Introduction
Human lifespan is significantly longer compared to the closely related primate species with shared common origin. For instance, our genetically closest relatives chimpanzees, when kept in captivity, have the mean life expectancy of only 32.5 years for males and 40.1 years for females, although some animals were reported to survive much longer, well into their 70s (Newsweek, 2017). The mean life expectancy of wild chimpanzees is even lower, approximately 33 years for both sexes combined (Wood et al., 2017). The mean human life expectancy, on the other hand, is 73.4 years (WHO, 2023), and it well exceeds 80 years in multiple countries (Worlddata, 2022). A significant proportion of the world’s population (up to 0.1% of total population in some countries like Malaysia and Japan) lives over 100 years these days. It is often claimed that the natural limit of human lifespan is approximately 120 years (Blagosklonny, 2021).
While the dramatic increase in human longevity is a relatively recent phenomenon closely related to the improved life quality and the advances in medicine in the last 200 years, multiple findings indicate that human lifespan has been gradually increasing during prehistoric time after our species separated from other primates. The human life expectancy at birth has doubled over an evolutionary span of about 300,000 generations from a great ape ancestor shared with chimpanzees (Finch, 2010). Approximately half of the lifespan difference between humans and other primates is associated with this increase.
Twin studies suggested a heritability of life span close to 25% (Dato et al., 2019). To date, only a few genetic variants in the following genes have been linked to longevity in different populations: APOE (APOE/TOMM40), FOXO3А, CDKN2B-AS1, and locus 5q33.3 (Kunizheva et al., 2022). These variants explain only a small part of the estimated heritability of human longevity (Dato et al., 2019). The mechanisms regulating the longevity on population levels remain poorly understood.
Telomeres and Longevity
It is generally accepted that inheriting longer telomeres from parents predisposes an individual to a longer lifespan (Scarabino et al., 2019, Rodríguez-Fernández et al., 2022). These chromosomal structures are known to be involved in cellular senescence (Victorelli and Passos, 2017) and their age-associated shortening is considered as an important contributor to organismal aging (McHugh and Gil, 2018, Vaiserman et al., 2021). A mechanism that can increase an average length of telomeres in a population would also contribute to a longer average lifespan and extended health span in this population.
Recently, it has emerged that the length of telomeres is associated with the paternal age at the time of conception (De Meyer et al., 2007). The length of telomeres in human spermatozoids increases with the age of male. Although the mechanism behind this phenomenon remains debatable (Stindl, 2016), its existence is well proven experimentally. The effect of paternal age at conception on telomere length may allow a gradual multi-generational adaptive calibration of reproductive lifespan to local demographic conditions (Eisenberg and Kuzawa, 2018). Moreover, the effect of paternal age at conception is detectable in both children and grandchildren (Eisenberg et al., 2012). The effect is transmitted through the matriline and patriline with similar strength and is characterized by a generational decay (Eisenberg et al., 2019).
The influence of maternal age on the length of telomeres in children is much smaller and probably not statistically significant (Nordfjäll et al., 2010). However, advanced maternal age is known to be associated with negative offspring health outcomes, which probably relates to the decrease of oocyte quality with age (Myrskylä and Fenelon, 2012). From this perspective, having an older father and younger mother is likely to increase the lifespan of offspring. Interestingly, in the majority (64%) of heterosexual couples, men are older than women, with the average age difference of 2.3 years. This age gap increases to almost 5 years in older couples (FiveThirtyEight, 2015). Although the routes of this phenomenon are considered mostly social, it may contribute to the increased longevity.
Human males remain fertile and capable of fathering children well into an advanced age. Extension of telomeres and associated extension of life span also means the extension of male reproductive span and increased possibility of fathering children at an older age. This may be an important consideration in social animals like humans and other primates in whom a higher position in social hierarchy and associated reproductive privileges often come with older age.
Unlike males, the reproductive span of females is limited by the onset of menopause relatively early in life. However, girls become sexually mature at younger age compared to boys. Moreover, the inheritance of shorter telomeres accelerates sexual maturation, which would allow reproduction at earlier age (Koss et al., 2020).
These observations provide a clear possibility for regulating the population lifespan in both directions in response to the natural selection pressure. When the pressure is low, a larger number of males survive well into the advanced age. These males will not only be able to conceive more children, but also pass longer telomeres to the children fathered at older age. Over time, these longer telomeres will contribute to the increased mean life span of the whole population. Moreover, a higher occurrence of longer telomeres will increase the chances of passing any longevity-associated genes that may occur in a person with longer telomeres. This could further accelerate the increase of longevity in a given population.
On the other hand, when the natural selection pressure is high, and the chances of surviving to an older age are low, the majority of children will be conceived by younger fathers and inherit shorter telomeres. These children will have a shorter lifespan, but will be able to reproduce earlier in life due to accelerated sexual maturation of girls. Earlier reproduction will help to produce more children during the shortened life span, which will improve the survival of population experiencing strong natural selection pressure.
Possible Role of Contraception
Under the constant selective pressure, any strategy resulting in delaying the age of reproduction may result in gradual increase of longevity. These days, contraception is a frequently used method of preventing undesirable pregnancy, which is commonly utilized to delay the parenthood. In 2016, the age of men’s entry into fatherhood in the US was 27.5 years. This is two years later compared to the same parameter measured in 1987/88 (Schweizer, 2019), and the trend for the increasing age of parenthood was observed for the most of 20
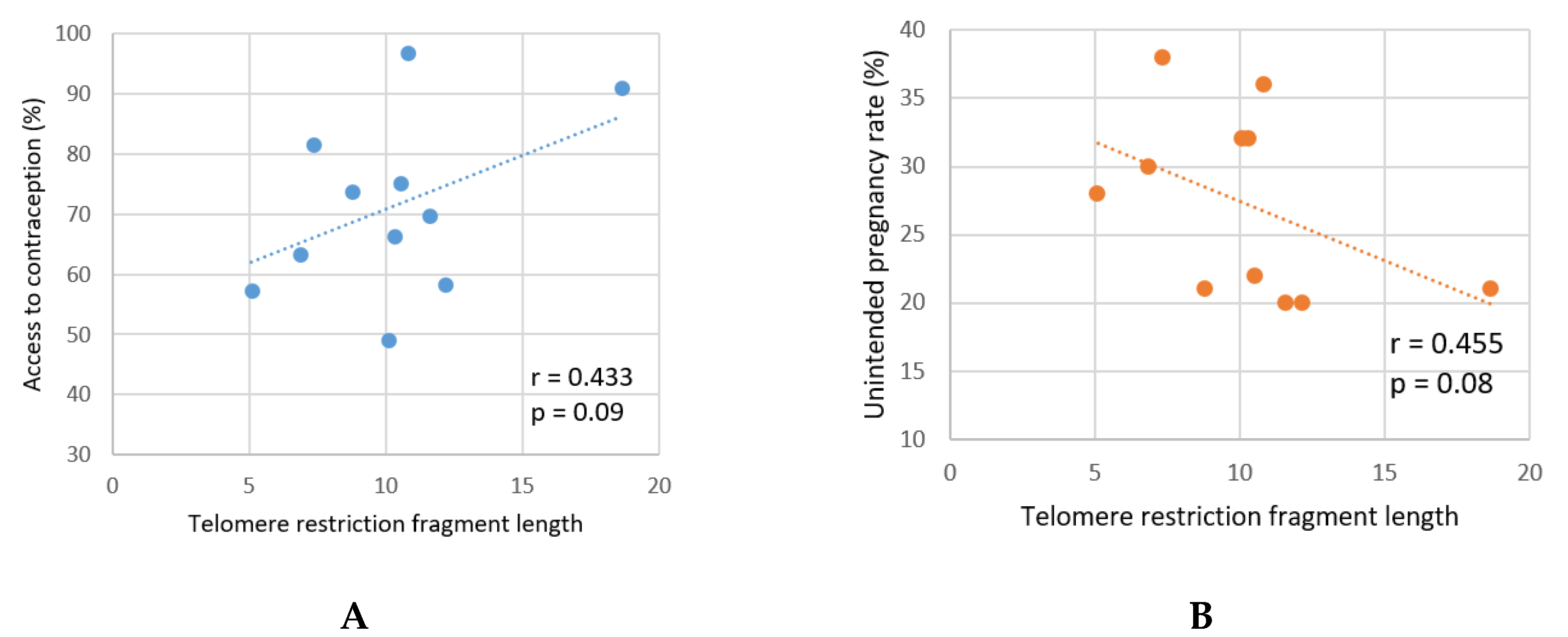
th century. The availability of effective contraception contributed to this trend significantly. However, the availability of contraception may differ very significantly between countries, as demonstrated by the recent analysis of contraceptive policy in Europe (Contraception Policy Atlas Europe 2023). Moreover, the availability of contraception does not necessarily mean that it is effectively utilized. The rate of unintended pregnancies remains very high in many European countries, and this parameter also shows significant regional variability (Bearak et al., 2020). The published data on the availability of contraception and the rate of unintended pregnancies (
Table 1) shows no correlation (r = 0.012, p = 0.361).
Effective contraception is a relatively novel phenomenon spanning just a few recent generations. Therefore, its effect on the telomere length in a population is expected to be small. The length of telomeres varies dramatically among people from different parts of the world. Even within the regions with relatively homogenous population, such as Europe, the mean telomere length can vary substantially between the countries (Eisenberg et al., 2011).
Still, the correlation between the availability or utilization of contraception and telomere lengths in population may exist. The data on telomere length in various geographic locations is very limited, which makes the analysis difficult. Eisenberg and co-authors (2011) measured the length of telomeres in young men aged 18-28 from 11 European countries (
Table 1). The results of these measurements can be used to evaluate if contraception’s availability/utilization and telomere length correlate on the population level.
Indeed, two indicators of contraception availability and utilization in Europe both revealed a weak correlation with the length of telomeres in younger males (r = 0.433 (p = 0.09) and r = 0.455 (p = 0.08)) (
Figure 1). Obviously, the statistical importance of these correlations is very low. The indicators of contraception use only reflect a current state of affairs and lack the historical perspective. These indicators are influenced by a large number of independent social and economic factors. Still, the analysis suggests that the trend may exist, and more research is needed to confirm or rule out this possibility.
Homosexuality as a Possible Contributor to Human Longevity
In the absence of contraceptive interventions or a significant degree of social control over reproduction, delaying reproduction is not a trivial task. Due to a high level of sex hormones, young males usually do not need additional encouragement to get engaged in sexual activities, which may easily result in a pregnancy as an unintended consequence. In a hierarchical social structures of various primate groups, including humans, the access of younger males to reproductive sex is often limited due to competition with dominant older males. But this exclusion is not full due to the difficulties in controlling the behavior of multiple group members, both males and females. If the selective pressure is strong, the relative number of older males is limited, which gives higher chances for younger males to reproduce earlier.
However, the situation becomes rather different if some “gay genes” are introduced in this population. Being sexually attracted to other males, gay males are not pursuing reproductive sex with females. Therefore, delaying of or exclusion from reproductive sex comes to them naturally. Being uninterested or less interested in sex with females, these males either don’t reproduce, or reproduce at much later age. The reproduction in the latter case may come as a result of an active decision to have children, rather than as an unintended consequence of sexual activity which is very common at younger age.
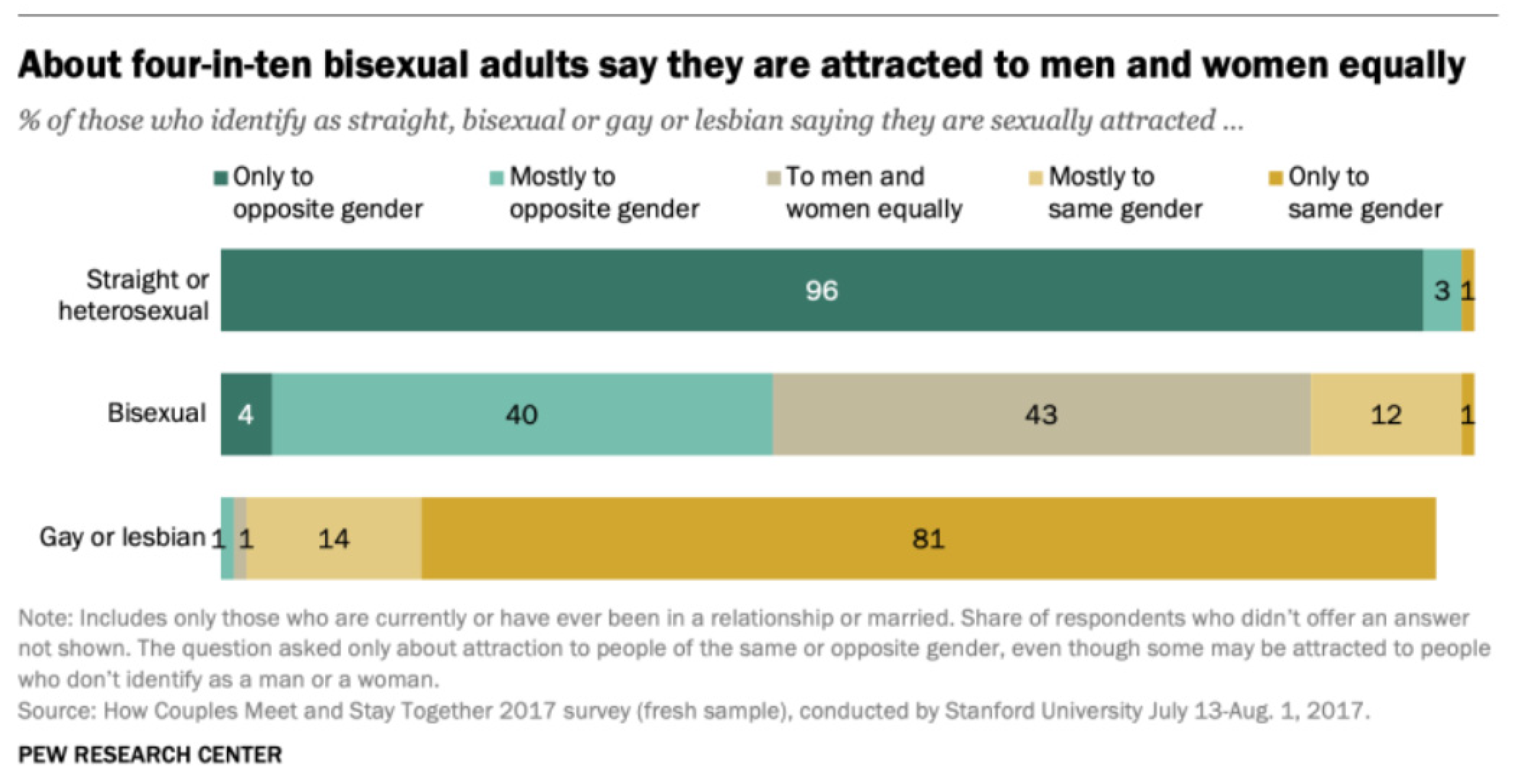
Simple statistical considerations demonstrate perfectly well how reproduction can be delayed in the presence of gay genes. Technically, homosexual males that engaged in reproductive sex should be considered bisexual. Bisexual population is very diverse. Some bisexuals only occasionally become sexually involved with the opposite sex, while others predominantly have sexual encounters with the opposite sex, with only few episodes of homosexual activity. The majority of bisexuals have fairly regular sexual encounters with both sexes (
Figure 2).
Let’s consider a society with no effective means of contraception (which would be the case for the most of human history). There is a finite number of sexual acts a single male can perform in certain period of time. Although the level of sexual activity is highly variable, the statistics suggests that an average male aged 18 to 29 has sex 112 times per year, or twice per week (Medical Daily, 2017). Very few sexual acts result in female becoming pregnant. On an average, couples have sex 78 times from the time they start trying to conceive to the time they get a positive result (New York Post, 2017). This means that the average time period between becoming sexually active and successfully impregnating a woman is 78/112 = 0.7 years (approximately 8.5 months).
Now let’s consider a bisexual male who engages in sex with other men 50% of time. Assuming that the average number of sexual intercourses per year remains unchanged, and only half of these intercourses (112/2 = 56) are potentially reproductive, the time period between becoming sexually active and successfully impregnating a woman is 78/56 = 1.4 years (~17 months). If a bisexual male in question is predominantly homosexual and engages in sex with women only 10% of time, the average time period between becoming sexually active and achieving a pregnancy becomes really large: 78/11.2 = ~7 years. Even this very simplified model shows that the statistically average age of fathering a child should increase very significantly among sexually active males who rarely have sex with women.
In the US, the mean age at first intercourse was reported at approximately 14.3 years (Cavazos-Rehg et al., 2009). In our model, we can assume for simplicity that the age of a sexual debut was approximately the same during the early history of our species. If this age was the same regardless of the sexual orientation, and there were no societal restrictions preventing early fatherhood, an average heterosexual male in this model could become a father just after 15, while a bisexual male with only 10% of male-female sex – at the age of 21. Such a significant difference in the age of reproduction can have a large impact on the length of telomeres passed to offspring.
In line with the less frequent involvement in the reproductive sex, bisexuals are significantly less likely to become parents. Canadian statistics demonstrates that only 13.4% of bisexual individuals live with at least one child under the age of 18. This number is even lower for gays and lesbians (6.2%). In comparison, nearly one-quarter (23.6%) of the heterosexual population was made up of parents living with at least one child under the age of 18 (Statistics Canada, 2021).
In almost all traditional societies, there is a strong pressure on boys to marry and produce children. Therefore, even purely homosexual (not bisexual) males may eventually father children, but typically later in life compared to their heterosexual peers. This phenomenon and its social and psychological consequences are well documented (for example, Zheng et al., 2020). Eventual reproduction in this part of population allows the passing of longer telomeres to the next generation, together with the genes predisposing for homosexuality.
Exclusive homosexual behavior is rather uncommon in primates (Cvorovic, 2006), but in human population approximately 5% of individuals are exclusively or almost exclusively gay. The quantitative estimates vary by large margins but, regardless of the source of statistics, this is a sizable proportion of the overall population. Still, 39% of gay men have had a female sexual partner sometime in their lives (Contexts, 2016).
It is interesting to note that in many conservative societies the percentage of LGBTQ+ people tends to exceed the global average, although the quantitative estimates vary by significant margins and aren’t fully reliable. According to one estimate, up to 17% of Indians and 15% of Brazilians are non-heterosexual, with bisexuals representing the largest part of these populations (9% and 7%, respectively) (Ipsos survey, 2021). These numbers well exceed the percentage of non-heterosexuals in all European countries provided by the same survey. In India, 70 to 80% of homosexual men are married, mostly to heterosexual women (The Time of India, 2015). The majority of homosexuals get married under pressure from family, to avoid social stigmatization. Such pressure is applied once the sexual orientation of involved individual becomes known (or suspected) by his family members. Usually this happens when young male remains single for a long time. As a result, such marriages happen at older age of males and result in fathering children later compared to their heterosexual peers. It’s easy to see how the transfer of “gay genes” to the next generation can be enforced under the intense social pressure and may even result in their higher occurrence. In contrast, the LGBTQ+ population in the countries such as the Netherlands, France, or New Zealand, with long-established tolerant attitude towards homosexual behavior is significantly smaller, rarely exceeding 10% mark in any survey.
Up to 25% of homosexual behavior is explained by genetic factors (Ganna et al., 2019). The nature of “gay genes” is poorly investigated, but it is clear that like with many other complex behavioral phenomena, the predisposition to homosexuality can be associated with multiple genetic variants (Sanders et al., 2017). The persistence of gay genes in human population represents an evolutionary puzzle due to obvious reproductive disadvantage. It is possible that being a carrier of gay genes provides some reproductive benefits (Chaladze, 2016), but the exact nature of these benefits remains to be established.
The majority of offspring of homosexual males are heterosexual, although an increased proportion of them inherits homosexual behavior. Between 16 and 57% of offspring of homosexuals inherit this behavioral trait (Schumm, 2010), while the mean frequency of homosexuals in human populations is usually in the single digits. This has two consequences. First, the presence of homosexual subpopulation results in a gradual increase of population lifespan, as their heterosexual children with extended telomeres will reproduce normally (i.e. at younger age compared to their gay fathers). Having on average longer telomeres, they are more likely to live longer and thus conceive more children. Homosexual offspring, however, by delaying the involvement in the reproductive sex, will contribute to further increase of telomere length in the consequent generations. Despite the lower reproduction rate by homosexuals, their presence can gradually increase the average lifespan of the whole population. This provides an obvious evolutionary advantage, as longer lifespan would also contribute to the larger overall number of offspring produced by individuals during their lifespan. As the length of telomeres thus becomes partially linked to sexual orientation, the “gay genes” can be successfully passed from one generation to another and remain sustained in populations over historically long periods of time. The effect of generational decay described by Eisenberg and co-authors (2019) will be reduced if longer telomeres are inherited together with genes predisposing for homosexuality.
Recent publications (for example, McNally et al., 2019, DeBoy et al., 2023) suggest that the length of telomeres and general health are connected. There is an optimal length of telomeres, and both too long and too short telomeres bring certain disadvantages. It would be safe to assume that in the early evolutionary history of our species the length of telomeres was shorter, in line with much shorter lifespan, and therefore their gradual elongation was linked to mostly positive effects. If this speculation is correct, having a certain proportion of gay genes in the population brought not only lifespan extension but also a better health. This would give the heterosexual carriers of gay genes an additional reproductive advantage, which would, in turn, further cement the presence of gay gene pool in the human population.
It is likely that this process brought the length of telomeres to the optimal average length. Extending the telomeres further would bring disadvantages, as well as increase the proportion of poorly reproducing gay sub-population to the level that would affect the overall reproduction rate. Eventually, a point of equilibrium may have been reached. Very large gay population can lead to a serious reduction of reproductive rate. Historical precedents include the ancient state of Sparta, where homosexuality was institutionalized. Despite the law requiring Spartan males to marry and produce children, the population of Sparta was gradually declining, which eventually led to the failure of state. The fact that this law was even introduced is a confirmation that the problem of poor reproduction was serious and well recognized.
Conclusion
The theory outlined in this article links together two phenomena that do not have fully satisfactory explanations at present time: why humans live much longer than other primates, and why homosexual behavior in our species persists despite its obvious reproductive disadvantages. Through delayed reproduction, homosexual and bisexual subpopulations contribute to the increasing mean length of telomeres in a population, which results in gradually increasing overall longevity. However, longer telomeres are passed together with the genes predisposing to homosexual behavior, which helps to sustain a substantial pool of “gay genes” despite their reproductive disadvantage. The described effects are likely to be small but may results in a significant demographical shifts over multiple generations. The inheritance of longer telomeres may also help to preserve and spread in populations other genes linked to longevity. The introduction of effective contraception (and the consequent serious increase of the mean age of fathering the children) may have reduced the importance of this effect, but it has likely played a significant role in the extension of human longevity in the past.
The theory provides a testable hypothesis: the average length of telomeres in homosexuals is likely to be longer than in heterosexuals. The difference should be more significant if the comparison is done for purely homosexual and purely heterosexual sub-populations of the same relatively young age and from the same geographic location, and if the use of contraception in the tested population is limited.
References
- Bearak J, Popinchalk A, Ganatra B, Moller A, Tunçalp Ö, Beavin C, Kwok L, Alkema L. (2020) Unintended pregnancy and abortion by income, region, and the legal status of abortion: estimates from a comprehensive model for 1990–2019. Lancet Glob Health 8:e1152–61. [CrossRef]
- Blagosklonny MV (2021) No limit to maximal lifespan in humans: how to beat a 122-year-old record. Oncoscience 8:110-119. [CrossRef]
- Cavazos-Rehg PA, Krauss MJ, Spitznagel EL, Schootman M, Bucholz KK, Peipert JF, Sanders-Thompson V, Cottler LB, Bierut LJ (2009) Age of sexual debut among US adolescents. Contraception 80(2):158-62. [CrossRef]
- Chaladze G (2016) Heterosexual Male Carriers Could Explain Persistence of Homosexuality in Men: Individual-Based Simulations of an X-Linked Inheritance Model. Arch Sex Behav 45(7):1705–1711. [CrossRef]
- Contexts (2016) Sexual orientation versus behavior—different for men and women? Available online: https://contexts.org/blog/sexual-orientation-versus-behavior-different-for-men-and-women/.
- Contraception Policy Atlas Europe 2023. European Parliamentary Forum for Sexual and Reproductive Rights. Available online: https://www.epfweb.org/sites/default/files/2023-02/Contraception_Policy_Atlas_Europe2023.pdf.
- Cvorovic J (2006) Nonhuman Primates Homosexual Behavior: A Critical Review of Literature. Antropologija 2:7-17.
- Dato S, Soerensen M, Rose G (2019) Untangling the Genetics of Human Longevity-A Challenging Quest. Genes (Basel) 10(8):585. [CrossRef]
- DeBoy EA, Tassia MG, Schratz KE, Yan SM, Cosner ZL, McNally EJ, Gable DL, Xiang Z, Lombard DB, Antonarakis ES, Gocke CD, McCoy RC, Armanios M (2023) Familial Clonal Hematopoiesis in a Long Telomere Syndrome. N Engl J Med 388(26):2422-2433. [CrossRef]
- De Meyer T, Rietzschel ER, De Buyzere ML, De Bacquer D, Van Criekinge W, De Backer GG, Gillebert TC, Van Oostveldt P, Bekaert S; Asklepios investigators (2007) Paternal age at birth is an important determinant of offspring telomere length. Hum Mol Genet 16(24):3097-102. [CrossRef]
- Eisenberg DTA, Salpea KD, Kuzawa CW, Hayes MG, Humphrie, SE (2011) Substantial variation in qPCR measured mean blood telomere lengths in young men from eleven European countries. Am J Hum Biol 23(2):228–231. [CrossRef]
- Eisenberg DTA, Hayes MG, Kuzawa CW (2012) Delayed paternal age of reproduction in humans is associated with longer telomeres across two generations of descendants. Proc Natl Acad Sci USA109(26):10251-6. [CrossRef]
- Eisenberg DTA, Kuzawa CW (2018) The paternal age at conception effect on offspring telomere length: mechanistic, comparative and adaptive perspectives. Philos Trans R Soc Lond B Biol Sci 373(1741):20160442. [CrossRef]
- Eisenberg DTA, Lee NR, Rej PH, Hayes MG, Kuzawa CW (2019) Older paternal ages and grandpaternal ages at conception predict longer telomeres in human descendants. Proc Biol Sci 286(1903):20190800. [CrossRef]
- Finch CE (2010) Evolution of the human lifespan and diseases of aging: Roles of infection, inflammation, and nutrition. Proc Natl Acad Sci USA 107(suppl_1):1718–1724. [CrossRef]
- FiveThirtyEight (2015) What’s The Average Age Difference In A Couple? Available online: https://fivethirtyeight.com/features/whats-the-average-age-difference-in-a-couple/.
- Ganna A, Verweij KJH, Nivard MG, Maier R, Wedow R, Busch AS, Abdellaoui A, Guo S, Sathirapongsasuti JF; 23andMe Research Team; Lichtenstein P, Lundström S, Långström N, Auton A, Harris KM, Beecham GW, Martin ER, Sanders AR, Perry JRB, Neale BM, Zietsch BP (2019) Large-scale GWAS reveals insights into the genetic architecture of same-sex sexual behavior. Science 365(6456):eaat7693. [CrossRef]
- Ipsos survey (2021) LGBT+ Pride 2021 Global Survey. Available online: https://www.ipsos.com/sites/default/files/ct/news/documents/2021-06/LGBT%20Pride%202021%20Global%20Survey%20Report_0.pdf.
- Koss KJ, Schneper LM, Brooks-Gunn J, McLanahan S, Mitchell C, Notterman DA (2020) Early Puberty and Telomere Length in Preadolescent Girls and Mothers. J Pediatr 222:193-199.e5. [CrossRef]
- Kunizheva SS, Volobaev VP, Plotnikova MY, Kupriyanova DA, Kuznetsova IL, Tyazhelova TV, Rogaev EI (2022) Current Trends and Approaches to the Search for Genetic Determinants of Aging and Longevity. Russ J Genet 58(12):1427-1443. [CrossRef]
- McHugh D, and Gil J (2018) Senescence and aging: Causes, consequences, and therapeutic avenues. J Cell Biol 217:65–77. [CrossRef]
- McNally EJ, Luncsford PJ, Armanios M (2019) Long telomeres and cancer risk: the price of cellular immortality. J Clin Invest 129(9):3474-3481. [CrossRef]
- Medical Daily (2017) Am I 'Normal?' Average Sex Frequency Per Week Linked To Age. 4213. Available online: https://www.medicaldaily.com/am-i-normal-average-sex-frequency-week-linked-age-421328.
- Myrskylä M, Fenelon A (2012) Maternal age and offspring adult health: evidence from the health and retirement study. Demography 49(4):1231-57. [CrossRef]
- Newsweek (2017) Zoo Animal Little Mama, World's Oldest Known Chimpanzee, Dies at 79 Years Old. 7126. 7126. Available online: https://www.newsweek.com/zoo-animal-little-mama-worlds-oldest-known-chimpanzee-dies-79-years-old-712622.
- New York Post (2017) Here’s how much sex you should have if you want to make a baby. Available online: https://nypost.com/2017/05/31/heres-how-much-sex-you-should-have-if-you-want-to-make-a-baby/?utm_campaign=SocialFlow&utm_source=NYPTwitter&utm_medium=SocialFlow&sr_share=twitter.
- Nordfjäll K, Svenson U, Norrback KF, Adolfsson R, Roos G (2010) Large-scale parent-child comparison confirms a strong paternal influence on telomere length. Eur J Hum Genet 18(3):385-9. [CrossRef]
- Pew Research Center (2019) Bisexual adults are far less likely than gay men and lesbians to be ‘out’ to the people in their lives. Available online: https://www.pewresearch.org/short-reads/2019/06/18/bisexual-adults-are-far-less-likely-than-gay-men-and-lesbians-to-be-out-to-the-people-in-their-lives/.
- Rodríguez-Fernández B, Gispert JD, Guigo R, Navarro A, Vilor-Tejedor N, Crous-Bou M (2022) Genetically predicted telomere length and its relationship with neurodegenerative diseases and life expectancy. Comput Struct Biotechnol J 20:4251-4256. [CrossRef]
- Sanders AR, Beecham GW, Guo S, Dawood K, Rieger G, Badner JA, Gershon ES, Krishnappa RS, Kolundzija AB, Duan J; MGS Collaboration; Gejman PV, Bailey JM, Martin ER (2017) Genome-Wide Association Study of Male Sexual Orientation. Sci Rep 7(1):16950. [CrossRef]
- Scarabino D, Peconi M, Pelliccia F, Corbo RM (2019) Analysis of the Association Between TERC and TERT Genetic Variation and Leukocyte Telomere Length and Human Lifespan-A Follow-Up Study. Genes 10:82. [CrossRef]
- Schumm WR (2010) Children of homosexuals more apt to be homosexuals? A reply to Morrison and to Cameron based on an examination of multiple sources of data. J Biosoc Sci 42(6):721-42. [CrossRef]
- Schweizer VJ (2019) 30 Years of Change in Men’s Entry into Fatherhood, 1987-2017. Family Profiles, FP-19-28. Bowling Green, OH: National Center for Family & Marriage Research. [CrossRef]
- Statistics Canada (2021) Family and household characteristics of lesbian, gay and bisexual people in Canada. Available online: https://www150.statcan.gc.ca/n1/pub/89-28-0001/2018001/article/00021-eng.htm.
- Stindl R (2016) The paradox of longer sperm telomeres in older men's testes: a birth-cohort effect caused by transgenerational telomere erosion in the female germline. Mol Cytogenet 9:12. [CrossRef]
- The Time of India (2015) Shame forcing gay men to get married to straight women. Available online: https://timesofindia.indiatimes.com/city/nagpur/shame-forcing-gay-men-to-get-married-to-straight-women/articleshow/48795234.cms.
- Vaiserman A, Krasnienkov D (2021) Telomere Length as a Marker of Biological Age: State-of-the-Art, Open Issues, and Future Perspectives. Front Genet 11:630186. [CrossRef]
- Victorelli S, Passos JF (2017) Telomeres and cell senescence - size matters not. EBioMedicine 21:14–20. [CrossRef]
- WHO (2023) GHE: Life expectancy and healthy life expectancy. Available online: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-life-expectancy-and-healthy-life-expectancy.
- Wood BM, Watts DP, Mitani JC, Langergraber KE (2017) Favorable ecological circumstances promote life expectancy in chimpanzees similar to that of human hunter-gatherers. J Hum Evol 105:41-56. [CrossRef]
- Worlddata (2022) Life expectancy. Available online: https://www.worlddata.info/life-expectancy.php.
- Zheng L, Hart TA, Noor SW, Wen G (2020) Stressors Based on Sexual Orientation and Mental Health Among Lesbian, Gay, and Bisexual Individuals in China: Minority Stress and Perceived Pressure to Get Married. Arch Sex Behav 49(5):1769-1782. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).