Submitted:
13 July 2023
Posted:
13 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Data Sources
2.2. Confounding or Interacting Factors
- Levels of urbanization in each country, as measured by the percentage of the total population residing in urban areas, provided by the World Bank’s database [76]. This information was available for 200 countries at both time points.
- The Gini coefficient, a measure of income inequality at a national level, from the World Bank’s database [77]. This information was available for 168 countries at both time points.
2.3. Data Analysis
3. Results
3.1. Incidence of Infectious Diseases and Mood Disorders in 204 Countries, 1990-2019
| Incidence | 1990 | 2019 | Change (%) | Significance |
|---|---|---|---|---|
| Major depressive disorder | 7.48 (3.04) | 7.10 (3.09) | -3.72 (10.56) | W = 15547.5, p < .001 |
| Bipolar disorder | 0.10 (0.04) | 0.10 (0.04) | 0 (6.26) | W = 9039.5, p = .464 |
| Upper respiratory infections | 42.01 (9.28) | 41.52 (8.51) | -0.80 (5.25) | W = 13013.5, p = .002 |
| Lower respiratory infections | 1.59 (0.76) | 1.14 (0.54) | -26.75 (12.13) | W = 20903.0, p < .001 |
| Enteric infections | 16.19 (7.49) | 19.08 (8.32) | 12.49 (24.99) | W = 3686.5, p < .001 |
| Intestinal nematode infections | 12.93 (33.16) | 7.28 (14.22) | -33.76 (54.66) | W = 10982.0, p < .001 |
| Tropical infectious diseases | 184.03 (1127.66) | 161.69 (812.85) | -20.70 (49.06) | W = 13051.5, p < .001 |
| Other infectious diseases | 1.33 (0.89) | 1.01 (0.28) | -21.77 (13.58) | W = 20904.0, p < .001 |
3.2. Cross-Sectional Associations between the Incidence of Infectious Diseases and Mood Disorders
| Year | Mood Disorder | Infectious Diseases | |||||
|---|---|---|---|---|---|---|---|
| URI | LRI | Enteric | Nematode | Tropical | Other | ||
| 1990 | MDD | -.02 (.814) | .07 (.323) | -.05 (.456) | -.02 (.814) | -.03 (.682) | .03 (.711) |
| BD | .40 (<.001)** | -.31 (<.001)** | -.47 (<.001)** | -.35 (<.001)** | -.29 (<.001)** | -.44 (<.001)** | |
| 2019 | MDD | -.05 (.525) | .11 (.115) | .07 (.309) | .06 (.362) | .02 (.747) | .09 (.214) |
| BD | .41 (<.001)** | -.35 (<.001)** | -.46 (<.001)** | -.33 (<.001)** | -.37 (<.001)** | -.43 (<.001)** | |
| Year | Mood Disorder | Infectious Diseases | |||||
|---|---|---|---|---|---|---|---|
| URI | LRI | Enteric | Nematode | Tropical | Other | ||
| 1990 | MDD | .12 (.209) | -.04 (.670) | -.20 (.036)* | -.16 (.094) | -.25 (.009)* | -.26 (.006)* |
| BD | .00 (.963) | .03 (.735) | -.07 (.481) | -.31 (<.001)** | -.21 (.026)* | -.42 (<.001)** | |
| 2019 | MDD | .04 (.650) | -.09 (.269) | -.04 (.607) | -.20 (.013)* | -.14 (.074) | -.08 (.307) |
| BD | .11 (.158) | -.18 (.026)* | -.23 (.003)* | -.17 (.033)* | -.29 (<.001)** | -.30 (<.001)** | |
3.3. Longitudinal Analyses
3.3.1. Cross-Lagged Regression Analyses
3.3.2. Relationships between Changes in the Incidence of Infectious Diseases and Mood Disorders over Time
3.3.3. Categorical Associations between Changes in the Incidence of Infectious Diseases and Mood Disorders over Time
3.4. Subgroup analyses
3.4.1. Analyses of Tropical and Nematode Infections
3.4.2. Analyses of Possible Interactions between Groups of Infectious Diseases
4. Discussion
4.1. Comparisons with the Existing Literature
4.2. Differential Associations of Infectious Diseases with Major Depression and Bipolar Disorder
4.3. Relationship between the Current Results and Existing Hypotheses
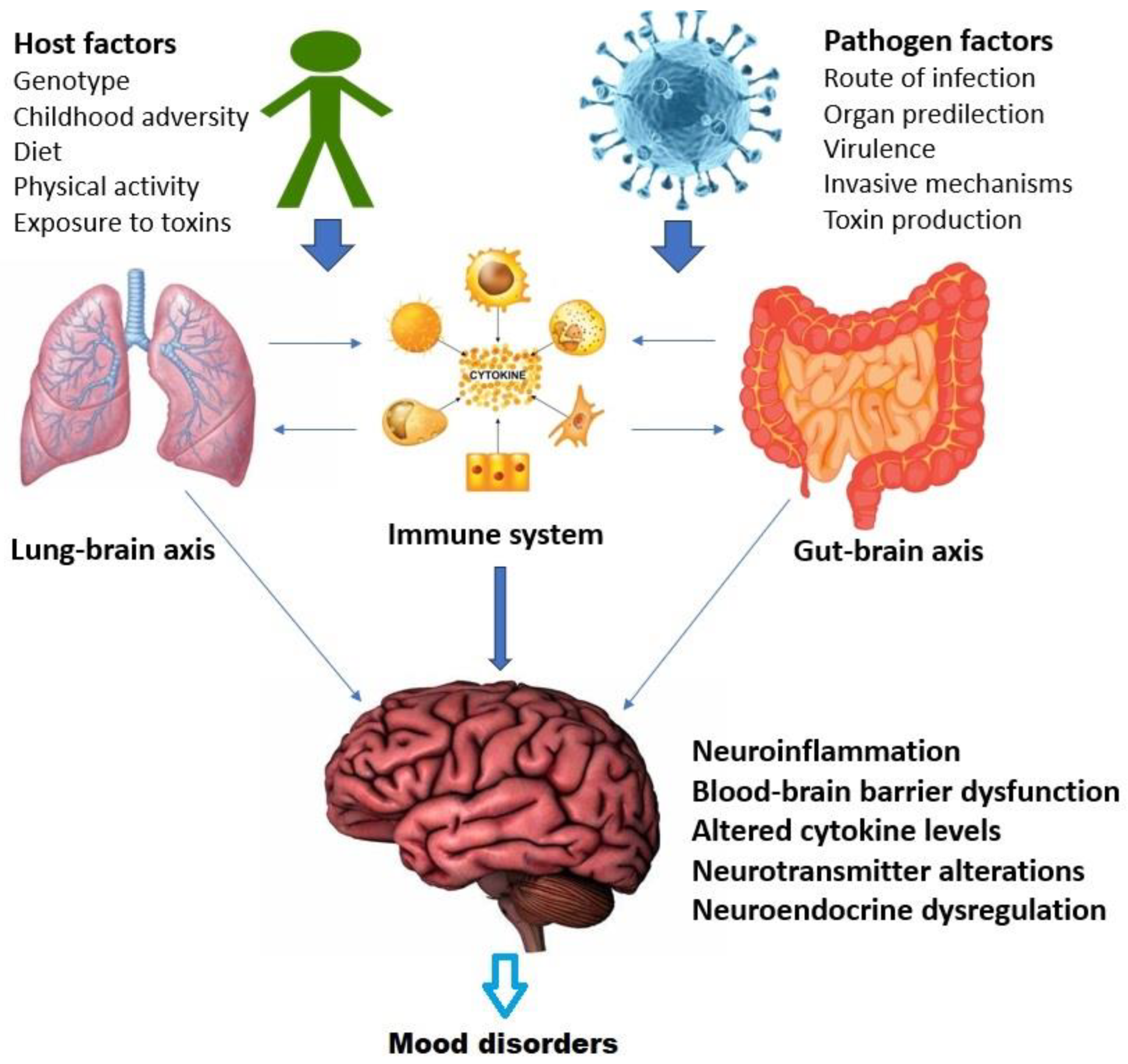
4.4. Possible Causal Mechanisms
4.5. Strengths and Limitations
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Depression and Other Common Mental Disorders: Global Health Estimates. Geneva: World Health Organization, 2017.
- Bromet, E.; Andrade, L.H.; Hwang, I.; Sampson, N.A.; Alonso, J.; di Girolamo, G.; de Graaf, R.; Demyttenaere, K.; Hu, C.; Iwata, N.; Karam, A.N.; Kaur, J.; Kostyuchenko, S.;…; Kessler, R.C. Cross-national epidemiology of DSM-IV major depressive episode. BMC Med. 2011, 9, 90. [CrossRef]
- GBD 2019 Mental Disorders Collaborators. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry 2022, 9, 137-150. [CrossRef]
- Judd, L.L.; Schettler, P.J.; Solomon, D.A.; Maser, J.D.; Coryell, W.; Endicott, J.; Akiskal, H.S. Psychosocial disability and work role function compared across the long-term course of bipolar I, bipolar II and unipolar major depressive disorders. J. Affect. Disord. 2008, 108, 49–58. [Google Scholar] [CrossRef]
- Ratheesh, A.; Davey, C.; Hetrick, S.; Alvarez-Jimenez, M.; Voutier, C.; Bechdolf, A.; McGorry, P.D.; Scott, J.; Berk, M.; Cotton, S.M. A systematic review and meta-analysis of prospective transition from major depression to bipolar disorder. Acta Psychiatr. Scand. 2017, 135, 273–284. [Google Scholar] [CrossRef]
- Vandeleur, C.L.; Merikangas, K.R.; Strippoli, M-P.F.; Castelao, E.; Preisig, M. Specificity of psychosis, mania and major depression in a contemporary family study. Mol. Psychiatry 2014, 19, 209-213. [CrossRef]
- Sowa-Kucma, M.; Styczen, K.; Siwek, M.; Misztak, P.; Nowak, R.J.; Dudek, D.; Rybakowski, J.K.; Nowak, G.; Maes, M. Are there differences in lipid peroxidation and immune biomarkers between major depression and bipolar disorder: effects of melancholia, atypical depression, severity of illness, episode number, suicidal ideation and prior suicide attempts. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 81, 372–383. [Google Scholar] [CrossRef]
- Swann, A.C. Mixed features: evolution of the concept, past and current definitions, and future prospects. CNS Spectr. 2017, 22, 161–169. [Google Scholar] [CrossRef]
- Merikangas, K.R.; Cui, L.; Heaton, L.; Nakamura, E.; Roca, C.; Ding, J.; Qin, H.; Guo, W.; Shugart, Y.Y.; Zarate, C.; Angst, J. Independence of familial transmission of mania and depression: results of the NIMH family study of affective spectrum disorders. Mol. Psychiatry 2014, 19, 214–219. [Google Scholar] [CrossRef]
- Kessler, R.C.; Bromet, E.J. The epidemiology of depression across cultures. Annu. Rev. Public Health 2013, 34, 119–138. [Google Scholar] [CrossRef] [PubMed]
- Stansfeld, S.A.; Clark, C.; Rodgers, B.; Caldwell, T.; Power, C. Repeated exposure to socioeconomic disadvantage and health selection as life course pathways to mid-life depressive and anxiety disorders. Soc. Psychiatry Psychiatr. Epidemiology 2011, 46, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Kavanaugh, S.A.; Neppl, T.K.; Melby, J.N.; Iowa State University. Economic pressure and depressive symptoms: testing the family stress model from adolescence to adulthood. J. Fam. Psychol. 2018, 32, 957-965. [CrossRef]
- Hande, S.H.; Krishna, S.M.; Sahote, K.K.; Dev, N.; Erl, T.P.; Ramakrishna, K.; Ravidhran, R.; Das, R. Population genetic variation of SLC6A4 gene, associated with neurophysiological development. J. Genet. 2021, 100, 16. [Google Scholar] [CrossRef] [PubMed]
- Cai, N.; Choi, K.W.; Fried, E.I. Reviewing the genetics of heterogeneity in depression: operationalizations, manifestations and etiologies. Hum. Mol. Genet. 2020, 29, R10–R18. [Google Scholar] [CrossRef] [PubMed]
- Kasof, J. Cultural variation in seasonal depression: cross-national differences in winter versus summer patterns of seasonal affective disorder. J. Affect. Disord. 2009, 115, 79–86. [Google Scholar] [CrossRef]
- Westover, A.N.; Marangell, L.B. A cross-national relationship between sugar consumption and major depression? Depress. Anxiety 2002, 16, 118–120. [Google Scholar] [CrossRef] [PubMed]
- Molendijk, M.; Molero, P.; Sanchez-Pedreno, F.O.; Van der Does, W.; Martinez-Gonzalez, M.A. Diet quality and depression risk: a systematic review and dose-response meta-analysis of prospective studies. J. Affect. Disord. 2018, 226, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Lambert, K.G.; Nelson, R.J.; Jovanovic, T.; Cerda, M. Brains in the city: neurobiological effects of urbanization. Neurosci. Biobehav. Rev. 2015, 58, 107–122. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.; Burns, J.K.; Dhingra, M.; Tarver, L.; Kohrt, B.A.; Lund, C. Income inequality and depression: a systematic review and meta-analysis of the association and a scoping review of mechanisms. World Psychiatry 2018, 17, 76–89. [Google Scholar] [CrossRef]
- Luthar, S.S. The culture of affluence: psychological costs of material wealth. Child Dev. 2003, 74, 1581–1593. [Google Scholar] [CrossRef]
- Way, B.M.; Lieberman, M.D. Is there a genetic contribution to cultural differences? Collectivism, individualism, and genetic markers of social sensitivity. Soc. Cogn. Affect. Neurosci. 2010, 5, 203–211. [Google Scholar] [CrossRef]
- Chiao, J.Y.; Blizinsky, K.D. Culture-gene coevolution of individualism-collectivism and the serotonin transporter gene. Proc. R. Soc. B. 2010, 277, 529–537. [Google Scholar] [CrossRef]
- Cole, S.W.; Arevalo, J.M.G.; Takahashi, R.; Sloan, E.K.; Lutgendorf, S.K.; Sood, A.K.; Sheridan, J.F.; Seeman, T.E. Computational identification of gene-social environment interaction at the human IL6 locus. Proc. Natl. Acad. Sci. U. S. A. 2010, 107, 5681–5686. [Google Scholar] [CrossRef]
- Tartter, M.; Hammen, C.; Bower, J.E.; Brennan, P.A.; Cole, S. Effects of chronic interpersonal stress exposure on depressive symptoms are moderated by genetic variation at IL6 and IL1β in youth. Brain Behav. Immun. 2015, 46, 104–111. [Google Scholar] [CrossRef]
- Dantzer, R.; Kelley, K.W. Twenty years of research on cytokine-induced sickness behavior. Brain Behav. Immun. 2007, 21, 153–160. [Google Scholar] [CrossRef]
- Maes, M.; Berk, M.; Goehler, L.; Song, C.; Anderson, G.; Galecki, P.; Leonard, B. Depression and sickness behavior are Janus-faced responses to shared inflammatory pathways. BMC Med. 2012, 10, 66. [Google Scholar] [CrossRef] [PubMed]
- Stieglitz, J.; Trumble, B.C.; Thompson, M.E.; Blackwell, A.D.; Kaplan, H.; Gurven, M. Depression as sickness behavior? A test of the host defense hypothesis in a high pathogen population. Brain Behav. Immun. 2015, 49, 130–139. [Google Scholar] [CrossRef]
- Lasselin, J. Back to the future of psychoneuroimmunology: studying inflammation-induced sickness behavior. Brain Behav. Immun. Health 2021, 18, 100379. [Google Scholar] [CrossRef] [PubMed]
- Kohler, C.A.; Freitas, T.H.; Maes, M.; de Andrade, N.Q.; Liu, C.S.; Fernandes, B.S.; Stubbs, B.; Solmi, M.; Veronese, N.; Herrmann, N.; Raison, C.L.; Miller, B.J.; Lanctot, K.L.; Carvalho, A.F. Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatr. Scand. 2017, 135, 373–387. [Google Scholar] [CrossRef] [PubMed]
- Raison, C.L.; Lowry, C.A.; Rook, G.A.W. Inflammation, sanitation, and consternation: loss of contact with coevolved, tolerogenic microorganisms and the pathophysiology and treatment of major depression. Arch. Gen. Psychiatry 2010, 67, 1211–1224. [Google Scholar] [CrossRef] [PubMed]
- Rook, G.A.W. Review series on helminths, immune modulation and the hygiene hypothesis: the broader implications of the hygiene hypothesis. Immunology 2008, 126, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Raison, C.L.; Miller, A.H. The evolutionary significance of depression in Pathogen Host Defense (PATHOS-D). Mol. Psychiatry 2013, 18, 15–37. [Google Scholar] [CrossRef] [PubMed]
- Raison, C.L.; Miller, A.H. Pathogen-host defense in the evolution of depression: insights into epidemiology, genetics, bioregional differences and female preponderance. Neuropsychopharmacology 2017, 42, 5–27. [Google Scholar] [CrossRef]
- Wittman, D. Darwinian depression. J. Affect. Disord. 2014, 168, 142–150. [Google Scholar] [CrossRef]
- Kovacs, D.; Eszlari, N.; Petschner, P.; Pap, D.; Vas, S.; Kovacs, P.; Gonda, X.; Bagdy, G.; Juhasz, G. Interleukin-6 polymorphism interacts with pain and life stress influencing depression phenotypes. J. Neural Transm. 2016, 123, 541–548. [Google Scholar] [CrossRef]
- Kovacs, D.; Eszlari, N.; Petschner, P.; Pap, D.; Vas, S.; Kovacs, P.; Gonda, X.; Juhasz, G.; Bagdy, G. Effects of IL1B single nucleotide polymorphisms on depressive and anxiety symptoms are determined by severity and type of life stress. Brain Behav. Immun. 2016, 56, 96–104. [Google Scholar] [CrossRef]
- Starr, L.R.; Hammen, C.; Conway, C.C.; Raposa, E.; Brennan, P.A. Sensitizing effect of early adversity on depressive reactions to later proximal stress: Moderation by polymorphisms in serotonin transporter and corticotropin releasing hormone receptor genes in a 20-year longitudinal study. Dev. Psychopathol. 2014, 26, 1241–1254. [Google Scholar] [CrossRef]
- Rook, G.A.W.; Raison, C.L.; Lowry, C.A. Childhood microbial experience, immunoregulation, inflammation and adult susceptibility to psychosocial stressors and depression in rich and poor countries. Evol. Med. Public Health 2013, 2013, 14–17. [Google Scholar] [CrossRef]
- Rook, G.A.W.; Raison, C.L.; Lowry, C.A. Microbial ‘old friends’, immunoregulation and socioeconomic status. Clin. Exp. Immunol. 2014, 177, 1–12. [Google Scholar] [CrossRef]
- Dawud, L.M.; Holbrook, E.M.; Lowry, C.A. Evolutionary aspects of diverse microbial exposures and mental health: focus on “old friends” and stress resilience. Curr. Top. Behav. Neurosci. 2023, 61, 93–117. [Google Scholar] [CrossRef]
- Pillay, R.K. Post-influenzal depression. Med. World 1959, 91, 119–121. [Google Scholar] [PubMed]
- Flewett, T.H. Postinfluenzal depression. Br. Med. J. 1976, 2, 815. [Google Scholar] [CrossRef] [PubMed]
- Meijer, A.; Zakay-Rones, Z.; Morag, A. Post-influenzal psychiatric disorder in adolescents. Acta Psychiatr. Scand. 1988, 78, 176–181. [Google Scholar] [CrossRef]
- Bornand, D.; Toovey, S.; Jick, S.S.; Meier, C.R. The risk of new onset depression in association with influenza – a population-based observational study. Brain Behav. Immun. 2016, 53, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Lam, M.H.; Wing, Y.K.; Yu, M.W.; Leung, C.M.; Ma, R.C.W.; Kong, A.P.S.; So, W.Y.; Fong, S.Y.; Lam, S.P. Mental morbidities and chronic fatigue in severe acute respiratory syndrome survivors: long-term follow-up. Arch. Intern. Med. 2009, 169, 2142–2147. [Google Scholar] [CrossRef] [PubMed]
- Mazza, M.G.; De Lorenzo, R.; Conte, C.; Poletti, S.; Vai, B.; Bolletini, I.; Melloni, E.M.T.; Furlan, R.; Ciceri, F.; Rovere-Querini, P.; COVID-19 BioB Outpatient Clinic Study Group; Benedetti, F. Anxiety and depression in COVID-19 survivors: Role of inflammatory and clinical predictors. Brain Behav. Immun. 2020, 89, 594-600. [CrossRef]
- Alacevich, C.; Thalmann, I.; Nicodemo, C.; de Lusignan, S.; Petrou, S. Depression and anxiety during and after episodes of COVID-19 in the community. Sci. Rep. 2023, 13, 8257. [Google Scholar] [CrossRef] [PubMed]
- Hashioka, S.; Inoue, K.; Miyaoka, T.; Hayashida, M.; Wake, R.; Oh-Nishi, A.; Inagaki, M. The possible causal link of periodontitis to neuropsychiatric disorders: more than psychosocial mechanisms. Int. J. Mol. Sci. 2019, 20, 3723. [Google Scholar] [CrossRef] [PubMed]
- Dare, L.O.; Bruand, P.-E.; Gerard, D.; Marin, B.; Lameyre, V.; Boumediene, F.; Preux, P.-M. Associations of mental disorders and neurotropic parasitic diseases: a meta-analysis in developing and emerging countries. BMC Public Health 2019, 19, 1645. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, P. Serious infection may systemically increase noradrenergic signaling and produce psychological effects. Med. Hypotheses 2020, 139, 109692. [Google Scholar] [CrossRef] [PubMed]
- Dugbartey, A.T.; Dugbartey, M.T.; Apedo, M.Y. Delayed neuropsychiatric effects of malaria in Ghana. J. Nerv. Ment. Dis. 1998, 186, 183–186. [Google Scholar] [CrossRef]
- Nevin, R.L.; Croft, A.M. Psychiatric effects of malaria and anti-malarial drugs: historical and modern perspectives. Malar. J. 2016, 15, 332. [Google Scholar] [CrossRef]
- McGuinness, A.J.; Davis, J.A.; Dawson, S.L.; Loughman, A.; Collier, F.; O’Hely, M.; Simpson, C.A.; Green, J.; Marx, W.; Hair, C.; Guest, G.; Mohebbi, M.; Berk, M.; Stupart, D.; Watters, D.; Jacka, F.N. A systematic review of gut microbiota composition in observational studies of major depressive disorder, bipolar disorder and schizophrenia. Mol. Psychiatry 2022, 27, 1920–1935. [Google Scholar] [CrossRef]
- Napolioni, V.; MacMurray, J. Infectious diseases, IL6 -174G>C polymorphism, and human development. Brain Behav. Immun. 2016, 51, 196–203. [Google Scholar] [CrossRef]
- Gal, Z.; Torok, D.; Gonda, X.; Eszlari, N.; Anderson, I.M.; Deakin, B.; Juhasz, G.; Bagdy, G.; Petschner, P. Inflammation and blood-brain barrier in depression: interaction of CLDN5 and IL6 gene variants in stress-induced depression. Int. J. Neuropsychopharmacol. 2023, 26, 189–197. [Google Scholar] [CrossRef]
- Roetker, N.S.; Yonker, J.A.; Lee, C.; Chang, V.; Basson, J.J.; Roan, C.L.; Hauser, T.S.; Hauser, R.M.; Atwood, C.S. Multigene interactions and the prediction of depression in the Wisconsin Longitudinal Study. BMJ Open 2012, 2, e000944. [Google Scholar] [CrossRef]
- Bowins, B. Hypomania: a depressive inhibition override defense mechanism. J. Affect. Disord. 2008, 109, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Rantala, M.J.; Luoto, S.; Borraz-Leon, J.I.; Krams, I. Bipolar disorder: an evolutionary psychoneuroimmunological approach. Neurosci. Biobehav. Rev. 2021, 122, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, J.; Ye, Y.; Zou, Y.; Chen, W.; Wang, Z.; Zou, Z. Peripheral cytokine levels across psychiatric disorders: A systematic review and network meta-analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry 2023, 125, 110740. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Lopez, A.D. Regional patterns of disability-free life expectancy and disability-adjusted life expectancy: global Burden of Disease Study. Lancet 1997, 349, 1347–1352. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.L.; Vos, T.; Lozano, R.; Naghavi, M.; Flaxman, A.D.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; Aboyans, V.; Abraham, J.; Ackerman, I.; Aggarwal, R.; Ahn, S.Y.; Ali, M.K.; AlMazroa, M.A. ;…; Lopez, A.D. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2197-2223. [Google Scholar] [CrossRef]
- Whiteford, H.A.; Degenhardt, L.; Rehm, J.; Baxter, A.J.; Ferrari, A.J.; Erskine, H.E.; Charlson, F.J.; Norman, R.E.; Flaxman, A.D.; Johns, N.; Burstein, R.; Murray, C.J.L.; Vos, T. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet 2013, 382, 1575–1586. [Google Scholar] [CrossRef] [PubMed]
- GBD 2013 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990–2013: quantifying the epidemiological transition. Lancet 2015, 386, 2145-2191. 2191. [CrossRef]
- Institute for Health Metrics and Evaluation. Protocol for the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD). Accessed online on 29/6/2023 at https://www.healthdata.org/gbd/about/protocol.
- Global Burden of Disease Collaborative Network. Global Burden of Disease 2019 (GBD 2019) Results. Seattle, United States: Institute for Health Metrics and Evaluation, 2020. Accessed online on 10/6/2023 at https://vizhub.healthdata.org/gbd-results/.
- Moriarty, A.S.; Meader, N.; Snell, K.I.E.; Riley, R.D.; Paton, L.W.; Dawson, S.; Hendon, J.; Chew-Graham, C.A.; Gilbody, S.; Churchill, R.; Phillips, R.S.; Ali, S.; McMillan, D. Predicting relapse or recurrence of depression: systematic review of prognostic models. Br. J. Psychiatry 2022, 221, 448–458. [Google Scholar] [CrossRef]
- Kishi, T.; Matsuda, Y.; Sakuma, K.; Okuya, M.; Mishima, K.; Iwata, N. Recurrence rates in stable bipolar disorder patients after drug discontinuation v. drug maintenance: a systematic review and meta-analysis. Psychol. Med. 2021, 51, 2721-2729. 2721. [CrossRef]
- Stranieri, G.; Carabetta, C. Socio-economic cultural transformations and Depression in elderly people. Psychiatr. Danub. 2015, 27, S212–215. [Google Scholar]
- Fischer, R.; Van de Vliert, E. Does climate undermine subjective well-being? A 58-nation study. Pers. Soc. Psychol. Bull. 2011, 37, 1031–1041. [Google Scholar] [CrossRef]
- Lecic-Tosevski, D. Is urban living good for mental health? Curr. Opin. Psychiatry 2019, 32, 204–209. [Google Scholar] [CrossRef]
- Cifuentes, M.; Sembajwe, G.; Tak, S.; Gore, R.; Kriebel, D.; Punnett, L. The association of major depressive episodes with income inequality and the human development index. Soc. Sci. Med. 2008, 67, 529–539. [Google Scholar] [CrossRef]
- Zumla, A.; Ustianowski, A. Tropical diseases: definition, geographic distribution, transmission, and classification. Infect. Dis. Clin. North. Am. 2012, 26, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Risquez, A.; Echezuria, L.; Rodriguez-Morales, A.J. Epidemiological transition in Venezuela: relationships between infectious diarrheas, ischemic heart diseases and motor vehicles accidents mortalities and the Human Development Index (HDI) in Venezuela, 2005-2007. J. Infect. Public Health 2010, 3, 95–97. [Google Scholar] [CrossRef] [PubMed]
- United Nations Development Programme. Human Development Report 1990. New York: Oxford University Press, 1990.
- United Nations Development Programme. Human Development Report 2019. Beyond income, beyond averages, beyond today: inequalities in human development in the 21st century. New York: United Nations Development Programme, 2019.
- The World Bank. Urban population (% of total population). Accessed online on 25/06/2023 at https://data.worldbank.org/indicator/SP.URB.TOTL.IN.ZS.
- The World Bank. Gini index. Accessed online on 25/06/2023 at https://data.worldbank.org/indicator/SI.POV.GINI.
- Saha, S.; Chant, D.C.; Welham, J.L.; McGrath, J.J. The incidence and prevalence of schizophrenia varies with latitude. Acta Psychiatr. Scand. 2006, 114, 36–39. [Google Scholar] [CrossRef]
- Patten, S.B.; Williams, J.V.A.; Lavorato, D.H.; Wang, J.L.; Bulloch, A.G.M. Major Depression Prevalence Increases with Latitude in Canada. Can. J. Psychiatry 2017, 62, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Kenny, D.A.; Harackiewicz, J. Cross-lagged panel correlation: practice and promise. J. Appl. Psychol. 1979, 64, 372–379. [Google Scholar] [CrossRef]
- Akoglu, H. User’s guide to correlation coefficients. Turk. J. Emerg. Med. 2018, 18, 91–93. [Google Scholar] [CrossRef]
- Hsu, T.-W.; Chu, C.-S.; Tsai, S.-J.; Bai, Y.-M.; Su, T.-P.; Chen, T.-J.; Chen, M.-H.; Liang, C.-S. Risk of major mental disorder after severe bacterial infections in children and adolescents: a nationwide longitudinal study. Neuropsychobiology 2022, 81, 539–549. [Google Scholar] [CrossRef]
- Yu, C.-P.; Lin, I.-J.; Wang, B.-L.; Tsao, C.-H.; Huang, S.-H.; Huang, Y.-C.; Sun, C.-A.; Chung, C.-H.; Hu, J.-M.; Chien, W.-C. Intestinal infectious diseases increase the risk of psychiatric disorders: a nationwide population-based cohort study. Medicine 2022, 101, e30959. [Google Scholar] [CrossRef]
- Chen, P.-H.; Tsai, S.-Y.; Pan, C.-H.; Chen, Y.-L.; Su, S.-S.; Chen, C.-C.; Kuo, C.-J. Prevalence and 5-year trend of incidence for medical illnesses after the diagnosis of bipolar disorder: a nationwide cohort study. Aust. N. Z. J. Psychiatry 2022, 56, 1164–1176. [Google Scholar] [CrossRef]
- Goodwin, R.D. Association between infection early in life and mental disorders among youth in the community: a cross-sectional study. BMC Public Health 2011, 11, 878. [Google Scholar] [CrossRef]
- Jayatilleke, N.; Hayes, R.D.; Chang, C.-K.; Stewart, R. Acute general hospital admissions in people with serious mental illness. Psychol. Med. 2018, 48, 2676–2683. [Google Scholar] [CrossRef] [PubMed]
- Elpers, H.; Teismann, H.; Wellmann, J.; Berger, K.; Karch, A.; Rubsamen, N. Major depressive disorders increase the susceptibility to self-reported infections in two German cohort studies. Soc. Psychiatry Psychiatr. Epidemiol. 2023, 58, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Mulugeta, A.; Zhou, A.; King, C.; Hypponen, E. Association between major depressive disorder and multiple disease outcomes: a phenome-wide Mendelian randomisation study in the UK Biobank. Mol. Psychiatry 2020, 25, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Nudel, R.; Hougaard, D.M.; Werge, T.; Benros, M.E. Genetic and epidemiological analyses of infection load and its relationship with psychiatric disorders. Epidemiol. Infect. 2023, 151, e93. [Google Scholar] [CrossRef] [PubMed]
- Shorter, J.R.; Meijsen, J.; Nudel, R.; Krebs, M.; Gadin, J.; Mikkelsen, D.H.; Avelar e Silva, R.N.; Benros, M.E.; Thompson, W.K.; Ingason, A.; Werge, T. Infection polygenic factors account for a small proportion of the relationship between infections and mental disorders. Biol. Psychiatry 2022, 92, 283–290. [Google Scholar] [CrossRef]
- Vindegaard, N.; Petersen, L.V.; Lyng-Rasmussen, B.I.; Dalsgaard, S.; Benros, M.E. Infectious mononucleosis as a risk factor for depression: a nationwide cohort study. Brain Behav. Immun. 2021, 94, 259–265. [Google Scholar] [CrossRef]
- Yen, Y.-F.; Chung, M.-S.; Hu, H.-Y.; Lai, Y.-J.; Huang, L.-Y.; Lin, Y.-S.; Chou, P.; Deng, C.-Y. Association of pulmonary tuberculosis and ethambutol with incident depressive disorder: a nationwide, population-based cohort study. Prim. Care Companion CNS Disord. 2023. [CrossRef]
- Korf, J.; Klein, H.C.; Versijpt, J.; den Boer, J.A.; Ter Horst, G.J. Considering depression as a consequence of activation of the inflammatory response system. Acta Neuropsychiatr. 2002, 14, 1–10. [Google Scholar] [CrossRef]
- van West, D.; Maes, M. Activation of the inflammatory response system: a new look at the etiopathogenesis of major depression. Neuro Endocrinol. Lett. 1999, 20, 11–17. [Google Scholar]
- Zhang, K.; Wang, X.; Tu, J.; Rong, H.; Werz, O.; Chen, X. The interplay between depression and tuberculosis. J. Leukoc. Biol. 2019, 106, 749–757. [Google Scholar] [CrossRef]
- Mellon, S.H.; Wolkowitz, O.M.; Schonemann, M.D.; Epel, E.S.; Rosser, R.; Burke, H.B.; Mahan, L.; Reus, V.I.; Stamatiou, D.; Liew, C.-C.; Cole, S.W. Alterations in leukocyte transcriptional control pathway activity associated with major depressive disorder and antidepressant treatment. Transl. Psychiatry 2016, 6, e821. [Google Scholar] [CrossRef] [PubMed]
- Grosse, L.; Hoogenboezem, T.; Ambree, O.; Bellingrath, S.; Jorgens, S.; de Wit, H.J.; Wijkhuijs, A.M.; Arolt, V.; Drexhage, H.A. Deficiencies of the T and natural killer cell system in major depressive disorder: T regulatory cell defects are associated with inflammatory monocyte activation. Brain Behav. Immun. 2016, 54, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Mendenhall, E.; Kohrt, B.A.; Norris, S.A.; Ndetei, D.; Prabhakaran, D. Non-communicable disease syndemics: poverty, depression, and diabetes among low-income populations. Lancet 2017, 389, 951–963. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.-J.; Heinrich, J.; Bloom, M.S.; Zhao, T.-Y.; Shi, T.-X.; Feng, W.-R.; Sun, Y.; Shen, J.-C.; Yang, Z.-C.; Yang, B.-Y.; Dong, G.-H. Ambient air pollution and depression: A systematic review with meta-analysis up to 2019. Sci. Total Environ. 2020, 701, 134721. [Google Scholar] [CrossRef]
- Abu-Ashour, W.; Twells, L.; Valcour, J.; Randell, A.; Donnan, J.; Howse, P.; Gamble, J.-M. The association between diabetes mellitus and incident infections: a systematic review and meta-analysis of observational studies. BMJ Open Diabetes Res. Care 2017, 5, e000336. [Google Scholar] [CrossRef]
- Auger, N.; Monnier, M.; Low, N.; Lee, G.E.; Bilodeau-Bertrand, M.; Luu, T.M. Maternal mental disorders and pediatric infectious diseases: a retrospective cohort study. Pediatr. Infect. Dis. J. 2021, 40, 697–703. [Google Scholar] [CrossRef]
- Okusaga, O.; Yolken, R.H.; Langenberg, P.; Lapidus, M.; Arling, T.A.; Dickerson, F.B.; Scrandis, D.A.; Severance, E.; Cabassa, J.A.; Balis, T.; Postolache, T.T. Association of seropositivity for influenza and coronaviruses with history of mood disorders and suicide attempts. J. Affect. Disord. 2011, 130, 220–225. [Google Scholar] [CrossRef]
- Steinberg, D.; Hirsch, S.R.; Marston, S.D.; Reynolds, K.; Sutton, R.N. Influenza infection causing manic psychosis. Br. J. Psychiatry 1972, 120, 531–535. [Google Scholar] [CrossRef]
- Maurizi, C.P. Influenza and mania: a possible connection with the locus ceruleus. South Med. J. 1985, 78, 207–209. [Google Scholar] [CrossRef]
- Russo, M.; Calisi, D.; De Rosa, M.A.; Evangelista, G.; Consoli, S.; Dono, F.; Santilli, M.; Gambi, F.; Onofrj, M.; Di Giannantonio, M.; Parruti, G.; Sensi, S.L. COVID-19 and first manic episodes: a systematic review. Psychiatry Res. 2022, 314, 114677. [Google Scholar] [CrossRef]
- Ayub, S.; Kanner, J.; Riddle, M.; Romano, G. Influenza-induced mania. J. Neuropsychiatry Clin. Neurosci. 2016, 28, e17–e18. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, C.R.; Corte-Real, B.R.; Saraiva, R.; Frey, B.N.; Kapczinski, F.; de Azevedo Cardoso, T. Triggers for acute mood episodes in bipolar disorder: a systematic review. J. Psychiatr. Res. 2023, 161, 237–260. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, F.B.; Severance, E.G.; Yolken, R.H. Non-SARS coronaviruses in individuals with psychiatric disorders. Curr. Top. Behav. Neurosci. 2023, 61, 265–278. [Google Scholar] [CrossRef]
- Luykx, J.J.; Lin, B.D. Are psychiatric disorders risk factors for COVID-19 susceptibility and severity? a two-sample, bidirectional, univariable, and multivariable Mendelian Randomization study. Transl. Psychiatry 2021, 11, 210. [Google Scholar] [CrossRef] [PubMed]
- Barcella, C.A.; Polcwiartek, C.; Mohr, G.H.; Hodges, G.; Sondergaard, K.; Bang, C.N.; Andersen, M.P.; Fosbol, E.; Kober, K.; Schou, M.; Torp-Pedersen, C.; Kessing, L.V.; Gislason, G.; Kragholm, K. Severe mental illness is associated with increased mortality and severe course of COVID-19. Acta Psychiatr. Scand. 2021, 144, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Kinney, D.K.; Tanaka, M. An evolutionary hypothesis of depression and its symptoms, adaptive value, and risk factors. J. Nerv. Ment. Dis. 2009, 197, 561–567. [Google Scholar] [CrossRef]
- Rantala, M.J.; Luoto, S.; Krams, I.; Karlsson, H. Depression subtyping based on evolutionary psychiatry: proximate mechanisms and ultimate functions. Brain Behav. Immun. 2018, 69, 603–617. [Google Scholar] [CrossRef]
- Oliveira, J.; Oliveira-Maia, A.J.; Tamouza, R.; Brown, A.S.; Leboyer, M. Infectious and immunogenetic factors in bipolar disorder. Acta Psychiatr. Scand. 2017, 136, 409–423. [Google Scholar] [CrossRef]
- Schiweck, C.; Claes, S.; Van Oudenhove, L.; Lafit, G.; Vaessen, T.; de Beeck, G.O.; Berghmans, R.; Wijkhuijs, A.; Muller, N.; Arolt, V.; Drexhage, H.; Vrieze, E. Childhood trauma, suicide risk and inflammatory phenotypes of depression: insights from monocyte gene expression. Transl. Psychiatry 2020, 10, 296. [Google Scholar] [CrossRef]
- Kaveladze, B.; Altman, A.D.; Niederhausen, M.; Loftis, J.M.; Teo, A.R. Social relationship quality, depression and inflammation: A cross-cultural longitudinal study in the United States and Tokyo, Japan. Int. J. Soc. Psychiatry 2022, 68, 253–263. [Google Scholar] [CrossRef]
- Felger, J.C.; Lotrich, F.E. Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience 2013, 246, 199–229. [Google Scholar] [CrossRef] [PubMed]
- Kappelmann, N.; Perry, B.I.; Khandaker, G.M. Prenatal and childhood immuno-metabolic risk factors for adult depression and psychosis. Harv. Rev. Psychiatry 2022, 30, 8–23. [Google Scholar] [CrossRef] [PubMed]
- Leone, M.; Kuja-Halkola, R.; Leval, A.; D’Onofrio, B.M.; Larsson, H.; Lichtenstein, P.; Bergen, P.E. Association of severe childhood infections with depression and intentional self-harm in adolescents and young adults. Brain Behav. Immun. 2022, 99, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Doney, E.; Cadoret, A.; Dion-Albert, L.; Lebel, M.; Menard, C. Inflammation-driven brain and gut barrier dysfunction in stress and mood disorders. Eur. J. Neurosci. 2022, 55, 2851–2894. [Google Scholar] [CrossRef] [PubMed]
- Obi-Azuike, C.; Ebiai, R.; Gibson, T.; Hernandez, A.; Khan, A.; Anugwom, G.; Urhi, A.; Prasad, S.; Souabni, S.A.; Olandunjoye, F. A systematic review on gut-brain axis aberrations in bipolar disorder and methods of balancing the gut microbiota. Brain Behav. 2023, 13, e3037. [Google Scholar] [CrossRef]
- Xi, C.; Li, A.; Lai, J.; Huang, X.; Zhang, P.; Yan, S.; Jiao, M.; Huang, H.; Hu, S. Brain-gut microbiota multimodal predictive model in patients with bipolar depression. J. Affect. Disord. 2023, 323, 140–152. [Google Scholar] [CrossRef]
- Huang, T.; Shang, Y.; Dai, C.; Zhang, Q.; Hu, S.; Xie, J. Gut microbiota and its relation to inflammation in patients with bipolar depression: a cross-sectional study. Ann. Gen. Psychiatry 2023, 22, 21. [Google Scholar] [CrossRef]
- Minuti, A.; Brufani, F.; Menculini, G.; Moretti, P.; Tortorella, A. The complex relationship between gut microbiota dysregulation and mood disorders: A narrative review. Curr. Res. Neurobiol. 2022, 3, 100044. [Google Scholar] [CrossRef]
- Carlessi, A.S.; Borba, L.A.; Zugno, A.I.; Quevedo, J.; Reus, G.Z. Gut microbiota-brain axis in depression: The role of neuroinflammation. Eur. J. Neurosci. 2021, 53, 222–235. [Google Scholar] [CrossRef]
- Janowska, M.; Rog, J.; Karakula-Juchnowicz, H. Disruptions within gut microbiota composition induced by improper antibiotics therapy as a probable trigger factor for development of depression - Case Reports. Ann. Agric. Environ. Med. 2021, 29, 713–718. [Google Scholar] [CrossRef]
- Sferrazza Papa, F.G.; Pellegrino, G.M.; Centanni, S. The lung-brain axis. In: Priori, A. (editor). Neurology of COVID-19. Milano: Milano University Press, 2021. Available online at: https://www.ncbi.nlm.nih.gov/books/NBK579773/.
- Bajinka, O.; Simbilyabo, L.; Tan, Y.; Jabang, J.; Saleem, S.A. Lung-brain axis. Crit. Rev. Microbiol. 2022, 48, 257–269. [Google Scholar] [CrossRef]
- Chen, J.; Li, T.; Ye, C.; Zhong, J.; Huang, J.-D.; Ke, Y.; Sun, H. The lung microbiome: a new frontier for lung and brain disease. Int. J. Mol. Sci. 2023, 24, 2170. [Google Scholar] [CrossRef]
- Villalba, N.; Ma, Y.; Gahan, S.A.; Joly-Amado, A.; Spence, S.; Yang, X.; Nash, K.R.; Yuan, S.Y. Lung infection by Pseudomonas aeruginosa induces neuroinflammation and blood-brain barrier dysfunction in mice. J. Neuroinflammation 2023, 20, 127. [Google Scholar] [CrossRef]
- Wu, S.; Yin, Y.; Du, L. Blood-brain barrier dysfunction in the pathogenesis of major depressive disorder. Cell. Mol. Neurobiol. 2022, 42, 2571–2591. [Google Scholar] [CrossRef]
- Zhao, N.O.; Topolski, N.; Tusconi, M.; Salarda, E.M.; Busby, C.W.; Lima, C.N.N.C.; Pillai, A.; Quevedo, J.; Barichello, T.; Fries, G.R. Blood-brain barrier dysfunction in bipolar disorder: Molecular mechanisms and clinical implications. Brain Behav. Immun. Health 2022, 21, 100441. [Google Scholar] [CrossRef]
- Sencio, V.; Machado, M.G.; Trottein, F. The lung-gut axis during viral respiratory infections: the impact of gut dysbiosis on secondary disease outcomes. Mucosal Immunol. 2021, 14, 296–304. [Google Scholar] [CrossRef]
- Raison, C.L.; Miller, A.H. Do cytokines really sing the blues? Cerebrum 2013, 2013, 10. URL: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3788165/. /: 10. URL: https.
- Mlynarska, E.; Gadzinowska, J.; Tokarek, J.; Forycka, J.; Szuman, A.; Franczyk, B.; Rysz, J. The role of the microbiome-brain-gut axis in the pathogenesis of depressive disorder. Nutrients 2022, 14, 1921. [Google Scholar] [CrossRef] [PubMed]
- Rogosa, D. A critique of cross-lagged correlation. Psychol. Bull. 1980, 88, 245–258. [Google Scholar] [CrossRef]
- Piantadosi, S.; Byar, D.P.; Green, S.B. The ecological fallacy. Am. J. Epidemiol. 1988, 127, 893–904. [Google Scholar] [CrossRef]
- Barnes, J.; Mondelli, V.; Pariante, C.M. Genetic contributions of inflammation to depression. Neuropsychopharmacology 2017, 42, 81–98. [Google Scholar] [CrossRef] [PubMed]
- Hidaka, B.H. Depression as a disease of modernity: explanations for increasing prevalence. J. Affect. Disord. 2012, 140, 205–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, L.; Lei, Y.; Liu, X.; Zhou, X.; Liu, Y.; Wang, M.; Yang, L.; Zhang, L.; Fan, S.; Xie, P. Meta-analysis of infectious agents and depression. Sci. Rep. 2014, 4, 4530. [Google Scholar] [CrossRef] [PubMed]
- Cossu, G.; Preti, A.; Gyppaz, D.; Gureje, O.; Carta, M.G. Association between toxoplasmosis and bipolar disorder: a systematic review and meta-analysis. J. Psychiatr. Res. 2022, 153, 284–291. [Google Scholar] [CrossRef] [PubMed]
| Infectious disease category | Cross-Correlation (Infectious Disease 1990 x Mood Disorder 2019) | Cross-Correlation (Mood Disorder 1990 x Infectious Disease 2019) | Cross-Lagged Regression Coefficient | Significance Level |
|---|---|---|---|---|
|
URI x MDD x BD |
-.08 -.34 |
-.05 -.32 |
-.029 .018 |
.681 .798 |
|
LRI x MDD x BD |
.12 -.34 |
.03 -.34 |
.097 .002 |
.168 .977 |
|
Enteric x MDD x BD |
.01 -.43 |
.01 -.44 |
.001 .008 |
.989 .910 |
|
Nematode x MDD x BD |
.09 -.38 |
-.02 -.36 |
.109 -.022 |
.121 .755 |
|
Tropical x MDD x BD |
.22 -.23 |
.14 -.27 |
.075 .038 |
.286 .589 |
|
Other x MDD x BD |
.11 -.43 |
.04 -.39 |
.065 -.037 |
.356 .599 |
| Infectious Disease Category | Cross-Correlation (Infectious Disease 1990 x Mood Disorder 2019) | Cross-Correlation (Mood Disorder 1990 x Infectious Disease 2019) | Cross-Lagged Regression Coefficient | Significance Level |
|---|---|---|---|---|
|
URI x MDD x BD |
-.26 .14 |
-.22 .14 |
-.040 -.001 |
.644 .991 |
|
LRI x MDD x BD |
.20 -.20 |
.07 -.26 |
.133 .056 |
.123 .517 |
|
Enteric x MDD x BD |
.16 -.31 |
.14 -.29 |
.022 -.017 |
.799 .844 |
|
Nematode x MDD x BD |
-.01 -.34 |
-.02 -.34 |
.006 -.006 |
.945 .945 |
|
Tropical x MDD x BD |
.27 -.19 |
.22 -.24 |
.048 .056 |
.579 .517 |
|
Other x MDD x BD |
.20 -.38 |
.16 -.34 |
.033 -.041 |
.703 .636 |
| Diagnostic Category | URI | LRI | Enteric | Nematode | Tropical | Other |
|---|---|---|---|---|---|---|
| MDD | .17 (.013)* | .29 (<.001)** | -.17 (.013)* | -.16 (.019)* | -.21 (.002)* | .13 (.066) |
|
MDD (adjusted) |
.06 (.423) | .27 (<.001)** | -.11 (.176) | -.11 (.154) | -.16 (.045)* | .13 (.108) |
| BD | .92 (<.001)** | .05 (.451) | -.82 (<.001)** | -.01 (.893) | -.29 (<.001)** | -.12 (.084) |
|
BD (adjusted) |
.88 (<.001)** | .00 (.962) | -.78 (<.001)** | .12 (.125) | -.23 (.003)* | -.08 (.289) |
| Change in the Incidence of Infectious Disease | Change in the Incidence of Major Depression | χ2 | Significance Level | |
|---|---|---|---|---|
| Decreased | Increased | |||
|
URI Decreased Increased |
91 (65.5%) 48 (34.5%) |
29 (44.6%) 36 (55.4%) |
7.95 |
.005* |
|
LRI Decreased Increased |
138 (99.3%) 1 (0.7%) |
64 (98.5%) 1 (1.5%) |
0.31 |
.537† |
|
Enteric Decreased Increased |
33 (23.7%) 106 (76.3%) |
22 (33.8%) 43 (66.2%) |
2.30 |
.130 |
|
Nematode Decreased Increased |
135 (97.1%) 4 (2.9%) |
63 (96.9%) 2 (3.1%) |
0.01 |
.999† |
|
Tropical Decreased Increased |
103 (74.1%) 36 (25.9%) |
54 (83.1%) 11 (16.9%) |
2.01 |
.156 |
|
Other Decreased Increased |
138 (99.3%) 1 (0.7%) |
63 (96.9%) 2 (3.1%) |
1.70 |
.239† |
| Change in the Incidence of Infectious Disease | Change in the Incidence of Bipolar Disorder | χ2 | Significance Level | |
|---|---|---|---|---|
| Decreased | Increased | |||
|
URI Decreased Increased |
102 (94.4%) 6 (5.6%) |
18 (18.8%) 78 (81.3%) |
120.22 |
<.001* |
|
LRI Decreased Increased |
107 (99.1%) 1 (0.9%) |
95 (99.0%) 1 (1.0%) |
0.01 |
.999† |
|
Enteric Decreased Increased |
5 (4.6%) 103 (95.4%) |
50 (52.1%) 46 (47.9%) |
58.12 |
<.001* |
|
Nematode Decreased Increased |
105 (97.2%) 3 (2.8%) |
93 (96.9%) 3 (3.1%) |
0.02 |
.999† |
|
Tropical Decreased Increased |
75 (69.4%) 33 (30.6%) |
82 (85.4%) 14 (14.6%) |
7.31 |
.007* |
|
Other Decreased Increased |
106 (98.1%) 2 (1.9%) |
95 (99.0%) 1 (1.0%) |
0.23 |
.999† |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





