1. Introduction
Currently, citrus fruits are in high demand in the global food industry [
1], their cultivation and consumption has been distributed in almost all countries of the world. In addition to direct consumption as fresh fruit, much of the world’s production is destined to the mainly food industry; however, its processing, generates solid waste, such as peels, which represent approximately 55- 60 % of the total fruit; these residues are usually not efficiently utilized.
It has been determined that citrus peels may contain bioactive compounds with industrially usable potential and the most feasible way is by extracting their essential oils (EO). Although there are many ways to extract EOs, such as maceration, hydrodistillation, Soxhlet and heating-relution, the use of green solvents [
2] has a growing trend to extract phenolic compounds from plants because it is associated with more sustainable aspects and environmental care [
3]. In addition to traditional techniques such as cold pressing and use of heat or steam [
4], ultrasound-assisted techniques, supercritical fluids and microwaves, have allowed increasing extraction yields and reduce damage to bioactive compounds [
5,
6]. Among many parameters, time, heating and water-plant material ratio are the main control elements in the EO extraction process [
7].
Previous works demonstrated that microwave-assisted extraction allows obtaining yields higher than 10.47% EO from citrus peels [
8,
9]. However, it should be noted that the yield may vary depending on the fruit species and peel characteristics [
10].
Lopresto et al. [
11] mention that the parameters to be taken into account in supercritical fluids are temperature and pressure, which should be kept above the critical point for the solvent, e.g. CO 2 (73.8 bar, 30.9 °C). CO2 has the advantage of being chemically inert, non-flammable, cost-effective and not prone to contamination [
12]. The relatively low viscosity and high diffusivity of supercritical fluids enhance diffusion and mass transfer, which reduces extraction time [
13].
Visakh et al. [
14] extracted essential oils from peels of four citrus fruits and found D-limonene to be the most abundant compound. [
15] in similar work obtained a yield of up to 0.99% D- limonene after 150 min and a solid to liquid ratio of 1:10. Freeze-drying and subsequent extraction with supercritical fluids [
16], allows obtaining higher yields, total phenol content and antioxidant activity of essential oils [
17,
18]. Twenty-five chemical constituents have been identified and quantified in lemon peel essential oil and limonene (70.02-71.76 %) and γ-terpinene (9.79-9.94 %) as major constituents [
19].
EOs from citrus peels, are characterized by possessing a strong and pleasant aroma with a refreshing sensation. They are used as flavoring in various foods, beverages and pharmaceuticals [
20]. They are rich in flavonoids, alkaloids, coumarins, limonoids, carotenoids, phenolic acids and antioxidants [
21,
22,
23].
Flavonoids are low molecular weight compounds and are responsible for the vivid color of citrus peels. They are composed of two benzene rings (A and B), linked through a heterocyclic pyran ring [
24].
The predominant phenolic compounds are the in citrus peel EO are l p -cinnamic acid, ferulic acid, isoferulic acid, 5-hydroxyvaleric acid, vanillic acid and 2-oxybenzoic acid [
25,
26] and heterocyclic oxygen compounds such as coumarin, furocoumarin and polymethoxyflavone (PMF) [
27].
The chemical composition of citrus essential oils is highly dependent on the maturity stage of the fruit and the geographical location in which they have been grown [
28]. According to Ajikumaran et al. [
29] limonene is the major component of essential oil of orange peel (84.75 %), mandarin (Citrus reticulata) (83.65 %), Kambili naragam (Citrus maxima) (87.54 %), lemon (36.70 %), mathala naragam (Citrus medica , round) (71.98 %) and Citrus medica (oblonga) (65.13 %) [
8,
9].
Furthermore, these EOs contain volatile aromatic compounds such as monoterpenes, sesquiterpenes and oxygenated compounds, limonene being a colorless aliphatic hydrocarbon with a pleasant citrus fragrance that can be used as a food flavoring. The main volatile compounds of C. bergamia EO are oxygenated monoterpenes (51.09 %), while monoterpene hydrocarbons represent the most abundant compounds in C. myrtifolia (82.15 %) and C. limon (80.33 %) EO. Other important compounds are β-pinene (9.31%), γ-terpinene (10.45%) and citronellol (8.19%) [
14].
Overall, monoterpene hydrocarbons, oxygenated monoterpenes and sesquiterpene hydrocarbons have been found to constitute 27.35; 71.52 and 0.9% of the aromatic volatile composition respectively of citrus EOs According to Cebi and Erarslan [
30] . Chromatographic techniques have been the most efficient techniques to determine the chemical composition of essential oils [
31] in addition to new techniques such as Raman spectroscopy with advantages such as rapid detection, low cost and does not destroy the sample [
32].
Consequently, in this research, the chemical properties of essential oils from peels of four citrus fruits extracted with green solvents were determined.
2. Materials and Methods
2.1. Obtaining peels from the four citrus fruits
Ripe fruits of lime (
Citrus limetta Risso), sweet lemon (
Citrus limetta sp.), mandarin or tangerine (
Citrus reticulata) and orange (
Citrus sinensis) were acquired from plantations in the provinces of Utcubamba, Rodriguez de Mendoza and juice vending centers in the city of Chachapoyas, Amazonas region, Peru (
Figure 1). The fruits were taken to the Engineering Laboratory of the Universidad Nacional Toribio Rodríguez de Mendoza de Amazonas for disinfection, conditioning and obtaining the respective peels.
2.2. Chemical reagents used
Folin-Ciocalteu reagent (Sigma Aldrich, USA), gallic acid (Sigma Aldrich, China, ≥ 98%), sodium carbonate (Na2CO3) (Spectrum Chemical Mfg. Corp, USA, 99.5%), methanol (CH3OH) (J.T.Baker, USA, 99. 98%), quercetin (C15H10O7), sodium acetate (C2H3Na2O2), aluminum chloride (ALCl3), 2,2-diphenyl-1-picrylhydrazyl (DPPH) (Sigma Aldrich, USA), 2,2’-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS+), potassium persulfate (K2S2O8), Coumarin 72609- 100 mg (Sigma aldrich, Switzerland, 99. 9%), Psoralen 89770-20mg (Sigma aldrich PhytoLab, Germany, ≥ 98%), Acetonitrile CH3CN (Scharlau).
2.3. Obtanining essential oil from citric peels
The procedure of Phat et al. [
33] was used for the extraction of citrus peel EOs. For hydrodistillation, a steam distiller (TECNAL, TE-2761, Brazil) was used and distilled water at an extraction temperature of 80°C was used as the green solvent. The distiller was started by adding 300 g of fruit peels (orange, sweet lemon, mandarin and lime) separately with 500 ml of distilled water. The extraction time was approximately 1 h for 300 g of sample; then, the water-essential oil mixture was refrigerated at 1°C to produce phase separation. The essential oils, purified by decanting, were stored in amber glass bottles with airtight lids at 4°C.
The EO yield was calculated using the following equation 1.
Where Y is the essential oil yield (%, v/w), V is the volume of essential oil obtained (ml) and W is the weight of citrus peel used (g).
2.3. Characteristics of the essential oils of citrus peels
2.3.1. Antioxidant capacity of essential oils by the DPPH (2,2-diphenyl-1-picrylhydrazyl) method
DPPH free radical scavenging activity was determined following the procedure described by Guo et al. [
34] and Smeriglio et al. [
35]. 1 ml of essential oil solution (10%) was mixed with 1 ml of 0.1 mM DPPH methanolic solution. The mixture was incubated in darkness at room temperature for 30 min. Then, in a spectrophotometer (EMC-11-UV SPECTROPHOTOMETER) the absorbances at 517 nm, (A_sample) were obtained. In addition to that a blank experiment was performed employing the same procedure, but without essential oil and the absorbance was recorded as (A blank). The measurements were carried out in quadruplicate.
DPPH scavenging activity was determined using equation 2.
2.3.2. Antioxidant capacity of essential oils with the ABTS.+ method
The procedure of Guo et al. [
34] was followed with some modifications. The radical cation ABTS
.+ was produced by reacting 7 mM aqueous ABTS
.+. To 88 μl of ABTS solution, 5 ml of 2.45 mM potassium persulfate was added. The mixture was kept in the dark at room temperature for 12 h. The ABTS
.+ solution was diluted with ethanol (approximately 1:80) to an absorbance of 0.700 ± 0.02 at 734 nm. To measure the antioxidant activity of AE, 30 μl of AE solution was added to 3000 μl of diluted ABTS
.+, proceeded to record the absorbance as at time zero after 30 min when the absorbance stabilized. To quantify the antioxidant activity of the essential oils, a curve was obtained with Trolox standard (y = -0.0004x + 0.8502) obtained from different concentrations (from 0 to 2000 mM).
2.3.3. Total phenolic content determination
Total phenolic content (TPC) was determined following the procedure of Ndayishimiye et al. [
36] and Smeriglio et al. [
35] with adjustments. For the reaction, 0.5 ml of diluted essential oil (2.5 mg/0.5ml ethanol) and 2.5 ml of diluted Folin-Ciocalteu’s reagent (1:10 ultrapure water) were added. Then 2 ml of sodium carbonate solution (7.5%) was added to the mixture, stirred vigorously and allowed to react for 60 min. The absorbance was measured at 764 nm in UV-Vis spectrophotometer (EMC-11-UV SPECTROPHOTOMETER). To quantify the total phenolic content, a standard curve for gallic acid (y = 0.0004x + 0.0212) with dilutions between 0 and 2500 mM was developed. The result was expressed in mg gallic acid equivalents per gram of EO (mgGAE/g).
2.3.4. Total flavonoid content determination
The method employed byNdayishimiye et al. [
36], was used to determine the total flavonoid content (TFC) with some modifications. A mixture consisting of 0.5 ml of essential oil (diluted in 4.5ml in ethanol), 0.5 ml of methanol, 50 μl of AlCl3 (10 %), 50 μl of 1 M potassium acetate and 1.4 ml of distilled water was made. It was left to stand for 30 min at room temperature. Afterwards, the absorbances were read at 415 nm in spectrophotometer. The quantification of TFC was based on the quercetin standard curve (y = 0.0001x + 0.0119) and expressed as mg quercetin equivalent (QE)/g AE.
2.3.5. Measurement of refractive index and density of essential oils
The refractive indices of essential oils extracted from citrus peels (orange, lime, mandarin and sweet lemon) were measured using an ABBE type refractometer (Brand, 2WAJ, USA). The instrument was thoroughly cleaned with distilled water and ethanol before each measurement. The n D values were measured to calibrate the refractometer and the accuracy of n D was ± 0.000.
On the other hand, the density of the essential oils was determined using the mass over volume formula [
37,
38].
2.3.6. Thermal properties of citrus peel essential oils
The thermal properties of citrus peel essential oils were measured using a differential scanning calorimeter DSC (DISCOVERY, DSC 2500). The measurements were performed in a dynamic nitrogen atmosphere (50 mL/min). Each of the oil samples analyzed had a mass of approximately 10 mg. The samples were heated between 25 and 245 °C at a heating rate of 10 °C/min. Instrument control and data analysis was carried out using TA Instruments TRIOS software, which allowed calculation of maximum and onset temperatures, enthalpy and heat flux [
37].
2.3.7. Raman mapping of essential oil composition
Samples were mapped using a Raman focal microscope system (Horiba Scientific, XploRA plus, Montpellier, France). Chemical maps were obtained using a 532 nm laser as excitation light with a filter (50%). Experimental conditions were: 100 nm slit width, 100 µm pinhole, x50/0.90 NA Vis-LWD air objective and 1 s acquisition time with 2 accumulations. The Raman signal was obtained using a 600 lines/mm grating centered between 800 and 3100 cm-1. The acquired spectra were corrected over a range from 1000 to 1800 cm-1, smoothed and baseline corrected using LabSpec 6 Suite software [
39].
2.3.8. Quantification of chemical compounds of essential oils by HPLC.
Essential oil samples were diluted with methanol (1-2 ml of sample in 1 ml of methanol) and centrifuged (30 min, 5000 rpm) before transferring to vials for analysis. For the characterization of phenolic compounds (coumarins and furanocoumarins), the procedure described by Cruz et al. [
40] was followed, with some modifications. The injection volume of each solution was 10 μl in a UHPLC system (Agilent Technologies 1290, model G7104A, Germany) equipped with a VI Flow injector and a C18 column (100 mm × 4.6 mm, ODS-2.3 μm). The mobile phase consisted of a linear gradient with a combination of solvent A: 2% acetic acid in water and solvent B: acetonitrile, water and acetic acid (400:90:10 v/v/v/v). The compounds were identified on a DAD detector set at 280 nm. The run time was 22 min and the temperature was 26°C. All standards used for quantitative determination were from Sigma-Aldrich, St.Louis, MO. The gradient program to be used was as follows: 0-2 min, linear 90% A and 10% B; 2-5 min, linear 88% A and 12%B; 5-8 min, linear 86% A and 14%B, 8-10 min, 84% A and 16%B; 10-12 min, linear 82% A and 18%B; 12-22 min, 90% A and 10%B. To quantify the phenolic compounds of the essential oils, standard curves were obtained for coumarin (y = 0.0000037x + 0.2154384), psoralen (y= 0.0000111x + 0.4174412) and p-coumaric acid (y = 0.00000518x + 0.07278492) at concentrations from 0 to 1mg/ml.
2.4. Data analysis
To determine the effect, an analysis of variance was performed using the Tukey statistical test at the level (p<0.05) and minimum significant differences, using the SPSS V. 25 statistical package.
3. Results and discussion
3.1. Yield of essential oils from citrus peels
The extraction of essential oil from peels of C. limetta Risso, C. limetta sp, C. reticulata and C. sinensis was performed, which, from 300 g of citrus peels used, 3, 2, 1.5 and 0.5 ml of essential oil were obtained, respectively.
Hydrodistillation is one of the most common methods for the extraction of essential oils and allows the use of environmentally friendly solvents [
41]. As evidenced by results, citrus peels have different essential oil yields (0.43% for orange, followed by lime 0.35%, mandarin 0.18% and sweet lemon 0.15%), which is conditioned by the skin volume unlike citrus species that have thinner skin [
42].
The essential oil yields were similar to those reported by other researchers,
C. lumia Risso with 1.75% [
35], 4.3% for orange and lemon (
C. lemon) [
8]. Extraction conditions and origin also influence yield according to published literature.
3.2. Antioxidant capacity of citrus peel essential oil
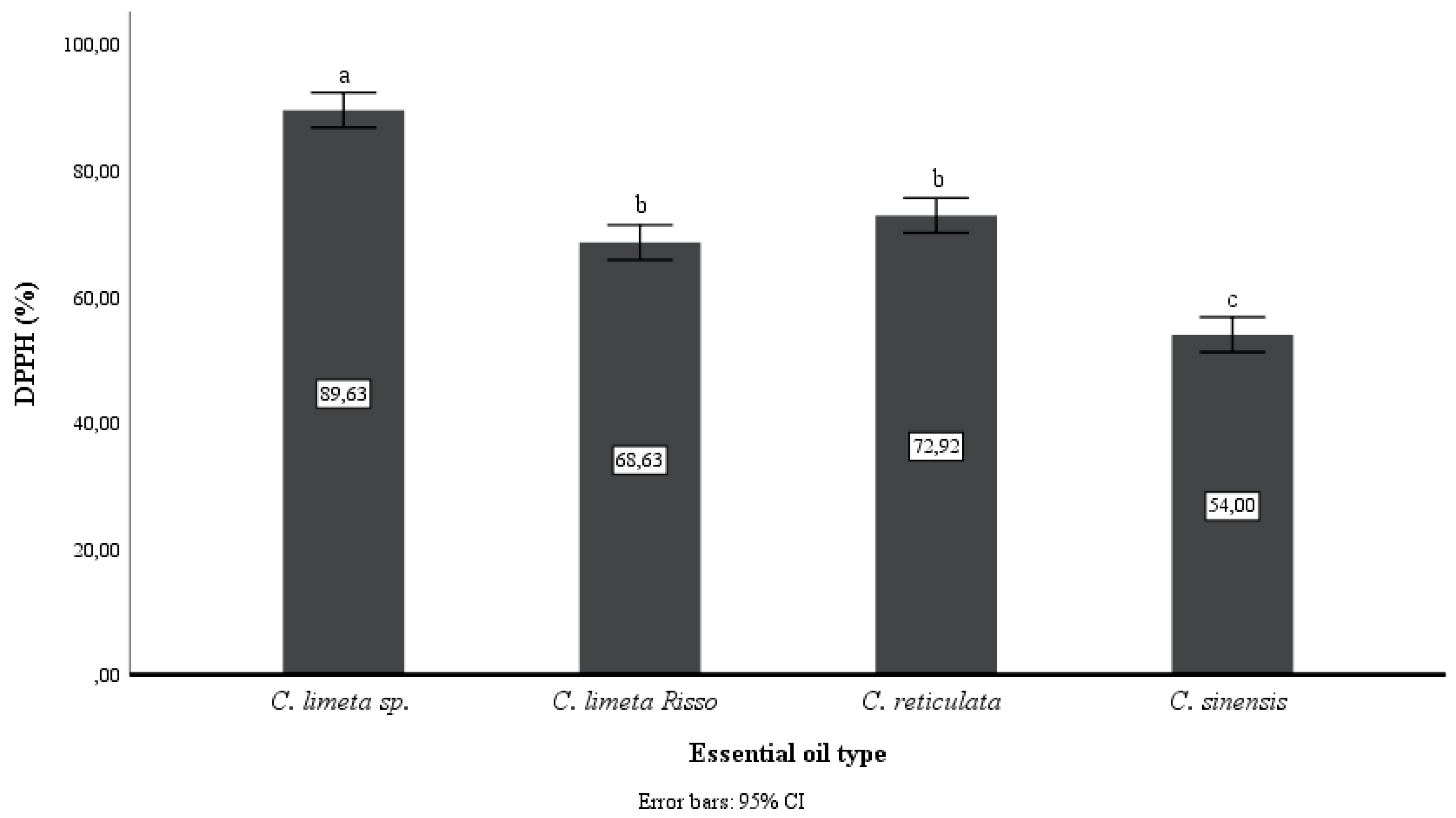
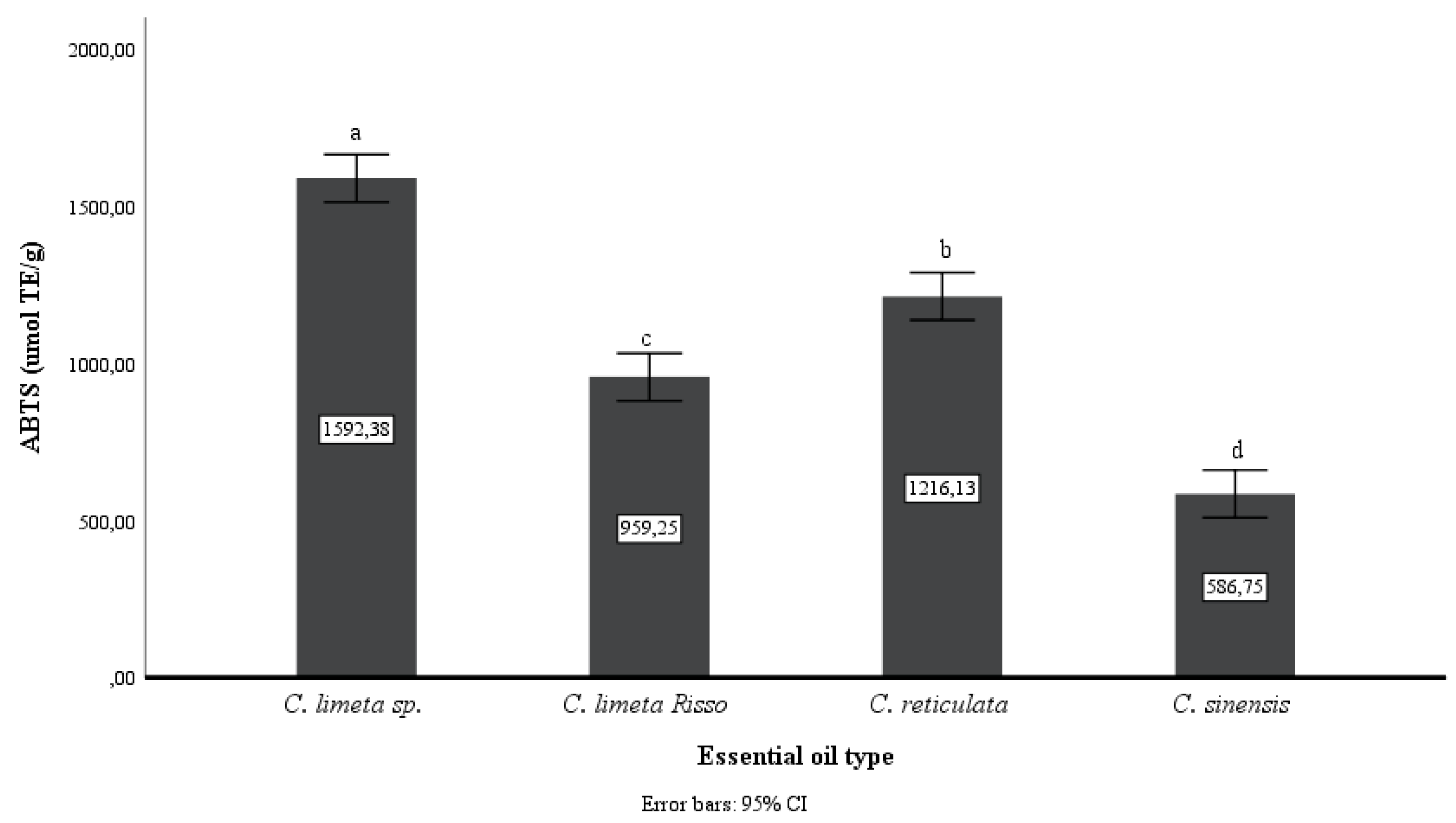
Figure 2 and
Figure 3 show that the EO of
C. limetta sp. has a higher antioxidant capacity than the other oils. In addition, there is a slight difference between the techniques used, however, it can be observed that C. reticulata EO is the second in antioxidant power in vitro and orange essential oil has the least antioxidant capacity (p<0.05).
Numerous antioxidants are commercially available for use in foods; however, natural rather than synthetic antioxidants are better to be used because of their safety, functional and sensory properties [
43]. The data obtained with higher values of antioxidant capacity in both methods as DPPH and ABTS+ were, 89.63% in the EO extracted from mandarin peel and 1592.38 (μmol TE/g of EO) in the EO from sweet lemon peel, respectively. The differences observed with the two techniques are due to the reaction specificity of each [
44]. By the DPPH method the EO extracted from lime peel was 68.63% and 54% from orange, while in the ABTS+ method the same results were obtained with the lime peel EO (959.25 μmol TE/g of EO) containing better antioxidant capacity compared to the orange peel EO (586.75 μmol TE/g of EO). In both methods, it can be seen that the EO extracted from lime peel showed higher antioxidant capacity as opposed to orange, which could be due to the higher content of limonene and linalool in the EO from the peel of this fruit. On the other hand, antioxidant activity values higher than those reported for Navel orange (44.45% DPPH) were obtained [
8]. In addition, the antioxidant capacity of the four studied EOs was higher than those reported by other authors for EOs of similar fruits [
28,
34]. Higher antioxidant capacity values in this type of essential oils have been related to high contents of γ-terpinene, terpinolene, geraniol, β-pinene and myrcene [
45], which could allow us to suppose that the EOs of citrus fruits grown in Amazonas, Peru, are rich in these compounds.
3.3. Total flavonoid and phenolic content
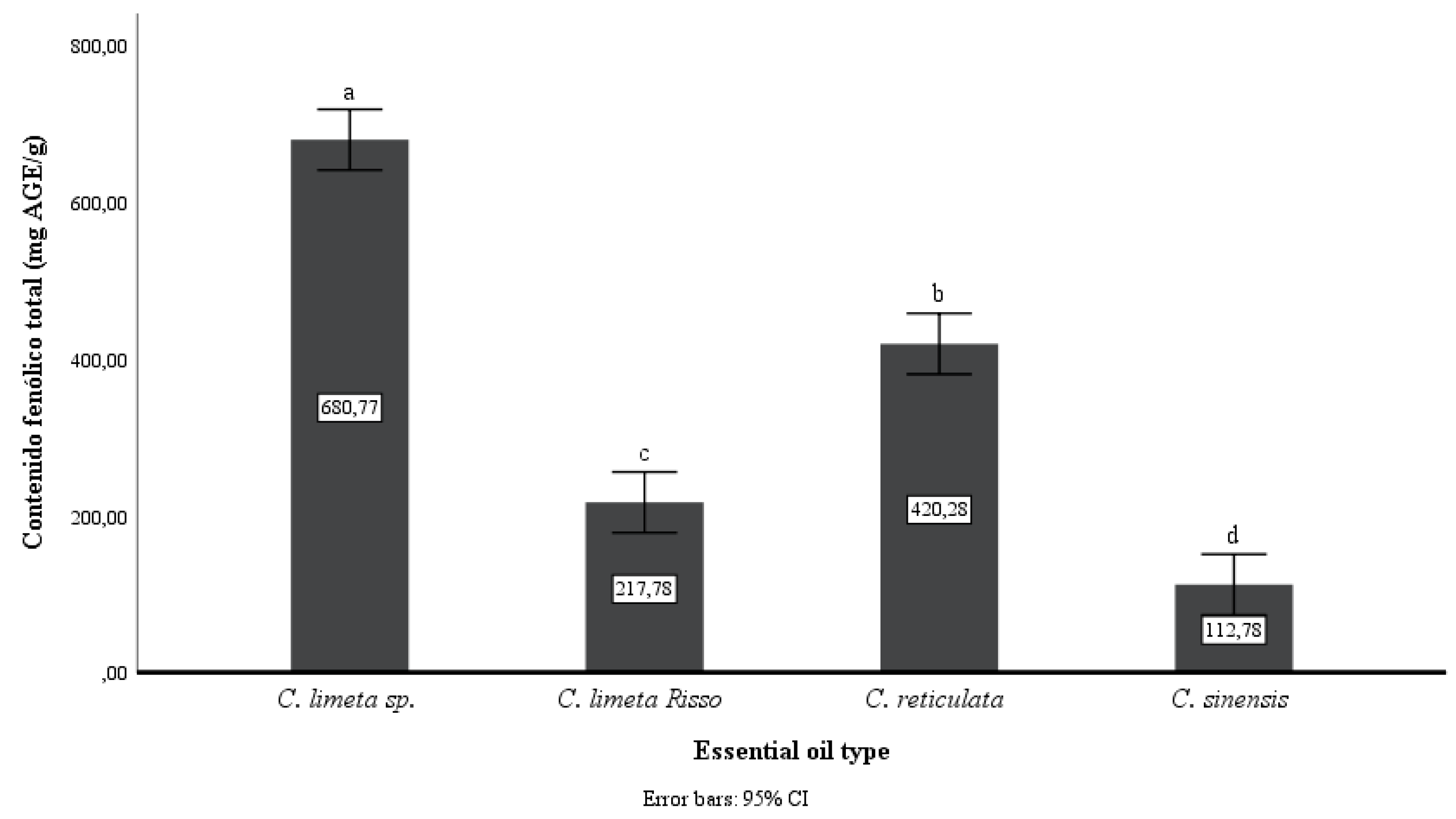
Although the flavonoid content of the four essential oils ranged between 18.92 and 23.18 mg QE/g, no significant differences were found among them (p>0.05), consequently, these EOs are very similar in total flavonoid content.
In
Figure 4, following the trend observed in the antioxidant capacity (
Figure 2 and
Figure 3) of the EOs studied,
C. limetta sp. had the highest total phenolic content (680.77 mg GAE/g), followed by the EOs of
C. reticulata,
C. limetta Risso and
C. sinensis.
It is also observed that the distances between the total phenolic content of the EOs for the four fruit skins are greater than in the antioxidant capacity, an indicator of the presence of other non-phenolic compounds in the EOs.
On the other hand, mandarin peel EO had 420.28 mg AGE/ g lower values than previous works (40.94 mg GAE/g) [
35,
36]. However, sweet lemon peel EO had high phenolic and flavonoid content (23.18 mg QE/g EO), as reported [
31].
3.4. Refractive index and density of essential oils
The refractive index and density values of the EOs studied are similar for the four fruits as shown in
Table 1. The refractive index value is highly dependent on the density and extraction time, as it indicates the change in the composition of each of the samples. The essential oils of citrus peels presented a refractive index in the range of 1.468–1.475. It has been determined that lower refractive indexes indicate a greater presence of lighter compounds such as terpenes and higher refractive indexes indicate the presence of heavier compounds such as terpenes [
46].
3.5. Thermal behavior of the essential oils (DSC)
Table 2 shows the differential scanning calorimetry (DSC) data of the four essential oils. The essential oil of
C. sinensis skin presented higher enthalpy (153.05 J/g) compared to the other three essential oils. However, the essential oil of
C. limetta Risso skin had a tighter onset temperature and maximum temperature (58.38 and 118.7°C, respectively) compared to the other essential oils, indicating better thermal stability. The onset temperature in the calorimetric profile of EOs is an indicator of their oxidative susceptibility. Samples with high initiation temperature have higher stability. Similarly, heating rate is another indicator of susceptibility [
47]. The essential oil of
C. limetta Risso, has higher oxidative stability since its onset temperature was the highest (58.38°C), followed by the EO of
C. sinensis (49.44°C),
C. limetta sp. (38.80°C) and
C. reticulata (26.19°C).
3.6. Raman spectroscopy of citrus peel essential oils
Table 3 shows the data of the Raman spectra of the four essential oils of citrus peels which were analyzed in the region between 200 to 3200 cm-1. The 800 -1200 cm-1 region shows seven Raman peaks for the essential oil of orange peel (803.15, 893.32, 923.38, 1028.57, 1082.24, 1116.59, 1157.38 cm-1), lime (805.30, 891.17, 923.38, 1019.98, 1084.39, 1118.74, 1161.67 cm-1) and sweet lemon (809. 59, 897.61, 931.96, 1035.01, 1084.39, 1127.32, 1165.97 cm-1) while for mandarin it shows only six Raman peaks (808.33, 892.21, 1014.8, 1087.93, 1126.64, 1163.2 cm-1), which are characteristic of the C-C skeleton. It is also notable that the strongest peaks of orange, lime, sweet lemon and tangerine essential oils are (1442.91, 1447.2, 1444.95, 1445.06 cm-1) between the region 1400- 1600 cm -1.
Regarding the possible composition of the ECs, as shown in the Raman spectrum studied (800 -1200 cm-1), there are seven Raman peaks for the skin EC of
C. sinensis,
C. limetta Risso and
C. limetta sp., while for the skin EC of
C. reticulata, only 6 peaks were found, indicating that it differs from the other three studied ECs, which are characteristic of the C-C skeleton. The spectra in the mentioned region agree with some investigations already carried out on peony seed essential oil, with four Raman peaks of the C-C skeleton [
48].
Table 3.
Raman peaks of essential oils from the peels of four citrus fruits.
Table 3.
Raman peaks of essential oils from the peels of four citrus fruits.
| Link type* |
Citrus essential oil source |
| C. sinensis |
C. limetta sp. |
C. reticulata |
C. limetta Risso |
| |
|
|
264.18 |
|
| |
|
|
283.54 |
|
| |
324.41 |
320.11 |
322.25 |
315.82 |
| |
|
343.73 |
343.76 |
|
| |
442.48 |
438.19 |
438.39 |
442.48 |
| |
496.16 |
468.25 |
481.41 |
478.98 |
| |
539.09 |
|
502.92 |
547.68 |
| |
|
|
548.08 |
|
| |
|
534.8 |
599.7 |
|
| |
646.43 |
639.99 |
647.02 |
646.43 |
| |
706.55 |
770.95 |
767.46 |
712.99 |
| |
766.66 |
|
|
766.66 |
| C-C |
803.15 |
809.59 |
808.33 |
805.3 |
| C1–C2 |
893.32 |
897.61 |
892.21 |
891.17 |
| δ(=C–H) |
923.38 |
931.96 |
|
923.38 |
| C-C |
1028.57 |
1035.01 |
1014.8 |
1019.98 |
| υ(C–C) |
1082.24 |
1084.39 |
1087.93 |
1084.39 |
| C-C |
1116.59 |
1127.32 |
1126.64 |
1118.74 |
| C-C |
1157.38 |
1165.97 |
1163.2 |
1161.67 |
| |
1213.2 |
|
|
|
| |
1245.4 |
1223.93 |
|
|
| C -H |
1303.37 |
1311.95 |
1309.45 |
1303.37 |
| |
1378.5 |
1384.95 |
1378.28 |
1384.95 |
| C -H |
1442.91 |
1447.2 |
1444.95 |
1445.06 |
| C -H |
|
|
1550.34 |
|
| |
|
|
1580.45 |
|
| C = C |
1651.15 |
1651.15 |
1651.43 |
1649.01 |
| C = C |
1683.36 |
1683.36 |
1683.69 |
1681.21 |
| |
|
|
|
2731.01 |
| C -H |
2887.73 |
2892.02 |
2883.82 |
2892.02 |
| C -H |
2919.93 |
2922.08 |
2920.38 |
2924.23 |
| C -H |
2969.31 |
2975.75 |
2974.15 |
2975.75 |
| =C-H |
|
|
|
3014.39 |
| |
|
|
3088.14 |
3089.53 |
Considering that limonene, has bending vibration = CH at 886 cm -1 for the di-substituted double bond [
49], the peaks obtained for the EO of
C. sinensis (893.32 cm-1),
C. limetta Risso (891.17 cm-1) and
C. limetta sp. (897.61 cm-1), and
C. reticulata (892.21 cm-1) skins, are indicative of the presence of this compound.
3.7. Quantification of phenolic compounds in UHPLC
The four citrus species contain coumarins in different concentrations, where the essential oil of
C. limetta Risso had the highest concentration of this compound. Psoralen is a compound that is part of the furanocoumarins group, this compound was found only in
C. limetta Risso and
C. reticulata while p-coumaric acid was only found in
C. reticulata (
Table 4). As reported by Mahato et al. [
50], coumarins and furanocoumarins are known to protect plants against fungal infections. According to Fan et al. [
51], citrus peel EOs contain these compounds and in this study,
C. limetta Risso and
C. reticulata EO contain coumarins and in
C. reticulata EO, p-coumaric acid was identified and quantified. This indicates that these essential oils could be used as natural antifungal agents.
4. Conclusions
The highest yield of essential oil was obtained from C. sinensis skin (0.43%).
The essential oil of C. limetta sp. has a higher antioxidant capacity than the essential oils of C. sinensis, C. limetta Risso and C. reticulata, which can be attributed to its high phenolic and total flavonoid content.
The essential oils of the four fruits studied had similar refractive indexes typical of this type of extracts.
Similarly, the essential oil of C. limetta Risso is more stable to oxidation by temperature.
The essential oils of C. limetta sp., C. sinensis, C. limetta Risso and C. reticulata have great potential to be used, in their entirety or in their fractions, in the food or pharmaceutical industry as inputs of natural origin.
Author Contributions
Conceptualization K.L.V., D.M., S.G.C., G.I, A.B.F., E.A.A., E.M.C.; methodology, K.L.V., D.M., S.G.C., R.C., L.D.M., C.R.B., L.T.; software, S.G.C., D.M.; validation, S.G.C., D.M., I.J.Y., E.M.C.; formal analysis, K.L.V., D.M., L.T., I.J.Y., C.Y.; investigation, K.L.V., D.M., S.G.C., E.M.C.; resources, K.L.V., D.M., S.G.C.; writing—original draft preparation, K.L.V., D.M., S.G.C. C.Y.; funding acquisition, L.D.M., G.I., R.C., E.A.A., A.B.F. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Program for Scientific Research and Advanced Studies (PROCIENCIA) - CONTRACT N° PE501080039-2022-PROCIENCIA in the project Development and characterization of bioactive bioplastics from coffee and cocoa by-products. The APC was financed by the Vice Rectorate of Research of the Universidad Nacional Toribio Rodríguez de Mendoza de Amazonas.
Data Availability Statement
Raw data can be provided by the corresponding author upon request.
Acknowledgments
To the National Council of Science and Technology (Concytec), National Program for Scientific Research and Advanced Studies (PROCIENCIA) - Contract N° PE501080039-2022-PROCIENCIA in the project “Nanoencapsulation of citrus peel essential oils extracted with green solvents to develop potentially functional fine aroma chocolates”.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rafiq, S.; Kaul, R.; Sofi, S.; Bashir, N.; Nazir, F.; Ahmad, G. Citrus Peel as a Source of Functional Ingredient: A Review. J. Saudi Soc. Agric. Sci. 2018, 17, 351–358. [Google Scholar] [CrossRef]
- Cvjetko, M.; Vidović, S.; Radojčić, I.; Jokić, S. New Perspective in Extraction of Plant Biologically Active Compounds by Green Solvents. Food Bioprod. Process. 2018, 109, 52–73. [Google Scholar] [CrossRef]
- Gómez-Mejía, E.; Rosales-Conrado, N.; León-González, M.; Madrid, Y. Citrus Peels Waste as a Source of Value-Added Compounds: Extraction and Quantification of Bioactive Polyphenols. Food Chem. 2019, 295, 289–299. [Google Scholar] [CrossRef]
- Satari, B.; Karimi, K. Citrus Processing Wastes: Environmental Impacts, Recent Advances, and Future Perspectives in Total Valorization. Resour. Conserv. Recycl. 2018, 129, 153–167. [Google Scholar] [CrossRef]
- Yingngam, B.; Brantner, A.; Treichler, M.; Brugger, N.; Navabhatra, A.; Nakonrat, P. Optimization of the Eco-Friendly Solvent-Free Microwave Extraction of Limnophila Aromatica Essential Oil. Ind. Crops Prod. 2021, 165, 113443. [Google Scholar] [CrossRef]
- Dao, P.; Tran, N.Y.T.; Tran, Q.; Bach, G.; Lam, T. Kinetics of Pilot-Scale Essential Oil Extraction from Pomelo (Citrus Maxima) Peels: Comparison between Linear and Nonlinear Models. Alexandria Eng. J. 2022, 61, 2564–2572. [Google Scholar] [CrossRef]
- Erandi, N.; Fan, M.; Choi, Y.; Kim, E. Citrus Peel as a Renewable Bioresource : Transforming Waste to Food Additives. J. Funct. Foods 2022, 95, 105163. [Google Scholar] [CrossRef]
- Brahmi, F.; Mokhtari, O.; Legssyer, B.; Hamdani, I.; Asehraou, A.; Hasnaoui, I.; Rokni, Y.; Diass, K.; Oualdi, I.; Tahani, A. Chemical and Biological Characterization of Essential Oils Extracted from Citrus Fruits Peels. Mater. Today Proc. 2021, 45, 7794–7799. [Google Scholar] [CrossRef]
- Wei, L.; Yu, X.; Li, H.; Zhu, M.; Pu, D.; Lu, Q.; Bao, Y.; Zu, Y. Optimization of Solvent-Free Microwave Extraction of Essential Oil from the Fresh Peel of Citrus Medica L. Var. Arcodactylis Swingle by Response Surface Methodology, Chemical Composition and Activity Characterization. Sci. Hortic. (Amsterdam). 2023, 309. [Google Scholar] [CrossRef]
- Teigiserova, D.; Tiruta-Barna, L.; Ahmadi, A.; Hamelin, L.; Thomsen, M. A Step Closer to Circular Bioeconomy for Citrus Peel Waste: A Review of Yields and Technologies for Sustainable Management of Essential Oils. J. Environ. Manage. 2021, 280, 111832. [Google Scholar] [CrossRef]
- Lopresto, C.; Meluso, A.; Di Sanzo, G.; Chakraborty, S.; Calabrò, V. Process-Intensified Waste Valorization and Environmentally Friendly d-Limonene Extraction. Euro-Mediterranean J. Environ. Integr. 2019, 4. [Google Scholar] [CrossRef]
- Long, T.; Lv, X.; Xu, Y.; Yang, G.; Xu, L.Y.; Li, S. Supercritical Fluid CO2 Extraction of Three Polymethoxyflavones from Citri Reticulatae Pericarpium and Subsequent Preparative Separation by Continuous High-Speed Counter-Current Chromatography. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2019, 1124, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Uwineza, P.; Waśkiewicz, A. Recent Advances in Supercritical Fluid Extraction of Natural Bioactive Compounds from Natural Plant Materials. Molecules 2020, 25. [Google Scholar] [CrossRef] [PubMed]
- Visakh, N.; Pathrose, B.; Chellappan, M.; Ranjith, M.; Sindhu, P.; Mathew, D. Chemical Characterisation, Insecticidal and Antioxidant Activities of Essential Oils from Four Citrus Spp. Fruit Peel Waste. Food Biosci. 2022, 50, 102163. [Google Scholar] [CrossRef]
- Negro, V.; Ruggeri, B.; Mancini, G.; Fino, D. Recovery of D-Limonene through Moderate Temperature Extraction and Pyrolytic Products from Orange Peels. J. Chem. Technol. Biotechnol. 2017, 92, 1186–1191. [Google Scholar] [CrossRef]
- Chua, L.Y.W.; Chong, C.H.; Chua, B.L.; Figiel, A. Influence of Drying Methods on the Antibacterial, Antioxidant and Essential Oil Volatile Composition of Herbs: A Review. Food Bioprocess Technol. 2019, 12, 450–476. [Google Scholar] [CrossRef]
- Farahmandfar, R.; Tirgarian, B.; Dehghan, B.; Nemati, A. Comparison of Different Drying Methods on Bitter Orange (Citrus Aurantium L.) Peel Waste: Changes in Physical (Density and Color) and Essential Oil (Yield, Composition, Antioxidant and Antibacterial) Properties of Powders. J. Food Meas. Charact. 2020, 14, 862–875. [Google Scholar] [CrossRef]
- Farahmandfar, R.; Tirgarian, B.; Dehghan, B.; Nemati, A. Changes in Chemical Composition and Biological Activity of Essential Oil from Thomson Navel Orange (Citrus Sinensis L. Osbeck) Peel under Freezing, Convective, Vacuum, and Microwave Drying Methods. Food Sci. Nutr. 2020, 8, 124–138. [Google Scholar] [CrossRef]
- Tunç, M.; Odabaş, H. Single-Step Recovery of Pectin and Essential Oil from Lemon Waste by Ohmic Heating Assisted Extraction/Hydrodistillation: A Multi-Response Optimization Study. Innov. Food Sci. Emerg. Technol. 2021, 74. [Google Scholar] [CrossRef]
- Shaw, D.; Dutt, A.; Paul, V.; Agarwal, A. Valorization of Essential Oils from Citrus Peel Powder Using Hydro- Distillation. Sustain. Chem. Pharm. 2023, 32, 101036. [Google Scholar] [CrossRef]
- Ademosun, A. Citrus Peels Odyssey: From the Waste Bin to the Lab Bench to the Dining Table. Appl. Food Res. 2022, 2, 100083. [Google Scholar] [CrossRef]
- Panwar, D.; Saini, A.; Panesar, P.S.; Chopra, H.K. Unraveling the Scientific Perspectives of Citrus By-Products Utilization: Progress towards Circular Economy. Trends Food Sci. Technol. 2021, 111, 549–562. [Google Scholar] [CrossRef]
- Wang, Z.; Mei, X.; Chen, X.; Rao, S.; Ju, T.; Li, J.; Yang, Z. Extraction and Recovery of Bioactive Soluble Phenolic Compounds from Brocade Orange (Citrus Sinensis) Peels: Effect of Different Extraction Methods Thereon. Lwt 2023, 173, 114337. [Google Scholar] [CrossRef]
- Koolaji, N.; Shammugasamy, B.; Schindeler, A.; Dong, Q.; Dehghani, F.; Valtchev, P. Citrus Peel Flavonoids as Potential Cancer Prevention Agents. Curr. Dev. Nutr. 2020, 4, 4005008. [Google Scholar] [CrossRef]
- Sharma, K.; Mahato, N.; Cho, M.H.; Lee, Y.R. Converting Citrus Wastes into Value-Added Products: Economic and Environmently Friendly Approaches. Nutrition 2017, 34, 29–46. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic Composition, Antioxidant Potential and Health Benefits of Citrus Peel. Food Res. Int. 2020, 132, 109114. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, S.; Chi-Tang, H. Dietary Bioactives and Essential Oils of Lemon and Lime Fruits. Food Sci. Hum. Wellness 2022, 11, 753–764. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.; Kaur, A.; Yadav, M. Insights into the Chemical Composition and Bioactivities of Citrus Peel Essential Oils. Food Res. Int. 2021, 143, 110231. [Google Scholar] [CrossRef]
- Ajikumaran, N.; Rajani, K.; Akhila, S.; Sabulal, B. Citrus Peels Prevent Cancer. Phytomedicine 2018, 50, 231–237. [Google Scholar] [CrossRef]
- Cebi, N.; Erarslan, A. Determination of the Antifungal, Antibacterial Activity and Volatile Compound Composition of Citrus Bergamia Peel Essential Oil. foods 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Maurya, A.; Mohanty, S.; Pal, A.; Chanotiya, C.; Bawankule, D. The Essential Oil from Citrus Limetta Risso Peels Alleviates Skin Inflammation: In-Vitro and in-Vivo Study. J. Ethnopharmacol. 2018, 212, 86–94. [Google Scholar] [CrossRef]
- Jin, H.; Li, H.; Yin, Z.; Zhu, Y.; Lu, A.; Zhao, D.; Li, C. Application of Raman Spectroscopy in the Rapid Detection of Waste Cooking Oil. Food Chem. 2021, 362, 130191. [Google Scholar] [CrossRef] [PubMed]
- Phat, D.; Quyen, N.; Truc, T.; Tan, V. Assessing the Kinetic Model on Extraction of Essential Oil and Chemical Composition from Lemon Peels (Citrus Aurantifolia) by Hydro-Distillation Process. Mater. Today Proc. 2021, 51, 172–177. [Google Scholar] [CrossRef]
- Guo, J.; Gao, Z.; Xia, J.; Ritenour, M.; Li, G.; Shan, Y. Comparative Analysis of Chemical Composition, Antimicrobial and Antioxidant Activity of Citrus Essential Oils from the Main Cultivated Varieties in China. Lwt 2018, 97, 825–839. [Google Scholar] [CrossRef]
- Smeriglio, A.; Alloisio, S.; Raimondo, F.; Denaro, M.; Xiao, J.; Cornara, L.; Trombetta, D. Essential Oil of Citrus Lumia Risso: Phytochemical Profile, Antioxidant Properties and Activity on the Central Nervous System. Food Chem. Toxicol. 2018, 119, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Ndayishimiye, J.; Jum, D.; Soo, B. Antioxidant and Antimicrobial Activity of Oils Obtained from a Mixture of Citrus By-Products Using a Modified Supercritical Carbon Dioxide. J. Ind. Eng. Chem. 2018, 57, 339–348. [Google Scholar] [CrossRef]
- Abedigamba, O.; Mndeme, F.; Mawire, A.; Bahadur, I. Thermo-Physical Properties and Thermal Energy Storage Performance of Two Vegetable Oils. J. Energy Storage 2023, 61, 106774. [Google Scholar] [CrossRef]
- Mora-Zúñiga, A.E.; Treviño-Garza, M.Z.; Amaya, C.A.; Galindo, S.A.; Castillo, S.; Martínez-Rojas, E.; Rodríguez-Rodríguez, J.; Báez-González, J.G. Comparison of Chemical Composition, Physicochemical Parameters, and Antioxidant and Antibacterial Activity of the Essential Oil of Cultivated and Wild Mexican Oregano Poliomintha Longiflora Gray. Plants 2022, 11. [Google Scholar] [CrossRef]
- Castro-Alayo, E.M.; Torrejón-Valqui, L.; Cayo-Colca, I.S.; Cárdenas-Toro, F.P. Evaluation of the Miscibility of Novel Cocoa Butter Equivalents by Raman Mapping and Multivariate Curve Resolution–Alternating Least Squares. Foods 2021, 10. [Google Scholar] [CrossRef]
- Cruz, J.F.M.; Leite, P.B.; Soares, S.E.; Bispo, E. da S. Bioactive Compounds in Different Cocoa (Theobroma Cacao, L) Cultivars during Fermentation. Food Sci. Technol. 2015, 35, 279–284. [Google Scholar] [CrossRef]
- Lubinska-szczygeł, M.; Kuczy, A.; Polkowska, Z.; Katrich, E.; Gorinstein, S. Determination of the Major By-Products of Citrus Hystrix Peel and Their Characteristics in the Context of Utilization in the Industry. Molecules 2023, 28, 3. [Google Scholar] [CrossRef] [PubMed]
- Dhakane-Lad, J.; Kar, A. Supercritical CO2 Extraction of Lycopene from Pink Grapefruit (Citrus Paradise Macfad) and Its Degradation Studies during Storage. Food Chem. 2021, 361, 130113. [Google Scholar] [CrossRef]
- Napoli, M. Di; Castagliuolo, G.; Badalamenti, N.; Maresca, V.; Basile, A.; Bruno, M.; Varcamonti, M.; Zanfardino, A. Citrus Aurantium ‘ Crispifolia ’ Essential Oil : A Promise for Nutraceutical Applications. Molecules 2023, 3, 153–164. [Google Scholar] [CrossRef]
- Raspo, M.; Vignola, M.; Andreatta, A.; Juliani, H. Antioxidant and Antimicrobial Activities of Citrus Essential Oils from Argentina and the United States. Food Biosci. 2020, 36, 100651. [Google Scholar] [CrossRef]
- Sarrou, E.; Chatzopoulou, P.; Dimassi-Theriou, K.; Therios, I. Volatile Constituents and Antioxidant Activity of Peel, Flowers and Leaf Oils of Citrus Aurantium L. Growing in Greece. Molecules 2013, 18, 10639–10647. [Google Scholar] [CrossRef] [PubMed]
- Padilla-De la Rosa, J.D.; Manzano-Alfaro, M.D.; Gómez-Huerta, J.R.; Arriola-Guevara, E.; Guatemala-Morales, G.; Cardador-Martínez, A.; Estarrón-Espinosa, M. Innovation in a Continuous System of Distillation by Steam to Obtain Essential Oil from Persian Lime Juice (Citrus Latifolia Tanaka). Molecules 2021, 26. [Google Scholar] [CrossRef]
- Mohammadi, N.; Ostovar, N. Essential Oil Composition of Polylophium Involucratum and Evaluation of Antioxidant Capacity of Seeds Ethanolic Extracts by DSC. Food Chem. Adv. 2022, 1, 100066. [Google Scholar] [CrossRef]
- Wang, H.; Xin, Y.; Ma, H.; Fang, P.; Li, C.; Wan, X.; He, Z.; Jia, J.; Ling, Z. Rapid Detection of Chinese-Specific Peony Seed Oil by Using Confocal Raman Spectroscopy and Chemometrics. Food Chem. 2021, 362, 130041. [Google Scholar] [CrossRef]
- Akolade, J.O.; Nasir-Naeem, K.O.; Swanepoel, A.; Yusuf, A.A.; Balogun, M.; Labuschagne, P. CO2-Assisted Production of Polyethylene Glycol / Lauric Acid Microparticles for Extended Release of Citrus Aurantifolia Essential Oil. J. CO2 Util. 2020, 38, 375–384. [Google Scholar] [CrossRef]
- Mahato, N.; Sinha, M.; Sharma, K.; Koteswararao, R.; Cho, M.H. Modern Extraction and Purification Techniques for Obtaining High Purity Food-Grade Bioactive Compounds and Value-Added Co-Products from Citrus Wastes. Foods 2019, 8, 1–81. [Google Scholar] [CrossRef]
- Fan, H.; Wu, Q.; Simon, J.; Shyi-Neng, L.; Chi -tang, H. Authenticity Analysis of Citrus Essential Oils by HPLC-UV-MS on Oxygenated Heterocyclic Components. J. Food Drug Anal. 2015, 23, 30–39. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).