1. Introduction
In the last 10 years, Latin America has substantially increased its contribution to world aquaculture, accounting for 18% of total fish production in 2020. Brazil is as the 8th largest producer of fish in continental waters of the world, reaching 552 thousand tons in 2020 (FAO 2022). Considering national statistical data, Brazilian aquaculture produced 860 thousand tons of fish in 2022 with tilapia (Oreochromis sp.), an exotic species, taking up 64% of Brazilian aquaculture. (Peixe BR 2022).
However, Brazil has other potential native genetic resources for national aquaculture that should be studied as viable options for fish farmers. The production of species from the Serrasalmidae family, such as pacu (Piaractus mesopotamicus), tambaqui (Colossoma macropomum), and some hybrids generated from the cross between these species, has a prominent position in aquaculture in Brazil. Together, these pure species and hybrids account for 38% of Brazilian production, with approximately 288 tons produced in 2019 (Peixe BR 2020).
The success of Neotropical fish from the Serrasalmidae family is not limited to Brazil, and according to Woynárovich (2019), these species have spread to most countries in South and Central America, some countries in the Caribbean, and several countries in Asia, including China, Indonesia, Malaysia, Myanmar, and Vietnam. In China, the production of tambaqui, pacu, pirapitinga (Piaractus brachypomus), tambatinga (Colossoma macropomum × Piaractus brachypomus), and tambacu (Colossoma macropomum × Piaractus mesopotamicus) combined account for 575 tons of fish (FAO FishStatJ 2018).
In regions that are characterized by low temperatures during the autumn and winter seasons, fish farmers cross tambaqui and pacu species in order to combine two important traits: the greater growth potential of tambaqui and the low temperature tolerance of pacu (Fernandes et al. 2018). However, although this strategy is widely used, there is still a lack of studies that prove the advantage of producing hybrid fish over “pure” parental species under different farming conditions (Reis Neto 2020).
In addition, some authors have reported that the use of some potentially fertile hybrids and allochthonous species in fish farming poses a potential risk to the environment (Del Pazo et al. 2021; Bradbeer et al. 2019; Frota et al. 2016; McKelvey et al. 2016; Silva et al. 2009; Alves et al. 2014; Hashimoto et al. 2014). Baggio et al. (2016) highlighted that many problems are caused by the use of hybrids, which due to frequent accidental escapes from fish farms, result in contamination of wild populations, generating a negative impact on pure genetic resources and consequent imbalance of biodiversity that can lead to extinction. In addition, the lack of identification control of breeding stocks causes mistaken mating in fish farms, generating post F1 animals with lower performance potentials than F1 hybrids. On the other hand, Frota et al (2016) also highlight the environmental risks of escapes from non-native pure species that enable antagonistic interactions with native genetic resources. Naturally, the use of hybrids in aquaculture can bring great benefits to the fish farmer, and hybridization is already a consolidated strategy in aquaculture. However, it is important to scientifically verify the advantages of farming hybrids before encouraging them.
Another important factor that must be considered when using hybrids for aquaculture is feed management, since commercial diets are basically formulated by varying the crude protein content according to the feeding habits of the fish, carnivores or omnivores, and sometimes for certain pure species. Commercially, there are no specific formulations for the hybrids, and these animals may respond differently to the nutritional requirements of their parents (Reis Neto et al. 2020).
An informative way of evaluating animal performance is using the growth curve, which is a graphical representation of weight as a function of the age of the individual. Santos et al. (2007) showed that the study of growth through the adjustment of functions that describe the entire life span of an animal can be used to summarize information from a series of data into a small set of interpretable biological parameters. Therefore, it is possible to identify populations or groups of animals that reach their growth potential at a younger age using growth curve parameters. According to Freitas (2007), this information can be obtained by observing parameters K and A of the growth curves, which express the growth velocity and limit or asymptotic weight of the animal, respectively.
In the present study, we evaluated weight as a function of age in four genetic groups, two purebreds and two hybrids, of Neotropical fish from the Serrasalmidae family fed with different feeding programs through nonlinear models. The presence of heterosis was also verified by the final weight and daily weight gain of the animals.
2. Methodology
2.1. Animals and Experimental Conditions
The experiment was conducted in Pariquera-açu (24°43ʹ14ʺ S, 47°52ʹ43″ W), at the Aquaculture Station of the Regional Pole of the Agribusiness Technology Agency of São Paulo, APTA Regional, Brazil.
From a diallel cross performed between pacu (5 males and 5 females) and tambaqui (5 males and 4 females) using the artificial reproduction protocol described by Criscuolo-Urbinati et al. (2012), four genetic groups were generated: pure pacu (female pacu × male pacu), pure tambaqui (female tambaqui × male tambaqui), hybrid tambacu (female tambaqui × male pacu), and hybrid paqui (female pacu × male tambaqui). The animals used in the crosses were previously evaluated using the Multiplex-PCR technique of nuclear (tpm1 and rag2) and mitochondrial (mt-co1 and mt-cyb) genes to confirm that they were from pure parents. More details on the reproduction and evaluation of the parents used to generate the genetic groups have been described by Reis Neto et al. (2020).
We evaluated 30 animals from each genetic group, previously identified with microchips, for 5 months (November 2016 to March 2017). At the beginning of the experiment, the fish were about 9 months old and weighed 186±22.3 g (tambaqui), 141±18.6 g (pacu), 128±16.3 g (tambacu) and 154±18.9 g (paqui). Juveniles were stored in three cages measuring 2 × 2 × 1.2 m with a useful volume of 4.8 m3, with 10 animals from each genetic group per cage. The cages were installed in an excavated pond measuring 600 m² with an average depth of 1.5 m and an average water renewal rate of 25%. To prevent the cages from touching the pond floor, the useful area was determined in the deepest region of the pond where the bottoms of the cages were at least 50 cm from the pond floor.
As previously proposed by Fernandes et al. (2018), different feeding programs were used for each cage. Program 1 (P1) consisted of only one phase, where a diet containing 24% crude protein was offered to fish throughout the experimental period. Feeding program 2 (P2) consisted of two phases: Phase 1 (1st month) and Phase 2 (2–5th month), when the fish received diets with 28% and 24% crude protein (CP), respectively. Feeding program 3 (P3) consisted of three phases: Phase 1 (1st month), Phase 2 (2nd month), and Phase 3 (3–5th month), when the fish received diets with 32%, 28%, and 24% CP, respectively.
All commercial diets were purchased from the same manufacturer, and their respective nutritional compositions were guaranteed by the manufacturer on the product label (
Table 1).
The energy value of the diets was calculated as the sum of the calories supplied by proteins, lipids, and non-nitrogen extract (ENN) multiplied by the Atwater factors (Brasil 2003). The ENN was obtained by subtracting the moisture, protein, ether extract, and mineral matter from the total weight.
Animals were fed twice daily, at 9:00 am and 3:30 pm, until apparent satiety. The cages were monitored daily for the presence of dead fish.
Limnological parameters were measured weekly between the two lines of the cages using a multiparameter water analyzer. (HI9146-04: Hanna Instruments, Brasil). Mean (± standard deviation) of dissolved oxygen values at 10 cm, 70 cm, and 150 cm depths were 7.9 ± 1.9 mg/L, 7.7 ± 1.7 mg/L, and 6.2 ± 1 .7 mg/L, respectively. At the same depths, the pH values were 6.15 ± 1.1, 5.9 ± 0.9, and 6.0 ± 0.7, respectively. The water temperature was measured daily from a depth of 50 cm, and ranged from 25.2 °C to 29.8 °C, with an average of 27.4 ± 3.8 °C. All recorded limnological parameters were within the ranges established by CONAMA (Resolution 357/05) for the farming of aquatic organisms in continental waters (Conama, 2005).
During the experimental period, all animals were weighed six times at 1, 30, 67, 96, 128, and 158 days of the experiment. Before weighing, the fish were anesthetized in a tank with water and eugenol at a concentration of 100 mg/L (Delbon & Ranzani Paiva 2012), and later identified and weighed with a precision balance. Average daily weight gain (DWG) was calculated for each individual using the following equation (Reis Neto et al. 2012):
All handling of animals was performed according to the standards established by the Ethics Committee on Animal Use of the Faculty of Agrarian and Veterinary Sciences of UNESP (CEUA, 12 April 2015, Protocol 23291/15).
2.2. Statistical analysis
Data obtained from the final weighing along with the average daily weight gain (DWG) were analyzed using a hierarchical model that considered the effects of the mating system (pure breed or cross breed) and the genetic groups as follows: tambaqui and pacu nested in the “purebreed” and tambacu and paqui nested in the “crossbreed” system. For these analyses, the effect of the nutritional plan was included as a block in the model and the initial weight of the fish was included as a covariate.
Heterosis was calculated for final weight and DWG from the adjusted averages, using the following formula:
H = heterosis;
= average of hybrids
= average of purebreeds.
Compiled data from all weighings were analyzed in a mixed linear model, with the fixed effects of food programs, genetic groups, and age in a 3 × 4 × 6 factorial scheme (nutritional plans × genetic groups × age), and, as a strategy to isolate individual effects, such as behavioral dominance, we included the random effect of each fish in the model.
When significant differences were detected between different groups or food programs, Tukey’s test was used for multiple comparisons of the means. When a significant effect was detected for the interaction between the age factor and genetic groups or food programs, a logistic model (1) was used to fit the growth curves according to the following reparameterization (2):
Parameter “A” represents the asymptotic or adult weight of the animal. The parameter “K” represents the maturity rate or the relationship between the relative growth rate and the adult weight of the animal; thus, a higher a value of K denotes a younger animal. “B” is a constant of the equation and “t” is the time in days.
When the logistic model did not fit the data set, the exponential model was used:
In this case, “A” represents the estimated initial weight, “K” the relative growth rate, and “t” is time.
The parameters of the logistic or exponential models were tested between the food programs and genetic groups using the likelihood ratio test.
All assumptions were evaluated, and when violated, logarithmic transformation was used. The significance level adopted was 5%.
All analyses were performed using R software version 4.2.3 (R Core Team 2010).
3. Results
No mortality was observed during the experimental period. The hierarchical model showed the superiority (p<0.05) of the pure groups in relation to the hybrids for final weight and DWG, resulting in negative heterosis for both traits (
Table 2). Still considering the hierarchical model, there was a difference between the pure groups only for the daily weight gain, with pacu (3.5 ± 0.98 g) presenting a higher average (p<0.05) than tambaqui (3.12 ± 1.1 g). Among the hybrid groups, there were no significant differences (p>0.05) in any trait.
The mixed model showed a significant effect (p<0.05) for the food programs and genetic groups, but not (p>0.05) for the interaction between these factors, demonstrating that the program feeding of pure and hybrid fish may be the same (
Table 3).
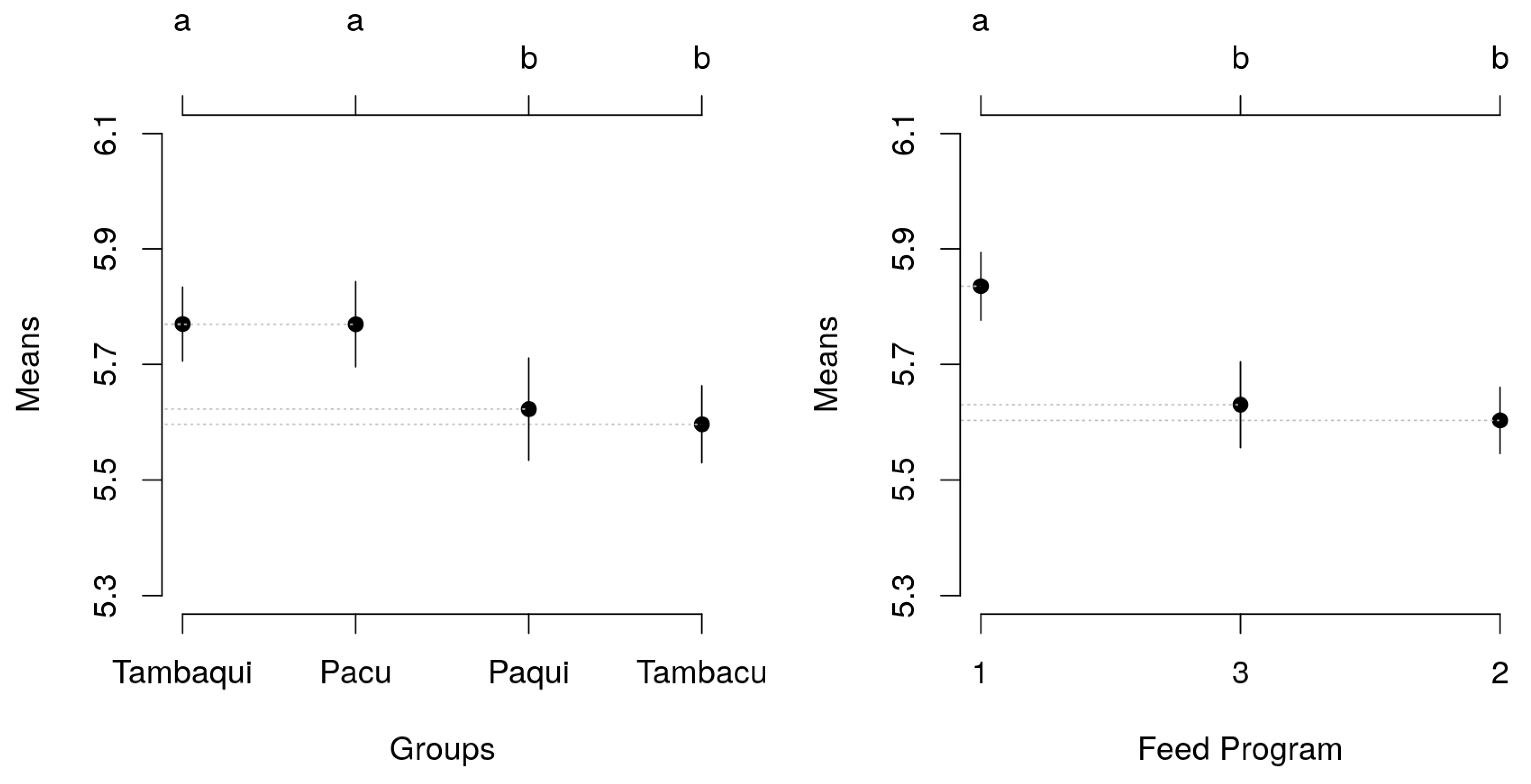
The comparison test applied to the average weight adjusted by the mixed model revealed that the pure animals differed (p<0.05) from the hybrids, but there was no difference (p>0.05) between the animals of the two pure groups (pacu × tambaqui) or between the animals of the two hybrid groups (tambacu × paqui). Regarding the feeding programs, the test of means indicated superiority (p<0.05) of the weight of the animals fed with Program 1 (
Figure 1).
Based on the significant interactions revealed by the mixed model (
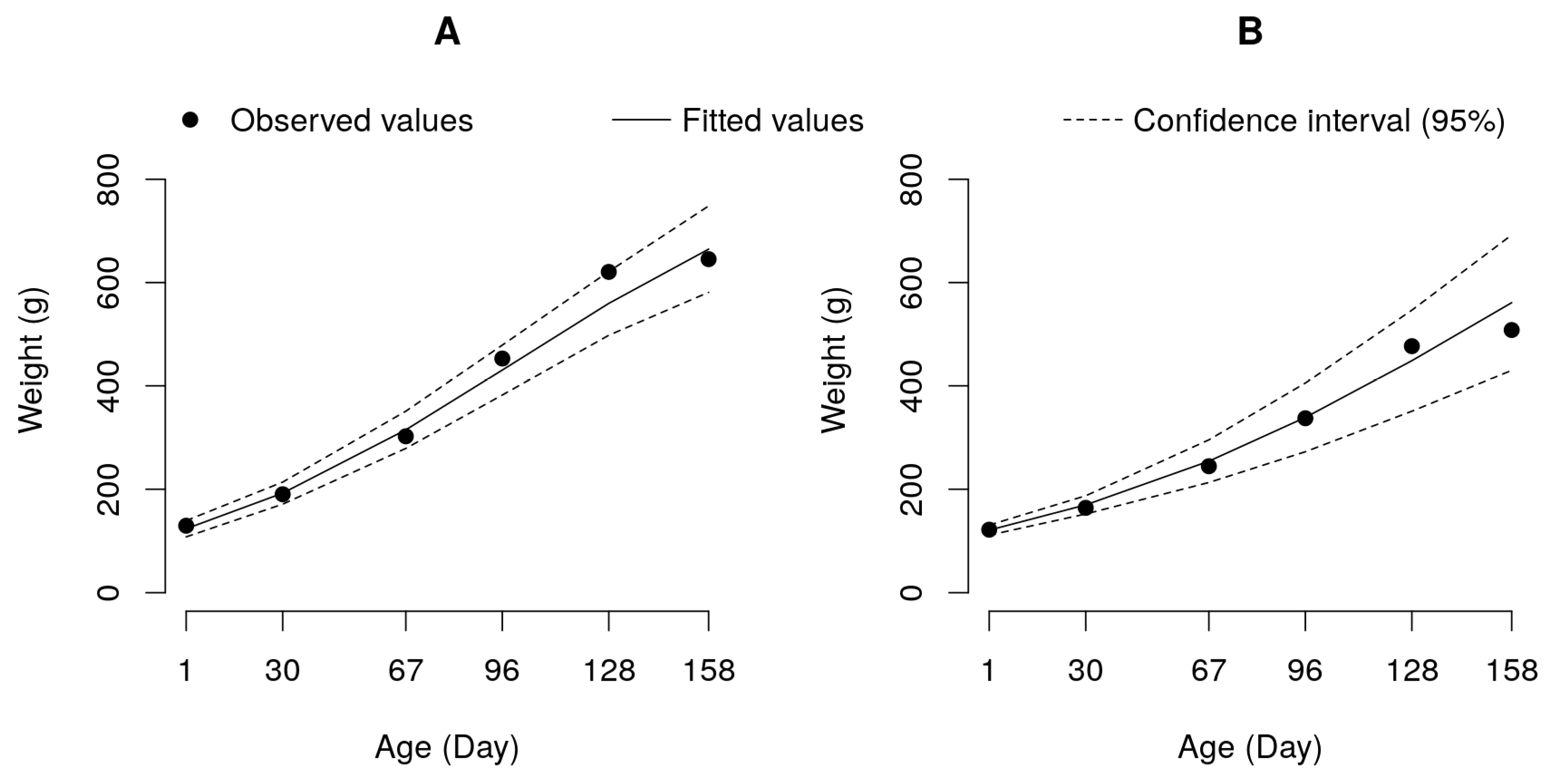
Table 3), the weight versus age curves were adjusted for each genetic group and feeding program. The logistic model was adjusted to the data of the pure pacu and the tambacu hybrid, which indicated a deceleration in the growth of these animals at the end of the experiment (
Figure 2).
The likelihood test applied to the parameters of the logistic model showed that pacu had a higher relative growth rate (parameter K = 0.0185) (p<0.05) than tambacu (k = 0.0134) (
Table 4), justifying the higher weight and daily weight gain in this group (
Table 2). In contrast, the asymptotic or estimated maximum weight (Parameter A) was higher (p<0.05) for the tambacu group (1137.12 g) than for pacu (889.12 g), showing that despite the slower growth, the hybrid group had a greater potential for final weight than the pure group (
Table 4 and
Figure 2).
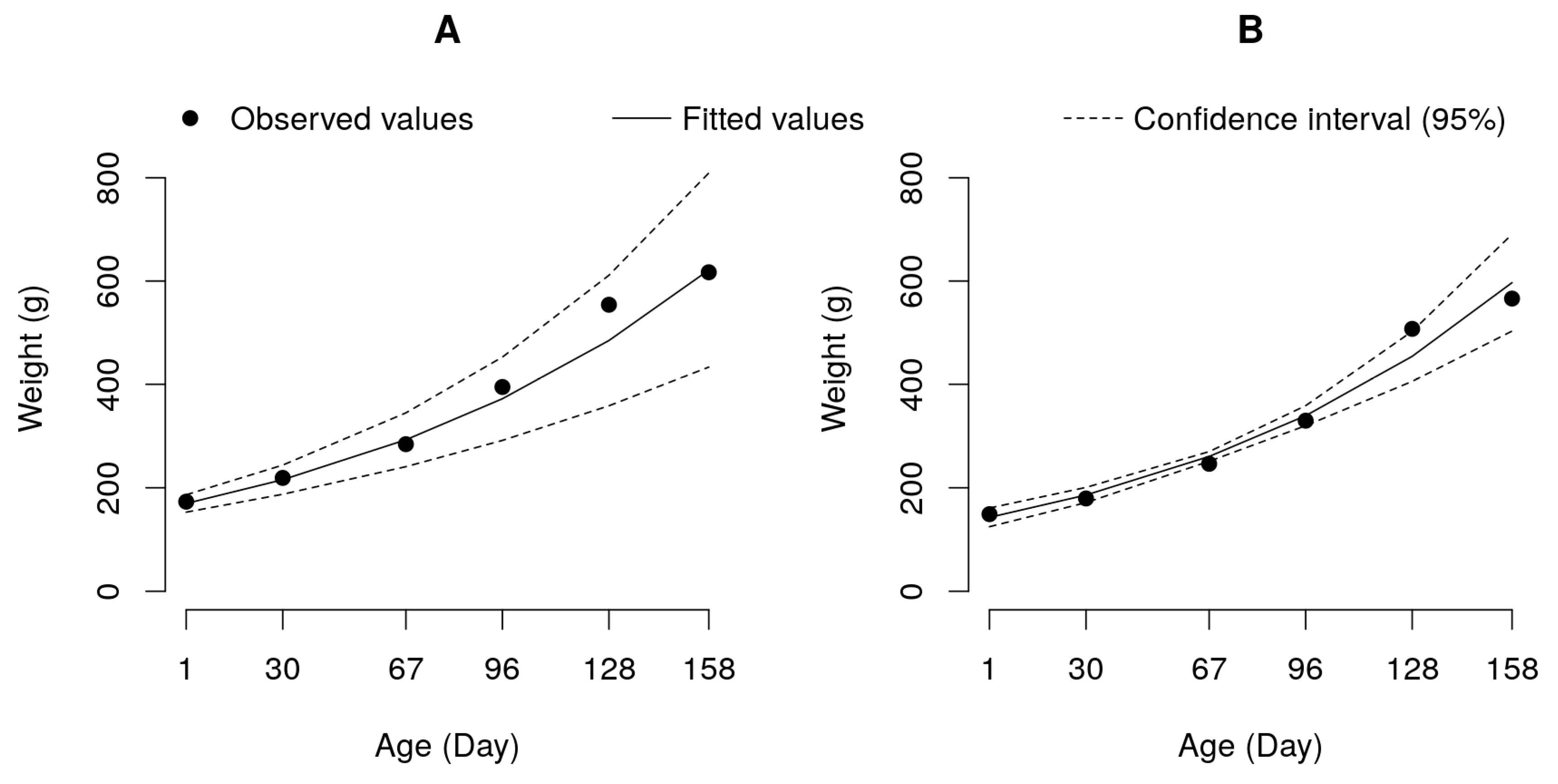
The growth of the pure tambaqui and hybrid paqui groups was better explained by the exponential model, suggesting that until the end of the experimental period, the animals in these groups still had accelerated growth (
Figure 3).
The exponential model has only two parameters: one describing the growth rate (Parameter K) corresponding to the same parameter of the logistic model, and the other describing the estimated initial weight (Parameter A). The K parameters of the paqui (0.0091) and tambaqui (0.0083) groups were similar (p>0.05), while the A parameter of the tambaqui group (168.28 g) was higher (p<0.05) than that of the paqui group (141.53 g) (
Table 5 and
Figure 3). For these two groups, it can be suggested that the estimated initial weight explains the better final performance of the pure group than that of the hybrid (
Table 2).
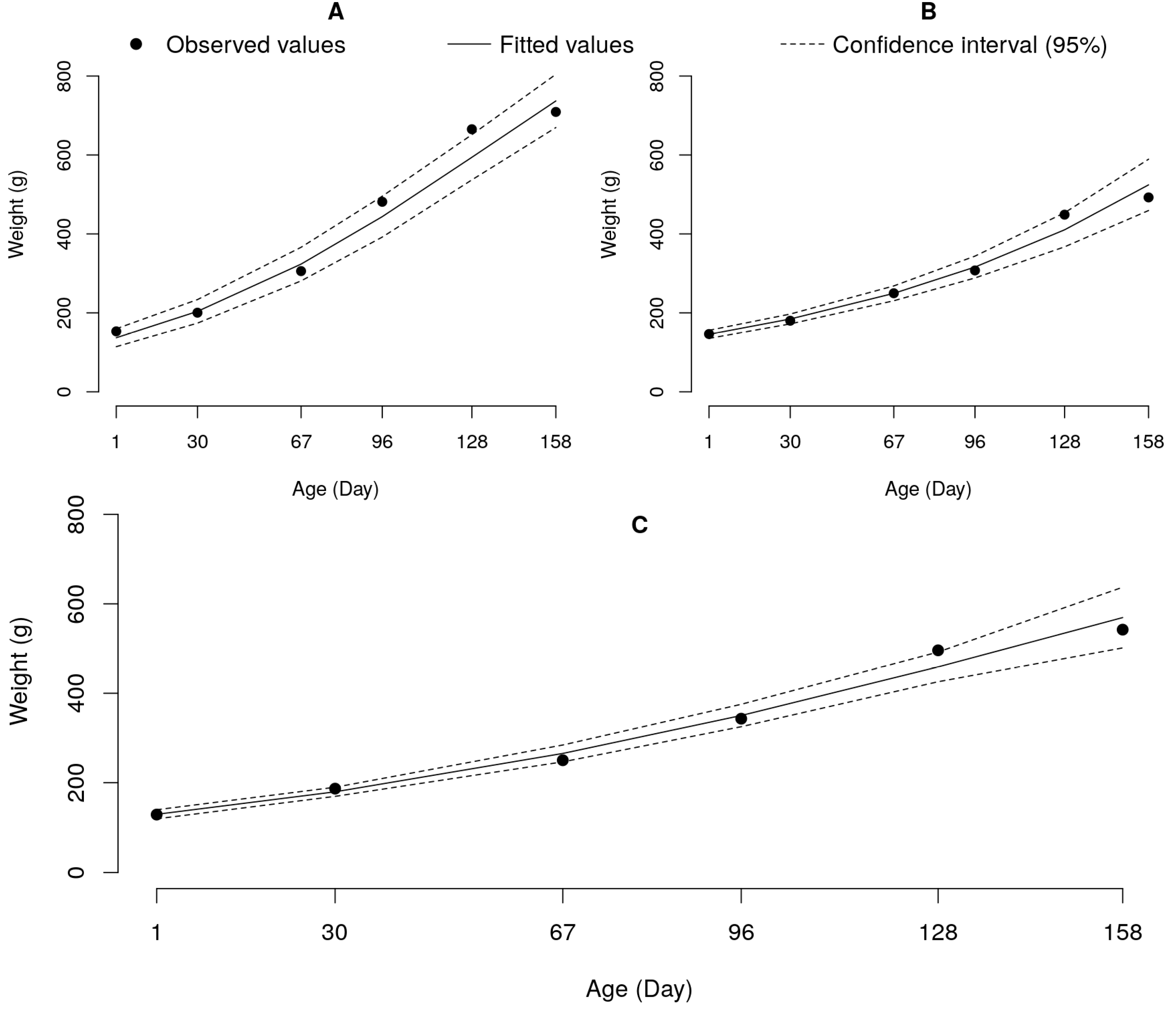
Considering the feeding programs, the logistic model was adjusted for the weight data of the animals fed Programs 1 and 2 (
Figure 4).
Animals fed the same commercial diet (P1) throughout the experimental period had a higher (p<0.05) velocity (K=0.0159) and higher (p<0.05) potential (A=1214.08) of growth than animals subjected to the program with one stratification (P2) of diets by age (K=0.0131; A=1137.42), suggesting that the higher protein diet (28% CP) offered to the animals during the first month of the experiment had no positive effect on growth (
Table 6 and
Figure 4).
The exponential model best described the growth of animals subjected to Program 3, which presented an estimated growth rate (Parameter K) and initial weight (Parameter A) of 0.0082 and 144.3, respectively (
Table 7). This may be an important result, since the exponential model indicates that at the end of the experiment, the animals continued to grow rapidly; thus, the stratification of diets according to the age of the animals in Program 3 may indicate a positive response for the growth of the evaluated genetic groups.
4. Discussion
Some authors have previously compared pure and hybrid fish from the Serrasalmidae family. Unlike the results found in the present study, Reis Neto et al. (2012) observed a considerable superiority for the final weight of the tambacu hybrid (1273.06 g) compared to pure animals of the pacu species (907.6 g), while Costa et al. (2018) determined the highest general combining ability for the final weight occurred with the pure tambaqui group when they evaluated the diallel cross between pacu, tambaqui, and pirapitinga (Piaractus brachypomus) species. In contrast, in another study by Reis Neto et al. (2020), pure pacu fish had a final weight (847.1 g) higher than that of tambacu hybrids (735.1 g).
An important difference between the cited studies, which can explain the divergence of results, is the farm system in which the animals were evaluated. Reis Neto et al. (2012) and Costa et al. (2018) carried out their experiments in ponds and a recirculation system respectively, while Reis Neto et al. (2020), as in the present work, evaluated the animals in cages, which could explain the similar results.
Other important factors that must be considered when interpreting the final weight results are the evaluation period. In the work of Reis Neto et al. (2012) and Costa et al. (2018), the animals reached close to the market weight (1500 g), whereas Reis Neto et al. (2020) did not evaluate the final period of growth of the animals when the growth potential of each genetic group could be determined. This highlights the importance of studying a growth curve that estimates not only the potential but also the speed of animal growth.
Mourad et al. (2018) compared the growth curves of pacu and tambacu by using a logistics model. In that study, the authors reported the superiority of the hybrid with a higher asymptotic weight (Parameter A) and the same relative growth rate (Parameter K) over pacu. The results presented in
Table 3 show that the same hybrid group also presented a higher asymptotic weight than the pacu, showing a greater growth potential; however, for the relative growth rate, the pure group was superior to the hybrid, which indicates greater early performing by the pacu in cages, since Mourad et al. (2018) evaluated the animals in ponds.
In practical terms, the parameter “K” of the logistic curve demonstrates how quickly the animals reach adult or asymptotic weight (Feitas 2005); thus, in the evaluation conditions the pacu group would be interesting for a fish industry that aims to process smaller animals. However, if the industry prefers to process larger fish, pacu is no longer a preferred option, because based on the asymptotic weight described by Parameter A of the logistic model (889.12 g), the animals of this group have decelerated growth, which reduces performance for weight gain. However, the tambacu hybrid group is a stronger alternative if the objective is to process larger fish, since the fish in this group have a higher asymptotic weight (1137.12 g); that is, the growth of tambacu remains accelerated until this weight.
With the data obtained for the two other genetic groups, tambaqui and paqui, the same type of inference on slaughter and processing weight was not possible, since the adjusted growth model was exponential (
Figure 3). The animals of these groups should be evaluated for a longer period of time or constant high temperatures to verify their growth potential.
In the study carried out by Mourad et al. (2018), the logistic model was adjusted for these two genetic groups and the pure tambaqui group showed a much higher growth potential (A = 1056.82 g) than the paqui hybrid (A = 797.82 g), whereas the growth rate was similar between groups (K = 0.034 for tambaqui and K = 0.033 for paqui). Based on these results, the authors indicated a better growth performance of tambaqui in relation to paqui.
Costa et al. (2018) also concluded that the tambaqui group was genetically superior to the other three genetic groups (pacu, tambacu, and paqui), as was observed by Silva et al. (2022) in a study comparing tambaqui with tambacu and another hybrid (Tambatinga) produced by species of the Serrasalmidae family (
Colossoma macropomum ×
Piaractus brachypomus). In the present work, Tukey test applied to the averages generated by the mixed model showed better performance for tambaqui than paqui and tambacu (
Figure 1). Similarly, although the hierarchical model used in this work does not allow for the comparison between groups of different mating systems, pure or crossed, the results in
Table 2 show a final weight of tambaqui (656.7 g) that is 10.2% higher than that of paqui (589.6 g) and 16.8% higher than that of tambacu (545.9 g). As previously mentioned, our experimental conditions (evaluation time) did not allow us to confirm the greater growth potential of the tambaqui group observed in other studies.
In general, the results discussed show the importance of studying growth curves. In a more superficial analysis, based only on the final performance of the animals (
Table 2), the pacu and tambaqui groups could be incorrectly recommended without considering the appropriate objectives. The growth curves provided relevant information that increased the chances of success in defining which genetic group would be most suitable according to the interests of the industry or fish farmer, in addition to revealing the need for a broader assessment of some groups to generate more consistent information.
The animals subjected to Feeding Programs 1 and 2 reached a final weight sufficient to estimate the parameters of the logistics model (Figure 5). Program 1 resulted in fish with higher potential (A = 1214.08 g) and faster (K = 0.0159) growth than Program 2 (A = 1137.42 g; K = 0.0131) (
Table 5). This result was unexpected, since the higher initial intake of protein offered by Program 2 was anticipated to have a positive effect on the growth of the animals. The Tukey test was used to compare the averages generated by the mixed model and revealed better performance for animals subjected to Feeding Program 1 (
Figure 4).
In the study by Reis Neto et al. (2020), the interaction between the same feeding programs and pacu and tambacu genetic groups was significant for final weight. Those authors observed that the higher protein supply offered to the tambacu hybrid in the early stages of life resulted in a positive effect on growth, whereas this effect was not observed in the pure pacu group.
Some hypotheses can be suggested to explain the results of our study on food programs. First, the 30-day period in which the animals were subjected to Food Program 2 with a greater amount of CP was not sufficient to generate a positive effect on the growth of these animals. Another hypothesis is that reducing the amount of protein offered to fish after 30 days of the experiment may have resulted in a negative effect on growth; thus, animals that started the experiment with a lower intake of CP did not suffer this negative effect. In addition, there may still be differences in the quality of the diets, even though they were from the same manufacturer. However, none of these hypotheses can be confirmed using the data obtained in this experiment.
Animals fed Food Program 3 did not reach a final weight sufficient to adjust the logistic growth model (
Figure 4). Thus, it was not possible to estimate the growth potential of these animals, which could be higher or lower than the growth potential of the animals subjected to Programs 1 and 2. The Tukey test showed a better performance of fish fed Program 1 (
Figure 2); however, in a longer evaluation of the experiment a positive response on the growth of animals from Program 3 that received a greater supply of CP in both initial months of the experiment could be observed. Reis Neto et al. (2020), evaluating the same feeding programs, observed superior performance of fish fed feeding programs with higher protein intake at the beginning of the experiment.
In addition to comparing the genetic groups, growth curves proved to be useful for the evaluation of feeding programs. In a less in-depth evaluation, Program 1 was more effective because it resulted in better performing animals; however, a broader evaluation is still needed before Program 3 can be discarded.
5. Conclusions
The short evaluation period did not allow to generate more informative parameters regarding the growth of the tambaqui and paqui genetic groups. The same occurred for animals fed the program that offered a greater initial intake of protein for the animals. Thus, it was not possible to compare the growth potential of these treatments with that of other treatments.
Among the other two genetic groups evaluated, in the evaluation conditions pacu proved to be a precocious species, since the animals in this group reached their weight at inflection, in cages, more quickly than tambacu. However, despite growing more slowly, the tambacu hybrid could reach a higher final weight without slowing down its growth. This information is useful for fish farmers and the fish industry when choosing one of these two genetic groups.
Among the feeding programs, animals that received less protein over the entire experiment showed a greater potential for growth; however, a longer evaluation is important to verify if the initial intake of this nutrient has no actual positive effect on fish growth.
Declaration of Competing Interest
No potential conflict of interest was reported by the authors.
Author Contributions
RVRN project coordination and manuscript writing; AFL coordination and participation in the growth experimente; DTH production of pure and hybrid genetic groups and molecular assessments of certification of groups; IBA data analysis; GRL and WRG data collection and manuscript writing.
References
- ALVES, A. L.; VARELA, E. S.; MORO, G. V.; KIRSCHNIK, L. N. G. 2014. Riscos genéticos da produção de híbridos de peixes nativos. Embrapa Pesca e Aquicultura-Documentos (INFOTECA-E). https://ainfo.cnptia.embrapa.br/digital/bitstream/item/131431/1/cnpasadoc3.pdf.
- BAGGIO, R. A.; MORETTI, C. B.; BIALETZKI, A.; BOEGER W., A. 2016. Hybrids between Pseudoplatystoma corruscans and P. reticulatum (Siluriformes: Pimelodidae) previously reported in the Upper Paraná River are likely escapes from aquaculture farms: evidence from microsatellite markers. Zoologia (Curitiba), v. 33, n. 2. [CrossRef]
- BRADBEER, S.J.; HARRINGTON, J.; WATSON, H.; et al. 2019. Limited hybridization between introduced and Critically Endangered indigenous tilapia fishes in northern Tanzania. Hydrobiologia, v. 832, n. 1, p. 257-268. [CrossRef]
- BRASIL. Resolução RDC ANVISA/MS nº. 360, de 23 de dezembro de 2003. 2003. Regulamento Técnico sobre Rotulagem Nutricional de Alimentos Embalados. Diário Oficial da União, Brasília, DF, 26 dez. Seção 1.
- CONAMA, 2005. CONAMA - National Environment Council. Resolution nº 357, of March 17 Ministry of the environment (2005) 23p.
- COSTA, A.C.; BOTELHO, H.A.; GOMES, R.C.D.S.; CAMPOS, S.A.S.; REIS NETO, R.V.; BALESTRE, M.; PREDO, F.D.; HASHIMOTO, D.T.; MARTINS, D.G.; PORTO-FOREST, F.; FREITAS, R.T.F. 2018. General and specific combining ability in Serrasalmidae Aquaculture Research., 00 pp. 1-. [CrossRef]
- URBINATI, E.C.; KURADOMI, R.Y.; BATLOUNI, S.R. 2012. The administration of exogenous prostaglandin may improve ovulation in pacu (Piaractus mesopotamicus). Theriogenology, 78, 2087 – 2094.
- DEL PAZO, F.; SÁNCHEZ, S.; POSNER, V.; SCIARA, A. A.; ARRANZ, S. E.; VILLANOVA, G. V. 2021. Genetic diversity and structure of the commercially important native fish pacu (Piaractus mesopotamicus) from cultured and wild fish populations: relevance for broodstock management. Aquaculture International, v. 29, n. 1, p. 289-305.
- DELBON, M.C.; RANZANI-PAIVA, M.J.T. 2012. Eugenol in tilapia juvenile: concentrations and successive administrations. Boletim do Instituto de Pesca, vol. 38, no. 1, pp. 43-52.
- FAO. 2022. The State of World Fisheries and Aquaculture 2022. Towards Blue Transformation. Rome. [CrossRef]
- FAO. 2018. The State of World Fisheries and Aquaculture 2020. Sustainability in action. Rome. [CrossRef]
- FERNANDES, E.M.; ALMEIDA, L.C.F.; HASHIMOTO, D.T.; LATTANZI, G.R.; GERVAZ, W.R.; LEONARDO, A.F.; REIS NETO, R.V. (2018) Survival of purebred and hybrid Serrasalmidae under low water temperature conditions. Aquaculture, v. 497, p. 97-102.
- FREITAS, A. R. 2005. Curvas de Crescimento na Produção Animal. Revista Brasileira de Zootecnia 34, 786–795. [CrossRef]
- FREITAS, A. R. 2007. Estimativas de curvas de crescimento na produção animal. Embrapa Pecuária Sudeste-Documentos (INFOTECA-E). https://www.embrapa.br/busca-de-publicacoes/-/publicacao/48050/estimativas-de-curvas-de-crescimento-na-producao-animal.
- FROTA, A.; DEPRÁ, G.C.; PETENUCCI, L.M.; GRAÇA, W.J. 2016. Inventory of the fish fauna from Ivaí River basin, Paraná State, Brazil. Biota Neotropica. 16(3): e20150151. [CrossRef]
- HASHIMOTO, DT; SENHORINI, JA; FORESTI, F; MARTÍNEZ, P; PORTO-FORESTI, F. 2014. Genetic identification of F1 and Post-F1 Serrasalmid juvenile hybrids in Brazilian aquaculture. PLoS: e89902. https://doirg.ez87.periodicos.capes.gov.br/10.1371/journal.pone.0089902.
- MCKELVEY, K.; YOUNG, M.; WILCOX, T.; DANIEL, M.; BINGHAM, K.; PILGRIM, M. 2016. Patterns of hybridization among cutthroat trout and rainbow trout in northern Rocky Mountain streams. Ecology and Evolution, v. 6, n. 3, p. 688-706. https://doi-org.ez87.periodicos.capes.gov.br/10.1002/ece3.1887.
- MOURAD, N. M. N.; COSTA, A. C.; FREITAS, R. T. F; SERAFINI, M. A.; REIS NETO, R.V.; FELIZARDO V., O. (2018) Weight and morphometric growth of Pacu (Piaractus mesopotamicus), Tambaqui (Colossoma macropumum) and their hybrids from spring to winter. Pesquisa Veterinária Brasileira (Online), v. 38, p. 544-550.
- PEIXE, B. R. (2023) Anuário Brasileiro Da Piscicutura PEIXE BR, 2023, 4. https://www.peixebr.com.br/anuario/.
- PEIXE, B. R. (2020) Anuário Brasileiro Da Piscicutura PEIXE BR, 2020, 1. https://www.peixebr.com.br/anuario/.
- R DEVELOPMENT CORE TEAM. 2010. R: A Language and Environment for Statistical Computing, Vienna, Austria R Foundation for Statistical Computing ISBN 3-900051-07-0.
- REIS NETO, R. V.; HASHIMOTO, D. T.; CORRÊA, C. F.; ENKE, D. B. S.; GERVAZ, W. R.; LATTANZI, G. R. 2020. Performance of tambacu hybrid (♂ Piaractus mesopotamicus x♀ Colossoma macropomum) and its parentais pacu (Piaractus mesopotamicus) evaluated in cages under different feeding programmes. Aquaculture Reports, v. 17, p. 100355.
- REIS NETO, R.V.; SERAFINI, M.A.; FREITAS, R.T.F.; ALLAMAN, I.B.; MOURAD, N.M.N.; LAGO, A.A. 2012. Performance and carcass traits in the diallel crossing of pacu and tambaqui. Revista Brasileira de Zootecnia / Brazilian Journal of Animal Science, v. 41, p. 2390-2395.
- SANTOS, V. B.; FREITAS, R. T. F.; SILVA, F. F.; FREATO, T. A. 2007. Avaliação de curvas de crescimento morfométrico de linhagens de tilápia do Nilo (Oreochromis niloticus). Ciência e Agrotecnologia, v. 31, n. 5, p. 1486-1492.
- SILVA, S.S.D.; DE SILVA, S.S.; NGUYEN, T.T.T.; TURCHINI, G.M.; AMARASINGHE, U.S.; ABERY, N.W. 2009. Alien species in aquaculture and biodiversity: a paradox in food production. AMBIO 38:24–28. https://doi-org.ez87.periodicos.capes.gov.br/10.1579/0044-7447-38.1.24.
- SILVA, A. C. C.; CORRÊA FILHO, R. A. C.; FORNARI, D. C.; ABREU, J. A.; BIGNARDI, A. B.; SEVERINO, M. F. G.; AMORIM, L. F. S.; ALBUQUERQUE, L. V.; CARNEIRO, I. L.; POVH, J. A. 2022. Production of tambaqui and of the tambatinga and tambacu hybrids: Performance, morphometric traits, and body yield. Aquaculture, p. 738107.
- WOYNÁROVICH, A.; VAN ANROOY, R. 2019. Field guide to the culture of tambaqui (Colossoma macropomum, Cuvier, 1816). FAO Fisheries and Aquaculture Technical Paper, n. 624, p. I-121.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).