1. Introduction
Visceral leishmaniasis (VL) is a serious public health and veterinary problem in Brazil [1–3], with territorial expansion that has reached all geographic regions of the country, including medium and large cities. The current epidemiological pattern differs from that initially constrained to the rural environment. The etiological agent Leishmania (Leishmania) infantum belongs to the Kinetoplastida order, Trypanosomatidae family, Leishmania genera (ROSS, 1903) and is transmitted by the bite of female dipterous insects of the Psychodidae family belonging to the Phlebotomus and Lutzomyia genera in the Old and New World, respectively [4]. Ninety percent of human VL cases in South America are reported in Brazil. Epidemiological data from 1990 to 2009 show an average incidence of VL in humans of approximately 1.8 cases/100,000 habitants. Between 1994 and 2005, the average fatality rate was 5.5% per year. The high lethality rate remains the main problem in VL, and its occurrence has been linked to the presence of other comorbidities such as hepatic, renal, and cardiac diseases and HIV infection [3].
Among VL-endemic countries, Brazil is the only country that conducts a regular government program to monitor and prevent further expansion of the disease [5] through The Program for Control of Visceral Leishmaniasis (PCVL). The Program comprises four basic public health measures: free referral for all reported human cases, euthanasia of seropositive domestic reservoirs (dogs), use of residual insecticides to control vector density (Lu. longipalpis), and rigorous epidemiological surveillance [1]. Subsequently, the Ministry of Health incorporated environmental management as a complementary recommendation for vector control and health education [6]. These actions have considerable operational and logistical costs, and are complex and laborious in practice [7–10]. The efficiency is questionable due to low technical and scientific support concerning the fragilities related to serum diagnosis, low cost-benefit ratio of canine euthanasia of serum-positive dogs, and rapid replacement with new dogs by owners [9,10]. The success of PCVL was observed only when the control measures were carried out in an integrated manner [11–13].
The present study evaluated the impact and efficiency of the fast and systematic removal of seropositive dogs as an isolated VL control action in an endemic area of intense disease transmission in Brazil.
2. Materials and Methods
2.1. Study area
Porteirinha is located in the northern region of the state of Minas Gerais (15°44’42’’S, 43°01’46’’W), 630 km from its capital, Belo Horizonte. The municipality occupies an area of 1788 km2 with an altitude of 567 m, within the “dry-land polygon”. The city comprises 12 neighborhoods distributed in hills in its higher region (São Judas Tadeu, Mato Verde, Vitória, and União) and wide openings or lower regions encompassing the Centro, Floresta, Morada do Parque, Ouro Branco, Renascença, São Sebastião, Kennedy, and Serranópolis neighborhoods (Suppl. 1). At the time of our study, the local population consisted of 35,465 inhabitants, with 41% living in the urban areas [14].
The geophysical characteristics of the municipality present a relief divided into three distinct parts: a high limestone hill (10%), a wavy section (50%), and a lower and flat region (40%) represented by the São Franciscan depression. The predominant phytogeography is the cerrado, with dense arboreal vegetation appearing in the humid parts of the valleys, especially on the banks of the main perennial (Gorutuba, Mosquito, Serra Branca, and Lages) and temporary (Mucambinho, Sítio Novo, Sanharol, and Cocos) rivers, all of which belong to the São Francisco River Basin. The climate is tropical and semi-humid, with an average temperature of 24ºC and a dry season lasting approximately 6 months per year. The rainy season extends from October to March, with an average annual rainfall of 600 mm [15].
2.2. Study design
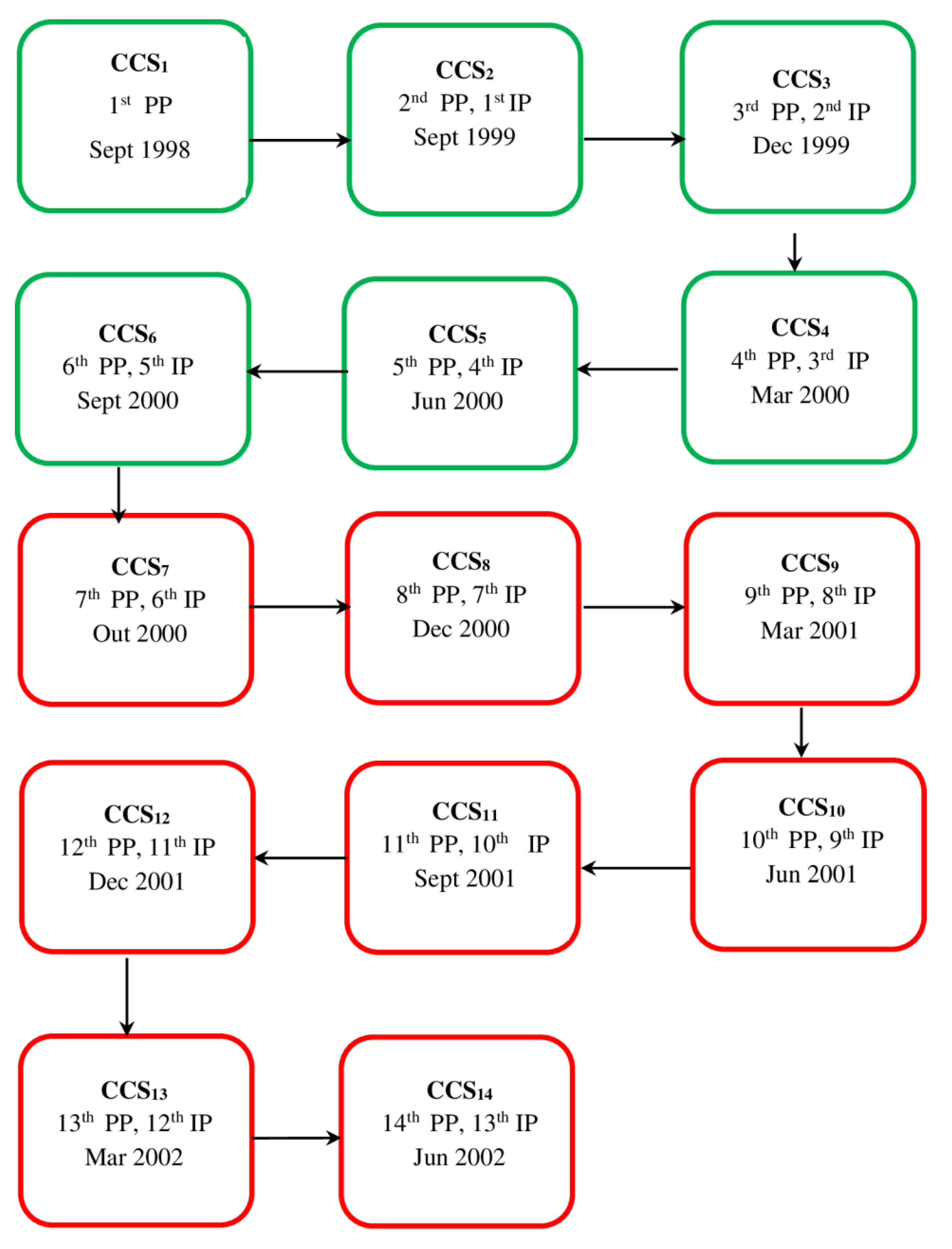
A prospective observational cohort was initiated in the September of 1998 through the first and only census survey (CCS) covering almost 100% of the canine population domiciled in Porteirinha. This study aimed to evaluate the epidemiological status of VL. This first stage was considered the study baseline and represented the first prevalence point (PP) in both urban and rural areas (
Figure 1). The second CCS was performed in September 1999 and corresponded to the second PP and the first incidence point (IP). From this point until the end of the study, only the canine population domiciled in urban areas was followed. The zootechnical profile of the native canine population was compiled during CCS
3.
2.3. Serological diagnosis of CVL by immunofluorescence antibody test (IFAT)
Canine blood was collected by filter paper impregnation [16,17] and IFAT was performed as described before [18]. Promastigotes from Leishmania (Leishmania) mexicana (strain MHOM/BR/1960/BH6) were used as antigens. Anti-dog IgG fluorescence-conjugated antibodies were produced by Biomanguinhos (FIOCRUZ, Brazil). Titers ≥1:40 were considered positive for CVL [19]. Seropositivity was confirmed by retesting a new sample collected via cephalic or jugular venipuncture [20]. Quality control was monitored by a reference laboratory (FUNED) of the National Network for Serodiagnosis of CVL.
2.4. Indicators of CVL morbidity
The comparative morbidity index (CMI) was used to stratify areas with the greatest risk of CVL [21].
where CMI values of 1 indicate medium risk, values >1 denote high risk, and values <1 indicate low risk. The incidence and prevalence rates were calculated as follows:
2.5. Follow-up of the population seropositive for CVL
In neighborhoods with great risk of VL transmission, the surviving seropositive dogs were maintained in their respective domiciles from CCS
1 to CCS
6 (
Figure 1). In the 2-year period, no intervention or control action was taken to avoid any interference with the local force of infection. The results of clinical examinations, suggestive clinical signs, and serological monitoring (serological titer) were recorded and transferred to a database.
The active search and systematic removal of dogs seropositive for VL started in CCS
7 and ended in CCS
14 (
Figure 1).
2.6. Clinical and serological diagnosis of human VL
VL diagnoses and treatment protocols were based on compatible clinical and laboratory findings [22]. Individuals with positive serology and clinical symptoms were considered symptomatic and those with positive serology but no clinical symptoms were considered inapparent for VL.
2.7. Statistical analysis
The database was constructed using ACCESS software. Proportions were compared by chi-square and Pearson correlation coefficient tests (p < 0.05). All households with cases of symptomatic or inapparent human VL between 1998 and 199922 or CVL were georeferenced using a GPS device (GARMIM-ETREX) and processed using MapInfo software.
3. Results
During our 4-year study, 40,387 indirect immunofluorescence reactions were performed, of which 556 samples yielded seropositive results for CVL, with an accumulated prevalence of 1.38% and accumulated incidence of 7.5 cases/1000 dogs/year.
In CCS1, 5071 dogs were examined (Suppl. 2). Two hundred-ninety-one dogs (5.7%) were seropositive for the disease. Statistical comparison of the prevalence ratios in urban (4.4%) and rural (6.1%) areas indicated that rural dogs had a higher chance of contracting VL.
The dynamics of CVL transmission were evaluated for 2 years, from CCS1 to CCS6, before the beginning of the screening-culling action (Suppl. 3). The number of dogs examined per survey varied from 1,398 (CCS2) to 5,071 (CCS1), in a total of 14,195 animals. The incidence rate ranged from 4.3 cases/1000/dogs/year in CCS7 to 15.9 cases/1000/dogs/year in CCS6.
Short-haired dogs had the highest chances of infection (Suppl. 4). Most animals from the survey (75%, 1,128 dogs) were mongrels, about 10% (150 dogs) belonged to 10 short-haired breeds (Chihuahua, Rottweiler, Weimaraner, Pinscher, American Pointer, Brazilian fila, Dachshund, Dobermann, Dog German, and Boxer), and 15% (226 dogs) were long-haired from six breeds (Poodle, Pekingese, Akita, Siberian Husky, German Shepherd, and Cocker). Among mongrel dogs, the average CVL seropositivity was 2.9%. The most affected breeds were Dobermann (22.2%) and Brazilian Fila (2.6%) (data not shown). The seropositivity rates for males (892 dogs) and females (612 dogs) did not differ significantly. Therefore, both sexes had the same probability of Leishmania infection.
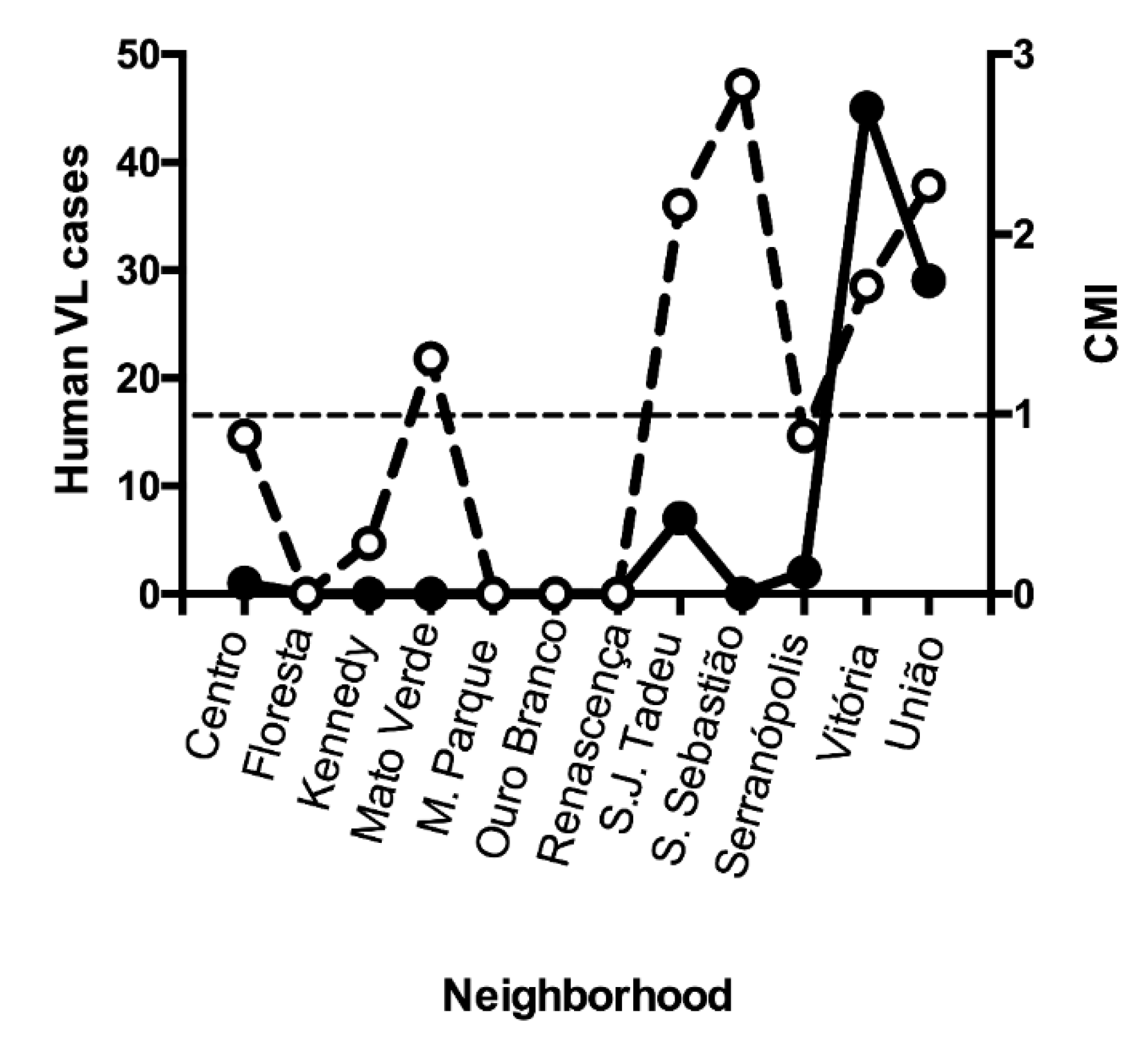
In the CCS3, CVL indicators were calculated for each neighborhood (Suppl. 5). CVL was detected in eight of the 12 neighborhoods of Porteirinha. Twenty-three new cases of CVL were diagnosed during the 2-year study period, with an average incidence of 15.2 cases/1000 dogs/year. The CVL incidence varied from 8.8 in Vila Vitória to 40.0 in Vila União. Six neighborhoods did not introduce new dogs, and the CVL incidence remained at zero. The CVL prevalence varied from zero to 4.38 cases. Five of the twelve neighborhoods had a CMI >1, indicating a high risk of CVL transmission.
In CCS4, at the end of the rainy season, we observed the highest rates of survival for seropositive dogs (86.6%), growth of the seropositive canine population (24.9%), seropositivity (3.0%), incidence (15.9 cases/1000/dogs/year), and number of new cases (30), and the lowest mortality rate (13.4%) for seropositive dogs. During follow-up, we observed several canine deaths and dogs missing from their former homes, suggesting an important migration rate of seropositive animals.
Almost all neighborhoods reported CVL cases between September 1998 and September 2001, except for Morada do Parque (Suppl. 6). Floresta and Mato Verde had the lowest numbers of cases (two each), whereas the largest number occurred in São Judas Tadeu (54 cases). During the same period, 84 human cases of VL (symptomatic or inapparent) were reported. The largest number of households with positive VL serology (45) was in Vitória, of which 42 were asymptomatic. In contrast, of the 29 patients in Vila União, 11 were symptomatic. Human cases occurred in neighborhoods with CMI >1, except for one single case in Centro (CMI=0.88) (
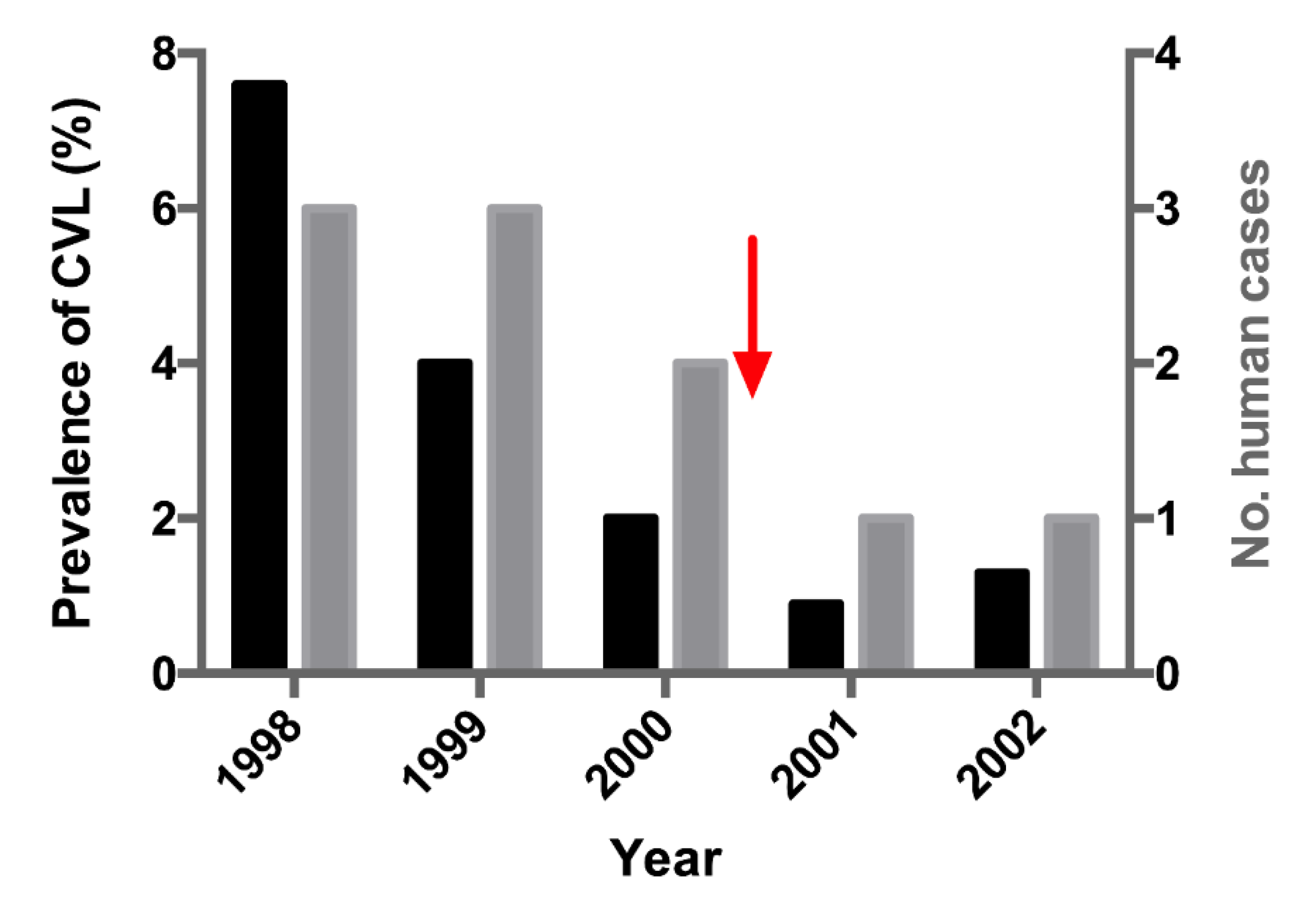
Figure 2). Three of the five high-risk neighborhoods registered human cases of VL. Among the 19 patients with symptomatic VL from all neighborhoods, 11 lived União. Because União had CMI=1.71 and 58% of the symptomatic cases of VL, we used this neighborhood as our model for the analysis of the screening-culling intervention. Notably, the incidence rate of human VL cases decreased by from eight to two cases after the intervention (
Figure 3). A concomitant reduction in the prevalence of CVL was noted.
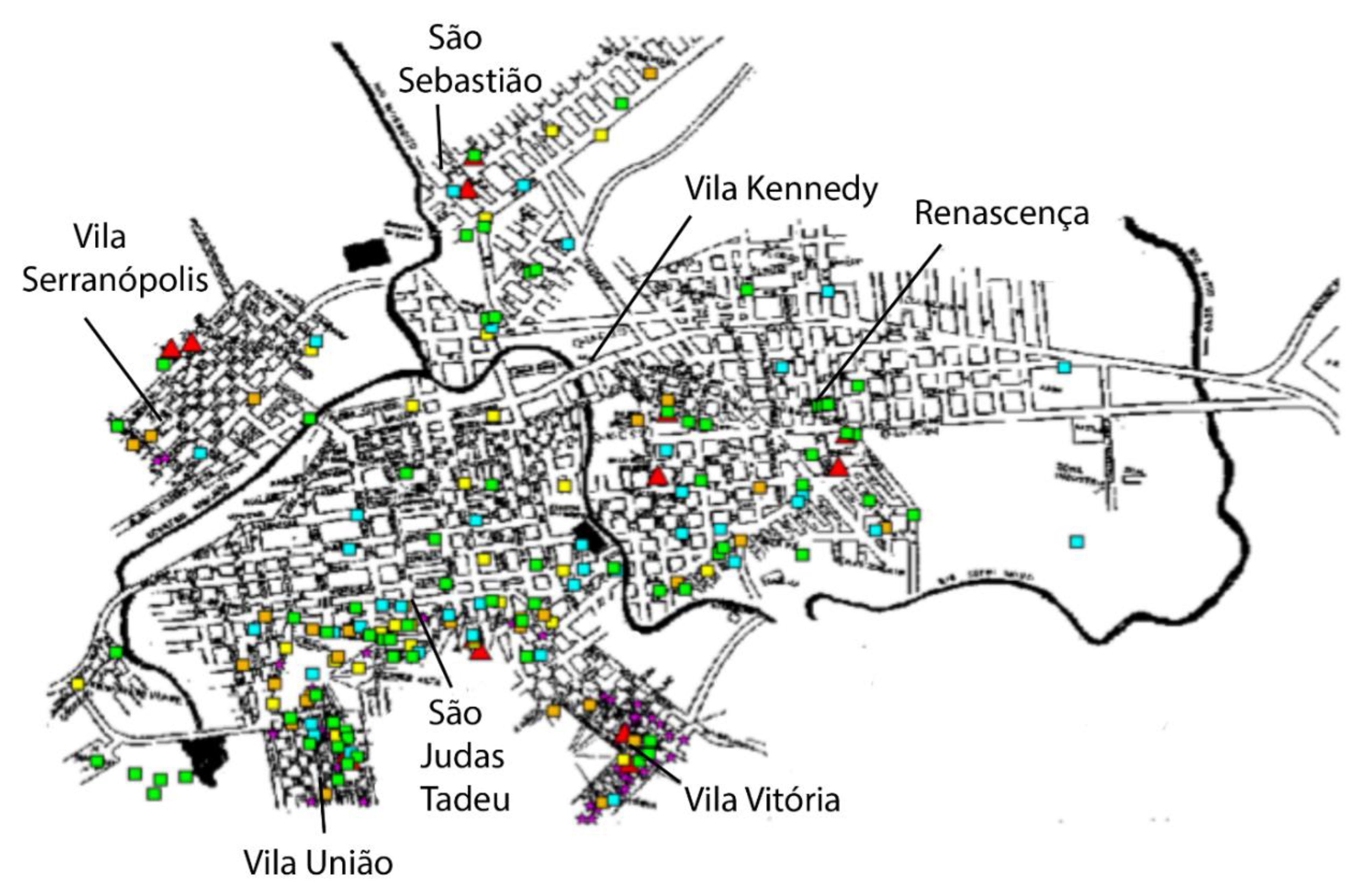
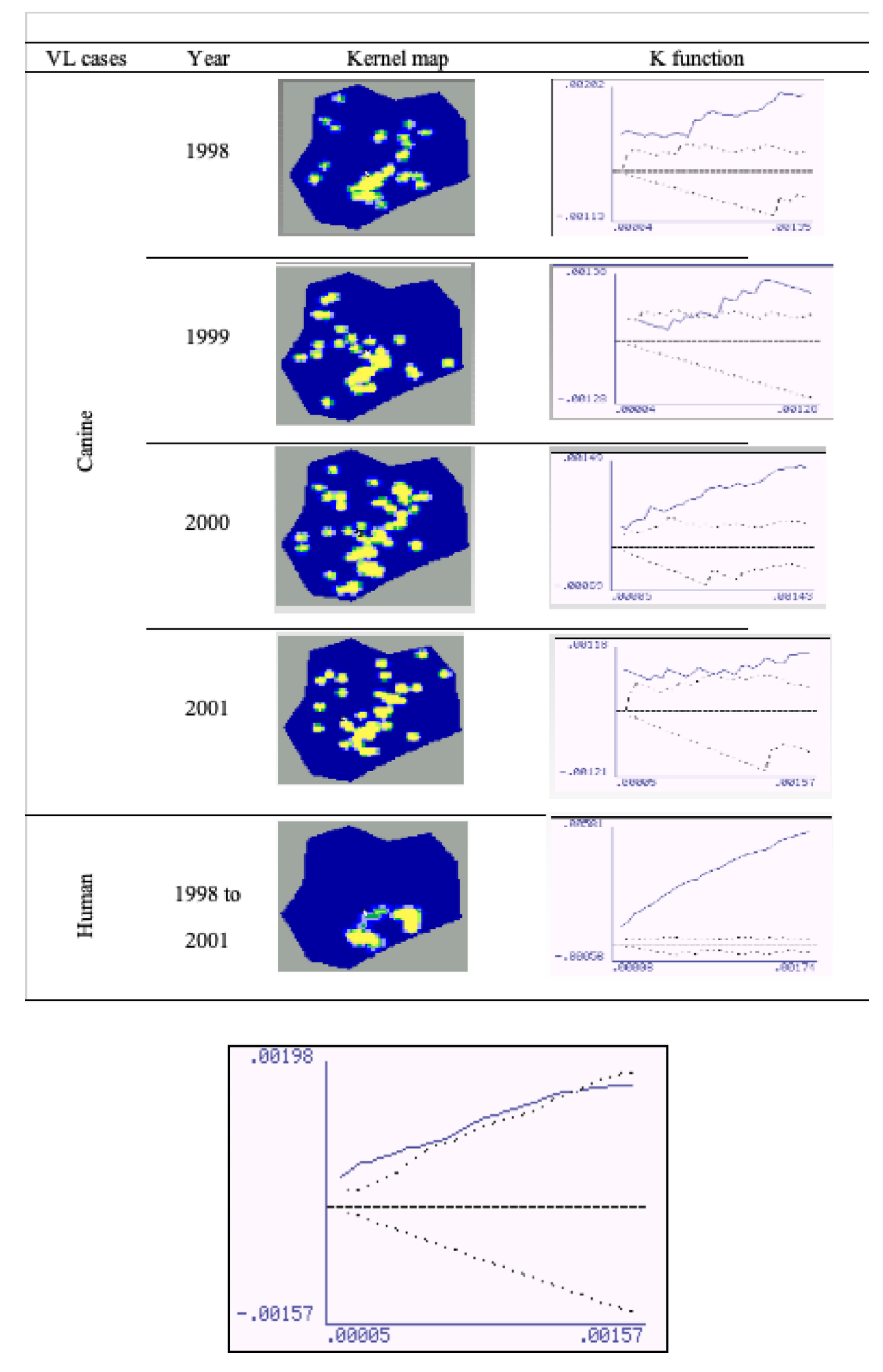
The spatial distribution of human and canine cases is shown in a geopolitical map of Porteirinha (
Figure 4). Kernel maps were evaluated using the respective K (univariate) function for the annual georeferencing of the spatial distribution of households with human and canine VL cases. The univariate K-function for each year of the spatial distribution of CVL cases was significant for 1998, 2000, and 2001, when spatial clusters were observed. K-function was not significant in 1999, when greater dispersion occurred in other regions of the city.
The univariate K-function for the spatial distribution of human VL cases (symptomatic and inapparent) was highly significant, confirming the existence of a restricted conglomerate in the neighborhoods of São Judas Tadeu, Vitória, and União.
Figure 5 shows the bivariate K-function for the spatial distribution of human and canine cases of, which dispersion patterns differed significantly. CVL was dispersed throughout the city and human VL was mostly concentrated in São Judas Tadeu, Vitória, and União.
4. Discussion
In Brazil, the CVL seropositivity in endemic areas ranges from 5% to 35% [15,17,23,24]. In Porteirinha, the CVL seropositivity (5.7%) was close to the lowest value in the range. Dogs in rural areas were more prone to contracting CVL than those living in urban areas. In urban areas, short-haired dogs had a higher risk of contracting CVL. Mongrel dogs (2.9%) and Fila Brasileira (2.6%) showed similar seropositivity rates, which differed from that of Dobermanns (22.2%), the most affected breed. Dobermann was also the most affected breed, with 35.3% seropositivity, in the Lisbon metropolitan region (Portugal). Sideris et al. [25] reported that this short-furred animal in Athens (Greece) was the most susceptible to CVL and was more easily bitten by sandflies. Boxers, together with German Shepherds, were also the most affected breeds in a study conducted in France [26]. The authors suggested that the high susceptibility was because both are working breeds, mostly acting as guard dogs; therefore, they are more exposed to Leishmania infection. In the context of Leishmania infection, dogs are distinguished more by their occupation than by their place of residence [27,28]. Working dog breeds in particular experience a greater force of infection compared to companion dogs. They usually sleep outside and are often exposed to sandfly bites. Companion dogs that generally stay inside their owners' homes are less exposed to bites. In addition, they are more often taken to veterinary clinics for grooming, and bathing, and exposed to products that drive flies away.
In the present study, male and female dogs were equally likely to show CVL seropositivity. Similar results were reported by others in Montes Claros in different countries [17,26,29,30]. In contrast, higher seropositivity rates were reported in male dogs in France [31].
In our study, IFAT was used as a screening and confirmatory test; therefore, we cannot rule out possible cross-reactions with other trypanosomatids, since the IFAT reaction does not distinguish infections, for example, between Le. (Le.) infantum, Leishmania (Leishmania) braziliensis, and even Trypanosoma cruzi [19].
Brazil has experienced a clear territorial expansion and a significant increase in the number of human VL cases [1–3]. The epidemic outbreaks recorded in important urban centers in Brazil demonstrated how the migration from the countryside to large cities influenced changes in the epidemiological profile of VL [37]. In the last three decades, many human cases were reported in several Brazilian capitals [1,3,33,34].
The factors affecting the epidemiology of VL in Porteirinha may be the same as those observed by several authors [35–38], including poverty, malnutrition, large numbers of infected dogs, high vector density in households and peridomiciles, large numbers of domestic animals, poor sanitary conditions, low socioeconomic indices and, possibly, the differentiated ecological valence of the species Lu. longipalpis.
Kernel maps for human and canine VL in Belo Horizonte showed a significant correlation between the occurrence of human and canine VL [39]. In Porteirinha, the pattern of dispersion of human VL and CVL were different, remaining restricted to the São Judas Tadeu, Vitória, and União, which are subnormal conglomerates without basic sanitary infrastructure, located in the foothills of the city. In these places, where a population of low socioeconomic status resides, high densities of Lu. longipalpis have been observed, along with numerous domestic animals raised in chicken coops, pigsties, and corrals, living with humans, and a high number of domiciled dogs [40,41].
In the present study, the existence of a well-defined spatial cluster of canine cases that spatially coincided with human cases was significant when evaluated using the bivariate K- function. While CVL was dispersed, human VL was restricted to the upper parts of the city, clearly suggesting the association of three main components (presence of seropositive dogs, association with the presence of susceptible humans, and high vector density) as determining factors involved in the eco-epidemiological chain. The Kernel maps showed a correlation between areas with active transmission of human infection and those with a significant prevalence of canine infection. The identification of areas with a greater risk of transmission is important, as it not only evidenced the local urbanization of VL, but is also useful in directing entomological studies and subsidizing protocols for integrated control actions in prioritized areas.
In general, euthanasia of seropositive dogs by PCVL takes an average of 120 days, enough time for transmission to other susceptible dogs and humans, justifying, in part, why the efficiency of the intervention has been questioned. Herein, seropositive dogs were excluded 30 days after serodiagnosis. In our model neighborhood (União), the intervention significantly reduced CVL prevalence and remained stable throughout the study period, always remaining <2.0% from the CCS7 onwards. The number of human cases, notably, decreased by 75%, mainly after the intervention suggesting that the systematic removal of seropositive dogs could be more efficient when adopted faster in endemic areas, especially when the force of infection is characterized by a high rate of transmission of human cases. The chi-square value for human cases in relation to the population residing in the União corresponded to a probability of error of 5%–10%. The low number of human cases reported in the study period may have caused bias and prevented it from reaching significance (p<0.005).
According Costa et al. [9], no high-evidence publications have established an association between the occurrence of human VL cases and the seroprevalence of CVL or the removal of infected dogs. Our results, as well as those reported by Ashford et al. [42], demonstrate that the systematic removal of the seropositive canine population may be insufficient as a measure to control VL but it could reduce the strength of infection among dogs and temporarily affect the cumulative incidence of seroconversion among them. Canine control alone may not be an effective methodology to reduce the number of infectious dogs or the consequent incidence of human diseases, but the use of a highly sensitive diagnostic method, as well as a reduction in the time between serodiagnosis and the consequent euthanasia of seropositive dogs, may lead to a significant reduction in the incidence of canine and human VL [10,42]. Five years of canine control intervention reduced the CLV seroprevalence in Ceará (Brazil) to between 0.5% and 1% [45]. In a case-control study conducted in the same state, a significant decrease in the incidence rate of human disease was observed in areas where seropositive dogs were euthanized [45].
In 2011, the Ministry of Health modified the serodiagnosis protocol for CVL by incorporating the dual-path platform chromatographic immunoassay (DPP® Bio-Manguinhos-FIOCRUZ-RJ) as a screening test and enzyme-linked immunosorbent assay (ELISA) as a confirmatory test [6], considerably speeding testing. In addition, the rapid removal of the seropositive domestic reservoir with a better level of sensitivity enabled the timely detection of CVL, which positively impacted the PCLV in Brazil.
Although 20 years have passed since the development of the present study, it remains a model for the evaluation of PCLV in endemic areas of intense VL transmission in Brazil It is among the first to apply geoprocessing to the spatial epidemiology of VL in Brazil. Kernel maps as well as the K-function clearly showed a positive and significant correlation between the occurrence of human and canine cases. An important point is that the screening-culling was the only control measure applied during our study. In addition, the same epidemiological setting (Porteirinha) was analyzed and compared, in contrast to other epidemiological studies that compare areas with different degrees of VL transmission and may introduce artifacts and bias the results.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
JCFS - Conceptualization, methodogy, investigation, formal analysis; data curation; GLLMC- data curation, formal analysis; JCFS, RMSM, CLFD, EDS- manuscript draft; CLFD, EDS- data curation, manuscript writing and review; RAB, RCG, EMM, LAS, MFR- investigation, formal analysis; MFR- logistical support; ESD- supervision, manuscript writing and review.
Funding
This research was financially supported through grants from the Research Support Foundation of the State of Minas Gerais (FAPEMIG - APQ-016944-10).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Monteiro SP, Lacerda MM, Arias JR. Controle da leishmaniose visceral no Brasil. Rev Soc Bras Med Trop. 1994, 27:67-72.
- Michalsky EM, França-Silva JC, Barata RA, Lara e Silva F de O, Loureiro AMF, Fortes-Dias CL, et al. Phlebotominae distribution in Janaúba, an area of transmission for visceral leishmaniasis in Brazil. Mem Inst Oswaldo Cruz 2009;104:56-61. [CrossRef]
- Morais MHF, Fiúza VOP, Araújo VEM, Carneiro A. Vigilância e controle da leishmaniose visceral no contexto urbano. Cad téc Vet Zootec. 2012;65:44-73.
- Lainson R, Shaw JJ. Evolution, classification and geographical distribution. In: Peters W, Killick-Kendrick R, editors. The Leishmaniasis in Biology and Medicine. London: Academic Press; 1987. p. 1-20.
- Palatnik-de-Sousa CB, dos Santos WR, França-Silva JC, da Costa RT, Reis AB, Palatnik M, et al. Impact of canine control on the epidemiology of canine and human visceral leishmaniasis in Brazil. Am J Trop Med Hyg. 2001;65(5):510-17. [CrossRef]
- Ministério da Saúde (MS). Brasil. Secretaria de Vigilância em Saúde. Manual de Vigilância e Controle da Leishmaniose Visceral. Brasília: MS; 2006. 120p.
- Tesh RB. Control of zoonotic visceral leishmaniasis: is it time to change strategies? Am J Trop Med Hyg. 1995; 52:287-92. [CrossRef]
- Dietze R, Barros GB, Teixeira L, Harris J, Michelson K, Falqueto A, et al. Effect of eliminating seropositive canines on the transmission of visceral leishmaniasis in Brazil. Clin Infect Dis. 1997; 25:1240-2. [CrossRef]
- Costa CHN, Pereira HF, Pereira FCA, Tavares JP, Araújo MV, Gonçalves MJO. Is the household dog a risk factor for American visceral leishmaniasis in Brazil? Trans R Soc Trop Med Hyg. 1999;93:464. [CrossRef]
- Courtenay O, Quinnell RJ, Garcez LM, Shaw JJ, Dye C. Infectiousness in a cohort of Brazilian dogs: why culling fails to control visceral leishmaniasis in areas of high transmission. J Infect Dis. 2002;186:1314-20. [CrossRef]
- Magalhaes PA, Mayrink W, da Costa CA, Melo MN, Dias M, Batista S, et al. [Kala-azar in the Rio Doce, Minas Gerais area. Results of prophylactic measures]. Rev Inst Med Trop Sao Paulo 1980;22:197-202.
- Barata RA, França-Silva JC, Da Silva JC, De Almeida SN, Almeida-Silva L, Dias, ES. Controle da leishmaniose visceral no município de Porteirinha, estado de Minas Gerais, no período de 1998 a 2003. Rev Soc Bras Med Trop. 2011;44(3):386-8.
- Rocha MF, Michalsky EM, Lara-Silva F de O, Pereira NCL, Lana, RS, França-Silva JC, et al. Impact of vector control actions in the abundance of Lutzomyia longipalpis in Montes Claros, Brazil. Acta Trop. 2022;228:106305. [CrossRef]
- Informe epidemiológico do SUS/Fundação Nacional da Saúde, Centro Nacional de Epidemiologia (Cenepi). Imprenta: Brasília, Fundação Nacional de Saúde, Cenepi, 1992.
- França-Silva JC, Barata RA, Costa RT, Monteiro EM, Machado-Coelho GL, Vieira EP, et al. Importance of Lutzomyia longipalpis in the dynamics of transmission of canine visceral leishmaniasis in the endemic area of Porteirinha municipality, Minas Gerais, Brazil. Vet Parasitol. 2005;13:213-20. [CrossRef]
- Coutinho SG, Nunes, MP, Marzochi MCA, Tramontano N. A urgery for American cutaneous and visceral leishmaniasis among 1,342 dogs from areas in Rio de Janeiro (Brazil) where the human diseases occur. Mem Inst Oswaldo Cruz 1985;80:17-22. [CrossRef]
- França-Silva JC, da Costa, RT, Siqueira AM, Machado-Coelho GLL, da Costa, CA, Mayrink W, et al. Epidemiology of canine visceral leishmaniosis in the endemic area of Montes Claros municipality, Minas Gerais State, Brazil. Vet Parasitol. 2003;111:161-73. [CrossRef]
- Camargo ME, Rebonato C. Cross-reactivity in fluorescence tests for Trypanosoma and Leishmania antibodies: a simple inhibition procedure to ensure specific results. Am J Trop Med Hyg. 1969;18:500-5. [CrossRef]
- Costa CA, Genaro O, Lana, M. de, Magalhães PA, Dias M, Michalick MSM, et al. Leishmaniose visceral canina: avaliação da metodologia sorológica utilizada em inquéritos epidemiológicos. Rev Soc Bras Med Trop. 1991;24:21-5.
- Rosário EY do, Genaro O, França-Silva JC, Costa, RT da, Mayrink W., Reis AB, et al. Evaluation of enzyme-linked immunosorbent assay using crude Leishmania and recombinant antigens as a diagnostic marker for canine visceral leishmaniasis. Mem Inst Oswaldo Cruz 2005;100:197-203. [CrossRef]
- Cesar CLG, Figueiredo GM, Westphal MF, Cardoso MRA, Costa, MZ de A, Gattás VL. Morbidade referida e utilização de serviços de saúde em localidades urbanas brasileiras: metodologia. Rev Saúde Pública 1996;30:153-60.
- Evans TG, Vasconcelos IAB, Lima JW, Teixeira JM, Mc. Ullife IT, Lopes UG, et al. Canine visceral leishmaniasis in northeast Brazil: Assessment of serodiagnostic methods. Am J Trop Med Hyg. 1990;42:118-23. [CrossRef]
- Nunes MP, Jackson JM, Carvalho RW, Furtado NJ, Coutinho SG. Serological survey for canine cutaneous and visceral leishmaniasis in areas at risk for transmission in Rio de Janeiro where prophylatic measures had been adopted. Mem Inst Oswaldo Cruz 1991;86:411-7. [CrossRef]
- Sideris V, Karagouni E, Papadopoulou G, Garifallou A, Dotsika E. Canine visceral leishmaniasis in the Great Athens area, Greece. Parasite 1996;3:125-30.
- Ranque JM, Quilici D. Les leishmanioses de la región provencale. Considerations epidemiologiques et ecologiques. Colloques Internationaux du CNRS, no. 239. Ecologie des leishmanioses. Centre National de la Recherche Scientifique 1977:285-93.
- Dye C, Killick-Kendrick R, Vitutia MM, Walton R, Killick-Kendrick M, Harith AE, et al. Epidemiology of canine leishmaniasis: prevalence, incidence and basic reproduction number calculated from a cross-sectional serological survey on the Island of Gozo, Malta. Parasitology 1992;105:35-41. [CrossRef]
- Hasibeder G, Dye C, Carpenter J. Mathematical modelling and theory for estimating the basic reproduction number of canine leishmaniasis. Parasitology 1992;105:43–53. [CrossRef]
- Pozio E, Gradoni L, Bettini S, Gramiccia M. Leishmaniasis in Tuscany (Italy): VI. Canine Leishmaniasis in the Focus of Monte Argentario (Grosseto). Acta Trop. 1981;38:383-93. [CrossRef]
- Abranches P, Silva-Pereira MC, Conceição-Silva FM, Santos-Gomes GM, Janz JG. Canine leishmaniasis: pathological and ecological factors influencing transmission of infection. J Parasitol. 1991:77:557-61. [CrossRef]
- Lanotte G, Rioux JA, Croset H, Vollhardt Y. [Ecology of leishmaniasis in the south of France. 8. Complement to the epidemiological application of the immunofluorescence technic: geometric and arithmetic mean titers in canine leishmaniasis]. Ann Parasitol Hum Comp. 1975; 50:1-5.
- Vieira JB, Coelho GE. [Visceral leishmaniasis or kala-azar: the epidemiological and control aspects]. Rev Soc Bras Med Trop. 1998;31(Suppl 2):85-92.
- Marzoch, MC de A, Marzochi KBF. Tegumentary and visceral leishmaniases in Brazil: emerging anthropozoonosis and possibilities for their control. Cad Saúde Pública 1994;10: S359-S375. [CrossRef]
- Genaro O, Costa CA da, Williams P, Silva JE, Rocha NM, Lima SL, et al. Ocorrência de calazar em área urbana da grande Belo Horizonte, MG. Rev Soc Bras Med Trop. 1990;23:121-121.
- Deane LM, Grimaldi G. Leishmaniasis in Brazil. In: Chang KP, Bray RS, editors. Leishmaniasis. Amsterdam: Elsevier; 1985. p. 247-81. [CrossRef]
- Badaro R, Jones TC, Lorenco R, Cerf BJ, Sampaio D, Carvalho EM, et al. A prospective study of visceral leishmaniasis in an endemic area of Brazil. J Infect Dis. 1986:154:639-49. [CrossRef]
- Dye C, Williams BG. Malnutrition, age and the risk of parasitic disease: visceral leishmaniasis revisited. Proc R Soc Lond B 1993;254:33-9. [CrossRef]
- Sherlock IA. Ecological interactions of visceral leishmaniasis in the state of Bahia, Brazil. Mem Inst Oswaldo Cruz 1996;91:671-83. [CrossRef]
- Oliveira CDL, Assunção RM, Reis IA, Proietti FA. Spatial distribution of human and canine visceral leishmaniasis in Belo Horizonte, Minas Gerais State, Brasil, 1994-1997. Cad Saúde Pública 2001;17:1231-39. [CrossRef]
- Barata RA, Silva JCF da, Costa RT da; Fortes-Dias CL, Silva JC da, Paula EV de, et al. Phlebotomine sand flies in Porteirinha, an area of American visceral leishmaniasis transmission in the state of Minas Gerais, Brazil. Mem Inst Oswaldo Cruz 2004;99:481-7. [CrossRef]
- Barata RA, França-Silva JC, Mayrink W, Silva JC da, Prata A, Lorosa ES, et al. Aspectos da ecologia e do comportamento de flebotomíneos em área endêmica de leishmaniose visceral, Minas Gerais. Rev Soc Bras Med Trop. 2005;38:421-5. [CrossRef]
- Ashford DA, David JR, Freire M, David R, Sherlock I., Eulálio MC, et al. Studies on control of visceral leishmaniasis: impact of dog control on canine and human visceral leishmaniasis in Jacobina, Bahia, Brazil. Am J Trop Med Hyg. 1998;59:53-7. [CrossRef]
- Dye C. The logic of visceral leishmaniasis control. Am J Trop Med Hyg. 1996;55:125-30. [CrossRef]
- Braga MDM, Coêlho ICB, Pompeu MML, Evans TG, MacAullife IT, Teixeira MJ, et al. Controle do calazar canino: comparação dos resultados de um programa de eliminação rápida de cães sororreagentes por ensaio imuno-enzimático com outro de eliminação tardia de cães sororreagentes por teste de imunofluorescência indireta de eluato de papel filtro. Rev Soc Bras Med Trop. 1998;31:419-24. [CrossRef]
- Alencar JE. Profilaxia do calazar no Ceará. Brazil. Rev Inst Med Trop São Paulo 1961;3: 175-80.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).