Submitted:
14 July 2023
Posted:
17 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
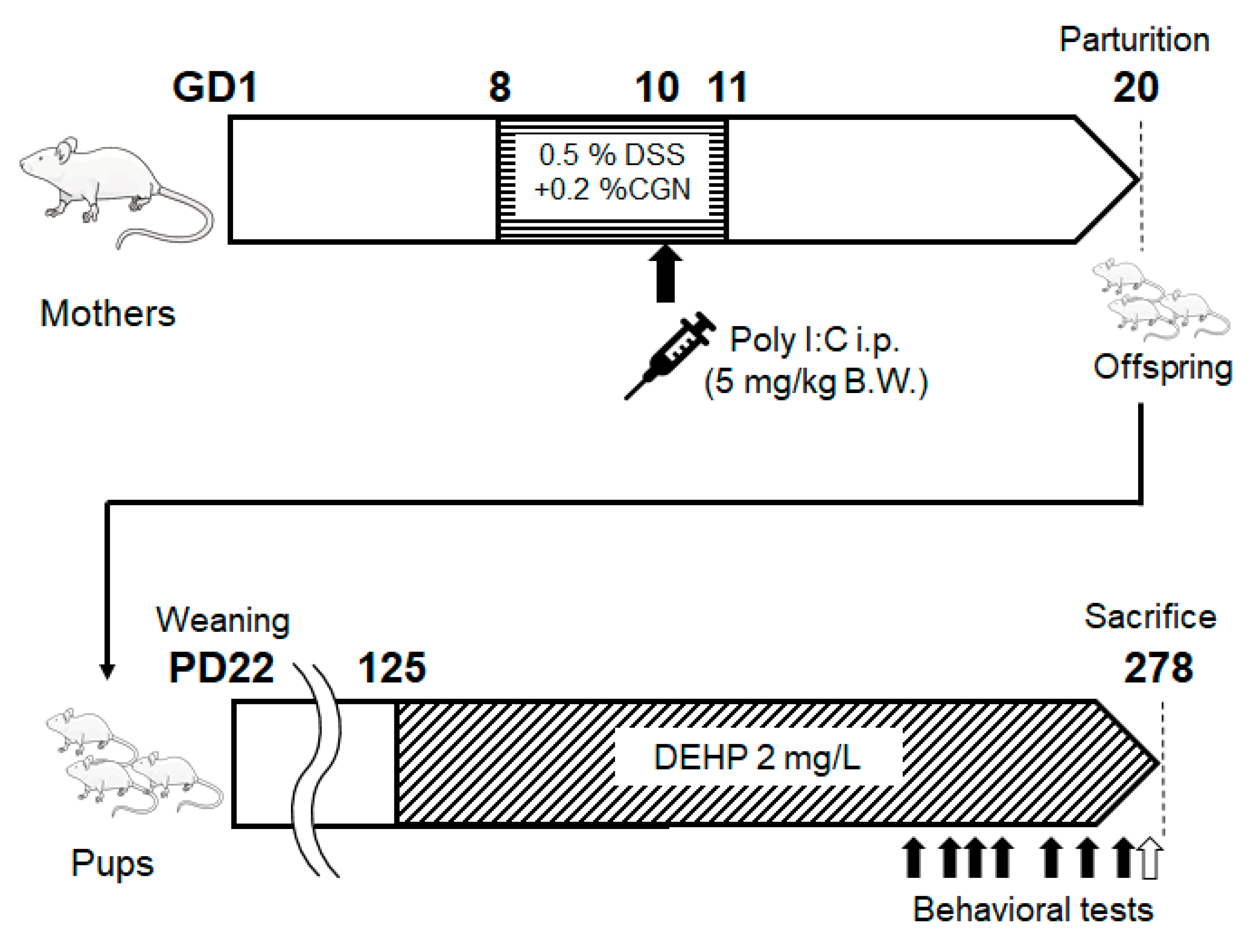
2.1. Mice
2.2. Materials
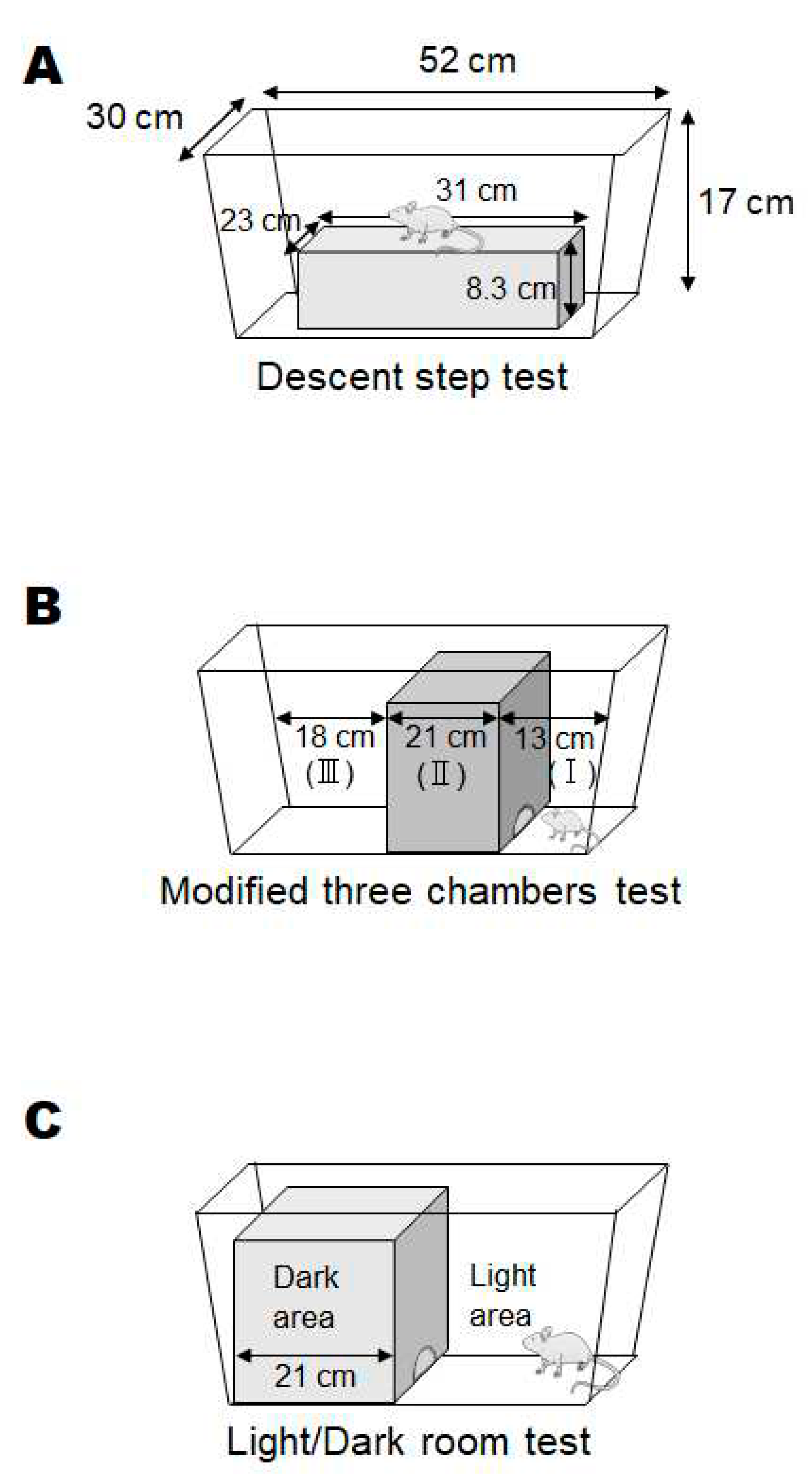
2.3. Behavioral Tests
- (1)
- Descent step test
- (2)
- Modified three chambers test
- (3)
- Light/dark room test
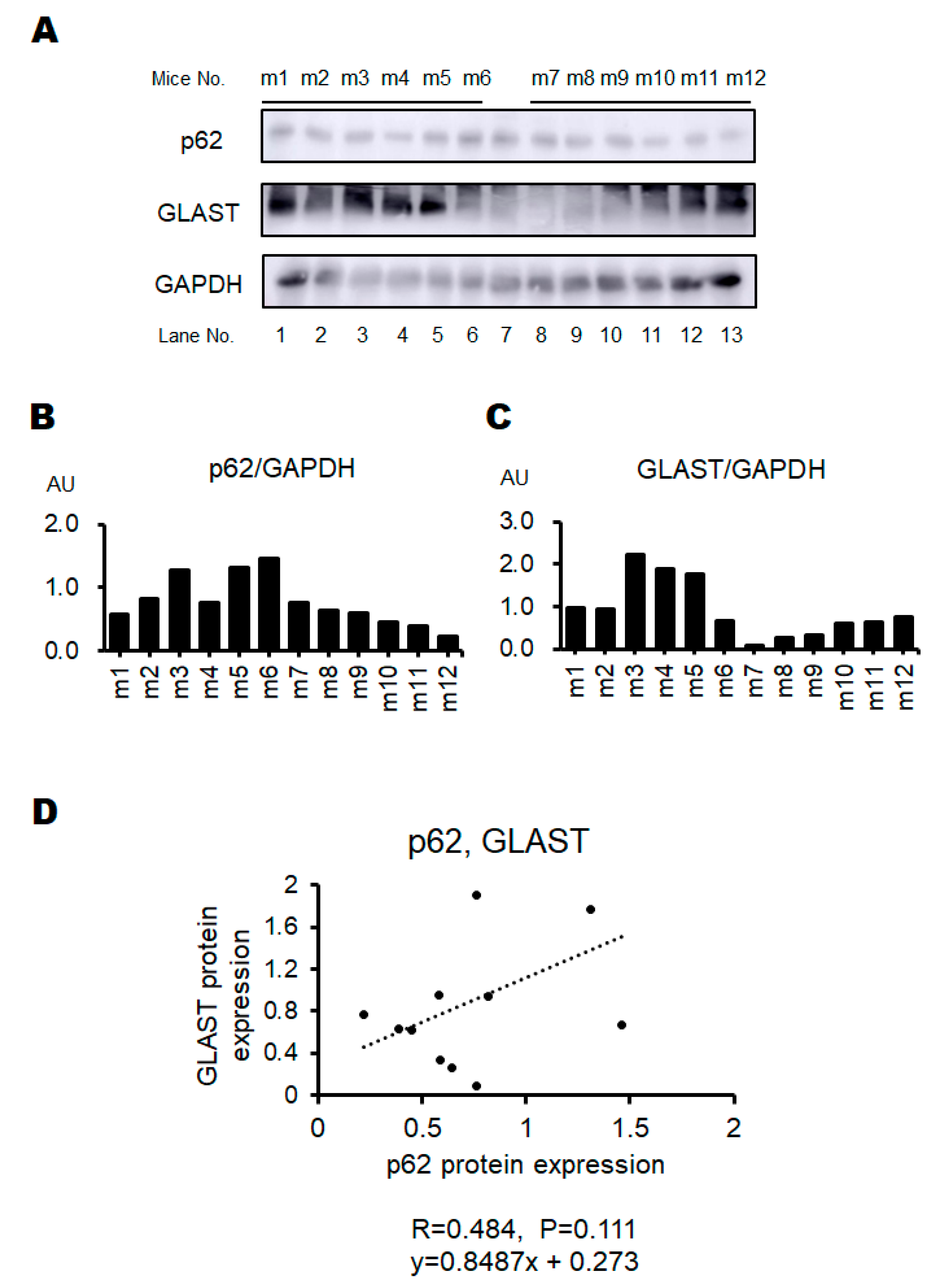
2.4. Western Blotting
2.5. Statistical Analyses
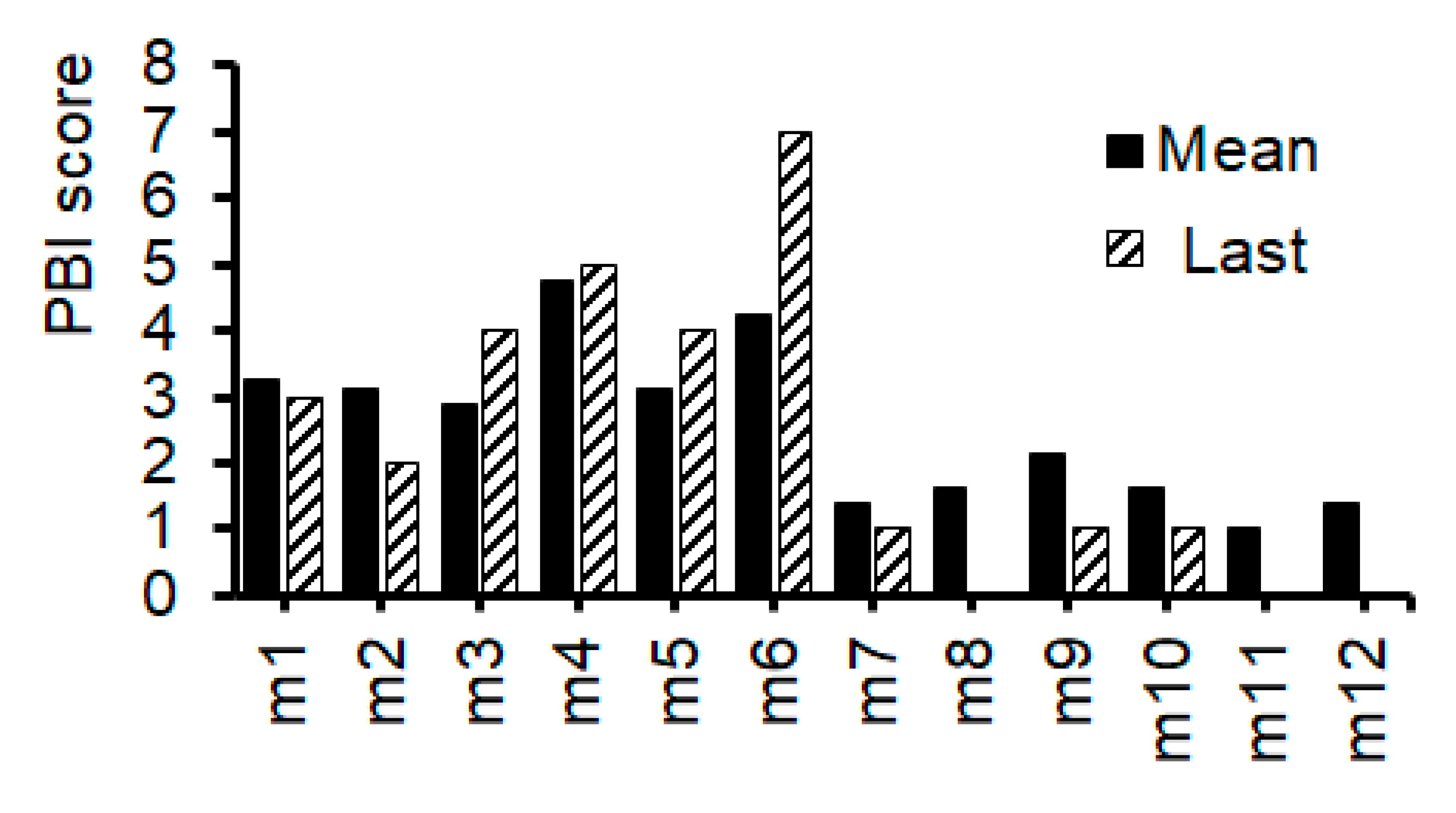
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Competing Interests Statement
Abbreviations
| CGN | carrageenan |
| CNS | central nervous system |
| DEHP | 2-ethylhexyl phthalate |
| DSS | sodium dextran sulfate |
| FMT | fecal microbiota transplantation |
| GAPDH | glyceraldehyde 3-phosphate dehydrogenase |
| GLAST | glutamate aspartate transporter |
| KO | knockout |
| LPS | lipopolysaccharide |
| mRNA | messenger RNA |
| NAC | N-acetylcysteine |
| NMDA | N-methyl-d-aspartate |
| PBI | psychological behavior index |
| poly I:C | polyriboinosinic-polyribocytidilic acid |
| QOL | quality of life |
References
- Singh, M.; Agarwal, V.; Jindal, D.; Pancham, P.; Agarwal, S.; Mani, S.; Tiwari, R.K.; Das, K.; Alghamdi, B.S.; Abujamel, T.S.; et al. Recent Updates on Corticosteroid-Induced Neuropsychiatric Disorders and Theranostic Advancements through Gene Editing Tools. Diagnostics 2023, 13, 337. [Google Scholar] [CrossRef]
- Khokhar, J.Y.; Dwiel, L.L.; Henricks, A.M.; Doucette, W.T.; Green, A.I. The link between schizophrenia and substance use disorder: A unifying hypothesis. Schizophr. Res. 2017, 194, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Owen, MJ.; Sawa, A.; Mortensen, PB. Schizophrenia. Lancet 2016, 388, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Picchioni, MM.; Murray, RM. Schizophrenia. BMJ 2007, 335, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Abel, K.M.; Drake, R.; Goldstein, J.M. Sex differences in schizophrenia. Int. Rev. Psychiatry 2010, 22, 417–428. [Google Scholar] [CrossRef]
- Ban, T.A. Fifty years chlorpromazine: a historical perspective. Neuropsychiatr. Dis. Treat. 2007, 3, 495–500. [Google Scholar] [CrossRef]
- Adler, C.; Goldberg, T.; Malhotra, A.; Pickar, D.; Breier, A. Effects of Ketamine on Thought Disorder, Working Memory, and Semantic Memory in Healthy Volunteers. Biol. Psychiatry 1998, 43, 811–816. [Google Scholar] [CrossRef]
- Weickert, C.S.; Fung, S.J.; Catts, V.S.; Schofield, P.R.; Allen, K.M.; Moore, L.T.; Newell, K.A.; Pellen, D.; Huang, X.-F.; Catts, S.V.; et al. Molecular evidence of N-methyl-D-aspartate receptor hypofunction in schizophrenia. Mol. Psychiatry 2013, 18, 1185–1192. [Google Scholar] [CrossRef]
- Del, Arco, A.; Segovia, G.; Mora, F. Blockade of NMDA receptors in the prefrontal cortex increases dopamine and acetylcholine release in the nucleus accumbens and motor activity. Psychopharmacology (Berl). 2008, 201, 325–338. [Google Scholar] [CrossRef]
- Hanson, K.L.; Grant, S.E.; Funk, L.H.; Schumann, C.M.; Bauman, M.D. Impact of Maternal Immune Activation on Nonhuman Primate Prefrontal Cortex Development: Insights for Schizophrenia. Biol. Psychiatry 2022, 92, 460–469. [Google Scholar] [CrossRef]
- Connor, C.M.; Dincer, A.; Straubhaar, J.; Galler, J.R.; Houston, I.B.; Akbarian, S. Maternal immune activation alters behavior in adult offspring, with subtle changes in the cortical transcriptome and epigenome. Schizophr. Res. 2012, 140, 175–184. [Google Scholar] [CrossRef]
- Garcia-Partida, J.A.; Torres-Sanchez, S.; MacDowell, K.; Fernández-Ponce, M.T.; Casas, L.; Mantell, C.; Soto-Montenegro, M.L.; Romero-Miguel, D.; Lamanna-Rama, N.; Leza, J.C.; et al. The effects of mango leaf extract during adolescence and adulthood in a rat model of schizophrenia. Front. Pharmacol. 2022, 13, 886514. [Google Scholar] [CrossRef]
- Romero-Miguel, D.; Casquero-Veiga, M.; MacDowell, K.S.; Torres-Sanchez, S.; Garcia-Partida, J.A.; Lamanna-Rama, N.; Romero-Miranda, A.; Berrocoso, E.; Leza, J.C.; Desco, M.; et al. A Characterization of the Effects of Minocycline Treatment During Adolescence on Structural, Metabolic, and Oxidative Stress Parameters in a Maternal Immune Stimulation Model of Neurodevelopmental Brain Disorders. Int. J. Neuropsychopharmacol. 2021, 24, 734–748. [Google Scholar] [CrossRef]
- Tanaka, M.; Bohár, Z.; Martos, D.; Telegdy, G.; Vécsei, L. Antidepressant-like effects of kynurenic acid in a modified forced swim test. Pharmacol. Rep. 2020, 72, 449–455. [Google Scholar] [CrossRef]
- Hadar, R.; Soto-Montenegro, M.L.; Götz, T.; Wieske, F.; Sohr, R.; Desco, M.; Hamani, C.; Weiner, I.; Pascau, J.; Winter, C. Using a maternal immune stimulation model of schizophrenia to study behavioral and neurobiological alterations over the developmental course. Schizophr. Res. 2015, 166, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Howland, J.; Cazakoff, B.; Zhang, Y. Altered object-in-place recognition memory, prepulse inhibition, and locomotor activity in the offspring of rats exposed to a viral mimetic during pregnancy. Neuroscience 2011, 201, 184–198. [Google Scholar] [CrossRef]
- Missig, G.; Mokler, E.L.; O Robbins, J.; Alexander, A.J.; McDougle, C.J.; A Carlezon, W. Perinatal Immune Activation Produces Persistent Sleep Alterations and Epileptiform Activity in Male Mice. Neuropsychopharmacology 2017, 43, 482–491. [Google Scholar] [CrossRef]
- Gröhn, C.; Norgren, E.; Eriksson, L. A systematic review of the neural correlates of multisensory integration in schizophrenia. Schizophr. Res. Cogn. 2022, 27, 100219. [Google Scholar] [CrossRef]
- Otero, A.M.; Antonson, A.M. At the crux of maternal immune activation: Viruses, microglia, microbes, and IL-17A. Immunol. Rev. 2022, 311, 205–223. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, V.; Afsharian, P.; Shahhoseini, M.; Kalantar, S.M.; Moini, A. A Review on Various Uses of N-Acetyl Cysteine. Cell J. 2017, 19, 11–17. [Google Scholar] [CrossRef]
- Stekelenburg, JJ.; Maes, JP.; Van, Gool, AR.; Sitskoorn, M.; Vroomen, J. Deficient multisensory integration in schizophrenia: an event-related potential study. Schizophr Res. 2013, 147, 253–261. [Google Scholar] [CrossRef]
- Tomoda, T.; Sumitomo, A.; Shukla, R.; Hirota-Tsuyada, Y.; Miyachi, H.; Oh, H.; French, L.; Sibille, E. BDNF controls GABAAR trafficking and related cognitive processes via autophagic regulation of p62. Neuropsychopharmacology 2021, 47, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Sumitomo, A.; Yukitake, H.; Hirai, K.; Horike, K.; Ueta, K.; Chung, Y.; Warabi, E.; Yanagawa, T.; Kitaoka, S.; Furuyashiki, T.; et al. Ulk2 controls cortical excitatory–inhibitory balance via autophagic regulation of p62 and GABAA receptor trafficking in pyramidal neurons. Hum. Mol. Genet. 2018, 27, 3165–3176. [Google Scholar] [CrossRef]
- Sumitomo, A.; Horike, K.; Hirai, K.; Butcher, N.; Boot, E.; Sakurai, T.; Nucifora, FC, Jr.; Bassett, AS.; Sawa, A.; Tomoda, T. A mouse model of 22q11.2 deletions: Molecular and behavioral signatures of Parkinson's disease and schizophrenia. Sci Adv. 2018, 4, eaar6637. [Google Scholar] [CrossRef] [PubMed]
- Yi, W.; Xiang-Liang, T.; Yu, Z.; Bin, L.; Lian-Ju, S.; Chun-Lan, L.; Tao, L.; Da-Wei, H.; Sheng-De, W.; Guang-Hui, W. DEHP exposure destroys blood-testis barrier (BTB) integrity of immature testes through excessive ROS-mediated autophagy. Genes Dis. 2018, 5, 263–274. [Google Scholar] [CrossRef]
- Wójtowicz, A.K.; Sitarz-Głownia, A.M.; Wnuk, A.; Kajta, M.; Szychowski, K.A. Involvement of the peroxisome proliferator-activated receptor gamma (Pparγ) and matrix metalloproteinases-2 and -9 (Mmp-2 and -9) in the mechanism of action of di(2-ethylhexyl)phthalate (DEHP) in cultured mouse brain astrocytes and neurons. Toxicol. Vitr. 2023, 92, 105639. [Google Scholar] [CrossRef] [PubMed]
- Pajarillo, E.; Rizor, A.; Lee, J.; Aschner, M.; Lee, E. The role of astrocytic glutamate transporters GLT-1 and GLAST in neurological disorders: Potential targets for neurotherapeutics. Neuropharmacology 2019, 161, 107559–107559. [Google Scholar] [CrossRef]
- Walsh, T.; McClellan, J.M.; McCarthy, S.E.; Addington, A.M.; Pierce, S.B.; Cooper, G.M.; Nord, A.S.; Kusenda, M.; Malhotra, D.; Bhandari, A.; et al. Rare Structural Variants Disrupt Multiple Genes in Neurodevelopmental Pathways in Schizophrenia. Science 2008, 320, 539–543. [Google Scholar] [CrossRef]
- Karlsson, RM.; Tanaka, K.; Heilig, M.; Holmes, A. Loss of glial glutamate and aspartate transporter (excitatory amino acid transporter 1) causes locomotor hyperactivity and exaggerated responses to psychotomimetics: rescue by haloperidol and metabotropic glutamate 2/3 agonist. Biol Psychiatry. 2008, 64, 810–814. [Google Scholar] [CrossRef]
- Karlsson, R.-M.; Tanaka, K.; Saksida, L.M.; Bussey, T.J.; Heilig, M.; Holmes, A. Assessment of Glutamate Transporter GLAST (EAAT1)-Deficient Mice for Phenotypes Relevant to the Negative and Executive/Cognitive Symptoms of Schizophrenia. Neuropsychopharmacology 2009, 34, 1578–1589. [Google Scholar] [CrossRef]
- Ikeda, Y.; Matsuda, S. Gut Protective Effect from D-Methionine or Butyric Acid against DSS and Carrageenan-Induced Ulcerative Colitis. Molecules 2023, 28, 4392. [Google Scholar] [CrossRef]
- Kang, J.S.; Baek, J.H.; Song, M.Y.; Rehman, N.U.; Chung, H.J.; Lee, D.K.; Yoo, D.Y.; Kim, H.J. Long-term exposure changes the environmentally relevant bis(2-ethylhexyl) phthalate to be a neuro-hazardous substance disrupting neural homeostasis in emotional and cognitive functions. Environ. Pollut. 2023, 324, 121387. [Google Scholar] [CrossRef]
- Chamera, K.; Kotarska, K.; Szuster-Głuszczak, M.; Trojan, E.; Skórkowska, A.; Pomierny, B.; Krzyżanowska, W.; Bryniarska, N.; Basta-Kaim, A. The prenatal challenge with lipopolysaccharide and polyinosinic:polycytidylic acid disrupts CX3CL1-CX3CR1 and CD200-CD200R signalling in the brains of male rat offspring: a link to schizophrenia-like behaviours. J. Neuroinflammation 2020, 17, 247. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, K.; Hashimoto, K.; Kishimoto, T.; Shimizu, E.; Ishikura, H.; Iyo, M. Immune Activation During Pregnancy in Mice Leads to Dopaminergic Hyperfunction and Cognitive Impairment in the Offspring: A Neurodevelopmental Animal Model of Schizophrenia. Biol. Psychiatry 2006, 59, 546–554. [Google Scholar] [CrossRef]
- Coyle, P.; Tran, N.; Fung, J.; Summers, B.; Rofe, A. Maternal dietary zinc supplementation prevents aberrant behaviour in an object recognition task in mice offspring exposed to LPS in early pregnancy. Behav. Brain Res. 2009, 197, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Schwendener, S.; Meyer, U.; Feldon, J. Deficient maternal care resulting from immunological stress during pregnancy is associated with a sex-dependent enhancement of conditioned fear in the offspring. J. Neurodev. Disord. 2009, 1, 15–32. [Google Scholar] [CrossRef]
- Bitanihirwe, B.K.; Peleg-Raibstein, D.; Mouttet, F.; Feldon, J.; Meyer, U. Late Prenatal Immune Activation in Mice Leads to Behavioral and Neurochemical Abnormalities Relevant to the Negative Symptoms of Schizophrenia. Neuropsychopharmacology 2010, 35, 2462–2478. [Google Scholar] [CrossRef]
- Sutcliffe, J.; Marshall, K.; Neill, J. Influence of gender on working and spatial memory in the novel object recognition task in the rat. Behav. Brain Res. 2007, 177, 117–125. [Google Scholar] [CrossRef]
- Stertz, L.; Di, Re, J. ; Pei, G.; Fries, GR.; Mendez, E.; Li, S.; Smith-Callahan, L.; Raventos, H.; Tipo, J.; Cherukuru, R.; et al. Convergent genomic and pharmacological evidence of PI3K/GSK3 signaling alterations in neurons from schizophrenia patients. Neuropsychopharmacology 2021, 46, 673–682. [Google Scholar] [CrossRef] [PubMed]
- Emamian, ES.; Hall, D.; Birnbaum, MJ.; Karayiorgou, M.; Gogos, JA. Convergent evidence for impaired AKT1-GSK3beta signaling in schizophrenia. Nat Genet. 2004, 36, 131–137. [Google Scholar] [CrossRef]
- Emamian, E.S. AKT/GSK3 signaling pathway and schizophrenia. Front. Mol. Neurosci. 2012, 5, 33. [Google Scholar] [CrossRef]
- Karege, F.; Perroud, N.; Burkhardt, S.; Schwald, M.; Ballmann, E.; La, Harpe, R.; Malafosse, A. Alteration in kinase activity but not in protein levels of protein kinase B and glycogen synthase kinase-3beta in ventral prefrontal cortex of depressed suicide victims. Biol Psychiatry. 2007, 61, 240–245. [Google Scholar] [CrossRef]
- Qu, L.; Liu, Y.; Deng, J.; Ma, X.; Fan, D. Ginsenoside Rk3 is a novel PI3K/AKT-targeting therapeutics agent that regulates autophagy and apoptosis in hepatocellular carcinoma. J. Pharm. Anal. 2023, 13, 463–482. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.S.; Lee, S.M.; Hwang, D.; Park, H.J.; Kim, J.W. Association between Unc-51-like autophagy activating kinase 2 gene polymorphisms and schizophrenia in the Korean population. Medicine 2022, 101, e28745. [Google Scholar] [CrossRef]
- Mizushima, N.; Yoshimori, T.; Ohsumi, Y. The Role of Atg Proteins in Autophagosome Formation. Annu. Rev. Cell Dev. Biol. 2011, 27, 107–132. [Google Scholar] [CrossRef]
- Sumitomo, A.; Tomoda, T. Autophagy in neuronal physiology and disease. Curr. Opin. Pharmacol. 2021, 60, 133–140. [Google Scholar] [CrossRef]
- Lippai, M.; Lőw, P. The Role of the Selective Adaptor p62 and Ubiquitin-Like Proteins in Autophagy. BioMed Res. Int. 2014, 2014, 832704. [Google Scholar] [CrossRef]
- Lech, M.A.; Leśkiewicz, M.; Kamińska, K.; Rogóż, Z.; Lorenc-Koci, E. Glutathione Deficiency during Early Postnatal Development Causes Schizophrenia-Like Symptoms and a Reduction in BDNF Levels in the Cortex and Hippocampus of Adult Sprague–Dawley Rats. Int. J. Mol. Sci. 2021, 22, 6171. [Google Scholar] [CrossRef] [PubMed]
- Shevelkin, A.V.; E Terrillion, C.; Hasegawa, Y.; A Mychko, O.; Jouroukhin, Y.; Sawa, A.; Kamiya, A.; Pletnikov, M.V. Astrocyte DISC1 contributes to cognitive function in a brain region-dependent manner. Hum. Mol. Genet. 2020, 29, 2936–2950. [Google Scholar] [CrossRef]
- Karlsson, R.-M.; Adermark, L.; Molander, A.; Perreau-Lenz, S.; Singley, E.; Solomon, M.; Holmes, A.; Tanaka, K.; Lovinger, D.M.; Spanagel, R.; et al. Reduced alcohol intake and reward associated with impaired endocannabinoid signaling in mice with a deletion of the glutamate transporter GLAST. Neuropharmacology 2012, 63, 181–189. [Google Scholar] [CrossRef]
- Uchida, M.; Hida, H.; Mori, K.; Yoshimi, A.; Kitagaki, S.; Yamada, K.; Hiraoka, Y.; Aida, T.; Tanaka, K.; Ozaki, N.; et al. Functional roles of the glial glutamate transporter (GLAST) in emotional and cognitive abnormalities of mice after repeated phencyclidine administration. Eur. Neuropsychopharmacol. 2019, 29, 914–924. [Google Scholar] [CrossRef] [PubMed]
- A Lewis, D.; González-Burgos, G. Neuroplasticity of Neocortical Circuits in Schizophrenia. Neuropsychopharmacology 2008, 33, 141–165. [Google Scholar] [CrossRef]
- Brown, A.S.; Vinogradov, S.; Kremen, W.S.; Poole, J.H.; Deicken, R.F.; Penner, J.D.; McKeague, I.W.; Kochetkova, A.; Kern, D.; Schaefer, C.A. Prenatal Exposure to Maternal Infection and Executive Dysfunction in Adult Schizophrenia. Am. J. Psychiatry 2009, 166, 683–690. [Google Scholar] [CrossRef]
- Bubenikova-Valesova, V.; Stuchlik, A.; Svoboda, J.; Bures, J.; Vales, K. Risperidone and ritanserin but not haloperidol block effect of dizocilpine on the active allothetic place avoidance task. Proc. Natl. Acad. Sci. USA 2008, 105, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Hardingham, G.E.; Do, K.Q. Linking early-life NMDAR hypofunction and oxidative stress in schizophrenia pathogenesis. Nat. Rev. Neurosci. 2016, 17, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Casquero-Veiga, M.; Romero-Miguel, D.; MacDowell, KS.; Torres-Sanchez, S.; Garcia-Partida, JA.; Lamanna-Rama, N.; Gómez-Rangel, V.; Romero-Miranda, A.; Berrocoso, E.; Leza, JC.; et al. Omega-3 fatty acids during adolescence prevent schizophrenia-related behavioural deficits: Neurophysiological evidences from the prenatal viral infection with PolyI:C. Eur Neuropsychopharmacol. 2021, 46, 14–27. [Google Scholar] [CrossRef]
- Felgel-Farnholz, V.; Hlusicka, E.B.; Edemann-Callesen, H.; Garthe, A.; Winter, C.; Hadar, R. Adolescent raloxifene treatment in females prevents cognitive deficits in a neurodevelopmental rodent model of schizophrenia. Behav. Brain Res. 2023, 441, 114276. [Google Scholar] [CrossRef]
- Tsuji, A.; Ikeda, Y.; Yoshikawa, S.; Taniguchi, K.; Sawamura, H.; Morikawa, S.; Nakashima, M.; Asai, T.; Matsuda, S. The Tryptophan and Kynurenine Pathway Involved in the Development of Immune-Related Diseases. Int. J. Mol. Sci. 2023, 24, 5742. [Google Scholar] [CrossRef]
- Yoshikawa, S.; Taniguchi, K.; Sawamura, H.; Ikeda, Y.; Tsuji, A.; Matsuda, S. A New Concept of Associations between Gut Microbiota, Immunity and Central Nervous System for the Innovative Treatment of Neurodegenerative Disorders. Metabolites 2022, 12, 1052. [Google Scholar] [CrossRef]
- Asai, T.; Yoshikawa, S.; Ikeda, Y.; Taniguchi, K.; Sawamura, H.; Tsuji, A.; Matsuda, S. Encouraging Tactics with Genetically Modified Probiotics to Improve Immunity for the Prevention of Immune-Related Diseases including Cardio-Metabolic Disorders. Biomolecules 2022, 13, 10. [Google Scholar] [CrossRef]
- Yoshikawa, S.; Taniguchi, K.; Sawamura, H.; Ikeda, Y.; Tsuji, A.; Matsuda, S. Encouraging probiotics for the prevention and treatment of immune-related adverse events in novel immunotherapies against malignant glioma. Explor. Target. Anti-tumor Ther. 2022, 3, 817–827. [Google Scholar] [CrossRef] [PubMed]
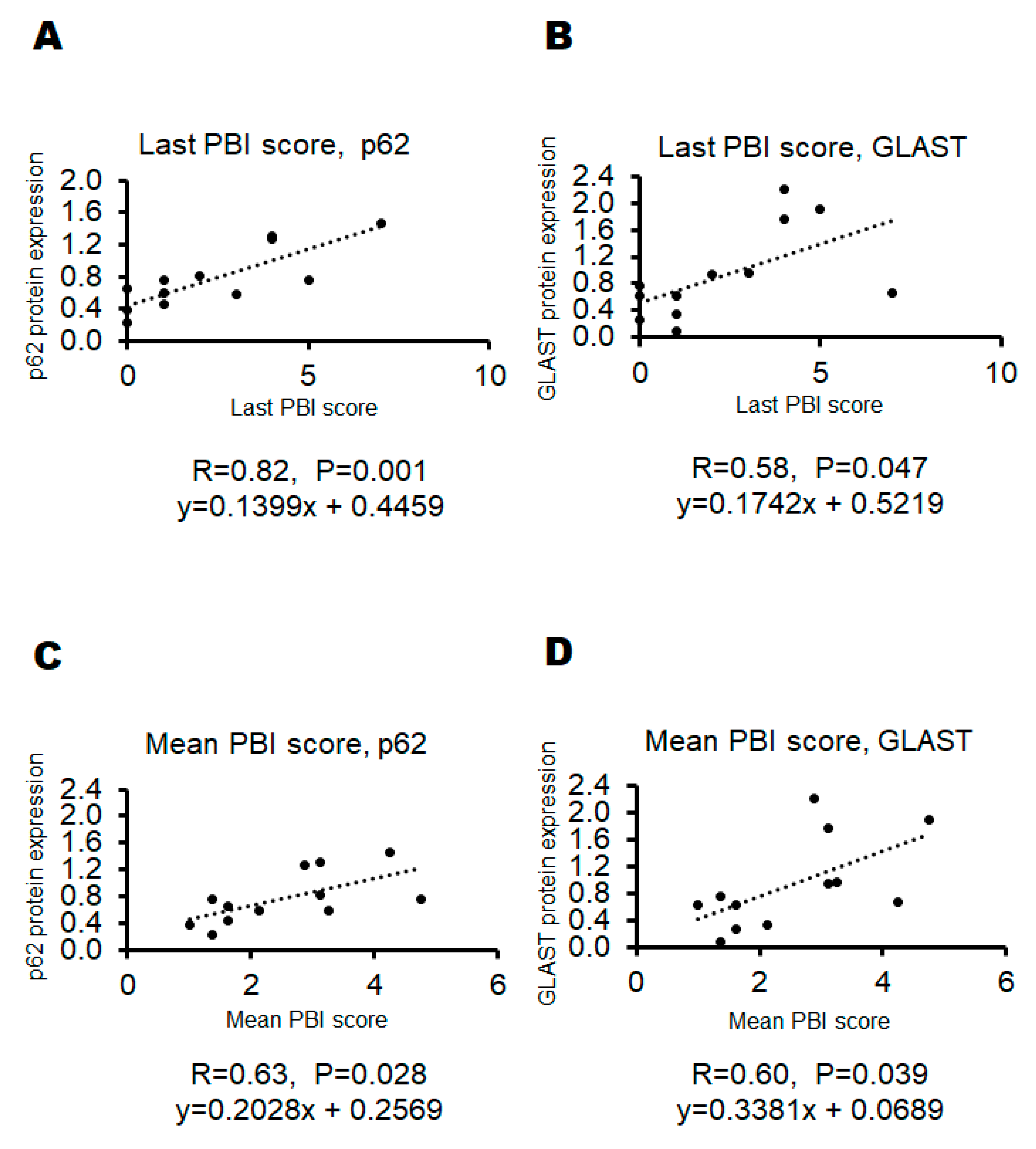
| Behavioral test | Score | |
|---|---|---|
| Descent step test | Non-descent descent |
0 1 |
| Modified three chambers test | In (III) room In (I) room In (II) room |
1 2 4 |
| Light/Dark room test | Time in light area ≧60 (sec) Time in light area 40-59 (sec) Time in light area 20-39 (sec) Time in light area 0-19 (sec) |
0 1 2 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





