1. Introduction
Acne vulgaris is a very common skin disorder and its prevalence is still increasing. The first symptoms usually occur at the age of 11-12 years old and eventually up to 85% of adolescents are affected. Acne vulgaris is a growing problem in developed country and is rare in developing country. The reason for the lower incidence of acne lesions in patients from less affluent countries is a diet containing less milk, dairy products and carbohydrates[
1]. Western diets, which contain food with high glycemic index, have a significant impact on disturbances in glucose and insulin serum levels and is associated with the development of insulin resistance (IR).
In severe cases, acne may have impact on skin condition in adulthood, especially when severe lesions do not heal properly, leading to discolorations and scars. Amount of adult patients with acne vulgaris is still increasing. Moreover, both acne and its complications may reduce self-confidence and induce mental health problems [
2].It is worth highlighting that the occurrence of extensive acne lesions affects the quality of life and is a factor influences psychosociological health. Correlation between psychosocial disorder and acne vulgaris is more significant problem among women than in men population [
3].
The development of acne lesions is strongly associated with metabolic and hormonal disorders. Acne vulgaris may be induced in the course of disorders characterized by abnormal levels not only of androgens, estrogens and progesterone, but also insulin and insulin-like growth factor-1 [
4,
5]. Acne vulgaris may be regarded as a civilization disease, likewise IR. Skin often reflects internal health, therefore acne formation may be associated with IR. IR is a condition characterized by the failure of insulin to provide the proper glucose transport into the tissues, which results in the development of hyperglycemia and hyperinsulinemia[
2].
The recently growing interest concerning a possible relationship between these diseases has been observed, although, thus far little data is available. What is already clear is that insulin serum levels were observed to be elevated in both disorders, and both acne and IR share the signal transduction pathways, including insulin-like growth factor – 1 (IGF-1) and mammalian target of rapamycin kinase 1 (mTORC1)[
6]. Furthermore, both acne and IR are observed in the course of syndromes such as HAIRAN -hyperandrogenism (HA), IR and acanthosis nigricans (AN) or polycystic ovary syndrome (PCOS)[
6]. Several studies have revealed noticeably higher insulin serum levels and HOMA values among acne patients compared to healthy controls, and therefore point to importance of considering IR to be a causative factor in acne formation. However, yet, no research was conducted in a Polish population and more data is required in order to include IR evaluation in standard acne diagnostics and treatment options.
In this project, we aimed to investigate IR in patients with acne vulgaris and compare our results with the data from different papers included in our systematic review.
2. Materials and Methods
2.1. Study design and participant recruitment
The study was conducted in the outpatient department of Dermatology and Venerology of Poznań University of Medical Sciences in Poland.
41 patients diagnosed with acne vulgaris and 47 controls were enrolled in the study. Out of the 41 patients 38 were female (93%) and 3 were male (7%). Their mean age was 30.63±10.57 and their mean BMI was 24.57±3.867. The control group comprised 47 people of whom 24 were female (51%) and 23 were male (49%). Their mean age was 25.87 ± 3.9 and their mean BMI was 22.9 ±2.53. Informed consent was received from each participant. The inclusion criteria for both groups were male and female patients aged 18-65 suffering from acne vulgaris. The inclusion criteria for study group was the presence of acne vulgaris, whereas the exclusion criteria in the study group were the presence of any hormonal disorder, including polycystic ovary syndrome, any thyroid dysfunction, Cushing syndrome, congenital adrenal hyperplasia, diabetes mellitus (or family history of DM), moreover any hormonal treatment, including hormonal contraceptive therapy or treatment with drugs that may decrease insulin sensitivity, including antipsychotic drugs e.g. olanzapine, clozapine, risperidone or quetiapine, statins and chronic treatment with niacin ( vitamin B3)[
7].The exclusion criterion in the control group was the presence of any chronic disease, including the acne vulgaris or any chronic treatment. The exclusion criteria in both groups was a high intake of high glycemic-load carbohydrates, milk and dairy products.
Patients were asked to complete a questionnaire, following which a physical examination was performed. The severity of the patients’ acne was evaluated using the Investigator’s Global Assessment Scale. A physical examination was performed in the control group and included measurements of height, weight, waist and hip circumference, heart rate and blood pressure.
The blood samples from the peripheral venous blood were collected after overnight fasting. The glucose serum level was evaluated in the central hospital laboratory. Insulin serum level was evaluated using the enzymatic Elisa DRG method. The normal ranges for these parameters were as follows: glucose 70-99, insulin 5-25 µIU/mL. The homeostasis model assessment of IR (HOMA-IR) was calculated using the formula: fasting insulin serum level (mU/ml) × fasting glucose serum level (mmol/l)/22.5. According to the criteria established for a Polish population by Szurkowska M. et al. in 2005, values over 2.1 indicated the presence of IR [
8]. The value was evaluated as the third quartile of HOMA-IR values calculated for the healthy population with BMI<25 kg/m2.
2.2. Analysis
Statistical analysis was performed using IBM SPSS Statistics 29.0.0.0. For continuous data, normal distribution was evaluated using the Shapiro-Wilk test and variance equality using Fisher-Snedecor test. For continuous data with a normal distribution and equal variances, the unpaired t-test was performed to compare the groups, when the variances were different the Cochran-Cox correction of the t-test, and when both assumptions were not met the Mann-Whitney test. A comparison of nominal data was made using the chi-square test. All numeric variables are presented as the mean ± standard deviation (SD) and nominal as frequency and percentages of each category. A p-value <0,05 was accepted as indicating statistical significance.
2.3. Database search for systematic review
The aim of our systematic review was to analyse and compare our results with other original papers describing IR in patients with acne. The PubMed, Google Scholar, Scopus and Cochrane Library databases were searched independently by two authors. The search strategy included controlled keywords and vocabulary, using the following terms: “insulin resistance”, acne vulgaris, “insulin resistance in patients with acne vulgaris”, “HOMA-IR”, “HOMA-IR in acne vulgaris”.
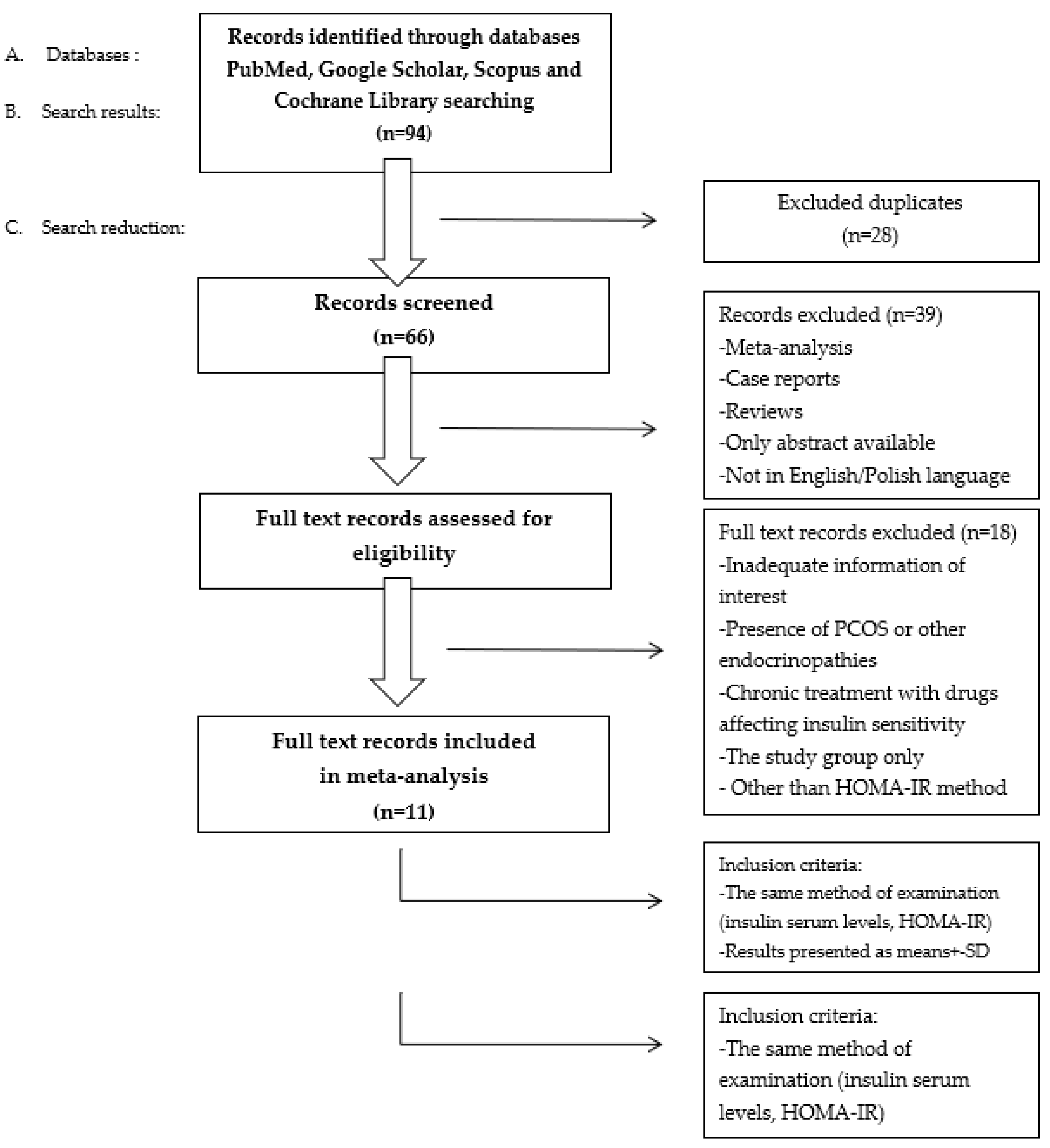
The initial selection of studies was based on the abstracts and keywords. Subsequently full text versions were obtained. The clinical importance of the papers obtained was considered, therefore case reports or papers presenting the study group only were excluded due to low clinical importance. Moreover, Greenhalgh’s evidence hierarchy was applied. Studies published in the past 11 years were considered for this review. The PRISMA list was followed during the construction of the review. the systematic literature research was developed according to PRISMA guidelines.
To include articles in our systematic review we decided to apply the following selection criteria: original paper, the same method of examination of insulin serum levels, HOMA-IR and results presented as mean ± SD or median with range. To exclude any possible impact of other abnormalities on IR development we decided to apply following exclusion criteria: the presence of PCOS or other endocrinopathies and treatment with drugs affecting insulin sensitivity. The process of study selection is presented in
Figure 1.
We highlighted 11 articles dealing with IR and acne vulgaris and which determine the connection between these conditions. Results presented as either mean ± SD or median (range) were considered separately and are presented in
Table 1 and
Table 2, respectively.
Most significant results, characterized by p-value <0.001 were obtained by Snight M. et al. and Nurhadi S. et al., who received HOMA-IR values in acne group (3.3±1.7) and (2.63±0.29) and in control group (1.5±1.9) and (1.71±0.26), respectively.
The study was approved by the Ethics Committee of Poznań University of Medical Sciences in Poland.
This research project was implemented with the use of funds for science, awarded by the Poznań University of Medical Sciences.
3. Results
3.1. Descriptive analysis
Groups of 41 patients and 47 healthy controls were age and BMI matched (p>0.05). The mean BMI in both groups was within the normal range (BMI<25 [kg/m2]). There were 18 (44%) acne patients and 9 controls (19%) with a BMI calculated as >=25[kg/m2].
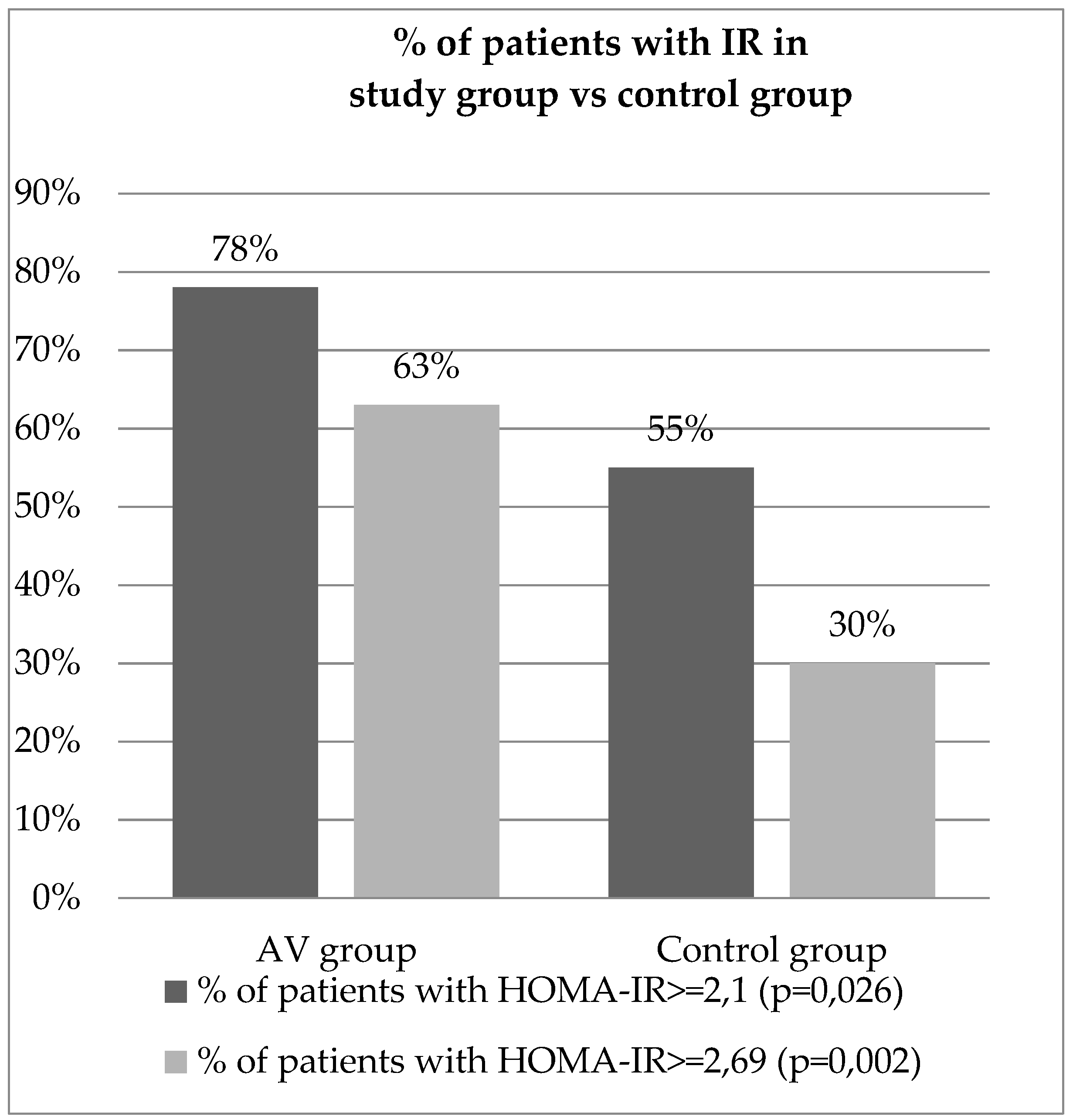
The mean glucose fasting serum level in the study group was 94.88 [mg/dl], whereas in control group was 79.51 [mg/dl], which was statistically significant (p<0.001). The mean insulin fasting serum level in the study group was 14.47 [µIU/mL] and 11.83 [µIU/mL] in the controls and it had no statistical significance (p=0.059). The HOMA-IR mean value calculated in the study group was 3.4 and in control group 2.34 with a strong statistically significant difference between the two groups (p<0.001). Assuming the cut-off value for IR determination is 2.1, 32 out of 41 patients were diagnosed with IR (78%) and out of the 47 controls 26 were diagnosed with IR (55%), which was statistically significant (p=0.026).
Regarding the calculation formula for determining the HOMA-IR cut-off value, we would like to emphasize, that in our study method, for our healthy control group, excluding people with BMI>=25kg/m2, the third quartile of HOMA-IR values estimated as 2.69. However, the study on a bigger group is necessary.
Nevertheless, if the HOMA-IR cut-off value for our study was established as 2.69, then 26 out of 41 patients would be diagnosed with IR (63%), and 14 out of 47 controls would be diagnosed with IR (30%) with the difference being even more significant. (p=0.002). The results of the calculated HOMA-IR are presented as the percentage of patients diagnosed with IR in both group are presented in
Figure 2.
Our results demonstrated that the glucose serum level and HOMA-IR were significantly higher in acne vulgaris group as compared to the control group, which means that IR might be an independent causative factor in acne vulgaris formation. All the results are presented in
Table 3.
3.2. Systematic review results.
3.2.1. Insulin serum levels
Analysing the data included in
Table 1 concerning serum levels of insulin, it is remarkable that in 7 out of 9 articles mean insulin serum levels were higher in the group of patients with acne, out of which 4 study results were statistically significant, which was assessed by p-value <0.05. The most significant difference between mean insulin serum levels in the study group (33.85±1.5) and the control group (7.14±2.4) was demonstrated in the study by Snight M. et al., for which the p-value was calculated as p<0.001. One of the studies did not include information about insulin serum levels. In
Table 2, only one of the selected studies contains information about insulin serum levels presented as a median with range. In this case, there was no significant difference between acne patients and healthy controls.
3.2.2. HOMA-IR
Comparing the data from
Table 1 on HOMA-IR values in the study group to the control group, it is noticeable that in 8 out of 9 articles HOMA-IR was higher in patients with acne compared to the controls. In 6 studies, the results were statistically significant, which was assessed by a p-value <0.05. The most significant results, characterized by a p-value <0.001 were obtained by Snight M. et al. and Nurhadi S. et al., who received HOMA-IR values in the acne group (3.3±1.7) and (2.63±0.29) and in the control group (1.5±1.9) and (1.71±0.26), respectively.
Table 2 contains 2 studies presenting data as median with range, only 1 of which showed higher HOMA-IR values calculated for the study group, compared to the controls. The results published by Mustafa AL. et al. showed a strong significant difference between acne patients – 6.02 (5.2-8.1) and the controls – 3.51 (2.2-4.3) with a calculated p-value <0.001.
4. Discussion
4.1. Acne vulgaris
Acne vulgaris is a chronic inflammatory skin disorder which involves the folliculopilosebaceous unit of the skin. It is a relatively common condition, as can be seen on the skin of 85% of teenagers and 33% of 15-44-year old patients. It, most often affect female adolescents and woman, although there is a high prevalence among males. Acne lesions, which include comedones, papules, pustules or nodules are most often located within the skin of face, shoulders, chest and back [
6]. Many different factors may lead to acne formation, including abnormal sebum production (hyperseborrhea), hyperkeratosis, inflammation or hormonal disturbances. Elevated levels of androgens, insulin and insulin-growth factor-1(IGF-1) lead to the promotion of hyperkeratosis and hyperseborrhea, resulting in acne formation. Moreover, these hormones affect each other, e.g. androgens lead to an increase in insulin and IGF-1 serum levels, whereas the androgen signal transduction is stimulated by IGF-1, moreover IGF-1 decreases serum level of sex hormone binding globulin (SHBG), leading to free androgens elevation.
4.2. Insulin resistance.
IR is a condition characterized by the failure of insulin to provide the proper glucose transport into the tissues, which results in the development of hyperglycemia and hyperinsulinemia [
20]. IR may be triggered by many factors, including genetic factors. However, environmental factors seem to play a major role in IR development.
Insulin and IGF-1 serum levels may be elevated in the course of many endocrine disorders, including e.g. acromegaly, hyperprolactinemia, hypercortisolism or congenital adrenal hyperplasia. Obesity, chronic inflammation, hypertriglyceridemia or activity of antagonistic hormones, including glucagon, cortisol and thyroxin seem to be major causative factors in IR development. Western diets, which include a high intake of high glycaemic-load carbohydrates and milk, promote sudden fluctuations in glucose and insulin serum levels. Moreover, milk contains IGF-1 itself, promoting IR development in various different ways. Increased milk and high glycaemic load products intake has been detected in acne patients compared to healthy controls, moreover no acne lesions were observed in populations with Paleolytic style of eating[
21]. An important fact is that high insulin and IGF-1 play a significant role in the development of type 2 diabetes and cardiovascular diseases. All of these are civilization diseases, which emphasize the importance of IR diagnosing and treatment. IR therapy includes both lifestyle changes – a low glycaemic load diet and physical activity and metformin therapy.
There is a wide range of methods to select from in evaluating IR. The euglycaemic metabolic clamp techniqe was the first to be designed by R. A. DeFronzo, J. D. Tobin and R. Andres in 1979, but due to its complicated process consisting of both glucose and insulin infusions, it is currently not widely used [
22]. The homeostasis model assessment scale (HOMA) is probably the most frequently used in IR calculations. Only the fasting serum levels of both glucose and insulin are required in the formula. However, the different cut-off values calculated in various populations, starting from 1.7 for the Japanese up to 3.8 for French, make it difficult to interpret [
23,
24]. Similar indexes include the Quantitative Insulin Sensitivity Check Index (QUICKI) or Matsuda Index, which requires three measurements of glucose and insulin serum levels during an oral glucose tolerance test (OGTT) [
25,
26]. An innovate idea for insulin sensitivity evaluation is to measure irisin serum levels, which were found to present a significant negative correlation with HOMA-IR values [
27].
4.3. Systematic review.
The connection between IR and acne vulgaris formation has already been the point of interest among several authors.
A study by Monica Singh et al. showed statistically higher mean HOMA-IR values in the group of 80 acne patients (3,3) compared to 80 healthy controls (1,5). However, there was no correlation found between HOMA-IR and acne severity [
9].
Sharma S et al. examined 100 adult patients and 100 controls. The groups were mixed-sex. The mean HOMA-IR value in the acne group (2,7) was found to be statistically higher than the control group (1,9) [
10].
Similarly, the study by Mustafa et al. proved the influence of IR in acne vulgaris formation when the median HOMA-IR value in a mixed-sex, 60-patient acne group (6.02) was statistically higher than in an identical non-acne group (3.51) [
19]. An interesting discovery was the negative correlation between the serum irisin level, which is a natural obesity, diabetes and IR protector, and HOMA-IR among acne patients, thereby the irisin serum level was lower in acne patients compared to the controls. Moreover, it had a negative correlation with acne severity. Physical exercise resulting in fat reduction and weight loss increases the irisin serum level [
28].
Nagpal et al. examined 100 male patients and 100 controls and received statistically higher HOMA-IR mean values in the acne group (2.0) than in non-acne controls (1.7). Any possible impact of hyperandrogenemia was excluded [
11].
Moreover, statistically higher mean HOMA-IR values were observed in the studies by Nurhadi et al. [
12] (acne patients 2.63 vs. non acne 1.71), in the study by Emiroglu N et al. [
15] (2.87 vs. 1.63), and in the study by Del Prete et al. [
17](1..7 vs. 1.1).In contrast, Cerman et al. did not observe any connection between IR and acne vulgaris formation [
14].
Aydin et al. [
13] and Cetinozman et al. [
16] obtained mean HOMA-IR values in study groups that were higher than in the controls, though with no statistical significance.
In the study by Nazik H. Hazrat et al. in 2023, an original method of IR evaluation was used. Serum levels of C-peptide, triglyceride and glucose were obtained, and later a TyG (triglyceride-glucose) index was calculated using the formula: {Ln (natural logarithm) (TG [mg/dL] × glucose [mg/dL]}. Values over 1.89ng/ml for C peptide and over 4.49 for the TyG index indicated a diagnosis of IR. As in other studies, these results suggested statistically more frequent IR development in the group of acne patients compared to the healthy controls [
29].
The study by Solanki et al. examined the study group only, although it investigated the original topic of recurrent acne and hirsutism. Patients were divided into groups based on the presence of acne, hirsutism or both. Moreover, each of the 3 groups was then divided into two, consisting of either naïve patients or patients with recurrent symptoms. In every group the percentage of IR patients was statistically higher among patients with recurrent symptoms compared to the naïve subjects. The results suggested a strong correlation between IR and recurrent acne and hirsutism [
30].
Another innovative study was conducted by Kaya İFK et al., in which researchers studied the influence of IR and visceral fat in acne formation. Apart from the HOMA-IR index the VAI (visceral adipose index) was used. For the VAI calculation the values of waist circumference, body mass index (BMI) and TG and HDL serum levels were required. However there was no significant difference found between the acne group and the control group in terms of HOMA-IR and VAI [
18].
Mustafa AI et al. studied the more limited topic of post-adolescent acne. They proved, that IR was more frequent in the acne group than the controls. Moreover, a strong predictor for post-adolescent acne formation could be serum fetuin-A concentration, which was statistically higher in the acne group. Furthermore HOMA-IR and fetuin-A serum levels were statistically higher in the case of patients who developed severe forms of acne, which was suggested to be a predictor of acne severity. Fetuin-A and HOMA-IR were found to be positively correlated, this could be because fetuin-A is a glycoprotein that acts as an antagonist to insulin receptors, and eventually leads to a decrease in muscle and hepatic insulin signaling and IR development [
31].
4.4. Metformin treatment.
IR evaluation is important in diagnosing acne vulgaris. In such cases, causal treatment could be applied, including both lifestyle changes and pharmacotherapy. Lifestyle changes, e.g. low glycaemic load diet, was found to reduce serum levels of IGF-1 and decrease acne lesions severity. Recently, growing interest in possible acne treatment modalities has observed, and metformin was noted to be an effective therapeutic method in acne treatment in such cases. Its effectiveness has already been proved in case of female patients with polycystic ovary syndrome (PCOS), however probably could be no less successful in male patients and females with no PCOS. Metformin not only increases insulin sensitivity, when inhibiting mTORC1 (mammalian target of rapamycin complex 1) and reducing IGF-1(insulin-like growth factor-1) serum levels leading to androgens signalling suppression, but also presents anti-inflammatory properties, which eventually causes a reduction in the GAGS score (The Global Acne Grading System) and disappearance of acne lesions [
32]. Metformin effectiveness in acne vulgaris treatment was described in the study of Robinson et al., who used a 12 weeks combination therapy consisting of 2,5% benzoyl peroxide and tetracycline in dose 250mg in both acne groups and extra metformin in dose 850mg in study group. Patients treated with metformin obtained better treatment results compared to no-metformin group, what proves metformin effectiveness in acne vulgaris therapy. [
33].
5. Conclusions
In our study, we found a positive correlation between IR and acne vulgaris development. In view the fact, that IR might be an independent factor in acne vulgaris formation, especially in forms of acne resistant to classical treatment methods, we highlight the importance of finding a possible relationship between these disorders when diagnosing acne and in such cases, of focusing more on causal rather than symptomatic treatment. Both lifestyle changes and pharmacological treatment, including e.g. metformin therapy, could be effective in such cases. However, more studies are necessary to prove its effectiveness in the treatment of acne vulgaris lesions.
Author Contributions
Conceptualization, K.L. ; methodology, M.G. and A.S-P.; formal analysis, M.G., W.S and B.W.; data curation, M.G. ; investigation, M.G. and A.S-P.; resources, M.G., W.S. and K.L. ; writing – original draft preparation, M.G. and W.S.; writing – review and editing A.S-P., B.W. and K.L. ; visualization, M.G. and W.S. ; supervision, K.L.; funding acquisition, M.G., W.S., and K.L. ; project administration, K.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of Poznań University of Medical Sciences in Poland..
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data obtained and analysed during the current study are available from the corresponding author upon request.
Acknowledgments
In this section, you can acknowledge any support given which is not covered by the author contribution or funding sections. This may include administrative and technical support, or donations in kind (e.g., materials used for experiments).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Melnik BC. Acne vulgaris: The metabolic syndrome of the pilosebaceous follicle. Clin Dermatol. 2018, 36(1), 29-40. [CrossRef]
- Dreno, B., & Poli, F. Epidemiology of acne. Dermatol. 2003,206, 7–10. [CrossRef]
- Kamangar, F.; Shinkai, K. Acne in the adult female patient: a practical approach. Int J Dermatol. 2012, 51(10), 1162-1174. [CrossRef]
- Elsaie, Mohamed L. Hormonal treatment of acne vulgaris: an update. Clin Cosmet Investig Dermatol. 2016, 9, 241-248. [CrossRef]
- Franik, G., Bizoń, A., Włoch, S., Kowalczyk, K., Biernacka-Bartnik, A., & Madej, P. Hormonal and metabolic aspects of acne vulgaris in women with polycystic ovary syndrome. Eur Rev Med Pharmacol Sci. 2018, 22(14), 4411-4418. [CrossRef]
- Napolitano, M., Megna, M., Monfrecola, G. Insulin resistance and skin diseases. ScientificWorldJournal. 2015. 2015, 479354. [CrossRef]
- Greabu, M., Badoiu, S. C., Stanescu-Spinu, I. I., Miricescu, D., Totan, A. R., Badoiu, S. E., Costagliola, M., Jinga, V. Drugs Interfering with Insulin Resistance and Their Influence on the Associated Hypermetabolic State in Severe Burns: A Narrative Review. Int J Mol Sci. 2021, 22(18), 9782. [CrossRef]
- Szurkowska, M., Szafraniec, K., Gilis-Januszewska, A., Szybiński, Z., Huszno, B Insulin resistance indices in population-based study and their predictive value in defining metabolic syndrome. Przegl. Epidemiol. 2005, 59, 743–51.
- Singh, M., & Shri, D. Insulin resistance in moderate to severe acne vulgaris. Indian J Dermatol. 2022 Mar-Apr, 67(2),205. [CrossRef]
- Sharma, S. , Goel, A., Kaur, J., Bassi, R., Tayade, A. Insulin resistance in adult Acne. IP Indian J Clin Exp Dermatology. 2019, 5(3), 202-205. [CrossRef]
- Nagpal, M., De, D., Handa, S., Pal, A., Sachdeva, N. Insulin Resistance and Metabolic Syndrome in Young Men With Acne. JAMA Dermatol. 2016, 152(4),399-404. [CrossRef]
- Nurhadi, S., Praharsini, IGAA., Wiraguna, AAGP. Elevated homeostatic model assessment of insulin resistance level increases the risk of acne. J Gen Proced Dermatol Venereol Indones. 2018, 3(1), 21-28. [CrossRef]
- Aydin K, Çetinözman F, Elcin G, Aksoy DY, Ucar F, Yildiz BO. Suppressed Adiponectin Levels and Increased Adiponectin Response to Oral Glucose Load in Lean Women with Severe Acne Normalizes after Isotretinoin Treatment. Dermatology. 2017,233(4), 314-319. [CrossRef]
- Çerman AA, Aktaş E, Altunay İK, Arıcı JE, Tulunay A, Ozturk FY. Dietary glycemic factors, insulin resistance, and adiponectin levels in acne vulgaris. J Am Acad Dermatol. 2016, 75(1),155-162. [CrossRef]
- Emiroğlu, N., Cengiz, FP., Kemeriz, F. Insulin resistance in severe acne vulgaris. Postepy Dermatol Alergol. 2015,32(4),281-285. [CrossRef]
- Cetinözman, F., Aksoy, DY., Elçin, G., Yıldız, BO. Insulin sensitivity, androgens and isotretinoin therapy in women with severe acne. J Dermatolog Treat. 2014, 25(2),119-122. [CrossRef]
- Del Prete, M., Mauriello, MC., Faggiano, A., Di Somma, C., Monfrecola, G., Fabbrocini, G., Colao,A. Insulin resistance and acne: a new risk factor for men? Endocrine. 2012, 42(3),555-560. [CrossRef]
- Kaya, İFK., Eryılmaz, MA., Pekgör, O., Külahçı, E. Evaluation of the relationship between insulin resistance and visceral adiposity index in patients with acne vulgaris. Turk J Med Sci. 2022, 52(2),477-483. [CrossRef]
- Mustafa, AI., El-Shimi, OS. Serum irisin: A prognostic marker for severe acne vulgaris. J Cosmet Dermatol. 2018, 17(5), 931-934. [CrossRef]
- Rogowicz-Frontczak, A., Majchrzak, A., Zozulińska-Ziółkiewicz, D. Insulin resistance in endocrine disorders - treatment options. Endokrynol Pol. 2017, 68(3), 334-351. [CrossRef]
- Sadowska-Przytocka, A., Gruszczyńska, M., Ostałowska, A., Antosik, P., Czarnecka-Operacz, M., Adamski, Z., & Łącka, K. Insulin resistance in the course of acne - literature review. Postepy Dermatol Alergol. 2022,39(2),231-238. [CrossRef]
- 22. Ferrannini E, Mari A. How to measure insulin sensitivity. J Hypertens. 1998, 16,895-906. [CrossRef]
- Bonora, E., Targher, G., Alberiche, M., Bonadonna, R. C., Saggiani, F., Zenere, M. B., Monauni, T., & Muggeo, M. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 2000, 23, 57-63. [CrossRef]
- Tang, Q., Li, X., Song, P., Xu, L. Optimal cut-off values for the homeostasis model assessment of insulin resistance (HOMA-IR) and pre-diabetes screening: Developments in research and prospects for the future. Drug Discov Ther. 2015, 9(6), 380-385. [CrossRef]
- Katz, A., Nambi, SS., Mather, K., Baron, A. D., Follmann, D. A., Sullivan, G., & Quon, M. J. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metabol. 2000, 85, 2402-10. [CrossRef]
- Gutch, M., Kumar, S., Razi, SM., Gupta, KK., Gupta, A. Assessment of insulin sensitivity/resistance. Indian J Endocrinol Metab. 2015, 19(1),160-164. [CrossRef]
- Tang, L., Yu, B., Liao, Y., Long, S., Yan, H., He, Q., & Li, C. Serum Irisin: A Potential Diagnostic Marker for Insulin Resistance in Acne Vulgaris. Indian J Dermatol. 2022, 67(4), 477. [CrossRef]
- Boström, P., Wu, J., Jedrychowski, M., Korde, A., Ye, L., Lo, J. C., Rasbach, K. A., et al. PGC1-alpha dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012, 481,463-468. [CrossRef]
- Hasrat, NH., Al-Yassen, AQ. The Relationship Between Acne Vulgaris and Insulin Resistance. Cureus. 2023, 15(1), e34241. [CrossRef]
- Solanki ,AD., Solanki, DKB., Banker, KK., Rangnani, TC., Patel, NM., Modi, KR. Role of Insulin Resistance in Patients of Acne Vulgaris and Hirsutism in the Western Part of India- A Cross-Sectional Study. Indian Dermatol Online J. 2022, 14(1), 38-43. [CrossRef]
- Mustafa, A. I., Kadah, A. S., Fawzy, E. M., & Mahmoud, G. M. A Novel Potential Link Between Post-adolescent Acne and Insulin Resistance. J Clin Aesthet Dermatol. 2022,15(12),33-37.
- Andreadi, A., Muscoli, S., Tajmir, R., Meloni, M., Minasi, A., Muscoli, C. et al. Insulin Resistance and Acne: The Role of Metformin as Alternative Therapy in Men. Pharmaceuticals (Basel). 2022, 16(1), 27. [CrossRef]
- Robinson S, Kwan Z, Tang MM. Metformin as an adjunct therapy for the treatment of moderate to severe acne vulgaris: A randomized open-labeled study. Dermatol Ther. 2019;32(4):e12953. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).