1. Introduction
Varicella caused by varicella zoster virus (VZV) is a highly contagious disease that often occurs during childhood. Although the disease is often benign and self-limiting, varicella may cause serious complications, such as bacterial infection of skin and soft tissue, encephalitis, pneumonia, and sepsis. Varicella may be fatal in high-risk individuals, such as newborns, pregnant women, and those with congenital or acquired immunosuppression [
1].
Varicella vaccination can substantially reduce the incidence of varicella. In countries in which varicella is an important public health concern, the WHO recommends use of one- or two-dose universal varicella vaccination (UVV) [
2]. Currently, about 40 countries have implemented UVV; some countries have introduced vaccination for high-risk groups. In some regions with high percentages of children, including China, India, and Southeast Asia, the varicella vaccine is available through the private sector [
3,
4,
5].
The use of varicella vaccine is increasing worldwide. Thus, a novel varicella vaccine, initially identified as NBP608, was developed by a Korean manufacturer. The NBP608 was approved by the Ministry of Food and Drug Safety (MFDS) of Korea in 2018 and acquired pre-qualification certification from the WHO in 2019. Named SKY VaricellaTM for commercial production, the NBP608 vaccine is in use in Korea and is distributed to South American countries by the Pan-American Health Organization.
In this study, we report results of a phase 3 clinical trial conducted as a pre-licensure requirement of the MFDS of Korea. We aimed to evaluate non-inferiority of immunogenicity and safety of NBP608 compared with those of VarivaxTM (Merck & Co., Inc., Kenilworth, NJ, USA).
2. Materials and Methods
2.1. Study Design and Participants
This phase 3, randomized, double blind, active controlled trial was conducted at 15 sites in Korea, two sites in the Philippines, and two sites in Mexico from July 2016 to June 2017. Eligible participants were healthy children aged 12 months to 12 years. The exclusion criteria were receipt of previous varicella vaccination, history of varicella infection or exposure to VZV within the past four weeks, body temperature of 38 ℃ or greater on the day of vaccination, hypersensitivity to gelatin or neomycin, history of Guillain Barre syndrome, congenital or acquired immunosuppression, receipt of blood derived products within five months of vaccination, receipt of other investigational products within one month of vaccination or plan to participate in another clinical trial during the study period, and ineligibility due to other reasons identified by the investigators.
This study was conducted in compliance with the International Conference on Harmonization Guidelines for Good Clinical Practice and Declaration of Helsinki. The study protocol was reviewed and approved by the MFDS of Korea; the national drug authorities of the Philippines and Mexico; and the Institutional Review Board of the participating site (St. Vincent’s Hospital, The Catholic University of Korea: VC16BDGT0078). This study was registered in Clinicaltrials.gov (NCT 03114943). Written informed consent was provided by parents or legal representatives of each participant prior to enrollment.
2.2. Randomization and Masking
The participants were randomly assigned in a 1:1 ratio to receive either the investigational NBP608 or control vaccine. The Interactive Web Response System was used to randomly stratify participants within three age groups:12–23 months, 24 months to 9 years, and 9 to 12 years. An unblinded pharmacist provided the vaccine according to the randomization code, and an unblinded staff member administered the subcutaneous deltoid injection. Participants and their parents/legal representatives remained blinded to the study allocation and vaccine preparation processes.
2.3. Vaccines
The NBP608 investigational vaccine is a live attenuated varicella vaccine manufactured by SK Bioscience (Seongnam, Korea). The vaccine was derived from the Oka strain of VZV. The virus was propagated in MRC-5 human diploid cells and contained ≥ 2400 plaque forming units (PFUs) of lyophilized virus. The VarivaxTM vaccine is also a live attenuated varicella vaccine derived from the Oka strain of VZV, was propagated in MRC-5 human diploid cells, and contained ≥1350 PFUs of lyophilized virus. Both vaccines were stored at 2–8℃ during the study period, and lyophilized pellets were reconstituted with 0.5 mL of sterile water immediately before injection.
2.4. Immunogenicity Endpoints
The primary and secondary immunogenicity endpoints were established under the guidance of MFDS of Korea. The primary endpoint was assessed using a fluorescent antibody to membrane antigen (FAMA) assay, noninferiority of the investigational vaccine was confirmed if the lower limit of the 95% confidence interval (95% LCL) for the postvaccination seroconversion rate (SCR) difference (investigational vaccine minus control vaccine) was greater than −15%. The secondary endpoints were SCR measured by glycoprotein-based enzyme-linked immunosorbent assay (gpELISA) and geometric mean titer (GMT) assessed by FAMA and gpELISA. After study completion, additional analysis was performed to test noninferiority of the difference in SCR (investigational vaccine minus control vaccine) measured by gpELISA, with a margin of -15%. Furthermore, post-vaccination GMT ratio analysis was conducted to assess whether the 95% LCL for GMT ratio (GMT of investigational vaccine divided by that of control vaccine), as measured by both FAMA assay and gpELISA, was equal to or greater than the non-inferiority margin of 0.5. Additionally, noninferiority in SCR, both by FAMA assay and gpELISA, was assessed in age strata.
2.5. Immunogenicity Assessment
The FAMA assay, the gold standard method for detecting neutralizing antibody, was performed according to a modified William’s method.6 Blood samples were collected before vaccination and within six to eight weeks after vaccination. Sera were stored at -70℃ until analysis. To produce VZV-infected target cells with cell-associated virus for use in FAMA assay antigen, the MRC 5 cell line was cultured, inoculated with VZV, and harvested when cytopathic effects reached 50–70%. Two-fold serial dilutions of the sera were performed from 1:2 to 1:1,024, and each diluted serum sample was mixed with FAMA assay antigen. Fluorescein-isothiocyanate (FITC)-conjugated goat anti-human immunoglobulin G (IgG) was used as the secondary antibody. Two investigators examined the antigen-serum mixtures with a fluorescence microscope. Samples with titer ≥1:4 were regarded as seropositive.
Measurement of antibody titers using gpELISA was performed with a Serion ELISA classic VZV IgG kit (Institut Virion/Serion GmbH, Wurzburg, Germany) according to the manufacturer’s instructions. Consistent with the manufacturer’s recommendations, anti-VZV IgG concentrations > 100 mIU/mL, 50–100 mIU/mL, or < 50 mIU/mL were interpreted as protective, equivocal, or susceptible, respectively. As in previous studies, both equivocal and positive groups were considered as seropositive [
7]. The FAMA assay and gpELISA were performed at SK Bioscience Research Laboratory.
2.6. Safety Assessment
For the safety endpoint, the proportions of participants with local solicited adverse events (AEs), systemic solicited AEs, and unsolicited AEs were assessed in the NBP608 and control groups. Participants were observed for 30 min after vaccination, and study staff recorded any local or systemic AEs and unsolicited AEs. Local and systemic solicited AEs were recorded by the child’s parents or legal guardians for seven days (Day 0 to Day 6), and participants were observed for six weeks (Day 0 to Day 41) for unsolicited AEs. Consistent with the recommendations of the MFDS of Korea, the intensities of local and systemic solicited and unsolicited AEs were graded as mild (grade 1), moderate (grade 2), severe (grade 3), or potentially life-threatening (grade 4) [
8].
Unsolicited AEs were recorded using terms from the Medical Dictionary for Regulatory Activities version 19.1.[
9] Serious adverse events (SAEs), defined as those considered life-threatening, requiring hospitalization, or resulting in significant disability or death, were recorded for 26 weeks.
Varicella-like rash was recorded for six weeks (Day 0 to Day 41). Samples from any newly appearing skin lesions were collected and underwent polymerase chain reaction (PCR) testing. PCR testing differentiated VZV from herpes simplex virus (HSV), another cause of blistering rash, and distinguished wild-type VZV from vaccine-derived VZV.
2.7. Statistical Analysis
Differences in categorical variables between the NBP608 and control groups were assessed using the Chi square or Fisher’s exact test. All P values < 0.05 were considered statistically significant. For immunogenicity assessment, the SCR and corresponding 95% CI were calculated using the Clopper-Pearson method. Two-sided 95% CIs of the SCR differences were calculated using the Wald method. The GMT and corresponding 95% CI were calculated using independent t-test on log-transformed antibody titers. The interval limits were re-transformed to original scale. For safety assessment, the Clopper-Pearson method was applied to calculate proportions of participants who experienced AEs and the corresponding 95% CI, and the Chi square or Fisher’s exact test was used for comparison. Based on previous data, the SCR in the NBP608 group was anticipated to be 76% or higher with a drop-out rate of approximately 30%. Therefore, a minimum of 244 participants in each vaccine group was required for a one-sided significance level of 0.025 and 90% power. All statistical analyses were conducted with SAS® software, version 9.4 (SAS Institute, Cary, NC, USA).
3. Results
3.1. Study Participants
A total of 515 participants was screened, 499 eligible participants were randomly assigned to receive the NBP608 (n = 251) or control (n = 248) vaccine, and 498 who received vaccinations were included in safety set. The flow of trial participants is shown in
Figure 1. After excluding participants who failed to complete the study or with protocol violations, the per-protocol (PP) set included 228 participants in the NBP608 group and 230 in the control group. The demographic characteristics of the participants are presented in
Table 1. Demographic characteristics of sex, age, weight, and height were comparable between the two groups.
3.2. Immunogenicity
The post-vaccination SCR as measured by FAMA is presented in
Table 2. The SCR was 99.53% (95% CI: 97.40, 99.99) and 96.38% (95% CI: 92.99, 98.42) in the NBP608 and control groups, respectively. The difference in SCR between the two groups (investigational group minus control group) was 3.15% (95% CI: 0.52, 5.78). The NBP608 group demonstrated non-inferiority to the control group, as the lower limit of the 95% CI of differences in SCR was greater than −15%. Given the wide age range (12 month to 12 years) of participants, SCR was analyzed by age subset. In the subgroup aged 12–23 months, the difference in SCR between the vaccine groups was 2.54% (95% CI: −0.08, 5.16), demonstrating NBP608 non-inferiority in this age group. In the 24 months to 12 years age group, the difference in SCR between the two vaccine groups was 8.0% (95% CI: −2.63, 18.63). The NBP608 group demonstrated non-inferiority compared to the control group in this age group as well.
The SCR measured by gpELISA after vaccination is presented in
Table 3. The SCR was 99.53% (95% CI: 97.42, 99.99) and 88.79% (95% CI: 83.90, 92.61) in the NBP608 and control groups, respectively. The difference in SCR between the two groups was 10.74% (95% CI: 6.50, 14.98). Therefore, the NBP608 group demonstrated non-inferiority to the control group. In the subgroup aged 12–23 months, the NBP608 group again demonstrated non-inferiority as the difference in SCR between the vaccine groups was 10.61% (95% CI: 6.32, 14.89). In the 24 months to 12 years age group, the difference in SCR between the two vaccine groups was 11.24% (95% CI: −5.78, 28.25), and NBP608 group non-inferiority in this age group was demonstrated.
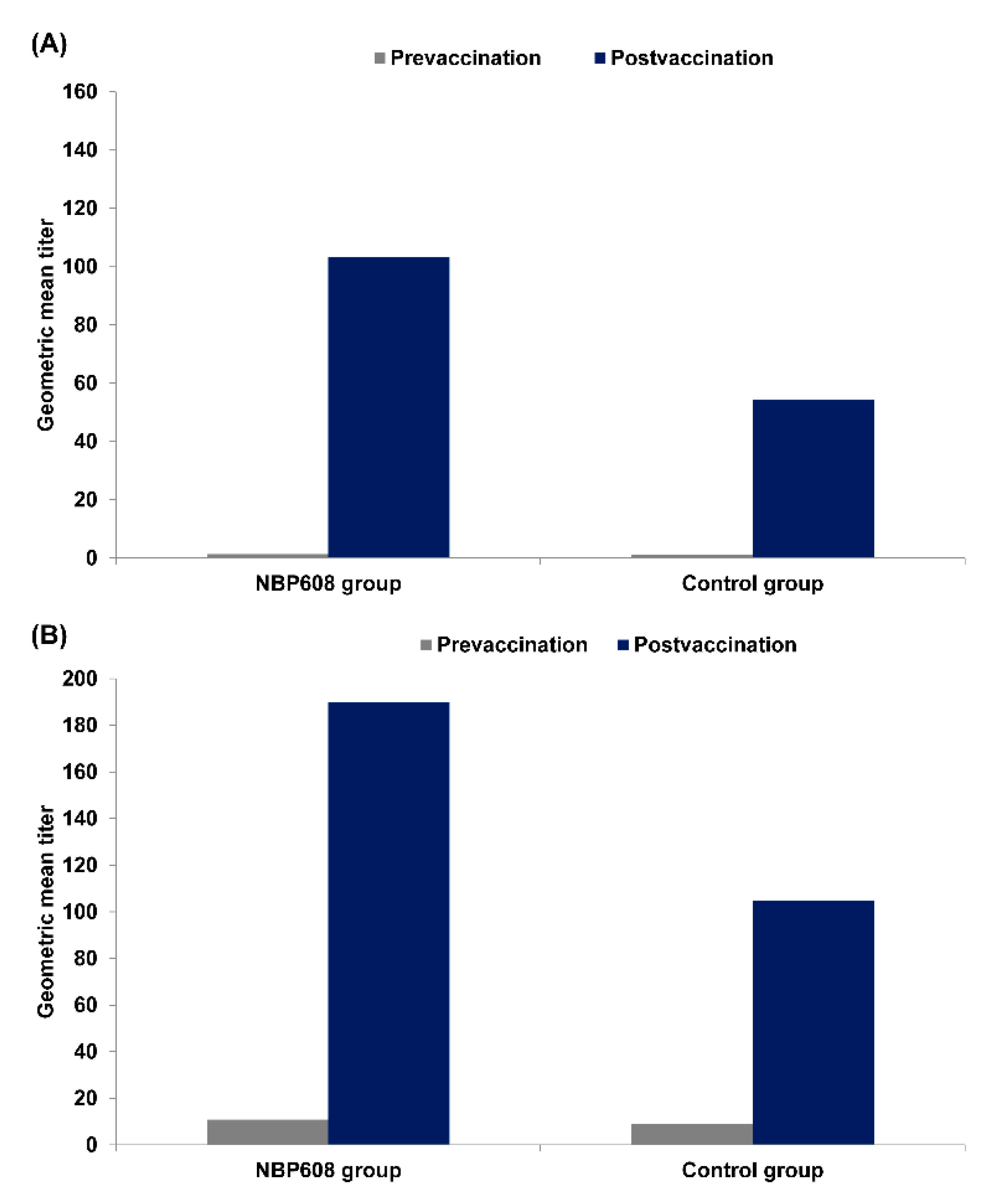
Figure 2 presents pre- and post-vaccination GMTs regardless of serostatus at baseline, including baseline seropositive participants. As measured by the FAMA assay, GMT increased from 1.37 to 103.15 in the NBP608 group and from 1.22 to 54.22 in the control group. As measured by gpELISA, GMT increased from 10.88 to 189.77 in the NBP608 group and from 9.16 to 104.84 in the control group.
Table 4 presents the post-vaccination GMT ratio analysis; baseline seropositive participants were excluded. When assessed using FAMA assay, the GMT ratio (GMT of investigational vaccine divided by that of the control vaccine) was 1.92 (95% CI: 1.55, 2.38). When assessed using gpELISA, GMT ratio was 1.73 (95% CI: 1.53, 1.95). The 95% LCL of GMT ratios, 1.55 by FAMA assay and 1.53 by gpELISA, met the non-inferiority criterion of 0.5.
3.3. Safety
The safety set included 498 participants, and the reported AEs are presented in
Table 5. There were no significant differences between the two groups in the proportions of participants who experienced local solicited AEs (P = 0.285), systemic solicited AEs (P = 0.756), or unsolicited AEs (P = 0.204). The safety profiles were, therefore, similar in the two groups. With respect to local solicited AEs, erythema was the most common, followed by pain/tenderness and swelling in both groups. For systemic solicited AEs, irritation was the most common in both groups; for unsolicited AEs, infection and infestation were the most common in both groups. Most AEs were grade 1 or 2, and there were no significant differences between the two groups with respect to the proportions of participants with grade 3 AEs (local solicited AEs, P = 0.075; systemic solicited AEs, P = 0.769; and unsolicited AEs, P = 0.686). None of the participants in either group demonstrated grade 4 AE.
There were no significant differences in the proportions of participants who had varicella-like rashes (P = 0.106; 3.19% of NBP806 group versus 0.81% of control group). None of the participants contracted vaccine-derived VZV as determined by the PCR test. The proportions of participants with SAEs were not significantly different between the vaccine groups (P = 0.285). Eight participants, seven children with common childhood infectious diseases and one with a burn, reported SAEs throughout the observation period. None of these were vaccine-related, and all eight children recovered.
4. Discussion
In this clinical trial, we compared the immunogenicity and safety of a newly developed varicella vaccine to those of VarivaxTM. For immunity testing against VZV, several methods have been developed to measure antibodies. The FAMA assay is regarded as the gold standard as FAMA titer has a high correlation with varicella protection. The antibody response measured by gpELISA also strongly correlates with neutralizing antibody response [
10,
11]. Thus, FAMA assay and gpELISA were chosen for immunity testing in this study.
The manufacture of varicella vaccines is complex, and individual manufacturers have developed unique techniques and formulations with the goal of producing a vaccine that is devoid of VZV pathogenicity and capable of inducing a protective immune response [
12,
13]. Previously published pre-licensure studies have reported SCRs ranging from 85% to greater than 90% [
10,
14]. The results for the NBP608 vaccine were consistent with previous studies and fulfilled the non-inferiority margin for immunogenicity in terms of SCR as measured by FAMA assay and gpELISA. GMT ratio analysis suggested non-inferiority, revealing a higher value in the NBP608 group than the control group; and the LCL of the 95% GMT ratio was higher than 0.5 in both FAMA assay and gpELISA.
Varicella vaccines are generally safe [
15,
16], and no SAEs reported in this study were related to the investigational or control vaccines. Most of the AEs in both groups were grade 1 or 2. The proportions of participants for whom local and systemic solicited or unsolicited AEs were reported were comparable in the groups. In previous published studies on other varicella vaccines, varicella-like rash was reported in 4-6% of vaccine recipients [
15]; in this study, varicella-like rash occurred in 3.19% of recipients of NBP608. Fever was reported to have occurred in 10−15% of varicella vaccine recipients, and most of these fever episodes were attributable to concurrent illness rather than vaccination [
15]. In this study, fever related to vaccine was not clearly documented. In the NBP608 group, consistent with previous reports, 9.56% of participants reported fever. These results indicate the acceptable safety profile of the investigational vaccine.
There are limitations to this study that need to be addressed. First, the immunogenicity was measured for only a short period, and additional studies evaluating long-term vaccine effectiveness and immune persistence are needed. Second, the study included a small number of children aged 24 months to 12 years, and future studies that include larger numbers of children in this age group are needed.
In conclusion, the NBP608 investigational vaccine satisfied the immunological non-inferiority and clinically acceptable safety profile criteria compared to the VarivaxTM control vaccine. The results will be informative to clinicians and healthcare authorities, particularly in regions that are currently in the process of implementing UVV or considering use of a varicella vaccine.
Author Contributions
Conceptualization, J.-H.K.; data curation, J.-H.K., K.H.K., H.-K.C., D.H.K., S.H.M., Y.Y.C., C.S.K., M.R.C. and I.A.R.K.; writing—original draft preparation, J.-H.K. and U.Y.C.; writing—review and editing, J.H.-K., U.Y.C., K.H.K., H.-K.C., D.H.K., S.H.M., Y.Y.C., C.S.K., M.R.C., I.A.R.K., H.K., J.H.R., S.J.L. and H.G.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by SK Bioscience (Seongnam, Korea); but the supporter was not involved in the management of study, data analyses, or preparation and publication of manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the MFDS of Korea; the national drug authorities of the Philippines and Mexico; and the Institutional Review Board of the participating site (St. Vincent’s Hospital, The Catholic University of Korea: VC16BDGT0078). This study was registered in Clinicaltrials.gov (NCT 03114943).
Informed Consent Statement
Informed consent was obtained by parents or legal representatives of each participant prior to enrollment.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
H Kim, JH Ryu, SJ Lee, and HG Park are employees of SK Bioscience.
References
- Gershon, A.A. Varicella-zoster virus. In: Cherry J, Harrison GJ, Kaplan SL, Steinbach WJ, Hotez P, editors. Feigin and Cherry’s Textbook of Pediatric Infectious Diseases. 8th ed. Philadelphia, PA: Elsevier; 2019, 1476–1484.
- World Health Organization. Varicella and herpes zoster vaccines: WHO position paper, June 2014 Recommendations. Vaccine 2016, 34, 198–199. [Google Scholar] [CrossRef] [PubMed]
- Varela, F.H.; Pinto, L.A.; Scotta, M.C. Global impact of varicella vaccination programs. Hum. Vaccines Immunother. 2019, 15, 645–657. [Google Scholar] [CrossRef] [PubMed]
- Suo, L.; Lu, L.; Zhao, D.; Pang, X. Impact of a 2-dose voluntary vaccination strategy on varicella epidemiology in Beijing, 2011–2017. Vaccine 2020, 38, 3690–3696. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, V. , Kaur, P., Azad, C. Varicella vaccination in India’s universal immunization program – is it time? Trop Doct 2022, 52, 547–549. [Google Scholar] [CrossRef] [PubMed]
- Williams V, Gershon A, Brunell PA. Serologic response to varicella-zoster membrane antigens measured by direct immunofluorescence. J Infect Dis 1974, 130, 669–672. [Google Scholar] [CrossRef] [PubMed]
- Sauerbrei, A.; Wutzler, P. Serological Detection of Varicella-Zoster Virus-Specific Immunoglobulin G by an Enzyme-Linked Immunosorbent Assay Using Glycoprotein Antigen. J. Clin. Microbiol. 2006, 44, 3094–3097. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Korean Food and Drug Safety. Multi-National Vaccine Clinical Trial Protocol. 1st ed. Cheongju, KR: Ministry of Korean Food and Drug Safety; 2016, 377–445.
- Medical Dictionary for Regular Activities. https://www.meddra.org/ Updated 2023. Accessed May 9, 2023.
- Gershon AA, Martin M, Seward JF. Varicella vaccine. In: Plotkin SA, Orenstein WA, Offit PA, Edwards KM, editors. Plotkin’s Vaccines. 7th ed. Philadelphia, PA: Elsevier; 2017, 1145–1180.
- Dollard, S.; Chen, M.-H.; Lindstrom, S.; Marin, M.; A Rota, P. Diagnostic and Immunologic Testing for Varicella in the Era of High-Impact Varicella Vaccination: An Evolving Problem. J. Infect. Dis. 2022, 226, S450–S455. [Google Scholar] [CrossRef] [PubMed]
- A Gershon, A.; Gershon, M.D.; Shapiro, E.D. Live Attenuated Varicella Vaccine: Prevention of Varicella and of Zoster. J. Infect. Dis. 2021, 224, S387–S397. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Rivailler, P.; Xu, S.; Xu, W. Comparison of the Whole-Genome Sequence of an Oka Varicella Vaccine from China with Other Oka Vaccine Strains Reveals Sites Putatively Critical for Vaccine Efficacy. J. Virol. 2019, 93. [Google Scholar] [CrossRef] [PubMed]
- Choi, U.Y.; Kim, K.H.; Lee, J.; Eun, B.W.; Kim, D.H.; Ma, S.H.; Kim, C.S.; Lapphra, K.; Tangsathapornpong, A.; Kosalaraksa, P.; et al. Immunogenicity and safety profiles of a new MAV/06 strain varicella vaccine in healthy children: A multinational, multicenter, randomized, double-blinded, active-controlled phase III study. Vaccine 2021, 39, 1758–1764. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Varicella. In: Hall E, Wodi AP, Hamborsky J, Morelli V, Schillie S, editors. Epidemiology and prevention of vaccine preventable diseases. 14th ed. Washington, DC: Public Health Foundation; 2021, 344–345.
- Moro, P.L.; Leung, J.; Marquez, P.; Kim, Y.; Wei, S.; Su, J.R.; Marin, M. Safety Surveillance of Varicella Vaccines in the Vaccine Adverse Event Reporting System, United States, 2006–2020. J. Infect. Dis. 2022, 226, S431–S440. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).