1. Introduction
The Arctic region is mainly occupied by the Arctic Ocean that is surrounded by land. On the other hand, the Antarctic region is dominated by the Antarctic Continent, with ice and snow covering the continent. Together, the Arctic and Antarctic regions are called the Polar Regions.
Antarctica is one of the extreme environments on the planet, and it is exposed to cold and dry conditions, with the lowest temperature recorded at –90 °C. Approximately 98% of its surface is covered by snow and ice, while 2% represents the ice-free area located in coastal areas and high mountains, wherein the snow melts in summer, thereby exposing the ground. Most of the terrestrial ecosystems of Antarctica are distributed in this ice-free area [
1]. Syowa Station, the headquarters for the Japanese Antarctic Research Expedition (JARE), is also an ice-free area.
The High Arctic, like Antarctica, is one of the most extreme environments, with a maximum temperature of below 0 °C for most of the year.
Despite exposure to conditions adversely affecting their survival, such as subzero temperatures and low nutrient and water availability, the fungi that inhabit cold environments can grow at near-subzero temperatures. Secretion of extracellular enzymes allows them to utilize complex materials as energy sources. Therefore, psychrophilic and psychrotolerant fungi play important roles in the nutrient cycle of the polar region ecosystems [
2].
This review presents a history of fungal research in Antarctica, followed by a report on fungal surveys near the Syowa Station. In addition, I summarize the impact of climate changes on fungal diversity based on fungal surveys in glacier retreat areas in the High Arctic. I also introduce the growth and enzyme activity of fungi inhabiting the polar regions at sub-zero temperatures. Finally, I discuss the prospects of fungal research in the Arctic and Antarctic regions.
2. History of fungal research in the Antarctic region and near Syowa Station
The Antarctica was discovered approximately during 1820 AD. The first report on fungi from the Antarctic region was approximately published in 1897–1899, when a Belgian expedition, the first to overwinter in the Antarctic region, collected
Sclerotium antarcticum from Danco Island near the Antarctic Peninsula [
3]. The late Roald Amundsen (1872–1928) who was the first to reach the South Pole participated in this expedition.
In the 1960s, many new fungal species from the Antarctic were reported by Fell et al. [
4] and di Menna [
5,
6], who is the first woman to receive a Doctor of Philosophy degree from New Zealand.
Bridge and Spooner (2012) created a "list of non-lichenized fungi from the Antarctic region," which enumerates who reported what species of fungi, when, and where in the Antarctic region. This list was valuable in understanding the fungal diversity in Antarctica; however, the list is no longer being updated [
7].
The first report on fungi around Syowa Station, the headquarters of JARE, was published in 1961 [
8], followed by three articles that reported 12 ascomycetes and 4 basidiomycetes in the span of four years until 1965 (
Table 1) [
9,
10,
11].
Table 1.
Number of fungal species near Syowa Station through 2012 and 2013–2022.
Table 1.
Number of fungal species near Syowa Station through 2012 and 2013–2022.
| |
through 2012 |
2013-2022 |
Total |
| Chytridiomycota |
0 |
0 |
0 |
| Zygomycota |
0 |
0 |
0 |
| Ascomycota |
12 |
49 |
61 |
| Basidiomycota |
4 |
12 |
16 |
| Total |
16 |
61 |
77 |
In the 1960s, JARE was temporarily suspended. During this period,
Moesziomyces antarcticus was reported from the Ross Island, separately from the JARE [
12].
M. antarcticus is known for its ability to secrete stable lipase and produce biosurfactants [
13,
14]. From the 1960s to 2013, no fungi were reported near the Syowa Station by JARE. Mycologists Dr. Tamotsu Hoshino and Dr. Takashi Osono participated in the 48th (2006–2007 austral summer) and 51st JARE (2009–2010 austral summer), respectively. Information from both these JARE activities were published in 2013 [
15,
16]).
3. Fungal diversity research near Syowa Station
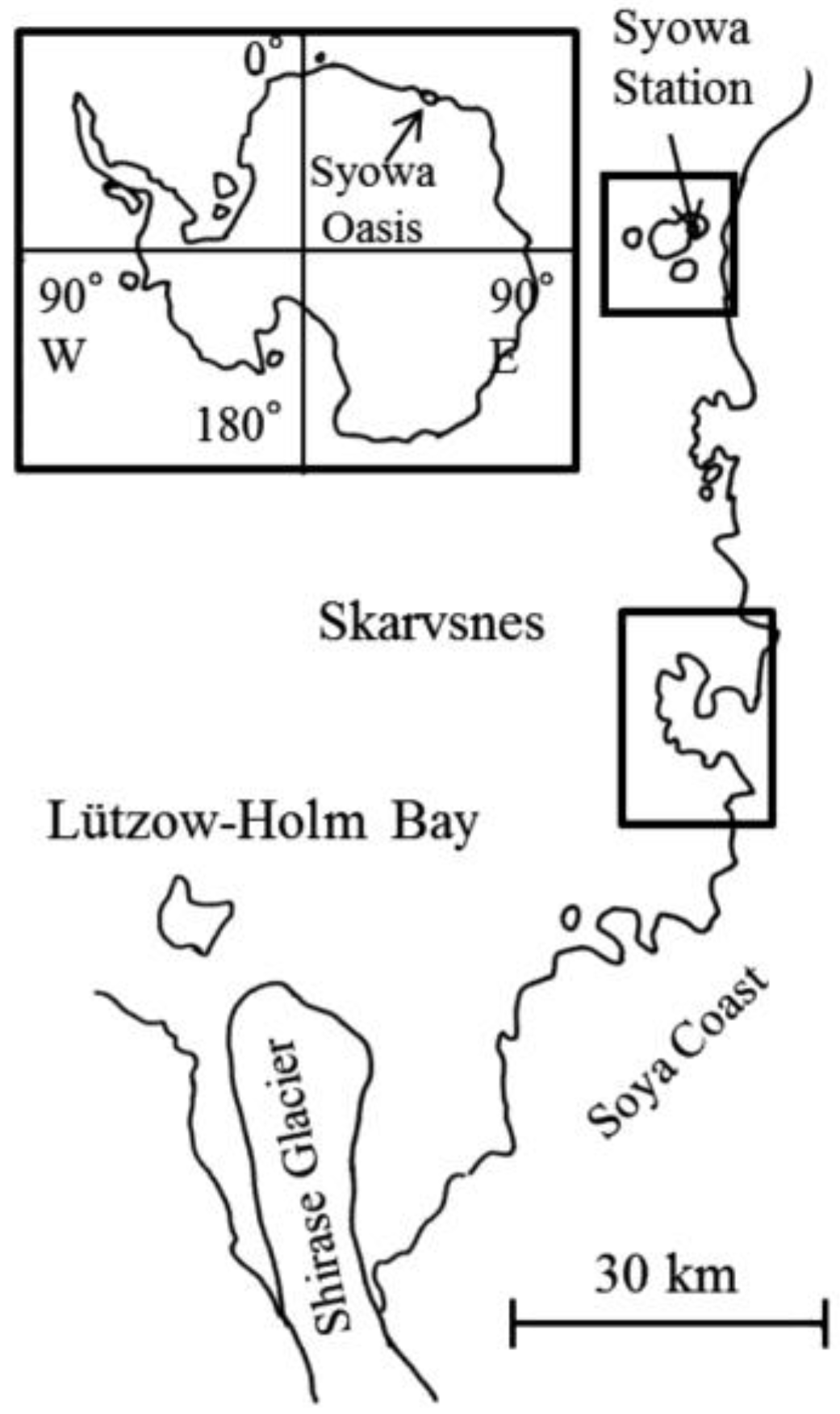
Using the samples collected by JARE–48 and JARE–49, we conducted a survey on the fungal diversity in the Skarvsnes ice-free area and the East Ongul Island, where Syowa Station is located (
Figure 1). Each species name was checked. Species name was updated using Index Fungorum (
http://www.indexfungorum.org). It should be noted that some isolates or strains have not been identified at the species level in the original article. I have attempted to reclassify these organisms if their DNA sequence was deposited in a DNA data bank. When reclassification at the species level was impossible, the isolates or strains were presented at the genus level.
Figure 1.
Map of Antarctica and the locations of the Skarvsnes ice-free area and Syowa Station (East Ongul Island).
Figure 1.
Map of Antarctica and the locations of the Skarvsnes ice-free area and Syowa Station (East Ongul Island).
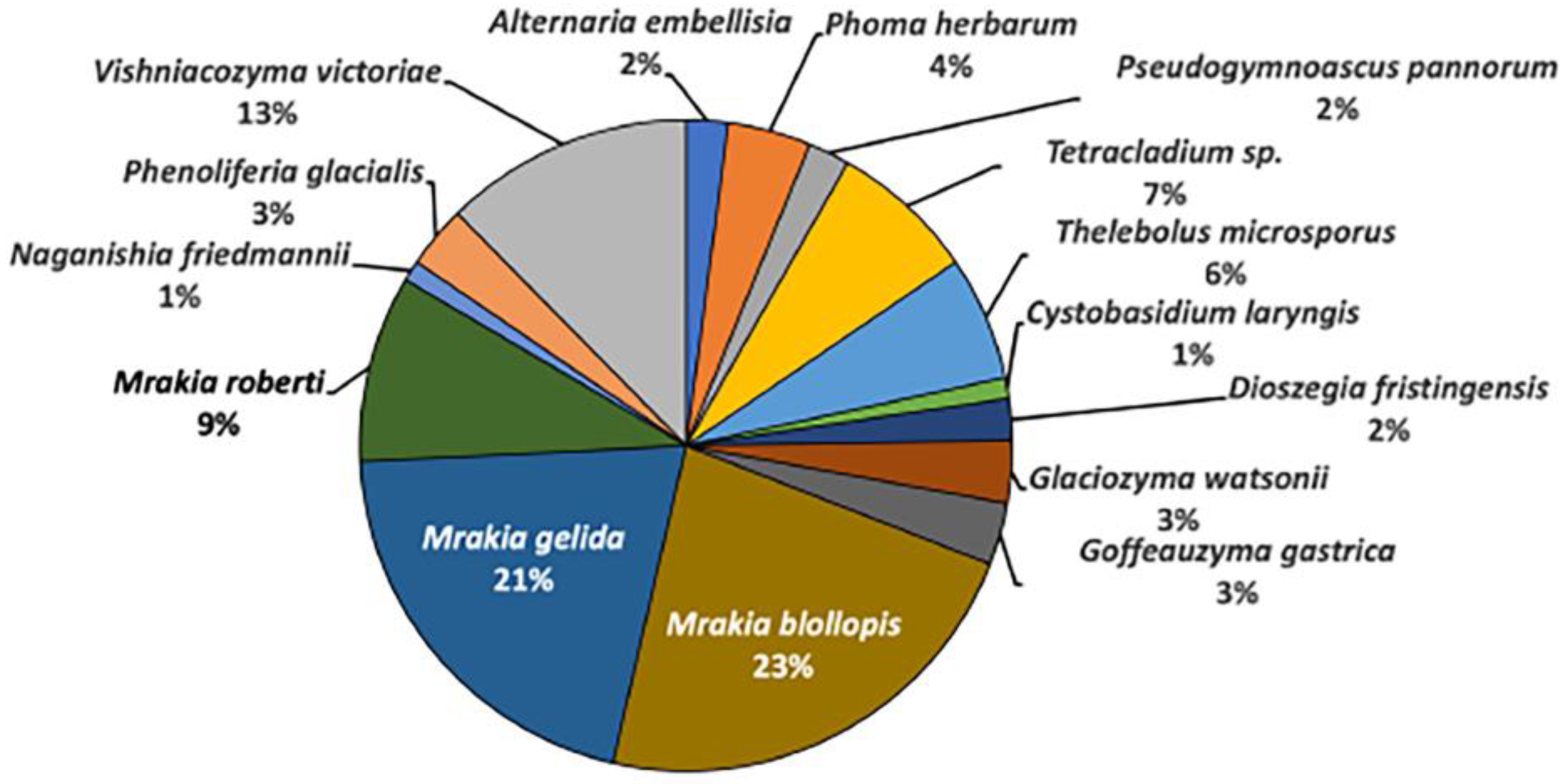
In the Skarvsnes ice-free area, 97 fungal strains were isolated and classified into five genera and five species of Ascomycota, as well as into eight genera and 10 species of Basidiomycota. At the genus level, Mrakia spp. accounted 53% of the fungal strains in the area, and the dominant fungal species were Mrakia blollopis (23%), Mrakia gelida (21%), and Mrakia robertii (9%) (
Figure 2) [
15]. The genus Mrakia is a basidiomycete yeast that has been reported from various cold environments around the world, including the Arctic region, Himalayas, Alps, and Antarctica [
17].
Figure 2.
Fungal diversities in the Skarvsnes ice-free area.
Figure 2.
Fungal diversities in the Skarvsnes ice-free area.
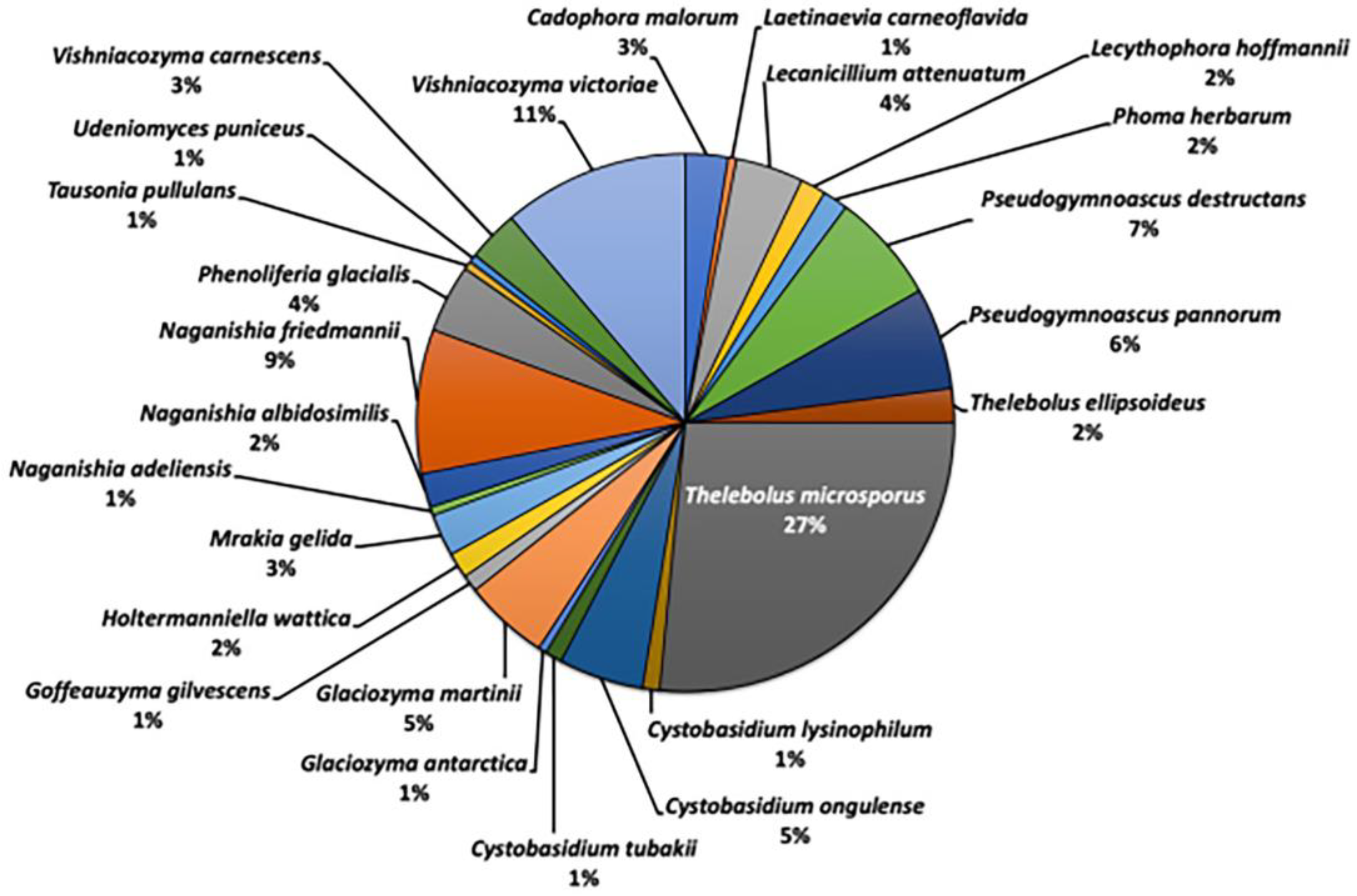
On East Ongul Island, 196 fungal strains were isolated and classified into eight genera and nine species of Ascomycota, as well as 10 genera and 16 species of Basidiomycota. The dominant fungal species on the island were Thelebolus microspores (27%), Vishniacozyma victoriae (11%), and Naganishia friedmannii (9%) (
Figure 3) [
18].
Three genera and three species of ascomycetous fungi (Phoma herbarum, Pseudogymnoascus pannorum, and Thelebolus microsporus), and four genera and four species of basidiomycetous fungi (Mrakia gelida, Naganishia friedmannii, Phenoliferia glacialis, and Vishniacozyma victoriae) were common between the East Ongul Island and Skarvsnes ice-free area. Although these two areas are only 60 km apart, the fungal diversity is quite different. This suggests that the fungal ecosystem near the Syowa Station is formed in a narrow space.
Figure 3.
Fungal diversities in the East Ongul island.
Figure 3.
Fungal diversities in the East Ongul island.
During the fungal surveys near the Syowa Station, two new fungal species, Cystobasidium tubakii and C. ongulense, were reported for the first time [
19]. Thus, the number of fungal species reported near the Syowa Station increased from 16 to 77 species (~4.8-fold) from 2013 to 2022 (
Table 1) [
20].
4. Fungal diversity research in the glacier retreat area of Svalbard, High Arctic
Austre Brøggerbreen (79°N, 12°E) is located in Ny-Ålesund in the Svalbard archipelago, Norway. The glacier has been markedly affected by climate change and is one of the most retreating glaciers in the world [
21]. Therefore, this review examined how the fungal flora changed over time.
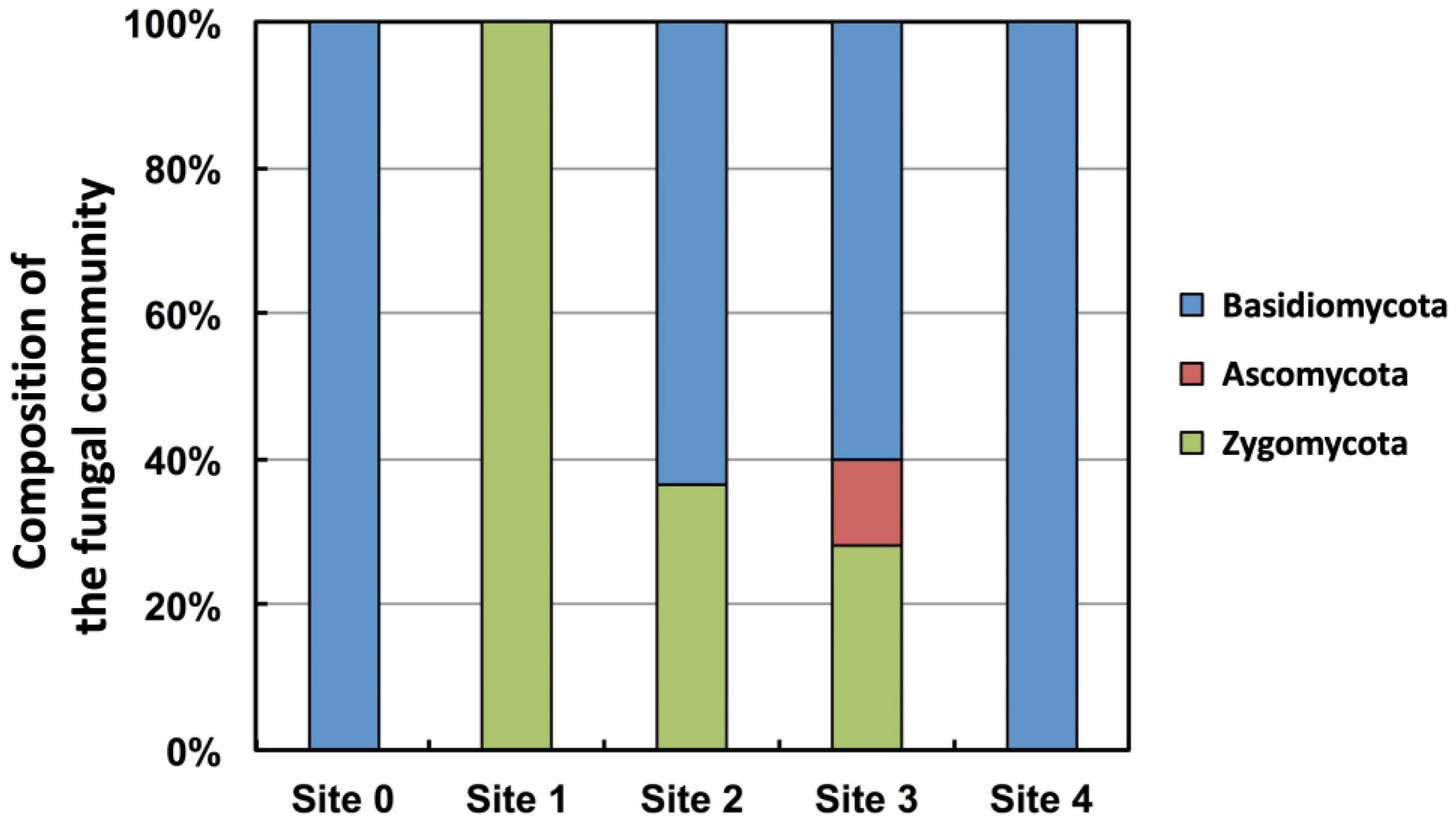
This study observed five sites: Site 0 was located upstream of the glacier, whereas Sites 1–4 were located in the deglaciation area of Austre Brøggerbreen. A total of 58 fungal strains were isolated and classified into two genera and two species of Ascomycota, four genera and seven species of Basidiomycota, and two genera and five species of Zygomycota. Only basidiomycete yeasts were isolated from Site 0, which is seven meters upstream the glacier terminus. Only zygomycetes were isolated from Site 1, where the ground was recently exposed approximately 10 y after the glacier retreated. At Site 2, where approximately 50 y have passed since the glacier retreated, the genus Mrakia, a basidiomycete yeast, was isolated, in addition to zygomycetes. At Site 3, where approximately 80 y have passed since the glacier retreated, a zygomycete, ascomycete, and basidiomycete yeast were isolated (
Figure 4).
Figure 4.
Composition of the fungal community at each sampling point in the Austre Brøggerbreen retreat area.
Figure 4.
Composition of the fungal community at each sampling point in the Austre Brøggerbreen retreat area.
Only basidiomycete yeasts were isolated at Site 0, 7 m upstream from the glacier terminus. Only zygomycetes were isolated at Site 1, where the ground was exposed relatively recently, about ten years after the glacier retreated. From Site 2, where about 50 years have passed since the glacier retreated, the genus Mrakia, a basidiomycete yeast, was isolated in addition to zygomycetes, and from Site 3, where about 80 y have passed since the glacier retreated, a zygomycete and basidiomycete yeast was isolated as well as an ascomycete yeast (Fig 4).
Based on these results, the changes in the fungal community in the Austre Brøggerbreen retreat area process can be inferred as follows.
Immediately after the glacier receded and exposed the ground, Mortierella spp. and Mucor spp., both of which belong to Zygomycota, colonized the area (Site 1).
Mrakia sp. colonized the area by utilizing the nutrients produced by zygomycetes (Site 2).
To colonize the area, ascomycete and basidiomycete yeasts (except for Mrakia sp.) utilized the nutrients accumulated by Mrakia sp. (Site 3).
Thus, the diversity of fungi in the Austre Brøggerbreen retreat area has changed significantly for more than 80 y [
22].
5. Fungal survey in the Ellesmere Island, Canadian High Arctic
The Ellesmere Island in Canada is known as the northernmost inhabited island in the world. Fungal surveys were conducted in the northern part of the Ellesmere Island on Ice Island (82°50′ N, 73° 40′ W) and Walker Glacier (unofficial name: 83°00′ N, 72°12′ W) (
Figure 5).
Figure 5.
Map of the Canadian High Arctic.
Figure 5.
Map of the Canadian High Arctic.
The Walker Glacier had been retreating its terminus at an average rate of 1.3 m/y from 1959 to 2013 due to glacial melting, thus exposing the ground. However, from 2013 to 2016, the glacier retreat rate increased to 3.3 m/y, suggesting that significant climate change also occurred in the High Arctic. In this glacial retreat area, fungi were cultured at nine sites, wherein two were isolated from the glacier and seven from the retreat area. Changes in the fungal community at each site were examined.
A total of 325 fungal strains were isolated, of which the DNA (ITS and D1/D2 regions) of 275 strains were successfully sequenced. These strains were classified into 11 genera and 11 species of Ascomycota, seven genera and 12 species of Basidiomycota, and two genera and six species of Zygomycota.
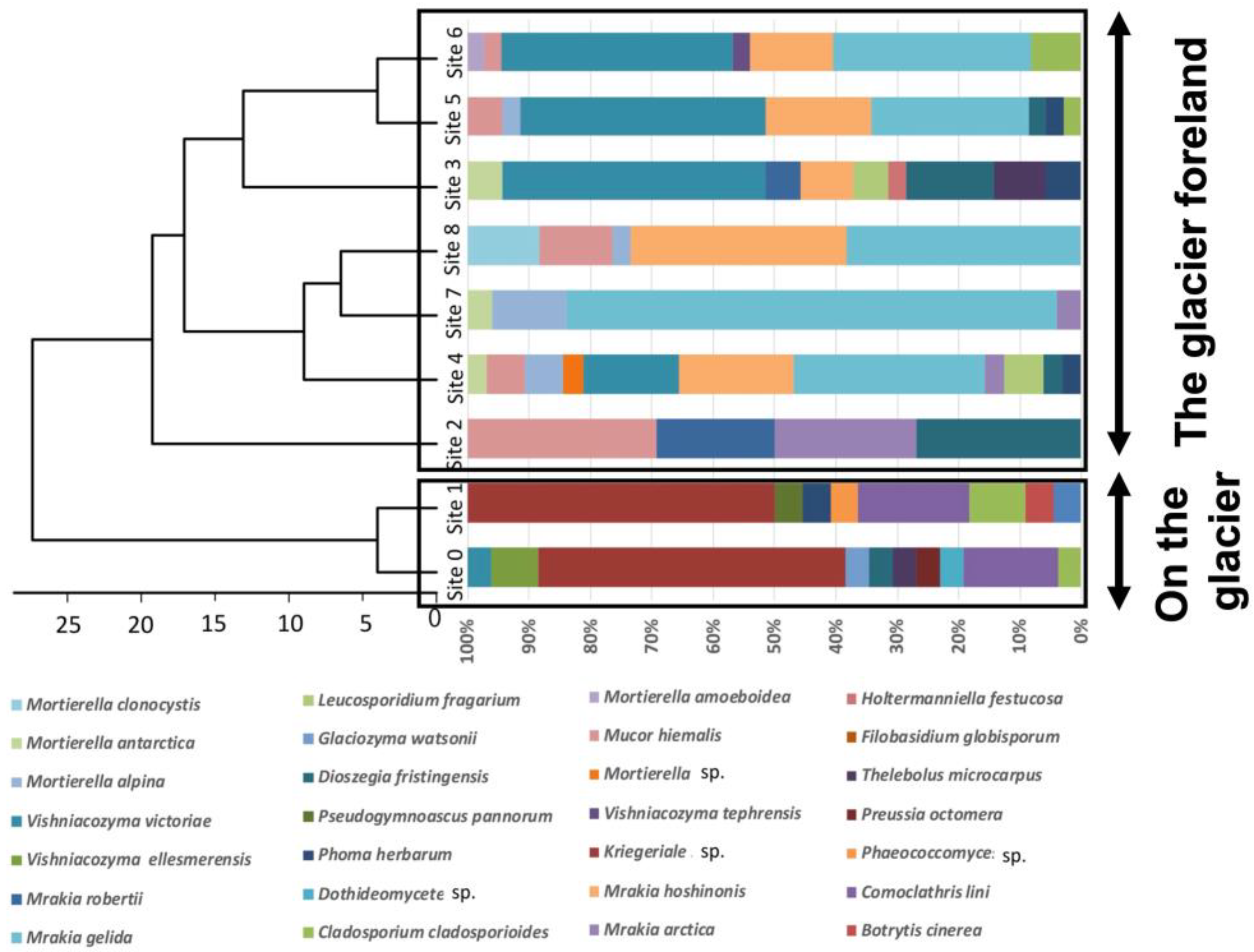
Cluster analysis showed that the fungal community was divided into two groups: on glaciers and in glacial retreat areas (
Figure 6). Among the fungi living on the Walker Glacier, several species appear to be novel and have not yet been reported; these species may be endemic to this region. This indicates that if climate change continues and the glaciers are entirely lost, many of the fungi living on the glaciers will lose their habitat and become extinct. Thus, global warming in the Arctic region affects not only animals, such as polar bears, but microorganisms, such as fungi, as well [
23].
During these fungal surveys, basidiomycete yeasts were reported as new fungal species:
Gelidatrema psychrophila and
Mrakia arctica from the Ice Island, and
Vishniacozyma ellesmerensis and
Mrakia hoshinonis from the Walker Glacier [
24,
25,
26,
27].
Figure 6.
Dendrogram of the fungal communities among the nine sites in the Walker Glacier and its foreland.
Figure 6.
Dendrogram of the fungal communities among the nine sites in the Walker Glacier and its foreland.
6. Growth and enzyme activities at sub-zero temperatures
Fungi cannot regulate their intracellular temperature, and thus their biological activities are affected by the extracellular environment, such as external and water temperatures. Therefore, it is difficult for fungi to maintain their vital activities in extreme environments, such as the Antarctic and Arctic regions, due to intracellular freezing and reduced enzyme activity caused by low temperatures. In the vicinity of Syowa Station and Ellesmere Island, the average maximum temperature is below 0℃ for most days of the year. However, 77 fungal species have been reported from the Syowa Station area, whereas more than 30 fungal species were reported from Ellesmere Island.
Fungi living in the polar regions were examined for their activity in sub-zero environments. Nineteen species of fungi isolated from the East Ongul Island and the Ellesmere Island were selected and cultured at −3°C to examine their ability to grow at sub-zero temperatures. −3°C is the lower temperature limit at which YPD liquid medium or potato dextrose agar medium will not freeze. Results confirmed growth at −3°C for all species, regardless of habitat, optimal growth temperature, or maximum growth temperature (
Table 2) [
24,
26,
27]. Furthermore, fungi living in the Antarctic and Arctic regions can degrade extracellular polymers even at −3°C and utilize these compounds for growth, indicating that these fungi harbor enzymes that are active at −3°C and have the ability to secrete these enzymes extracellularly. The ability of the same strains to secrete enzymes at −3°C were examined and calculated using the following formula:
Secretion ability was assessed as follows: ++, strongly positive, for values >2.0; +, positive, for values between 1.0 and 2.0; w, weakly positive, for values < 1.0; and –, negative, no clear zone.
Table 2.
Growth ability of fungi isolated from the East Ongul Island in Antarctica and the Ellesmere Island in the Canadian High Arctic.
Table 2.
Growth ability of fungi isolated from the East Ongul Island in Antarctica and the Ellesmere Island in the Canadian High Arctic.
| Species |
Habitat |
growth at -3 °C |
Optimum growth temperature |
Maximum growth temperature |
| Cystobasidium lysinophilum |
East Ongul Island |
+ |
25 °C |
30 °C |
| Cystobasidium ongulense |
East Ongul Island |
+ |
20 °C |
30 °C |
| Cystobasidium tubakii |
East Ongul Island |
+ |
15-17 °C |
25 °C |
| Glaciozyma Antarctica |
East Ongul Island |
+ |
10 °C |
15 °C |
| Glaciozyma martinii |
East Ongul Island |
+ |
15 °C |
17 °C |
| Goffeauzyma gilvescens |
East Ongul Island |
+ |
20 °C |
25 °C |
| Holtermanniella wattica |
East Ongul Island |
+ |
15 °C |
25 °C |
| Mrakia arctica |
Ellesmere Island |
+ |
15 °C |
20 °C |
| Mrakia gelida |
East Ongul Island |
+ |
15 °C |
20 °C |
| Mrakia hoshinonis |
Ellesmere Island |
+ |
15 °C |
20 °C |
| Naganishia adeliensis |
East Ongul Island |
+ |
25 °C |
30 °C |
| Naganishia albidosimilis |
East Ongul Island |
+ |
25 °C |
30 °C |
| Naganishia friedmannii |
East Ongul Island |
+ |
20 °C |
25 °C |
| Phenoliferia glacialis |
East Ongul Island |
+ |
15 °C |
17 °C |
| Tausonia pullulans |
East Ongul Island |
+ |
15 °C |
25 °C |
| Udeniomyces puniceus |
East Ongul Island |
+ |
20 °C |
25 °C |
| Vishniacozyma carnescens |
East Ongul Island |
+ |
20 °C |
25 °C |
| Vishniacozyma ellesmerensis |
Ellesmere Island |
+ |
15-17 °C |
20 °C |
| Vishniacozyma victoriae |
East Ongul Island |
+ |
17 °C |
25 °C |
Culture experiments were performed on potato dextrose agar medium. + indicates a clear growth within seven days after culture.
Table 3 summarizes the results of the enzyme secretion tests for lipase, cellulase, and protease from the fungal strains isolated from the East Ongul Island and the Ellesmere Island.
Table 3.
Enzyme secretion ability of fungi isolated from the Antarctic and Canadian High Arctic at -3°C.
Table 3.
Enzyme secretion ability of fungi isolated from the Antarctic and Canadian High Arctic at -3°C.
| Species |
Habitat |
Lipase |
Cellulase |
Protease |
| Cystobasidium lysinophilum |
East Ongul Island |
- |
- |
- |
| Cystobasidium ongulense |
East Ongul Island |
0.43±0.05 |
0.17±0.06 |
- |
| Cystobasidium tubakii |
East Ongul Island |
0.19±0.07 |
0.13±0.01 |
- |
| Glaciozyma Antarctica |
East Ongul Island |
0.41±0.13 |
- |
- |
| Glaciozyma martinii |
East Ongul Island |
- |
- |
- |
| Goffeauzyma gilvescens |
East Ongul Island |
2.50±0.20 |
- |
- |
| Holtermanniella wattica |
East Ongul Island |
2.27±0.09 |
- |
- |
| Mrakia arctica |
Ellesmere Island |
6.15±0.68 |
5.34±0.78 |
0.75 ± 0.12 |
| Mrakia gelida |
East Ongul Island |
- |
0.35±0.07 |
- |
| Mrakia hoshinonis |
Ellesmere Island |
4.29±0.34 |
2.55±0.20 |
1.53±0.05 |
| Naganishia adeliensis |
East Ongul Island |
1.23±0.41 |
- |
0.40±0.12 |
| Naganishia albidosimilis |
East Ongul Island |
0.84±0.16 |
- |
- |
| Naganishia friedmannii |
East Ongul Island |
- |
- |
1.11±0.08 |
| Phenoliferia glacialis |
East Ongul Island |
- |
- |
- |
| Tausonia pullulans |
East Ongul Island |
2.60±0.33 |
1.88±0.21 |
- |
| Udeniomyces puniceus |
East Ongul Island |
- |
2.92±0.37 |
0.86±0.35 |
| Vishniacozyma carnescens |
East Ongul Island |
0.56±0.31 |
0.49±0.22 |
- |
| Vishniacozyma ellesmerensis |
Ellesmere Island |
1.56±0.16 |
- |
- |
| Vishniacozyma victoriae |
East Ongul Island |
0.62±0.03 |
- |
0.49±0.18 |
The values represent the difference between the diameters of the zone of clearance and the colony, expressed as a proportion of the colony size (means ± SD for triplicates).-; no activity.
Holtermanniella wattica,
Tausonia pullulans, and
Goffeauzyma gilvescens isolated from East Ongul Island, as well as
Mrakia arctica and
M. hoshinonis isolated from Ellesmere Islandshowed high lipase secretion ability at −3°C. Meanwhile,
Udeniomyces puniceus isolated from East Ongul Island, and
M. arctica and
M. hoshinonis isolated from Ellesmere Island showed high cellulase secretion ability at −3°C [
24,
26,
27]. None of the fungi isolated from the Antarctic and Arctic regions showed high protease secretion ability at −3°C. Interestingly,
M. arctica and
M. hoshinonis isolated from Ellesmere Island showed higher secretory ability for cellulase and lipase than the fungal strains from Antarctica [
24,
27].
These results indicate that fungi in the Antarctic and Arctic regions can secrete active enzymes even at sub-zero temperatures. This suggests that these fungi play an essential role in decomposing organic materials in environments where the temperature is below freezing most days of the year, such as in polar regions.
7. Future research prospects
In addition to the fungal survey using the culture-based method, a long-read next-generation sequencer, such as MinION and PacBio Sequel2 sequencing, can be used to conduct a comprehensive fungal survey at the species level. Fungal surveys not been conducted in many areas in the vicinity of Syowa Station in Antarctica and Ellesmere Island in the Canadian High Arctic. Investigation on the fungal diversity of these areas and the effects of climate change on the fungal community should be continued.
Because Antarctic and Arctic fungal strains are at risk of habitat loss and extinction due to climate change, systematic preservation for the future generations of these strains should be attempted. In addition, the genome sequence of Antarctic fungi has attracted attention as a novel genetic resource because it provides information on active enzymes even at sub-zero temperatures and new pharmaceutical raw materials. By predicting gene sequences from the whole genome sequences of Antarctic fungi and publishing the results in a genome database, I would like to make genome information on Antarctic fungi available to everyone, even if they lack knowledge on bioinformatics. The Antarctic fungi should be actively utilized by universities, research institutes, and private companies. This will allow Japan to lead the world in establishing a new research field using Antarctic fungi.
Author Contributions
Wrote the manuscript: MT.
Funding
This work was supported by the NIPR Research Project (KP-309), a JSPS Grant-in-Aid for Scientific Research(B) to M. Tsuji (no. 23H03590), Institution for Fermentation, Osaka, for General Research Grant to M. Tsuji (no. G-2022-1-007), and the ArCS2 (Arctic Challenge for Sustainability) II project (Program Grant Number JPMXD1420318865) provided by the Ministry of Education, Culture, Sports, Science and Technology, Japan. This work was also supported by "Strategic Research Projects " grant from ROIS (Research Organization of Information and Systems) (no. 2022-SRP-01).
Acknowledgments
The author would like to thank the National Institute of Polar Research, Japan, and The National Institute of Technology, Asahikawa College, Japan, for their support in obtaining these research results. This work is contributions to the National Institute of Polar Research, Japan mission and the International Arctic Science Committee (IASC) project T-MOSAiC (Terrestrial Multidisciplinary distributed Observatories for the Study of Arctic Connections).
Conflicts of Interest
The author declares no conflict of interest.
References
- Onofri, S.; Zucconi, L.; Tosi, S. Continental Antarctic Fungi. IHW Verlag, München, Germany, 2007.
- Tsuji, M.; Kudoh, S. Soil Yeasts in the Vicinity of Syowa Station, East Antarctica: Their Diversity and Extracellular Enzymes, Cold Adaptation Strategies, and Secondary Metabolites. Sustainability 2020, 12, 4518. [CrossRef]
- Boomer, E.; Rousseau, M. Note préliminaire sur les champignons recueillis par l’Expedition Antarctique Belge. Bulletin de l'Académie Royale des Sciences de Belgique Classe des Sciences 1900, 8, 640–646.
- Fell, J.W.; Statzell, A.C.; Hunter, I.L.; Phaff, H.J. Leucosporidium gen. n., the heterobasidiomycetous stage of several yeasts of the genus Candida. Antonie van Leeuwenhoek, 1969, 35, 433–462. [CrossRef]
- di Menna, M.E. Three new yeasts from Antarctica soils: Candida nivalis, Candida gelida and Candida frigida spp. n. Antonie van Leeuenwoek, 1966, 32, 25–28. [CrossRef]
- di Menna, M.E. Yeasts in Antarctic soil. Antonie van Leeuwenhoek , 1966, 32, 29–38. [CrossRef]
- Bridge, P.D.; Spooner, B.M. (2012) Non-lichenized Antarctic fungi: transient visitors or members of a cryptic ecosystem? Fungal Ecol. 2012, 5, 381–394. [CrossRef]
- Tubaki, K. On some fungi isolated from the Antarctic materials. Biological results of the Japanese Antarctic Research Expedition,1961, 14, 1- 9.
- Tubaki, K. (1961b). Note on some fungi and yeasts from Antarctica. Antarctic Record (Tokyo), 1961, 11, 161 162.
- Soneda, M. (1961). On some yeasts from the Antarctic region. Biological results of the Japanese Antarctic Research Expedition,1961, 15, 3-10.
- Tubaki, K. ;Asano, I. (1965). Additional species of fungi isolated from the Antarctic materials. JARE scientific reports. Series E: Biology, 27, 1–27.
- Goto, S.; Sugiyama, J.; Iizuka, H. A Taxonomic Study of Antarctic Yeasts. Mycologia, 1969, 61, 784–774. [CrossRef]
- Kitamoto, D.; Yanagishita, H.; Shinbo, T.; Nakane, T.; Kamisawa, C.; Nakahara, T. Surface active properties and antimicrobial activities of mannosylerythritol lipids as biosurfactants produced by Candida Antarctica. Journal of Biotechnology, 1993, 29, 91–96. [CrossRef]
- Kitamoto, D.; Ikegami, T.; Suzuki, G.T.; Sasaki, A.; Takeyama, Y.; Idemoto, Y.; Koura, N.; Yanagishita, H. Microbial conversion of n-alkanes into glycolipid biosurfactants, mannosylerythritol lipids, by Pseudozyma (Candida Antarctica). Biotechnology Letters, 2001, 23, 1709–1714. [CrossRef]
- Tsuji, M.; Fujiu, S.; Xiao, N.; Hanada, Y.; Kudoh, S.; Kondo, H.; Tsuda, S.; Hoshino, T. Cold adaptation of fungi obtained from soil and lake sediment in the Skarvsnes ice-free area, Antarctic. FEMS Microbiol. Lett. 2013, 346, 121–130.
- Hirose, D.; Tanabe, Y.; Uchida, M.; Kudoh, S.; Osono, T. Microfungi associated with withering willow wood in ground contact near Syowa Station, East Antarctica for 40 years. Polar Biology, 2013,36, 919–924. [CrossRef]
- Tsuji, M. A catalog of fungi recorded from the vicinity of Syowa Station. Mycoscience, 2018, 59, 319–324. [CrossRef]
- Tsuji, M. Genetic diversity of yeasts from East Ongul Island, East Antarctica and their extracellular enzymes secretion. Polar Biol. 2018, 41, 249–258. [CrossRef]
- Tsuji, M.; Tsujimoto, M.; Imura, S. (2017) Cystobasidium tubakii and Cystobasidium ongulense, new basidiomycetous yeast species isolated from East Ongul Island, East Antarctica. Mycoscience, 2017, 58, 103 –107. [CrossRef]
- Tsuji, M. An index of non-lichenized fungi recorded in the vicinity of Syowa Station, East Antarctica. In Fungi in Polar Regions; Tsuji, M.; Hoshino, T. Eds.; CRC press, Oxford, United Kingdom, 2019; pp.1–16.
- Nowak, A.; Hodson, A. Changes in meltwater chemistry over a 20-year period following a thermal regime switch from polythermal to cold-based glaciation at Austre Brøggerbreen, Svalbard. Polar Research, 2014, 33, 22779.
- Tsuji, M.; Uetake, J.; Tanabe, Y. Changes in the fungal community of Austre Brøggerbreen deglaciation area, Ny-Ålesund, Svalbard, High Arctic. Mycoscience, 2016, 57, 448–451. [CrossRef]
- Tsuji, M.; Vincent, W.F.; Tanabe, Y.; Uchida, M. Glacier Retreat Results in Loss of Fungal Diversity. Sustainability 2022, 14, 1617. [CrossRef]
- Tsuji, M.; Tanabe, Y.; Vincent, W.F.; Uchida, M. Mrakia arctica sp. nov., a new psychrophilic yeast isolated from an ice island in the Canadian High Arctic. Mycoscience, 2018, 59, 54–58. [CrossRef]
- Tsuji, M.; Tanabe, Y.; Vincent, W.F.; Uchida, M. Gelidatrema psychrophila sp. nov., a novel yeast species isolated from an ice island in the Canadian High Arctic. Mycoscience, 2018, 59, 67–70. [CrossRef]
- Tsuji, M.; Tanabe, Y.; Vincent, W.F.; Uchida, M. Vishniacozyma ellesmerensis sp. nov., a new psychrophilic yeast isolated from a retreating glacier in the Canadian High Arctic. International Journal of Systematic and Evolutionary Microbiology, 2019, 69, 696–700. [CrossRef]
- Tsuji, M.; Tanabe, Y.; Vincent, W.F.; Uchida, M. Mrakia hoshinonis sp. nov., a novel psychrophilic yeast isolated from a retreating glacier on Ellesmere Island in the Canadian High Arctic. International Journal of Systematic and Evolutionary Microbiology, 2019, 69, 944–948. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).