oxygen reduction reaction: A minireview. Catalysts, 2020, 10, 1472. [CrossRef]
- Y. Zhao, Y. Liu, Y. Chen, X. Liu, X. Li, S. Gao. A treasure map for nonmetallic catalysts: optimal nitrogen and fluorine distribution of biomass-derived carbon materials for high-performance oxygen reduction catalysts. J. Mater. Chem. A, 2021, 9, 18251. [CrossRef]
- Shenghui Jiao, Yutong Yao, Junliu Zhang, Liqiong Zhang, Changwei Li, Huixin Zhang, Xin Zhao, Honglei Chen, Jianchun Jiang: Nano-flower-like porous carbon derived from soybean straw for efficient N-S co-doped supercapacitors by coupling in-situ heteroatom doping with green activation method. Appl. Surf. Sci. 2023, 615, 156365.
- H. Jin, X. Wang, Z. Gu, J. Polin. Carbon materials from high ash biochar for supercapacitor and improvement of capacitance with HNO3 surface oxidation. J. Power Sources, 2013, 236, 285–292. [CrossRef]
- F. Chen, J. Yang, T. Bai, B. Long, X. Zhou. Biomass waste-derived honeycomb-like nitrogen and oxygen dual-doped porous carbon for high performance lithium-sulfur batteries. Electrochim. Acta, 2016, 192, 99–109. [CrossRef]
- Y. Qiu, J. Huo, F. Jia, B.H. Shanks, W. Li. N- and S-doped mesoporous carbon as metal-free cathode catalysts for direct bio renewable alcohol fuel cells. Mater. Chem. A, 2016, 4, 83–95. [CrossRef]
- J-H. Lee, S-J. Park. Recent advances in preparations and applications of carbon aerogels: A review. Carbon, 2020, 163, 1-18. [CrossRef]
- M. Bakierska, M. Lis, J. Pacek, M. Świętosławski, M. Gajewska, A. Tąta, E. Proniewicz, M. Molenda. Bio-derived carbon nanostructures for high-performance lithium-ion batteries. Carbon, 2019, 145, 426–432. [CrossRef]
- B. Jaleh, M. Nasrollahzadeh, M. Eslamipanah, A. Nasri, E. Shabanlou, N.R. Manwar, R. Zboril, P. Fornasiero, M.B. Gawande. The Role of Carbon-Based Materials for Fuel Cells Performance. Carbon, 2022, 198, 301–352. [CrossRef]
- H. Song, R. Na, C. Hong, G. Zhang, X. Li, Y. Kang, Q. Zhang, H. Xie. In situ measurement and mechanism analysis of the lithium storage behavior of graphene electrodes. Carbon, 2022, 188, 146–154. [CrossRef]
- L. Li, D. Zhang, J. Deng, Y. Gou, J. Fang, H. Cui, Y. Zhao, M. Cao. Carbon-based materials for fast charging lithium-ion batteries. Carbon, 2021, 183, 721–734. [CrossRef]
- S. Wu, H. Wu, M. Zou, X. Shi, Y. Yuan, W. Bai, A. Cao. Short-range ordered graphitized-carbon nanotubes with large cavity as high-performance lithium-ion battery anodes. Carbon, 2020, 158, 642–650. [CrossRef]
- J.L. Gómez-Urbano, G. Moreno-Fernández, M. Arnaiz, J. Ajuria, T. Rojo, D. Carriazo. Graphene-coffee waste derived carbon composites as electrodes for optimized lithium ion capacitors. Carbon, 2020, 162, 273–282. [CrossRef]
- T. Zhang, F. Zhang, L. Zhang, Y. Lu, Y. Zhang, X. Yang, Y. Ma, Y. Huang. High energy density Li-ion capacitor assembled with all graphene-based electrodes. Carbon, 2015, 92, 106–118. [CrossRef]
- M.B. Lim, M. Hu, S. Manandhar, A. Sakshaug, A. Strong, L. Riley, P.J. Pauzauskie. Ultrafast sol–gel synthesis of graphene aerogel materials. Carbon, 2015, 95, 616–624. [CrossRef]
- Y. Wu, J. Zhu, L. Huang. A review of three-dimensional graphene-based materials: Synthesis and applications to energy conversion/storage and environment. Carbon, 2019, 143, 610–640. [CrossRef]
- S. Yang, L. Zhi, K. Tang, X. Feng, J. Maier, K. Mullen. Efficient synthesis of heteroatom (N or S)-doped graphene based on ultrathin graphene oxide-porous silica sheets for oxygen reduction reaction. Adv. Funct. Mater., 2012, 22 (17), 3634-3640. [CrossRef]
- Z. Lin, G. Waller, Y. Liu, M. Liu, C.P. Wong. Facile synthesis of nitrogen-doped graphene via pyrolysis of graphene oxide and urea, and its electrocatalytic activity toward the oxygen-reduction reaction. Adv. Energy Mater., 2012, 2 (7), 884-888. [CrossRef]
- Z. Lin, G.H. Waller, Y. Liu, M. Liu, C.P. Wong. 3D nitrogen-doped graphene prepared by pyrolysis of graphene oxide with polypyrrole for electrocatalysis of oxygen reduction reaction. Nano Energy, 2013, 2, 241-248. [CrossRef]
- M. Enterría, F.J. Martí-Jimeno, F. Suárez-García, J.I. Paredes, M.F.R. Pereira, J.I. Martins, A. Martínez-Alonso, J.M.D. Tascón, J.L. Figueiredo. Effect of nanostructure on the supercapacitor performance of activated carbon xerogels obtained from hydrothermally carbonized glucose-graphene oxide hybrids. Carbon, 2016, 105, 474-483. [CrossRef]
- F.J. Martín-Jimeno, F. Suárez-García, J.I. Paredes, A. Martínez-Alonso, J.M.D. Tascón. Activated carbon xerogels with cellular morphology derived from hydrothermally carbonized glucose-graphene oxide hybrids and their performance towards CO2 and dye adsorption. Carbon, 2015, 81, 137-147. [CrossRef]
- M. Canal-Rodríguez, A. Arenillas, N. Rey-Raap, G. Ramos-Fernández, I. Martín-Gullón, J.A. Menéndez. Graphene-doped carbon xerogel combining high electrical conductivity and surface area for optimized aqueous supercapacitors. Carbon, 2017, 118, 291-298. [CrossRef]
- G. Ramos-Fernández, M. Canal-Rodríguez, A. Arenillas, J.A. Menéndez, I. Rodríguez-Pastor, I. Martín-Gullon. Determinant influence of the electrical conductivity versus surface area on the performance of graphene oxide-doped carbon xerogel supercapacitors. Carbon, 2018, 126, 456-463. [CrossRef]
- K. Sato, M. Noguchi, A. Demachi, N. Oki, M. Endo. A mechanism of lithium storage in disordered carbons. Science. 1994, 264, 556−558. [CrossRef]
- J.S. Xue, J.R. Dahn. Dramatic effect of oxidation on lithium insertion in carbons made from epoxy resin. J. Electrochem. Soc. 1995, 142, 3668−3677. [CrossRef]
- D.Y. Pan, S. Wang, B. Zhao, M.H. Wu, H.J. Zhang, Y. Wang, Z. Jiao. Li storage properties of disordered graphene nanosheets. Chem. Mater., 2009, 21, 3136−3142. [CrossRef]
- R. Yazami, M. Deschamps. High reversible capacity carbon-lithium negative electrode in polymer electrolyte. J. Power Sources. 1995, 54, 411−415. [CrossRef]
- J-C. Li, P-X. Hou, M. Cheng, C. Liu, H-M. Cheng, M. Shao. Carbon nanotube encapsulated in nitrogen and phosphorus co-doped carbon as a bifunctional electrocatalyst for oxygen reduction and evolution reactions. Carbon, 2018, 139, 156-163. [CrossRef]
- Y. Li, J. Yang, J. Huang, Y. Zhou, K. Xu, N. Zhao, X. Cheng. Soft template-assisted method for synthesis of nitrogen and sulfur co-doped three-dimensional reduced graphene oxide as an efficient metal free catalyst for oxygen reduction reaction. Carbon, 2017, 122, 237-246. [CrossRef]
- S.K. Samaniego Andrade, I. Bakos, G. Dobos, A. Farkas, G. Kiss, S. Klébert, J. Madarász, K. László. Biomass related highly porous metal free carbon for gas storage and electrocatalytic applications. Materials, 2021, 14 (13), 3488. [CrossRef]
- B. Berke, L. Sós, V. Bérczes, A. Domján, L. Porcar, O. Czakkel, K. László. Graphene derivatives in responsive hydrogels: Effect of concentration and surface chemistry. European Polymer Journal, 2017, 93, 717–725. [CrossRef]
- W.S. Hummers Jr., R.E Offeman. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339−1339.
- D.C. Marcano, D.V. Kosynkin, J.M. Berlin, A. Sinitskii, Z. Sun, A. Slesarev, et al. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–14. [CrossRef]
- S. Brunauer, P.H. Emmett, E. Teller. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60 (2), 309–319. [CrossRef]
- M.M. Dubinin, L.V. Radushkevich. The Equation of the characteristic curve of activated charcoal. Dokl. Akad. Nauk. SSSR., 1947, 55, 327-329.
- J. Landers, G.Y. Gor, A.V. Neimark. Density functional theory methods for characterization of porous materials. Colloids Surf. A Physicochem. Eng., 2013, 437, 3-32. [CrossRef]
- Bertóti, M. Mohai, K. László. Surface modification of graphene and graphite by nitrogen plasma: Determination of chemical state alterations and assignments by quantitative X-ray photoelectron spectroscopy. Carbon, 2015, 84, 185-196. [CrossRef]
- M. Mohai. XPS MultiQuant: multimodel XPS quantification software. Surf. Interface Anal., 2004, 36 (8), 828-832. [CrossRef]
- S. Evans, R.G. Pritchard, J.M. Thomas. Relative differential subshell photoionization cross-sections (MgKα) from lithium to uranium. J. Electron Spectrosc. Relat. Phenom., 1978, 14 (5), 341-358. [CrossRef]
- R.F. Reilman, A. Msezane, S.T. Manson. Relative intensities in photoelectron spectroscopy of atoms and molecules. J. Electron Spectrosc. Relat. Phenom., 1976, 8 (5), 389-394. [CrossRef]
- B. Nagy, A. Domán, A. Menyhárd, K. László. Influence of graphene oxide incorporation on resorcinol-formaldehyde polymer and carbon aerogels. Periodica Polytechnica Chemical Engineering, 2018, 62 (4), 441–449. [CrossRef]
- M. Thommes, K. Kaneko, A.V. Neimark, J.P. Oliver, F. Rodriguez-Reinoso, J. Rouquerol, K.S.W. Sing. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem., 2015, 87 (9-10), 1051-1069. [CrossRef]
- Y. Wang, D.C. Alsmeyer, R.L. McCreery. Raman spectroscopy of carbon materials: Structural basis of observed spectra. Chem. Mater., 1990, 2 (5), 557-563. [CrossRef]
- M.A. Pimenta, G. Dresselhaus, M.S. Dresselhaus, L.G. Cançado, A. Jorio, R. Saito. Studying disorder in graphite-based systems by Raman spectroscopy. Phys. Chem. Chem. Phys., 2007, 9 (11), 1276-1290. [CrossRef]
- Cuesta, P. Dhamelincourt, J. Laureyns, A. Martínez-Alonso, J.M.D. Tascón. Raman microprobe studies on carbon materials. Carbon, 1994, 32, 1523-1532. [CrossRef]
- S. Claramunt, A. Varea, D. López-Díaz, M.M. Velázquez, A. Cornet, A. Cirera. The importance of interbands on the interpretation of the Raman spectrum of graphene oxide. J. Phys. Chem. C, 2015, 119, 10123-10129. [CrossRef]
- M. Couzi, J-L. Bruneel, D. Talaga, L. Bokobza. A multi wavelength Raman scattering study of defective graphitic carbon materials: The first order Raman spectra revisited. Carbon, 2016, 107, 388-394. [CrossRef]
- J. McDonald-Wharry, M. Manley-Harris, K. Pickering. Carbonization of biomass-derived chars and the thermal reduction of a graphene oxide sample studied using Raman spectroscopy. Carbon, 2013, 59, 383-405. [CrossRef]
- Y-R. Rhim, D. Zhang, D. H. Fairbrother, K. A. Wepasnick, K.J. Livi, R.J. Bodnar, D.C. Nagle. Changes in electrical and microstructural properties of microcrystalline cellulose as function of carbonization. Carbon, 2010, 48 (4), 1012-1024. [CrossRef]
- N. Gavrilov, I.A. Pasti, M. Mitric, J. Trava-Sejdic, G. Ciric-Marjanonic, S.V. Mantus. Electrocatalysis of oxygen reduction reaction on polyaniline-derived nitrogen-doped carbon nanoparticle surfaces in alkaline media. J. Power Sources. 2012, 220, 306-316. [CrossRef]
- D. Yin, N. Lu, Z.-Y. Li, J.-L. Yang. A computational infrared spectroscopic study of graphene oxide. J. Chem. Phys., 2013, 139, 084704. [CrossRef]
- X. Jiao, Y. Qiu, L. Zhangab, X. Zhang. Comparison of the characteristic properties of reduced graphene oxides synthesized from natural graphites with different graphitization degrees. RSC Adv., 2017, 7, 52337–52344. [CrossRef]
- Z. Ling, G. Wang, Q. Dong, B. Qian, M. Zhang, C. Lia, J. Qiu. An ionic liquid template approach to graphene–carbon xerogel composites for supercapacitors with enhanced performance. J. Mater. Chem. A, 2014, 2 (35), 14329–14333. [CrossRef]
- H. Lu, C. Yang, J. Chen, J. Li, H. Jin, J. Wang, S. Wang. Tailoring hierarchically porous nitrogen-, sulfur-codoped carbon for high-performance supercapacitors and oxygen reduction. Small, 2020, 16 (17), 1906584. [CrossRef]
- L. Ge, D. Wang, P. Yang, H. Xu, L. Xiao, G-X. Zhang, X. Lu, Z. Duan, F. Meng, J. Zhang, M. An. Graphite N-C-P dominated three-dimensional nitrogen and phosphorus co-doped holey graphene foams as high-efficiency electrocatalyst for Zn-air batteries. Nanoscale, 2019, 11, 17010-17017. [CrossRef]
- Y. Zhao, S. Huang, M. Xia, S. Rehman, S. Mu, Z. Kou, Z. Zhang, Z. Chen, F. Gao, Y. Hou. N-P-O co-doped high performance 3D graphene prepared through red phosphorus-assisted “cutting-thing” technique: A universal synthesis and multifunctional applications. Nano Energy, 2016, 28, 346-355. [CrossRef]
- J. Tong, W. Ma, W. Wang, J. Ma, W. Li, L. Bo, H. Fan. Nitrogen/phosphorus dual-doped hierarchically porous graphitic biocarbon with greatly improved performance on oxygen reduction reaction in alkaline media. J. Electroanal. Chem., 2018, 809, 163-170. [CrossRef]
- J. Zhang, L. Qu, G. Shi, J. Liu, J. Chen, L. Dai. N, P-codoped carbon networks as efficient metal-free bifunctional catalysts for oxygen reduction and hydrogen evolution reactions. Angew. Chem., 2015, 128 (6), 2270-2274. [CrossRef]
- Y-N. Zhu, C-Y. Cao, W-Y. Jiang, S-L. Yang, J-S. Hu, W-G. Song, L-J. Wan. Nitrogen, phosphorus, and sulfur co-doped ultrathin carbon nanosheets as a metal-free catalyst for selective oxidation of aromatic alkanes and the oxygen reduction reaction. J. Mater. Chem. A, 2016, 4 (47), 18470-18477. [CrossRef]
- J. Wu, X. Zheng, C. Jin, J. Tian, R. Yang. Ternary doping of phosphorus, nitrogen, and sulfur into porous carbon for enhancing electrocatalytic oxygen reduction. Carbon, 2015, 92, 327-338. [CrossRef]
- X. Zheng, X. Cao, J. Wu, J. Tian, C. Jin, R. Yang. Yolk-shell N/P/B ternary-doped biocarbon derived from yeast cells for enhanced oxygen reduction reaction. Carbon, 2016, 107, 907-916. [CrossRef]
- P. Chandran, A. Ghosh, S. Ramaprabhu. High-performance platinum-free oxygen reduction reaction and hydrogen oxidation reaction catalyst in polymer electrolyte membrane fuel cell. Sci. Rep., 2018, 8, 3591. [CrossRef]
- C. Moreno-Castilla, M.B. Dawidziuk, F. Carrasco-Marín, E. Morallón. Electrochemical performance of carbon gels with variable surface chemistry and physics. Carbon, 2012, 50 (9), 3324-3332. [CrossRef]
- B. Lobato, L. Suárez, L. Guardia, T. A. Centeno. Capacitance and surface of carbons in supercapacitors. Carbon, 2017, 122, 434-445.
- Y. Lu, Z. Ye, Y. Zhao, Q. Li, M. He, C. Bai, X. Wang, Y. Han, X. Wan, S. Zhang, Y. Ma, Y. Chen. Graphene supported double-layer carbon encapsulated silicon for high-performance lithium-ion battery anode materials. Carbon, 2023, 201, 962–971. [CrossRef]
- T.J. Yokokura, J.R. Rodriguez, V.G. Pol, Waste biomass-derived carbon anode for enhanced lithium storage, ACS Omega, 2020, 5 (31), 19715–19720. [CrossRef]
- J. Wang, P. Nie, B. Ding, S. Dong, X. Hao, H. Dou, X. Zhang. Biomass derived carbon for energy storage devices. J. Mater. Chem. A, 2017, 5 (6), 2411–2428. [CrossRef]
- A.S. Alex, M.S. Ananda Lekshmi, V. Sekkar, B. John, C. Gouri, S.A. Ilangovan. Microporous carbon aerogel prepared through ambient pressure drying route as anode material for lithium ion cells. Polym. Adv. Technol., 2017, 28 (12), 1945–1950. [CrossRef]
- S. T. Taleghani, B. Marcos, K. Zaghib, G. Lantagne. A study on the effect of porosity and particles size distribution on Li-ion battery performance. J. Electrochem. Soc., 2017, 164, E3179.
- E. Duraisamy, A. Prasath, V.S. Devi, M.N.M. Ansari, P. Elumalai. Sustainably-derived hierarchical porous carbon from spent honeycomb for high-performance lithium-ion battery and ultracapacitors. Energy Storage, 2020, 2 (4), e136. [CrossRef]
- K. Kim, R.A. Adams, P.J. Kim, A. Arora, E. Martinez, J.P. Youngblood, V.G. Pol. Li-ion storage in an amorphous, solid, spheroidal carbon anode produced by dry-autoclaving of coffee oil. Carbon, 2018, 133, 62−68. [CrossRef]
- A.M. Stephan, T.P. Kumar, R. Ramesh, S. Thomas, S.K. Jeong, K.S. Nahm. Pyrolitic carbon from biomass precursors as anode materials for lithium batteries. Mater. Sci. Eng. A, 2006, 430 (1-2), 132− 137. [CrossRef]
- R.R. Gaddam, D. Yang, R. Narayan, KVSN. Raju, N.A. Kumar, X.S. Zhao. Biomass derived carbon nanoparticle as anodes for high performance sodium and lithium ion batteries. Nano Energy, 2016, 26, 346−352. [CrossRef]
- W. Xing, J.S. Xue, J.R. Dahn. Optimizing pyrolysis of sugar carbons for use as anode materials in lithium-ion batteries. J. Electrochem. Soc., 1996, 143, 3046−3052. [CrossRef]
- W. Wang, Y. Sun, B. Liu, S. Wang, M. Cao. Porous carbon nanofiber webs derived from bacterial cellulose as an anode for high-performance lithium-ion batteries. Carbon, 2015, 91, 56–65. [CrossRef]
- Paudics, S. Farah, I. Bertóti, K. László, M. Mohai, A. Szilágyi, M. Kubinyi. Fluorescence probing of binding sites on graphene oxide nanosheets with Oxazine 1 dye. Appl. Surf. Sci. 2021, 541, 148451.
Figure 1.
Typical images of CA (a), the GO cryogel (b), and the CAGO50 sample (c).
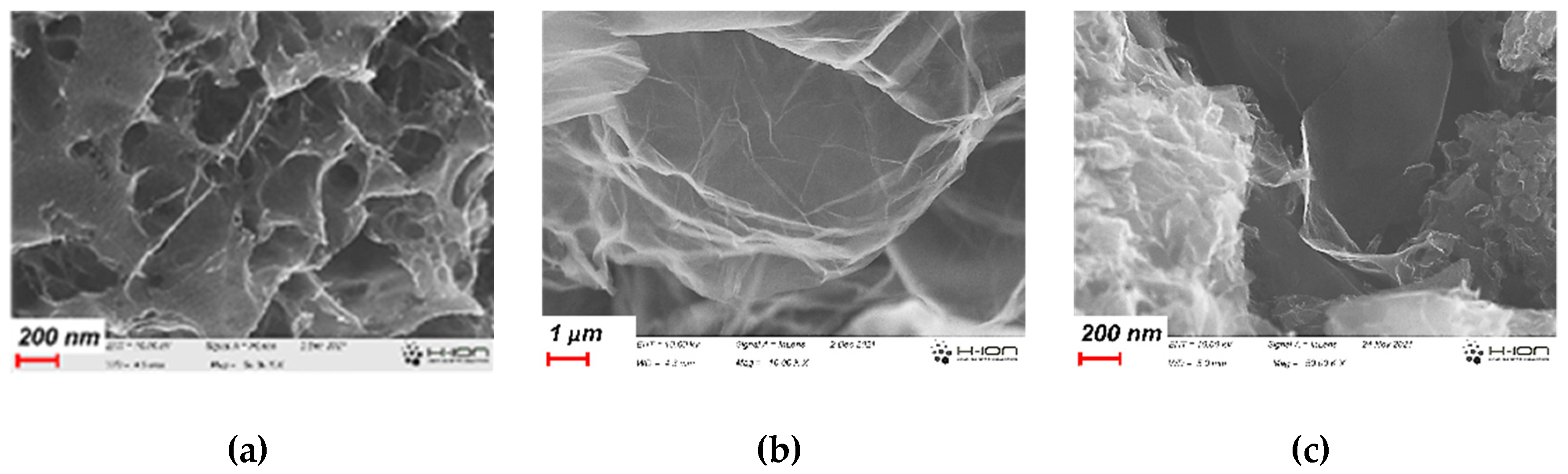
Figure 2.
(a) Low temperature N2 adsorption/desorption isotherms of annealed carbons; (b) Combined pore size distribution functions of annealed carbons. Functions were estimated by quenched solid density functional theory QSDFT, slit/cylinder geometry for N2 and NLDFT for CO2, respectively.
Figure 2.
(a) Low temperature N2 adsorption/desorption isotherms of annealed carbons; (b) Combined pore size distribution functions of annealed carbons. Functions were estimated by quenched solid density functional theory QSDFT, slit/cylinder geometry for N2 and NLDFT for CO2, respectively.
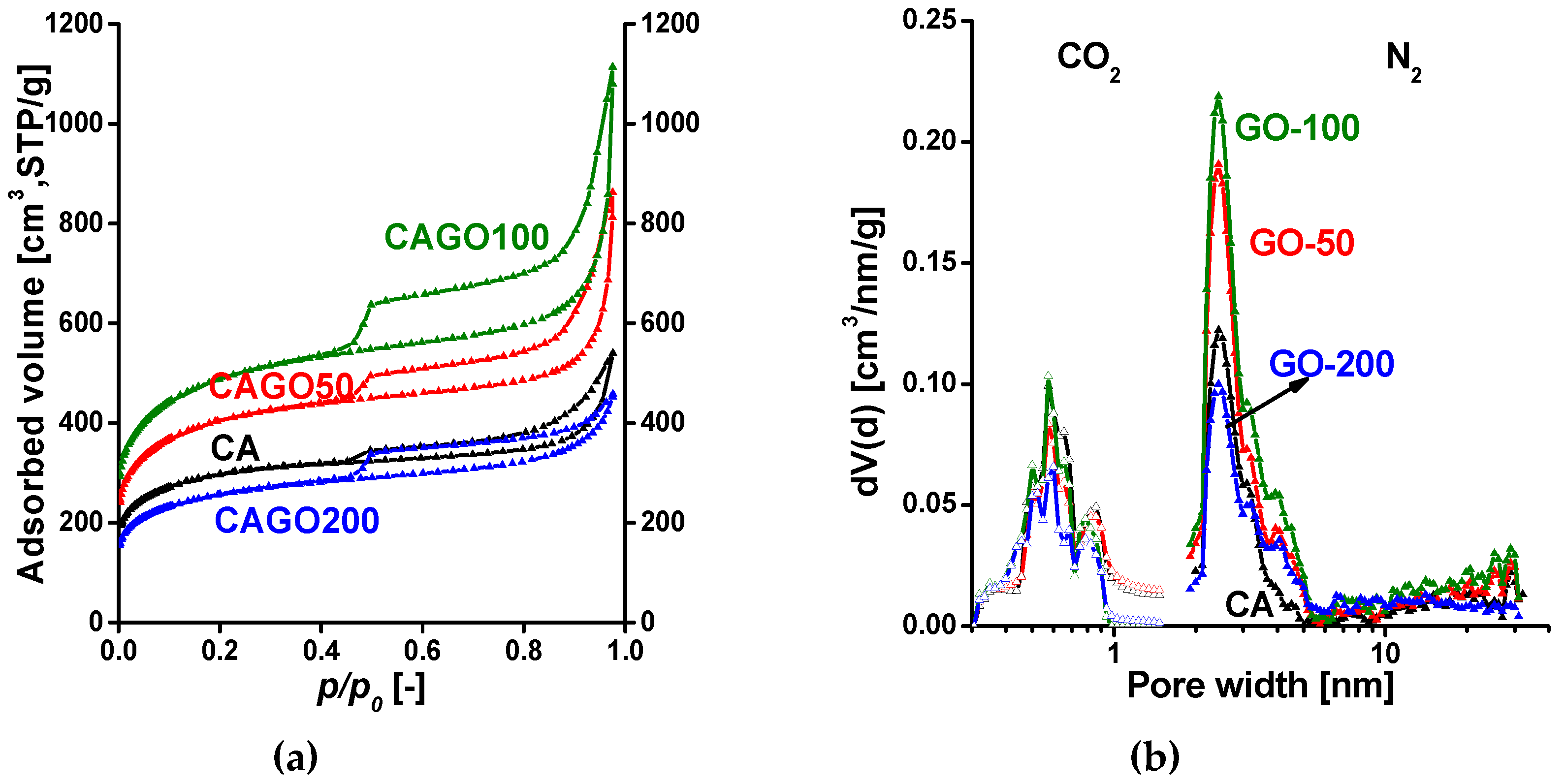
Figure 3.
Decomposition of C1s, O1s, N1s and S2p regions of photoelectron spectra of the CAGO100 sample.
Figure 3.
Decomposition of C1s, O1s, N1s and S2p regions of photoelectron spectra of the CAGO100 sample.
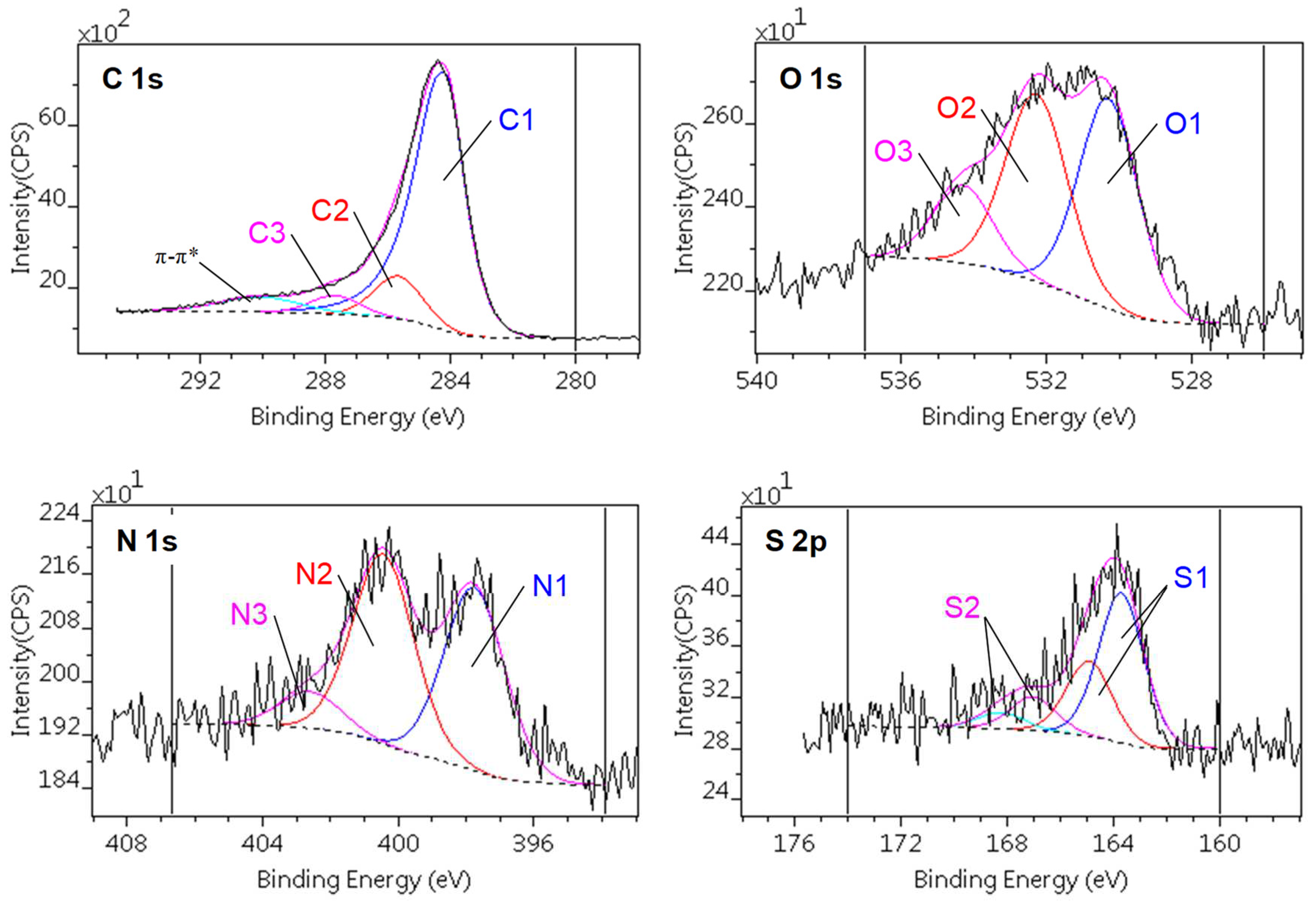
Figure 4.
Cathodic linear potential sweep of the carbon xerogel loaded electrodes in oxygen saturated 0.1 M KOH. Loading: 75 μg/cm2, sweep rate: 5 mV/s. Rotation rate: 400 (blue), 625 (black), 900 (red), 1250 (green) rpm, (increasing downwards). Rotation rates were applied in the following order: 625, 900, 1250, 400 rpm. LSVs on Pt/C were measured for comparison.
Figure 4.
Cathodic linear potential sweep of the carbon xerogel loaded electrodes in oxygen saturated 0.1 M KOH. Loading: 75 μg/cm2, sweep rate: 5 mV/s. Rotation rate: 400 (blue), 625 (black), 900 (red), 1250 (green) rpm, (increasing downwards). Rotation rates were applied in the following order: 625, 900, 1250, 400 rpm. LSVs on Pt/C were measured for comparison.
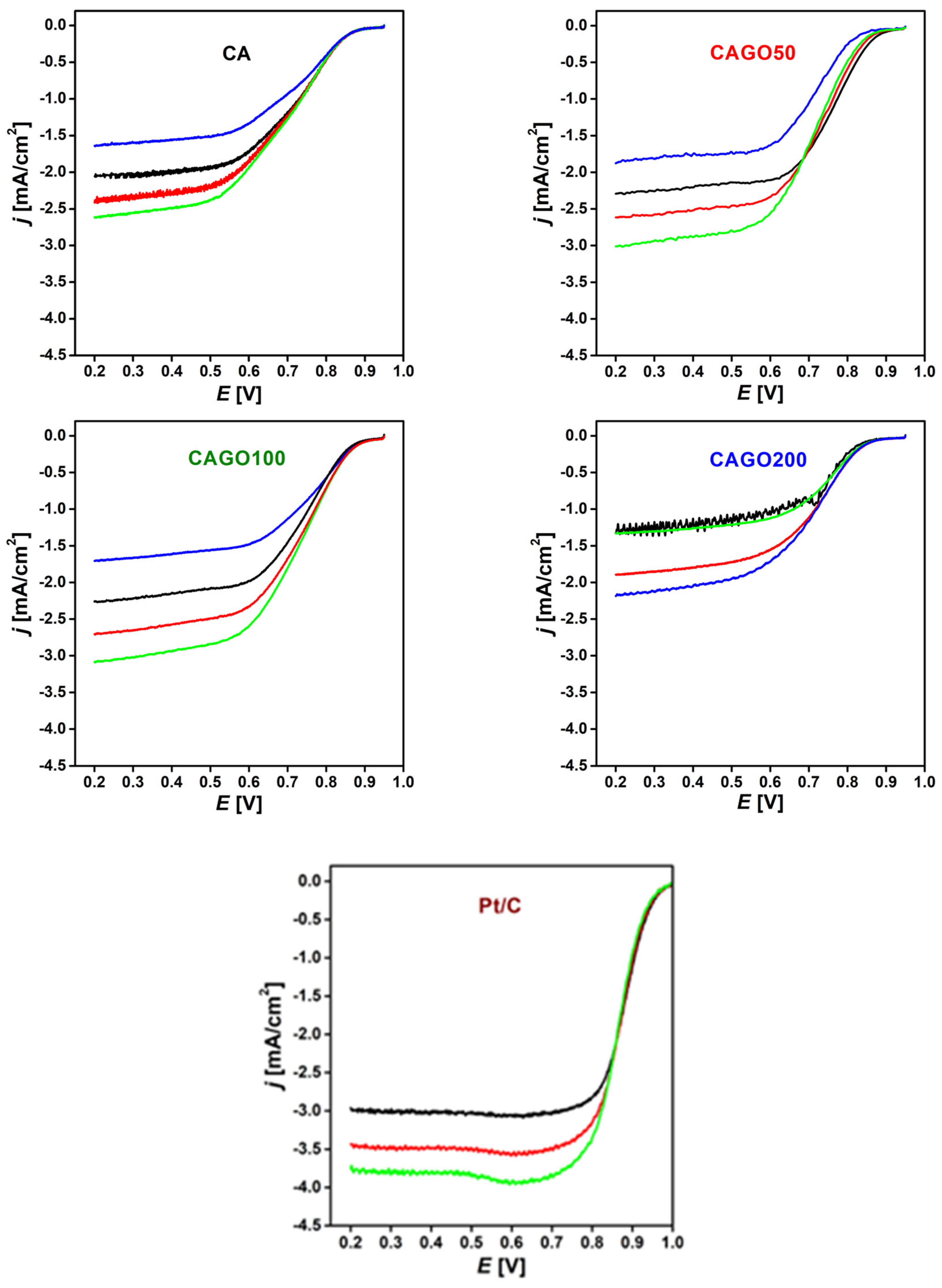
Figure 5.
Koutecky-Levich (KL) plots of the carbon electrodes at E = 0.3 V. The theoretical 2 e- and 4 e- KL plots are shown for comparison.
Figure 5.
Koutecky-Levich (KL) plots of the carbon electrodes at E = 0.3 V. The theoretical 2 e- and 4 e- KL plots are shown for comparison.
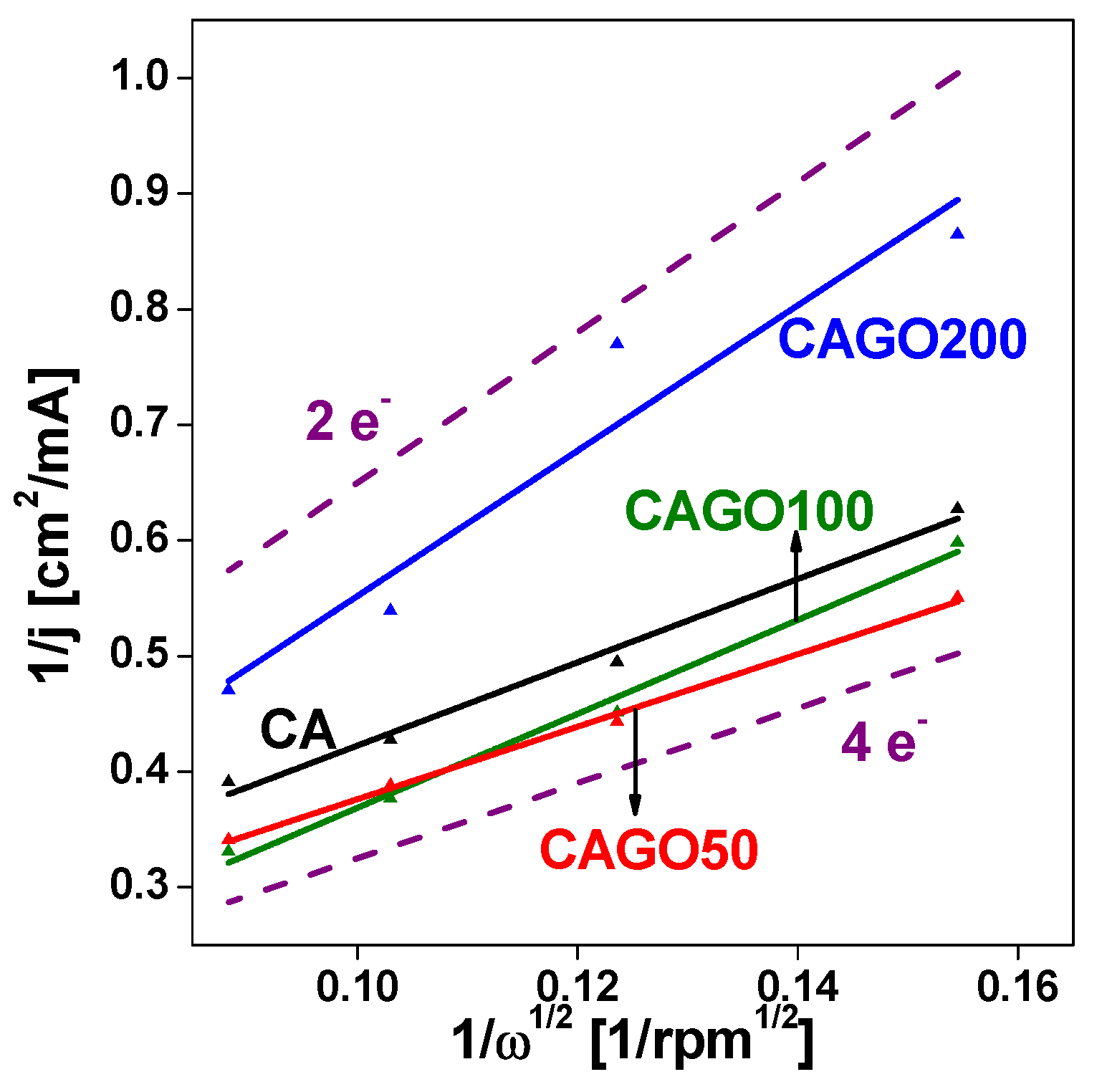
Figure 6.
Cyclic voltammograms (1) of the virgin electrode; (2) after 50 cycles in oxygen-free 0.1 M KOH electrolyte; (3) after additional 50 cycles in oxygen-saturated electrolytes; (4) after an additional ORR test with rotating electrode (sweep rate: 5 mV/s, four rotation rates). The column graph compares the gravimetric capacitances of the carbons corresponding to CV 1 (black), 2 (red), 3 (green) and 4 (blue), respectively.
Figure 6.
Cyclic voltammograms (1) of the virgin electrode; (2) after 50 cycles in oxygen-free 0.1 M KOH electrolyte; (3) after additional 50 cycles in oxygen-saturated electrolytes; (4) after an additional ORR test with rotating electrode (sweep rate: 5 mV/s, four rotation rates). The column graph compares the gravimetric capacitances of the carbons corresponding to CV 1 (black), 2 (red), 3 (green) and 4 (blue), respectively.

Figure 7.
Galvanostatic charge – discharge profiles of CA, CAGO50, CAGO100 and CAGO200 at constant current density 100 mA/g, cycle 1 grey, cycle 10 red, cycle 20 blue, cycle 30 green, cycle 40 purple, cycle 50 gold.
Figure 7.
Galvanostatic charge – discharge profiles of CA, CAGO50, CAGO100 and CAGO200 at constant current density 100 mA/g, cycle 1 grey, cycle 10 red, cycle 20 blue, cycle 30 green, cycle 40 purple, cycle 50 gold.
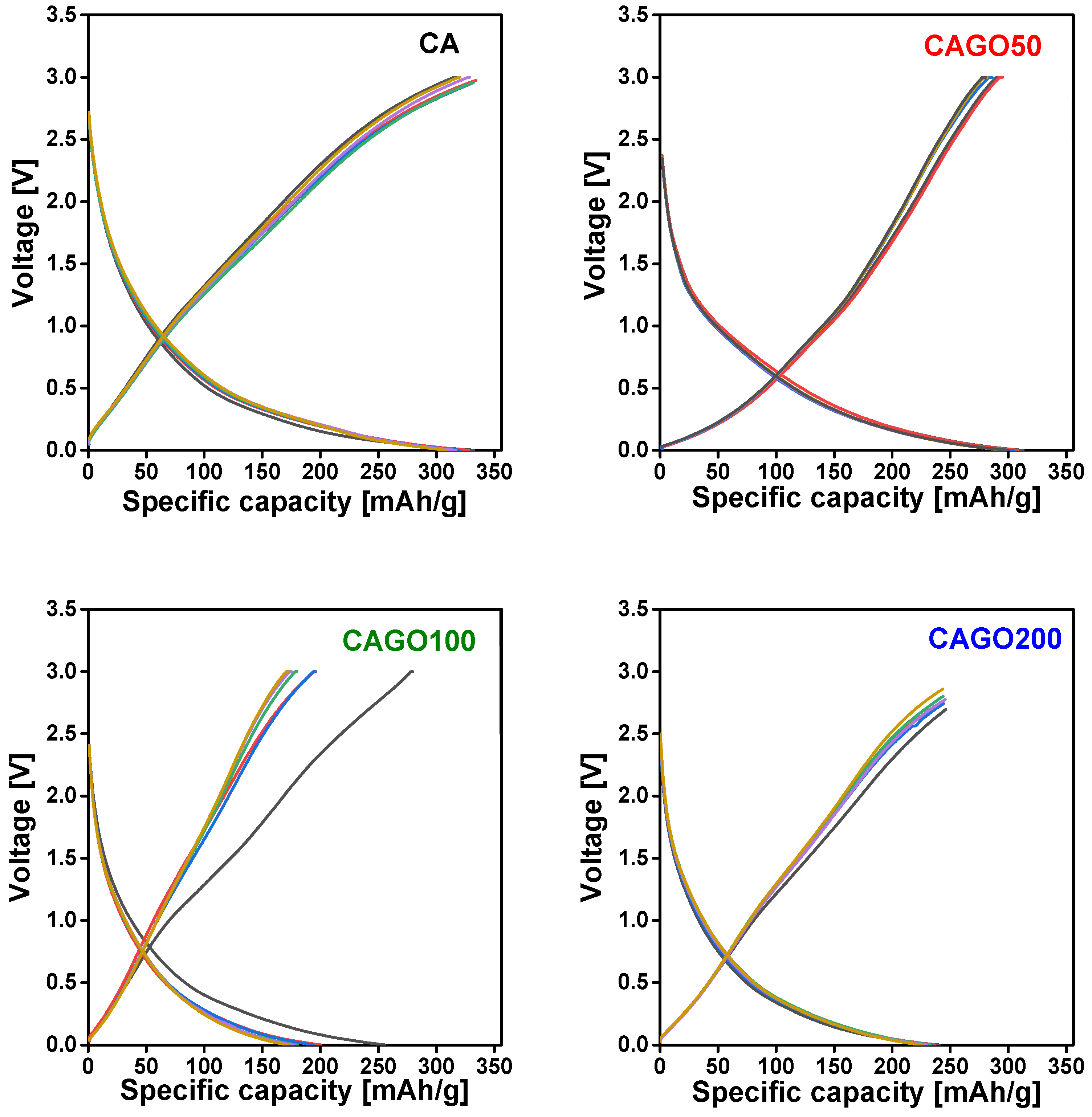
Figure 8.
Long-term cycling performance of CA, CAGO50, CAGO100, and CAGO200.
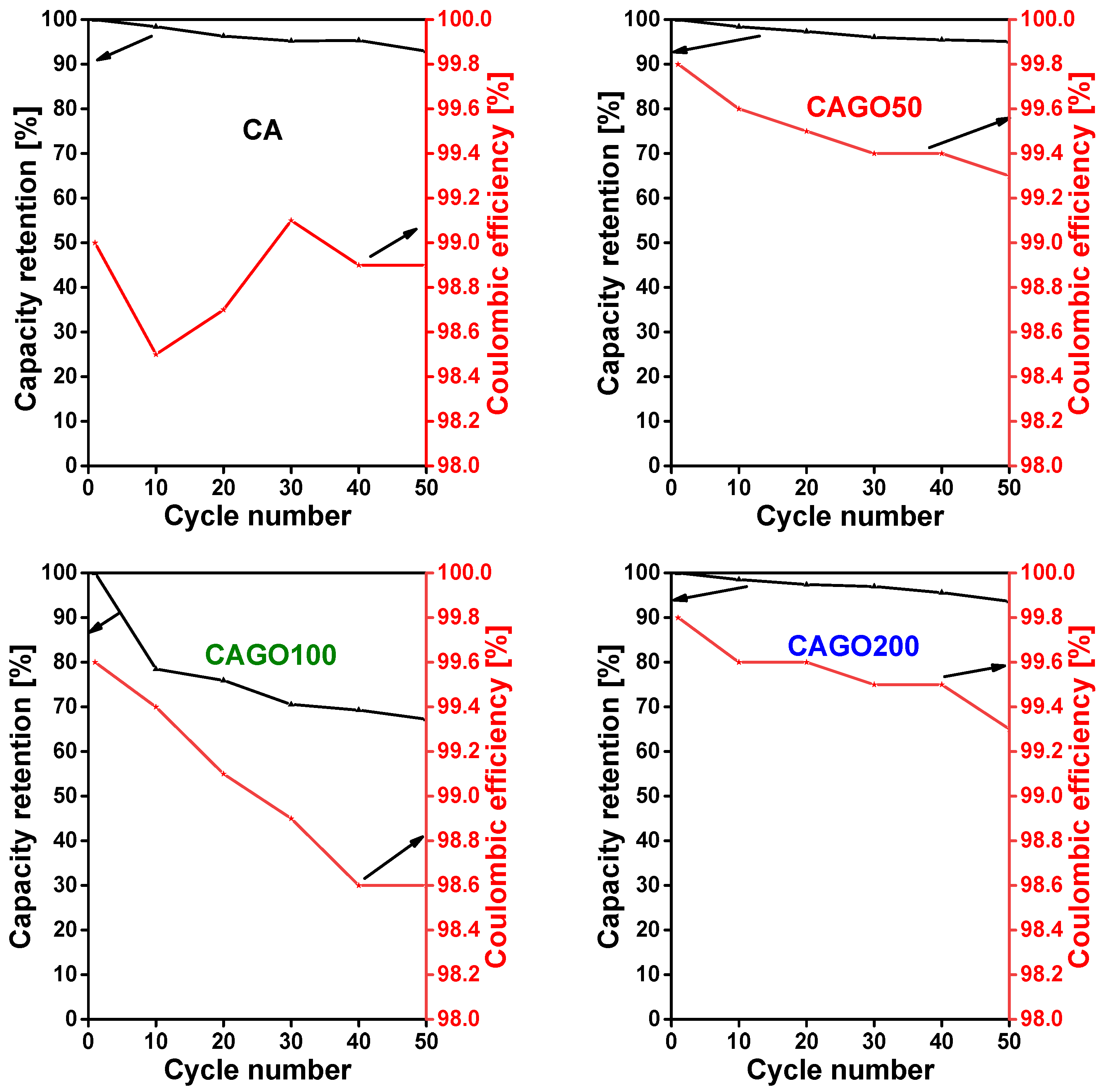
Table 1.
Porous characteristics of annealed carbon aerogel samples from gas adsorptionmeasurements*.
Table 1.
Porous characteristics of annealed carbon aerogel samples from gas adsorptionmeasurements*.
| Method | Parameter | Units | CA | CAGO50 | CAGO100 | CAGO200 |
|---|---|---|---|---|---|---|
| From N2 | SBET | [m2/g] | 1070 | 1479 | 1779 | 933 |
| V0.98 | [cm3/g] | 0.83 | 1.33 | 1.72 | 0.71 | |
| Vmicro,DR | [cm3/g] | 0.42 | 0.54 | 0.64 | 0.34 | |
| [%] | 51 | 40 | 37 | 48 | ||
| Vmicro,DFT | [cm3/g] | 0.31 | 0.40 | 0.48 | 0.25 | |
| [%] | 37 | 30 | 28 | 35 | ||
| From CO2 | Vumicro,DR | [cm3/g] | 0.073 | 0.062 | 0.059 | 0.041 |
| Vumicro,DFT | [cm3/g] | 0.042 | 0.039 | 0.030 | 0.025 |
*SBET: apparent surface area from BET model, V0.98: liquid equivalent of the gas adsorbed at p/p0 = 0.98, Vmicro,DR: micropore volume from DR model, Vmicro,DFT: micropore volume from DFT model; Vumicro,DR: ultra-micropore volume from DR model; Vumicro,DFT: ultra-micropore volume from DFT model.
Table 2.
Surface composition (atomic %) measured by XPS.
| Sample | C | O | N | S | O / C | N / C | S / C | O+N+S C |
S / N |
|---|---|---|---|---|---|---|---|---|---|
| CA | 90.6 | 3.3 | 5.1 | 1.0 | 0.036 | 0.056 | 0.011 | 0.104 | 0.196 |
| CAGO50 | 92.0 | 3.1 | 3.7 | 1.3 | 0.034 | 0.039 | 0.014 | 0.087 | 0.361 |
| CAGO100 | 90.7 | 4.1 | 4.1 | 1.2 | 0.045 | 0.045 | 0.013 | 0.104 | 0.293 |
| CAGO200 | 90.4 | 3.7 | 4.4 | 1.4 | 0.041 | 0.049 | 0.015 | 0.105 | 0.318 |
| GO-film | 67.4 | 32.1 | - | 0.5 | 0.476 | - | 0.007 | 0.484 | - |
Table 3.
Decomposition of C1s and O1s regions of photoelectron spectra: binding energy ranges, chemical state assignations and surface compositions (atomic %).
Table 3.
Decomposition of C1s and O1s regions of photoelectron spectra: binding energy ranges, chemical state assignations and surface compositions (atomic %).
| C1s | O1s | |||||
|---|---|---|---|---|---|---|
| C1 | C2 | C3 | O1 | O2 | O3 | |
| Chemical state | sp2 C=C | C–O C–N C–S |
C=O O–C–O N–C–O |
S–O | C–O–C C–OH C=O |
OC–O–CO (H2O) |
| Binding energy [eV] | 284.3 – 284.4 |
285.7 – 285.8 |
287.5 – 287.9 |
530.2 – 530.6 | 532.1 – 532.5 | 533.9 – 534.3 |
| CA | 74.0 | 10.9 | 5.4 | 1.5 | 1.7 | |
| CAGO50 | 78.8 | 7.4 | 5.5 | 1.9 | 1.3 | |
| CAGO100 | 74.7 | 11.0 | 4.8 | 1.8 | 1.7 | 0.7 |
| CAGO200 | 75.9 | 9.4 | 4.8 | 1.8 | 1.6 | 0.5 |
Table 4.
Decomposition of N1s and S2p regions of photoelectron spectra: binding energy ranges, chemical state assignations and surface compositions (atomic %).
Table 4.
Decomposition of N1s and S2p regions of photoelectron spectra: binding energy ranges, chemical state assignations and surface compositions (atomic %).
| N1s | S2p | ||||
|---|---|---|---|---|---|
| N1 | N2 | N3 | S1 | S2 | |
| Chemical state | C–N | OO–C–N | C–N+ | C–S | C–SO3 |
| Binding energy [eV] | 397.8 – 398.0 |
400.4 – 400.5 | 402.4 – 402.7 | 164.9 – 165.0 | 168.3 – 168.6 |
| CA | 2.3 | 2.3 | 0.8 | 0.9 | 0.2 |
| CAGO50 | 1.6 | 1.7 | 0.6 | 1.2 | n.d. |
| CAGO100 | 1.9 | 1.9 | 0.4 | 1.0 | 0.2 |
| CAGO200 | 2.0 | 2.0 | 0.7 | 1.1 | 0.3 |
Table 5.
Comparison of the onset potentials and half wave potentials of double doped carbon aero/xero/cryogel electrodes in 0.1 M KOH, vs. RHE.
Table 5.
Comparison of the onset potentials and half wave potentials of double doped carbon aero/xero/cryogel electrodes in 0.1 M KOH, vs. RHE.
| Sample | BET surface area [m2/g] |
Onset potential [mV] |
E1/2 [mV] |
Number of e- transferred | Ref. |
|---|---|---|---|---|---|
| SWCNT@N,P doped carbon | 616 | 920 | 850 | 3.91 | [69] |
| N,S co-doped 3D rGO | 392 | 895 | 732 | 3.87 | [70] |
| N,S porous carbon materials | 732 | 940 | 840 | - | [95] |
| N,P-holey graphene foams | 758 | 983 | 865 | 3.70 | [96] |
| 3D-high performance graphene | 1406 | 928 | 836 | 3.83 | [97] |
| N,P porous graphitic biocarbon | 845 | -14 (vs. Ag/AgCl) |
-115 (vs. Ag/AgCl) |
3.9 | [98] |
| N,P co-doped carbon | 375 | 950 | 820 | 3.7 | [99] |
| N,P,S co-doped carbon nanosheets | 1198 | 938 | 800 | 3.8 – 4.0 | [100] |
| P,N,S-porous carbon | 711 | 905 | 780 | 3.68 – 3.96 | [101] |
| N,P,B biocarbon | 1155 | 904 | 790 | 3.78 – 3.90 | [102] |
| Pt/C (20 wt.% Pt on Vulcan XC-72) | - | 960 | 869 | 3.96 | [102] |
| CA | 1070 | 855 | 700 | 3.5 |
Our work |
| CAGO50 | 1479 | 850 | 760 | 4.0 | |
| CAGO100 | 1779 | 845 | 730 | 3.1 | |
| CAGO200 | 933 | 825 | 700 | 2.0 | |
| Pt/C | 957 | 886 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
supplementary.pdf (1.15MB )
Submitted:
12 July 2023
Posted:
13 July 2023
You are already at the latest version
Alerts
A peer-reviewed article of this preprint also exists.
supplementary.pdf (1.15MB )
This version is not peer-reviewed
Submitted:
12 July 2023
Posted:
13 July 2023
You are already at the latest version
Alerts
Abstract
High surface area carbon was obtained from -carrageenan – urea cryogels doped with 1.25 – 5 wt% graphene oxide (GO). Incorporation of GO resulted in multifaceted modification in the textural and chemical properties of the carbon cryogel. The thermal decomposition of GO facilitated the formation of micropores resulted in a substantial increase in the apparent surface area (up to 1780 m2/g). The annealing at 1000 °C removed part of the heteroatoms but left behind a high number of defects. While the volatile thermal degradation products of GO affected the porous texture and the surface chemistry in a complex way, its reduced residue resulted in a gradually increasing electrical conductivity. The influence of GO was studied in oxygen reduction reaction (ORR) and in Li-ion storage application. GO affected the electron transfer mechanism in the ORR tests and challenged the stability of the electrodes. Even if the electrode has been stabilized under oxygen-free conditions, later may undergo destabilization processes during the ORR. The 5 wt% GO doped sample was the most sensitive under oxidative conditions, but finally it exhibited the highest capacitance. In the Li-ion battery tests the coulombic efficiency of all the samples was consistently above 98 %, indicating the great potential of such carbons for efficient Li-ion insertion and reinsertion during the charge – discharge process, thereby providing a promising alternative for graphite-based anodes. The cell from the 1.25% sample showed an initial discharge capacity of 313 mAh/g, 95.1 % capacity retention and 99.3 % coulombic efficiency after 50 charge – discharge cycles.

Keywords:
Subject: Chemistry and Materials Science - Materials Science and Technology
1. Introduction
The fascinating microstructural and surface properties of biomass-based carbon materials justify the extensive interest in their application in various fuel cells [1,2,3], supercapacitors [4,5] and alkaline ion batteries [6,7,8]. Compared to terrestrial biomass the potential of marine-based sources, despite their abundance, is far less explored. Crustaceans rich in N-containing chitin, or edible red seaweed with reasonable sulfated polysaccharide content also may serve as precursors for N or S containing porous carbon materials [9,10,11].
Porous carbons with high surface area, rich porosity and tuned surface chemistry are essential to boost their electrochemical properties. The high surface area, which goes hand in hand with microporous texture and facilitates the exposure of active sites, might, however, limit the diffusion-controlled processes. In contrast, macroporous materials exhibit better transport kinetics, but their correspondingly lower surface area hampers the number of the exposed active sites, and therefore results in poorer surface area related performance. Well-tailored porous carbons with interconnected pore morphology make it possible to fabricate high-performance electrodes in fuel cells, as battery anodes [12] and high-rate electrochemical capacitive energy storage devices [13]. In fuel cells the ultra-micro and micropores are pseudo active sites for adsorption and cleavage of O2 molecules, while the meso- and macropores promote the oxygen reduction reaction (ORR) by facilitating the mass transfer [14,15,16]. Ultra-micropores of similar size to O2 molecules act as pseudo catalytic centers. They stimulate the O2 adsorption and consequently promote O = O bond splitting, fostering the ORR through the 4 e- reduction mechanism [17]. The enhanced interface between the electrolyte and the electrodes shortens the ion transport pathways, fosters electrolyte penetration, and facilitates mass transport and ion diffusion in Li-ion batteries as well as in supercapacitors [18,19,20,21]. Excessive widening of the pores decreases the coulombic efficiency and thus the reversible capacity of batteries; however, pores that are too narrow restrict the access of the bulky solvated Li-ions. Fine tuning of the carbon microstructure is therefore necessary for an effective balance between rate performance and storage capacity [22]. The bulky Li-ions solvated in a tetrahedral arrangement within the electrolyte [23] become intercalated and stored in the nanopores of the electrode in de-solvated form. The maximum Li-ion storage capacity can be achieved when the actual size of the Li-ion fits perfectly into the pore size. The benefits of carbon aerogels as anode materials in lithium-ion batteries can be manifested in three aspects: i) the carbon skeleton is a good electronic conductor that can ensure efficient electron transport; ii) the 3D interconnected and open porous framework facilitates the electrolyte penetration and provide fast Li ion diffusion channels; iii) the open interconnected pores offer enough space to accommodate the volume changes (expansion and contraction during repeated charge – discharge processes) of the electroactive materials, thus helping to maintain the structural and thereby electrochemical stability during cell cycling [24,25,26].
Besides the pore structure the other key factor is the surface chemistry of the carbons. The fundamentals of both texture and surface chemistry design and the corresponding challenges of nanostructured mesoporous carbon materials including carbon gels and their application were summarized by Enterría et al [27]. While the parameters of the sol-gel synthesis technique can be used to tune the adsorption and diffusion characteristics of carbon aerogels, non-metallic heteroatoms (e.g., B, N, O, P, S) generate additional active sites and defects, increase the activity and/or selectivity of the electrochemical processes, and construct more channels for electron and ion transfer [28,29,30].
Although the role of the various N species in the electrocatalytic oxygen reduction process is not fully elucidated, the graphitic and/or pyridinic nitrogen atoms seem to be the most efficient ones in the ORR [31,32,33,34]. Pyridinic nitrogens present in the carbon structure are Lewis bases, while graphitic or quaternary N may act as n-type dopants in amorphous carbon structures. The higher electronegativity of N (3.04 vs. 2.55 of C) facilitates the electron transfer and increases the charge density on the neighboring carbon atom, thus fostering the electrical conductivity, polarity, and surface wettability. In consequence, N doping enhances the high-rate capability in Li-ion battery anode applications and the pseudo-capacitance in supercapacitor applications [35,36,37,38].
The large covalent radius of the S atoms alters the electronic and metallic properties of the carbon matrix [39]. Sulfoxide, sulfones, and sulfonic acids located in the mesopores foster the access of the electrolyte delivering dissolved oxygen into the pore system [40]. The thiophenic sulfur compounds appear to be particularly active in enhancing the physisorption of the oxygen from electrolytes in small carbon pores [40]. The S atom incorporated in aromatic rings causes a slightly positive charge on the neighboring C atoms [40,41] and brings additional hydrophobicity to the surface, thus promoting the adsorption of molecular oxygen [34].
DFT calculations revealed that dual S and N doping results in a large number of active sites through the redistribution of spin and charge densities, thus synergistically improving the ORR performance [41]. Atomic level distribution of (hetero)atoms in the porous carbon matrix significantly improves the rate of the ORR, which is otherwise too slow for practical applications [42,43]. They also affect the performance of supercapacitors [44]. They reduce or may even prevent the need for Pt loading, which is an economic obstacle in commercializing fuel cells. N, P, and S atoms in biomass-based carbon electrodes act as extra active Li-ion storage sites [45,46]. The kinetics of the electrochemical reaction in heteroatom doped carbons are much faster, resulting in higher capacity and better rate performance. The C-S defect sites induce torsion in the graphitic layer structure and the extended interlayer spacing facilitates the insertion of lithium ions during the charge – discharge cycling of the cells [47].
Nanocarbons (carbon nanotubes, carbide and carbonitride graphene) themselves may form electrically conductive interconnected aerogel matrix through van der Waals interactions [48]. Graphene, due to its outstanding properties (high electrical and thermal conductivity, good mechanical strength) plays a particularly important role in the development of a new generation of batteries. Their surface area, mechanical flexibility, and broad electrochemical window [49] make graphene-based electrodes promising for fuel cells [50], batteries [51,52,53], supercapacitors [54,55,56,57], etc. Graphene oxide (GO) has been used as a platform [58] for the facile synthesis of N-doped graphene-like catalysts using e.g., urea [59], or polypyrrole [60] as doping agents. The thermal/annealing treatment during the synthesis converts the GO to reduced GO (rGO) and at least partly restore the desired sheet-like structure and physico-chemical properties of the electrocatalytically more attracting graphene.
Although thermal insulation and electric conductivity are uniquely combined in carbon aerogels, incorporation of carbon nanoparticles can considerably improve their electric properties. These particles can be also used as templating agents [61] and/or conductive additives [62]. Incorporation of 0.23 – 0.46 wt% well dispersed GO into the resorcinol – formaldehyde (RF) polymer xerogel precursor resulted in a carbon xerogel of high porosity and of excellent electrical conductivity: when used as electrode in aqueous supercapacitors at high current density, the capacitance and the power were enhanced by 25 and 100 %, respectively, compared of the undoped carbon xerogel [63]. It was also concluded that the electrical conductivity seems to play a more important role than the specific surface area in the electrode performance of mesoporous carbon xerogels. The optimum GO loading was below 2 wt% as higher loadings resulted in lower energy and power densities in spite of the increased electric conductivity [64]. In Li-ion batteries, graphene-doped carbon/carbon aerogel electrodes can accommodate lithium more easily than the common graphite anode [49]: in addition to the intercalation mechanism, they can exhibit fast lithium adsorption [23,65,66], defect trapping [67], build-up of faradaic capacitance [68], etc.
In spite of the intensive studies on the performance of electrodes fabricated from carbons doped with two heteroatoms or carbon nanoparticles, their simultaneous application is rare [69,70] and none of them addressed the influence of the nanoparticle concentration. Recently our group studied the oxygen reduction activity of a N, S co-doped metal free carbon synthesized from a ι-carreageenan – urea polymer cryogel precursor [71]. In this work we use the same matrix to synthesize GO-doped carbons with interconnected porous structure and high surface area to study the influence of the added GO on the morphology and chemistry of the carbon cryogel obtained. In RF based carbon xerogels developed for electric application the optimal GO content was found below 2 wt % [64]. Li et al [69] incorporated 4 wt% SWCNT, while the other group [70] added 13 wt% GO in the polymer precursor. Earlier we found that in responsive 3D poly(N-isopropylacrylamide) networks the percolation threshold was around 5 wt% related to the polymer matrix [72]. Based on these references the GO content was varied between 1.25 and 5 wt%. The texture of the carbon cryogels was characterized by electron microscopic imaging, N2 and CO2 adsorption measurements, Raman spectroscopy and powder X-ray diffraction (XRD). X-ray photoelectron spectroscopy (XPS), SEM supported electron dispersive spectroscopy (EDS), ultimate elemental analysis and FTIR were used to study the surface and bulk chemistry. The samples were probed as electrodes i) in a fuel cell under conditions, similar to alkaline anion exchange membrane fuel cells and ii) in Li-ion batteries in order to reveal the effect of the rGO in these applications.
2. Materials and methods
2.1. Synthesis
The rGO containing N and S double-doped carbon aerogels were synthesized according to the method of Li et al [11], using ι-carrageenan, urea (both from Sigma Aldrich) and aqueous GO suspension. The aqueous GO suspension (0.96 wt%) was prepared from natural graphite using an improved Hummers’ method [73,74]. Hydrogels were obtained by mixing 2 g urea and 2 g ι-carrageenan with 100 mL aqueous GO suspension (containing 50, 100 and 200 mg GO, respectively) at 80 °C. Thus, polymer gels with 1.25, 2.5, and 5 % GO were obtained. A GO-free gel was also prepared for comparison. The cooled hydrogels were freeze dried and pyrolyzed in a rotary quartz reactor at 700 °C (20 °C/min) in dry N2 flow (25 L/h) for 1 h. After washing with aq. 1.0 M HCl, the samples were annealed at 1000 ˚C in Ar flow for 1 h [71]. The resulting carbon aerogels are labeled as CA, CAGO50, CAGO100 and CAGO200.
2.2. Characterization methods
Scanning electron micrographs were taken by a Zeiss Sigma 300 FESEM field emission scanning electron microscope (Carl Zeiss QEC GmbH, Germany). Low temperature (-196.15 ˚C) nitrogen adsorption measurements were performed after 24 h degassing at 110 ˚C on a NOVA 2000e (Quantachrome, USA) automatic volumetric instrument. The apparent surface area SBET was determined using the Brunauer-Emmett-Teller (BET) model [75]. A pore volume V0.98 was estimated from the amount of vapor adsorbed at p/p0 = 0.98, assuming that the adsorbed gas fills the pores as liquid. The Dubinin-Radushkevich (DR) plot [76] was used to calculate the micropore volume Vmicro. The pore size distribution was computed by the Quenched Solid Density Functional Theory (QSDFT) for slit/cylindrical pore geometry [77]. Carbon dioxide adsorption was measured at 0 °C up to atmospheric pressure with an AUTOSORB-1 (Quantachrome, USA) computer-controlled analyzer. Evaluation of the primary adsorption data was performed with the Quantachrome ASiQwin software (version 3.0). Raman spectra were obtained using a LabRAM (Horiba Jobin Yvon) instrument. The laser source was a λ = 532 nm Nd-YAG (laser power at the focal point 15 mW). A 0.6 OD filter was used to reduce the power of the beam. Parameter optimization and data analysis were performed by a LabSpec 5 software. Powder X-ray diffractograms (XRD) were obtained in the range 2𝜃 = 10˚ - 130˚ with an X’Pert Pro MPD (PANalytical Bv., Almelo, The Netherlands) X-ray diffractometer using an X’celerator type detector and monochromatic Cu Kα radiation with a Ni filter foil (λ = 1.5406 Å).
The ultimate elemental analysis was performed in triplicates on an Elementar Vario Micro (CHNS) instrument. The lack of metal traces was confirmed with SEM/EDS (JEOL JSM 6380LA, Tokyo, Japan). X-ray photoelectron spectra were recorded on a Kratos XSAM 800 spectrometer operating in fixed analyzer transmission mode, using Mg Kα1,2 (1253.6 eV) excitation. The pressure of the analysis chamber was lower than 1⋅10-7 Pa. Survey spectra were recorded in the 150–1300 eV range in 0.5 eV steps. The photoelectron lines of C1s, O1s, N1s and S2p were measured in 0.1 eV steps with 1 s dwell time. The spectra were referenced to the energy of the C1s line of the sp2 type graphitic carbon, set at 284.3 ± 0.1 eV binding energy (BE). Peak decomposition was performed after Shirley-type background removal using Gaussian–Lorentzian peak shape with 70:30 ratio. Details of the applied fitting procedure are described elsewhere [78]. Quantitative analysis, based on integrated peak intensity, was performed by the XPS MultiQuant program [79], applying the conventional infinitely thick layer model using the experimentally determined photoionization cross-section data of Evans et al. [80] and the asymmetry parameters of Reilman et al. [81]. Attenuated total reflectance Fourier transform infrared spectroscopy (FTIR-ATR) were recorded on powdered carbons in the 4000 - 400 cm−1 wavelength range at a resolution of 4 cm-1 by 32 scans using a Tensor 27 (Bruker Optik GmbH, Leipzig, Germany) spectrophotometer equipped with a Platinum ATR unit A225. The crystal was made of diamond having refractive index of 2.4. For the background signal, the measured medium was air. Since absorption by the powders was very strong, a moderate polynomial baseline correction and smoothing were required.
2.3. Electrochemical performance tests
The electrical conductivity was estimated by a laboratory made instrument. The powdered samples were gradually compressed (0.5-5 MPa) in a rigid polytetrafluoroethylene (PTFE) tube (1 cm2 internal cross section) with two copper bolts (one on each side of the tube). The conductivity was estimated from the pressure dependence of the resistance [82]. The electrocatalytic ORR tests were performed using a glassy carbon (GC) rotating disc electrode (RDE, Pine Research Instrumentation, Durham, NC, USA). The ink for the working electrodes was prepared by dispersing 2 mg powdered annealed carbon (CA, CAGO50, CAGO100, or CAGO200) in a mixture of 1.6 mL MilliQ water, 0.4 mL isopropyl alcohol and 8 µL 5% Nafion® solution. After 30 min sonication, the ink was pipetted on to the dry mirror-polished GC and dried at room temperature. The loading varied between 50 and 400 μg/cm2. Measurements were implemented in 0.1 M KOH electrolyte using three-electrode systems with a hydrogen electrode as a reference and Pt wire as a counter electrode in a three-compartment PTFE cell [71]. All potentials are given vs. the reversible hydrogen electrode (RHE).
The working electrodes for the Li-ion storage studies were prepared by mixing the carbon samples and polyvinylidene fluoride binder (PVDF 99.9 %, Solvay) in a weight ratio of 95:5 in a ball mill for 1 hour. After adding an adequate amount of N-methyl-2-pyrrolidone (NMP) the slurry was cast on to a copper foil using an automatic film applicator (BYK Gardner GmbH, Germany). 14 mm diameter circular discs were cut from the dried film (70 °C in a vacuum oven, overnight). CR2032 coin-type cells were assembled in an argon-filled glove box using the as-prepared coated disc and Li metal disc as working and counter electrode, respectively, Whatman glass fiber as the separator, and 1.0 M lithium hexafluorophosphate (LiPF6) in ethylene carbonate and diethyl carbonate (EC: DEC 1:1 vol %) as electrolyte and employing a manual crimping machine (MTI MSK 110). The electrochemical performance of the half-cell was performed using an electrochemical workstation (Biologic VMP 300) at room temperature. The galvanostatic charge – discharge cycling of the half-cell was done in a potential window of 0-3 V (vs. Li+/Li).
3. Results and Discussion
3.1. Development of the morphology during the annealing
The FESEM images demonstrate the complexity of the pore structure of the annealed carbon gels (Figure 1). The early inclusion of GO already in the precursor suspension influences the morphology developing during gelation and cryogenic drying and is reflected also in the final annealed state (Figure 1c). It is apparent that the reduced GO formed during the heat treatments is evenly distributed in the carbon matrix. The typical nanopores within the CA matrix are of 2-3 nm (Figure S1). The yield of the synthesis steps is reported in Table S1.
The low temperature (-196 ˚C) N2 adsorption isotherms of the annealed carbon samples having various GO content are shown in Figure 2a. All the isotherms are of composite Type IV + Type II with an H4 hysteresis loop displaying a sharp step at p/p0 = 0.45 [83]. The shape and type of the hysteresis loops suggest an interconnected pore network of micro-, meso- and macropores. Numerical data deduced from the measured isotherms are presented in Table 1. Since the macropores are not totally filled with condensed nitrogen, the liquid equivalent volume V0.98 was determined at p/p0 = 0.98.
The effect of the GO is most spectacular in the micropore region of the isotherm. Incorporation of GO increases not only the carbon content of the polymer matrix, but also the volatile products that are released during the thermal treatments. The 1.25 - 2.5 % GO (samples CAGO50 and CAGO100) increased the pore volumes and the apparent surface area almost proportionally, while addition of 5 % GO (CAGO200) resulted in a drop both in surface area and pore volume. The ultra-micropore region was revealed by CO2 adsorption measurements performed at 0 °C. The combined pore size distribution curves in Figure 2b indicate the presence of ultra-micropores as well as significant micro- and narrow mesoporosity in the range 1.7 – 6 nm. The increasing amount of oxidative volatile gases evolving during the thermal decomposition of the GO may be responsible for the decreasing trend in ultra-microporosity.
The Raman spectra in Figure S2 exhibit the iconic D (~1350 cm-1; defects, edges, and disordered carbon sites) and G (~1580 cm-1; vibration of sp2-hybridized graphitic carbon) band regions typical of carbon materials [84]. The first and second order regions of the spectra were deconvoluted for further analysis (Figure S2b, Tables S2 and S3 [85,86,87,88]). The D band (around 1350 cm-1) indicates the aromatic clusters with edges/borders of mainly amorphous carbon (sp3 -bonded) structures [89]. The blue shift of the G band may be caused by an internal stress developing when the incorporated GO and/or newly developing clusters collide during the annealing treatment at 1000 °C [89,90]. The D' band, associated with disordered graphenic lattices (around 1620 cm-1) appeared only in the GO-free CA sample. On the other hand, the second order region was detected only in the GO doped samples. The trend in the ID/IG ratio along with the relative sharpening of the G band and the reduced intensity D” in the GO doped samples confirms an ordering effect of the added GO in the carbon matrix [86].
3.2. Surface chemistry of the samples
The effect of GO on the surface composition was studied by XPS (Table 2). The successful removal of the metal impurities was confirmed by SEM supported ED spectroscopy (Figure S3). As expected, the O content of the incorporated GO affects not only the porous texture but also the surface chemistry of the annealed samples. The complexity of the thermal treatments explains the change in the overall C or O content and the trend of the heteroatom/carbon ratios.
The composite photoelectron lines (C1s, O1s, N1s and S2p) were decomposed into different chemical states. The self-oxidation alters the surface composition as well as the chemical environment. Due to the complicated structure of the samples, however, the chemical states cannot be resolved exactly. The suggested states refer to a broad range of chemical bonds. The shape of the C1 (sp2 C) component is asymmetric, as in pure and low heteroatom-containing graphite and graphene. The tail of the asymmetric peak overlaps with the peaks of the C–O(N) components; using this line-shape also gives satisfactory oxygen + nitrogen balance (the ratio of the measured and calculated concentrations) for all samples.
Figure 3 presents a typical decomposition scheme of the C1s, O1s, N1s and S2p spectral regions, while Table 3 and Table 4 show the quantitative results of the decomposition, together with the binding energy ranges of the components and their assignations to various chemical states.
The decomposition of the C1s signal revealed three different carbon species. The most abundant is the sp2 form (C1). The presence of the π-π* shake-up satellite also confirms the assignation of this component. The concentration of C1 is slightly higher in the GO-doped samples. The other components can be assigned to carbon atoms connected to one (C2) or two (C3) heteroatoms.
The O1 and O2 oxygen components are found in all samples; they can be assigned to S–O and to various C–O bonds, respectively. The O3 component, present in the high GO-containing samples, can be connected to either highly oxidized carbon (anhydride, carbonate). Owing to their low concentration, the corresponding carbon species cannot be resolved in the spectra. Nitrogen is also present in three chemical states. N1 is the simple C–N bond, while N2 is bonded to carbon with further heteroatoms. The quantities of these nitrogens are commensurable. The low concentration, high binding energy N3 component is a kind of quaternary ammonium. Due to the synthesis procedure, it is assumed that the nitrogen atoms are homogeneously distributed in the matrix and also decorate the walls of the micropores and ultra-micropores [91]. The sulfur content, present as C–S sulfide (S1) and in oxidized forms (S2; sulfone, sulfate), is the least affected by the treatments, but it should be noted that all the sulfur in the CAGO50 sample is sulfidic, while in the others the contribution of the S2 forms reaches 20-30 %.
The FTIR measurements give complementary information about the chemical structures of the samples with diverse GO content (Figure S4, Table 4). From the rich spectra three main regions were considered to monitor the chemical changes in the bulk [92,93]. All spectra display a strong adsorption band at 1554 cm-1, assigned to the –C=C– skeletal vibration of aromatic regions. The 1749 cm-1 band of the GO-loaded samples implies the presence of carbonyl groups (–C=O), stemming from aldehydes, ketones, esters and carboxylic acids. The band at 1372 cm-1, which is absent in the starting material, is ascribed to the –OH in plane bending of phenols. If we compare the amount of the latter two signals to the corresponding carbon skeleton peak at 1554 cm-1, we can establish that the ratio of oxygen-containing groups increases with increasing GO content.
In summary, addition of GO to the carrageenan – urea carbon xerogel results in multifaceted modification of the textural and chemical properties of the samples. The thermal decomposition of GO enhances self-activation and facilitates the formation of micropores which, for the CAGO50 and CAGO100 samples, also result in a considerable increase in the apparent surface area. With 5 wt% GO probably too much oxygen was introduced to the matrix which destroyed the finer porosity. The annealing at 1000 °C certainly removed part of the heteroatoms but left behind a high number of defects. It is expected that these new active sites (N-S-C) “reconstructed” from edged thiophene S, graphitic N, and pentagon defects and the 3D porous architecture result in an outstanding electrocatalytic activity [11]. There is a significant difference between the bulk and surface compositions: the population of the heteroatoms is much richer in the bulk. They are present in the expected chemical forms in the surface of all the samples.
3.3. Effect of GO on ORR performance
Likewise other groups we also found that incorporation of GO improved the electric conductivity of the cryogels [64,94]. In our samples the improvement was proportional to the amount of GO added, in spite of the lower apparent surface area of CAGO200 (Figure S5).
The ORR performance of the GO doped samples was investigated under conditions similar to alkaline anion exchange membrane fuel cells. The linear sweep voltammograms (LSVs) of the four samples are plotted in Figure 4. The high potential regions reveal not only the slight differences among the various samples but also their alteration during the consecutive cycles. CAGO200 was found to be particularly unstable. The addition of GO gradually shifted the onset potential. CAGO50 exhibited the highest half-wave potential, which gradually decreased back to the value observed with the GO-free CA carbon. In Table 5 we compare the onset and half-wave potentials (E1/2) of these curves with the results of other groups.
The Koutecky-Levich (KL) equation [103] was used to estimate the correlation between the current density j and the rotation rate ω
where jk is the kinetic current density, jlim is the limiting diffusion current density, n is the number of electrons transferred in ORR per oxygen molecule, F is the Faraday constant, D is the diffusion coefficient of oxygen in the electrolyte, ν is the kinematic viscosity of the electrolyte, C is the concentration of oxygen in the electrolyte. KL plots of CAGO50 (Figure 5) display a slope closest to the theoretical curve corresponding to the 4 e- mechanism, i.e., incorporation of 1.25% GO improved the performance, whereas further added GO has the opposite impact. At higher GO loadings the lines become steeper, nearer to the theoretical 2 e- slope. Based on these findings from our samples CAGO50 seems to have the optimum pore texture and chemistry for ORR under the investigated conditions. Although the electrical conductivity increased linearly with increasing GO loading, no similar change in ORR by adding GO is apparent. This agrees well with the work by Ramos-Fernández et al. who also observed that increasing GO content improved the electric conductivity of carbon xerogels. They also observed that enhanced porosity may spoil the electrochemical performance by disrupting the GO network throughout the carbon matrix [64].
The (in)stability of the electrodes was further explored with CV responses measured after a series of treatments simulating application conditions. Based on the KL evaluation the GO-free CA and the CAGO50 and CAGO200, showing the lowest and highest slopes were selected for stability studies. The fresh electrodes made from the carbons, after running a CV cycle (curve 1) were exposed to further 50 cycles in argon treated oxygen-free KOH electrolyte and then another 50 cycles in oxygen-saturated KOH, finally an ORR test was run (sweep rate: 5 mV/s, rotation rates: 400, 625, 900, 1250 rpm). The voltammograms, curves 2, 3 and 4, obtained after each set of treatment are compared in Figure 6. (The effect of the electrode loading is discussed in the Supplement.) The slightly distorted rectangular shapes may stem from the pseudo-faradaic reactions of the corresponding functional groups [104]. The width of the hysteresis is not correlated with the BET surface area (Table 1), but the presence of the GO apparently reduces the gravimetric capacitance. The coincidence of curves 1 and 2 in CA carbon (Figure 6) reveal that this sample is stable under oxygen-free conditions. After an additional 50 cycles in oxygen-saturated electrolyte (curve 3) the widening of the CV curve implies that the electrocatalytically active surface area increases in the presence of oxygen. The consecutive four ORR cycles further modify the electrode surface (curve 4). The performance of the CAGO50 carbon is very similar, however, it seems somehow even less stable under oxygenic conditions. The CAGO200 electrode was instable already in oxygen-free conditions (curves 1 and 2). The change in the surface was even more expressed after being cycled in oxygen-saturated electrolyte, which apparently stabilized the surface: no further changes were seen after these ORR cycles (curve 4). These observations indicate that the electron transfer mechanism deduced from the KL plot can only be considered as an estimation. The kinetics of the reduction reaction in the presence of the heteroatoms is indeed more sophisticated, and further influenced by the rGO. The chemical species locally evolving in the ORR may initiate various processes including restructuring, activation, degradation, etc. of the electrode surface. The plots in Figure 6 clearly show that even if the electrode has been stabilized under oxygen-free conditions, later may undergo destabilization processes during the ORR. Nevertheless, the widening of the CV curves mark an increase in the electrocatalytically active surface area. The deviation of the corresponding curves confirms that addition of GO to the precursor affect the stability of the electrodes. As expected from the KL plot, the CAGO200 sample is more sensitive to the oxidative conditions, but after stabilization this sample exhibited the highest capacitance. The trend of the gravimetric capacitance in this figure is different from the trend expected from the apparent surface area (1070, 1479 and 933 m2/g for CA, CAGO50 and CAGO200, respectively) implying that the accessibility of the nitrogen molecules is different from that of the species involved in the electrocatalytic processes [105].
3.4. Effect of GO on Li-ion battery application
In order to reveal the eff+ect of GO on the performance of the carbon xerogel as anode material in Li-ion batteries galvanostatic charge – discharge cycling tests were performed. In the half-cell setup the CA, CAGO50, CAGO100, or CAGO200 sample was the working electrode and the Li metal disc served as the counter and reference electrode, i.e., no further conductive agent was used. In the galvanostatic test a constant current I is applied to the cell and the potential is evaluated as a function of time t. The specific capacity Cs during complete charge – discharge is given by
Cs = I⋅t / m
The nature of the charge – discharge plots largely depends on the surface chemistry (microstructure, porosity, heteroatom content, etc.) and the adsorption capacity of the carbon host. Figure 7 shows the cell voltage vs. specific capacity charge – discharge cycling plots of the samples under investigation. The cyclability of the materials is presented here as the total charge – discharge capacity (or capacity retention) as a function of the number of cycles (Figure 8).
Figure 7 shows the cell voltage vs. specific capacity charge – discharge cycling plots of the samples under investigation. While highly ordered graphitized carbon anodes show a steep charge – discharge curve [106], sloping shapes similar to Figure 8 are distinctive for amorphous biomass-derived carbon anodes in Li-ion battery applications [25,107,108]. The broad peaks in the XRD patterns (Figure S7) reveal that the carbon cryogels studied in this work are typically amorphous, therefore charge – discharge curves with this shape expected, i.e., the CA, CAGO50, CAGO100, and CAGO200 have the potential to store the Li-ions gradually and consistently over their operating potential. A comparable charge – discharge plateau was measured also on RF polymer based carbon xerogels [109]. The latter microporous carbon xerogels were successfully tested as anode material in lithium-ion cells exhibiting a specific capacity 288 mAh/g and retaining 97% of the initial capacity after 100 charge–discharge cycles.
The cyclability of the carbons is presented here as the total charge – discharge capacity (or capacity retention) as a function of the number of cycles (Figure 9). The undoped carbon, CA shows an initial discharge capacity 332 mAh/g at a constant current density 100 mA/g. The cell shows good capacity retention and high coulombic efficiency (92.9% and 99.7%, respectively) even after 50 charge – discharge cycles.
For comparison, 218 mAh/g at a current density 0.372 A/g was reported by Wang et al. [6]. Among the GO doped carbons CAGO50 exhibits the best performance in terms of cycling stability. The cell shows an initial discharge capacity of 313 mAh/g and a highly reversible capacity of 297 mAh/g after 50 cycles, corresponding to 95.1 % capacity retention. This good stability agrees well with the impedance analysis results: this carbon has the lowest impedance. The enhanced performance of CAGO50 compared to pristine CA can be explained in terms of the surface area and porosity [110]. The higher surface area of CAGO50 improves the electrolyte accessibility and ion diffusion in the pores of the electrode, which in turn results in higher coulombic efficiency and cell stability [22,110]. The charge – discharge plateau recorded in the 1st and 50th cycle is almost identical confirming the resilient stability of the electrode [111]. Following this, CAGO200 showed an initial discharge capacity of 240 mAh/g and a capacity retention of 93.6% after 50 cycles (Figure 8).
The overall electrochemical performance of CAGO200 in the Li-ion battery tests was better than undoped CA and slightly lower than CAGO50. CAGO100 presented the poorest electrochemical performance in spite of having the largest surface area. This apparent surface area, however, is also combined with a larger pore volume in the wider pore size region, which seems to decrease the initial coulombic efficiency and reduces the reversible capacity of the battery [22]. The initial discharge capacity of CAGO100 is 255 mAh/g, but the cell poorly maintained its stability. The low retention capacity (67.2% after 50 cycles) might be due to a kinetic limitation in the porous electrode [110]. The compatibility of the pore and the Li+ ion sizes is also responsible for the charge capacity. CAGO100 and CAGO200 showed the highest and lowest pore volume, respectively, both in the micro- and mesopore regions. On the one hand wider pores may not be completely filled by the Li+ ions, and on the other, low porosity limits the entrance of Li+ containing electrolyte into the pores. CAGO50, which seems to have the optimum pore size compatible with Li+ and is capable to effectively accommodate the Li+ ions, exhibits the highest overall capacity among the GO doped samples.
As shown in Figure 8, the coulombic efficiency of all the samples was consistently maintained above 98.5 % indicating the efficient Li-ion insertion and reinsertion potential of these carbons during the charge – discharge process [111]. CA and CAGO100 displayed lower coulombic efficiency than CAGO50 and CAGO200 (>99.3 %) indicating that addition of GO is clearly beneficial for more stable electrochemical performance in Li ion batteries, but the GO doping level must be carefully tuned.
It may be concluded that the carbons investigated here can be utilized as potential anodes in Li-ion batteries. According to our results the pore structure and the chemical composition corresponding to CAGO50 proved to be the best to achieve an acceptably high reversible discharge capacity and electrode stability under prolonged cycling. The electrochemical performance of these carbon electrodes proved to be comparable to the results previously reported on carbon electrode anode in Li-ion batteries [112,113,114,115,116].
Conclusions
Addition of GO successfully increases the surface area and the porosity of the carbon material up to 2.5% (up to SBET 1780 m2/g and V0.98 1.7 cm3/g), but 5% is destroyed the finer pores. The self-activating effect of the GO influences the surface chemistry in a complex way, but sufficient O, N, and S heteroatoms were preserved in various chemical forms. The highest concentration of sp2 carbons was detected on the surface of sample CAGO50 (1.25 wt% GO). Although the volatile thermal degradation products of GO affected the porous texture and the surface chemistry in a subtle way, the residual rGO results in monotonically increasing electrical conductivity. GO affected the electron transfer mechanism and challenged the stability of the electrodes in ORR tests. TheCAGO50 sample displayed the advantageous 4e- mechanism. The CAGO200 (5 wt% GO) sample was the most sensitive under oxidative conditions, but after conditioning it exhibited the highest capacitance in ORR. In the Li-ion battery tests the coulombic efficiency of all the samples was consistently above 98%. The cell made from CAGO50 showed the lowest impedance, an initial discharge capacity of 313 mAh/g, 95.1 % capacity retention and 99.3 % coulombic efficiency after 50 charge – discharge cycles. Despite having the largest surface area sample CAGO100 performed more poorly than CAGO50 in both application tests. This is a clear indication that concerted fine-tuning of the pore morphology and surface chemistry is required for both the advanced electrocatalytic and electrochemical performance. The as-prepared GO-doped carbon samples with their ultra-micropores and high surface area performed well and can be a promising electrode material for both applications.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Acknowledgments
We extend our warm thanks to A. Farkas and J. Madarász for their invaluable assistance in Raman spectroscopy and XRD, as well as to G. Bosznai for his technical assistance. We are thankful to B. Pinke and A. Bulátkó for their help with SEM-EDS measurements. Financial support from the Hungarian Scientific Research Funds OTKA K128410 and K 143571 is acknowledged. The research is part of project no. BME-NVA-02, implemented with the support of the Ministry of Innovation and Technology of Hungary from the National Research, Development and Innovation Fund, and financed under the TKP2021 funding scheme. SKSA is grateful to the Stipendium Hungaricum scholarship program of the Hungarian Government. SSL and RK thank the financial support of project no. RRF-2.3.1-21-2022-00009, titled National Laboratory for Renewable Energy, implemented with support from the Recovery and Resilience Facility of the European Union within the framework of Programme Széchenyi Plan Plus.
References
- P. Chen, L.K. Wang, G. Wang, M.R. Gao, J. Ge, W.J. Yuan, Y.H. Shen, A.J. Xie, S.H. Yu. Nitrogen-doped nanoporous carbon nanosheets derived from plant biomass: an efficient catalyst for oxygen reduction reaction. Energy Environ. Sci., 2014, 7 (12), 4095–4103. [CrossRef]
- J. Zhao, Y. Liu, X. Quan, S. Chen, H. Yu, H. Zhao. Nitrogen-doped carbon with high degree of graphitization derived from biomass as high-performance electrocatalyst for oxygen reduction reaction. Appl. Surf. Sci. 2017, 396, 986–993. [CrossRef]
- Q. Zhao, Q. Ma, F. Pan, Z. Wang, B. Yang, J. Zhang, J. Zhang. Facile synthesis of nitrogen-doped carbon nanosheets as metal-free catalysts with excellent oxygen reduction performance in alkaline and acidic media. J Solid State Electrochem., 2016, 20, 1469-1479. [CrossRef]
- P. Cheng, T. Li, H. Yu, L. Zhi, Z. Liu, Z. Lei. Biomass-derived carbon fiber aerogel as a binder-free electrode for high-rate supercapacitors. J. Mater. Chem. C, 2016, 120 (4), 2079–2086. [CrossRef]
- ZT You, L Zhao, KH Zhao, HX Liao, SJ Wen, YH Xiao, BC Cheng, SJ Lei. Highly tunable three-dimensional porous carbon produced from tea seed meal crop by-products for high performance supercapacitors. Appl. Surf. Sci. 2023, 607, 155080.
- L. Wang, Z. Schnepp, M.M. Titirici. Rice husk-derived carbon anodes for lithium-ion batteries. J. Mater. Chem. A, 2013, 1 (17), 5269-5273. [CrossRef]
- T. Liu, X. Li. Biomass-derived nanostructured porous carbons for sodium ion batteries: a review. Mater. Technol., 2018, 34 (4), 232–245. [CrossRef]
- H. Li, Z. Cheng, Q. Zhang, A. Natan, Y. Yang, D. Cao, H. Zhu. Bacterial-derived, compressible, and hierarchical porous carbon for high-performance potassium-ion batteries. Nano Lett., 2018, 18 (11), 7407–7413. [CrossRef]
- Z. Sebestyén, E. Jakab, A. Domán, P. Bokrossy, I. Bertóti, J. Madarász, K. László. Thermal degradation of crab shell biomass, a nitrogen-containing carbon precursor. J. Therm. Anal. Calorim., 2020, 142, 301-308. [CrossRef]
- K.M. Zia, S. Tabasum, M. Nasif, N. Sultan, N. Aslam, A. Noreen, M. Zuber. A review on synthesis, properties and applications of natural polymer based carrageenan blends and composites. Int. J. Biol. Macromol., 2017, 96, 282-301. [CrossRef]
- D. Li, Y. Jia, G. Chang, J. Chen, H. Liu, J. Wang, Y. Hu, Y. Xia, D. Yang, X. Yao. A defect-driven metal-free electrocatalyst for oxygen reduction in acidic electrolyte. Chem, 2018, 4, 2345–2356. [CrossRef]
- J. Hou, C. Cao, F. Idrees, X. Ma. Hierarchical porous nitrogen-doped carbon nanosheets derived from silk for ultrahigh-capacity battery anodes and supercapacitors. ACS Nano, 2015, 9 (3), 2556–2564. [CrossRef]
- D.W. Wang, F. Li, M. Liu, G.Q. Lu, H.M. Cheng. 3D aperiodic hierarchical porous graphitic carbon material for high-rate electrochemical capacitive energy storage. Angew. Chem. Int. Ed., 2007, 47 (2), 373–376. [CrossRef]
- Gabe, R. Ruiz-Rosas, C. González-Gaitán, E. Morallón, D. Cazorla-Amorós. Modeling of oxygen reduction reaction in porous carbon materials in alkaline medium. Effect of microporosity. J. Power Sources, 2019, 412, 451-464. [CrossRef]
- Y. Liu, K. Li, B. Ge, L. Pu, Z. Liu. Influence of micropore and mesoporous in activated carbon air-cathode catalysts on oxygen reduction reaction in microbial fuel cells. Electrochim. Acta, 2016, 214, 110-118. [CrossRef]
- J. Cao, C. Zhu, Y. Aoki, H. Habazaki. Starch-derived hierarchical porous carbon with controlled porosity for high performance supercapacitors. ACS Sustainable Chem. Eng., 2018, 6 (6), 7292-7303. [CrossRef]
- T.J. Bandosz. Revealing the impact of small pores in oxygen reduction on carbon electrocatalysts: A journey through recent findings. Carbon, 2022, 188, 289-304. [CrossRef]
- M. Huang, S.J. Yoo, J.S. Lee, T.H. Yoon. Electrochemical properties of an activated carbon xerogel monolith from resorcinol-formaldehyde for supercapacitor electrode applications. RSC Adv., 2021, 11, 33192-33201. [CrossRef]
- X. Feng, Y. Bai, M. Liu, Y. Li, H. Yang, X. Wang, C. Wu. Untangling the respective effects of heteroatom-doped carbon materials in batteries, supercapacitors and the ORR to design high performance materials. Energy Environ. Sci., 2021, 14, 2036-2089. [CrossRef]
- Y. Zhai, Y. Dou, D. Zhao, P.F. Fulvio, R.T. Mayes, S. Dai. Carbon materials for chemical capacitive energy storage. Adv. Mater., 2011, 23, (42), 4828-4850. [CrossRef]
- W. Tang, Y. Zhang, Y. Zhong, T. Shen, X. Wang, X. Xia, J. Tu. Natural biomass-derived carbons for electrochemical energy storage. Mater. Res. Bull., 2017, 88, 234–241. [CrossRef]
- Z. Zhu, Z. Xu. The rational design of biomass-derived carbon materials towards next-generation energy storage: A review. Renewable and Sustainable Energy Reviews, 2020, 134, 110308. [CrossRef]
- J.R. Dahn, T. Zheng, Y.H. Liu, J.S. Xue. Mechanisms for lithium insertion in carbonaceous materials. Science, 1995, 270, 590−593. [CrossRef]
- S. Sun, Q. Yan, M. Wu, X. Zhao. Carbon aerogel based materials for secondary batteries. Sustain. Mater. Technol., 2021, 30, e00342. [CrossRef]
- D. Li, Y. Wang, Y. Sun, Y. Lu, S. Chen, B. Wang, H. Zhang, Y. Xia, D. Yang. Turning gelidium amansii residue into nitrogen-doped carbon nanofiber aerogel for enhanced multiple energy storage. Carbon, 2018, 137, 31–40. [CrossRef]
- Q.Q. Xiong, Z.G. Ji. Controllable growth of MoS2/C flower-like microspheres with enhanced electrochemical performance for lithium ion batteries. J Alloys Compd., 2016, 673, 215–219. [CrossRef]
- M. Enterría, J.L. Figueiredo. Nanostructured mesoporous carbons: Tuning texture and surface chemistry. Carbon, 2016, 108, 79-102. [CrossRef]
- J. Zhang, J. Zhang, F. He, Y. Chen, J. Zhu, D. Wang, M. Shichun, H.Y. Yang. Defect and doping co-engineered non-metal nanocarbon ORR electrocatalyst. Nano-Micro Lett., 2021, 13, 65. [CrossRef]
- X Wang, LW Yang, R Li, YX Chen, ZG Wu, BH Zhong, XD Guo. Heteroatom-doped Ginkgo Folium porous carbon modified separator for high-capacity and long-cycle lithium-sulfur batteries. Appl. Surf. Sci. 2022, 602, 154342.
- B Yan, L. Feng, JJ Zheng, Q. Zhang, YZ Dong, YC Ding, WS Yang, JQ Han, SH Jiang, SJ He. Nitrogen-doped carbon layer on cellulose derived free-standing carbon paper for high-rate supercapacitors. Appl. Surf. Sci. 2023, 608, 155144.
- Z. Duan, G. Henkelman. Identification of active sites of pure and nitrogen-doped carbon materials for oxygen reduction reaction using constant-potential calculations. J. Phys. Chem. C., 2020, 124 (22), 12016-12023. [CrossRef]
- B. Nagy, S. Villar-Rodil, J.M.D. Tascón, I. Bakos, K. László. Nitrogen doped mesoporous carbon aerogels and implications for electrocatalytic oxygen reduction reactions. Microporous and Mesoporous Mater., 2016, 230, 135-144. [CrossRef]
- D. Guo, R. Shibuya, C. Akiba, S. Saji, T. Kondo, J. Nakamura. Active sites of nitrogen-doped carbon materials for oxygen reduction reaction clarified using model catalysts. Science, 2016, 351 (6271), 361–365. [CrossRef]
- Dewang Kong, Wenjing Yuan, Cun Li, Jiming Song, Anjian Xie, Yuhua Shen: Synergistic effect of nitrogen-doped hierarchical porous carbon/graphene with enhanced catalytic performance for oxygen reduction reaction. Appl. Surf. Sci., 2017, 393, 144-150. https://doi.org/10.1016/j.apsusc.2016.10.019.
- K. Lee, L. Shabnam, S.N. Faisal, V.C. Hoang, V.G. Gomes. Aerogel from fruit biowaste produces ultracapacitors with high energy density and stability. J. Energy Storage, 2020, 27, 101152. [CrossRef]
- Gopalakrishnan, S. Badhulika. Effect of self-doped heteroatoms on the performance of biomass-derived carbon for supercapacitor applications. J. Power Sources, 2020, 480, 228830. [CrossRef]
- Donglin He , Wang Zhao, Ping Li, Zhiwei Liu, Haoyang Wu, Luan Liu, Kun Han, Lang Liu, Qi Wan, Faheem K. Butt, Xuanhui Qu: Bifunctional biomass-derived 3D nitrogen-doped porous carbon for oxygen reduction reaction and solid-state supercapacitor. Appl. Surf. Sci., 2019, 465, 303-312.
- HQ Gao, D Zhang, HT Zhou, JC Wu, GJ Xu, ZL Huang, MH Liu, JH Yang, D Chen. Boosting gravimetric and volumetric energy density of supercapacitors by 3D pomegranate-like porous carbon structure design. Appl. Surf. Sci. 2020, 534, 147613.
- S. Ito, T. Murata, M. Hasegawa, Y. Bito, Y. Toyoguchi. Study on CxN and CxS with disordered carbon structure as the anode materials for secondary lithium batteries. J. Power Sources, 1997, 68 (2), 245-248. [CrossRef]
- M. Seredych, K. László, E. Rodríguez-Castellón, T.J. Bandosz. S-doped carbon aerogels/GO composites as oxygen reduction catalysts. J. Energy Chem., 2016, 25 (2), 236-245. [CrossRef]
- J. Liang, Y. Jiao, M. Jaroniec, S.Z. Qiao. Sulfur and nitrogen dual-doped mesoporous graphene electrocatalyst for oxygen reduction with synergistically enhanced performance. Angew. Chem. Int. Ed., 2012, 51 (46), 11496-11500. [CrossRef]
- M. Jiang, X. Yu, H. Yang, S. Chen. Optimization strategies of preparation of biomass-derived carbon electrocatalyst for boosting oxygen reduction reaction: A minireview. Catalysts, 2020, 10, 1472. [CrossRef]
- Y. Zhao, Y. Liu, Y. Chen, X. Liu, X. Li, S. Gao. A treasure map for nonmetallic catalysts: optimal nitrogen and fluorine distribution of biomass-derived carbon materials for high-performance oxygen reduction catalysts. J. Mater. Chem. A, 2021, 9, 18251. [CrossRef]
- Shenghui Jiao, Yutong Yao, Junliu Zhang, Liqiong Zhang, Changwei Li, Huixin Zhang, Xin Zhao, Honglei Chen, Jianchun Jiang: Nano-flower-like porous carbon derived from soybean straw for efficient N-S co-doped supercapacitors by coupling in-situ heteroatom doping with green activation method. Appl. Surf. Sci. 2023, 615, 156365.
- H. Jin, X. Wang, Z. Gu, J. Polin. Carbon materials from high ash biochar for supercapacitor and improvement of capacitance with HNO3 surface oxidation. J. Power Sources, 2013, 236, 285–292. [CrossRef]
- F. Chen, J. Yang, T. Bai, B. Long, X. Zhou. Biomass waste-derived honeycomb-like nitrogen and oxygen dual-doped porous carbon for high performance lithium-sulfur batteries. Electrochim. Acta, 2016, 192, 99–109. [CrossRef]
- Y. Qiu, J. Huo, F. Jia, B.H. Shanks, W. Li. N- and S-doped mesoporous carbon as metal-free cathode catalysts for direct bio renewable alcohol fuel cells. Mater. Chem. A, 2016, 4, 83–95. [CrossRef]
- J-H. Lee, S-J. Park. Recent advances in preparations and applications of carbon aerogels: A review. Carbon, 2020, 163, 1-18. [CrossRef]
- M. Bakierska, M. Lis, J. Pacek, M. Świętosławski, M. Gajewska, A. Tąta, E. Proniewicz, M. Molenda. Bio-derived carbon nanostructures for high-performance lithium-ion batteries. Carbon, 2019, 145, 426–432. [CrossRef]
- B. Jaleh, M. Nasrollahzadeh, M. Eslamipanah, A. Nasri, E. Shabanlou, N.R. Manwar, R. Zboril, P. Fornasiero, M.B. Gawande. The Role of Carbon-Based Materials for Fuel Cells Performance. Carbon, 2022, 198, 301–352. [CrossRef]
- H. Song, R. Na, C. Hong, G. Zhang, X. Li, Y. Kang, Q. Zhang, H. Xie. In situ measurement and mechanism analysis of the lithium storage behavior of graphene electrodes. Carbon, 2022, 188, 146–154. [CrossRef]
- L. Li, D. Zhang, J. Deng, Y. Gou, J. Fang, H. Cui, Y. Zhao, M. Cao. Carbon-based materials for fast charging lithium-ion batteries. Carbon, 2021, 183, 721–734. [CrossRef]
- S. Wu, H. Wu, M. Zou, X. Shi, Y. Yuan, W. Bai, A. Cao. Short-range ordered graphitized-carbon nanotubes with large cavity as high-performance lithium-ion battery anodes. Carbon, 2020, 158, 642–650. [CrossRef]
- J.L. Gómez-Urbano, G. Moreno-Fernández, M. Arnaiz, J. Ajuria, T. Rojo, D. Carriazo. Graphene-coffee waste derived carbon composites as electrodes for optimized lithium ion capacitors. Carbon, 2020, 162, 273–282. [CrossRef]
- T. Zhang, F. Zhang, L. Zhang, Y. Lu, Y. Zhang, X. Yang, Y. Ma, Y. Huang. High energy density Li-ion capacitor assembled with all graphene-based electrodes. Carbon, 2015, 92, 106–118. [CrossRef]
- M.B. Lim, M. Hu, S. Manandhar, A. Sakshaug, A. Strong, L. Riley, P.J. Pauzauskie. Ultrafast sol–gel synthesis of graphene aerogel materials. Carbon, 2015, 95, 616–624. [CrossRef]
- Y. Wu, J. Zhu, L. Huang. A review of three-dimensional graphene-based materials: Synthesis and applications to energy conversion/storage and environment. Carbon, 2019, 143, 610–640. [CrossRef]
- S. Yang, L. Zhi, K. Tang, X. Feng, J. Maier, K. Mullen. Efficient synthesis of heteroatom (N or S)-doped graphene based on ultrathin graphene oxide-porous silica sheets for oxygen reduction reaction. Adv. Funct. Mater., 2012, 22 (17), 3634-3640. [CrossRef]
- Z. Lin, G. Waller, Y. Liu, M. Liu, C.P. Wong. Facile synthesis of nitrogen-doped graphene via pyrolysis of graphene oxide and urea, and its electrocatalytic activity toward the oxygen-reduction reaction. Adv. Energy Mater., 2012, 2 (7), 884-888. [CrossRef]
- Z. Lin, G.H. Waller, Y. Liu, M. Liu, C.P. Wong. 3D nitrogen-doped graphene prepared by pyrolysis of graphene oxide with polypyrrole for electrocatalysis of oxygen reduction reaction. Nano Energy, 2013, 2, 241-248. [CrossRef]
- M. Enterría, F.J. Martí-Jimeno, F. Suárez-García, J.I. Paredes, M.F.R. Pereira, J.I. Martins, A. Martínez-Alonso, J.M.D. Tascón, J.L. Figueiredo. Effect of nanostructure on the supercapacitor performance of activated carbon xerogels obtained from hydrothermally carbonized glucose-graphene oxide hybrids. Carbon, 2016, 105, 474-483. [CrossRef]
- F.J. Martín-Jimeno, F. Suárez-García, J.I. Paredes, A. Martínez-Alonso, J.M.D. Tascón. Activated carbon xerogels with cellular morphology derived from hydrothermally carbonized glucose-graphene oxide hybrids and their performance towards CO2 and dye adsorption. Carbon, 2015, 81, 137-147. [CrossRef]
- M. Canal-Rodríguez, A. Arenillas, N. Rey-Raap, G. Ramos-Fernández, I. Martín-Gullón, J.A. Menéndez. Graphene-doped carbon xerogel combining high electrical conductivity and surface area for optimized aqueous supercapacitors. Carbon, 2017, 118, 291-298. [CrossRef]
- G. Ramos-Fernández, M. Canal-Rodríguez, A. Arenillas, J.A. Menéndez, I. Rodríguez-Pastor, I. Martín-Gullon. Determinant influence of the electrical conductivity versus surface area on the performance of graphene oxide-doped carbon xerogel supercapacitors. Carbon, 2018, 126, 456-463. [CrossRef]
- K. Sato, M. Noguchi, A. Demachi, N. Oki, M. Endo. A mechanism of lithium storage in disordered carbons. Science. 1994, 264, 556−558. [CrossRef]
- J.S. Xue, J.R. Dahn. Dramatic effect of oxidation on lithium insertion in carbons made from epoxy resin. J. Electrochem. Soc. 1995, 142, 3668−3677. [CrossRef]
- D.Y. Pan, S. Wang, B. Zhao, M.H. Wu, H.J. Zhang, Y. Wang, Z. Jiao. Li storage properties of disordered graphene nanosheets. Chem. Mater., 2009, 21, 3136−3142. [CrossRef]
- R. Yazami, M. Deschamps. High reversible capacity carbon-lithium negative electrode in polymer electrolyte. J. Power Sources. 1995, 54, 411−415. [CrossRef]
- J-C. Li, P-X. Hou, M. Cheng, C. Liu, H-M. Cheng, M. Shao. Carbon nanotube encapsulated in nitrogen and phosphorus co-doped carbon as a bifunctional electrocatalyst for oxygen reduction and evolution reactions. Carbon, 2018, 139, 156-163. [CrossRef]
- Y. Li, J. Yang, J. Huang, Y. Zhou, K. Xu, N. Zhao, X. Cheng. Soft template-assisted method for synthesis of nitrogen and sulfur co-doped three-dimensional reduced graphene oxide as an efficient metal free catalyst for oxygen reduction reaction. Carbon, 2017, 122, 237-246. [CrossRef]
- S.K. Samaniego Andrade, I. Bakos, G. Dobos, A. Farkas, G. Kiss, S. Klébert, J. Madarász, K. László. Biomass related highly porous metal free carbon for gas storage and electrocatalytic applications. Materials, 2021, 14 (13), 3488. [CrossRef]
- B. Berke, L. Sós, V. Bérczes, A. Domján, L. Porcar, O. Czakkel, K. László. Graphene derivatives in responsive hydrogels: Effect of concentration and surface chemistry. European Polymer Journal, 2017, 93, 717–725. [CrossRef]
- W.S. Hummers Jr., R.E Offeman. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339−1339.
- D.C. Marcano, D.V. Kosynkin, J.M. Berlin, A. Sinitskii, Z. Sun, A. Slesarev, et al. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–14. [CrossRef]
- S. Brunauer, P.H. Emmett, E. Teller. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60 (2), 309–319. [CrossRef]
- M.M. Dubinin, L.V. Radushkevich. The Equation of the characteristic curve of activated charcoal. Dokl. Akad. Nauk. SSSR., 1947, 55, 327-329.
- J. Landers, G.Y. Gor, A.V. Neimark. Density functional theory methods for characterization of porous materials. Colloids Surf. A Physicochem. Eng., 2013, 437, 3-32. [CrossRef]
- Bertóti, M. Mohai, K. László. Surface modification of graphene and graphite by nitrogen plasma: Determination of chemical state alterations and assignments by quantitative X-ray photoelectron spectroscopy. Carbon, 2015, 84, 185-196. [CrossRef]
- M. Mohai. XPS MultiQuant: multimodel XPS quantification software. Surf. Interface Anal., 2004, 36 (8), 828-832. [CrossRef]
- S. Evans, R.G. Pritchard, J.M. Thomas. Relative differential subshell photoionization cross-sections (MgKα) from lithium to uranium. J. Electron Spectrosc. Relat. Phenom., 1978, 14 (5), 341-358. [CrossRef]
- R.F. Reilman, A. Msezane, S.T. Manson. Relative intensities in photoelectron spectroscopy of atoms and molecules. J. Electron Spectrosc. Relat. Phenom., 1976, 8 (5), 389-394. [CrossRef]
- B. Nagy, A. Domán, A. Menyhárd, K. László. Influence of graphene oxide incorporation on resorcinol-formaldehyde polymer and carbon aerogels. Periodica Polytechnica Chemical Engineering, 2018, 62 (4), 441–449. [CrossRef]
- M. Thommes, K. Kaneko, A.V. Neimark, J.P. Oliver, F. Rodriguez-Reinoso, J. Rouquerol, K.S.W. Sing. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem., 2015, 87 (9-10), 1051-1069. [CrossRef]
- Y. Wang, D.C. Alsmeyer, R.L. McCreery. Raman spectroscopy of carbon materials: Structural basis of observed spectra. Chem. Mater., 1990, 2 (5), 557-563. [CrossRef]
- M.A. Pimenta, G. Dresselhaus, M.S. Dresselhaus, L.G. Cançado, A. Jorio, R. Saito. Studying disorder in graphite-based systems by Raman spectroscopy. Phys. Chem. Chem. Phys., 2007, 9 (11), 1276-1290. [CrossRef]
- Cuesta, P. Dhamelincourt, J. Laureyns, A. Martínez-Alonso, J.M.D. Tascón. Raman microprobe studies on carbon materials. Carbon, 1994, 32, 1523-1532. [CrossRef]
- S. Claramunt, A. Varea, D. López-Díaz, M.M. Velázquez, A. Cornet, A. Cirera. The importance of interbands on the interpretation of the Raman spectrum of graphene oxide. J. Phys. Chem. C, 2015, 119, 10123-10129. [CrossRef]
- M. Couzi, J-L. Bruneel, D. Talaga, L. Bokobza. A multi wavelength Raman scattering study of defective graphitic carbon materials: The first order Raman spectra revisited. Carbon, 2016, 107, 388-394. [CrossRef]
- J. McDonald-Wharry, M. Manley-Harris, K. Pickering. Carbonization of biomass-derived chars and the thermal reduction of a graphene oxide sample studied using Raman spectroscopy. Carbon, 2013, 59, 383-405. [CrossRef]
- Y-R. Rhim, D. Zhang, D. H. Fairbrother, K. A. Wepasnick, K.J. Livi, R.J. Bodnar, D.C. Nagle. Changes in electrical and microstructural properties of microcrystalline cellulose as function of carbonization. Carbon, 2010, 48 (4), 1012-1024. [CrossRef]
- N. Gavrilov, I.A. Pasti, M. Mitric, J. Trava-Sejdic, G. Ciric-Marjanonic, S.V. Mantus. Electrocatalysis of oxygen reduction reaction on polyaniline-derived nitrogen-doped carbon nanoparticle surfaces in alkaline media. J. Power Sources. 2012, 220, 306-316. [CrossRef]
- D. Yin, N. Lu, Z.-Y. Li, J.-L. Yang. A computational infrared spectroscopic study of graphene oxide. J. Chem. Phys., 2013, 139, 084704. [CrossRef]
- X. Jiao, Y. Qiu, L. Zhangab, X. Zhang. Comparison of the characteristic properties of reduced graphene oxides synthesized from natural graphites with different graphitization degrees. RSC Adv., 2017, 7, 52337–52344. [CrossRef]
- Z. Ling, G. Wang, Q. Dong, B. Qian, M. Zhang, C. Lia, J. Qiu. An ionic liquid template approach to graphene–carbon xerogel composites for supercapacitors with enhanced performance. J. Mater. Chem. A, 2014, 2 (35), 14329–14333. [CrossRef]
- H. Lu, C. Yang, J. Chen, J. Li, H. Jin, J. Wang, S. Wang. Tailoring hierarchically porous nitrogen-, sulfur-codoped carbon for high-performance supercapacitors and oxygen reduction. Small, 2020, 16 (17), 1906584. [CrossRef]
- L. Ge, D. Wang, P. Yang, H. Xu, L. Xiao, G-X. Zhang, X. Lu, Z. Duan, F. Meng, J. Zhang, M. An. Graphite N-C-P dominated three-dimensional nitrogen and phosphorus co-doped holey graphene foams as high-efficiency electrocatalyst for Zn-air batteries. Nanoscale, 2019, 11, 17010-17017. [CrossRef]
- Y. Zhao, S. Huang, M. Xia, S. Rehman, S. Mu, Z. Kou, Z. Zhang, Z. Chen, F. Gao, Y. Hou. N-P-O co-doped high performance 3D graphene prepared through red phosphorus-assisted “cutting-thing” technique: A universal synthesis and multifunctional applications. Nano Energy, 2016, 28, 346-355. [CrossRef]
- J. Tong, W. Ma, W. Wang, J. Ma, W. Li, L. Bo, H. Fan. Nitrogen/phosphorus dual-doped hierarchically porous graphitic biocarbon with greatly improved performance on oxygen reduction reaction in alkaline media. J. Electroanal. Chem., 2018, 809, 163-170. [CrossRef]
- J. Zhang, L. Qu, G. Shi, J. Liu, J. Chen, L. Dai. N, P-codoped carbon networks as efficient metal-free bifunctional catalysts for oxygen reduction and hydrogen evolution reactions. Angew. Chem., 2015, 128 (6), 2270-2274. [CrossRef]
- Y-N. Zhu, C-Y. Cao, W-Y. Jiang, S-L. Yang, J-S. Hu, W-G. Song, L-J. Wan. Nitrogen, phosphorus, and sulfur co-doped ultrathin carbon nanosheets as a metal-free catalyst for selective oxidation of aromatic alkanes and the oxygen reduction reaction. J. Mater. Chem. A, 2016, 4 (47), 18470-18477. [CrossRef]
- J. Wu, X. Zheng, C. Jin, J. Tian, R. Yang. Ternary doping of phosphorus, nitrogen, and sulfur into porous carbon for enhancing electrocatalytic oxygen reduction. Carbon, 2015, 92, 327-338. [CrossRef]
- X. Zheng, X. Cao, J. Wu, J. Tian, C. Jin, R. Yang. Yolk-shell N/P/B ternary-doped biocarbon derived from yeast cells for enhanced oxygen reduction reaction. Carbon, 2016, 107, 907-916. [CrossRef]
- P. Chandran, A. Ghosh, S. Ramaprabhu. High-performance platinum-free oxygen reduction reaction and hydrogen oxidation reaction catalyst in polymer electrolyte membrane fuel cell. Sci. Rep., 2018, 8, 3591. [CrossRef]
- C. Moreno-Castilla, M.B. Dawidziuk, F. Carrasco-Marín, E. Morallón. Electrochemical performance of carbon gels with variable surface chemistry and physics. Carbon, 2012, 50 (9), 3324-3332. [CrossRef]
- B. Lobato, L. Suárez, L. Guardia, T. A. Centeno. Capacitance and surface of carbons in supercapacitors. Carbon, 2017, 122, 434-445.
- Y. Lu, Z. Ye, Y. Zhao, Q. Li, M. He, C. Bai, X. Wang, Y. Han, X. Wan, S. Zhang, Y. Ma, Y. Chen. Graphene supported double-layer carbon encapsulated silicon for high-performance lithium-ion battery anode materials. Carbon, 2023, 201, 962–971. [CrossRef]
- T.J. Yokokura, J.R. Rodriguez, V.G. Pol, Waste biomass-derived carbon anode for enhanced lithium storage, ACS Omega, 2020, 5 (31), 19715–19720. [CrossRef]
- J. Wang, P. Nie, B. Ding, S. Dong, X. Hao, H. Dou, X. Zhang. Biomass derived carbon for energy storage devices. J. Mater. Chem. A, 2017, 5 (6), 2411–2428. [CrossRef]
- A.S. Alex, M.S. Ananda Lekshmi, V. Sekkar, B. John, C. Gouri, S.A. Ilangovan. Microporous carbon aerogel prepared through ambient pressure drying route as anode material for lithium ion cells. Polym. Adv. Technol., 2017, 28 (12), 1945–1950. [CrossRef]
- S. T. Taleghani, B. Marcos, K. Zaghib, G. Lantagne. A study on the effect of porosity and particles size distribution on Li-ion battery performance. J. Electrochem. Soc., 2017, 164, E3179.
- E. Duraisamy, A. Prasath, V.S. Devi, M.N.M. Ansari, P. Elumalai. Sustainably-derived hierarchical porous carbon from spent honeycomb for high-performance lithium-ion battery and ultracapacitors. Energy Storage, 2020, 2 (4), e136. [CrossRef]
- K. Kim, R.A. Adams, P.J. Kim, A. Arora, E. Martinez, J.P. Youngblood, V.G. Pol. Li-ion storage in an amorphous, solid, spheroidal carbon anode produced by dry-autoclaving of coffee oil. Carbon, 2018, 133, 62−68. [CrossRef]
- A.M. Stephan, T.P. Kumar, R. Ramesh, S. Thomas, S.K. Jeong, K.S. Nahm. Pyrolitic carbon from biomass precursors as anode materials for lithium batteries. Mater. Sci. Eng. A, 2006, 430 (1-2), 132− 137. [CrossRef]
- R.R. Gaddam, D. Yang, R. Narayan, KVSN. Raju, N.A. Kumar, X.S. Zhao. Biomass derived carbon nanoparticle as anodes for high performance sodium and lithium ion batteries. Nano Energy, 2016, 26, 346−352. [CrossRef]
- W. Xing, J.S. Xue, J.R. Dahn. Optimizing pyrolysis of sugar carbons for use as anode materials in lithium-ion batteries. J. Electrochem. Soc., 1996, 143, 3046−3052. [CrossRef]
- W. Wang, Y. Sun, B. Liu, S. Wang, M. Cao. Porous carbon nanofiber webs derived from bacterial cellulose as an anode for high-performance lithium-ion batteries. Carbon, 2015, 91, 56–65. [CrossRef]
- Paudics, S. Farah, I. Bertóti, K. László, M. Mohai, A. Szilágyi, M. Kubinyi. Fluorescence probing of binding sites on graphene oxide nanosheets with Oxazine 1 dye. Appl. Surf. Sci. 2021, 541, 148451.
Figure 1.
Typical images of CA (a), the GO cryogel (b), and the CAGO50 sample (c).

Figure 2.
(a) Low temperature N2 adsorption/desorption isotherms of annealed carbons; (b) Combined pore size distribution functions of annealed carbons. Functions were estimated by quenched solid density functional theory QSDFT, slit/cylinder geometry for N2 and NLDFT for CO2, respectively.
Figure 2.
(a) Low temperature N2 adsorption/desorption isotherms of annealed carbons; (b) Combined pore size distribution functions of annealed carbons. Functions were estimated by quenched solid density functional theory QSDFT, slit/cylinder geometry for N2 and NLDFT for CO2, respectively.

Figure 3.
Decomposition of C1s, O1s, N1s and S2p regions of photoelectron spectra of the CAGO100 sample.
Figure 3.
Decomposition of C1s, O1s, N1s and S2p regions of photoelectron spectra of the CAGO100 sample.

Figure 4.
Cathodic linear potential sweep of the carbon xerogel loaded electrodes in oxygen saturated 0.1 M KOH. Loading: 75 μg/cm2, sweep rate: 5 mV/s. Rotation rate: 400 (blue), 625 (black), 900 (red), 1250 (green) rpm, (increasing downwards). Rotation rates were applied in the following order: 625, 900, 1250, 400 rpm. LSVs on Pt/C were measured for comparison.
Figure 4.
Cathodic linear potential sweep of the carbon xerogel loaded electrodes in oxygen saturated 0.1 M KOH. Loading: 75 μg/cm2, sweep rate: 5 mV/s. Rotation rate: 400 (blue), 625 (black), 900 (red), 1250 (green) rpm, (increasing downwards). Rotation rates were applied in the following order: 625, 900, 1250, 400 rpm. LSVs on Pt/C were measured for comparison.

Figure 5.
Koutecky-Levich (KL) plots of the carbon electrodes at E = 0.3 V. The theoretical 2 e- and 4 e- KL plots are shown for comparison.
Figure 5.
Koutecky-Levich (KL) plots of the carbon electrodes at E = 0.3 V. The theoretical 2 e- and 4 e- KL plots are shown for comparison.

Figure 6.
Cyclic voltammograms (1) of the virgin electrode; (2) after 50 cycles in oxygen-free 0.1 M KOH electrolyte; (3) after additional 50 cycles in oxygen-saturated electrolytes; (4) after an additional ORR test with rotating electrode (sweep rate: 5 mV/s, four rotation rates). The column graph compares the gravimetric capacitances of the carbons corresponding to CV 1 (black), 2 (red), 3 (green) and 4 (blue), respectively.
Figure 6.
Cyclic voltammograms (1) of the virgin electrode; (2) after 50 cycles in oxygen-free 0.1 M KOH electrolyte; (3) after additional 50 cycles in oxygen-saturated electrolytes; (4) after an additional ORR test with rotating electrode (sweep rate: 5 mV/s, four rotation rates). The column graph compares the gravimetric capacitances of the carbons corresponding to CV 1 (black), 2 (red), 3 (green) and 4 (blue), respectively.

Figure 7.
Galvanostatic charge – discharge profiles of CA, CAGO50, CAGO100 and CAGO200 at constant current density 100 mA/g, cycle 1 grey, cycle 10 red, cycle 20 blue, cycle 30 green, cycle 40 purple, cycle 50 gold.
Figure 7.
Galvanostatic charge – discharge profiles of CA, CAGO50, CAGO100 and CAGO200 at constant current density 100 mA/g, cycle 1 grey, cycle 10 red, cycle 20 blue, cycle 30 green, cycle 40 purple, cycle 50 gold.

Figure 8.
Long-term cycling performance of CA, CAGO50, CAGO100, and CAGO200.

Table 1.
Porous characteristics of annealed carbon aerogel samples from gas adsorptionmeasurements*.
Table 1.
Porous characteristics of annealed carbon aerogel samples from gas adsorptionmeasurements*.
| Method | Parameter | Units | CA | CAGO50 | CAGO100 | CAGO200 |
|---|---|---|---|---|---|---|
| From N2 | SBET | [m2/g] | 1070 | 1479 | 1779 | 933 |
| V0.98 | [cm3/g] | 0.83 | 1.33 | 1.72 | 0.71 | |
| Vmicro,DR | [cm3/g] | 0.42 | 0.54 | 0.64 | 0.34 | |
| [%] | 51 | 40 | 37 | 48 | ||
| Vmicro,DFT | [cm3/g] | 0.31 | 0.40 | 0.48 | 0.25 | |
| [%] | 37 | 30 | 28 | 35 | ||
| From CO2 | Vumicro,DR | [cm3/g] | 0.073 | 0.062 | 0.059 | 0.041 |
| Vumicro,DFT | [cm3/g] | 0.042 | 0.039 | 0.030 | 0.025 |
*SBET: apparent surface area from BET model, V0.98: liquid equivalent of the gas adsorbed at p/p0 = 0.98, Vmicro,DR: micropore volume from DR model, Vmicro,DFT: micropore volume from DFT model; Vumicro,DR: ultra-micropore volume from DR model; Vumicro,DFT: ultra-micropore volume from DFT model.
Table 2.
Surface composition (atomic %) measured by XPS.
| Sample | C | O | N | S | O / C | N / C | S / C | O+N+S C |
S / N |
|---|---|---|---|---|---|---|---|---|---|
| CA | 90.6 | 3.3 | 5.1 | 1.0 | 0.036 | 0.056 | 0.011 | 0.104 | 0.196 |
| CAGO50 | 92.0 | 3.1 | 3.7 | 1.3 | 0.034 | 0.039 | 0.014 | 0.087 | 0.361 |
| CAGO100 | 90.7 | 4.1 | 4.1 | 1.2 | 0.045 | 0.045 | 0.013 | 0.104 | 0.293 |
| CAGO200 | 90.4 | 3.7 | 4.4 | 1.4 | 0.041 | 0.049 | 0.015 | 0.105 | 0.318 |
| GO-film | 67.4 | 32.1 | - | 0.5 | 0.476 | - | 0.007 | 0.484 | - |
Table 3.
Decomposition of C1s and O1s regions of photoelectron spectra: binding energy ranges, chemical state assignations and surface compositions (atomic %).
Table 3.
Decomposition of C1s and O1s regions of photoelectron spectra: binding energy ranges, chemical state assignations and surface compositions (atomic %).
| C1s | O1s | |||||
|---|---|---|---|---|---|---|
| C1 | C2 | C3 | O1 | O2 | O3 | |
| Chemical state | sp2 C=C | C–O C–N C–S |
C=O O–C–O N–C–O |
S–O | C–O–C C–OH C=O |
OC–O–CO (H2O) |
| Binding energy [eV] | 284.3 – 284.4 |
285.7 – 285.8 |
287.5 – 287.9 |
530.2 – 530.6 | 532.1 – 532.5 | 533.9 – 534.3 |
| CA | 74.0 | 10.9 | 5.4 | 1.5 | 1.7 | |
| CAGO50 | 78.8 | 7.4 | 5.5 | 1.9 | 1.3 | |
| CAGO100 | 74.7 | 11.0 | 4.8 | 1.8 | 1.7 | 0.7 |
| CAGO200 | 75.9 | 9.4 | 4.8 | 1.8 | 1.6 | 0.5 |
Table 4.
Decomposition of N1s and S2p regions of photoelectron spectra: binding energy ranges, chemical state assignations and surface compositions (atomic %).
Table 4.
Decomposition of N1s and S2p regions of photoelectron spectra: binding energy ranges, chemical state assignations and surface compositions (atomic %).
| N1s | S2p | ||||
|---|---|---|---|---|---|
| N1 | N2 | N3 | S1 | S2 | |
| Chemical state | C–N | OO–C–N | C–N+ | C–S | C–SO3 |
| Binding energy [eV] | 397.8 – 398.0 |
400.4 – 400.5 | 402.4 – 402.7 | 164.9 – 165.0 | 168.3 – 168.6 |
| CA | 2.3 | 2.3 | 0.8 | 0.9 | 0.2 |
| CAGO50 | 1.6 | 1.7 | 0.6 | 1.2 | n.d. |
| CAGO100 | 1.9 | 1.9 | 0.4 | 1.0 | 0.2 |
| CAGO200 | 2.0 | 2.0 | 0.7 | 1.1 | 0.3 |
Table 5.
Comparison of the onset potentials and half wave potentials of double doped carbon aero/xero/cryogel electrodes in 0.1 M KOH, vs. RHE.
Table 5.
Comparison of the onset potentials and half wave potentials of double doped carbon aero/xero/cryogel electrodes in 0.1 M KOH, vs. RHE.
| Sample | BET surface area [m2/g] |
Onset potential [mV] |
E1/2 [mV] |
Number of e- transferred | Ref. |
|---|---|---|---|---|---|
| SWCNT@N,P doped carbon | 616 | 920 | 850 | 3.91 | [69] |
| N,S co-doped 3D rGO | 392 | 895 | 732 | 3.87 | [70] |
| N,S porous carbon materials | 732 | 940 | 840 | - | [95] |
| N,P-holey graphene foams | 758 | 983 | 865 | 3.70 | [96] |
| 3D-high performance graphene | 1406 | 928 | 836 | 3.83 | [97] |
| N,P porous graphitic biocarbon | 845 | -14 (vs. Ag/AgCl) |
-115 (vs. Ag/AgCl) |
3.9 | [98] |
| N,P co-doped carbon | 375 | 950 | 820 | 3.7 | [99] |
| N,P,S co-doped carbon nanosheets | 1198 | 938 | 800 | 3.8 – 4.0 | [100] |
| P,N,S-porous carbon | 711 | 905 | 780 | 3.68 – 3.96 | [101] |
| N,P,B biocarbon | 1155 | 904 | 790 | 3.78 – 3.90 | [102] |
| Pt/C (20 wt.% Pt on Vulcan XC-72) | - | 960 | 869 | 3.96 | [102] |
| CA | 1070 | 855 | 700 | 3.5 |
Our work |
| CAGO50 | 1479 | 850 | 760 | 4.0 | |
| CAGO100 | 1779 | 845 | 730 | 3.1 | |
| CAGO200 | 933 | 825 | 700 | 2.0 | |
| Pt/C | 957 | 886 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
Pseudocapacitive Effect of Carbons Doped with Different Functional Groups as Electrode Materials for Electrochemical Capacitors
Mojtaba Mirzaeian
et al.
Energies,
2020
Reduced Graphene Oxide Aerogels with Functionalization-Mediated Disordered Stacking for Sodium-Ion Batteries
Jaehyeung Park
et al.
Batteries,
2022
Supercapacitor Properties of rGO-TiO2 Nanocomposite in Two-component Acidic Electrolyte
Yury Volfkovich
et al.
Materials,
2022
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated






