Introduction
Atopic dermatitis (AD) is a complex, chronic disease characterised by skin inflammation, erythema, dryness and pruritic lesions (1, 2). AD can be highly heterogenous in presentation and is marked by intermittent disease activity, which fluctuates between spontaneous flares and remissions (3-5). The prevalence of AD is currently increasing, affecting up to 20% of children and 10% of adults (6).
Treatment for AD aims to establish long-term control by targeting flares and minimising the frequency and severity of flare episodes (7). Although there is currently no universal strategy for flare management, there are several treatment approaches that emphasise daily emollient use as part of a maintenance treatment plan, with anti-inflammatory therapies, such as topical corticosteroids (TCS) or topical calcineurin inhibitors (TCI), used for diseases flares (7-9).
Dermoflan AD emollient plus cream (Meda Pharma, S.p.A., a Mylan Company; referred to hereafter as EC) is an example of a next-generation emollient plus cream, which specifically targets AD pathophysiology via multiple mechanisms (9, 10). Previous studies have shown that EC can restore epidermal homeostasis and improve skin hydration following clinical remission, indicating that EC could be a valid adjuvant to pharmacological therapy in patients with mild-to-moderate AD (11). In addition, EC has been shown to be effective as maintenance therapy for AD, maintaining regression of flare for up to four months in almost all patients, when used after anti-inflammatory pharmacological therapies such as the TCI pimecrolimus (12). Regular use of EC with topical anti-inflammatory therapies introduced at disease flare to induce remission may therefore be an effective approach for the treatment of patients with mild-to-moderate AD. In this report, we present a case study of a patient with mild-to-moderate AD treated with a complementary approach of EC and pimecrolimus for flare management.
Case presentation
A 24-year-old male patient, previously diagnosed with AD at the age of three years, visited the outpatient clinic at a central hospital after worsening of AD symptoms that were non-responsive to low- and medium-potency TCS. He reported worsening pruritus, erythema, scaling and lichenification, mainly of the face. He was also suffering from disturbed sleep, due to pruritus, and psychological and social impairment. He had a history of atopy (allergic rhinitis and mild asthma) and a body mass index (BMI) of 26.7 kg/m2. The patient was otherwise healthy with no known systemic disease. Regarding family history of other conditions, his father had mild asthma. Prior treatments for AD consisted of regular emollient application, low- and medium-potency TCS and antihistamines. He had also been treated with bilastine and inhaled corticosteroids for allergic rhinitis and asthma, respectively.
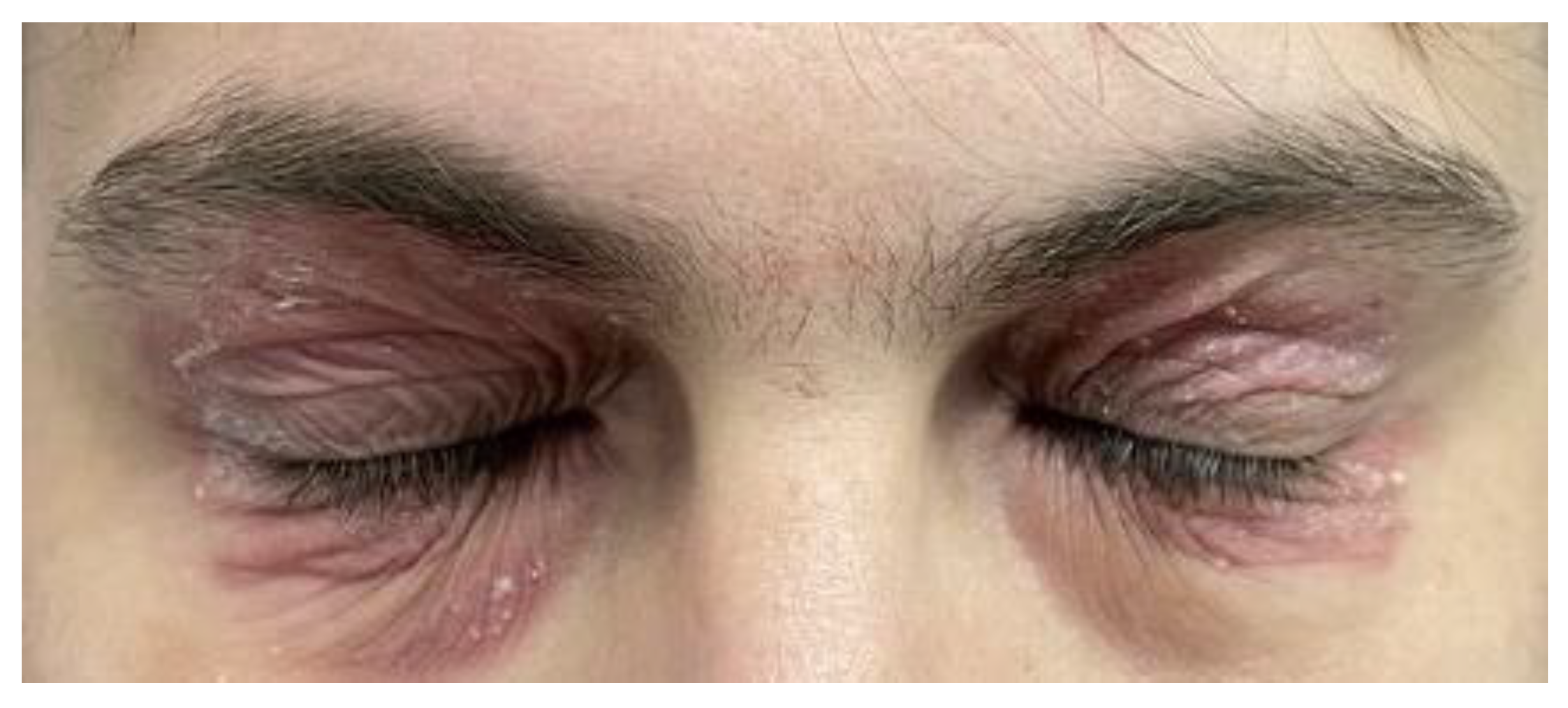
Physical examination findings included moderate lesions localised around the eyes (
Figure 1) and mild lesions affecting the upper limb flexures. The Investigator’s Global Assessment (IGA) score was 3, the Eczema Area and Severity Index (EASI) was 5.5, body surface area (BSA) was 5% and the Pruritus Numerical Rating Scale (Pruritus-NRS) was 7/10, indicating mild-to-moderate AD. Epicutaneous patch testing showed negative results; other allergy tests, such as blood and skin prick tests, were not conducted. In addition, laboratory results showed normal eosinophil count and immunoglobulin E levels of 1760 IU/mL. Diagnosis of AD, mainly periocular AD, was confirmed clinically based on the signs and symptoms.
After the visit to the clinic, the patient was prescribed pimecrolimus twice daily for 12 days and EC twice daily for AD-affected skin. He was advised to continue applying EC indefinitely to AD-affected skin and to re-initiate pimecrolimus after the 12 days if the AD symptoms relapsed or worsened. He also continued to take bilastine 20 mg for allergic rhinitis.
At the 3-week follow-up visit, the AD lesions had improved and were categorised as very mild. The IGA score was 1, the EASI was 0.9, BSA was <1% and the Pruritus-NRS was 1/10. The patient reported that between Day 12 and Week 3, he applied only EC to AD-affected skin and did not use pimecrolimus. From Week 3 onwards, he continued to apply only EC to AD-affected skin.
At the 8-week follow-up visit, the clinical signs of AD and the disease severity evaluation scores remained unchanged. Between Weeks 3 and 8, the patient confirmed that he continued to use only EC.
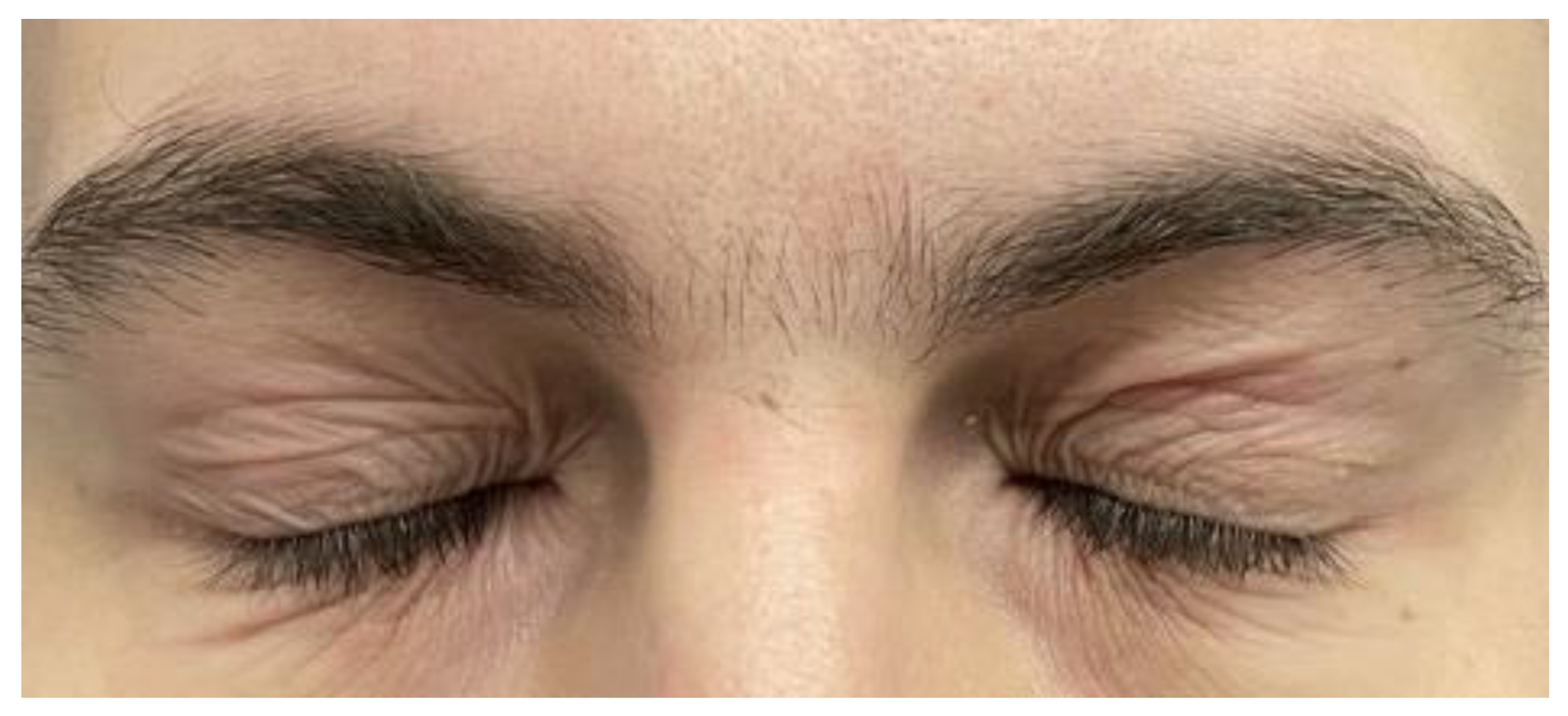
At the 16-week follow-up visit, the severity of AD remained unchanged, similar to Week 3 and Week 8 (
Figure 2). However, the patient reported mild worsening of AD symptoms on his face at Week 12, resulting in the application of pimecrolimus twice a day for five days. Overall, the patient reported a flare-free period between Weeks 3 and 12 and between Weeks 13 and 16. Throughout the 16 weeks, no tolerability issues were reported.
Discussion
Management of AD is often challenging as it needs to address both the intermittent and chronic nature of AD; treatment strategies aim to achieve long-term control by preventing flares and minimising flare episodes (3, 7). This case report demonstrates a complementary approach of daily maintenance EC and intermittent pimecrolimus for flare management of mild-to-moderate AD that is resistant to TCS. Within three weeks of treatment, the mean IGA score was improved from a baseline score of 3 to 1, the EASI decreased from 5.5 to 0.9, BSA reduced from 5% to <1% and the Pruritus-NRS was reduced from 7/10 to 1/10. These conditions remained unchanged between Week 3 and Week 12. Although the patient did experience worsening of AD symptoms at Week 12, the clinical signs and symptoms of AD had improved by Week 16 following re-initiation of pimecrolimus for five days and continuous use of EC.
A previous study that employed a similar synergistic approach, with pimecrolimus for flare treatment and EC for maintenance therapy between flares, maintained AD remission (12). In the open-label, multi-centre study, application of EC after induction of remission of AD symptoms with pimecrolimus therapy maintained the regression of flare for up to four months. The flare-free period experienced by the patient in this case study is broadly in line with those results (flare-free period of three months). However, in contrast with the previous study, the patient required re-initiation of pimecrolimus due to worsening of AD, which reduced the symptoms within three weeks. As no disease severity assessments were obtained between Week 12 and Week 16, the flare severity was unknown and thus the potential benefit of the complementary treatment approach could not be quantified. However, this case study illustrates that a complementary approach using daily administration of maintenance EC and intermittent application of pimecrolimus for flare management was effective for controlling flare in this patient with mild-to-moderate AD.
Basic emollient therapy is crucial for AD treatment (13). Guidelines advise the frequent use of emollients as primary treatment for mild disease, and as part of the treatment plan for moderate-to-severe disease to restore skin barrier and prevent flares (2, 14, 15). The next generation of emollients, known as emollients plus, are vehicle-type substances with additional non-medicated, active ingredients (2, 9, 10). The emollient plus EC, for example, contains key active ingredients that target multiple mechanisms involved in AD pathophysiology: liquorice extract that has anti-inflammatory properties (16); niacinamide, sterols, glycosphingolipid and Linum seed oil for barrier enhancement (9, 17, 18); xylitol and galacto-oligosaccharide for selective antibacterial and prebiotic activity to maintain the skin microbiome (19-21); and laureth-9-polydocanol to reduce itching (22). Evidence from in vitro and clinical trials suggest that EC is able to help restore epidermal homeostasis and improve the skin of patients with mild-to-moderate AD (11). In this case study, the use of maintenance EC and intermittent pimecrolimus was effective for preventing flares between Weeks 0 and 12.
Anti-inflammatory therapies such as TCI or TCS are crucial for long-term flare management in AD (15). Although TCS are the recommended first-line pharmacological treatment for patients with AD who have failed to respond to emollients alone, prolonged exposure has been associated with side effects including skin atrophy, telangiectasia and striae (15, 23). The degree of corticosteroid exposure can also impact the progression of AD disease, including the worsening and spreading of AD symptoms, and the development of new conditions, such as anxiety, depression and food or environmental allergies (23). TCI are corticosteroid-free alternatives to TCS. Importantly, TCI do not have the same risks of skin atrophy, impaired epidermal barrier function or enhanced percutaneous absorption associated with TCS usage (24). Furthermore, it has been demonstrated that TCI exhibit significantly improved efficacy, as measured by physician's global assessment, compared with TCS of varying potencies (25). However, the use of TCI can be associated with a burning sensation (25), which can be mitigated by pre-cooling the tube (26).
Given the risks associated with anti-inflammatory therapies, guidelines recommend corticosteroid-sparing strategies in certain situations (e.g., in patients who have received long-term uninterrupted TCS, in patients with steroid recalcitrance, for use on sensitive skin areas and for steroid-induced atrophy) (15). Emollient therapies have been shown to provide a significant corticosteroid-sparing effect, decreasing the need for TCS (9). Thus, the complementary approach used in this study may be a viable alternative for the long-term management of AD, offering a corticosteroid-sparing approach and reducing the need for topical anti-inflammatory treatment while maintaining efficacy for up to three months. However, prospective clinical trials are required to confirm this hypothesis.
Conclusion
In this case study, a complementary approach using daily administration of maintenance EC and intermittent application of pimecrolimus for flare management was effective for treating mild-to-moderate AD. This approach may be particularly beneficial for patients who exhibit resistance to TCS. Furthermore, implementing EC and pimecrolimus in this manner could limit the need for topical anti-inflammatory treatment. Comparative studies are required to further understand the value of this treatment approach.
Author Contributions
Tiago Torres: Investigation, Writing - Reviewing and Editing
Funding
Medical writing support was funded by Meda Pharma S.p.A., a Viatris company.
Informed Consent Statement
Written informed consent was obtained from the patient.
Acknowledgements
Medical writing support for the development of this manuscript, under the direction of the author, was provided by Keerthi Sivan, MSc, of Ashfield MedComms, an Inizio company, and funded by Meda Pharma S.p.A., a Viatris company.
Conflicts of Interest
Tiago Torres has received research grants and/or consulting fees from AbbVie, Almirall, Amgen, Arena Pharmaceuticals, Biocad, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Eli Lilly, Janssen, LEO Pharma, MSD, Novartis, Pfizer, Samsung Bioepis, Sandoz, Sanofi and Viatris.
References
- Hon KL, Kung JSC, Ng WGG, Leung TF. Emollient treatment of atopic dermatitis: latest evidence and clinical considerations. Drugs Context. 2018, 7, 212530. [Google Scholar] [CrossRef]
- Wollenberg A, Barbarot S, Bieber T, Christen-Zaech S, Deleuran M, Fink-Wagner A, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J Eur Acad Dermatol Venereol. 2018, 32, 657–682. [Google Scholar] [CrossRef] [PubMed]
- Abuabara K, Margolis DJ, Langan SM. The Long-Term Course of Atopic Dermatitis. Dermatol Clin. 2017, 35, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Chovatiya R, Silverberg JI. The Heterogeneity of Atopic Dermatitis. J Drugs Dermatol. 2022, 21, 172–176. [Google Scholar] [CrossRef]
- Sidbury R, Alikhan A, Bercovitch L, Cohen DE, Darr JM, Drucker AM, et al. Guidelines of care for the management of atopic dermatitis in adults with topical therapies. J Am Acad Dermatol. 2023. [Google Scholar]
- Global Atopic Dermatitis Atlas. Global Atopic Dermatitis Atlas 2022 Report. London: International League of Dermatological Societies 2022.
- Girolomoni G, Busa VM. Flare management in atopic dermatitis: from definition to treatment. Ther Adv Chronic Dis. 2022, 13, 20406223211066728. [Google Scholar] [CrossRef]
- Sidbury R, Tom WL, Bergman JN, Cooper KD, Silverman RA, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: Section 4. Prevention of disease flares and use of adjunctive therapies and approaches. J Am Acad Dermatol. 2014, 71, 1218–1233. [Google Scholar] [CrossRef]
- Araviiskaia E, Pincelli C, Sparavigna A, Luger T. The Role of a Novel Generation of Emollients, 'Emollients Plus', in Atopic Dermatitis. Clin Cosmet Investig Dermatol. 2022, 15, 2705–2719. [Google Scholar] [CrossRef]
- Sparavigna A, Tenconi B, Penna L. Efficacy of a novel emollient plus cream in atopic dermatitis: a randomised, vehicle-controlled, double-blind study. J Plastic Pathol Dermatol 2019, 15, 85–93. [Google Scholar]
- Quadri M, Lotti R, Bonzano L, Ciardo S, Guanti MB, Pellacani G, et al. A Novel Multi-Action Emollient Plus Cream Improves Skin Barrier Function in Patients with Atopic Dermatitis: In vitro and Clinical Evidence. Skin Pharmacol Physiol. 2021, 34, 8–18. [Google Scholar] [CrossRef]
- Sparavigna A, Trischitta A. Open multi-centre study on the use of a novel emollient plus cream (EC) for maintaining eczema regression after pimecrolimus therapy in atopic dermatitis. J Plastic Pathol Dermatol. 2020, 16, 3–14. [Google Scholar]
- Wollenberg A, Kinberger M, Arents B, Aszodi N, Avila Valle G, Barbarot S, et al. European guideline (EuroGuiDerm) on atopic eczema - part II: non-systemic treatments and treatment recommendations for special AE patient populations. J Eur Acad Dermatol Venereol. 2022, 36, 1904–1926. [Google Scholar] [CrossRef]
- Maliyar K, Sibbald C, Pope E, Gary Sibbald R. Diagnosis and Management of Atopic Dermatitis: A Review. 2018, 31, 538–550. [CrossRef]
- Eichenfield LF, Tom WL, Berger TG, Krol A, Paller AS, Schwarzenberger K, et al. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014, 71, 116–132. [Google Scholar] [CrossRef] [PubMed]
- Saeedi M, Morteza-Semnani K, Ghoreishi MR. The treatment of atopic dermatitis with licorice gel. J Dermatolog Treat. 2003, 14, 153–157. [Google Scholar] [CrossRef]
- Bissett D. Topical niacinamide and barrier enhancement. Cutis. 2002, 70 (6 Suppl), 8––12; discussion 21-3.
- Kildaci I, Budama-Kilinc Y, Kecel-Gunduz S, Altuntas E. Linseed Oil Nanoemulsions for treatment of Atopic Dermatitis disease: Formulation, characterization, in vitro and in silico evaluations. Journal of Drug Delivery Science and Technology. 2021, 64, 102652. [Google Scholar] [CrossRef]
- Davani-Davari D, Negahdaripour M, Karimzadeh I, Seifan M, Mohkam M, Masoumi SJ, et al. Prebiotics: Definition, Types, Sources, Mechanisms, and Clinical Applications. Foods. 2019, 8.
- Katsuyama M, Ichikawa H, Ogawa S, Ikezawa Z. A novel method to control the balance of skin microflora. Part 1. Attack on biofilm of Staphylococcus aureus without antibiotics. J Dermatol Sci. 2005, 38, 197–205. [Google Scholar] [CrossRef]
- Krutmann, J. Pre- and probiotics for human skin. J Dermatol Sci. 2009, 54, 1–5. [Google Scholar] [CrossRef]
- Freitag G, Hoppner T. Results of a postmarketing drug monitoring survey with a polidocanol-urea preparation for dry, itching skin. Curr Med Res Opin. 1997, 13, 529–537. [Google Scholar] [CrossRef]
- Barta K, Fonacier LS, Hart M, Lio P, Tullos K, Sheary B, et al. Corticosteroid exposure and cumulative effects in patients with eczema: Results from a patient survey. Ann Allergy Asthma Immunol. 2023, 130, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Luger T, Boguniewicz M, Carr W, Cork M, Deleuran M, Eichenfield L, et al. Pimecrolimus in atopic dermatitis: consensus on safety and the need to allow use in infants. Pediatr Allergy Immunol. 2015, 26, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Abedz N, Pawliczak R. Efficacy and safety of topical calcineurin inhibitors for the treatment of atopic dermatitis: meta-analysis of randomized clinical trials. Postepy Dermatol Alergol. 2019, 36, 752–759. [Google Scholar] [CrossRef] [PubMed]
- Al-Khenaizan, S. Practical tip: Precooling topical calcineurin inhibitors tube; reduces burning sensation. Dermatol Online J. 2010, 16, 16. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).