Submitted:
13 July 2023
Posted:
14 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Primary Cell Culture
2.2. Characterization of Primary Cell Cultures
2.3. Gene Expression Analysis
2.4. Statistical Analysis
3. Results
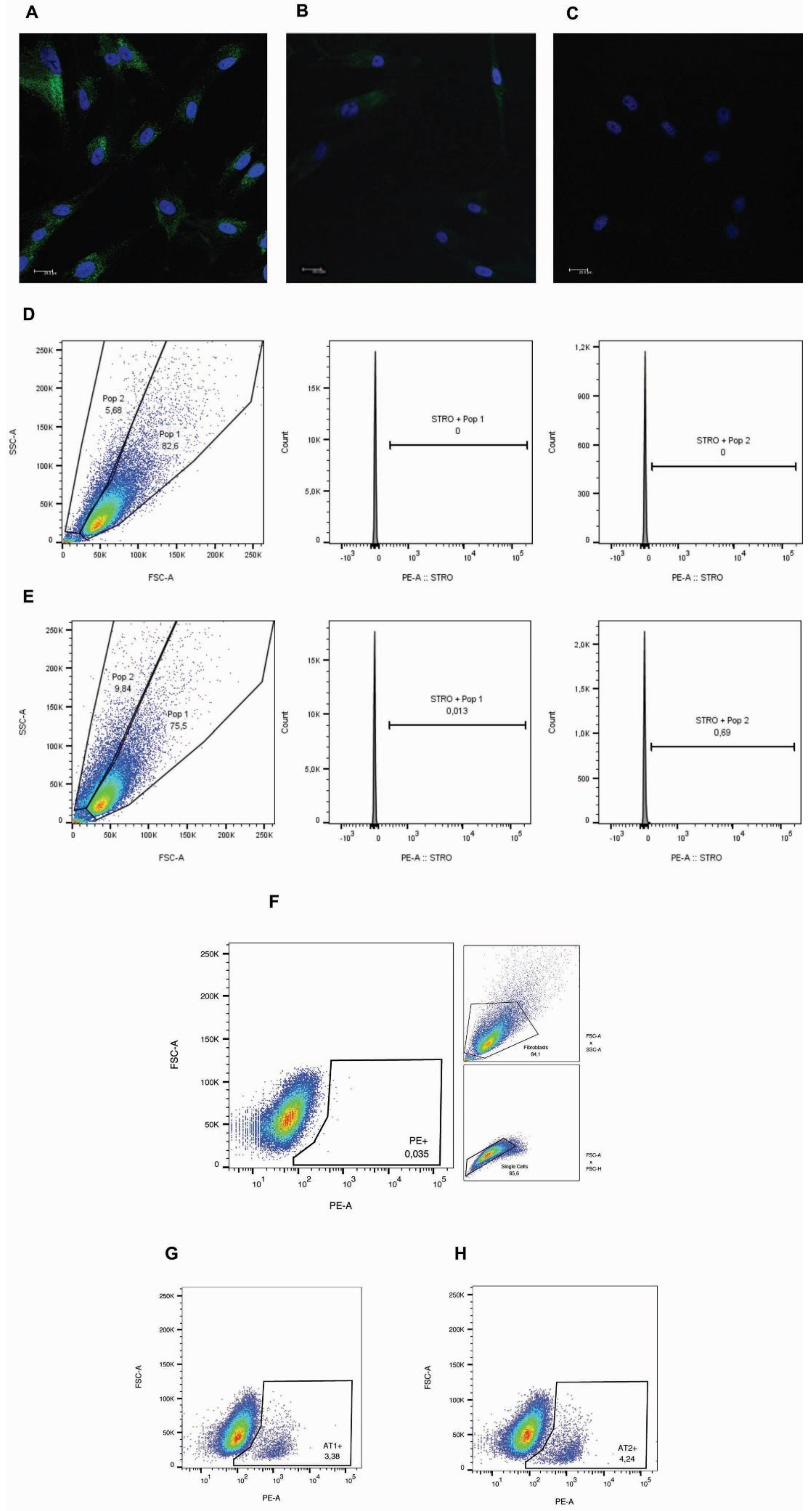
3.1. Characterization of Primary Cells
3.2. mRNA Expression of Inflammatory Mediators in Primary Human Cells from Periodontal Tissues
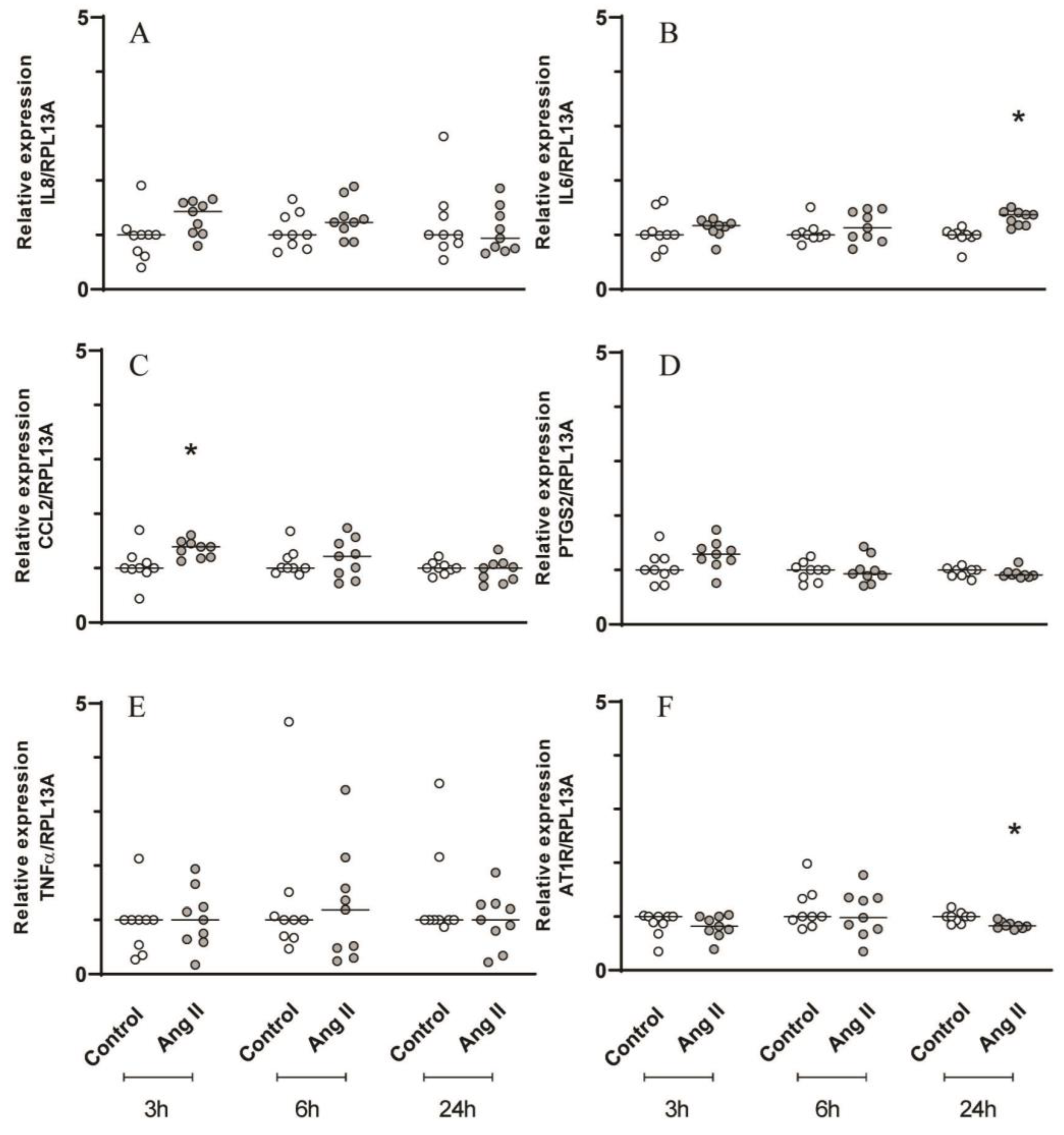
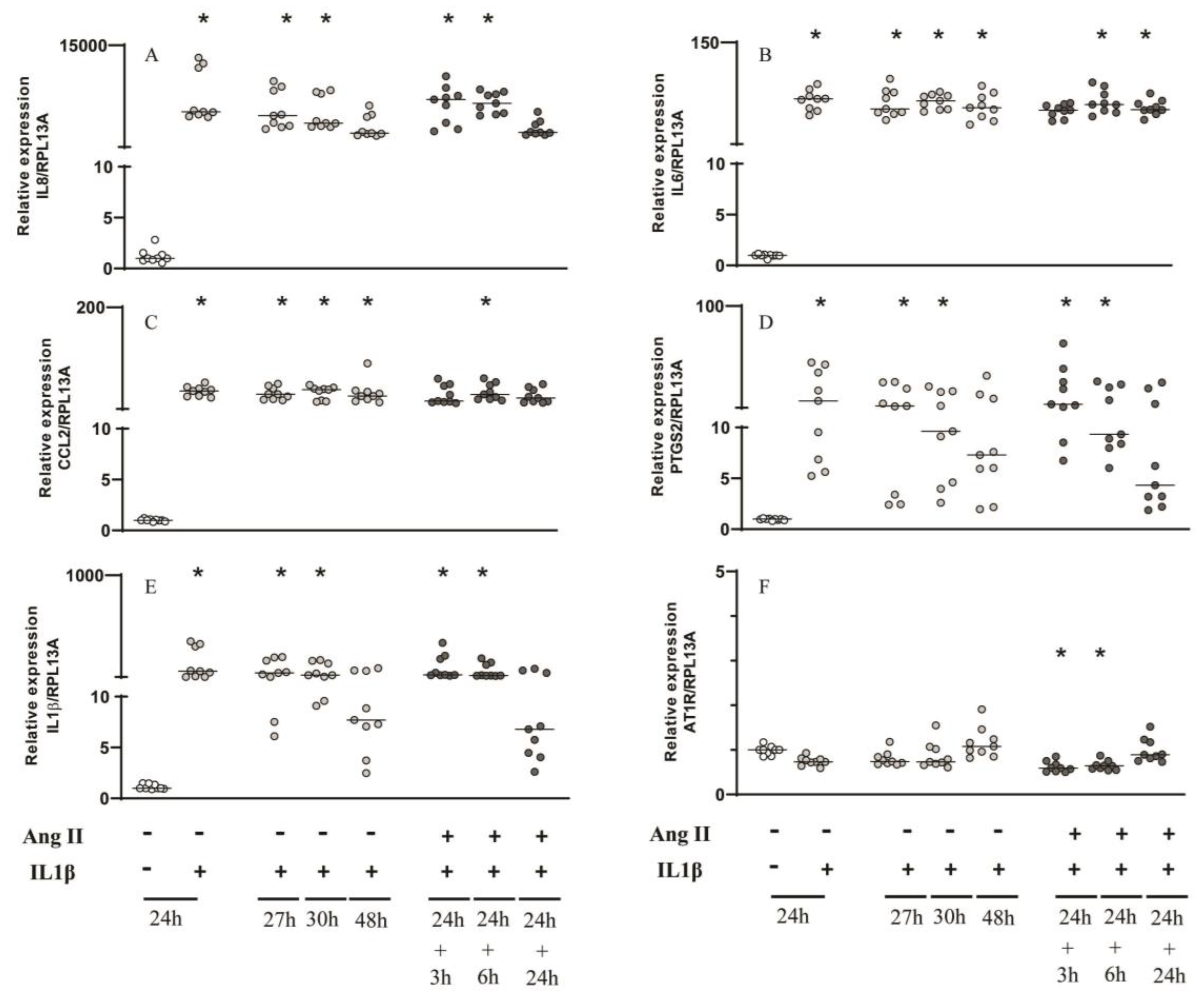
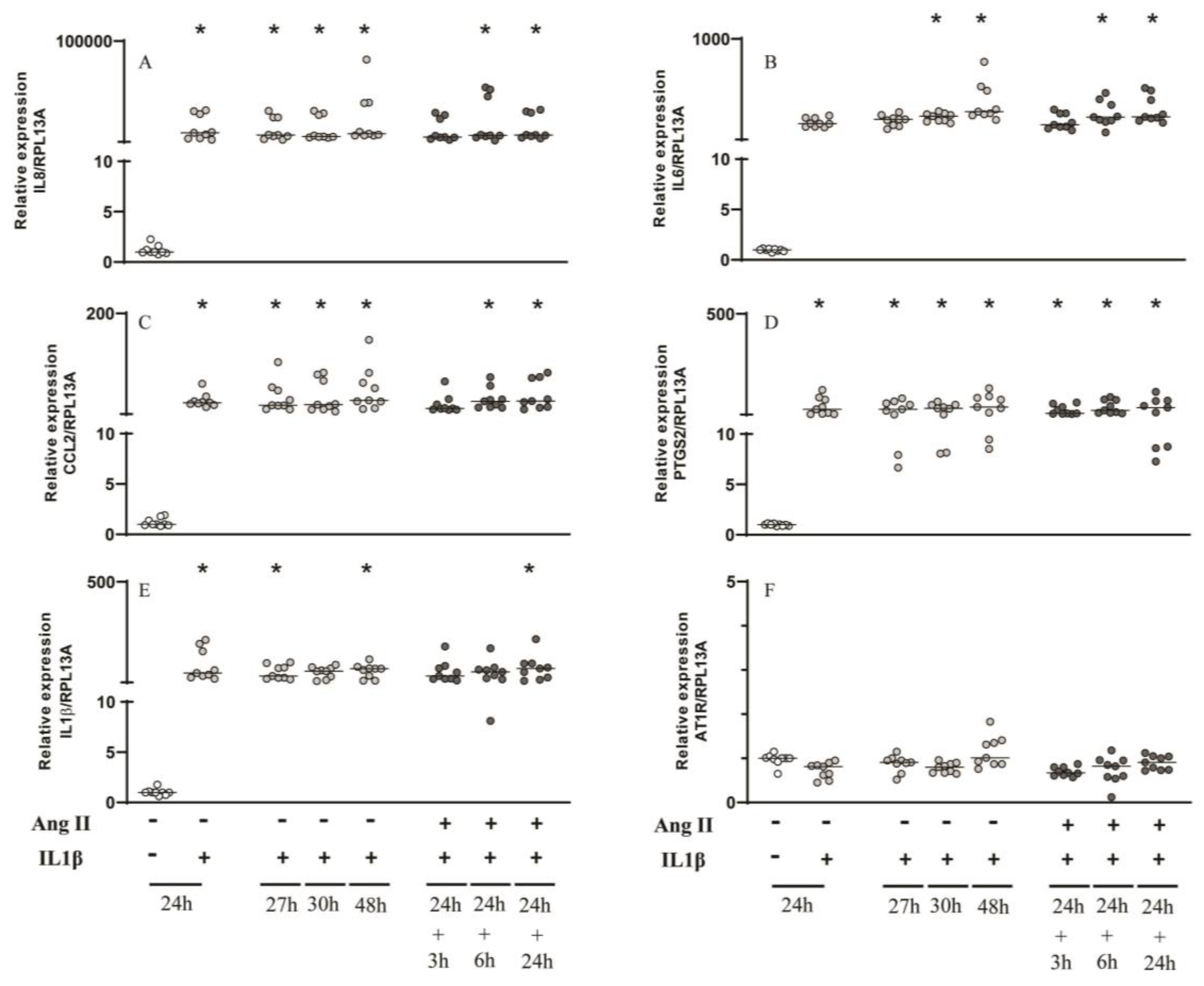
3.2.1. Ang II Slightly Increased IL6 and CCL2/MCP1 Expression in Gingival Primary Cells and IL8 and PTGS2/COX2 in Periodontal Ligament Cells
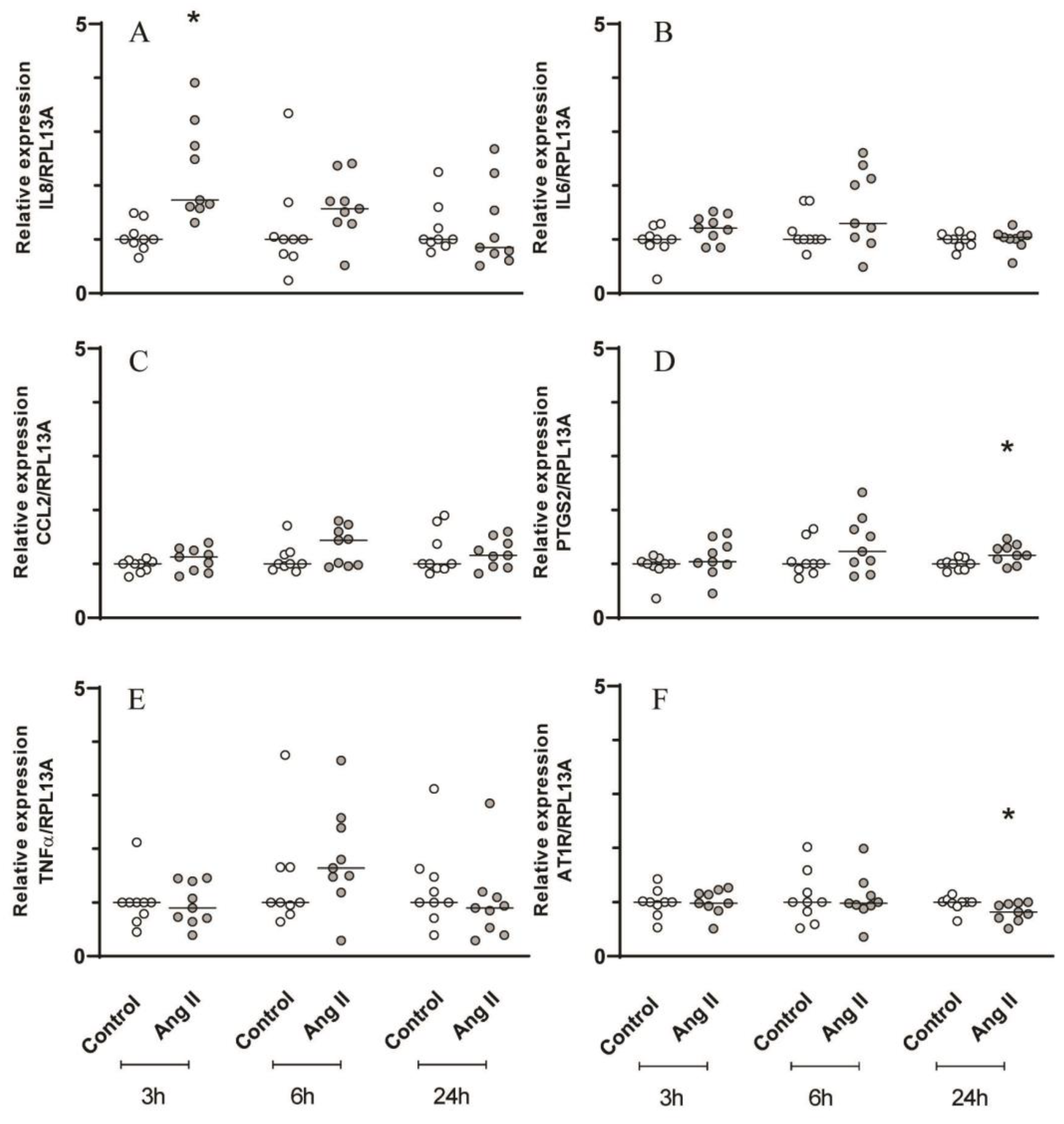
3.2.2. IL1β for 24h Induced mRNA Upregulation of Several Inflammatory Mediators in Gingival and Periodontal Ligament Cells and Ang II Did Not Cause a Synergistic Effect
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benigni, A.; Cassis, P.; Remuzzi, G. Angiotensin II Revisited: New Roles in Inflammation, Immunology and Aging. EMBO Mol. Med. 2010, 2, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Saravi, B.; Lang, G.; Ülkümen, S.; Burchard, T.; Weihrauch, V.; Patzelt, S.; Boeker, M.; Li, Z.; Woelber, J.P. The Tissue Renin-Angiotensin System (Tras) and the Impact of Its Inhibition on Inflammation and Bone Loss in the Periodontal Tissue. Eur. Cells Mater. 2020, 40, 203–226. [Google Scholar] [CrossRef]
- Santos, C.F.; Akashi, A.E.; Dionísio, T.J.; Sipert, C.R.; Didier, D.N.; Greene, A.S.; Oliveira, S.H.P.; Pereira, H.J.V.; Becari, C.; Oliveira, E.B.; et al. Characterization of a Local Renin-Angiotensin System in Rat Gingival Tissue. J. Periodontol. 2009, 80, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.F.; Morandini, A.C.; Dionísio, T.J.; Faria, F.A.; Lima, M.C.; Figueiredo, C.M.; Colombini-Ishikiriama, B.L.; Sipert, C.R.; Maciel, R.P.; Akashi, A.P.; et al. Functional Local Renin-Angiotensin System in Human and Rat Periodontal Tissue. PLoS ONE 2015, 10, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Gabriele, L.G.; Morandini, A.C.; Dionísio, T.J.; Santos, C.F. Angiotensin II Type 1 Receptor Knockdown Impairs Interleukin-1β-Induced Cytokines in Human Periodontal Fibroblasts. J. Periodontol. 2017, 88, e1–e11. [Google Scholar] [CrossRef] [PubMed]
- Dionísio, T.J.; Souza, G.P.; Colombini-Ishikiriama, B.L.; Garbieri, T.F.; Parisi, V.A.; Oliveira, G.M.; Cano, I.P.; Rodini, C.O.; Oliveira, S.H.P.; Greene, A.S.; et al. AT1 Receptor Antagonism Promotes Bone Loss Attenuation in Experimental Periodontitis, Blocks Inflammatory Mediators, and Upregulates Antioxidant Enzymes and Bone Formation Markers. J. Periodontol. 2020, 91, 533–544. [Google Scholar] [CrossRef]
- Garbieri, T.F.; Martin, V.; dos Santos, C.F.; Gomes, P.d.S.; Fernandes, M.H. The Embryonic Chick Femur Organotypic Model as a Tool to Analyze the Angiotensin II Axis on Bone Tissue. Pharmaceuticals 2021, 14. [Google Scholar] [CrossRef]
- Valente, A.J.; Yoshida, T.; Murthy, S.N.; Sakamuri, S.S.V.P.; Katsuyama, M.; Clark, R.A.; Delafontaine, P.; Chandrasekar, B. Angiotensin II Enhances AT 1-Nox1 Binding and Stimulates Arterial Smooth Muscle Cell Migration and Proliferation through AT 1, Nox1, and Interleukin-18. Am. J. Physiol. - Hear. Circ. Physiol. 2012, 303, 282–296. [Google Scholar] [CrossRef]
- Vogiatzi, K.; Apostolakis, S.; Vlata, Z.; Krabovitis, E.; Spandidos, D.A. Opposite Effect of Angiotensin Receptor Blockade on CXCL8 Production and CXCR1/2 Expression of Angiotensin II-Treated THP-1 Monocytes. Exp. Ther. Med. 2013, 5, 987–991. [Google Scholar] [CrossRef]
- ZHANG, F.; LU, M.; WANG, H.; REN, T. Aspirin Attenuates Angiotensin II-Induced Inflammation in Bone Marrow Mesenchymal Stem Cells via the Inhibition of ERK1/2 and NF-ΚB Activation. Biomed. Reports 2013, 1, 930–934. [Google Scholar] [CrossRef]
- Zhang, F.; Sun, D.; Chen, J.; Guan, N.; Huo, X.; Xi, H. Simvastatin Attenuates Angiotensin IIInduced Inflammation and Oxidative Stress in Human Mesangial Cells. Mol. Med. Rep. 2015, 11, 1246–1251. [Google Scholar] [CrossRef] [PubMed]
- Sauter, N.S.; Thienel, C.; Plutino, Y.; Kampe, K.; Dror, E.; Traub, S.; Timper, K.; Bédat, B.; Pattou, F.; Kerr-Conte, J.; et al. Angiotensin II Induces Interleukin-1b-Mediated Islet Inflammation and β-Cell Dysfunction Independently of Vasoconstrictive Effects. Diabetes 2015, 64, 1273–1283. [Google Scholar] [CrossRef]
- Skurk, T.; Van Harmelen, V.; Hauner, H. Angiotensin II Stimulates the Release of Interleukin-6 and Interleukin-8 from Cultured Human Adipocytes by Activation of NF-ΚB. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1199–1203. [Google Scholar] [CrossRef] [PubMed]
- Scheuren, N.; Jacobs, M.; Ertl, G.; Schorb, W. Cyclooxygenase-2 in Myocardium Stimulation by Angiotensin-II in Cultured Cardiac Fibroblasts and Role at Acute Myocardial Infarction. J. Mol. Cell. Cardiol. 2002, 34, 29–37. [Google Scholar] [CrossRef]
- Han, Y.; Runge, M.S.; Brasier, A.R. Angiotensin II Induces Interleukin-6 Transcription in Vascular Smooth Muscle Cells through Pleiotropic Activation of Nuclear Factor-Κb Transcription Factors. Circ. Res. 1999, 84, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Hasegawa-Nakamura, K.; Sakoda, K.; Matsuyama, T.; Noguchi, K. Involvement of Angiotensin II Type 1 Receptors in Interleukin-1β-Induced Interleukin-6 Production in Human Gingival Fibroblasts. Eur. J. Oral Sci. 2011, 119, 345–351. [Google Scholar] [CrossRef]
- Morandini, A.C.F.; Sipert, C.R.; Gasparoto, T.H.; Greghi, S.L.A.; Passanezi, E.; Rezende, M.L.R.; Sant’ana, A.P.; Campanelli, A.P.; Garlet, G.P.; Santos, C.F. Differential Production of Macrophage Inflammatory Protein-1α, Stromal-Derived Factor-1, and IL-6 by Human Cultured Periodontal Ligament and Gingival Fibroblasts Challenged With Lipopolysaccharide From P. Gingivalis. J. Periodontol. 2010, 81, 310–317. [Google Scholar] [CrossRef]
- Sipert, C.R.; Morandini, A.C.; Dionísio, T.J.; Machado, M.A.A.M.; Oliveira, S.H.P.; Campanelli, A.P.; Kuo, W.P.; Santos, C.F. In Vitro Regulation of CCL3 and CXCL12 by Bacterial By-Products Is Dependent on Site of Origin of Human Oral Fibroblasts. J. Endod. 2014, 40, 95–100. [Google Scholar] [CrossRef]
- Colombini-Ishikiriama, B.L.; Dionisio, T.J.; Garbieri, T.F.; Da Silva, R.A.; Machado, M.A.A.M.; De Oliveira, S.H.P.; Lara, V.S.; Greene, A.S.; Santos, C.F. What Is the Response Profile of Deciduous Pulp Fibroblasts Stimulated with E. Coli LPS and E. Faecalis LTA? BMC Immunol. 2020, 21, 1–9. [Google Scholar] [CrossRef]
- Morandini, A.C.F.; Chaves Souza, P.P.; Ramos-Junior, E.S.; Brozoski, D.T.; Sipert, C.R.; Souza Costa, C.A.; Santos, C.F. Toll-Like Receptor 2 Knockdown Modulates Interleukin (IL)-6 and IL-8 but Not Stromal Derived Factor-1 (SDF-1/CXCL12) in Human Periodontal Ligament and Gingival Fibroblasts. J. Periodontol. 2013, 84, 535–544. [Google Scholar] [CrossRef]
- Sipert, C.R.; Morandini, A.C.d.F.; Modena, K.C.d.S.; Dionisio, T.J.; Machado, M.A.A.M.; de Oliveira, S.H.P.; Campanelli, A.P.; Santos, C.F. CCL3 and CXCL12 Production in Vitro by Dental Pulp Fibroblasts from Permanent and Deciduous Teeth Stimulated by Porphyromonas Gingivalis LPS. J. Appl. Oral Sci. 2013, 21, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Segawa, M.; Nakao, S.; Ogata, Y.; Sugiya, H.; Furuyama, S. Angiotensin II Induces Prostaglandin E2 Release in Human Gingival Fibroblasts. Life Sci. 2003, 72, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Monnouchi, S.; Maeda, H.; Fujii, S.; Tomokiyo, A.; Kono, K.; Akamine, A. The Roles of Angiotensin II in Stretched Periodontal Ligament Cells. J. Dent. Res. 2011, 90, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Pizzatto, L.N.; Meneses, C.C.B.; Diniz, E.A.; Dionísio, T.J.; Santos, C.F.; Sipert, C.R. Angiotensin II Regulates Proliferation and Function of Stem Cells of Apical Papilla. J. Endod. 2020, 46, 810–817. [Google Scholar] [CrossRef] [PubMed]
- Morinelli, T.A.; Raymond, J.R.; Baldys, A.; Yang, Q.; Lee, M.-H.; Luttrell, L.; Ullian, M.E. Identification of a Putative Nuclear Localization Sequence within ANG II AT(1A) Receptor Associated with Nuclear Activation. Am. J. Physiol. Cell Physiol. 2007, 292, C1398–C1408. [Google Scholar] [CrossRef]
- Denu, R.A.; Nemcek, S.; Bloom, D.D.; Goodrich, A.D.; Kim, J.; Mosher, D.F.; Hematti, P. Fibroblasts and Mesenchymal Stromal/Stem Cells Are Phenotypically Indistinguishable. Acta Haematol. 2016, 136, 85–97. [Google Scholar] [CrossRef]
- Du, L.; Yang, P.; Ge, S. Isolation and Characterization of Human Gingiva-Derived Mesenchymal Stem Cells Using Limiting Dilution Method. J. Dent. Sci. 2016, 11, 304–314. [Google Scholar] [CrossRef]
- Matsuzuka, T.; Miller, K.; Pickel, L.; Doi, C.; Ayuzawa, R.; Tamura, M. The Synergistic Induction of Cyclooxygenase-2 in Lung Fibroblasts by Angiotensin II and pro-Inflammatory Cytokines. Mol. Cell. Biochem. 2009, 320, 163–171. [Google Scholar] [CrossRef]
- Yokoyama, T.; Sekiguchi, K.; Tanaka, T.; Tomaru, K.; Arai, M.; Suzuki, T.; Nagai, R. Angiotensin II and Mechanical Stretch Induce Production of Tumor Necrosis Factor in Cardiac Fibroblasts. Am. J. Physiol. Circ. Physiol. 1999, 276, H1968–H1976. [Google Scholar] [CrossRef]
- Sipert, C.R.; Moraes, I.G.; Bernardinelli, N.; Garcia, R.B.; Bramante, C.M.; Gasparoto, T.H.; Figueira, E.A.; Dionísio, T.J.; Campanelli, A.P.; Oliveira, S.H.P.; et al. Heat-Killed Enterococcus Faecalis Alters Nitric Oxide and CXCL12 Production but Not CXCL8 and CCL3 Production by Cultured Human Dental Pulp Fibroblasts. J. Endod. 2010, 36, 91–94. [Google Scholar] [CrossRef]
- Morandini, A.C.; Chaves Souza, P.P.; Ramos-Junior, E.S.; Souza Costa, C.A.; Santos, C.F. MyD88 or TRAM Knockdown Regulates Interleukin (IL)-6, IL-8, and CXCL12 MRNA Expression in Human Gingival and Periodontal Ligament Fibroblasts. J. Periodontol. 2013, 84, 1353–1360. [Google Scholar] [CrossRef] [PubMed]
- Gurantz, D.; Cowling, R.T.; Varki, N.; Frikovsky, E.; Moore, C.D.; Greenberg, B.H. IL-1β and TNF-α Upregulate Angiotensin II Type 1 (AT 1) Receptors on Cardiac Fibroblasts and Are Associated with Increased AT1 Density in the Post-MI Heart. J. Mol. Cell. Cardiol. 2005, 38, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Imaizumi, T.; Tanji, K.; Sakaki, H.; Metoki, N.; Sato, Y.; Wakabayashi, K.; Kimura, H.; Satoh, K. Interleukin-1β Enhances the Angiotensin-Induced Expression of Plasminogen Activator Inhibitor-1 through Angiotensin Receptor Upregulation in Human Astrocytes. Brain Res. 2006, 1073–1074, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Alique, M.; Sánchez-López, E.; Rayego-Mateos, S.; Egido, J.; Ortiz, A.; Ruiz-Ortega, M. Angiotensin II, via Angiotensin Receptor Type 1/Nuclear Factor-ΚB Activation, Causes a Synergistic Effect on Interleukin-1-β-Induced Inflammatory Responses in Cultured Mesangial Cells. JRAAS - J. Renin-Angiotensin-Aldosterone Syst. 2015, 16, 23–32. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




