2.1. Basic composition
The basic composition (protein, fat, water, and solids-non-fat content) of the sous-vide-heated meat samples is shown in
Table 1.
The raw meat had the highest water content. Its values were significantly different from the other variants of heated meat. On the other hand, the meat heated for 30 min at 121.1°C in an autoclave had the lowest water content. It was statistically significantly different from the samples heated with the sous-vide method and the raw meat. The sous-vide heating decreased the water content in the samples heated up to 8h. At further heating stages, i.e. 8h, 10h, 12h, 14h, 16h, and 18h, the water content remained at the same level. The lower water content in the samples heated in the autoclave resulted from much greater contraction of muscle fibres caused by the high temperature of thermal treatment, which resulted in a greater loss of water from the muscles.
The analysis of the results showed that the meat sample heated in the autoclave for 30 minutes had the highest protein content, which was statistically significantly different from the protein content in the other variants. The raw meat had the lowest protein content, which was statistically significantly different from the other variants. The protein content in the sous-vide samples heated for 6h, 8h, 10h, and 12h ranged from 26.50% to 27.22%. There was a statistically significant increase in the protein content in the sample heated for 14h. This value was similar in the samples heated for 16h, whereas the protein content in the sample heated for 18h was slightly lower.
Changes in the protein content in meat are also caused by the loss of water. Since the water loss in the samples heated in the autoclave was higher, they had higher protein content than the raw meat or meat heated for 6 hours (
Table 1). The sample heated for 6h had the lowest fat content, which was significantly different from the fat content in the other variants. As the sous-vide heating time increased, so did the fat content in the samples heated for 6-10h (statistically significant differences). Further heating up to 12h resulted in a slight increase in the fat content, but the difference was not statistically significant. There was a statistically significant decrease in the fat content in the successive meat heating variants – it amounted to 3.31% in the sample heated for 16h. The total meat composition and fat content are the main determinants of meat quality. Differences in the fat content between the samples may have been caused by the heterogeneous structure of the muscle. As the heating time of the meat increased, the fat melted and decomposed, whereas the weight loss of the product increased. The raw meat had the lowest fat-free dry matter content, which was significantly different from the other variants. The meat heated in the autoclave at 121.1°C for 30 minutes had the highest fat-free dry matter content, which was significantly different from the sous-vide-cooked and raw meat samples. This effect was caused by a greater loss of water and other meat components (myofibrillar and sarcoplasmic proteins, collagen, lipids, vitamins, minerals, and flavour compounds).
2.2. Cooking loss
Cooking loss resulting from heat treatment is an important factor in the meat industry due to its role in overall processing efficiency. It is mainly caused by the loss of water as well as other meat components such as myofibrillar and sarcoplasmic proteins, collagen, lipids, vitamins, minerals, and flavour compounds [
21,
22]. Cooking loss always takes place, regardless of the thermal treatment technique.
Table 1 shows the mass losses in the meat samples after sous-vide cooking at 60°C for 6-18h, and in the meat samples cooked in the autoclave (121.1°C, 0.2MPa).
Within the whole range of sous-vide heating times, the weight loss in the meat samples ranged from 23.19% to 26.72% and tended to increase slightly along with the cooking time (P<0.05). The highest weight loss of 33.87% was observed in the meat samples cooked in the autoclave.
The loss of meat mass during cooking is caused by the contraction of muscle fibres and connective tissue as well as the direction of heat conduction. At about 60℃ the direction of thermal shrinkage to the muscle fibre axis mostly changes from transverse to longitudinal [
23]. Transverse contraction, which is attributed to the expansion of the space between the muscle fibres and the endomysium, has minimal effect on the loss of water and other meat components. Most of the meat weight loss during cooking is caused by the longitudinal contraction of the muscles as a result of denaturation of connective tissue proteins, mainly myofibrillar proteins and collagen. Myofibrillar contraction reduces the muscle ability to retain water. The contraction of the connective tissue of the perimysium is caused by the compression of muscle fibre bundles, which leads to the expulsion of a larger volume of fluid from the muscle [
17,
24].
The duration and temperature of cooking clearly influence the capacity of the meat to retain water. Many researchers observed greater weight losses in meat as the heating temperature increased, regardless of the meat type and thermal processing method [
20,
25].
In our study the weight loss in the sous-vide-cooked meat samples (Longissimus lumborum pork muscle) tended to increase within the temperature range of 60-85°C (the results have not been presented in this manuscript). The lowest weight loss was observed in the variant heated at 60°C. This value differed significantly from the other variants of the experiment.
The amount of weight loss in the sous-vide heat treatment is also influenced by the heating time.
Hwang et al. (2019) [
15] analysed the effect of temperature (50℃, 55℃, and 60 ℃) and time (12h and 24h) on the weight loss in sous-vide-cooked pork loin. The study showed that the weight loss during cooking tended to increase along with temperature, but longer cooking times at 50°C resulted in a smaller loss. When the cooking temperature was higher than 55℃, the cooking time had no effect on the weight loss in the meat samples. However, after the cooking at 60℃ for 24h the weight loss was usually higher than after 12h. Jeong et al. [
25] analysed the effect of sous-vide cooking conditions (temperature and time) on the properties of pork ham and observed a similar dependence. The amount of meat weight loss was influenced by temperature to a greater extent than by the cooking time. The study also showed that when the cooking time was shortened to 2h, the weight loss was lower despite the temperature increase to 100°C.
In our study the weight losses in the sous-vide meat samples cooked at 60°C were equal and lower than in the other heating variants (60-85°C). The results of our experiment are in line with the observations made by other researchers.
As mentioned above, most of the water in the muscles can be found in myofibrils, between myofibrils as well as between myofibrils and the sarcolemma [
23]. Heating the muscle causes denaturation of meat proteins, including myofibrillar and collagen proteins. It results in the contraction and subsequent solubilisation of collagen. These heat-induced structural changes in the muscles result in fluid loss during cooking [
26].
The denaturation and shrinkage of meat protein largely depend on the heating temperature. The weight losses in the sous-vide-cooked muscle samples were smaller than in the autoclave-cooked samples mostly because of the lower temperature of the heat treatment (60°C). In consequence, both protein coagulation and collagen solubilisation were limited [
27].
High temperature (121.1°C) causes structural disintegration of myofibrils. Their structure is considerably loosened during cooking due to progressive changes in the intramuscular connective tissue caused by the much higher intensity of denaturation of myofibrillar and collagen meat proteins. As a result, muscle contraction is much greater than at lower temperatures. Such changes in the muscle structure cause a much greater loss of fluids. This effect is manifested by the increase in the dry matter content, especially the share of proteins in the meat composition (
Table 1) [
26].
2.3. Colour analysis
The change of the meat colour during cooking is an important effect influencing the assessment of its quality during consumption. The colour of a piece of meat cooked at a certain temperature depends on how quickly it reaches that temperature and how long it is held at that temperature. The faster it reaches the temperature, the redder it is; the longer it is held at a certain temperature, the lighter it becomes [
16].
The colour changes that occur during cooking reflect heat-induced changes in the muscle pigment, myoglobin. Denaturation and aggregation of myofibrillar and sarcoplasmic proteins are also important factors affecting the assessment of the colour of cooked meat. Changes in these proteins significantly affect the amount of light scattered [
28].
Our research showed that both the sous-vide and autoclave heating of pork significantly influenced its colour. The raw meat had the lowest value of the L parameter, determining the brightness of the finished product, and its values were statistically significantly different from the other variants. The time of sous-vide heating of pork at 60°C did not significantly affect the colour parameters, i.e. L* brightness, a*red and b*yellow. Only the samples heated in the autoclave at 121.1°C had higher a* and b* values but a lower L* value (
Table 2).
The raw meat had a negative value of the a* parameter. This may have been caused by the presence of Pseudomonas fluorescens bacteria, which thrive in refrigerated meat. The bacteria can sometimes be seen as a green fluorescent layer on the meat surface, but they are not harmful to healthy people. By contrast, the value of the a* parameter in all other time variants (6-18h) ranged from 0.16 to 0.70. The values of this parameter did not differ significantly between the samples. On the other hand, the largest statistically significant difference from the other variants was observed in the meat cooked in the autoclave.
The meat heated in the autoclave at 121.1°C for 30 min had the largest share of the yellow colour, whereas the raw meat had the lowest. The sous-vide meat samples heated at 60°C for 6h, 8h, 10h, 12h, 14h, 16h, and 18h did not differ statistically significantly in the share of yellow.
Sanchez del Pulgar et al. (2012) [
29] observed that the cooking of meat at moderate temperatures, even for a very long time, did not affect the proportion of the red colour. Our analysis showed that the sous vide samples heated at 60°C for 5h and 12h had higher values of the L* parameter than those heated at 80°C for the same time or cooked traditionally. Similarly, Roldán et al. (2013) [
30] observed that lamb samples heated at 60°C for different time periods had slightly higher L* values than those heated at higher temperatures (70°C or 80°C).
Jeong et al. (2018) [
25] used the sous-vide method and different percentages of vacuum (98.81% or 96.58%) to heat pork ham at 61°C and 71°C for 45 and 90 minutes. The researchers observed higher values of the L* parameter as the temperature and heating time increased. This may have been caused by the fact that the higher water content in the heated meat samples allowed light to penetrate deeper into the tissue and resulted in a darker surface of the product. Also, as the temperature increased, the process of protein denaturation and aggregation intensified, which resulted in an increase in the L* parameter [
25,
31]. Sun et al. [
32] found that as the temperature and heating time of beefsteaks increased, so did the values of the L* and b* parameters, whereas the value of the a*parameter decreased.
In our study there were no statistically significant differences between the time variants (6, 8, 10, 12, 14, 16, 18h) in the colour parameters a* and b*, but the autoclave-heated samples had higher values of the a* and b* parameters than the other samples (
Table 2). These results were different from those obtained by other researchers because the proportion of the red colour (a*) in the heated meat was inversely proportional to the degree of myoglobin denaturation. The myoglobin denaturation process starts at 60°C. Sanchez del Pulgar et al. (2012) [
29], examined the effect of heating temperature on the meat colour and found that the meat heated at higher temperatures had a lower proportion of the red colour at 80°C than at 60°C in various time variants. According to Sanchez del Pulgar et al. (2012) [
29], the colour saturation depends on the concentration of myoglobin, the level of degradation, and the degree of protein denaturation in meat. Vaudagna et al. (2002) [
33] found a similar relationship of the colour parameter a* in sous-vide-cooked beef heated at 50-65°C for 90-360 min.
In our study there were lower values of the L parameter but higher values of the a* and b* parameters in the pork cooked in the autoclave. The changes in these parameters may have been caused by the higher pressure inside the vacuum bag (about 0.2MPa) and higher temperature (121.1°C).
As mentioned before, changes in meat redness (a*) after cooking mainly result from the content of myoglobin and the degree of its denaturation. However, the colour of the muscle tissue is determined not only by the amount of myoglobin, but also by the direction and scope of its chemical transformations. During thermal processing myoglobin and its derivatives are transformed into myochromogens. Myochromogen, which is a denatured form of myoglobin, is red. Heating muscles at high temperature under elevated pressure increased the degree of myoglobin denaturation and reduced its solubility. As a result, there was less myoglobin in thermal leakage. Despite the high temperature but short heating time (0.5 h), the myoglobin may not have been completely denatured. The lower water content resulting from the increased weight loss (
Table 1) caused an increase in the dry matter content and, consequently, a higher concentration of pigments on the muscle surface than in the sous-vide-cooked meat.
The higher values of the b* parameter in the cooked meat can be attributed to an elevated level of metmyoglobin (the oxidised form of myoglobin), which results in a more brownish colour [
34]. Obviously, oxidation had a limited effect on the colour due to the use of vacuum bags. The differences in the intensity of this process in the SV-cooked meat and the AC-cooked meat were caused by pressure and temperature. The degree of oxidation is also influenced by pH. Higher temperature and higher pH accelerate the oxidation process. Our research (the results have not been presented in this manuscript) showed that the meat cooked under increased pressure and temperature had a higher pH than both the raw meat and the SV-cooked meat heated at 60°C for 4 hours (AC – 6.07; raw – 5.78; SV – 5.89). Generally, an increase in pH was caused by greater protein denaturation.
Brewer et al. (2001) [
35] observed that the higher pH of the meat treated under high pressure was correlated with the lower L* value, which resulted in a darker colour of the meat. The results obtained by these researchers may explain why our instrumental colour analysis showed a lower L* value in the AC-cooked muscles than in the SV-cooked muscles.
Generally, there are hardly any studies investigating the effect of cooking meat under elevated pressure and at high temperature on its physical properties. There have been various publications on the properties of meat subjected to non-thermal High Hydrostatic Pressure (HHP) treatment followed by heat treatment. However, there are differences in the molecular mechanisms responsible for changes in the properties of cooked meat which has been subjected to the HPP treatment and the properties of pressure-cooked meat. The explanation of our results is hypothetical and requires further research.
2.4. Thiamine
Apart from the taste, pork loin is a good source of various nutrients affecting the functions of the human body. It is not only rich in protein, micronutrients such as easily absorbable iron, selenium, zinc, as well as bioactive compounds (coenzyme Q10 and creatine), but it is also the best source of B vitamins (B1, B2, B6 and B12) among all types of meat. The content of vitamin B1 in pork is 4-5 times greater than in other types of meat [
36].
As cooking is an integral part of the processing and preparation of meat products, it significantly affects not only the quality and sensory characteristics, such as the texture and taste of finished products [
21,
22], but it may also cause undesirable changes, reducing the nutritional value and bioavailability of various components, including vitamins. The loss of vitamins and minerals in cooked meat is caused by molecular interactions which take place during heat treatment.
The temperature and duration of heat treatment are the main factors affecting the loss of vitamins [
19]. Therefore, some cooking methods, such as prolonged SV cooking may cause a large loss of vitamins, whereas shorter cooking time may reduce this loss [
38], as results from the analysis of the data in our experiment (
Table 3).
As can be seen, as the pork sous-vide heating time increased, the retention of vitamin B1 in the muscle decreased. The highest content of thiamine was found in the sample heated for 6h, whereas the lowest – in the one heated for 18h.
The fact that the content of vitamin B1 in the thermal leakage was increasing up to 12h and remained at the same level up to 14h shows that thiamine was released from the muscle. The decrease in the content of thiamine within the time range of 14-18h was most likely caused by its thermal degradation as a result of longer heating time [
37,
38].
The effect of high temperature on the vitamin B1 content was clearly visible in muscles cooked under pressure and at high temperature. The content of thiamine in the meat cooked in the autoclave was relatively lower than its content in the sous-vide meat cooked for up to 14h.
In consequence of the loss of vitamin B1 in the muscles as a result of the thermal leakage of liquids, its content in dry matter also decreased throughout the heating time range. The highest content of thiamine in the dry non-fat matter was found in the sous-vide-cooked meat heated for 6h. The content of vitamin B1 in the muscle heated in the autoclave was comparable to its content in the samples heated for 14h.
The loss of vitamins during cooking cannot be avoided. However, some thermal processing techniques particularly significantly influence the content of B vitamins, including thiamine (B1) [
39,
40]. As results from reference publications, in comparison with traditional cooking, the sous-vide method enables greater retention of B vitamins, including thiamine (B1) [
24,
41] due to lower losses. However, the greatest loss of vitamin B1 takes place during the sterilisation of canned food. During traditional cooking and stewing, the loss is about 50-70% [
42]. Some of the vitamin contained in the product diffuses into the thermal leakage. When fat is used for frying, the loss of thiamine is greater due to the formation of fat oxidation products. This effect can be eliminated during grilling [
43].
2.5. Rheology
In our study the effect of the time of sous-vide heating of pork at 60°C on its rheomechanical properties was also analysed. Dynamical Mechanical Analysis (DMA) was applied to determine the rheological properties. In this method no samples are destroyed and measurements are not repetitive. It seems to be one of the most reliable sources of information on the properties and quality of meat at different stages of technological processing leading to the end product. Especially the rheomechanical properties of meat products at different technological stages indicate both their physicochemical state and their structure [
44].
Meat is the muscle tissue which consists of muscle cells: muscle fibres – myofibrils and connective tissue. These two structural components of the muscle mainly affect its rheological and textural properties [
45]. The colloidal solution of sarcoplasmic proteins, which is muscle juice filling the interior of the fibres and the spaces between the myofibrils, is an equally important structural element affecting the rheological and textural properties of meat. Physically, muscles can be treated as a dispersion system composed of two phases, i.e. a hydrocolloid continuous phase (an aqueous colloidal solution of proteins and real low-molecular soluble compounds) and a dispersed phase, which is composed of muscle fibres containing insoluble myofibrillar proteins and connective tissue proteins, mainly collagen.
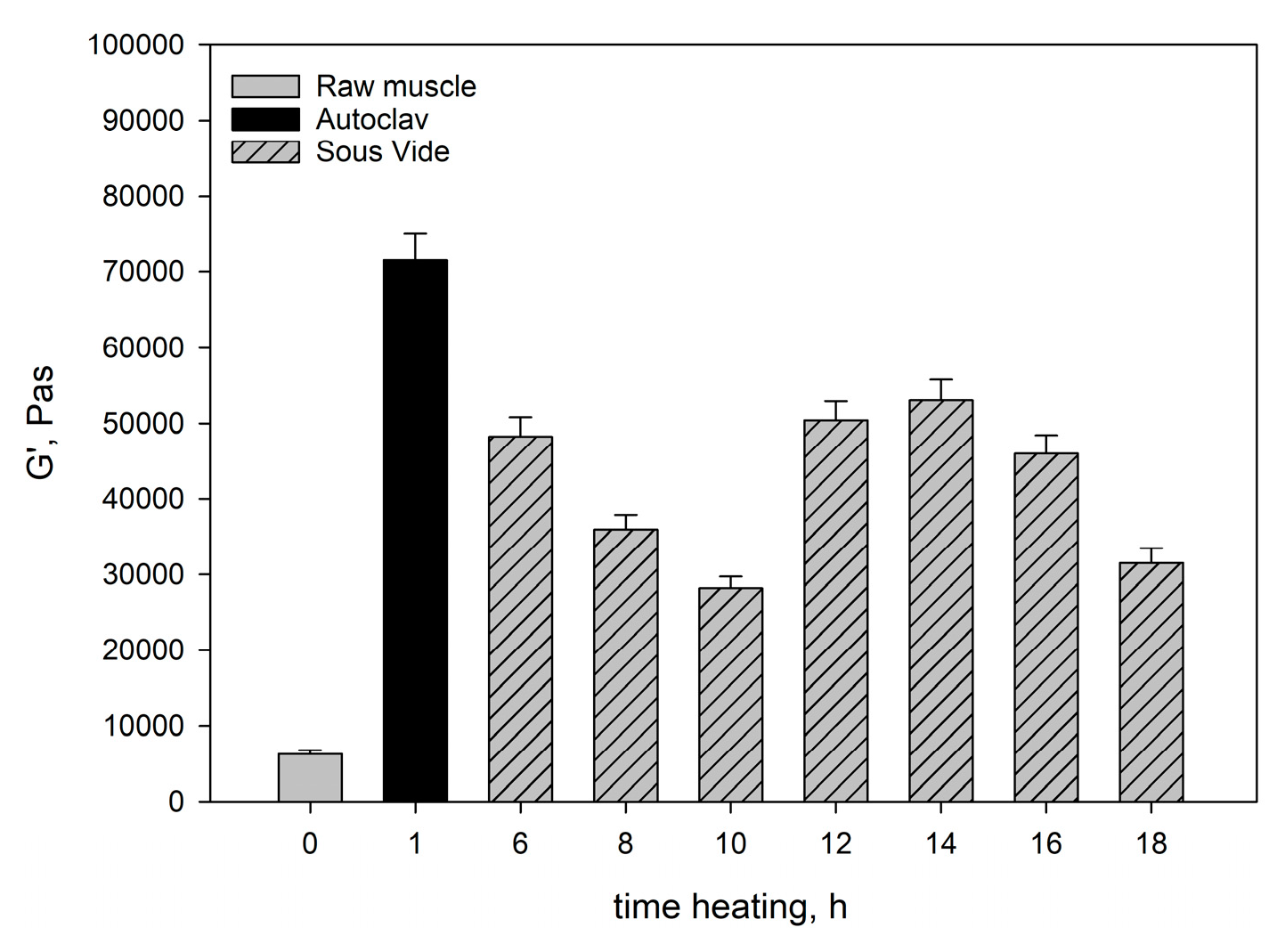
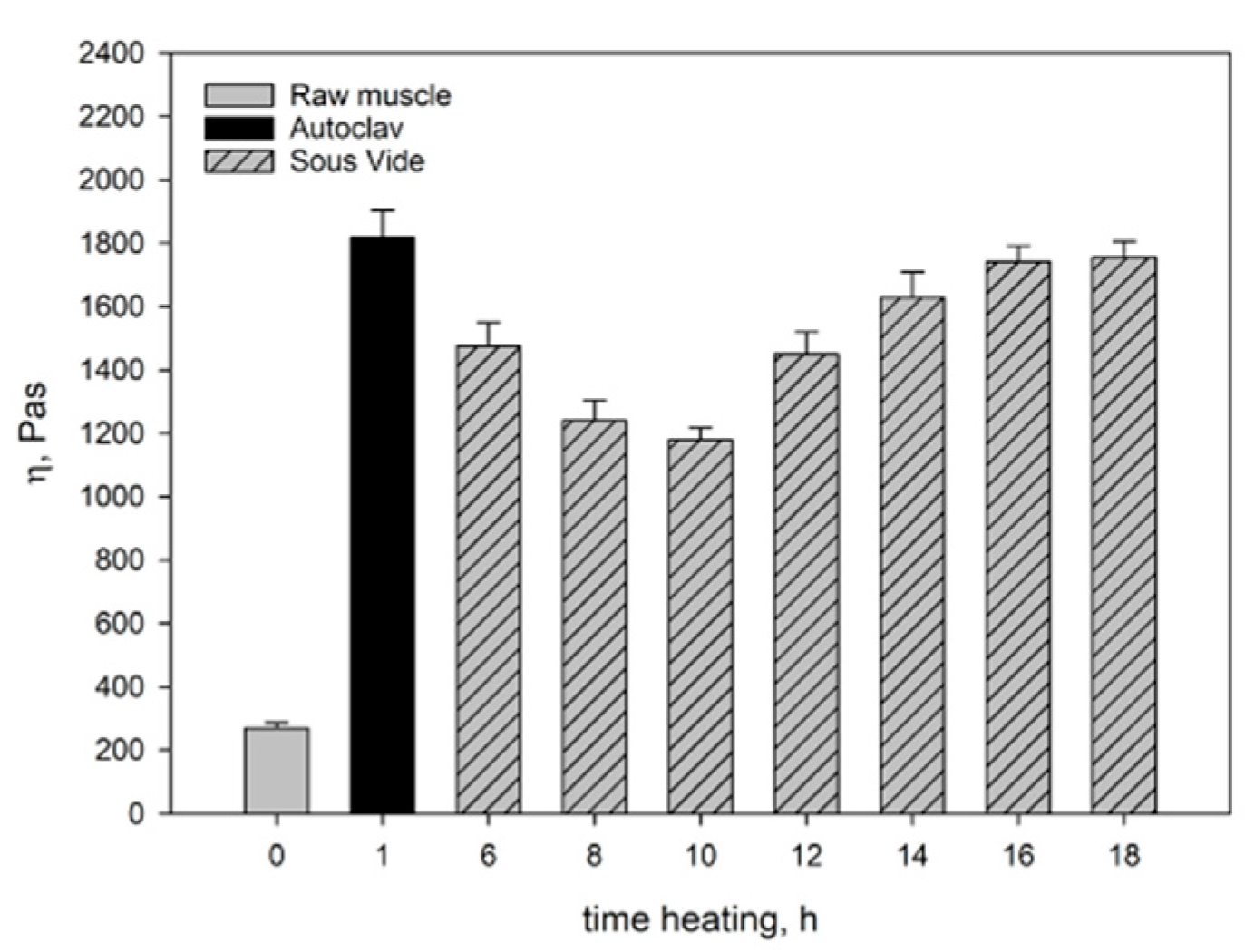
Figure 1 and
Figure 2 show the moduli of elasticity (G’) and dynamic viscosity (η) of: raw pork, sous-vide-heated pork, and pork heated in the autoclave. The modulus of elasticity and dynamic viscosity of the sous-vide-heated meat were determined as a function of heating time.
The rheological tests showed that the raw muscle (longissimus dorsi muscle) had a lower G’ value than the heated muscles (
Figure 1), because a viscous colloidal solution of sarcoplasmic proteins filling the spaces inside and between the cells in the raw muscle dissipated mechanical energy and limited its spread. The connective tissue and myofibrils containing undenatured myofibrillar proteins (myosin and actin) and collagen contained in the connective tissue were characterised by low stiffness. This resulted not only in lower G’ values (
Figure 1), but also in a lower value of dynamic viscosity (η) (
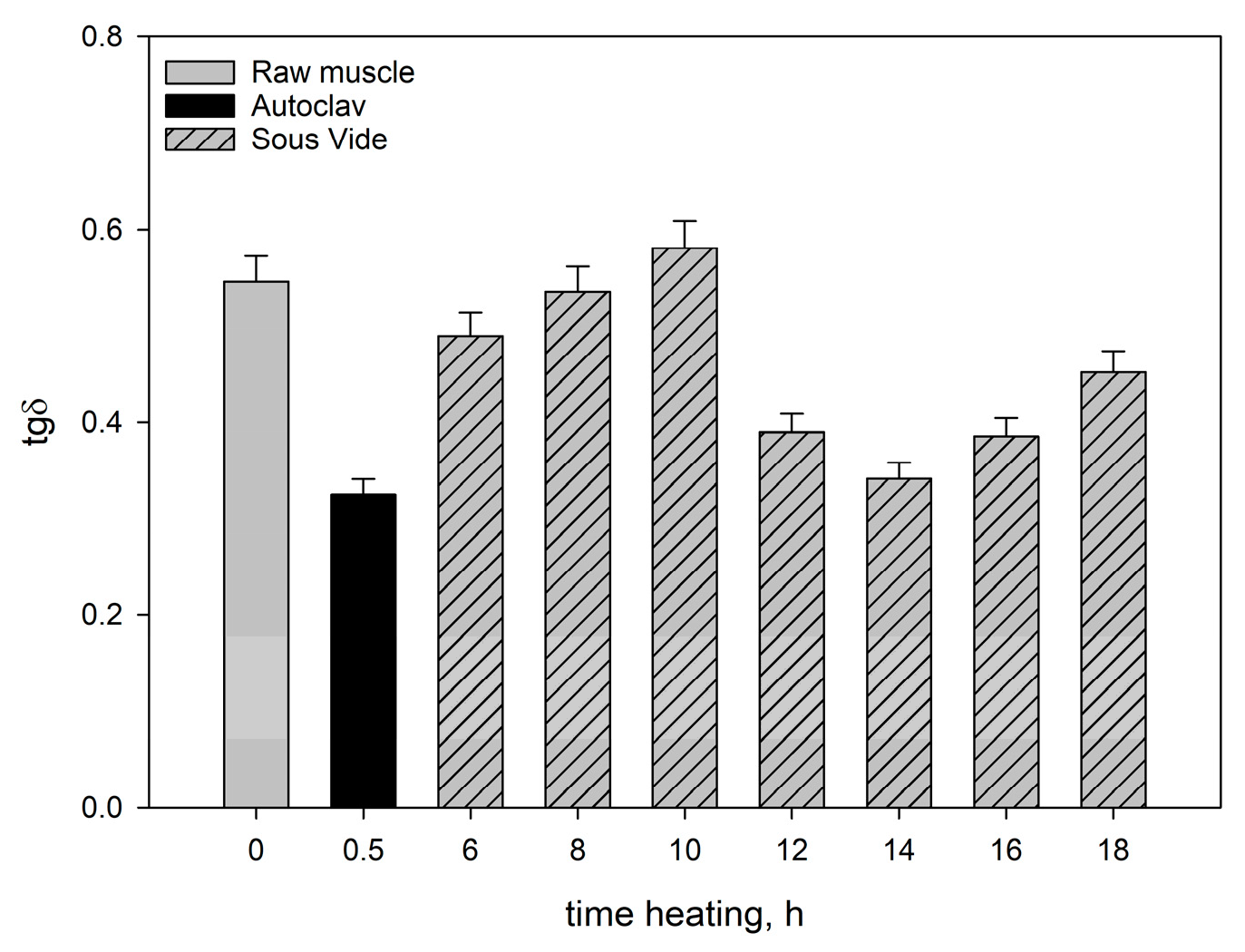
Figure 2). In consequence, there was an increase in the ability to dissipate mechanical energy tgδ (
Figure 3).
The rheological properties of dispersion systems are determined by the rheological characteristics of the continuous phase, the deformability of the dispersed phase, as well as the interactions between these phases [
46]. Therefore, during the heating of the muscles, the molecular changes occurring within the structural units of the muscle tissue, both in the myofibrillar and connective tissue, are extremely important for the rheological properties. These changes are caused by the denaturation and solubilisation of the protein components in muscles [
17]. In our study they occurred as soon as the final temperature of 60°C was reached during heating.
During thermal denaturation at 30-35°C, native tropomyosin dissociates from F-actin. At 40-45°C, myosin dissociates into light and heavy chains. Simultaneously, particularly light myosin chains become partially unfolded in the thermal process [
26]. At 45-50°C, dissociation of the actomyosin complex takes place [
47]. A further increase in temperature above 50°C causes conformational changes within both free myosin molecules and actomyosin chains. At the same time, there are structural changes in the connective tissue. Purslow et al. (2016) [
23] observed that the connective tissue, especially collagen, denatured at 53-63°C, and gelled at 60-70°C. Tornberg (2005) [
17] studied structural changes in meat proteins at different temperatures and found that most sarcoplasmic proteins aggregated at 40-60°C. The intramuscular adipose tissue also melts as a result of heating.
The aforementioned changes occurring during muscle heating result in structuring within the hydrocolloid continuous phase filling the spaces inside and between cells, the aggregation and cross-linking of myofibrillar proteins extracted into hydrocolloid, and the gelation of partially dissolved collagen. The denaturation of myofibrillar proteins in muscle fibres causes their stiffening. In consequence, after heating muscles have a more compact structure than raw muscles, whereas all dispersion components (hydrocolloid and muscle tissue) are more strongly bound to each other. Thanks to these changes, after heating muscles more effectively respond to dynamic mechanical impacts affecting their elastic properties. This is manifested not only by the increase in the value of rheomechanical parameters (G’ and η) (
Figure 1 and
Figure 2), but also by the values of texture determinants (
Table 4).
Spatial structures formed by the elastic associations of myosin and actomyosin have a greater capacity for an elastic reaction under the mechanical influence than the hydrogel resulting from the thermal transformations of soluble proteins, mainly sarcoplasmic ones [
48].
The rheological and textural properties of heated muscles are shaped by the processes of protein denaturation and the resulting spatial structures with temperature-dependent density of the network segments.
The approximate relationship between the modulus of stiffness of highly elastic spatial networks of polymers and the concentration of segments in these networks is determined by the following relation [
49]:
where:
This relationship implies that at a given protein concentration, the increase in stiffness of the system (hydrocolloid) at a given temperature occurs as a result of the expansion of the spatial network nodes. New molecule segments are bound as a result of the interaction between protein polypeptide chains.
After heating the muscle systems for 6-10h there was a noticeable decrease in their elastic properties (G’) and a corresponding increase in the ability to dissipate mechanical energy (tgδ) (
Figure 1 and
Figure 3) in consequence of the change in the saturation of the hydrocolloid with sarcoplasmic and myofibrillar proteins. As a result, the cross-linking of spatial protein matrices decreased.
The change in the protein saturation of hydrocolloids was caused by the structural changes after the heating of mainly the muscle fibres (the shrinkage and swelling of myofibrils) and partly the connective tissue [
23]. As a result, as the heating time increased, the water-holding capacity of the muscles decreased, whereas the exudation of the solution containing mainly sarcoplasmic proteins and partially solubilised collagen increased (
Table 1).
As results from the analysis of the data obtained in our study, the heating time at constant temperature was one of the main factors responsible for the rheological properties of cooked muscles. It significantly influenced the course and intensity of the molecular processes occurring within the structural units of the muscle tissue, which affect these properties. Generally, up to 10 hours of heating myofibrils contract transversely and swell, whereas sarcoplasmic proteins gelate [
50]. Collagenase, an enzyme contained in sarcoplasmic proteins, causes partial solubilisation of collagen [
45] and a major part of the connective tissue. Proteolytic enzymes also contribute to changes in the structure of the connective tissue. After 10h there are further transformations in the connective tissue structures and muscle fibres. The connective tissue network and muscle fibres contract longitudinally. As a result, proteins in myofibrils have a more aggregated, dense structure, whereas the elasticity of muscle fibres increases. This is reflected by the values of rheological determinants. Within the range of the meat heating time analysed in our study, the moduli of elasticity (G’) (
Figure 1) were greater than in the meat heated for 10h. The ability to dissipate mechanical energy (tgδ) decreased noticeably (
Figure 3). At the same time, the degree of cross-linking of the hydrocolloid phase increased. This effect was caused by the progressing gelatinisation of solubilised collagen as the heating time increased from 12h to 16h and by the increase in the content of myofibrillar proteins extracted into the hydrogel [
48,
51]. These proteins not only compensate for the loss of sarcoplasmic proteins and dissolved collagen due to thermal leakage but also improve the elastic properties of the hydrocolloid. This was manifested by an increase in the value of dynamic viscosity η (
Figure 2) of the muscle systems, observed within that time interval. The slight decrease in the elastic properties of the meat cooked for 18 hours may have been caused by the expulsion of some non-aggregated proteins contained in the hydrocolloid into the extracellular spaces as a result of the pressure exerted by the shrinking connective tissue. Thus, these proteins did not participate in the formation of the protein matrix nor did they have any effect on its elastic properties.
The rheomechanical properties of the meat heated under elevated pressure (0.2MPa) and at high temperature (121.1°C) were compared with those of the sous-vide-cooked meat. The values of the modulus of elasticity (G’) and dynamic viscosity (η) in the meat cooked in the autoclave were significantly greater than in the sous-vide-cooked meat, regardless of the heating time (
Figure 1 and
Figure 2). The high values of the modulus of elasticity (G’) but significantly lower energy dissipation capacity (
Figure 3) show that the elastic properties of the meat heated in the autoclave were much greater than those of the sous-vide-cooked meat. This was due to the fact that the rheological properties of meat are influenced by molecular changes occurring within the structural units of the muscle tissue. Their intensity depends not only on the heating time but also on temperature [
23,
25].
In the muscles cooked at high temperature (121.1°C), the changes in the myofibrils and in the intramuscular connective tissue were greater than in the sous-vide-cooked muscles at 60°C. These changes were caused by the denaturing processes/transitions of myofibrillar and connective tissue proteins, mainly collagen. As a result of their gelation, the cross-linking density of the intermuscular hydrocolloid continuous phase increased significantly. Due to the greater aggregation of proteins in the muscle fibres, they were stiffer than the sous-vide-cooked muscles at 60°C. This effect was manifested by higher values of the rheomechanical parameters (G’, and η) (
Figure 1 and
Figure 2) and lower energy dissipation capacity (
Figure 3).
The marked increase in the G’ value after 30 minutes of isothermal heating at 121.1°C also resulted from the denaturation of actin, the second main myofibrillar protein, which probably forms a gel network between denatured actin and myosin and with other actin molecules, which additionally increases the elasticity of the spatial network of proteins.
Due to the fact that actin begins to denature at 74°C [
51,
52], these proteins were in their native state in the sous-vide-cooked muscles at 60°C and did not have any influence on their elastic properties.
2.6. Texture analysis
2.6.1. WBSF
Texture is probably the most important quality factor affecting consumers’ satisfaction with meat products. The International Organization for Standardization (ISO) defines texture as all the rheological and structural properties of a food product which can be perceived by humans through tactile, mechanical and, if possible, visual and auditory receptors. Therefore, meat texture is a characteristic which can be defined by certain homogeneous properties that are detected by the human senses of vision, hearing, somesthesis, and kinaesthesia. These properties are perceived as: hardness, gumminess, elasticity, cohesiveness, adhesion, and stickiness.
The shear force test (Warner-Bratzler shear force – WBSF) and texture profile analysis are classic instrumental methods of meat tenderness (hardness) assessment.
The most common instrumental procedure of meat tenderness assessment is the WBSF test. The test measures the maximum force (N) as a function of knife movement (mm) and compression to shear (cut off) a meat sample (MPa). The result of this measurement indicates the hardness (toughness). of meat.
The maximum force observed during the shear test was selected to characterise the texture of the samples (
Table 4).
In the shear force and shear work tests, all the samples were characterised by similar dependencies in relation to the time variants under analysis. Among the sous-vide-cooked samples, the pork heated for 6 hours was characterised by the greatest shear force and shear work values, which were significantly different from the values observed in the other heated samples.
As the heating time increased up to 12h, there was a statistically significant decrease in the values of both parameters (shear force and shear work). During heating for 14 hours there was a noticeable increase in the shear force and shear work. When the heating time was extended to 18 hours, the values of both parameters decreased significantly (
Table 3).
The shear force values noted in our tests were similar to the results of the experiment conducted by Christensen et al. (2011) [
53], where the shear force values of the pork Longissimus dorsi muscle ranged from 12.6 to 41.1N and decreased as the cooking temperature increased. The influence of the cooking time was not uniform and depended on the cooking temperature. However, in an experiment conducted by Vaudagna et al. (2002) [
33], the mean shear force values decreased as the cooking temperature increased, but the meat cooking time did not have significant effect on the mean shear force values. The observations made by Vaudagna et al. in an experiment on the influence of beef heating time on the shear force values were different from the observations made in our study. This may have been caused by the fact that our pork heating times were much longer than the beef heating times used by Vaudagna and his team.
Bertola et al. (1994) [
54] noted the lowest shear force values (WBSF) during the heating of small pieces of beef (diameter – 1.5 cm; length – 2 cm) at 60°C and 64°C. Low values of shear force are desirable in cooked meat because they reflect greater tenderness of the ready-to-eat product. Meat tenderness is influenced by myofibrils and connective tissue proteins and their transformations during heat treatment, mainly the denaturation of myofibrillar proteins (myosin and actomyosin) and the solubilisation of collagen [
24].
Hwang et al. (2019) [
15] observed that the shear force of pork tenderloin was affected by the process temperature, whereas the process time had significant effect on the meat tenderness only at 50°C. The researchers suggested that the tenderness of meat cooked for a longer period of time (24h vs. 12h) was enhanced by the activity of intrinsic proteases which remained active at such a low temperature. The thermal inactivation of proteases at temperatures higher than 55°C may have been the reason why the heat-induced structural changes in meat proteins had significant influence on meat tenderness.
Similarly, Jeong et al. (2018) [
25] observed that the cooking temperature had significant effect on the shear force of sous-vide-cooked pork ham at 61°C and 71°C, whereas the effect of the cooking time was observed at 71°C. Increased meat tenderness during cooking is caused by the conversion of collagen to gelatine [
51]. Ismail et al. (2019) [
55] observed increasing collagen solubility in beef cooked at 45°C, 65°C, and 75°C. Vasanthi et al. (2007) [
56] observed that collagen solubility tended to increase along with cooking temperature (80-100°C) and time of (30-60 min). The increase in the degree of collagen dissolution as well as the degree of denaturation of muscle proteins are important factors affecting the textural properties of muscles, as evidenced by the analysis of the texture parameters of the muscles heated in the autoclave.
In the muscles cooked in the autoclave at 121.1°C collagen was dissolved and myofibrillar proteins were denatured to a much greater extent than in the sous-vide-cooked muscles heated at 60°C. As a result, the myofibrillar fibres were stiffer. Gelled collagen increases the cross-linking density of the intermuscular hydrocollidal phase. As a result, the muscles heated in the autoclave had a more compact structure than the sous-vide-cooked muscles, whereas all dispersion components (hydrocolloid and muscle tissue) were more strongly bound to each other, as evidenced by an increase in the shear force and shear work (
Table 4).
2.6.2. Texture Profile Analysis (TPA)
TPA provides more information on the texture of meat products than shear force, which is a useful indicator of initial meat tenderness [
34]. The texture profile attributes are listed in
Table 4.
During the sous-vide heating of pork, hardness I and II decreased gradually as the cooking time increased from 6h to 10h. The decrease in both parameters was statistically significant. Then, their values were increasing until the 14-hour heating time. After 14 hours of heating hardness I and II tended to deteriorate again until the moment of the 18-hour meat heating time (
Table 4). Changes in meat tenderness and hardness during sous-vide cooking are caused by heat-induced changes in the connective tissue and muscle fibre proteins. When the heat contained inside the vacuum bag comes into contact with the moist content of the bag, the connective tissue begins to dissolve. It results in partial solubilisation and gelation of collagen, which increases meat tenderness. The denaturation of myofibrillar proteins causes the stiffening of muscle fibres, which increases meat hardness [
51].
As was the case with the shear force and work, during the heating of pork for 14 hours there was an increase in hardness I and II (
Table 4). This may have been caused by the heterogeneous structure of the muscle tissue, the amount of connective tissue (collagen), tendons, and intramuscular fat in the muscle under analysis. Roldan et al. [
30] observed that when lamb was heated for a longer period of time at the same temperature, the hardness of the sample decreased. The meat was heated at 60°C, 70°C, and 80°C for 6h, 12h, and 24h.
Polak et al. (2019) [
57] studied the effect of sous-vide technology on beef quality. The researchers observed that the meat samples heated for 30 hours at 64°C, 68°C, and 72°C were less hard than the beef samples heated for 24 hours. They concluded that a longer sous-vide heating time contributed to the softening of the muscle, which may have been caused by the complete dissolution of collagen [
51].
The highest chewiness was observed in the sous-vide-cooked pork after 6 hours of heating. As the cooking time increased from 6 to 10 hours, the chewiness of the pork decreased. It remained at a similar level up to 18 hours of heating and there were no statistically significant differences. This may have been caused by the fact that many enzymes are denatured during sous-vide cooking at 55-60°C, but some collagenases are active and can significantly increase meat tenderness after more than 6 hours of heating [
58]. Roldán et al. (2013) [
30] observed a similar effect of the heating time on chewiness. They found that cooking time reduced chewiness. However, when meat was heated at 60°C for 6, 12, and 24 hours, chewiness decreased only after the heating time was extended to 24 hours.
In our study, the meat heated in the autoclave had the highest elasticity, and its values differed statistically significantly from the other variants of the experiment. The meat samples heated for 12, 14, and 18 hours had lower elasticity than the meat heated for 8 and 10 hours and they did not differ statistically significantly from each other. The highest elasticity was observed in the sous-vide-cooked pork heated for 6 hours. The value of this parameter did not differ statistically significantly from that of the meat heated for 10 hours. There were no statistically significant differences in elasticity between the meat samples heated for 12, 14, 16, and 18 hours.
Elasticity is defined as the ability of a deformed product to quickly return to its natural shape [
44]. Sánchez del Pulgar et al. (2012) [
29] observed that the heating of pork at 60°C for 5 or 12 hours did not cause any significant changes in hardness, elasticity, cohesiveness, or chewiness. The values of these parameters decreased only in the samples heated for 12h at 80°C. Jeong et al. (2018) [
25] found a slight difference in the elasticity and consistency of the sous-vide-heated meat samples as the temperature increased from 61°C to 71°C. They also observed that when the heating time was extended from 45 to 90 min, there were slight changes in springiness and elasticity. Similarly, although in our study, the heating times were much longer, the changes in elasticity were minimal.
In comparison with the sous-vide-cooked muscles, those heated in the autoclave (121.1°C) had higher values of textural parameters determined both with the WBFS and TPA methods within the entire range of times.
The molecular mechanisms responsible for rheological (described above) and textural changes occurring in muscles during isothermal heating at specific temperatures are similar. At high temperature (121.1°C) and elevated pressure, changes in myofibrils, connective tissue proteins and their transformations during heat treatment, mainly denaturation of myofibrillar proteins (myosin, actomyosin, and actin), solubilisation of collagen and its conversion to gelatine occur with much greater intensity than at the temperature used for sous-vide cooking (60°C). These changes lead to the structuring of the intermuscular hydrocolloid continuous phase, which results in the formation of spatial structures with temperature-dependent density of network segments. They also lead to the structuring of the dispersed phase, i.e. myofibrils. In consequence of heating the muscles at different temperatures, they differ in the degree of bonding of the dispersion components (hydrocolloid and muscle tissue). This is manifested not only by differences in the values of texture determinants (
Table 4), but also in the values of rheomechanical and parameters (G’ and η) (
Figure 1 and
Figure 2).