Submitted:
14 July 2023
Posted:
17 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
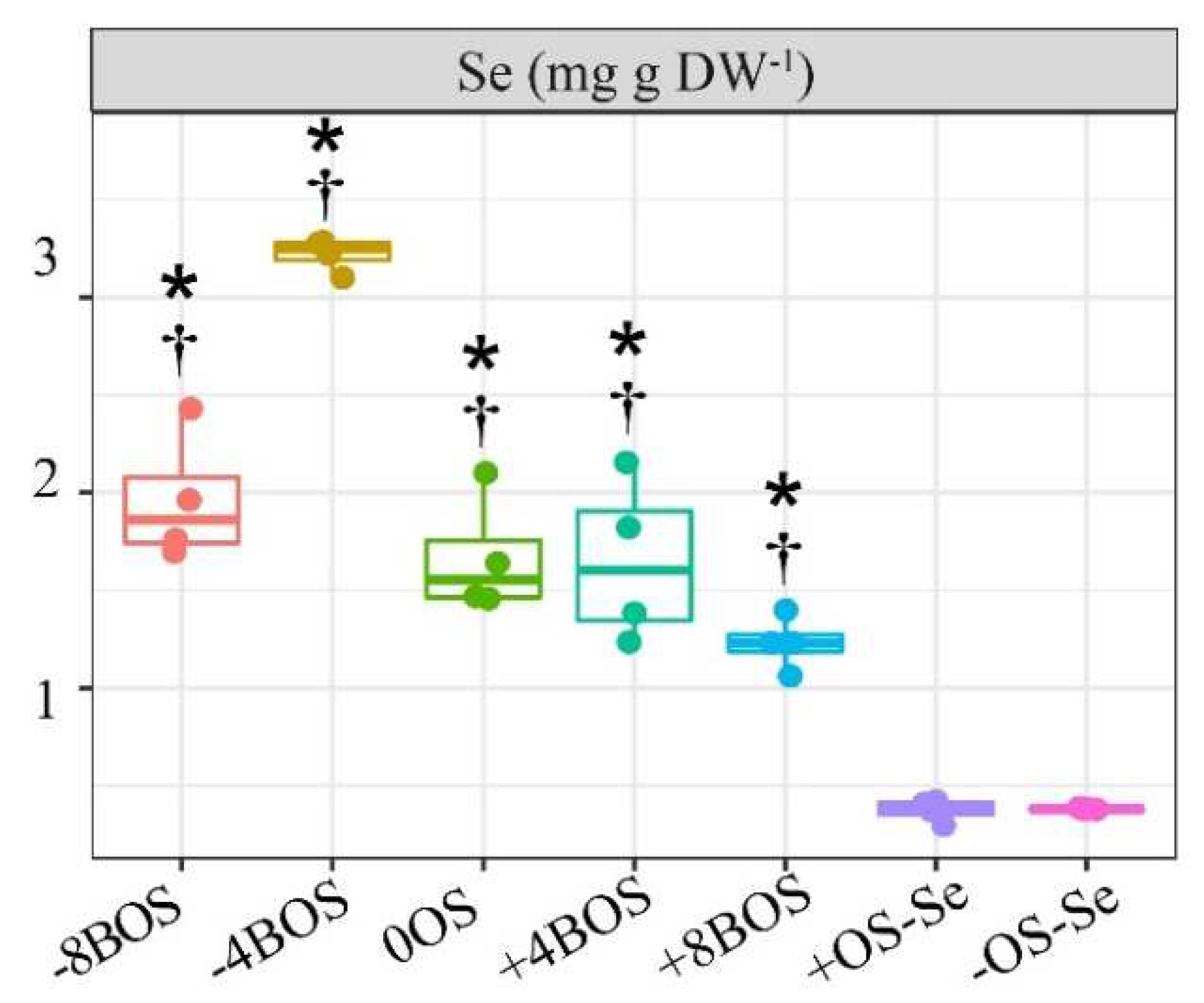
2.1. Analysis of Se content
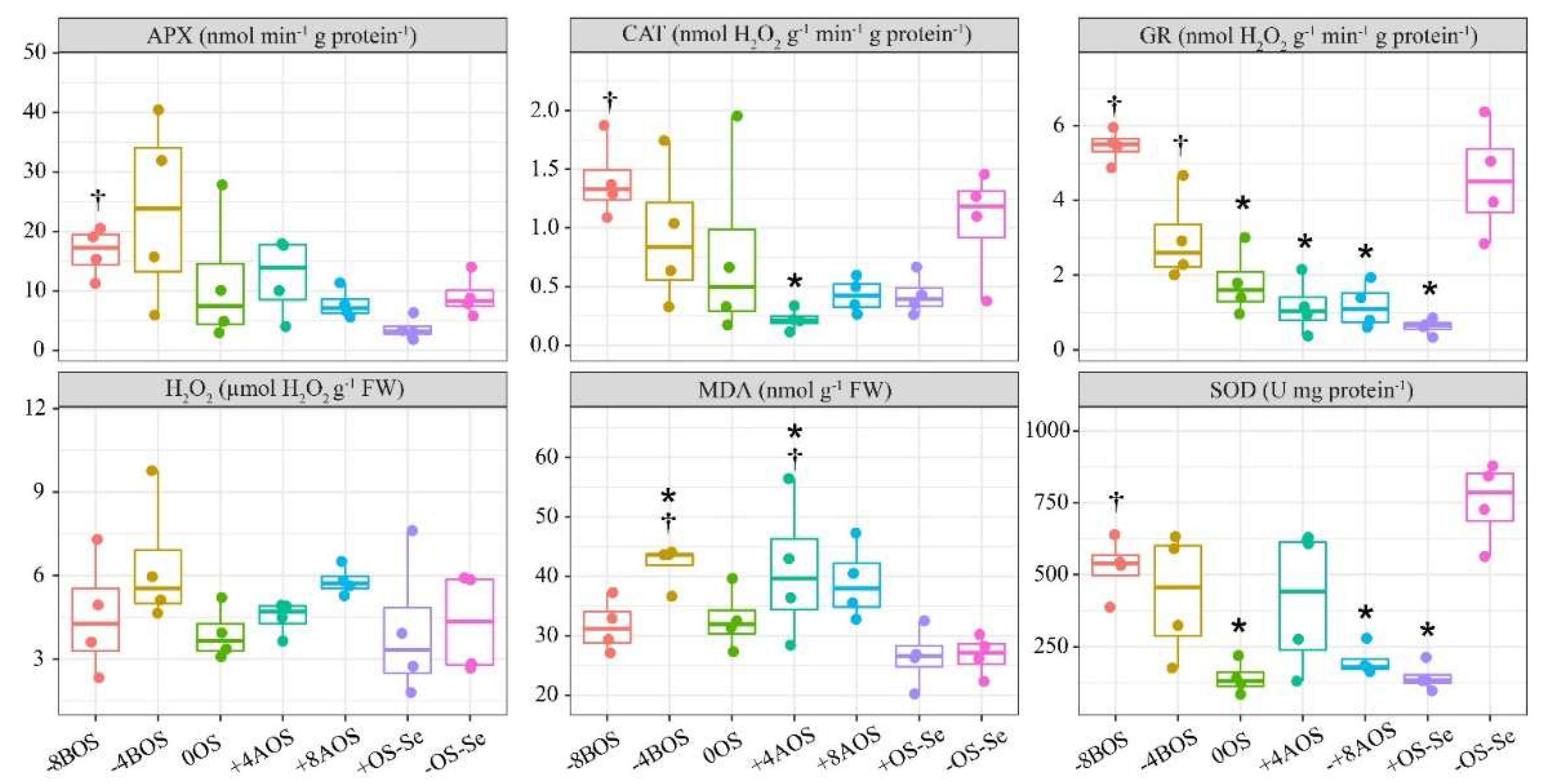
2.2. H2O2, MDA, and antioxidant enzymes (SOD, CAT, APX, GR)
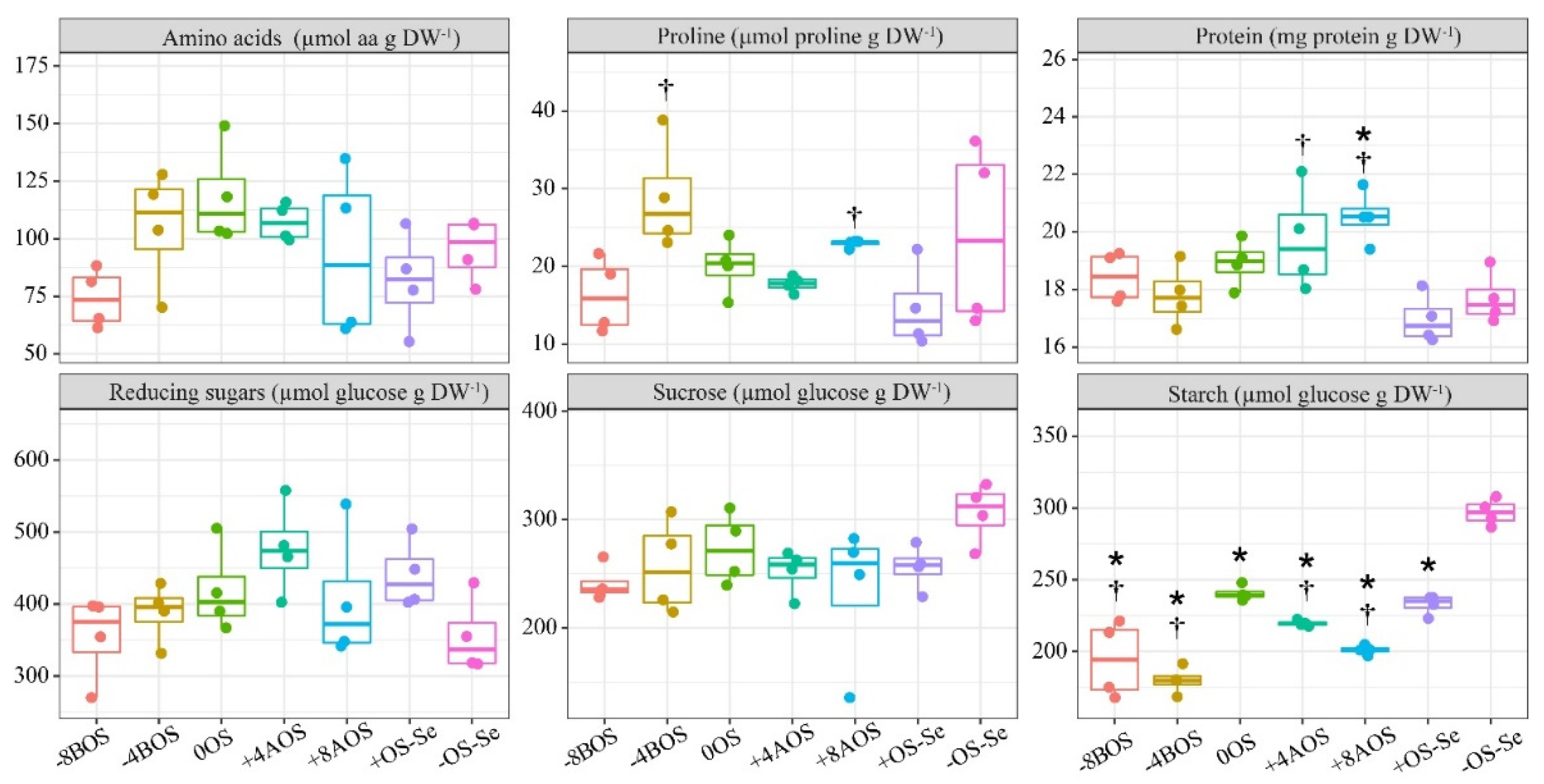
2.3. Carbohydrates, Protein, Amino Acids, Proline
2.4. Chlorophyll Fluorescence parameters (MultispeQ®)
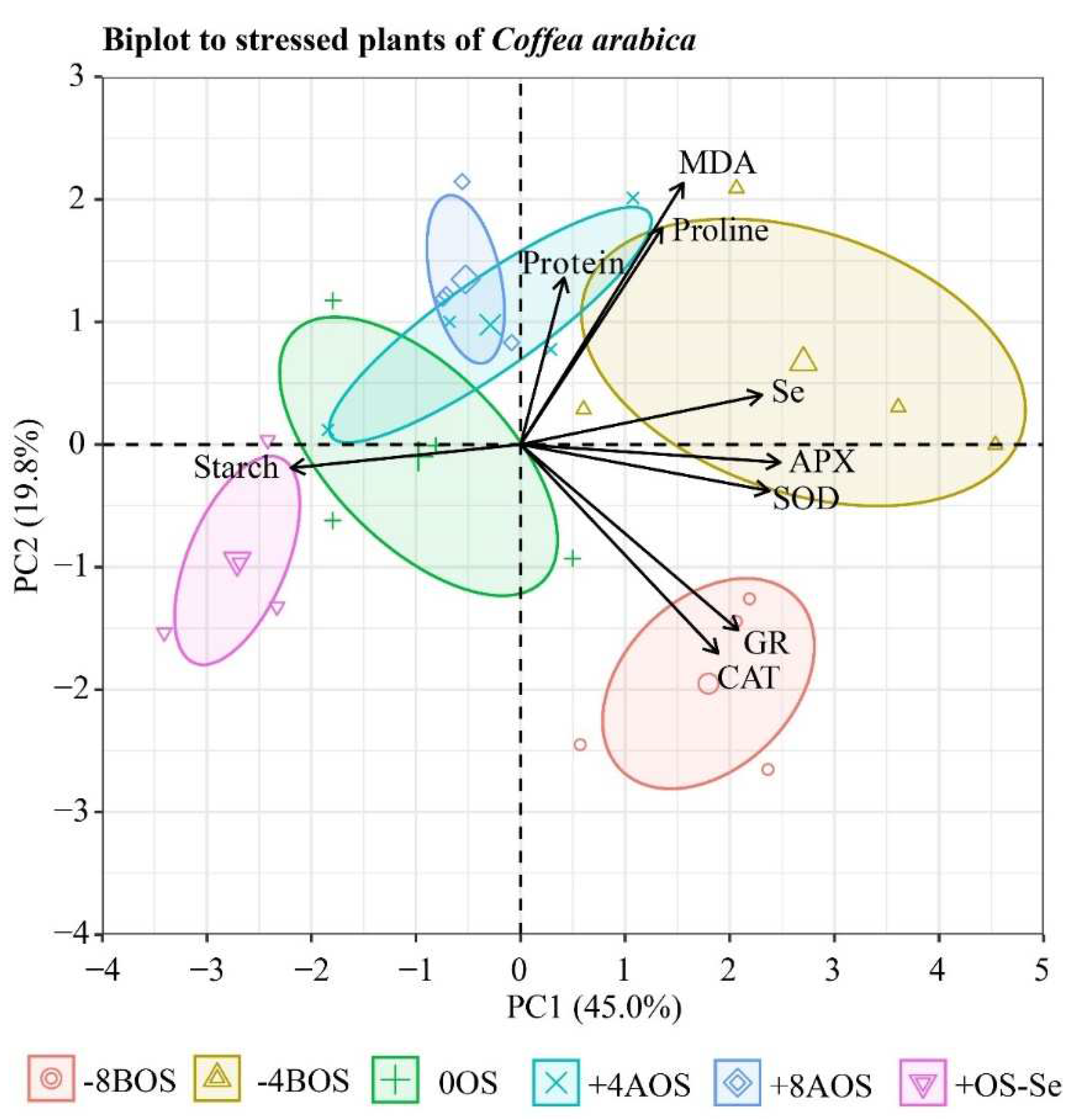
2.5. Principal component analysis (PCA)
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Study Site
4.2. Experimental design and treatments
4.3. Application of foliar treatments
4.4. Osmotic stress imposition and leaf water status
4.5. Leaf sample collection and preparation
4.6. Assessments
4.6.1. Selenium content in leaves and detection limit (LOD and LOQ)
4.6.2. Carbohydrates, total protein, total free amino acids
4.6.3. Proline
4.6.4. Antioxidant enzymes (SOD, CAT, APX, GR)
4.6.5. Hydrogen peroxide and lipid peroxidation (malondialdehyde)
4.6.6. Chlorophyll fluorescence parameters (MultispeQ®)
4.7. Statistical analysis and PCA
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change 2014, 151.
- Ortiz-Bobea, A.; Ault, T.R.; Carrillo, C.M.; Chambers, R.G.; Lobell, D.B. Anthropogenic Climate Change Has Slowed Global Agricultural Productivity Growth. Nat. Clim. Chang. 2021, 11, 306–312. [Google Scholar] [CrossRef]
- Kotz, M.; Levermann, A.; Wenz, L. The Effect of Rainfall Changes on Economic Production. Nature 2022, 601, 223–227. [Google Scholar] [CrossRef]
- Cerdán, C.R.; Rebolledo, M.C.; Soto, G.; Rapidel, B.; Sinclair, F.L. Local Knowledge of Impacts of Tree Cover on Ecosystem Services in Smallholder Coffee Production Systems. Agric. Syst. 2012, 110, 119–130. [Google Scholar] [CrossRef]
- Chain-Guadarrama, A.; Martínez-Salinas, A.; Aristizábal, N.; Ricketts, T.H. Ecosystem Services by Birds and Bees to Coffee in a Changing Climate: A Review of Coffee Berry Borer Control and Pollination. Agric. Ecosyst. Environ. 2019, 280, 53–67. [Google Scholar] [CrossRef]
- Davis, A.P.; Govaerts, R.; Bridson, D.M.; Stoffelen, P. An Annotated Taxonomic Conspectus of the Genus Coffea (Rubiaceae). Bot. J. Linn. Soc. 2006, 152, 465–512. [Google Scholar] [CrossRef]
- DaMatta, F.M.; Ramalho, J.D.C. Impacts of Drought and Temperature Stress on Coffee Physiology and Production: A Review. Brazilian J. Plant Physiol. 2006, 18, 55–81. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Manivannan, P.; Wahid, A.; Farooq, M.; Al-Juburi, H.J.; Somasundaram, R.; Panneerselvam, R. Drought Stress in Plants: A Review on Morphological Characteristics and Pigments Composition. Int. J. Agric. Biol. 2009, 11, 100–105. [Google Scholar]
- Zhu, J.K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Z.; Waraich, E.A.; Akhtar, S.; Anjum, S.; Ahmad, T.; Mahboob, W.; Hafeez, O.B.A.; Tapera, T.; Labuschagne, M.; Rizwan, M. Physiological Responses of Wheat to Drought Stress and Its Mitigation Approaches. Acta Physiol. Plant. 2018, 40, 1–13. [Google Scholar] [CrossRef]
- Nawaz, F.; Ahmad, R.; Ashraf, M.Y.Y.; Waraich, E.A.A.; Khan, S.Z.Z. Effect of Selenium Foliar Spray on Physiological and Biochemical Processes and Chemical Constituents of Wheat under Drought Stress. Ecotoxicol. Environ. Saf. 2015, 113, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, G.; Sun, H.; Ma, L.; Guo, Y.; Zhao, Z.; Gao, H.; Mei, L. Effects of Drought Stress on Photosynthesis and Photosynthetic Electron Transport Chain in Young Apple Tree Leaves. Biol. Open 2018, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Semedo, J.N.; Rodrigues, W.P.; Dubberstein, D.; Martins, M.Q.; Martins, L.D.; Pais, I.P.; Rodrigues, A.P.; Leitão, A.E.; Partelli, F.L.; Campostrini, E.; et al. Coffee Responses to Drought, Warming and High [CO2] in a Context of Future Climate Change Scenarios. In Climate Change Management; Alves, F., Leal Filho, W., Azeiteiro, U., Eds.; Climate Change Management; Springer International Publishing: Cham, 2018; pp. 465–477. ISBN 978-3-319-72873-5. [Google Scholar]
- Feng, R.; Wei, C.; Tu, S. The Roles of Selenium in Protecting Plants against Abiotic Stresses. Environ. Exp. Bot. 2013, 87, 58–68. [Google Scholar] [CrossRef]
- Bhadwal, S.; Sharma, S. Selenium Alleviates Carbohydrate Metabolism and Nutrient Composition in Arsenic Stressed Rice Plants. Rice Sci. 2022, 29, 385–396. [Google Scholar] [CrossRef]
- Prasad, R.; Shivay, Y.S. Agronomic Biofortification of Plant Foods with Minerals, Vitamins and Metabolites with Chemical Fertilizers and Liming. J. Plant Nutr. 2020, 43, 1534–1554. [Google Scholar] [CrossRef]
- Abul-Soud, M.; Abd-Elrahman, S. Foliar Selenium Application to Improve the Tolerance of Eggplant Grown under Salt Stress Conditions. Int. J. Plant Soil Sci. 2016, 9, 1–10. [Google Scholar] [CrossRef]
- Ravello, R.A.V.; de Oliveira, C.; Lessa, J.; Boas, L.V.V.; de Castro, E.M.; Guilherme, L.R.G.; Lopes, G. Selenium Application Influenced Selenium Biofortification and Physiological Traits in Water-Deficit Common Bean Plants. Crop Pasture Sci. 2021, 73, 44–55. [Google Scholar] [CrossRef]
- Sousa, G.F. de; Silva, M.A.; de Morais, E.G.; Van Opbergen, G.A.Z.; Van Opbergen, G.G.A.Z.; de Oliveira, R.R.; Amaral, D.; Brown, P.; Chalfun-Junior, A.; Guilherme, L.R.G. Selenium Enhances Chilling Stress Tolerance in Coffee Species by Modulating Nutrient, Carbohydrates, and Amino Acids Content. Front. Plant Sci. 2022, 13, 1–15. [Google Scholar] [CrossRef]
- Luo, H.; Xing, P.; Liu, J.; Pan, S.; Tang, X.; Duan, M. Selenium Improved Antioxidant Response and Photosynthesis in Fragrant Rice (Oryza Sativa L.) Seedlings during Drought Stress. Physiol. Mol. Biol. Plants 2021, 27, 2849–2858. [Google Scholar] [CrossRef]
- Thuc, L.V.; Sakagami, J.I.; Hung, L.T.; Huu, T.N.; Khuong, N.Q.; Vi, L.L.V. Foliar Selenium Application for Improving Drought Tolerance of Sesame (Sesamum Indicum L.). Open Agric. 2021, 6, 93–101. [Google Scholar] [CrossRef]
- Becerra-Vázquez, Á.G.; Coates, R.; Sánchez-Nieto, S.; Reyes-Chilpa, R.; Orozco-Segovia, A. Effects of Seed Priming on Germination and Seedling Growth of Desiccation-Sensitive Seeds from Mexican Tropical Rainforest. J. Plant Res. 2020, 133, 855–872. [Google Scholar] [CrossRef]
- WANG, X.; LIU, F.; JIANG, D. Priming: A Promising Strategy for Crop Production in Response to Future Climate. J. Integr. Agric. 2017, 16, 2709–2716. [Google Scholar] [CrossRef]
- Franzoni, G.; Cocetta, G.; Prinsi, B.; Ferrante, A.; Espen, L. Biostimulants on Crops: Their Impact under Abiotic Stress Conditions. Horticulturae 2022, 8. [Google Scholar] [CrossRef]
- Nephali, L.; Piater, L.A.; Dubery, I.A.; Patterson, V.; Huyser, J.; Burgess, K.; Tugizimana, F. Biostimulants for Plant Growth and Mitigation of Abiotic Stresses: A Metabolomics Perspective. Metabolites 2020, 10, 1–26. [Google Scholar] [CrossRef]
- Jolayemi, O.L.; Malik, A.H.; Ekblad, T.; Fredlund, K.; Olsson, M.E.; Johansson, E. Protein-Based Biostimulants to Enhance Plant Growth—State-of-the-Art and Future Direction with Sugar Beet as an Example. Agronomy 2022, 12. [Google Scholar] [CrossRef]
- Upadhyaya, H.; Sahoo, L.; Panda, S.K. Molecular Physiology of Osmotic Stress in Plants. In Molecular Stress Physiology of Plants; Springer India: India, 2013; ISBN 9788132208075. [Google Scholar]
- Reddy, Y.A.N.; Reddy, Y.N.P.; Ramya, V.; Suma, L.S.; Reddy, A.B.N.; Krishna, S.S. Drought Adaptation: Approaches for Crop Improvement; Elsevier Inc., 2020; ISBN 9780128200896.
- Moustakas, M.; Sperdouli, I.; Moustaka, J. Early Drought Stress Warning in Plants: Color Pictures of Photosystem II Photochemistry. Climate 2022, 10, 179. [Google Scholar] [CrossRef]
- Suma, L.S. Characterization of Selected Germplasm Accessions for Drought Tolerance in Finger Millet (Eleusine Coracana (L.) Gaertn, University of Agricultural Sciences, GKVK., 2014.
- Bartlett, M.K.; Scoffoni, C.; Sack, L. The Determinants of Leaf Turgor Loss Point and Prediction of Drought Tolerance of Species and Biomes: A Global Meta-Analysis. Ecol. Lett. 2012, 15, 393–405. [Google Scholar] [CrossRef]
- Read, J.; Sanson, G.D.; Garine-Wichatitsky, M. d.; Jaffre, T. Sclerophylly in Two Contrasting Tropical Environments: Low Nutrients vs. Low Rainfall. Am. J. Bot. 2006, 93, 1601–1614. [Google Scholar] [CrossRef]
- Kostopoulou, P.; Barbayiannis, N.; Noitsakis, B. Water Relations of Yellow Sweetclover under the Synergy of Drought and Selenium Addition. Plant Soil 2010, 330, 65–71. [Google Scholar] [CrossRef]
- Ribeiro, C.; Stitt, M.; Hotta, C.T. How Stress Affects Your Budget—Stress Impacts on Starch Metabolism. Front. Plant Sci. 2022, 13, 1–9. [Google Scholar] [CrossRef]
- Thalmann, M.; Santelia, D. Starch as a Determinant of Plant Fitness under Abiotic Stress. New Phytol. 2017, 214, 943–951. [Google Scholar] [CrossRef]
- Thalmann, M.; Pazmino, D.; Seung, D.; Horrer, D.; Nigro, A.; Meier, T.; Kölling, K.; Pfeifhofer, H.W.; Zeeman, S.C.; Santelia, D. Regulation of Leaf Starch Degradation by Abscisic Acid Is Important for Osmotic Stress Tolerance in Plants. Plant Cell 2016, 28, 1860–1878. [Google Scholar] [CrossRef] [PubMed]
- Kempa, S.; Krasensky, J.; Dal Santo, S.; Kopka, J.; Jonak, C. A Central Role of Abscisic Acid in Stress-Regulated Carbohydrate Metabolism. PLoS One 2008, 3. [Google Scholar] [CrossRef]
- Danso, O.P.; Asante-badu, B.; Zhang, Z.; Song, J.; Wang, Z.; Yin, X. Selenium Biofortification : Strategies, Progress and Challenges. 2023, 1–29.
- Sitnicka, D.; Orzechowski, S. Cold-Induced Starch Degradation in Potato Leaves — Intercultivar Differences in the Gene Expression and Activity of Key Enzymes. Biol. Plant. 2014, 58, 659–666. [Google Scholar] [CrossRef]
- Mateus, M.P. de B.; Tavanti, R.F.R.; Tavanti, T.R.; Santos, E.F.; Jalal, A.; Reis, A.R. dos Selenium Biofortification Enhances ROS Scavenge System Increasing Yield of Coffee Plants. Ecotoxicol. Environ. Saf. 2021, 209. [Google Scholar] [CrossRef]
- Silva, M.A.; Sousa, G.F. De; Bañuelos, G.; Amaral, D.; Brown, P.H. Selenium Speciation in Se-Enriched Soybean Grains from Biofortified Plants Grown under Different Methods of Selenium Application. 2023, 1–13.
- Silva, M.A.; de Sousa, G.F.; Corguinha, A.P.B.; de Lima Lessa, J.H.; Dinali, G.S.; Oliveira, C.; Lopes, G.; Amaral, D.; Brown, P.; Guilherme, L.R.G. Selenium Biofortification of Soybean Genotypes in a Tropical Soil via Se-Enriched Phosphate Fertilizers. Front. Plant Sci. 2022, 13, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Liu, K.; Li, M.; Zhang, W.; Zhao, X.; Zhao, Z.; Liu, X. Difference of Selenium Uptake and Distribution in the Plant and Selenium Form in the Grains of Rice with Foliar Spray of Selenite or Selenate at Different Stages. F. Crop. Res. 2017, 211, 165–171. [Google Scholar] [CrossRef]
- Manojlović, M.S.; Lončarić, Z.; Cabilovski, R.R.; Popović, B.; Karalić, K.; Ivezić, V.; Ademi, A.; Singh, B.R. Biofortification of Wheat Cultivars with Selenium. Acta Agric. Scand. Sect. B Soil Plant Sci. 2019, 69, 715–724. [Google Scholar] [CrossRef]
- Pilon-Smits, E.A.H.; Winkel, L.H.E.; Lin, Z.-Q. Selenium in Plants: Molecular, Physiological, Ecological and Evolutionary Aspects; Pilon-Smits, E.A.H., Winkel, L.H.E., Lin, Z.-Q., Eds.; Plant Ecophysiology; 1st ed.; Springer International Publishing: Cham, 2017; ISBN 978-3-319-56248-3. [Google Scholar]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Al Mahmud, J.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 1–52. [Google Scholar] [CrossRef]
- Gupta, M.; Gupta, S. An Overview of Selenium Uptake, Metabolism, and Toxicity in Plants. Front. Plant Sci. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Zhang, L.; Chu, C. Selenium Uptake, Transport, Metabolism, Reutilization, and Biofortification in Rice. Rice 2022, 15. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, Z.; Luo, L.Y.; Fu, P.N.; Wang, Q.; Li, H.F. Selenium Uptake and Biotransformation in Brassica Rapa Supplied with Selenite and Selenate: A Hydroponic Work with HPLC Speciation and RNA-Sequencing. J. Agric. Food Chem. 2019. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Lin, W.; Jiao, H.; Liu, J.; Chan, L.; Liu, X.; Wang, R.; Chen, T. Uptake, Transport, and Metabolism of Selenium and Its Protective Effects against Toxic Metals in Plants: A Review. Metallomics 2021, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sors, T.G.; Ellis, D.R.; Salt, D.E. Selenium Uptake, Translocation, Assimilation and Metabolic Fate in Plants. Photosynth. Res. 2005, 86, 373–389. [Google Scholar] [CrossRef]
- Lanza, M.G.D.B.; Reis, A.R. dos Roles of Selenium in Mineral Plant Nutrition: ROS Scavenging Responses against Abiotic Stresses. Plant Physiol. Biochem. 2021, 164, 27–43. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.W.; Pilon-Smits, E.A.H.; Schiavon, M. Mechanisms of Selenium Hyperaccumulation in Plants: A Survey of Molecular, Biochemical and Ecological Cues. Biochim. Biophys. Acta - Gen. Subj. 2018, 1862, 2343–2353. [Google Scholar] [CrossRef]
- Konze, J.R.; Schilling, N.; Kende, H. Enhancement of Ethylene Formation by Selenoamino Acids. Plant Physiol. 1978, 62, 397–401. [Google Scholar] [CrossRef]
- Tangjaidee, P.; Swedlund, P.; Xiang, J.; Yin, H.; Quek, S.Y. Selenium-Enriched Plant Foods: Selenium Accumulation, Speciation, and Health Functionality. Front. Nutr. 2023, 9, 1–19. [Google Scholar] [CrossRef]
- Akladious, S.A. Influence of Different Soaking Times with Selenium on Growth, Metabolic Activities of Wheat Seedlings under Low Temperature Stress. African J. Biotechnol. 2012, 11, 14792–14804. [Google Scholar] [CrossRef]
- Schiavon, M.; Lima, L.W.; Jiang, Y.; Hawkesford, M.J. Effects of Selenium on Plant Metabolism and Implications for Crops and Consumers BT - Selenium in Plants: Molecular, Physiological, Ecological and Evolutionary Aspects. In; Pilon-Smits, E.A.H., Winkel, L.H.E., Lin, Z.-Q., Eds.; Springer International Publishing: Cham, 2017; pp. 257–275. ISBN 978-3-319-56249-0. [Google Scholar]
- Trippe, R.C.; Pilon-Smits, E.A.H. Selenium Transport and Metabolism in Plants: Phytoremediation and Biofortification Implications. J. Hazard. Mater. 2021, 404, 124178. [Google Scholar] [CrossRef]
- Elkelish, A.A.; Soliman, M.H.; Alhaithloul, H.A.; El-Esawi, M.A. Selenium Protects Wheat Seedlings against Salt Stress-Mediated Oxidative Damage by up-Regulating Antioxidants and Osmolytes Metabolism. Plant Physiol. Biochem. 2019, 137, 144–153. [Google Scholar] [CrossRef]
- Banerjee, A.; Roychoudhury, A. Role of Glutathione in Plant Abiotic Stress Tolerance. React. Oxyg. Nitrogen Sulfur Species Plants Prod. Metab. Signal. Def. Mech. 2019, 1, 159–172. [Google Scholar] [CrossRef]
- Rahman, M.; Rahman, K.; Sathi, K.S.; Alam, M.M.; Nahar, K.; Fujita, M.; Hasanuzzaman, M. Supplemental Selenium and Boron Mitigate Salt-induced Oxidative Damages in Glycine Max L. Plants 2021, 10, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Adnan, M. Application of Selenium A Useful Way to Mitigate Drought Stress: A Review. Open Access J. Biog. Sci. Res. 2020, 3. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Akbar, A.; Parveen, A.; Rasheed, R.; Hussain, I.; Iqbal, M. Phenological Application of Selenium Differentially Improves Growth, Oxidative Defense and Ion Homeostasis in Maize under Salinity Stress. Plant Physiol. Biochem. 2018, 123, 268–280. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; García-Caparrós, P.; Parvin, K.; Zulfiqar, F.; Ahmed, N.; Fujita, M. Selenium Supplementation and Crop Plant Tolerance to Metal/Metalloid Toxicity. Front. Plant Sci. 2022, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.M.; Rimoldi Tavanti, R.F.; Gratão, P.L.; Alcock, T.D.; Reis, A.R. dos Selenate and Selenite Affect Photosynthetic Pigments and ROS Scavenging through Distinct Mechanisms in Cowpea (Vigna Unguiculata (L.) Walp) Plants. Ecotoxicol. Environ. Saf. 2020, 201, 110777. [Google Scholar] [CrossRef] [PubMed]
- DaMatta, F.M.; Ronchi, C.P.; Maestri, M.; Barros, R.S. Ecophysiology of Coffee Growth and Production. Brazilian J. Plant Physiol. 2007, 19, 485–510. [Google Scholar] [CrossRef]
- van der Vossen, H.; Bertrand, B.; Charrier, A. Next Generation Variety Development for Sustainable Production of Arabica Coffee (Coffea Arabica L.): A Review. Euphytica 2015, 204, 243–256. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The Water-Culture Method for Growing Plants without Soil. Calif. Agr. Expt. Sta. Circ. 1950, 347, 1–32. [Google Scholar]
- Salgado, O.G.G.; Teodoro, J.C.; Alvarenga, J.P.; de Oliveira, C.; de Carvalho, T.S.; Domiciano, D.; Marchiori, P.E.R.; Guilherme, L.R.G. Cerium Alleviates Drought-Induced Stress in Phaseolus Vulgaris. J. Rare Earths 2020, 38, 324–331. [Google Scholar] [CrossRef]
- Villela, F.A.; Doni Filho, L.; Sequeira, E.L. Tabela de Potencial Osmótico Em Função Da Concentração de Polietileno Glicol 6.000 e Da Temperatura. Pesqui. Agropecuária Bras. 1991, 26, 1957–1968. [Google Scholar]
- Fernandes, I.; Marques, I.; Paulo, O.S.; Batista, D.; Partelli, F.L.; Lidon, F.C.; Damatta, F.M.; Ramalho, J.C.; Ribeiro-Barros, A.I. Understanding the Impact of Drought in Coffea Genotypes: Transcriptomic Analysis Supports a Common High Resilience to Moderate Water Deficit but a Genotype Dependent Sensitivity to Severe Water Deficit. Agronomy 2021, 11, 1–27. [Google Scholar] [CrossRef]
- Scholander, P.F.; Bradstreet, E.D.; Hemmingsen, E.A.; Hammel, H.T. Sap Pressure in Vascular Plants. Science (80-. ). 1965, 148, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Kuhlgert, S.; Austic, G.; Zegarac, R.; Osei-Bonsu, I.; Hoh, D.; Chilvers, M.I.; Roth, M.G.; Bi, K.; TerAvest, D.; Weebadde, P.; et al. MultispeQ Beta: A Tool for Large-Scale Plant Phenotyping Connected to the Open PhotosynQ Network. R. Soc. Open Sci. 2016, 3. [Google Scholar] [CrossRef]
- 74. U.S. EPA Method 3051A (SW-846): Microwave Assisted Acid Digestion of Sediments, Sludges, and Oils. Ятыатат, 2007; 30, 75.
- Silva Junior, E.C.; Wadt, L.H.O.; Silva, K.E.; Lima, R.M.B.; Batista, K.D.; Guedes, M.C.; Carvalho, G.S.; Carvalho, T.S.; Reis, A.R.; Lopes, G.; et al. Natural Variation of Selenium in Brazil Nuts and Soils from the Amazon Region. Chemosphere 2017, 188, 650–658. [Google Scholar] [CrossRef]
- Zanandrea, I.; Alves, J.D.; Deuner, S.; Goulart, P. de F.P.; Henrique, P.D.C.; Silveira, N.M. Tolerance of Sesbania Virgata Plants to Flooding. Aust. J. Bot. 2010, 57, 661. [Google Scholar] [CrossRef]
- DISCHE, Z. General Color Reactions. In Carbohydrate chemistry; WHISTLER, R.L.., WOLFRAM, M.L., Eds.; New York: Academic: New York:, 1962; pp. 477–512. [Google Scholar]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Yemm, E.W.; Willis, A.J. The Estimation of Carbohydrates in Plant Extracts by Anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid Determination of Free Proline for Water-Stress Studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Biemelt, S.; Keetman, U.; Albrecht, G. Re-Aeration Following Hypoxia or Anoxia Leads to Activation of the Antioxidative Defense System in Roots of Wheat Seedlings. Plant Physiol. 1998, 116, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Giannopolitis, C.N.; Ries, S.K. Superoxide Dismutases. 1977, 309–314.
- Havir, E.A.; McHale, N.A. Biochemical and Developmental Characterization of Multiple Forms of Catalase in Tobacco Leaves. Plant Physiol. 1987, 84, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Asada, K. Hydrogen Peroxide Is Scavenged by Ascorbate-Specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Schaedle, M.; Bassham, J.A. Chloroplast Glutathione Reductase. Plant Physiol. 1977, 59, 1011–1012. [Google Scholar] [CrossRef] [PubMed]
- García-Limones, C.; Hervás, A.; Navas-Cortés, J.A.; Jiménez-Díaz, R.M.; Tena, M. Induction of an Antioxidant Enzyme System and Other Oxidative Stress Markers Associated with Compatible and Incompatible Interactions between Chickpea (Cicer Arietinum L.) and Fusarium Oxysporum f. Sp. Ciceris. Physiol. Mol. Plant Pathol. 2002, 61, 325–337. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative Stress and Some Antioxidant Systems in Acid Rain-Treated Bean Plants. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Loreto, F.; Velikova, V. Isoprene Produced by Leaves Protects the Photosynthetic Apparatus against Ozone Damage, Quenches Ozone Products, and Reduces Lipid Peroxidation of Cellular Membranes. Plant Physiol. 2001, 127, 1781–1787. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal Lipid Peroxidation. In Methods in Enzymology; 1978; Vol. 52, pp. 302–310 ISBN 9780121819521.
- Silva, V.M.; Rimoldi Tavanti, R.F.; Gratão, P.L.; Alcock, T.D.; Reis, A.R. dos Selenate and Selenite Affect Photosynthetic Pigments and ROS Scavenging through Distinct Mechanisms in Cowpea (Vigna Unguiculata (L.) Walp) Plants. Ecotoxicol. Environ. Saf. 2020, 201, 110777. [Google Scholar] [CrossRef]
- Chambers, J.M.; Hastie, T.J. Statistical Models in S; Wadsworth & Brooks: Pacific Grove, CA. , 1992; ISBN 9780534167646.
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biometrical J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- R Core Team R: A Language and Environment for Statistical Computing 2022.
- Pinheiro, J.; Bates, D. Linear and Nonlinear Mixed Effects Models Available online: https://cran.r-project.org/package=nlme.
- Kassambara, A.; Mundt, F. Extract and Visualize the Results of Multivariate Data Analyses Available online: https://cran.r-project.org/package=factoextra.
- Lê, S.; Josse, J.; Husson, F. FactoMineR : An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




