Submitted:
13 July 2023
Posted:
14 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
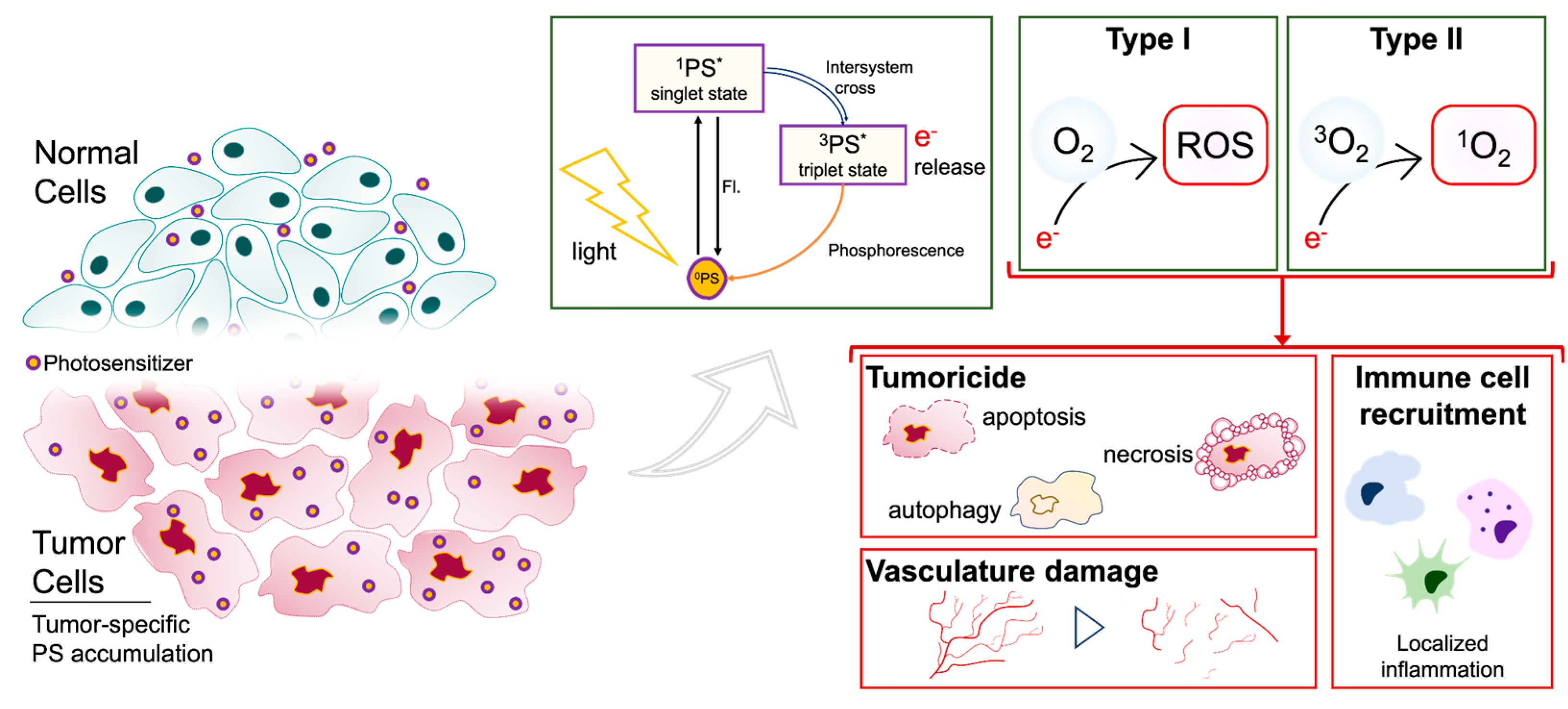
2. Photodynamic therapy mediated tumoricidal effect
2.1. PDT induces cell death following uptake and accumulation
2.2. PDT controls glioma stem cell (GSC) processes
2.3. PDT modulates neurovasculature: disruption of the blood brain barrier (BBB) and destruction of tumor vasculature
2.4. PDT stimlates anti-tumor immunity
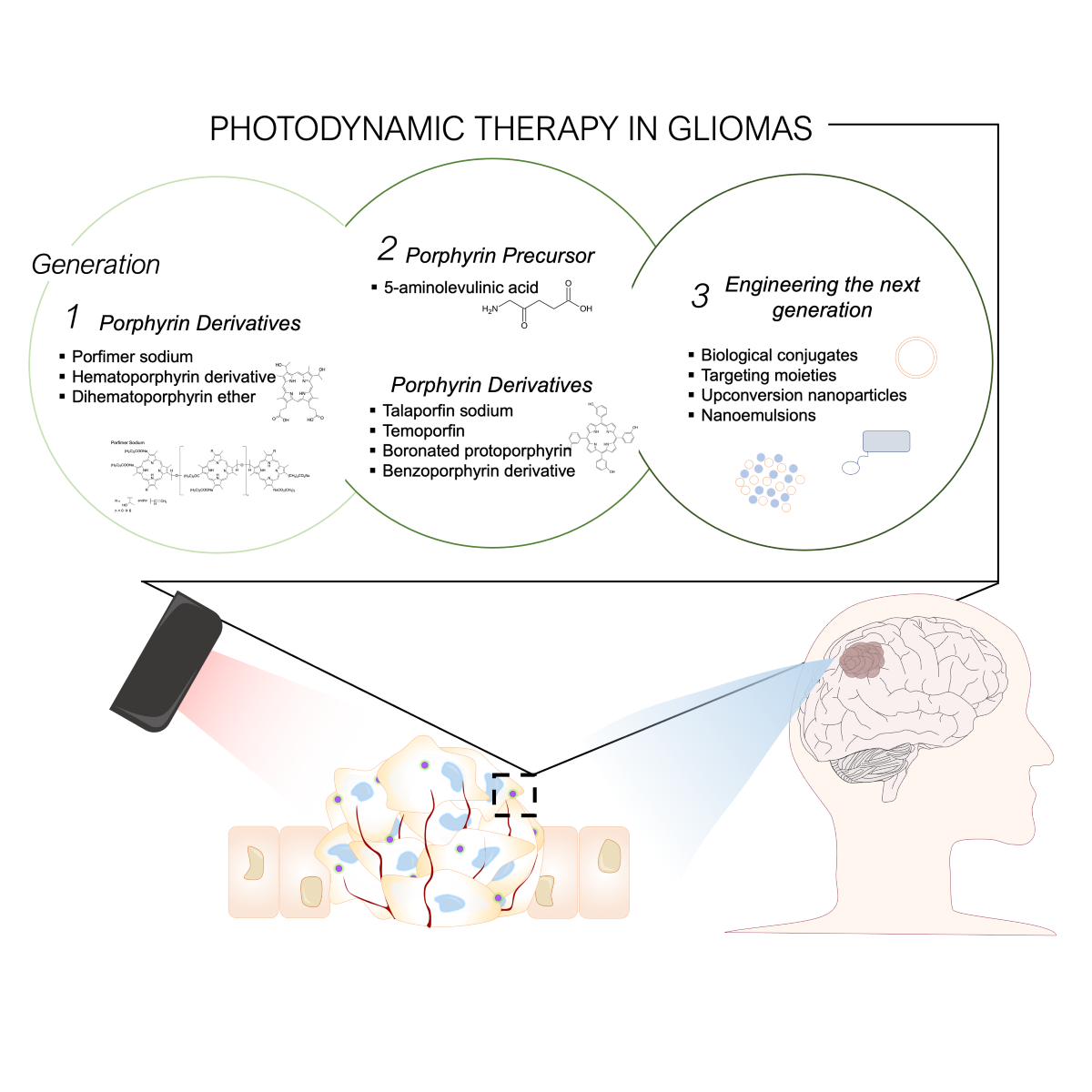
3. Photosensitizers
3.1. First Generation: naturally occurring porphyrins
3.2. Second Generation: increased singlet oxygen potency
3.2.1. Combining second-generation PDT with standard therapies
3.3. Third Generation PS: increased tumor selectivity
4. Optimizing Light Delivery
5. PDT in other CNS tumors
6. Limitations
6.1. Limitations of PDT and its synergistic agents
6.2. PDT efficacy negatively influenced by harsh glioma microenvironment
6.3. Innate PDT resistance
6.4. Peri-tumor edema limits PDT efficacy
6.5. PDT drug interactions and synergistic agents
7. Conclusions and future directions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30.
- Das, S. & Marsden, P. A. Angiogenesis in Glioblastoma. New England Journal of Medicine vol. 369 1561–1563. 2013. [CrossRef]
- Stupp, R. Stupp, R. et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. The Lancet Oncology vol. 10 459–466. 2009. [Google Scholar] [CrossRef]
- Stupp, R. et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [CrossRef] [PubMed]
- Majewska, P. et al. Postprogression survival in patients with glioblastoma treated with concurrent chemoradiotherapy: a routine care cohort study. CNS Oncol 2017, 6, 307–313. [CrossRef]
- Parker, N. R. , Khong, P., Parkinson, J. F., Howell, V. M. & Wheeler, H. R. Molecular Heterogeneity in Glioblastoma: Potential Clinical Implications. Front. Oncol. 0, (2015).
- DeCordova, S. et al. Molecular Heterogeneity and Immunosuppressive Microenvironment in Glioblastoma. Front. Immunol. 0, (2020).
- Management of glioblastoma after recurrence: A changing paradigm. J. Egypt. Natl. Canc. Inst. 2016, 28, 199–210. [CrossRef] [PubMed]
- Gallego, O. Nonsurgical treatment of recurrent glioblastoma. Curr. Oncol. 22, (2015).
- Weller, M. , Cloughesy, T., Perry, J. R. & Wick, W. Standards of care for treatment of recurrent glioblastoma--are we there yet? Neuro. Oncol. 15, (2013).
- dos Santos, M. A. et al. Systematic review and meta-analysis of phase I/II targeted therapy combined with radiotherapy in patients with glioblastoma multiforme: quality of report, toxicity, and survival. J. Neurooncol. 2015, 123, 307–314. [CrossRef]
- Su, J. et al. Molecularly Targeted Drugs Plus Radiotherapy and Temozolomide Treatment for Newly Diagnosed Glioblastoma: A Meta-Analysis and Systematic Review. 2016. [Google Scholar] [CrossRef]
- Laws, E. R., Jr, Cortese, D. A., Kinsey, J. H., Eagan, R. T. & Anderson, R. E. Photoradiation therapy in the treatment of malignant brain tumors: a phase I (feasibility) study. Neurosurgery. 1981, 9, 672–678.
- Dougherty, T. J. et al. Photoradiation therapy for the treatment of malignant tumors. Cancer Res. 1978, 38, 2628–2635.
- da Silva, B. A. et al. Novel Scintillating Nanoparticles for Potential Application in Photodynamic Cancer Therapy. Pharmaceutics 14, (2022).
- Vedunova, M. et al. DC vaccines loaded with glioma cells killed by photodynamic therapy induce Th17 anti-tumor immunity and provide a four-gene signature for glioma prognosis. Cell Death Dis. 2022, 13, 1062.
- Plaetzer, K., Krammer, B., Berlanda, J., Berr, F. & Kiesslich, T. Photophysics and photochemistry of photodynamic therapy: fundamental aspects. Lasers Med. Sci. 2009, 24, 259–268.
- Hirschberg, H. , Berg, K. & Peng, Q. Photodynamic therapy mediated immune therapy of brain tumors. Neuroimmunol Neuroinflamm 5, (2018).
- Cramer, S. W. & Chen, C. C. Photodynamic Therapy for the Treatment of Glioblastoma. Front Surg. 2019, 6, 81.
- Castano, A. P., Mroz, P. & Hamblin, M. R. Photodynamic therapy and anti-tumour immunity. Nature Reviews Cancer 2006, vol. 6 535–545 . [CrossRef]
- Dougherty, T. J. et al. Photodynamic therapy. J. Natl. Cancer Inst. 1998, 90, 889–905. [CrossRef] [PubMed]
- Cramer, S. W. & Chen, C. C. Photodynamic Therapy for the Treatment of Glioblastoma. Front Surg. 2019, 6, 81.
- Agostinis, P. et al. Photodynamic therapy of cancer: an update. CA Cancer J. Clin. 2011, 61, 250–281. [CrossRef] [PubMed]
- Miki, Y., Akimoto, J., Moritake, K., Hironaka, C. & Fujiwara, Y. Photodynamic therapy using talaporfin sodium induces concentration-dependent programmed necroptosis in human glioblastoma T98G cells. Lasers Med. Sci. 2015, 30, 1739–1745.
- Castano, A. P., Demidova, T. N. & Hamblin, M. R. Mechanisms in photodynamic therapy: part one-photosensitizers, photochemistry and cellular localization. Photodiagnosis Photodyn. Ther. 2004, 1, 279–293.
- Castano, A. P., Demidova, T. N. & Hamblin, M. R. Mechanisms in photodynamic therapy: part two—cellular signaling, cell metabolism and modes of cell death. Photodiagnosis and Photodynamic Therapy 2005, vol. 2 1-23. [CrossRef]
- Castano, A. P., Demidova, T. N. & Hamblin, M. R. Mechanisms in photodynamic therapy: Part three—Photosensitizer pharmacokinetics, biodistribution, tumor localization and modes of tumor destruction. Photodiagnosis and Photodynamic Therapy 2005, vol. 2 91–106. [CrossRef]
- Stylli, S. S. & Kaye, A. H. Photodynamic therapy of cerebral glioma – A review Part I – A biological basis. Journal of Clinical Neuroscience 2006, vol. 13 615-625 . [CrossRef]
- Miki, Y., Akimoto, J., Hiranuma, M. & Fujiwara, Y. Effect of talaporfin sodium-mediated photodynamic therapy on cell death modalities in human glioblastoma T98G cells. J. Toxicol. Sci. 2014, 39, 821–827.
- Schimanski, A. et al. Human glioblastoma stem-like cells accumulate protoporphyrin IX when subjected to exogenous 5-aminolaevulinic acid, rendering them sensitive to photodynamic treatment. Journal of Photochemistry and Photobiology B: Biology 2016, vol. 163 203–210 . [CrossRef]
- Fujishiro, T. et al. 5-Aminolevulinic acid-mediated photodynamic therapy can target human glioma stem-like cells refractory to antineoplastic agents. Photodiagnosis Photodyn. Ther. 2018, 24, 58–68. [CrossRef]
- Auffinger, B. et al. Conversion of differentiated cancer cells into cancer stem-like cells in a glioblastoma model after primary chemotherapy. Cell Death & Differentiation 2014, vol. 21 1119–1131 . [CrossRef]
- Jackson, M., Hassiotou, F. & Nowak, A. Glioblastoma stem-like cells: at the root of tumor recurrence and a therapeutic target. Carcinogenesis. 2015, 36, 177–185.
- Fisher, C. et al. Liposomal Lapatinib in Combination with Low-Dose Photodynamic Therapy for the Treatment of Glioma. J. Clin. Med. Res. 8, (2019).
- Madsen, S. J. et al. Increased nanoparticle-loaded exogenous macrophage migration into the brain following PDT-induced blood-brain barrier disruption. Lasers Surg. Med. 2013, 45, 524–532. [CrossRef]
- Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell. 2011, 144, 646–674.
- Dolmans, D. E. J. G. J., Fukumura, D. & Jain, R. K. Photodynamic therapy for cancer. Nat. Rev. Cancer. 2003, 3, 380–387.
- Yi, W. et al. Photodynamic therapy mediated by 5-aminolevulinic acid suppresses gliomas growth by decreasing the microvessels. J. Huazhong Univ. Sci. Technolog. Med. Sci. 2015, 35, 259–264. [CrossRef] [PubMed]
- Etminan, N. et al. Modulation of migratory activity and invasiveness of human glioma spheroids following 5-aminolevulinic acid-based photodynamic treatment. Laboratory investigation. J. Neurosurg. 2011, 115, 281–288. [CrossRef] [PubMed]
- Li, F. et al. Photodynamic therapy boosts anti-glioma immunity in mice: a dependence on the activities of T cells and complement C3. J. Cell. Biochem. 2011, 112, 3035–3043. [CrossRef] [PubMed]
- Etminan, N. et al. Heat-shock protein 70-dependent dendritic cell activation by 5-aminolevulinic acid-mediated photodynamic treatment of human glioblastoma spheroids in vitro. Br. J. Cancer 2011, 105, 961–969. [CrossRef] [PubMed]
- Shibata, S. et al. Photo-immune therapy with liposomally formulated phospholipid-conjugated indocyanine green induces specific antitumor responses with heat shock protein-70 expression in a glioblastoma model. Oncotarget vol. 10 175–183. 2019. [CrossRef]
- Turubanova, V. D. et al. Immunogenic cell death induced by a new photodynamic therapy based on photosens and photodithazine. J Immunother Cancer 2019, 7, 350. [CrossRef]
- Helbig, D., Simon, J. C. & Paasch, U. Photodynamic therapy and the role of heat shock protein 70. Int. J. Hyperthermia. 2011, 27, 802–810.
- Zhou, F., Xing, D. & Chen, W. R. Regulation of HSP70 on activating macrophages using PDT-induced apoptotic cells. Int. J. Cancer. 2009, 125, 1380–1389.
- Kammerer, R. et al. Induction of immune mediators in glioma and prostate cancer cells by non-lethal photodynamic therapy. PLoS One 6, e21834 (2011).
- Xie, W. et al. The Destruction Of Laser-Induced Phase-Transition Nanoparticles Triggered By Low-Intensity Ultrasound: An Innovative Modality To Enhance The Immunological Treatment Of Ovarian Cancer Cells. Int. J. Nanomedicine 2019, 14, 9377–9393. [CrossRef]
- Yang, W. et al. Smart Nanovesicle-Mediated Immunogenic Cell Death through Tumor Microenvironment Modulation for Effective Photodynamic Immunotherapy. ACS Nano 2020, 14, 620–631. [CrossRef]
- Ni, J. et al. Dendritic cell vaccine for the effective immunotherapy of breast cancer. Biomed. Pharmacother. 2020, 126, 110046. [CrossRef] [PubMed]
- He, H. et al. Tumor-targeted nanoplatform for in situ oxygenation-boosted immunogenic phototherapy of colorectal cancer. Acta Biomater. 2020, 104, 188–197. [CrossRef] [PubMed]
- Wang, H. et al. Engineering antigen as photosensitiser nanocarrier to facilitate ROS triggered immune cascade for photodynamic immunotherapy. Biomaterials 2020, 244, 119964. [CrossRef] [PubMed]
- Wang, T. et al. Light-Enhanced O2-Evolving Nanoparticles Boost Photodynamic Therapy To Elicit Antitumor Immunity. ACS Applied Materials & Interfaces vol. 11 16367–16379. 2019. [CrossRef]
- Doix, B., Trempolec, N., Riant, O. & Feron, O. Low Photosensitizer Dose and Early Radiotherapy Enhance Antitumor Immune Response of Photodynamic Therapy-Based Dendritic Cell Vaccination. Front. Oncol. 2019, 9, 811.
- Liu, D. et al. Correction to Redox-Activated Porphyrin-Based Liposome Remote-Loaded with Indoleamine 2,3-Dioxygenase (IDO) Inhibitor for Synergistic Photoimmunotherapy through Induction of Immunogenic Cell Death and Blockage of IDO Pathway. Nano Lett. 2020, 20, 1476. [CrossRef]
- Olzowy, B. et al. Photoirradiation therapy of experimental malignant glioma with 5-aminolevulinic acid. J. Neurosurg. 2002, 97, 970–976. [CrossRef]
- Kostron, H., Obwegeser, A., Jakober, R., Zimmermann, A. & Rueck, A. C. Experimental and clinical results of mTHPC (Foscan)-mediated photodynamic therapy for malignant brain tumors. Optical Methods for Tumor Treatment and Detections: Mechanisms and Techniques in Photodynamic Therapy VII. Optical Methods for Tumor Treatment and Detections, 1998. [CrossRef]
- Callahan, D. E. et al. Boronated protoporphyrin (BOPP): localization in lysosomes of the human glioma cell line SF-767 with uptake modulated by lipoprotein levels. Int. J. Radiat. Oncol. Biol. Phys. 1999, 45, 761–771. [Google Scholar] [CrossRef]
- Aveline, B., Hasan, T. & Redmond, R. W. Photophysical and photosensitizing properties of benzoporphyrin derivative monoacid ring A (BPD-MA). Photochem. Photobiol. 1994, 59, 328–335.
- Schmidt, M. H. et al. Evaluation of photodynamic therapy near functional brain tissue in patients with recurrent brain tumors. J. Neurooncol. 2004, 67, 201–207. [Google Scholar] [CrossRef]
- Fingar, V. H. et al. Analysis of acute vascular damage after photodynamic therapy using benzoporphyrin derivative (BPD). Br. J. Cancer 1999, 79, 1702–1708. [Google Scholar] [CrossRef]
- Fingar, V. H., Wieman, T. J. & Haydon, P. S. The effects of thrombocytopenia on vessel stasis and macromolecular leakage after photodynamic therapy using photofrin. Photochem. Photobiol. 1997, 66, 513–517.
- Sharman, W. M., Allen, C. M. & van Lier JE. Photodynamic therapeutics: basic principles and clinical applications. Drug Discov. Today. 1999, 4, 507–517.
- Gao, S.-G. et al. [Absorption and elimination of photofrin-II in human immortalization esophageal epithelial cell line SHEE and its malignant transformation cell line SHEEC]. Ai Zheng 2009, 28, 1248–1254. [Google Scholar] [CrossRef]
- Schweitzer, V. G. Photodynamic therapy for treatment of head and neck cancer. Otolaryngol. Head Neck Surg. 1990, 102, 225–232. [Google Scholar] [CrossRef]
- Kim, M. M. & Darafsheh, A. Light Sources and Dosimetry Techniques for Photodynamic Therapy. Photochem. Photobiol. 2020, 96, 280–294.
- Shimizu, K. et al. Intraoperative Photodynamic Diagnosis Using Talaporfin Sodium Simultaneously Applied for Photodynamic Therapy against Malignant Glioma: A Prospective Clinical Study. Front. Neurol. 2018, 9, 24. [Google Scholar] [CrossRef]
- Tetard, M.-C., Vermandel, M., Mordon, S., Lejeune, J.-P. & Reyns, N. Experimental use of photodynamic therapy in high grade gliomas: a review focused on 5-aminolevulinic acid. Photodiagnosis Photodyn. Ther. 2014, 11, 319–330.
- Mahmoudi, K. et al. 5-aminolevulinic acid photodynamic therapy for the treatment of high-grade gliomas. J. Neurooncol. 2019, 141, 595–607. [Google Scholar] [CrossRef]
- Cramer, S. W. & Chen, C. C. Photodynamic Therapy for the Treatment of Glioblastoma. Front Surg. 2019, 6, 81.
- Lipson, R. L., Baldes, E. J. & Olsen, A. M. HEMATOPORPHYRIN DERIVATIVE: A NEW AID FOR ENDOSCOPIC DETECTION OF MALIGNANT DISEASE. The Journal of Thoracic and Cardiovascular Surgery vol. 42 623–629. 1961. [CrossRef]
- Lipson, R. L. & Baldes, E. J. The photodynamic properties of a particular hematoporphyrin derivative. Arch. Dermatol. 1960, 82, 508–516.
- Kessel, D. Hematoporphyrin and HPD: photophysics, photochemistry and phototherapy. Photochem. Photobiol. 1984, 39, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Tomio, L. et al. Elimination pathway of hematoporphyrin from normal and tumor-bearing rats. Tumori 1982, 68, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.-X. et al. Underlying mechanism of the photodynamic activity of hematoporphyrin induced apoptosis in U87 glioma cells. Int. J. Mol. Med. 2018, 41, 2288–2296. [Google Scholar] [CrossRef] [PubMed]
- Perria, C. et al. Fast attempts at the photodynamic treatment of human gliomas. J. Neurosurg. Sci. 1980, 24, 119–129. [Google Scholar]
- McCulloch, G. A. et al. Phototherapy in malignant brain tumors. Prog. Clin. Biol. Res. 1984, 170, 709–717. [Google Scholar]
- Kaye, A. H., Morstyn, G. & Brownbill, D. Adjuvant high-dose photoradiation therapy in the treatment of cerebral glioma: a Phase 1–2 study. Journal of Neurosurgery vol. 67 500–505. 1987. [CrossRef]
- Muller, P. J. & Wilson, B. C. Photodynamic therapy: cavitary photoillumination of malignant cerebral tumours using a laser coupled inflatable balloon. Can. J. Neurol. Sci. 1985, 12, 371–373.
- Kostron, H., Weiser, G., Fritsch, E. & Grunert, V. Photodynamic therapy of malignant brain tumors: clinical and neuropathological results. Photochem. Photobiol. 1987, 46, 937–943.
- Muller, P. J. & Wilson, B. C. Photodynamic therapy of malignant primary brain tumours: clinical effects, post-operative ICP, and light penetration of the brain. Photochem. Photobiol. 1987, 46, 929–935.
- Kostron, H., Fritsch, E. & Grunert, V. Photodynamic therapy of malignant brain tumours: a phase I/II trial. Br. J. Neurosurg. 1988, 2, 241–248.
- Kostron, H., Plangger, C., Fritsch, E. & Maier, H. Photodynamic treatment of malignant brain tumors. Wien. Klin. Wochenschr. 1990, 102, 531–535.
- Powers, S. K. , Cush, S. S., Walstad, D. L. & Kwock, L. Stereotactic intratumoral photodynamic therapy for recurrent malignant brain tumors. Neurosurgery 29, 688–95; discussion 695–6 (1991).
- Muller, P. J. & Wilson, B. C. Photodynamic therapy of malignant brain tumours. Lasers Med. Sci. 1990, 5, 245–252.
- Origitano, T. C. & Reichman, O. H. Photodynamic therapy for intracranial neoplasms: development of an image-based computer-assisted protocol for photodynamic therapy of intracranial neoplasms. Neurosurgery 32, 587–95; discussion 595–6 (1993).
- Muller, P. J. & Wilson, B. C. Photodynamic therapy for recurrent supratentorial gliomas. Semin. Surg. Oncol. 1995, 11, 346–354.
- Popovic, E. A., Kaye, A. H. & Hill, J. S. Photodynamic therapy of brain tumors. Seminars in Surgical Oncology vol. 11 335–345. [CrossRef]
- Muller, P. J. & Wilson, B. C. Photodynamic therapy of supratentorial gliomas. in Optical Methods for Tumor Treatment and Detection: Mechanisms and Techniques in Photodynamic Therapy VI vol. 2972 14–26 (SPIE, 1997).
- Muller, P. J. et al. Photofrin photodynamic therapy for malignant brain tumors. Optical Methods for Tumor Treatment and Detection: Mechanisms and Techniques in Photodynamic Therapy X. 2001. [CrossRef]
- Stylli, S. S., Howes, M., MacGregor, L., Rajendra, P. & Kaye, A. H. Photodynamic therapy of brain tumours: evaluation of porphyrin uptake versus clinical outcome. J. Clin. Neurosci. 2004. 2004, 11, 584–596.
- Stylli, S. S., Kaye, A. H., MacGregor, L., Howes, M. & Rajendra, P. Photodynamic therapy of high grade glioma - long term survival. 2005, 12, 389–398.
- Muller, P. J. & Wilson, B. C. Photodynamic therapy of brain tumors--a work in progress. Lasers Surg. Med. 2006, 38, 384–389.
- Kaneko, S. Recent Advances in PDD and PDT for Malignant Brain Tumors. The Review of Laser Engineering 2008, 36, 1351–1354. [Google Scholar] [CrossRef]
- Perria, C. et al. Photodynamic therapy of malignant brain tumors: clinical results of, difficulties with, questions about, and future prospects for the neurosurgical applications. Neurosurgery 1988, 23, 557–563. [Google Scholar] [CrossRef]
- Forbes, I. J. et al. PHOTOTHERAPY OF HUMAN TUMOURS USING HAEMATOPORPHYRIN DERIVATIVE. Medical Journal of Australia vol. 2 489–493. 1980. [CrossRef]
- Kostron, H., Obwegeser, A. & Jakober, R. Photodynamic therapy in neurosurgery: a review. J. Photochem. Photobiol. B. 1996, 36, 157–168.
- Quirk, B. J. et al. Photodynamic therapy (PDT) for malignant brain tumors – Where do we stand? Photodiagnosis and Photodynamic Therapy 2015, vol. 12 530–544 . [CrossRef]
- Kostron, H. , Hochleitner, B. W., Obwegeser, A. & Seiwald, M. Clinical and experimental results of photodynamic therapy in neurosurgery. in 5th International Photodynamic Association Biennial Meeting (ed. Cortese, D. A.) vol. 2371 126–128 (SPIE, 1994).
- Gunaydin, G., Gedik, M. E. & Ayan, S. Photodynamic Therapy for the Treatment and Diagnosis of Cancer-A Review of the Current Clinical Status. Front Chem. 2021, 9, 686303.
- Du, P. et al. Photodynamic therapy leads to death of C6 glioma cells partly through AMPAR. Brain Res. 2012, 1433, 153–159. [Google Scholar] [CrossRef]
- Hu, S.-L., Du, P., Hu, R., Li, F. & Feng, H. Imbalance of Ca2+ and K+ fluxes in C6 glioma cells after PDT measured with scanning ion-selective electrode technique. Lasers Med. Sci. 2014, 29, 1261–1267.
- Dąbrowski, J. M. & Arnaut, L. G. Photodynamic therapy (PDT) of cancer: from local to systemic treatment. Photochem. Photobiol. Sci. 2015, 14, 1765–1780.
- Tirapelli, L. F. et al. Apoptosis in glioma cells treated with PDT. Photomed. Laser Surg. 2011, 29, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Park, J. , Lee, Y.-K., Park, I.-K. & Hwang, S. R. Current Limitations and Recent Progress in Nanomedicine for Clinically Available Photodynamic Therapy. Biomedicines 9, (2021).
- Baskaran, R., Lee, J. & Yang, S.-G. Clinical development of photodynamic agents and therapeutic applications. Biomater Res. 2018, 22, 25.
- Kostron, H., Fiegele, T. & Akatuna, E. Combination of FOSCAN® mediated fluorescence guided resection and photodynamic treatment as new therapeutic concept for malignant brain tumors. Medical Laser Application 2006, vol. 21 285–290 . [CrossRef]
- Rosenthal, M. A. et al. Phase I and pharmacokinetic study of photodynamic therapy for high-grade gliomas using a novel boronated porphyrin. J. Clin. Oncol. 2001, 19, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, M. A., Kavar, B., Uren, S. & Kaye, A. H. Promising survival in patients with high-grade gliomas following therapy with a novel boronated porphyrin. J. Clin. Neurosci. 2003, 10, 425–427.
- Beck, T. J. et al. Interstitial photodynamic therapy of nonresectable malignant glioma recurrences using 5-aminolevulinic acid induced protoporphyrin IX. Lasers Surg. Med. 2007, 39, 386–393. [Google Scholar] [CrossRef]
- Eljamel, M. S., Goodman, C. & Moseley, H. ALA and Photofrin fluorescence-guided resection and repetitive PDT in glioblastoma multiforme: a single centre Phase III randomised controlled trial. Lasers Med. Sci. 2008, 23, 361–367.
- Stepp, H. G. et al. Fluorescence-guided resections and photodynamic therapy for malignant gliomas using 5-aminolevulinic acid. in Photonic Therapeutics and Diagnostics vol. 5686 547–557 (SPIE, 2005).
- Stepp, H. et al. ALA and malignant glioma: fluorescence-guided resection and photodynamic treatment. J. Environ. Pathol. Toxicol. Oncol. 2007, 26, 157–164. [Google Scholar] [CrossRef]
- Akimoto, J., Haraoka, J. & Aizawa, K. Preliminary clinical report on safety and efficacy of photodynamic therapy using talaporfin sodium for malignant gliomas. Photodiagnosis Photodyn. Ther. 2012, 9, 91–99.
- Lyons, M., Phang, I. & Eljamel, S. The effects of PDT in primary malignant brain tumours could be improved by intraoperative radiotherapy. Photodiagnosis Photodyn. Ther. 2012, 9, 40–45.
- Johansson, A. et al. Protoporphyrin IX Fluorescence and Photobleaching During Interstitial Photodynamic Therapy of Malignant Gliomas for Early Treatment Prognosis. Lasers in Surgery and Medicine vol. 45 225–234. 2013. [CrossRef]
- Muragaki, Y. et al. Phase II clinical study on intraoperative photodynamic therapy with talaporfin sodium and semiconductor laser in patients with malignant brain tumors. J. Neurosurg. 2013, 119, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, C., Rühm, A., Tonn, J.-C., Kreth, S. & Kreth, F.-W. SURG-25INTERSTITIAL PHOTODYNAMIC THERAPY OF DE-NOVO GLIOBLASTOMA MULTIFORME WHO IV. Neuro-Oncology vol. 17 v219.5–v220. 2015. [CrossRef]
- Vanaclocha, V. et al. Photodynamic therapy in the treatment of brain tumours. A feasibility study. Photodiagnosis Photodyn. Ther. 2015, 12, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Nitta, M. et al. Role of photodynamic therapy using talaporfin sodium and a semiconductor laser in patients with newly diagnosed glioblastoma. J. Neurosurg. 1–8 (2018).
- Lietke, S. et al. Interstitial Photodynamic Therapy Using 5-ALA for Malignant Glioma Recurrences. Cancers 13, (2021).
- Vermandel, M. et al. Standardized intraoperative 5-ALA photodynamic therapy for newly diagnosed glioblastoma patients: a preliminary analysis of the INDYGO clinical trial. J. Neurooncol. 2021, 152, 501–514. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T. et al. Therapeutic Options for Recurrent Glioblastoma-Efficacy of Talaporfin Sodium Mediated Photodynamic Therapy. Pharmaceutics 14, (2022).
- Kozlikina, E. I. , Trifonov, I. S., Sinkin, M. V., Krylov, V. V. & Loschenov, V. B. The Combined Use of 5-ALA and Chlorin e6 Photosensitizers for Fluorescence-Guided Resection and Photodynamic Therapy under Neurophysiological Control for Recurrent Glioblastoma in the Functional Motor Area after Ineffective Use of 5-ALA: Preliminary Results. Bioengineering (Basel) 9, (2022).
- Neagu, M. et al. Toxicological and efficacy assessment of post-transition metal (Indium) phthalocyanine for photodynamic therapy in neuroblastoma. Oncotarget 2016, 7, 69718–69732. [Google Scholar] [CrossRef]
- Velazquez, F. N. et al. Effectiveness of ZnPc and of an amine derivative to inactivate Glioblastoma cells by Photodynamic Therapy: an in vitro comparative study. Sci. Rep. 2019, 9, 3010. [Google Scholar] [CrossRef]
- Stylli, S., Hill, J., Sawyer, W. & Kaye, A. Aluminium phthalocyanine mediated photodynamic therapy in experimental malignant glioma. J. Clin. Neurosci. 1995, 2, 146–151.
- de Paula, L. B., Primo, F. L., Pinto, M. R., Morais, P. C. & Tedesco, A. C. Evaluation of a chloroaluminium phthalocyanine-loaded magnetic nanoemulsion as a drug delivery device to treat glioblastoma using hyperthermia and photodynamic therapy. RSC Adv. 2017, 7, 9115–9122.
- Chelakkot, V. S. et al. MEK reduces cancer-specific PpIX accumulation through the RSK-ABCB1 and HIF-1α-FECH axes. Sci. Rep. 2020, 10, 22124. [Google Scholar] [CrossRef]
- Hagiya, Y. et al. Pivotal roles of peptide transporter PEPT1 and ATP-binding cassette (ABC) transporter ABCG2 in 5-aminolevulinic acid (ALA)-based photocytotoxicity of gastric cancer cells in vitro. Photodiagnosis Photodyn. Ther. 2012, 9, 204–214. [Google Scholar] [CrossRef]
- Kobuchi, H. et al. Mitochondrial localization of ABC transporter ABCG2 and its function in 5-aminolevulinic acid-mediated protoporphyrin IX accumulation. PLoS One 7, e50082 (2012).
- Ishizuka, M. et al. Novel development of 5-aminolevurinic acid (ALA) in cancer diagnoses and therapy. Int. Immunopharmacol. 2011, 11, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Markwardt, N. A. et al. 405 nm versus 633 nm for protoporphyrin IX excitation in fluorescence-guided stereotactic biopsy of brain tumors. J. Biophotonics 2016, 9, 901–912. [Google Scholar] [CrossRef] [PubMed]
- Akimoto, J., Fukami, S., Ichikawa, M., Mohamed, A. & Kohno, M. Intraoperative Photodiagnosis for Malignant Glioma Using Photosensitizer Talaporfin Sodium. Front Surg. 2019, 6, 12.
- Tsutsumi, M. et al. Photodynamic therapy with talaporfin sodium induces dose-dependent apoptotic cell death in human glioma cell lines. Photodiagnosis Photodyn. Ther. 2013, 10, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Miki, Y. et al. Photodynamic therapy in combination with talaporfin sodium induces mitochondrial apoptotic cell death accompanied with necrosis in glioma cells. Biol. Pharm. Bull. 2013, 36, 215–221. [Google Scholar] [CrossRef]
- Jia, Y. et al. Photodynamic therapy combined with temozolomide inhibits C6 glioma migration and invasion and promotes mitochondrial-associated apoptosis by inhibiting sodium-hydrogen exchanger isoform 1. Photodiagnosis Photodyn. Ther. 2019, 26, 405–412. [Google Scholar] [CrossRef]
- Miki, Y. et al. Concomitant treatment with temozolomide enhances apoptotic cell death in glioma cells induced by photodynamic therapy with talaporfin sodium. Photodiagnosis Photodyn. Ther. 2014, 11, 556–564. [Google Scholar] [CrossRef]
- Cong, D. et al. Upregulation of NHE1 protein expression enables glioblastoma cells to escape TMZ-mediated toxicity via increased H+ extrusion, cell migration and survival. Carcinogenesis 2014, 35, 2014–2024. [Google Scholar] [CrossRef]
- Zhang, X., Guo, M., Shen, L. & Hu, S. Combination of photodynamic therapy and temozolomide on glioma in a rat C6 glioma model. Photodiagnosis and Photodynamic Therapy 2014, vol. 11 603–612 . [CrossRef]
- Tzerkovsky, D. A., Osharin, V. V., Istomin, Y. P., Alexandrova, E. N. & Vozmitel, M. A. Fluorescent diagnosis and photodynamic therapy for C6 glioma in combination with antiangiogenic therapy in subcutaneous and intracranial tumor models. Exp. Oncol. 2014, 36, 85–89.
- Josefsen, L. B. & Boyle, R. W. Photodynamic therapy: novel third-generation photosensitizers one step closer? British journal of pharmacology vol. 154 1–3 (2008).
- Luiza Andreazza, N. et al. Berberine as a photosensitizing agent for antitumoral photodynamic therapy: Insights into its association to low density lipoproteins. Int. J. Pharm. 2016, 510, 240–249. [Google Scholar] [CrossRef]
- Zhu, X. et al. Transferrin/aptamer conjugated mesoporous ruthenium nanosystem for redox-controlled and targeted chemo-photodynamic therapy of glioma. Acta Biomater. 2018, 82, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Sudheesh, K. V. et al. A Cyclometalated Ir Complex as a Lysosome-Targeted Photodynamic Therapeutic Agent for Integrated Imaging and Therapy in Cancer Cells. Chemistry 2018, 24, 10999–11007. [Google Scholar] [CrossRef] [PubMed]
- Ibarra, L. E. et al. Metallated porphyrin-doped conjugated polymer nanoparticles for efficient photodynamic therapy of brain and colorectal tumor cells. Nanomedicine 2018, 13, 605–624. [Google Scholar] [CrossRef] [PubMed]
- Boreham, E. M. et al. A cyclometallated fluorenyl Ir(iii) complex as a potential sensitiser for two-photon excited photodynamic therapy (2PE-PDT). Dalton Trans. 2015, 44, 16127–16135. [Google Scholar] [CrossRef]
- Tang, X.-L. et al. pH-Responsive Magnetic Mesoporous Silica-Based Nanoplatform for Synergistic Photodynamic Therapy/Chemotherapy. ACS Appl. Mater. Interfaces 2018, 10, 15001–15011. [Google Scholar] [CrossRef] [PubMed]
- Jamali, Z. et al. Evaluation of targeted curcumin (CUR) loaded PLGA nanoparticles for in vitro photodynamic therapy on human glioblastoma cell line. Photodiagnosis Photodyn. Ther. 2018, 23, 190–201. [Google Scholar] [CrossRef]
- Xu, J. et al. High Affinity of Chlorin e6 to Immunoglobulin G for Intraoperative Fluorescence Image-Guided Cancer Photodynamic and Checkpoint Blockade Therapy. ACS Nano 2019, 13, 10242–10260. [Google Scholar] [CrossRef]
- Wang, Q. et al. Fluorinated polymeric micelles to overcome hypoxia and enhance photodynamic cancer therapy. Biomater Sci 2018, 6, 3096–3107. [Google Scholar] [CrossRef]
- Lu, L. et al. An iRGD-conjugated prodrug micelle with blood-brain-barrier penetrability for anti-glioma therapy. Biomaterials 2020, 230, 119666. [Google Scholar] [CrossRef]
- de Paula, L. B., Primo, F. L. & Tedesco, A. C. Nanomedicine associated with photodynamic therapy for glioblastoma treatment. Biophys. Rev. 2017, 9, 761–773.
- Pellosi, D. S., Paula, L. B., de Melo, M. T. & Tedesco, A. C. Targeted and Synergic Glioblastoma Treatment: Multifunctional Nanoparticles Delivering Verteporfin as Adjuvant Therapy for Temozolomide Chemotherapy. Mol. Pharm. 2019, 16, 1009–1024.
- Yan, L. et al. Dextran-Benzoporphyrin Derivative (BPD) Coated Superparamagnetic Iron Oxide Nanoparticle (SPION) Micelles for T2-Weighted Magnetic Resonance Imaging and Photodynamic Therapy. Bioconjug. Chem. 2019, 30, 2974–2981. [Google Scholar] [CrossRef] [PubMed]
- Bœuf-Muraille, G. et al. Evaluation of mTHPC-loaded PLGA nanoparticles for in vitro photodynamic therapy on C6 glioma cell line. Photodiagnosis Photodyn. Ther. 2019, 25, 448–455. [Google Scholar] [CrossRef]
- Castilho-Fernandes, A., Lopes, T. G., Primo, F. L., Pinto, M. R. & Tedesco, A. C. Photodynamic process induced by chloro-aluminum phthalocyanine nanoemulsion in glioblastoma. Photodiagnosis Photodyn. Ther. 2017, 19, 221–228.
- Davanzo, N. N., Pellosi, D. S., Franchi, L. P. & Tedesco, A. C. Light source is critical to induce glioblastoma cell death by photodynamic therapy using chloro-aluminiumphtalocyanine albumin-based nanoparticles. Photodiagnosis Photodyn. Ther. 2017, 19, 181–183.
- Lv, Z. et al. A multiphoton transition activated iron based metal organic framework for synergistic therapy of photodynamic therapy/chemodynamic therapy/chemotherapy for orthotopic gliomas. J. Mater. Chem. B Mater. Biol. Med. 2023. [Google Scholar] [CrossRef]
- Tsai, Y.-C. et al. Targeted Delivery of Functionalized Upconversion Nanoparticles for Externally Triggered Photothermal/Photodynamic Therapies of Brain Glioblastoma. Theranostics 2018, 8, 1435–1448. [Google Scholar] [CrossRef] [PubMed]
- Chen, G., Qiu, H., Prasad, P. N. & Chen, X. Upconversion nanoparticles: design, nanochemistry, and applications in theranostics. Chem. Rev. 2014, 114, 5161–5214.
- Tang, X.-L. et al. Near-infrared light-activated red-emitting upconverting nanoplatform for T-weighted magnetic resonance imaging and photodynamic therapy. Acta Biomater. 2018, 74, 360–373. [Google Scholar] [CrossRef]
- Wang, L.-X. et al. Antitumor activity of photodynamic therapy with a chlorin derivative in vitro and in vivo. Tumour Biol. 2015, 36, 6839–6847. [Google Scholar] [CrossRef]
- Hiramatsu, R. et al. Tetrakis(p-carboranylthio-tetrafluorophenyl)chlorin (TPFC): application for photodynamic therapy and boron neutron capture therapy. J. Pharm. Sci. 2015, 104, 962–970. [Google Scholar] [CrossRef]
- Song, R., Hu, D., Chung, H. Y., Sheng, Z. & Yao, S. Lipid-Polymer Bilaminar Oxygen Nanobubbles for Enhanced Photodynamic Therapy of Cancer. ACS Appl. Mater. Interfaces. 2018, 10, 36805–36813.
- Wang, X. et al. Enhancing selective photosensitizer accumulation and oxygen supply for high-efficacy photodynamic therapy toward glioma by 5-aminolevulinic acid loaded nanoplatform. J. Colloid Interface Sci. 2020, 565, 483–493. [Google Scholar] [CrossRef] [PubMed]
- Huang, X. et al. Combined Cancer Chemo-Photodynamic and Photothermal Therapy Based on ICG/PDA/TPZ-Loaded Nanoparticles. Mol. Pharm. 2019, 16, 2172–2183. [Google Scholar] [CrossRef] [PubMed]
- Dupont, C., Mordon, S., Deleporte, P., Reyns, N. & Vermandel, M. A novel device for intraoperative photodynamic therapy dedicated to glioblastoma treatment. Future Oncol. 2017, 13, 2441–2454.
- Madsen, S. J., Sun, C. H., Tromberg, B. J. & Hirschberg, H. Development of a novel indwelling balloon applicator for optimizing light delivery in photodynamic therapy. Lasers Surg. Med. 2001, 29, 406–412.
- Jamali, Z., Hejazi, S. M., Ebrahimi, S. M., Moradi-Sardareh, H. & Paknejad, M. Effects of LED-Based photodynamic therapy using red and blue lights, with natural hydrophobic photosensitizers on human glioma cell line. Photodiagnosis Photodyn. Ther. 2018, 21, 50–54.
- Whelan, H. T. High-grade glioma/glioblastoma multiforme: is there a role for photodynamic therapy? J. Natl. Compr. Canc. Netw. 10 Suppl 2, S31–4 (2012).
- Curnow, A. & Bown, S. G. The role of reperfusion injury in photodynamic therapy with 5-aminolaevulinic acid – a study on normal rat colon. British Journal of Cancer vol. 86 989–992. 2002. [CrossRef]
- Tetard, M.-C. et al. Interstitial 5-ALA photodynamic therapy and glioblastoma: Preclinical model development and preliminary results. Photodiagnosis Photodyn. Ther. 2016, 13, 218–224. [Google Scholar] [CrossRef]
- Vermandel, M. et al. Comparison of different treatment schemes in 5-ALA interstitial photodynamic therapy for high-grade glioma in a preclinical model: An MRI study. Photodiagnosis Photodyn. Ther. 2019, 25, 166–176. [Google Scholar] [CrossRef]
- Leroy, H.-A. et al. MRI assessment of treatment delivery for interstitial photodynamic therapy of high-grade glioma in a preclinical model. Lasers Surg. Med. 2018, 50, 460–468. [Google Scholar] [CrossRef]
- Leroy, H.-A. et al. Interstitial photodynamic therapy and glioblastoma: Light fractionation in a preclinical model. Lasers Surg. Med. 2017, 49, 506–515. [Google Scholar] [CrossRef]
- Hirschberg, H. et al. Repetitive photodynamic therapy of malignant brain tumors. J. Environ. Pathol. Toxicol. Oncol. 2006, 25, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Davies, N. & Wilson, B. C. Interstitial in vivo ALA-PpIX mediated metronomic photodynamic therapy (mPDT) using the CNS-1 astrocytoma with bioluminescence monitoring. Photodiagnosis Photodyn. Ther. 2007, 4, 202–212.
- Guo, H.-W. et al. Low-fluence rate, long duration photodynamic therapy in glioma mouse model using organic light emitting diode (OLED). Photodiagnosis Photodyn. Ther. 2015, 12, 504–510. [Google Scholar] [CrossRef]
- van Zaane, F. et al. A telemetric light delivery system for metronomic photodynamic therapy (mPDT) in rats. J. Biophotonics 2010, 3, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Plattner, M. , Bernwick, W. & Kostron, H. Hematoporphyrin-derivative photodynamic in-vitro sensitivity testing for brain tumors. in International Conference on Photodynamic Therapy and Laser Medicine vol. 1616 182–185 (SPIE, 1993).
- Chen, Z.-Q. , Wu, S.-E. & Zhu, S.-G. Adjuvant photodynamic therapy in surgical management of cerebral tumors. in International Conference on Photodynamic Therapy and Laser Medicine vol. 1616 94–97 (SPIE, 1993).
- Marks, P. V. , Furneaux, C. & Shivvakumar, R. An in vitro study of the effect of photodynamic therapy on human meningiomas. Br. J. Neurosurg. 1992, 6, 327–332. [Google Scholar]
- Neurosurgery. https://academic.oup.com/neurosurgery/article-abstract/32/3/357/2755000.
- Malham, G. M., Thomsen, R. J., Finlay, G. J. & Baguley, B. C. Subcellular distribution and photocytotoxicity of aluminium phthalocyanines and haematoporphyrin derivative in cultured human meningioma cells. Br. J. Neurosurg. 1996, 10, 51–57.
- Tsai, J. C., Hsiao, Y. Y., Teng, L. J., Chen, C. T. & Kao, M. C. Comparative study on the ALA photodynamic effects of human glioma and meningioma cells. Lasers Surg. Med. 1999, 24, 296–305.
- Sam Eljamel, M. Which intracranial lesions would be suitable for fluoresce guided resection?: A prospective review of 110 consecutive lesions. in Photodynamic Therapy: Back to the Future vol. 7380 112–125 (SPIE, 2009).
- El-Khatib, M. et al. Aminolevulinic acid-mediated photodynamic therapy of human meningioma: an in vitro study on primary cell lines. Int. J. Mol. Sci. 2015, 16, 9936–9948. [Google Scholar] [CrossRef]
- Hefti, M., Holenstein, F., Albert, I., Looser, H. & Luginbuehl, V. Susceptibility to 5-Aminolevulinic Acid Based Photodynamic Therapy in WHO I Meningioma Cells Corresponds to Ferrochelatase Activity. Photochemistry and Photobiology vol. 87 235–241. 2011. [CrossRef]
- Sun, W. et al. Gefitinib enhances the efficacy of photodynamic therapy using 5-aminolevulinic acid in malignant brain tumor cells. Photodiagnosis Photodyn. Ther. 2013, 10, 42–50. [Google Scholar] [CrossRef]
- Cornelius, J. F. et al. Enhancing the effect of 5-aminolevulinic acid based photodynamic therapy in human meningioma cells. Photodiagnosis Photodyn. Ther. 2014, 11, 1–6. [Google Scholar] [CrossRef]
- Ichikawa, M. et al. Photodynamic therapy with talaporfin sodium induces dose- and time-dependent apoptotic cell death in malignant meningioma HKBMM cells. Photodiagnosis Photodyn. Ther. 2019, 25, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T. et al. Photodynamic therapy using talaporfin sodium induces heme oxygenase-1 expression in rat malignant meningioma KMY-J cells. J. Toxicol. Sci. 2018, 43, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T. et al. Possible mechanism of heme oxygenase-1 expression in rat malignant meningioma KMY-J cells subjected to talaporfin sodium-mediated photodynamic therapy. Photodiagnosis Photodyn. Ther. 2020, 32, 102009. [Google Scholar] [CrossRef] [PubMed]
- Kirollos, R. W., Marks, P. V., Igbaseimokumo, U. & Chakrabarty, A. A preliminary experimental in vivo study of the effect of photodynamic therapy on human pituitary adenoma implanted in mice. Br. J. Neurosurg. 1998, 12, 140–145.
- Marks, P. V., Buxton, T. & Furneaux, C. E. In vitro study of the effect of photodynamic therapy on pituitary adenomas. Br. J. Neurosurg. 1993, 7, 401–406.
- Igbaseimokumo, U. Quantification of in vivo Photofrin uptake by human pituitary adenoma tissue. J. Neurosurg. 2004, 101, 272–277. [Google Scholar] [CrossRef]
- Marks, P. V. et al. Effect of photodynamic therapy on recurrent pituitary adenomas: clinical phase I/II trial--an early report. Br. J. Neurosurg. 2000, 14, 317–325. [Google Scholar] [CrossRef]
- Nemes, A. et al. 5-ALA Fluorescence in Native Pituitary Adenoma Cell Lines: Resection Control and Basis for Photodynamic Therapy (PDT)? PLoS One 11, e0161364 (2016).
- Neumann, L. M. et al. Efficacy of 5-aminolevulinic acid based photodynamic therapy in pituitary adenomas-experimental study on rat and human cell cultures. Photodiagnosis Photodyn. Ther. 2016, 14, 77–83. [Google Scholar] [CrossRef]
- Cornelius, J. F. et al. 5-Aminolevulinic acid-based photodynamic therapy of chordoma: In vitro experiments on a human tumor cell line. Photodiagnosis Photodyn. Ther. 2017, 20, 111–115. [Google Scholar] [CrossRef]
- Gull, H. H. et al. Ciprofloxacin enhances phototoxicity of 5-aminolevulinic acid mediated photodynamic treatment for chordoma cell lines. Photodiagnosis Photodyn. Ther. 2021, 35, 102346. [Google Scholar] [CrossRef]
- Briel-Pump, A. et al. Accumulation of protoporphyrin IX in medulloblastoma cell lines and sensitivity to subsequent photodynamic treatment. J. Photochem. Photobiol. B 2018, 189, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Schwake, M. et al. In-Vitro Use of 5-ALA for Photodynamic Therapy in Pediatric Brain Tumors. Neurosurgery 2018, 83, 1328–1337. [Google Scholar] [CrossRef] [PubMed]
- Kast, R.E.; Michael, A.P.; Sardi, I.; Burns, T.C.; Heiland, T.; Karpel-Massler, G.; Kamar, F.G.; Halatsch, M.-E. A New Treatment Opportunity for DIPG and Diffuse Midline Gliomas: 5-ALA Augmented Irradiation, the 5aai Regimen. Brain Sci. 2020, 10, 51. [CrossRef]
- Chiba, K. et al. Photodynamic therapy for malignant brain tumors in children and young adolescents. Front. Oncol. 2022, 12, 957267. [Google Scholar] [CrossRef] [PubMed]
- Stepp, H. & Stummer, W. 5-ALA in the management of malignant glioma. Lasers Surg. Med. 2018, 50, 399–419.
- Kawai, N. et al. ABCG2 expression is related to low 5-ALA photodynamic diagnosis (PDD) efficacy and cancer stem cell phenotype, and suppression of ABCG2 improves the efficacy of PDD. PLoS One 14, e0216503 (2019).
- Blake, E. & Curnow, A. The hydroxypyridinone iron chelator CP94 can enhance PpIX-induced PDT of cultured human glioma cells. Photochem. Photobiol. 2010, 86, 1154–1160.
- Chen, X. et al. Calcitriol enhances 5-aminolevulinic acid-induced fluorescence and the effect of photodynamic therapy in human glioma. Acta Oncol. 2014, 53, 405–413. [Google Scholar] [CrossRef]
- Wang, C. et al. Low-dose arsenic trioxide enhances 5-aminolevulinic acid-induced PpIX accumulation and efficacy of photodynamic therapy in human glioma. J. Photochem. Photobiol. B 2013, 127, 61–67. [Google Scholar] [CrossRef]
- Coupienne, I. et al. NF-kappaB inhibition improves the sensitivity of human glioblastoma cells to 5-aminolevulinic acid-based photodynamic therapy. Biochem. Pharmacol. 2011, 81, 606–616. [Google Scholar] [CrossRef]
- Albert, I., Hefti, M. & Luginbuehl, V. Physiological oxygen concentration alters glioma cell malignancy and responsiveness to photodynamic therapy in vitro. Neurol. Res. 2014, 36, 1001–1010.
- Dereski, M. O., Madigan, L. & Chopp, M. The Effect of Hypothermia and Hyperthermia on Photodynamic Therapy of Normal Brain. Neurosurgery 1995, vol. 36 141-146 . [CrossRef]
- Fisher, C. J. et al. ALA-PpIX mediated photodynamic therapy of malignant gliomas augmented by hypothermia. PLoS One 12, e0181654 (2017).
- Christie, C. Synergistic chemotherapy by combined moderate hyperthermia and photochemical internalization. Biomed. Opt. Express 2016, 7, 1240–1250. [Google Scholar] [CrossRef]
- Fisher, C. J. et al. Modulation of PPIX synthesis and accumulation in various normal and glioma cell lines by modification of the cellular signaling and temperature. Lasers Surg. Med. 2013, 45, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Ghogare, A. A., Rizvi, I., Hasan, T. & Greer, A. ‘Pointsource’ delivery of a photosensitizer drug and singlet oxygen: eradication of glioma cells in vitro. Photochem. Photobiol. 2014, 90, 1119–1125.
- Girotti, A. W. et al. Upregulation of nitric oxide in tumor cells as a negative adaptation to photodynamic therapy. Lasers Surg. Med. 2018, 50, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J. M., Emmer, J. V., Korytowski, W., Hogg, N. & Girotti, A. W. Antagonistic Effects of Endogenous Nitric Oxide in a Glioblastoma Photodynamic Therapy Model. Photochem. Photobiol. 2016, 92, 842–853.
- Fahey, J. M., Stancill, J. S., Smith, B. C. & Girotti, A. W. Nitric oxide antagonism to glioblastoma photodynamic therapy and mitigation thereof by BET bromodomain inhibitor JQ1. J. Biol. Chem. 2018, 293, 5345–5359.
- Lee, S. Y. et al. TP53 regulates human AlkB homologue 2 expression in glioma resistance to Photofrin-mediated photodynamic therapy. Br. J. Cancer 2010, 103, 362–369. [Google Scholar] [CrossRef]
- Li, B. O. et al. Effect of photodynamic therapy combined with torasemide on the expression of matrix metalloproteinase 2 and sodium-potassium-chloride cotransporter 1 in rat peritumoral edema and glioma. Oncol. Lett. 2016, 11, 2084–2090. [Google Scholar] [CrossRef]
- Zhang, X. et al. The effect of bumetanide on photodynamic therapy-induced peri-tumor edema of C6 glioma xenografts. Lasers Surg. Med. 2014, 46, 422–430. [Google Scholar] [CrossRef]
- Gupta, S., Dwarakanath, B. S., Muralidhar, K., Koru-Sengul, T. & Jain, V. Non-monotonic changes in clonogenic cell survival induced by disulphonated aluminum phthalocyanine photodynamic treatment in a human glioma cell line. J. Transl. Med. 2010, 8, 43.
- Hefti, M., Albert, I. & Luginbuehl, V. Phenytoin reduces 5-aminolevulinic acid-induced protoporphyrin IX accumulation in malignant glioma cells. J. Neurooncol. 2012, 108, 443–450.
- Misuth, M., Horvath, D., Miskovsky, P. & Huntosova, V. Synergism between PKCδ regulators hypericin and rottlerin enhances apoptosis in U87 MG glioma cells after light stimulation. Photodiagnosis Photodyn. Ther. 2017, 18, 267–274.
- Zheng, X. et al. Atorvastatin reduces functional deficits caused by photodynamic therapy in rats. Int. J. Oncol. 2011, 39, 1133–1141. [Google Scholar] [PubMed]
- Pridham, K. J. et al. Connexin 43 confers chemoresistance through activating PI3K. Oncogenesis 2022, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.-P. et al. A novel role of Cx43-composed GJIC in PDT phototoxicity: an implication of Cx43 for the enhancement of PDT efficacy. Int. J. Biol. Sci. 2019, 15, 598–609. [Google Scholar] [CrossRef]
| Photosensitizer | Intracellular Localization | Excitation Wavelength (nm) | Treatment Windowa | Clearance Time | Tumor : Normal Fluorescence Ratiob | Administration | Side effects | |
|---|---|---|---|---|---|---|---|---|
| First Generation | Porfimer Sodium | Inner mitochondrial membrane | 630 | 48 – 150 h | 4 – 8 weeks | 2.5 – 4 : 1 | Systemic | Skin sensitization, thrombocytopenia |
| Hematoporphyrin derivative [HpD] | 408, 510, 630c | 24 – 48 h | 4 – 6 weeks | Systemic | ||||
| Dihematoporphyrin ether [DHE] | 395, 630c | 24 – 72 h | 4 – 6 weeks | Systemic | ||||
| Second Generation | 5-Aminolevulinic Acid (Levulin®, Gliolan®) | Early: mitochondria Late: plasma membrane, lysosomes | 410, 510, 635c | 4 – 8 h | 2 days | 10 – 20 : 1 | Oral | Skin sensitization, nausea, elevated liver enzymes, anemia |
| Talaporfin sodium (Laserphyrin, AptocineTM, LS11, PhotoIon®) | Lysosomes | 664 | 12 – 26 h | 15 days | ND | Systemic | Skin sensitization | |
| Temoporfin [m-THPC; m-tetrahydroxyphenylchlorin] (Foscan®, liquid formulation; Foslip®, liposomal formulation) | Strong: golgi apparatus, Endoplasmic reticulum Weak: mitochondria, lysosomes | 652 | 48 – 110 h | 15 days | 150 : 14 | Systemic | Skin sensitization | |
| Boronated protoporphyrin [BOPP] | Lysosomes | 630 | 24 h | 4 – 6 weeks | 400 : 1 | Systemic | Skin sensitization, thrombocytopenia | |
| Benzoporphyrin derivative [BPD] | Lysosomes | 680-690 | 15 – 30 min. | 1 – 5 days | ND | Systemic | Vascular damage |
| Study Groupa (n, number of GBM patients in study) |
Mean Age | PSb | Dosec | Routed | Time prior to Photoillumin-ation | Photoillumi-nation Methode | Laser / Light Wavelengthf (nm) |
Photoillumination Energy (ED unless otherwise specified) |
Reported survivalg | Survival Statistics | Adverse Events | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Perria et al. (1980) | GBM | 2 | n/a | HpD | 5 mg/kg | IV | n/a | n/a | 628 | 720 – 2400 J/cm2 | MS | 6.9 mo | n/a | |
| Laws et al. (1981) | rMG | 5 | 14-75 | HpD | 5 mg/kg | IV | 48 – 72 h | Interstitial | 630 | 30 – 60 mW/cm2 § | TTP | 1-6 mo | Increased skin photosenstivity | |
| McCulloch et al. (1984) | GBM | 9 | n/a | HpD | 5 mg/kg | IV | n/a | n/a | 627.8 (>1 laser) | n/a | OS | GBM (n=3) | 17-42 mo | Increase in P/O cerebral edema |
| Muller & Wilson (1985)h | GBM rGBM |
1 2 |
53 32,44 |
HpD | 2.5 mg/kg 2 mg/kg |
IV | 24 h | Cavitary (balloon) | 630 | 8 – 68 J/cm2 | n/a | None | ||
| Kaye et al. (1987)i | GBM rGBM |
13 6 |
45*** 40*** |
HpD | 5 mg/kg | IV | 24 h | Interstitial | AI (9) GMVL (14) |
70 – 120 J/cm2 120 – 230 J/cm2 |
PFS | GBM rGBM |
3-13 mo 12-16 wk |
No AEs |
| Kostron et al. (1987)j | GBM rGBM (1x) rGBM (mult) |
6 5 3 |
63.3 50.8 57.0 |
HpD | 1.0 mg/cm3 | IV IA Direct tumor |
3 d | LED (n = 9) Cavitary (n = 5) |
620-640 632 |
422 J/cm2 § < 1600 J/cm2 § |
MS/OS | GBM rGBM (1x) rGBM (mult) |
12 mo 2-7 mo 5 mo |
IA/Direct tolerated without skin phototoxicity |
| Muller & Wilson (1987)h |
[HpD] GBM rGBM [DHE] GBM rGBM |
1 1 7 7 |
52 32 58.3 39.4 |
HpD (8) DHE (24) Total dose |
2.14 mg/kg 2.08 mg/kg 150 mg |
IV | 18 – 24 h | Cavitary | 630 | HpD: 32 J/cm2 DHE: 23 J/cm2 |
MS | [HpD] GBM rGBM [DHE] GBM rGBM |
2.9 mo 5.8 mo 1.1-13.6 mo 0.2-10.7 mo |
Skin photosensitivity (n = 3) |
| Kostron et al. (1988)j | GBM rGBM (1x) rGBM (mult) |
8 9 3 |
55** | HpD | 1 mg/cm3 | IV, IA and/or Direct | 3 d | LED Cavitary |
590-750 632 |
422 J/cm2 § 60 – 200 J/cm2 |
OS | GBM rGBM (1x) rGBM (mult) |
0.5-19 m 3-14 mo 1-6 mo |
Skin phototoxicity (IA/IV only) |
| HPD only (n=9), [HPD + single dose radiation of 4 Gy fast electrons] (n=10), [HPD + single dose radiation + conventional radiotherapy] (n=4); 3 cases of recurrence and subsequent re-treatment. | ||||||||||||||
| Kostron et al. (1990)j | GBM rGBM |
9 18 |
n/a | HpD | n/a | IV, IA and/or Direct | n/a | Interstitial | 630 | 40 – 220 J/cm2 | OS | GBM rGBM |
0.5-29 mo 4-13 mo |
Increased phototoxicity of the skin |
| Muller & Wilson (1990)h | GBM rGBM |
9 14 |
48 | HpD DHE |
5 mg/kg 2 mg/kg |
IV | 18 – 24 h | Cavitary | 630 | 24 J/cm2 | MS | GBM + rGBM | 6.3 mo | Increased skin photosensitivity |
| Powers et al. (1991) | rGBM rMG |
1 5 |
42-61 | HPE | 2.0 mg/kg | IV | 24 h | Interstitial | 630 | 1000 J§§ | TTP | rGBM rMG |
2-27 wk 6-45 wk |
Edema, increased intracranial pressure and skin photosensitivity |
| Origitano et al. (1993) |
rGBM | 8 | 42.2 | PNa | 2.0 mg/kg | IV | 48 – 72 h | Cavitary Interstitial + post-resection cavitary |
630 630 |
50 J/cm2 100 J/cm per fiber |
TTP | 5-22 mo | Increased skin photosensitivity | |
| Muller & Wilson (1995)h | rGBM | 32 | 41** | HpD PNa HPE |
5 mg/kg 2 mg/kg 2 mg/kg |
IV IV IV |
12 – 36 h | Cavitary | 630 | 38 J/cm2 | MS | [Stratify by light dose] Energy: > 1700 J < 1700 J |
28 wk 29 wk |
Edema, increased skin photosensitivity |
| Popovic et al. (1995)i | GBM rGBM |
38 40 |
n/a | HpD | 2.0-2.5 mg/kg | IV | 24 h | Cavitary | AI (1986-1987) GMVL (1987-1994) |
240 – 260 J/cm2 (initial pts: 70) |
MS | GBM rGBM |
24 mo 9 mo |
n/a |
| Muller & Wilson (1997)h | GBM rGBM |
11 32 |
40 58 |
PNa | 2 mg/kg | IV | 12 – 36 h | Cavitary | 630 | GBM: 30 J/cm2 * rGBM: 43 J/cm2 * |
MS | GBM rGBM |
37 wk 30 wk |
Increased P/O cerebral edema |
| Muller et al. (2001) [Phase II]h | rGBM (ED ≤ 50) (ED ≥ 50) |
37 (22) (15) |
41** | PNa | 2 mg/kg | IV | 12 – 36 h | Cavitary | 630 | 8 – 110 J/cm2 | MS | rGBM (ED ≤ 50) (ED ≥ 50) |
avg 29 wk (29 wk) (34 wk) |
Increased P/O cerebral edema |
| Muller et al. (2001) [Phase III]h |
GBM High light rGBM Low light High light |
20 26 26 |
54 48 52 |
PNa | 2 mg/kg | IV | 12 – 36 h | Cavitary | 630 | 30 – 50 J/cm2 (low) 110 – 130 J/cm2 (high) |
MS |
GBM High rGBM Low High |
92 wk 29 wk 51 wk |
n/a |
| Schmidt et al. (2004) | Recurrent brain tumors (including GBM) |
NS | n/a | PNa | 0.75 mg/kg 1.20 mg/kg 1.60 mg/kg 2.00 mg/kg |
IV | 18 – 24 h | Laser/LED + Cavitary balloon | Laser: 630 LED: 20 – 25 |
100 J/cm2 | Not specified | No neurotoxicity | ||
| Stylli et al. (2004)i | GBM rGBM |
31 27 |
44*** | HpD | 5 mg/kg | IV | 24 h | Cavitary | AI (1986-1987) GMVL (1987-1994) KTP (1994-2000) |
240 J/cm2 §§ | MS | GBM/rGBM | 24 mo | n/a |
| Stylli et al. (2005)i | GBM rGBM |
31 55 |
47* 42* |
HpD | 5 mg/kg | IV | 24 h | Cavitary | AI (1986-1987) GMVL (1987-1994) KTP (1994-2000) |
240 J/cm2 §§ | MS | GBM rGBM |
14.3 mo 13.5 mo |
Increased cerebral edema (n = 3) |
| Muller et al. (2006)h | GBM rGBM |
12 37 |
59 41 |
PNa | 2 mg/kg | IV | 12 – 36 h | Cavitary (balloon or continuous filling with Intralipid) +/- interstitial |
AI KTP |
58 J/cm2 §§§ | MS | GBM rGBM |
33 wk 29 wk |
Skin photosensitivity |
| Kaneko (2008) | GBM | 26 | HPE | 3 mg/kg | IV | 2 d | Interstitial | 640 | 180 J/cm | n/a | n/a | |||
| Study Groupa (n, number of patients with disease & treatment) |
Mean Age | PSb | Dosec | Routed | Time prior to Photoillumination | Photoillumination Methode | Laser / Light Wavelengthf (nm) |
Photoillumination Energyg (ED unless otherwise specified) |
Reported survivalh | Survival Statistics | Adverse Events | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kostron et al. (1998) | GBM rGBM |
2 8 |
61-72 34-72 |
mTHPCx | 0.15 mg/kg | IV | 4 d | Interstitial I/O cavitary |
KTP 652 |
300 mW/cm2 § 20 J/cm2 |
TTP MS |
rGBM rGBM |
4 mo 6 mo |
Phototoxicity |
| Rosenthall et al. (2001/2003)i | GBM rGBM |
7 9 |
51* | BOPP | 0.25-8.0 mg/kg | IV | 24 h | I/O fiber diffuser for focused surface irradiation | 630 | 25 J/cm2 | MS | GBM rGBM |
5 mo 11 mo |
n/a |
| Schmidt et al. (2004) | Recurrent brain tumors (including GBM) |
NS | n/a | BPD | 0.25 mg/kg | IV | 3–6 h | Laser fiber optic catheter/balloon LED balloon |
680 | 1,800 J§§ (at 100 J/cm2) |
Not specified | No cytotoxic effects | ||
| Kostron et al. (2006) | GBM | 26 | n/a | mTHPCx | 0.15 mg/kg | IV | 4 d | I/O cavitary I/O fiber diffuser |
KTP 652 |
300 mW/cm2 § 20 J/cm2 |
MS | GBM | 15 mo | Increased skin sensitivity |
| Beck et al. (2007) | rGBM | 10 | 51.7 | 5-ALA | 20 mg/kg | Oral | 1 h | I/O fiber diffuser for focused surface irradiation | 633 | 100 J/cm2 | MS | 15 m | n/a | |
| Elijamel et al. (2007) | GBM, PDT(+) GBM, PDT(-) |
13 14 |
59.6 60.1 |
5-ALA -- |
20 mg/kg -- |
Oral -- |
3 h -- |
P/O cavitary balloon | 630 | 100 J/cm2 | PFS MS |
GBM | 8.6 mo 52.8 wk |
Deep venous thrombosis (n=2) |
| Stepp et al. (2005) | GBM | 5 | n/a | 5-ALA | 20 mg/kg | Oral | 3 h | I/O fiber diffuser for focused surface irradiation | 633 | 100 – 200 J/cm2 | n/a | n/a | ||
| Stepp et al. (2007) | GBM (a) GBM (b) GBM (c) |
5 8 7 |
n/a | 5-ALA | 20 mg/kg | Oral | 3 h | I/O fiber diffuser for focused surface irradiation | 633 | (a) 100 J/cm2 (b) 150 J/cm2 (c) 200 J/cm2 |
n/a | No AEs | ||
| Akimoto et al. (2012) | GBM rGBM |
6 8 |
49-82 41-61 |
TS | 40 mg/m2 | IV | 24 h | I/O fiber diffuser for focused surface irradiation (1.0 cm diameter) |
664 | 27 J/cm2 | PFS | GBM | 24.8 mo | Increased photosenstivity |
| Lyons et al. (2012) | Total (GBM) PDT(+) PDT(-) [a], [b] [c], [d] |
73 30 43 17, 13 18, 25 |
59** | 5-ALA | 20 mg/kg | Oral | 3 h | [a] IORT, I/O cavitary, MSR [b] I/O cavitary, MSR [c] IORT, MSR [d] MSR only |
630 | 100 J/cm2 | PFS MS |
[a] [b] PDT+ PDT- |
79 wk 39.7 wk 62.9 wk 20.6 wk |
n/a |
| Johansson et al. (2013) | GBM rGBM |
1 4 |
42 56 |
5-ALA | 20-30 mg/kg | Oral | 5–8 h | Interstitial | 635 | 720 J/cm2 | TTP | 3-36 mo | n/a | |
| Muragaki et al. (2013) | GBM | 13 | 47.1** | TS | 40 mg/m2 | IV | 22–27 h | I/O fiber diffuser for focused surface irradiation (1.5 cm diameter) |
664 | 27 J/cm2 | PFS MS |
12 mo 27.9 mo |
Increased photosensitivity | |
| Schwartz et al. (2015) | GBM | 15 | n/a | 5-ALA | 20 mg/kg 30 mg/kg |
Oral | n/a | Interstitial | 633 | 12.96 J §§ | PFS MS |
16 m 34 m |
Transient aphasia, pulmonary embolism | |
| Vanaclocha et al. (2015) | GBM | 20 | 49*** | DHE mTHPCx |
2 mg/kg 0.15 mg/kg |
IV | 48 h 96 h |
I/O cavitary | 630 652 |
75 J/cm2 20 J/cm2 |
PFS MS MS (from 1st diagnosis) |
10 mo 9 mo 17 mo |
Skin photosensitivity Dermatitis |
|
| Nitta et al. (2018) | GBM | 11 | 54 | TS | 40 mg/m2 | IV | 22–26 h | I/O fiber diffuser for focused surface irradiation (1.5 cm diameter) | 664 | 27 J/cm2 | PFS MS |
19.6 mo 27.5 mo |
Asymptomatic transient peripheral edema | |
| Shimizu et al. (2018) | GBM rGBM |
7 7 |
45-74 40-69 |
TS | 40 mg/m2 | IV | 22– 26 h | I/O fiber diffuser for focused surface irradiation (1.5 cm diameter) | 664 | 100 J/cm2 | n/a | Pulmonary embolism (if vessels are not shielded) |
||
| Lietke et al. (2021) | rGBM | 37 | 49.4* | 5-ALA | 20 mg/kg | Oral | 3 – 5 h | Interstitial | 635 | 8,883 J §§ | TTP MS (from 1st diagnosis) |
Study combines GBM & AA | 7.1 mo 39.7 mo |
Transient worsening of pre-existing neurological deficits |
| Vermandel et al. (2021) | GBM | 10 | 57.1* | 5-ALA | 20 mg/kg | Oral | 6 h | I/O cavitary | 635 | 200 J/cm2 | PFS MS |
17.1 mo 23.1 mo |
No AEs | |
| Kobayashi et al. (2022) | GBM | 43 | 46.7** | TS | 40 mg/m2 | IV | 22 – 26 h | I/O fiber diffuser for surface irradiation (1.5 cm diameter) |
664 | 27 J/cm2 | PFS MS |
6.3 mo 15.4 mo |
No AEs | |
| Kozlikina et al. (2022) | GBM | CR | 29 | 5-ALA + Ce6 | 20 mg/kg 1 mg/kg |
Oral IV |
4 – 4.5 h 3 – 3.5 h |
I/O fiber | 660 | 60 J/cm2 §§§ | n/a | n/a | ||
| Study Name | Clinical Trial Phase (Study ID) |
Type of Cancer | Drug | Principal Investigator |
|---|---|---|---|---|
| Photodynamic Therapy (PDT) for malignant brain tumor in children | Phase I/II (UMIN000030883) |
Brain Tumor (Pediatric) |
Talaporfin sodium (Leserphyrin) |
Kawamata Takakazu (Tokyo Women’s Medical University Hospital) |
| Clinical Safety Study on 5-Aminolevulinic Acid (5-ALA) in Children and Adolescents With Supratentorial Brain Tumors | Phase II (NCT04738162) |
Brain Tumor (Pediatric) |
5-ALA (Gliolan) |
Walter Stummer (University Hospital, Münster) |
| Stereotactical Photodynamic Therapy With 5-aminolevulinic Acid (Gliolan®) in Recurrent Glioblastoma | Phase II (NCT04469699) |
GBM | 5-ALA (Gliolan) |
Walter Stummer (University Hospital, Münster) |
| PD L 506 for Stereotactic Interstitial Photodynamic Therapy of Newly Diagnosed Supratentorial IDH Wild-type Glioblastoma | Phase II (NCT03897491) |
GBM | 5-ALA (PD L 506) |
Niklas Thon (Klinikum der Universität München) |
| Dose Finding for Intraoperative Photodynamic Therapy of Glioblastoma | Phase II (NCT04391062) |
GBM | 5-ALA (Gliolan) |
Nicholas Reyns (University Hospital, Lille) |
| Study to Evaluate 5-ALA Combined With CV01 Delivery of Ultrasound in Recurrent High Grade Glioma | Phase I (NCT05362409) |
High Grade Glioma | 5-ALA (Gliolan) |
Alpheus Medical (Wash. Univ. St. Louis, Dent Institute, Northwell Health) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





