Submitted:
12 July 2023
Posted:
14 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
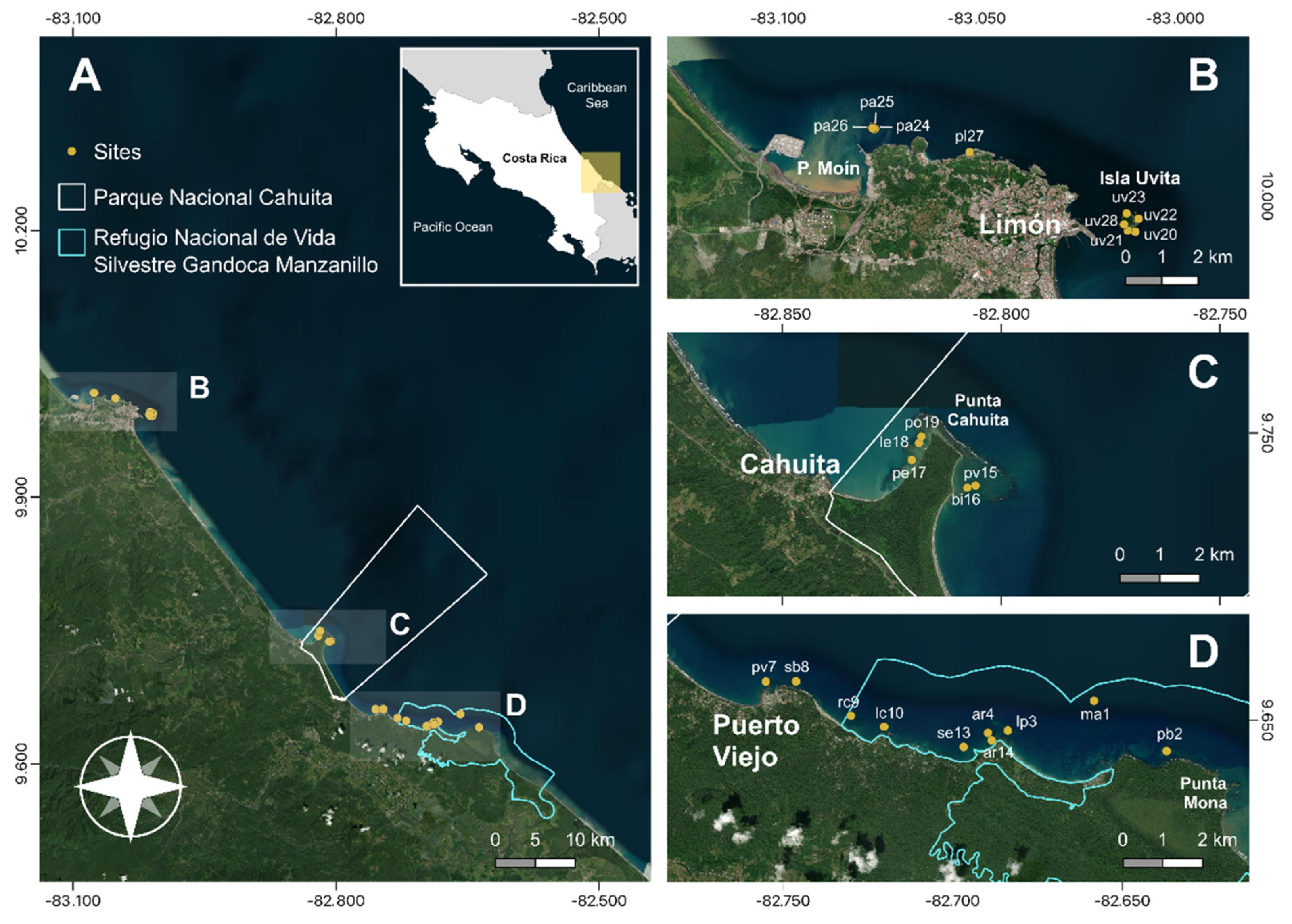
2.1. Study area
2.2. Survey sites and data collection
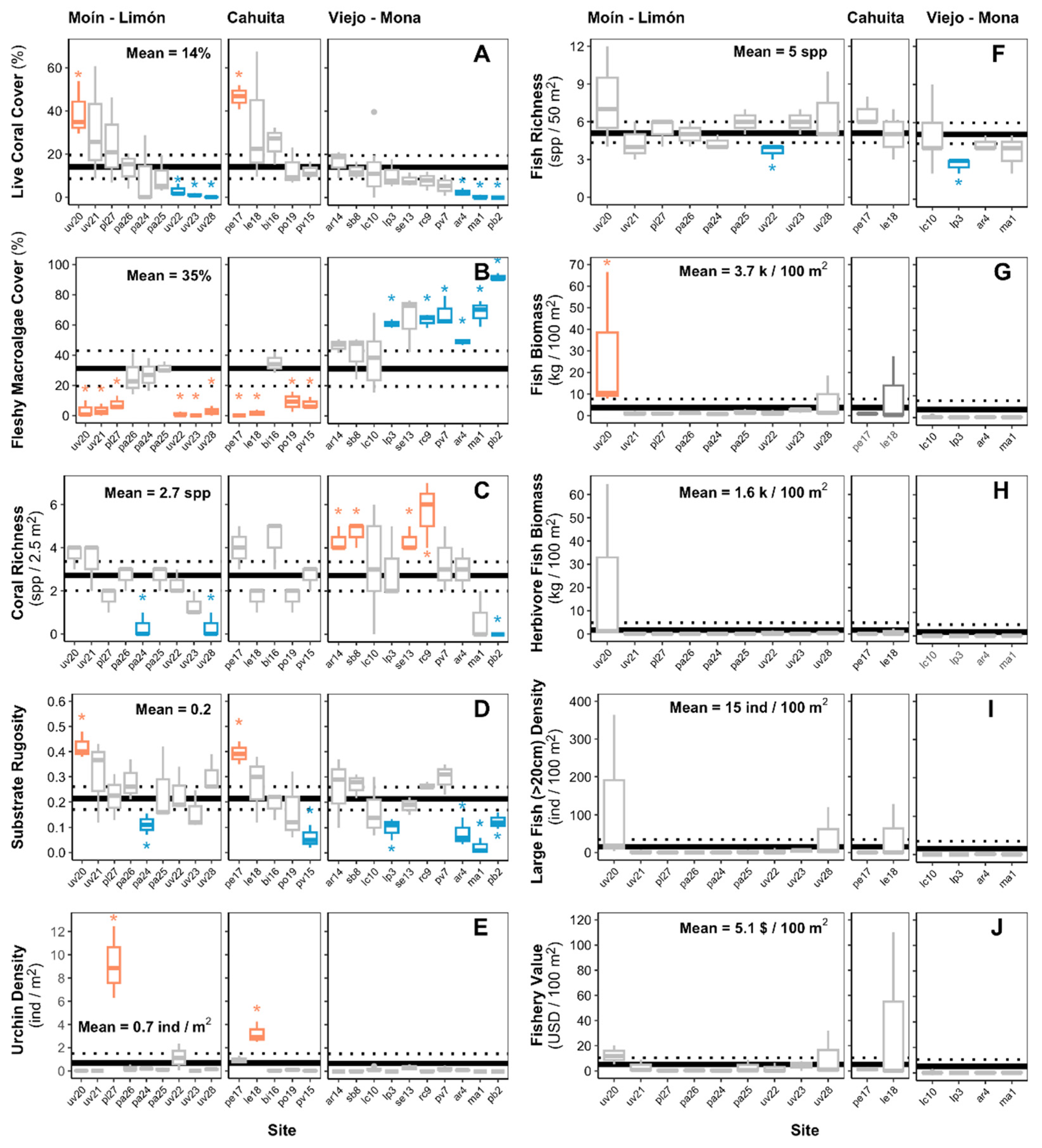
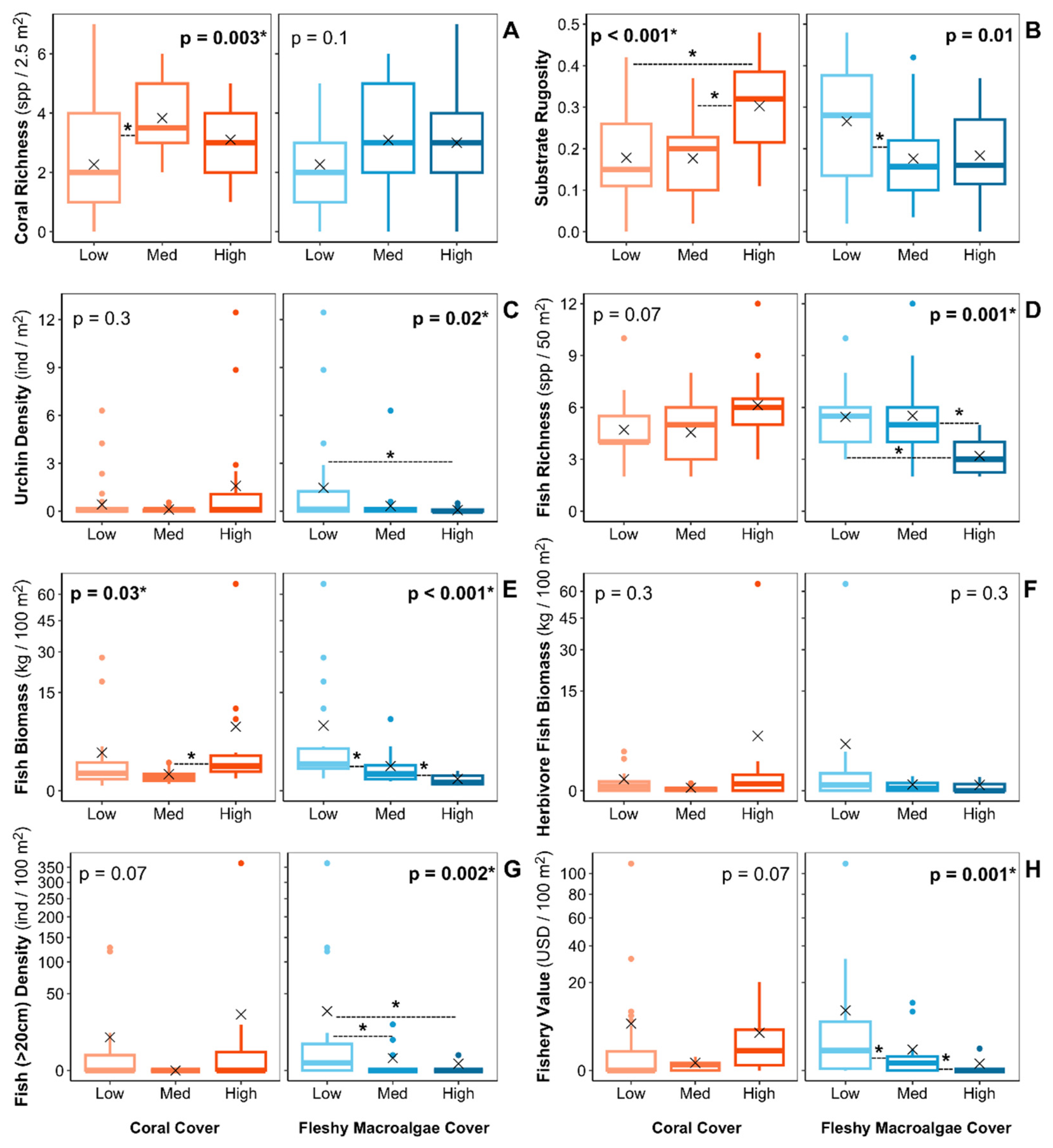
2.3. Ecosystem metrics
- Coral richness: Number of coral species encountered at each benthic transect (spp. 2.5 m-2). This metric is a component of biodiversity and acts as an important supporting service [16]. Thus, coral richness can enhance coral growth and reef resilience and may influence dive tourism as it’s related to the aesthetic value of the reef [50,51,52,53].
- Fish richness: Number of fish species encountered at each transect (spp. 50 m-2). This metric is a component of biodiversity, acting as an important supporting service [16]. Thus, fish richness can enhance reef resilience, support fisheries productivity, and influence dive tourism as it’s related to the aesthetic value of the reef [50,52,57,58].
- Large fish density: Number of fishes greater than 20 cm in total length counted at each transect per area (ind 100 m-2). Lester et al. [17] used a > 40 cm cutoff to measure this metric. However, we didn’t count any individual with an estimated total length greater than 40 cm, so we decided to use the > 20 cm cutoff. Larger fishes may increase the fishery value, improve the aesthetic value of the reef, and influence dive tourism [57,58,61,62].
- Potential fishery value: To quantify the potential fishery value of the reef fish assemblage, we multiplied the species-specific biomass estimates for each transect by the species-specific price estimates reported by Lester et al. [17] (USD 100 m-2). The price estimates are based on fishery statistics from Puerto Rico that provided an estimate of value in USD per pound of fish landed by species or by family Lester et al. [17]. For species that didn’t appeared in the Lester et al. [17] list, we first determined if they had or not a fishery value based on the information listed in FishBase under the human uses category [63]. If “commercial fisheries” was listed as a human use of the specific species, we then assigned it the price estimate of a similar species included in the Lester et al. [17] list (i.e. of the same genus or family). Of the total fish species encountered on the surveys, four species of commercial fishery importance were not listed in the species-specific price estimates reported by Lester et al. [17] (Supplementary material S4).
2.4. Data analysis
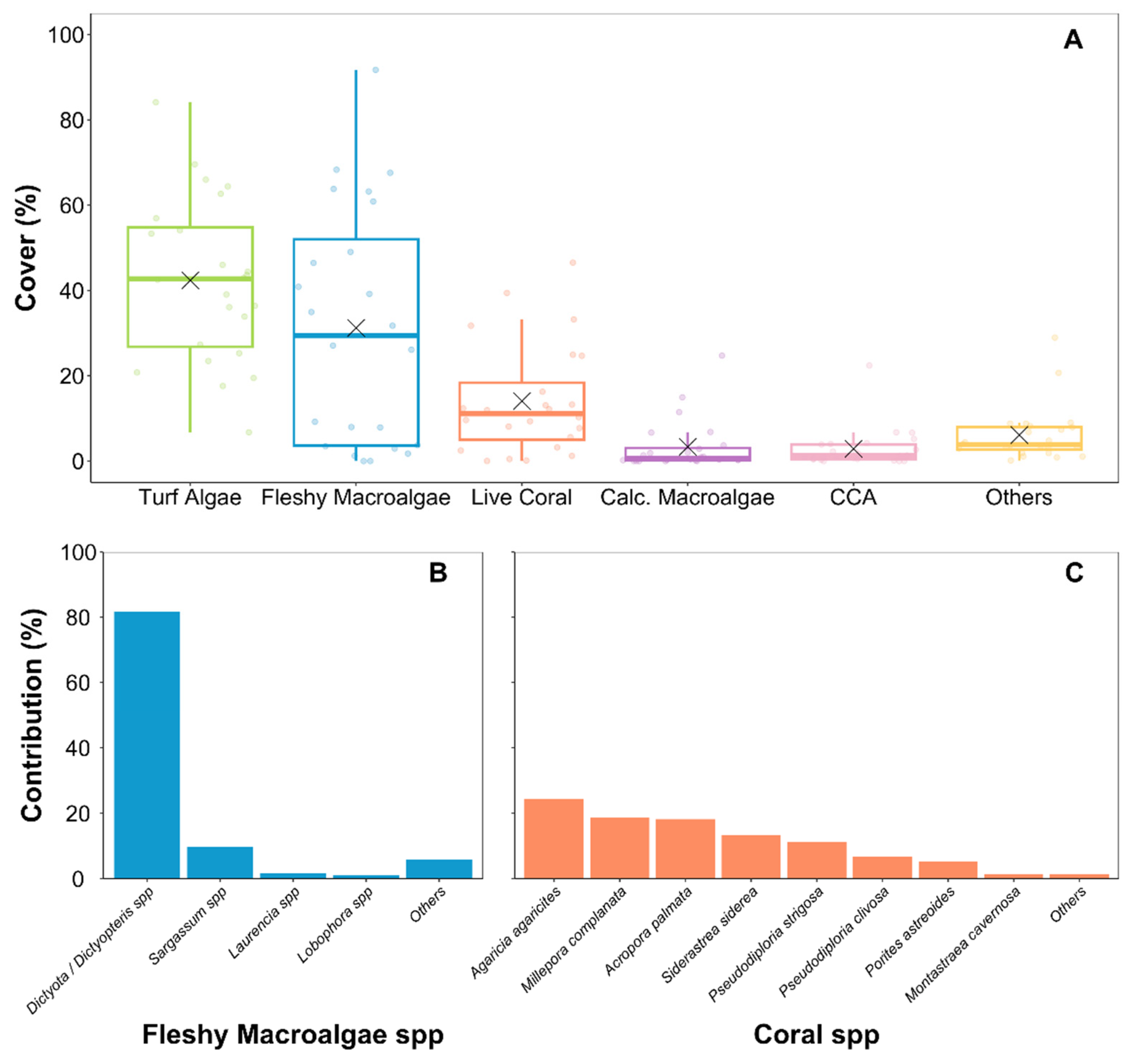
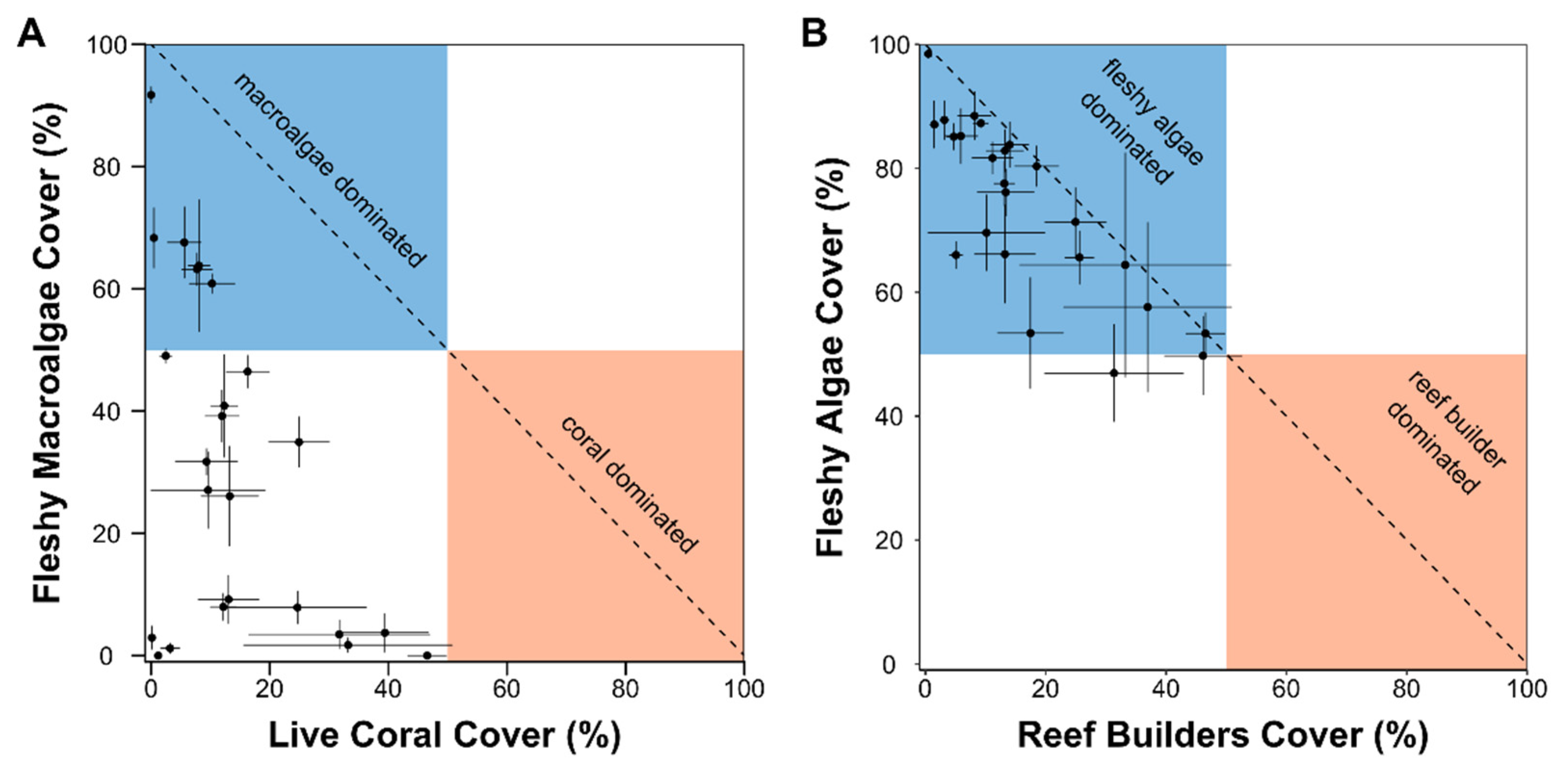
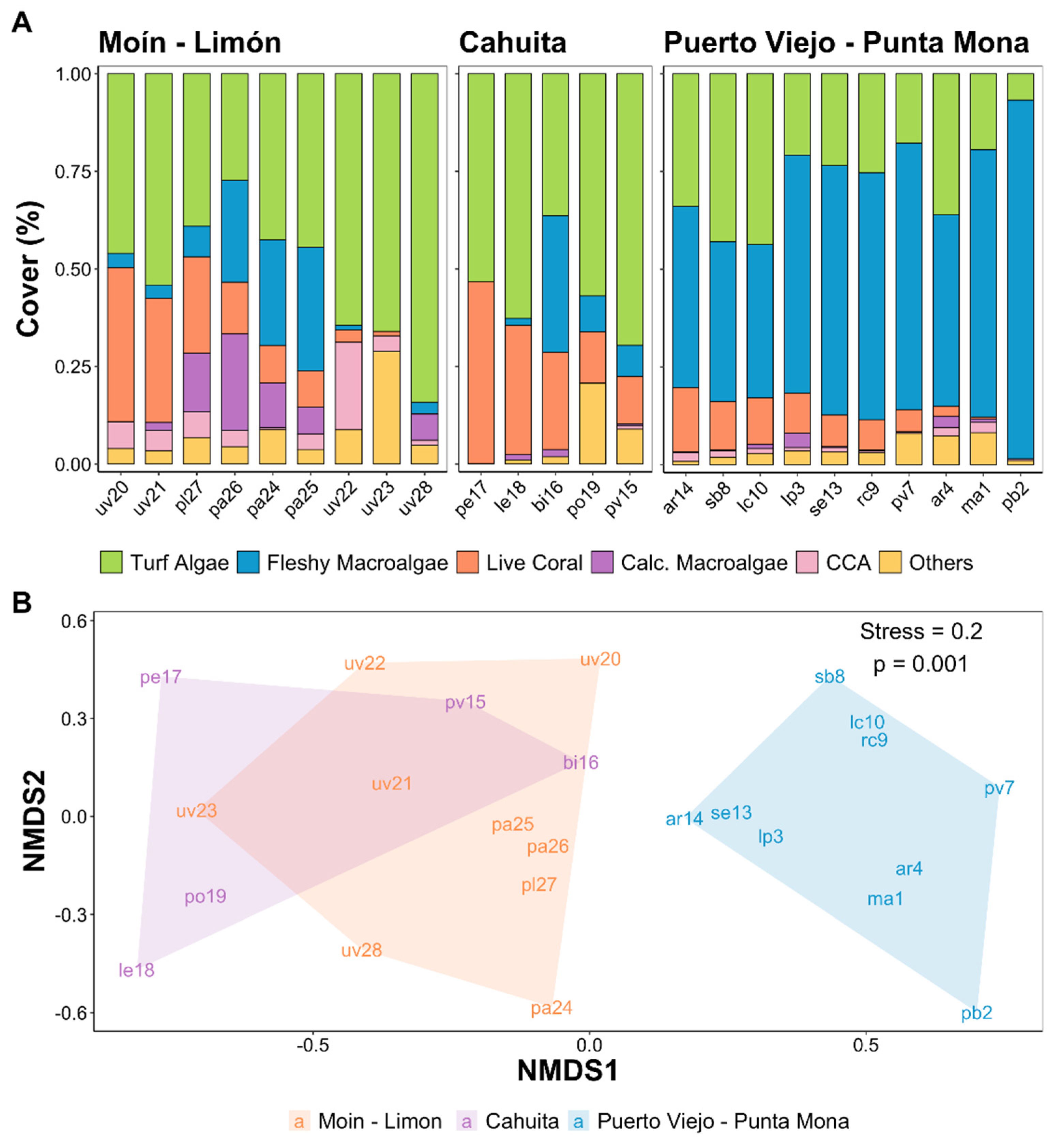
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hughes, T.P.; Barnes, M.L.; Bellwood, D.R.; Cinner, J.E.; Cumming, G.S.; Jackson, J.B.C.; Kleypas, J.; van de Leemput, I.A.; Lough, J.M.; Morrison, T.H.; et al. Coral Reefs in the Anthropocene. Nature 2017, 546, 82–90. [Google Scholar] [CrossRef]
- Aronson, R.B.; Precht, W.F. White-Band Disease and the Changing Face of Caribbean Coral Reefs. In The Ecology and Etiology of Newly Emerging Marine Diseases; Porter, J.W., Ed.; Developments in Hydrobiology; Springer Netherlands: Dordrecht, 2001; pp. 25–38. ISBN 978-94-017-3284-0. [Google Scholar]
- Lapointe, B.E. Nutrient Thresholds for Bottom-up Control of Macroalgal Blooms on Coral Reefs in Jamaica and Southeast Florida. Limnol. Oceanogr. 1997, 42, 1119–1131. [Google Scholar] [CrossRef]
- Mumby, P.J.; Hedley, J.D.; Zychaluk, K.; Harborne, A.R.; Blackwell, P.G. Revisiting the Catastrophic Die-off of the Urchin Diadema Antillarum on Caribbean Coral Reefs: Fresh Insights on Resilience from a Simulation Model. Ecol. Model. 2006, 196, 131–148. [Google Scholar] [CrossRef]
- Muñiz-Castillo, A.I.; Rivera-Sosa, A.; Chollett, I.; Eakin, C.M.; Andrade-Gómez, L.; McField, M.; Arias-González, J.E. Three Decades of Heat Stress Exposure in Caribbean Coral Reefs: A New Regional Delineation to Enhance Conservation. Sci. Rep. 2019, 9, 11013. [Google Scholar] [CrossRef] [PubMed]
- Randazzo-Eisemann, Á.; Garza-Pérez, J.R.; Penié-Rodriguez, I.; Figueroa-Zavala, B. 25 Years of Multiple Stressors Driving the Coral-Algae Phase Shift in Akumal, Mexico. Ocean Coast. Manag. 2021, 214, 105917. [Google Scholar] [CrossRef]
- Contreras-Silva, A.I.; Tilstra, A.; Migani, V.; Thiel, A.; Pérez-Cervantes, E.; Estrada-Saldívar, N.; Elias-Ilosvay, X.; Mott, C.; Alvarez-Filip, L.; Wild, C. A Meta-Analysis to Assess Long-Term Spatiotemporal Changes of Benthic Coral and Macroalgae Cover in the Mexican Caribbean. Sci. Rep. 2020, 10, 8897. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.P. Catastrophes, Phase Shifts, and Large-Scale Degradation of a Caribbean Coral Reef. Science 1994, 265, 1547–1551. [Google Scholar] [CrossRef]
- Mumby, P.J. Phase Shifts and the Stability of Macroalgal Communities on Caribbean Coral Reefs. Coral Reefs 2009, 28, 761–773. [Google Scholar] [CrossRef]
- Alvarez-Filip, L.; Dulvy, N.K.; Gill, J.A.; Côté, I.M.; Watkinson, A.R. Flattening of Caribbean Coral Reefs: Region-Wide Declines in Architectural Complexity. Proc. R. Soc. B Biol. Sci. 2009, 276, 3019–3025. [Google Scholar] [CrossRef]
- Alvarez-Filip, L.; Gill, J.A.; Dulvy, N.K.; Perry, A.L.; Watkinson, A.R.; Côté, I.M. Drivers of Region-Wide Declines in Architectural Complexity on Caribbean Reefs. Coral Reefs 2011, 30, 1051–1060. [Google Scholar] [CrossRef]
- Newman, S.P.; Meesters, E.H.; Dryden, C.S.; Williams, S.M.; Sanchez, C.; Mumby, P.J.; Polunin, N.V.C. Reef Flattening Effects on Total Richness and Species Responses in the Caribbean. J. Anim. Ecol. 2015, 84, 1678–1689. [Google Scholar] [CrossRef] [PubMed]
- Chong-Seng, K.M.; Mannering, T.D.; Pratchett, M.S.; Bellwood, D.R.; Graham, N.A.J. The Influence of Coral Reef Benthic Condition on Associated Fish Assemblages. PLOS ONE 2012, 7, e42167. [Google Scholar] [CrossRef]
- Pratchett, M.S.; Hoey, A.S.; Wilson, S.K. Reef Degradation and the Loss of Critical Ecosystem Goods and Services Provided by Coral Reef Fishes. Curr. Opin. Environ. Sustain. 2014, 7, 37–43. [Google Scholar] [CrossRef]
- Smith, J.E.; Brainard, R.; Carter, A.; Grillo, S.; Edwards, C.; Harris, J.; Lewis, L.; Obura, D.; Rohwer, F.; Sala, E.; et al. Re-Evaluating the Health of Coral Reef Communities: Baselines and Evidence for Human Impacts across the Central Pacific. Proc. R. Soc. B Biol. Sci. 2016, 283, 20151985. [Google Scholar] [CrossRef] [PubMed]
- Woodhead, A.J.; Hicks, C.C.; Norström, A.V.; Williams, G.J.; Graham, N.A.J. Coral Reef Ecosystem Services in the Anthropocene. Funct. Ecol. 2019, 33, 1023–1034. [Google Scholar] [CrossRef]
- Lester, S.E.; Rassweiler, A.; McCoy, S.J.; Dubel, A.K.; Donovan, M.K.; Miller, M.W.; Miller, S.D.; Ruttenberg, B.I.; Samhouri, J.F.; Hay, M.E. Caribbean Reefs of the Anthropocene: Variance in Ecosystem Metrics Indicates Bright Spots on Coral Depauperate Reefs. Glob. Change Biol. 2020, 26, 4785–4799. [Google Scholar] [CrossRef]
- Robinson, J.P.W.; Wilson, S.K.; Robinson, J.; Gerry, C.; Lucas, J.; Assan, C.; Govinden, R.; Jennings, S.; Graham, N.A.J. Productive Instability of Coral Reef Fisheries after Climate-Driven Regime Shifts. Nat. Ecol. Evol. 2019, 3, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Bruno, J.F.; Sweatman, H.; Precht, W.F.; Selig, E.R.; Schutte, V.G.W. Assessing Evidence of Phase Shifts from Coral to Macroalgal Dominance on Coral Reefs. Ecology 2009, 90, 1478–1484. [Google Scholar] [CrossRef] [PubMed]
- Norström, A.V.; Nyström, M.; Lokrantz, J.; Folke, C. Alternative States on Coral Reefs: Beyond Coral–Macroalgal Phase Shifts. Mar. Ecol. Prog. Ser. 2009, 376, 295–306. [Google Scholar] [CrossRef]
- Mcleod, E.; Anthony, K.R.N.; Mumby, P.J.; Maynard, J.; Beeden, R.; Graham, N.A.J.; Heron, S.F.; Hoegh-Guldberg, O.; Jupiter, S.; MacGowan, P.; et al. The Future of Resilience-Based Management in Coral Reef Ecosystems. J. Environ. Manage. 2019, 233, 291–301. [Google Scholar] [CrossRef]
- Williams, S.M.; Chollett, I.; Roff, G.; Cortés, J.; Dryden, C.S.; Mumby, P.J. Hierarchical Spatial Patterns in Caribbean Reef Benthic Assemblages. J. Biogeogr. 2015, 42, 1327–1335. [Google Scholar] [CrossRef]
- Spalding, M.D.; Fox, H.E.; Allen, G.R.; Davidson, N.; Ferdaña, Z.A.; Finlayson, M.; Halpern, B.S.; Jorge, M.A.; Lombana, A.; Lourie, S.A.; et al. Marine Ecoregions of the World: A Bioregionalization of Coastal and Shelf Areas. BioScience 2007, 57, 573–583. [Google Scholar] [CrossRef]
- Aronson, R.B.; Hilbun, N.L.; Bianchi, T.S.; Filley, T.R.; McKee, B.A. Land Use, Water Quality, and the History of Coral Assemblages at Bocas Del Toro, Panamá. Mar. Ecol. Prog. Ser. 2014, 504, 159–170. [Google Scholar] [CrossRef]
- Cortés, J.N.; Risk, M.J. A Reef under Siltation Stress: Cahuita, Costa Rica. Bull. Mar. Sci. 1985, 36, 339–356. [Google Scholar]
- Cramer, K.L.; O’Dea, A.; Leonard-Pingel, J.S.; Norris, R.D. Millennial-Scale Change in the Structure of a Caribbean Reef Ecosystem and the Role of Human and Natural Disturbance. Ecography 2020, 43, 283–293. [Google Scholar] [CrossRef]
- Restrepo, J.D.; Park, E.; Aquino, S.; Latrubesse, E.M. Coral Reefs Chronically Exposed to River Sediment Plumes in the Southwestern Caribbean: Rosario Islands, Colombia. Sci. Total Environ. 2016, 553, 316–329. [Google Scholar] [CrossRef]
- Cortés, J.; Jiménez, C.E.; Fonseca, A.C.; Alvarado, J.J. Status and Conservation of Coral Reefs in Costa Rica. Rev. Biol. Trop. 2010, 58, 33–50. [Google Scholar] [CrossRef]
- Guzmán, H.M. Caribbean Coral Reefs of Panama: Present Status and Future Perspectives. In Latin American Coral Reefs; Cortés, J., Ed.; Elsevier Science: Amsterdam, 2003; pp. 241–274. ISBN 978-0-444-51388-5. [Google Scholar]
- Rivas, N.; Gómez, C.E.; Millán, S.; Mejía-Quintero, K.; Chasqui, L. Coral Reef Degradation at an Atoll of the Western Colombian Caribbean. PeerJ 2023, 11, e15057. [Google Scholar] [CrossRef]
- Cortés, J.; Jiménez, C. Past, Present and Future of the Coral Reefs of the Caribbean Coast of Costa Rica. In Latin American Coral Reefs; Cortés, J., Ed.; Elsevier Science: Amsterdam, 2003; pp. 223–239. ISBN 978-0-444-51388-5. [Google Scholar]
- G.m, W. The Benthic Flora of Punta Cahuita: An Annotated List of Species with New Additions to the Costa Rican Atlantic Flora [Algae]. Brenesia 1974. [Google Scholar]
- Risk, M.J.; Murillo, M.M.; Cortés, J. Observaciones biológicas preliminares sobre el arrecife coralino en el Parque Nacional de Cahuita, Costa Rica. Rev. Biol. Trop. 1980, 28, 361–382. [Google Scholar]
- Cortés, J. A Reef under Siltation Stress: A Decade of Degradation. Biol. Conserv. 1996, 76, 215–215. [Google Scholar] [CrossRef]
- Cortés, J.; Soto, R.; Jimenez, C.; Astorga, A. Earthquake Associated Mortality of Intertidal and Coral Reef Organisms (Caribbean of Costa Rica). Proc. 7th Int. Coral Reef Symp. 1992, 1, 235–240. [Google Scholar]
- Guzmán, H.M.; Cortés, J. Mortandad de Gorgonia flabellum Linnaeus (Octocorallia: Gorgoniidae) en la Costa del Caribe de Costa Rica. Rev. Biol. Trop. 1984, 32, 305–308. [Google Scholar]
- Guzmán, H.M.; Jiménez, C.E. Contamination of Coral Reefs by Heavy Metals along the Caribbean Coast of Central America (Costa Rica and Panama). Mar. Pollut. Bull. 1992, 24, 554–561. [Google Scholar] [CrossRef]
- Murillo, M.M.; Cortés, J. Alta mortalidad en la población del erizo de mar Diadema antillarum Philippi (Echinodermata: Echinoidea), en el Parque Nacional Cahuita, Limón,Costa Rica. Rev. Biol. Trop. 1984, 32, 167–169. [Google Scholar]
- Cortés, J.; Fonseca, A.C.; Nivia-Ruiz, J.; Nielsen-Muñoz, V.; Samper-Villarreal, J.; Salas, E.; Martínez, S.; Zamora-Trejos, P. Monitoring Coral Reefs, Seagrasses and Mangroves in Costa Rica (CARICOMP). Rev. Biol. Trop. 2010, 58, 1–22. [Google Scholar] [PubMed]
- Cortés, J.; Oxenford, H.A.; van Tussenbroek, B.I.; Jordán-Dahlgren, E.; Cróquer, A.; Bastidas, C.; Ogden, J.C. The CARICOMP Network of Caribbean Marine Laboratories (1985–2007): History, Key Findings, and Lessons Learned. Front. Mar. Sci. 2019, 5. [Google Scholar] [CrossRef]
- Cortés-Núñez, J.; Guzmán-Espinal, H.M. Arrecifes Coralinos de La Costa Atlántica de Costa Rica. Brenesia. 1985, 275–292. [Google Scholar]
- Fernández, C.; Alvarado, J.J. El arrecife coralino de Punta Cocles, costa Caribe de Costa Rica. Rev. Biol. Trop. 2004, 52, 121–129. [Google Scholar]
- Fonseca, A. A Rapid Assessment at Cahuita National Park, Costa Rica, 1999 (Part 1: Stony Corals and Algae); Smithsonian Institution Press, 2003. [Google Scholar]
- Araya-Vargas, A.; Nova-Bustos, N. Health Status Evaluation of Shallow Coral Reefs in Cahuita and Manzanillo, Costa Rica. Biodivers. Nat. Hist. 2017, 3, 48–55. [Google Scholar]
- Williams, S.; Mumby, P.; Chollett, I.; Cortés, J. Importance of Differentiating Orbicella Reefs from Gorgonian Plains for Ecological Assessments of Caribbean Reefs. Mar. Ecol. Prog. Ser. 2015, 530. [Google Scholar] [CrossRef]
- Trygonis, V.; Sini, M. PhotoQuad: A Dedicated Seabed Image Processing Software, and a Comparative Error Analysis of Four Photoquadrat Methods. J. Exp. Mar. Biol. Ecol. 2012, 424–425, 99–108. [Google Scholar] [CrossRef]
- Aronson, R.B.; Precht, W.F. Landscape Patterns of Reef Coral Diversity: A Test of the Intermediate Disturbance Hypothesis. J. Exp. Mar. Biol. Ecol. 1995, 192, 1–14. [Google Scholar] [CrossRef]
- Alvarado, J.J.; Beita-Jiménez, A.; Mena, S.; Fernández-García, C.; Guzman-Mora, A.G.; Cortés, J. Ecosistemas coralinos del Parque Nacional Isla del Coco, Costa Rica: estructura y comparación 1987-2014. Rev. Biol. Trop. 2016, 64, 153–175. [Google Scholar] [CrossRef]
- Moberg, F.; Folke, C. Ecological Goods and Services of Coral Reef Ecosystems. Ecol. Econ. 1999, 29, 215–233. [Google Scholar] [CrossRef]
- Uyarra, M.C.; Watkinson, A.R.; Côté, I.M. Managing Dive Tourism for the Sustainable Use of Coral Reefs: Validating Diver Perceptions of Attractive Site Features. Environ. Manage. 2009, 43, 1–16. [Google Scholar] [CrossRef]
- Imamura, K.; Takano, K.T.; Kumagai, N.H.; Yoshida, Y.; Yamano, H.; Fujii, M.; Nakashizuka, T.; Managi, S. Valuation of Coral Reefs in Japan: Willingness to Pay for Conservation and the Effect of Information. Ecosyst. Serv. 2020, 46, 101166. [Google Scholar] [CrossRef]
- Rogers, C.S. Coral Reef Resilience through Biodiversity. Int. Sch. Res. Not. 2013, 2013, e739034. [Google Scholar] [CrossRef]
- Clements, C.S.; Hay, M.E. Biodiversity Enhances Coral Growth, Tissue Survivorship and Suppression of Macroalgae. Nat. Ecol. Evol. 2019, 3, 178–182. [Google Scholar] [CrossRef]
- Graham, N.A.J.; Nash, K.L. The Importance of Structural Complexity in Coral Reef Ecosystems. Coral Reefs 2013, 32, 315–326. [Google Scholar] [CrossRef]
- Ferrario, F.; Beck, M.W.; Storlazzi, C.D.; Micheli, F.; Shepard, C.C.; Airoldi, L. The Effectiveness of Coral Reefs for Coastal Hazard Risk Reduction and Adaptation. Nat. Commun. 2014, 5, 3794. [Google Scholar] [CrossRef]
- Francis, F.T.; Filbee-Dexter, K.; Yan, H.F.; Côté, I.M. Invertebrate Herbivores: Overlooked Allies in the Recovery of Degraded Coral Reefs? Glob. Ecol. Conserv. 2019, 17, e00593. [Google Scholar] [CrossRef]
- Grafeld, S.; Oleson, K.; Barnes, M.; Peng, M.; Chan, C.; Weijerman, M. Divers’ Willingness to Pay for Improved Coral Reef Conditions in Guam: An Untapped Source of Funding for Management and Conservation? Ecol. Econ. 2016, 128, 202–213. [Google Scholar] [CrossRef]
- Williams, I.D.; Polunin, N.V.C. Differences between Protected and Unprotected Reefs of the Western Caribbean in Attributes Preferred by Dive Tourists. Environ. Conserv. 2000, 27, 382–391. [Google Scholar] [CrossRef]
- Arias-González, J.E.; Fung, T.; Seymour, R.M.; Garza-Pérez, J.R.; Acosta-González, G.; Bozec, Y.-M.; Johnson, C.R. A Coral-Algal Phase Shift in Mesoamerica Not Driven by Changes in Herbivorous Fish Abundance. PLOS ONE 2017, 12, e0174855. [Google Scholar] [CrossRef]
- Cheal, A.J.; MacNeil, M.A.; Cripps, E.; Emslie, M.J.; Jonker, M.; Schaffelke, B.; Sweatman, H. Coral–Macroalgal Phase Shifts or Reef Resilience: Links with Diversity and Functional Roles of Herbivorous Fishes on the Great Barrier Reef. Coral Reefs 2010, 29, 1005–1015. [Google Scholar] [CrossRef]
- Gill, D.A.; Schuhmann, P.W.; Oxenford, H.A. Recreational Diver Preferences for Reef Fish Attributes: Economic Implications of Future Change. Ecol. Econ. 2015, 111, 48–57. [Google Scholar] [CrossRef]
- Graham, N.; Dulvy, N.; Jennings, S.; Polunin, N. Size-Spectra as Indicators of the Effects of Fishing on Coral Reef Fish Assemblages. Coral Reefs 2005, 24, 118–124. [Google Scholar] [CrossRef]
- Froese, R.; Pauly, D. FishBase. World Wide Web Electronic Publication. Available online: https://www.fishbase.se/summary/citation.php (accessed on 13 June 2023).
- Fox, J.; Weisberg, S. An R Companion to Applied Regression; SAGE Publications, 2018; ISBN 978-1-5443-3648-0. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing 2022.
- Souter, D.; Planes, S.; Wicquart, J.; Logan, M.; Obura, D. Status of Coral Reefs of the World: 2020 Report. Global Coral Reef Monitoring Network (GCRMN)/International Coral Reef Initiative (ICRI) 2021.
- McField, M.; Soto, M.; Craig, N.; Giro, A.; Drysdale, I.; Guerrero, C.; Rueda, M.; Kramer, P.; Canty, S.; Muñiz, I. 2022 Mesoamerican Reef Report Card. Healthy Reefs Initiative 2022.
- Huntington, B.E.; Miller, M.W.; Pausch, R.; Richter, L. Facilitation in Caribbean Coral Reefs: High Densities of Staghorn Coral Foster Greater Coral Condition and Reef Fish Composition. Oecologia 2017, 184, 247–257. [Google Scholar] [CrossRef]
- Newman, M.J.H.; Paredes, G.A.; Sala, E.; Jackson, J.B.C. Structure of Caribbean Coral Reef Communities across a Large Gradient of Fish Biomass. Ecol. Lett. 2006, 9, 1216–1227. [Google Scholar] [CrossRef] [PubMed]
- Santavy, D.L.; Courtney, L.A.; Fisher, W.S.; Quarles, R.L.; Jordan, S.J. Estimating Surface Area of Sponges and Gorgonians as Indicators of Habitat Availability on Caribbean Coral Reefs. Hydrobiologia 2013, 707, 1–16. [Google Scholar] [CrossRef]
- Wulff, J. Sponge Contributions to the Geology and Biology of Reefs: Past, Present, and Future. In Coral Reefs at the Crossroads; Hubbard, D.K., Rogers, C.S., Lipps, J.H., Stanley, Jr., George, D., Eds.; Coral Reefs of the World; Springer Netherlands: Dordrecht, 2016; pp. 103–126. ISBN 978-94-017-7567-0. [Google Scholar]
- Bell, J.J.; Rovellini, A.; Davy, S.K.; Taylor, M.W.; Fulton, E.A.; Dunn, M.R.; Bennett, H.M.; Kandler, N.M.; Luter, H.M.; Webster, N.S. Climate Change Alterations to Ecosystem Dominance: How Might Sponge-Dominated Reefs Function? Ecology 2018, 99, 1920–1931. [Google Scholar] [CrossRef]
- Mumby, P.J.; Hastings, A.; Edwards, H.J. Thresholds and the Resilience of Caribbean Coral Reefs. Nature 2007, 450, 98–101. [Google Scholar] [CrossRef]
- Valdez, M.F.; Villalobos, C.R. Distribución espacial, correlación con el substrato y grado de agregación en Diadema antillarum Philippi (Echinodermata: Echinoidea). Rev. Biol. Trop. 1978, 26, 237–245. [Google Scholar]
- Alvarado, J.J.; Cortes, J.; Salas, E. Population Densities of Diadema Antillarum Philippi at Cahuita National Park (1977-2003), Costa Rica. Carib J Sci 2004, 40, 257–259. [Google Scholar]
- Carpenter, R.C.; Edmunds, P.J. Local and Regional Scale Recovery of Diadema Promotes Recruitment of Scleractinian Corals. Ecol. Lett. 2006, 9, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Edmunds, P.J.; Carpenter, R.C. Recovery of Diadema Antillarum Reduces Macroalgal Cover and Increases Abundance of Juvenile Corals on a Caribbean Reef. Proc. Natl. Acad. Sci. 2001, 98, 5067–5071. [Google Scholar] [CrossRef] [PubMed]
- Idjadi, J.A.; Haring, R.N.; Precht, W.F. Recovery of the Sea Urchin Diadema Antillarum Promotes Scleractinian Coral Growth and Survivorship on Shallow Jamaican Reefs. Mar. Ecol. Prog. Ser. 2010, 403, 91–100. [Google Scholar] [CrossRef]
- Myhre, S.; Acevedo-Gutiérrez, A. Recovery of Sea Urchin Diadema Antillarum Populations Is Correlated to Increased Coral and Reduced Macroalgal Cover. Mar. Ecol. Prog. Ser. 2007, 329, 205–210. [Google Scholar] [CrossRef]
- Williams, S.M. The Reduction of Harmful Algae on Caribbean Coral Reefs through the Reintroduction of a Keystone Herbivore, the Long-Spined Sea Urchin Diadema Antillarum. Restor. Ecol. 2022, 30, e13475. [Google Scholar] [CrossRef]
- Lessios, H. a. The Great Diadema Antillarum Die-Off: 30 Years Later. Annu. Rev. Mar. Sci. 2016, 8, 267–283. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Barreras, R.; Montañez-Acuña, A.; Otaño-Cruz, A.; Ling, S.D. Apparent Stability of a Low-Density Diadema Antillarum Regime for Puerto Rican Coral Reefs. ICES J. Mar. Sci. 2018, 75, 2193–2201. [Google Scholar] [CrossRef]
- Spadaro, A.J.; Butler, M.J. Herbivorous Crabs Reverse the Seaweed Dilemma on Coral Reefs. Curr. Biol. 2021, 31, 853–859.e3. [Google Scholar] [CrossRef]
- Jackson, J.B.C.; Kirby, M.X.; Berger, W.H.; Bjorndal, K.A.; Botsford, L.W.; Bourque, B.J.; Bradbury, R.H.; Cooke, R.; Erlandson, J.; Estes, J.A.; et al. Historical Overfishing and the Recent Collapse of Coastal Ecosystems. Science 2001, 293, 629–637. [Google Scholar] [CrossRef]
- Pandolfi, J.M.; Bradbury, R.H.; Sala, E.; Hughes, T.P.; Bjorndal, K.A.; Cooke, R.G.; McArdle, D.; McClenachan, L.; Newman, M.J.H.; Paredes, G.; et al. Global Trajectories of the Long-Term Decline of Coral Reef Ecosystems. Science 2003, 301, 955–958. [Google Scholar] [CrossRef]
- Laguna Cruz, M.; Pereira-Chaves, J.; Ríos Duarte, R. Influencia del pez León (Pterois miles y Pterois volitans) en la cadena de valor de la pesca artesanal, Caribe sur de Costa Rica. UNED Res. J. 2019, 11. [Google Scholar] [CrossRef]
- Suchley, A.; McField, M.D.; Alvarez-Filip, L. Rapidly Increasing Macroalgal Cover Not Related to Herbivorous Fishes on Mesoamerican Reefs. PeerJ 2016, 4, e2084. [Google Scholar] [CrossRef]
- González-De Zayas, R.; Rossi, S.; Hernández-Fernández, L.; Velázquez-Ochoa, R.; Soares, M.; Merino-Ibarra, M.; Castillo-Sandoval, F.S.; Soto-Jiménez, M.F. Stable Isotopes Used to Assess Pollution Impacts on Coastal and Marine Ecosystems of Cuba and México. Reg. Stud. Mar. Sci. 2020, 39, 101413. [Google Scholar] [CrossRef]
- Lapointe, B.E.; Brewton, R.A.; Herren, L.W.; Porter, J.W.; Hu, C. Nitrogen Enrichment, Altered Stoichiometry, and Coral Reef Decline at Looe Key, Florida Keys, USA: A 3-Decade Study. Mar. Biol. 2019, 166, 108. [Google Scholar] [CrossRef]
- Lapointe, B.E.; Tewfik, A.; Phillips, M. Macroalgae Reveal Nitrogen Enrichment and Elevated N:P Ratios on the Belize Barrier Reef. Mar. Pollut. Bull. 2021, 171, 112686. [Google Scholar] [CrossRef]
- Wakwella, A.; Mumby, P.J.; Roff, G. Sedimentation and Overfishing Drive Changes in Early Succession and Coral Recruitment. Proc. R. Soc. B Biol. Sci. 2020, 287, 20202575. [Google Scholar] [CrossRef] [PubMed]
- Sura, S.A.; Bell, A.; Kunes, K.L.; Turba, R.; Songer, R.; Fong, P. Responses of Two Common Coral Reef Macroalgae to Nutrient Addition, Sediment Addition, and Mechanical Damage. J. Exp. Mar. Biol. Ecol. 2021, 536, 151512. [Google Scholar] [CrossRef]
- Gordon, S.E.; Goatley, C.H.R.; Bellwood, D.R. Low-Quality Sediments Deter Grazing by the Parrotfish Scarus Rivulatus on Inner-Shelf Reefs. Coral Reefs 2016, 35, 285–291. [Google Scholar] [CrossRef]
- Wenger, A.S.; Harvey, E.; Wilson, S.; Rawson, C.; Newman, S.J.; Clarke, D.; Saunders, B.J.; Browne, N.; Travers, M.J.; Mcilwain, J.L.; et al. A Critical Analysis of the Direct Effects of Dredging on Fish. Fish Fish. 2017, 18, 967–985. [Google Scholar] [CrossRef]
- Samper-Villarreal, J.; Sagot-Valverde, J.G.; Gómez-Ramírez, E.H.; Cortés, J. Water Quality as a Potential Factor Influencing Seagrass Change Over Time at Cahuita National Park, Costa Rica. Caribb. J. Sci. 2021, 51, 72–85. [Google Scholar] [CrossRef]
- Alfaro-Sandí, J.; Piedra-Marín, G.; Saravia-Arguedas, A.Y.; Piedra-Castro, L.; Alfaro-Sandí, J.; Piedra-Marín, G.; Saravia-Arguedas, A.Y.; Piedra-Castro, L. Evaluación de los parámetros físicos y químicos del agua de mar en los alrededores de la Isla Uvita, Limón, Costa Rica. Rev. Tecnol. En Marcha 2021, 34, 88–95. [Google Scholar] [CrossRef]
- D’Croz, L.; Rosario, J.B. del; Gondola, P. The Effect of Fresh Water Runoff on the Distribution of Dissolved Inorganic Nutrients and Plankton in the Bocas Del Toro Archipelago, Caribbean Panama. Caribb. J. Sci. 2005. [Google Scholar]
- D’Croz, L.; Robertson, D.R. Coastal Oceanographic Conditions Affecting Coral Reefs on Both Sides of the Isthmus of Panama. In Proceedings of the Proceedings of the 8th International Coral Reef Symposium; 1997; Volume 2, pp. 3–205. [Google Scholar]
- Roder, C.; Cortés, J.; Jiménez, C.; Lara, R. Riverine Input of Particulate Material and Inorganic Nutrients to a Coastal Reef Ecosystem at the Caribbean Coast of Costa Rica. Mar. Pollut. Bull. 2009, 58, 1937–1943. [Google Scholar] [CrossRef]
- Muller-Parker, G.; Cortés, J. Spatial Distribution of Light and Nutrients in Some Coral Reefs of Costa Rica during January 1997. Rev. Biol. Trop. 2001, 49, 251–263. [Google Scholar]
- Badilla-Aguilar, A.; Mora-Alvarado, D. Calidad sanitaria de las aguas superficiales en litorales de Costa Rica: situación del 2012 al 2018. Rev. Tecnol. En Marcha 2019. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




