Introduction
Globally, people are becoming more concerned about antibiotic pollution of the environment in general and aquatic ecosystems in particular [
1,
2]. Antibiotics are necessary medications in hospitals, and significant volumes of antibiotics are excreted and disposed of in hospital wastewater. The extensive and unauthorized use of antibiotics in Bangladesh has become an issue in addressing the presence of residual antibiotics in wastewater. Wastewater is of two types; urban wastewater includes shops, offices and factories, farms, transport, fuel depots, and mines [
3]. Domestic wastewater is used in homes and offices’ toilets, showers, baths, kitchen sinks, and laundries. Wastewater can be a significantly focusing reservoir and environmental supplier of antibiotics resistance organisms [
4]. They have been proposed as hotspots for gene transfer and can spread antibiotic-resistant genes between bacterial species [
5]. This wastewater contains antibiotics which can form a selection pressure for antibiotic resistance even in low concentrations [
6]. Antibiotics come from a variety of sources, including humans, animals, agriculture, aquaculture, and the pharmaceutical industry. The majority of antibiotics are used by humans, who are also largely to blame for the amount of antibiotics that end up in sewage. The problem with the water cycle and food chain is made worse by reusing treated wastewater containing partially decomposed antibiotic residues [
7]. Other sources, disposal of unused drugs which are date expired, hospital effluent, pharmaceutical wastes, and animal waste contribute to increasing the higher concentration of wastewater.
A wide range of research has been conducted in Europe and other developed countries on residual antibiotics and identifying resistant microorganisms [
8]. Only a few review works have been focused on residual antibiotics in Bangladesh [
9,
10]. Yet, there needs to be more knowledge in Bangladesh about the residual antibiotics that remain in wastewater before it is released into the environment and the sewerage system. There needs to be comprehensive information on residual antibiotics and resistant microorganisms. Minimal work has been done in the plant, fish, and poultry feed [
11,
12]. This study aims to develop quick methods for detecting mainly used residual antibiotics Doxycycline, Ciprofloxacin, and Tetracycline in wastewater collected from the treatment plant of Pagla, Kadamtali, Dhaka from eight different treatment steps. The extension of the study also identifies the bacterial species present in the waste water and transformed resistant to two significant antibiotics.
Materials
All three standard antibiotics were purchased from Sigma-Aldrich (Germany). All other solvents of HPLC grade were purchased from Sigma Aldrich (Germany) through local certified vendors; Deionized water was used for standard and sample preparation. 0.45µm cellulose acetate Millipore microfilter was purchased from Pall ® Corporation, India, and the PTFE syringe filter from Membrane Solution.
Methods
Pagla Wastewater Treatment Plant: Dhaka has only one wastewater treatment plant located at Pagla, Kadamtali, Dhaka. The factory can produce 130,000 m3/day. The facility has 49,000 household sewer connections, 778 km of sewer lines with various diameters, 20 lift stations for sewers, one pump station in the center, and three truck interceptor sewers. The neighboring river Buriganga receives the treated wastewater for discharge [
13].
Figure 1.
Waste water Treatment Plant.
Figure 1.
Waste water Treatment Plant.
Sample Collection:
Collection jars were washed per the laboratory’s washing protocol and dried in an oven for wastewater collection. About 200mL of wastewater was collected from 8 different points of the treatment plant at Pagla, Kadamtali. Samples were collected and named as A-Raw sewage (bar screen chamber), B-Equalization collection tank, C-primary settling tank, D-aeration tank, E-Secondary settling tank, F-chlorination dosing tank, G-dual media filter tank and H-treated water for reuse
Table 1. Samples of aliquots were made and preserved at 4oC for further study. One part was sent for microbiological analysis, and the other was used to detect the presence of residual antibiotics by HPLC. All wastewater samples were tested for temperature, pH, TDS, EC, Time, and Location, shown in
Table 2.
Sample Preparation: All collected samples, about 200 mL of water, were filtered with 0.45µm of nylon membrane filter to remove impurities. Samples were air-dried for several hours and reached a volume of 180mL.
Instrument: Devices used included the HPLC high gradient pump system, autosampler, degasser, heated oven, and photodiode array (PDA) detector
. HPLC analysis of antibiotic content in the wastewater samples was performed according to previously published method [
14,
15]; the mobile phase used was 5% acetic acid, Methanol, and Acetonitrile (v/v 55:20:25) for Doxycycline at a flow rate of 1.0 mL/min, with a wavelength of 347. At the same time, Ciprofloxacin and Tetracycline were run separately with 10% acetic acid and Acetonitrile (90:10) at a flow rate of 1 mL/min and wavelength at 280 nm. Chromatography used 20 μL of the injected liquid at ambient temperature on a reverse column C18 (250 mm × 4.6 mm). HPLC condition is shown in
Table 3.
Standard Preparation: Weighing the approved standard (Doxycycline, Ciprofloxacin, and Tetracycline) and dissolving it in double-distilled water produced stock solutions of 10 mg L-1 [
16]. All working solutions and standards were created using these solutions. At -20°C, all solutions were kept in a brown glass bottle. By diluting sections of the stock with double distilled water and filtering through 0.45 m nylon membrane micro-filters, the standards (1, 2, 4, 8, and 10 ppm) were created [
17].
Analysis of wastewater for antibiotics by HPLC: Shimadzu Model SPD-M20A High-Performance Liquid Chromatography (HPLC) was employed. The material was injected into the HPLC column at about 20 L. Antibiotics in wastewater samples were determined by contrasting the HPLC peaks with the appropriate standard solution peaks. A calibration curve from conventional solutions and retention times was used to calculate the concentration.
Standard Preparation
Microbiological Analysis: Using the accepted techniques for examining water and wastewater, water sample analysis for bacterial analysis was performed [
18]. The total heterotrophic bacterial count and total coliform were determined as part of the bacteriological examination utilizing the serial dilution method and pour plate procedures [
19]. Aerobic heterotrophic bacteria were counted and isolated using tryptone soy agar (TSA; Oxoid Ltd., Basingstoke, UK). The identification and isolation of coliform bacteria were carried out using MacConkey agar (Difco), SS agar (Diagnostic Pasteur), and Thiosulphate citrate bile-salt sucrose (TCBS) agar media. Fecal coliform Plates were incubated at 44.5 °C for 24 hours, while inoculated Petri dishes were incubated at 37 °C. Immediately following the counting, isolated bacterial colonies were discovered. Gram-positive aerobic heterotrophic bacterial isolates were identified using Bergey’s Manual for Systematic Bacteriology [
20]. The Manual for Laboratory Investigations of Acute Enteric Infections was used to determine enteric bacteria, on the other hand [
21]. Following the manufacturer’s instructions, an API 20E kit was used to identify and separate the Gram-negative bacteria of the Enterobacteriaceae family.
Antibiotic susceptibility testing: According to CLSI’s advice, antimicrobial susceptibility testing was performed using the disk diffusion method on Muller Hinton agar (MHA) after the Kirby-Bauer procedure [
22]. According to Panda et al.’s instructions, the bacteria were aerobically cultivated in TSB at 37 °C for 24 hours before being suspended in sterile saline at a density corresponding to the 0.5 McFarland standard [
23]. Mueller Hinton agar plates were inoculated by spreading 100 l of the bacterial suspensions evenly across the surface of the agar, letting the plates dry, and then covering them with antibiotic discs. Three antibiotics—Ciprofloxacin (5 mg), Doxycycline (30 mg), and Tetracycline (30 mg]—were examined. The plates were then incubated at 37°C for 24 hours, after which the zones of inhibition were quantified and categorized as susceptible (S), intermediate (I), or resistant (R) using the CLSI chart [
24].
Result
For the quantitative evolution of residual antibiotics and microbial resistance and sensitivity test in the raw and finished water, HPLC was used on all eight samples obtained from the Pagla wastewater plant’s eight distinct treatment phases. The natural and finished wastewater were numbered A to H. Qualitative assessment of all eight water samples shows acidic to neutral (4.19 to 7.07) pH and temperature ranging about 31oC to 34
oC. Both Electric Conductivity (EC) and Total Dissolve Solids (TDS) are above average in all steps and gradually decrease in the finished water sample (H)
Table 2.
Doxycycline and Ciprofloxacin were frequently detected in all eight samples, but no Tetracycline was seen in any of the samples. The statistical analysis shows a significant difference (p=0.02) between antibiotics detected in raw and finished water samples
Table 4. And Figure 2. shows the HPLC peaks of all three antibiotics in eight water samples.
The study also determines the bacterial load in eight different steps of water samples.
Table 5 shows the average number of heterotrophic bacteria and total coliforms.
The viable aerobic heterotrophic bacteria ranged between 5.421 and 4.754 log cfu/ml. The highest load was observed in a sample of Secondary setting tank-E (5.421 log cfu/ml), while the lowest count (4.754 log cfu/ml) was found in the sample of dual media filter-G. The maximum values of total coliforms were (3.402 log cfu/ml) in the sample of primary setting tank-E, and a minimum count (1.014 log cfu/ml) was observed for the sample in finished water for re-use-H. The highest load for fecal coliform was observed in a sample of equalization collection tank B (1.716 log cfu/ml), and no fecal coliform was found in the treated water sample for re-use-H. However, the total bacterial load decreases from the E step (9.3 log cfu/mL) to H (5.9 log cfu/mL).
During this study, 160 discrete bacterial colonies were primarily isolated, and finally, 30 selected bacterial isolates were studied in detail for identification and their drug resistance pattern to Doxycycline, Tetracycline, and Ciprofloxacin. All of the chosen isolates were catalase-positive and aerobic. Most organisms could hydrolyze starch, were motile, and were methyl red positive. Tests on the isolated organisms’ physiological and biochemical makeup revealed that they belonged to nine different genera,
viz., Bacillus, Alcaligenes, Escherichia coli, Enterobacter cloacae, Klebsiella, Salmonella, Pseudomonas, Citrobacter freundii, C. youngae and Staphylococcus (
Table 6).
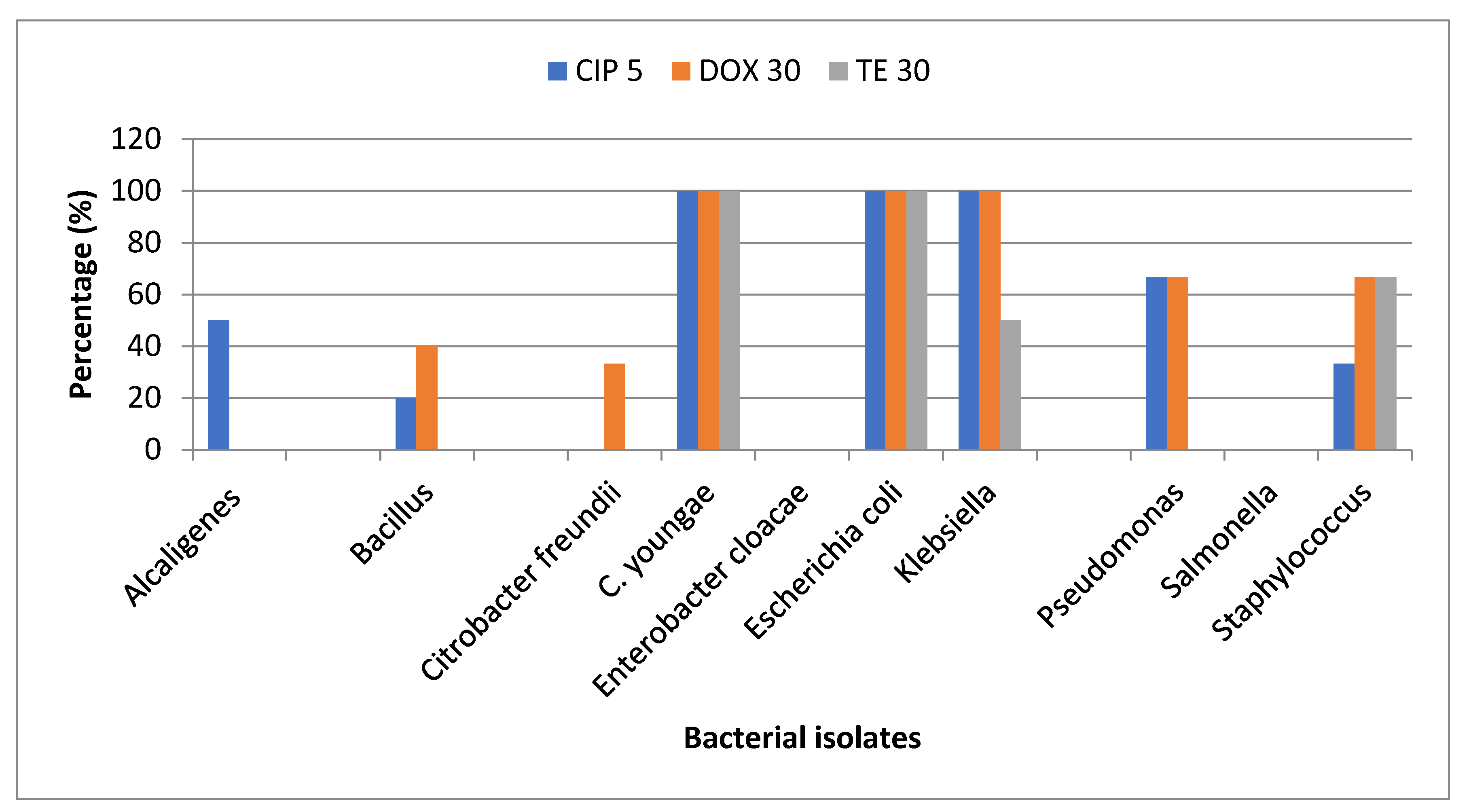
Antibiotic susceptibility testing: Antibiotic susceptibility analysis to detect resistant bacteria was performed using Doxycycline (30 µg), Ciprofloxacin (5 µg), and Tetracycline (30 µg). Any bacterium presenting a resistance phenotype in 3 classes of antibiotics is a multi-drug resistant (MDR) bacterium [
25]. According to the resistant pattern definition,
Escherichia coli, Klebsiella, and
C. youngae were MDR (
Table 7).
Most of the isolated Bacillus sp. were resistant to Doxycycline, and all were sensitive to Tetracycline. All the selected E. coli isolated from Site-A, C, D, E, and F were resistant to the tested three antibiotics. Klebsiella isolated from site-A, Staphylococcus isolated from site-C, and C. youngae isolated from site-C and D were found resistant to all the tested antibiotics. Enterobacter cloacae and Salmonella isolated from different sites were susceptible to all the tested antibiotics. (Figure 2).
Figure 2.
Percentage of selected isolates resistant to tested antibiotics.
Figure 2.
Percentage of selected isolates resistant to tested antibiotics.
Discussion
The “Pagla” wastewater plant’s antibiotic residual levels have been measured in the current investigation. Samples were taken at eight different treatment stages. The frequency of antibiotic prescriptions in hospital inpatient wards, the stability of the environment, and the effects of antibiotics on the environment are known or suspected. The antibiotic prescription pattern in hospital inpatient wards, the environmental stability, and the suspected and known environmental impact of an antibiotic.
In this study, all eight collected samples were subjected to HPLC for qualitative and quantitative evaluation of residual antibiotics in raw and finished water. Physical analysis of the samples; temperature, pH, TDS, EC, time, and location were also analyzed. The higher value of TDS and ES indicates the presence of impurities and solid particles in the water. Water’s TDS concentration indicates the presence of inorganic salts and trace amounts of organic matter, while its EC value indicates how well it can conduct an electric current [
16]. Natural phenomena, including geological conditions and seawater, as well as human activities like residential and industrial waste and agriculture, are the material sources for TDS and EC [
17,
18,
19], which shows the existence of impurities and solid particles in the water.
Although pH has no direct effect on the consumer, it is one of the most crucial operational water quality characteristics because it affects the aquatic life, solubility of solids, taste and odor of solids, and the ability of solids to dissolve oxygen [
26]. The pH of the water should range from 6.5 to 8.5, as suggested by the WHO, [
27], which is consistent with this finding for G and H steps. Increased concentration of Doxycycline and Ciprofloxacin is used not only for humans but also for animals and fish. Doxycycline and Tetracycline are used for many types of infection, including respiratory tract infection. The high concentration of Doxycycline in the finished wastewater indicates the effective use of Doxycycline rather than Tetracycline and Ciprofloxacin. Another reason is that the pandemic facilitated Doxycycline and Ciprofloxacin use. Studies show hospital and pharmaceutical waste also contribute to these residual values. It has been suggested that its lack of detection in the last phase of the treatment plant is due to the easy destruction of the -lactam ring, its high metabolic rate, and the process of decarboxylation [
28].
Only used for animal therapies, enrofloxacin can occasionally be converted to Ciprofloxacin under specific circumstances [
28]. Therefore, it is important to consider the likelihood and severity of pollution from animal sources.
To lessen toxicity and increase biodegradability, complex organic molecules cannot be broken down by conventional wastewater treatment facilities. New technologies, such as sophisticated oxidation processes, must be created to meet this need.
It is found that the studied wastewater treatment plant efficiently reduces the total coliform and total bacterial load except for Aerobic heterotrophic bacteria. This might be brought on by aerobic heterotrophic bacteria that are Doxycycline- and other antibiotic-resistant in the absence of doxycycline residue.
In this study, the maximum total viable count was found in Secondary setting tank-E (9.3 log cfu/ml), and the lowest count (5.999 log cfu/ml) was found in the treated water. A similar observation was also reported by Saha et al [
29]. In Saidabad Water Treatment Plant, 3.78 log10 cfu/100 ml was the average of the maximum and least values of total coliforms in raw water. Coliforms were not detected in treated water in Saidabad Water Treatment Plant [
30]. The present study found coliforms in treated water (1.014 log cfu/ml), but fecal coliforms were not detected in treated water. The study showed that the Pagla sewage treatment plant significantly reduced bacterial counts in the final treatment. There was no fecal coliform found in site H. However, coliform and heterotrophic bacterial counts in effluent from the treatment plant exceeded according to WHO [
31].
Three different antibiotics were used to assess the susceptibility of the isolates, and from the results, their antibiotic resistance profiles and numerous antibiotic resistance phenotypes were compiled. Antibiotic resistance is one of the world’s most burning public health concerns. Antibiotic-resistant bacteria can spread swiftly and consequently threaten the environment with new strains of infectious disease.
Among bacterial isolates,
E. coli and
C. youngae were 100% resistant to Tetracycline, Doxycycline, and Ciprofloxacin, following the study conducted by many research groups where most isolates were also resistant to Doxycycline, erythromycin and Tetracycline (92.9%) [
32,
33]. This is not in agreement with yet another observation, where all the isolates were found to be susceptible to Ciprofloxacin in
E. coli and
Pseudomonas was observed [
34].
Conclusions
According to the study’s findings, Ciprofloxacin and Doxycycline were found in both the finished and raw water. This can lead to the emergence of bacteria that are resistant to antibiotics, which has grave consequences. Due to the adverse effects of lifetime exposure to Ciprofloxacin and Doxycycline, even at low levels, the potential persistence of these drugs in water sources is a serious concern. As traditional antibiotics cannot treat some diseases caused by pathogenic resistant bacteria, antibiotic contamination of drinking water must be addressed immediately. It was a good sign that, Tetracycline was not detected in the Pagla wastewater treatment plant. This study has underlined the significance of a sewage treatment facility in lowering environmental bacterial contamination. However, cleaned wastewater may still contain germs that are resistant to antibiotics. Additionally, the wastewater treatment plant’s high bacterial numbers and antibiotic-resistant bacteria may contribute to the spread of water-borne illnesses and the broader spreading of antimicrobial resistance. This study thus emphasizes the demand for a thorough strategy to address antibiotics. Hospital wastewater is a significant source of antibiotic contamination in nations like Bangladesh. Further analysis is required to determine the relationship between the amounts of antibiotics prescribed in hospitals and the amounts of antibiotic residues discovered in hospital effluent. Further investigation is needed into this impact on environmental bacterial resistance development and its eventual public health implications.
Author Contributions
Conceptualization, Gazi Sultana; Data curation, GNNS, TH; Formal analysis TA, AR; Funding acquisition, GNNS; Investigation, GNNS, TH; Methodology, Gazi Sultana, TH, AAS; Project administration, Gazi Sultana, AAS; Resources, TH, NH; Writing—original draft, GNNS, TH, AAS; Writing—review & editing, Gazi Sultana, NH.
Conflicts of Interest
The authors declare no conflict of interest.
References
- J.-L. Liu and M.-H. Wong, “Pharmaceuticals and personal care products (PPCPs): A review on environmental contamination in China,” Environment International, vol. 59, pp. 208–224, 2013. [CrossRef]
- M. Walczak and W. Donderski, “Antibiotic sensitivity of neustonic bacteria in Lake Jeziorak May,” Polish Journal of Environmental Studies, vol. 13, no. 4, pp. 429–434, 2004.
- S. Manzetti and R. Ghisi, “The environmental release and fate of antibiotics,” Marine Pollution Bulletin, vol. 79, no. 1-2, pp. 7–15, 2014. [CrossRef]
- A. Swiecilo and I. Zych-Wezyk, “Bacterial Stress response as an adaptive to life in a soil environment,” Polish Journal of Environmental Studies, vol. 22, no. 6, p. 157, 2013.
- S. T. Glassmeyer, E. K. Hinchey, S. E. Boehme et al., “Disposal practices for unwanted residential medications in the United States,” Environment International, vol. 35, no. 3, pp. 566-572, 2009. [CrossRef]
- D. K. Brown, Pharmaceutically active compounds in residential and hospital effluent, municipal waste water, and the Rio Grande in Albuquerque, New Mexico, The University of New Mexico, New Mexico, 2009.
- S. Hussain, M. Naeem, and M. N. Chaudhry, “Estimation of residual antibiotics in pharmaceutical effluents and their fate in affected areas,” Polish Journal of Environmental Studies, vol. 25, no. 2, pp. 607–614, 2016. [CrossRef]
- J. P. Bound, K. Kitsou, and N. Voulvoulis, “Household disposal of pharmaceuticals and perception of risk to the environment,” Environmental Toxicology and Pharmacology, vol. 21, no. 3, pp. 301–307, 2006. [CrossRef]
- Md. Khalid A, Abrar S, Kudrat U. J. Water pollution in Bangladesh and its impact on public health (2019), Heliyon 5; e021 45.
- Hassan M, Hassan R, Mahmud MA, Pia HI, Hassa MA, Uddin MJ. Sewage waste water Characteristics and its management in urban areas-A case study at Pagla sewage treatment plant, Dhaka. (2017). Urban and Regional Planing, 2 (3): 13-16. [CrossRef]
- S. Abuin, R. Codony, R. Compa˜ n’ o, M. Granados, and M. D. Prat, “Analysis of macrolide antibiotics in river water by solidphase extraction and liquid chromatography-mass spectrometry,” Journal of Chromatography A, vol. 1114, no. 1, pp. 73–81, 2006.
- M. Seifrtov´ a, L. Nov´akov´ a, C. Lino, A. Pena, and P. Solich, “An overview of analytical methodologies for the determination of antibiotics in environmental waters,” Analytica Chimica Acta, vol. 649, no. 2, pp. 158–179, 2009.
- Hassen A, Saidi N, Cherif M, Boudabous A (1998) Resistance of environmental bacteria to heavy metals. Bioresour Technol 64:7–15. [CrossRef]
- A. Bia?k-Bieli´ nska, J. Kumirska, M. Borecka et al., Selected analytical challenges in the determination of pharmaceuticalsin drinking/marine waters and soil/sediment samples, Journal of Pharmaceutical and Biomedical Analysis, vol. 121, pp. 271–296,2016. [CrossRef]
- I. Taverniers, M. De Loose, and E. Van Bockstaele, Trends in quality in the analytical laboratory. II. Analytical methodvalidation and quality assurance, TrAC Trends in Analytical Chemistry, vol. 23, no. 8, pp. 535–552, 2004. [CrossRef]
- Haq A. K., (2006) Water management in Dhaka. Water Resources Development, 22(2), pp.
- Zuane, J.D., 1996. Handbook of Drinking Water Quality, second ed. John Wiley & Sons, New York.
- American Public Health Association, Standard Methods for Examination of Water and Waste Water, American Public Health Association, 1999.
- Clesceri, L.S., A.E. Greenberg and A.D. Eaton. 1998. Standard methods for examination of water and waste water. APHA. Washington DC. Chapter 9, pp. 140.
- Sneath, P.H.A., N.S. Mair, M.E. Sharpe and J.G. Holt. 1986. Bergey’s manual of systematic bacteriology. Vol. 2. Williams and Wilkins Co., Baltimore. pp. 965-1599.
- WHO. 1987. Manual for laboratory investigations of acute enteric infections. CDD/83.3 Rev. 1. pp. 113.
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically. Approved Standard. CLSI document M07-A10; Wayne, PA, USA, 2015, 10th ed.
- Panda SK, Das R, Leyssen P, Neyts J, Luyten W. Assessing medicinal plants traditionally used in the Chirang Reserve Forest, Northeast India for antimicrobial activity. Journal of ethno pharmacology. 2018 Oct 28; 225:220-33. [CrossRef]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing. CLSI Document M100-S24; Wayne, PA, USA, 2014.
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [CrossRef]
- WHO, 2011. Guidelines for Drinking-Water Quality, fourth ed. World Health Organization.
- S. Manzetti and R. Ghisi, 2014 “The environmental release and fate of antibiotics,” Marine Pollution Bulletin, vol. 79, no. 1-2, pp. 7–15. [CrossRef]
- USP. Veterinary Pharmaceutical Information Monographs Antibiotics. Journal of Veterinary Pharmacology and Therapeutics, vol. 26, supplement, 2003.
- Saha, M.L., Alam, A., Khan, M.R. and Hoque, S., 2012. Bacteriological, Physical And Chemical Properties Of The Pagla Sewage Treatment Plant s Water. Dhaka University Journal of Biological Sciences, 21(1), pp.1-8.
- Hossain, M.A., Begum, T., Fakhruddin, A.N.M. and Khan, S.I., 2006. Bacteriological and physicochemical analyses of the raw and treated water of Saidabad water treatment plant, dhaka. Bangladesh Journal of Microbiology, 23(2), pp.133-136. [CrossRef]
- Cotruvo, J.A., 2017. 2017 WHO guidelines for drinking water quality: first addendum to the fourth edition. Journal?American Water Works Association, 109(7), pp.44-51. [CrossRef]
- Katouli, M., Thompson, J.M., Gündo?du, A. and Stratton, H.M., 2012, June. Antibiotic resistant bacteria in hospital waste waters and sewage treatment plants. In Science forum and stakeholder engagement: Building linkages, collaboration and science quality (pp. 225-229). Brisbane, Australia: Griffith University.
- Moore, J.E., Moore, P.J., Millar, B.C., Goldsmith, C.E., Loughrey, A., Rooney, P.J. and Rao, J.R., 2010. The presence of antibiotic resistant bacteria along the River Lagan. Agricultural Water Management, 98(1), pp.217-221. [CrossRef]
- Mulamattathil, S.G., Bezuidenhout, C., Mbewe, M. and Ateba, C.N., 2014. Isolation of environmental bacteria from surface and drinking water in Mafikeng, South Africa, and characterization using their antibiotic resistance profiles. Journal of pathogens, 2014. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).