1. Introduction
Cervical cancer is one of the most common cancers affecting women worldwide with an estimated 604,127 new cases and 341,831 deaths in 2020 alone [
1]. It is primarily caused by infection with high-risk Human Papillomaviruses (HPVs) which can lead to persistent lesions and the progression of cervical cancer [
2,
3]. Insufficient immune clearance of HPV infected cells prevents the effective elimination of the virus, contribution to the development and progression of cervical cancer [
3]. One of the key factors responsible for this immune clearance failure is the E5 protein of HPV. We have previously shown that E5 is expressed during the early stages of infection and down-regulates the transport of MHC class 1 complex to the cell surface [
4,
5]. The downregulation of cell surface MHC class I may allow the virus to establish infection by avoiding immune clearance of virus-infected cells by cytotoxic T cells (CTLs) [
6]. Therapeutic at-tempts have been made to treat malignant disease caused by HPV through vaccines/immunotherapy. However, it has met with limited success due to local and systemic immunosuppressive factors in HPV positive tumors [
7,
8,
9]. Therefore, there is a growing demand for novel and safer therapeutic approaches against HPV- related cervical cancer [
10,
11].
In recent years, natural products derived from plants have gained considerable attention for their potential therapeutic properties against various types of cancers, including cervical cancer [
12,
13]. Many natural compounds have been identified as potential candidates for cancer therapy due to their ability to induce apoptosis, inhibit cell proliferation, and modulate cellular signaling pathways [
14]. Among these, fig latex derived from the
Ficus Carica has exhibited promising anticancer properties. Recent studies have highlighted the cytotoxic effects of fig latex against various cancer cell lines, including cervical, stomach and colorectal cancer [
15,
16,
17]. Notably, our previous publication demonstrated the effectiveness of fig latex in suppressing cervical cancer cell growth and inducing apoptosis, thereby substantiating its potential as a valuable candidate for cancer therapy [
17].
Based on the above highlights, we investigated the effects of fig latex on the immune response related genes in HPV related cervical cancer by using RNA Sequencing (RNA-seq). Here we show that fig latex effectively inhibited the growth of HPV positive cervical cancer cell lines, namely CaSki and HeLa while having no cytotoxic effects on normal/non-cancerous cervical cells (HCKT1). Analysis of RNA-seq data revelated that fig latex regulates the expression of genes associated with immune surveillance, specifically those involved in “Class I MHC-mediated antigen presentation" as well as "Antigen processing: Ubiquitination and Proteasome degradation". These findings shed light on new therapeutic avenues against HPV-associated cervical cancers. The outcomes of this study hold promise for expedited resolution of HPV infection, particularly benefiting individuals with early-stage HPV-related cancer.
2. Results
2.1. Fig latex inhibits the growth of HPV positive human cervical cancer cell lines
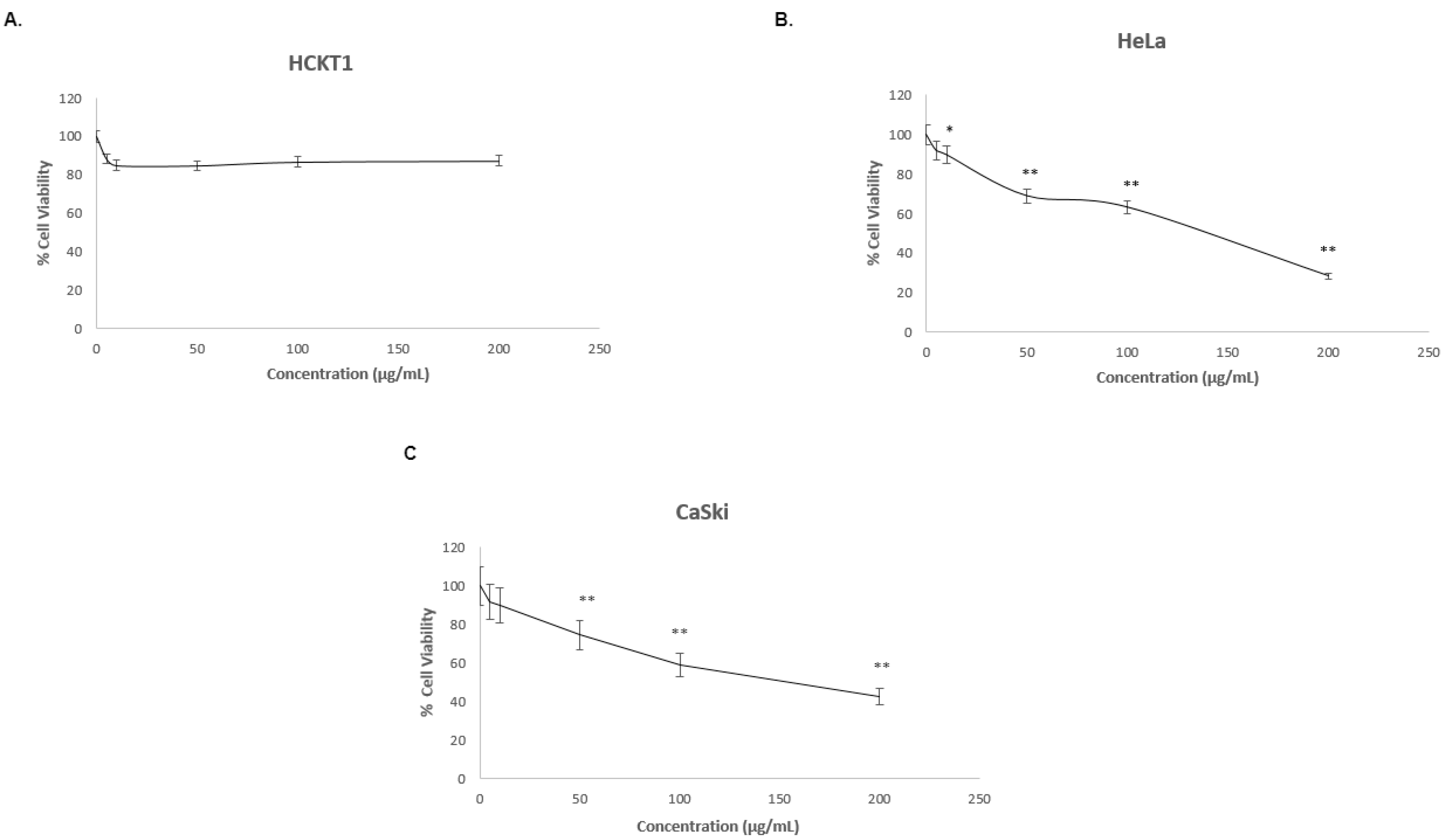
To investigate whether fig latex affects the growth of three distinct cervical cell lines, one normal human cervical keratinocytes (HCKT1) and two HPV-positive cervical cancer cell lines (HeLa HPV18+, CaSki HPV16+) were treated with various concentrations of fig latex (5 μg/ml, 10 μg/ml, 50 μg/ml, 100 μg/ml, 200 μg/ml) for 72 h. The viability of the cells was analyzed by Sulforhodamine B (SRB) colorimetric assay. Results revealed that The IC50 values of fig latex at 72h on HeLa and CaSki were found to be 106 μg/ml, 110 μg/ml and (
Figure 1B-C). Moreover, fig latex did not induce cytotoxicity in normal human cervical keratinocytes compared to cervical cancer cells (
Figure 1A). These findings suggest that certain concentrations of fig latex exhibit selective cytotoxicity towards HPV-positive cervical cancer cells while sparing normal cells.
2.2. Transcriptomic profiling of different HPV positive cervical cancer cells upon fig latex treatment
To study the gene expression profile of HPV positive human cervical cancer cells under fig latex treatment, cervical cancer cell lines, HPV 18 type positive HeLa and HPV 16 type positive CaSki were treated with 100 ug/ml of fig latex that was closest to the IC50 value of fig latex. The gene expression analysis was performed by using RNA-seq. Differential gene expression analysis showed 149 significantly differentially expressed genes, which were expressed in HPV-positive cancer cell lines. 64 differentially expressed genes were consistently downregulated while 85 of them were consistently upregulated in HPV-positive cervical cancer cell lines upon fig latex treatment (
Supplementary Figure S2).
2.3. Analysis of differential expressed genes in HPV positive cervical cancer cell lines upon fig latex treatment using pathway enrichment analysis
Differentially expressed genes in HPV-positive cervical cancer cell lines following fig latex treatment were analysed by pathway enrichment analysis. The results demonstrated that the genes regulated by fig latex treatment were prominently involved in immune surveillance pathways, including “Class I MHC-mediated antigen presentation” and “processing through ubiquitination and proteasome degradation” (
Table 1). Importantly, we observed a set of common genes that overlapped across these pathways, suggesting their pivotal role in viral immune response. These common genes included RPS27A, RNF111, CUL5, FBXO4, KLHL22, FBXL4, TRIP12. The findings from this pathway enrichment analysis suggest that fig latex may modulate key signaling pathways involved in viral immune response, highlighting its therapeutic potential in the management of HPV-associated cervical cancers.
3. Discussion
Cervical cancer, primarily caused by high-risk human papilloma virus HPV infection represents a significant global burden [
1,
18]. A major challenge in the treatment of cervical cancer lies in the tumor cells’ ability to evade immune surveillance, mainly mediated by HPV oncoprotein E5[
4,
5,
6,
7]. This immune evasion not only allows the tumors to evade and destruction by immune system but also contributes to the progression of the disease leading to poor clinical outcomes [
3,
7]. Consequently, the development of novel therapeutic interventions is crucial [
18]. Natural products have gained considerable attention in cancer research due to their potential as sources of novel therapeutic agents [
14]. Our previous study demonstrated the potential therapeutic properties of
Ficus Carica latex, a natural product derived from fig, when applied to HPV-positive cervical cancer cell lines [
17]. However, the specific molecular mechanisms underlying its action in HPV-positive cervical cancer cells remain largely unknown. In this study, we aimed to investigate the potential of fig latex as a therapeutic intervention to counteract immune evasion and enhance the immune response against HPV-positive cancer cells.
To achieve this, we subjected HPV-positive cervical cancer cells to fig latex treatment and examined gene expression profile and signaling pathways involved in immune surveillance. Through comprehensive RNA-seq analysis, we thoroughly examined the transcriptome of the treated cells and compared it to the control group. This approach allowed us to identify differentially expressed genes and gain insights into the molecular changes induced by fig latex treatment.
Our study demonstrated the growth inhibitory effects of fig latex on cervical cancer cells, which aligns with our previous findings [
17]. Treatment with fig latex significantly inhibited cell growth in HeLa and CaSki cells, with calculated IC50 values of 106 and 110 μg/ml, respectively. Importantly, normal human cervical keratinocytes (HCKT1) were unaffected by fig latex treatment, indicating its selective cytotoxicity towards cervical cancer cells. These findings suggest that fig latex possesses potent anti-cancer properties specifically targeting cervical cancer cells while sparing normal/non-cancerous cells.
In addition to its growth inhibitory effect, this study reports, for the first time, that fig latex exerts anticancer effects by modulating key signaling pathways involved in immune surveillance, including the Class I MHC-mediated anti-gen processing and presentation pathway (p-value: 7.82E-08) and the Antigen processing: Ubiquitination & Proteasome degradation pathway (p-value: 1.12E-06). Class I MHC-mediated antigen processing pathway is crucial for immune recognition as by presenting antigens to T cells. Notably, several key genes involved in antigen presentation, including RPS27A, RNF111, CUL5, FBXO4, FBXL4, CALR were upregulated upon fig latex treatment [
19,
20,
21,
22,
23,
24]. It is well documented that HPV E5 protein hinders major histocompatibility complex (MHC) class 1 antigen presentation by impeding the transport of MHC class 1 molecules to cell surface, thereby compromising the recognition of HPV infected cells by cytotoxic T lymphocytes (CTLs) and dampening the antiviral immune response [
4,
5,
6]. The restoration of antigen presentation by fig latex treatment in HPV positive cervical cancer cells signifies its ability to overcome the inhibitory effects of HPV E5 and reinstate immune recognition of infected cells. By modulating the Class I MHC mediated antigen processing and presentation pathway, fig latex treatment potentially enhances immune recognition and promotes the clearance of HPV-infected cells. This is consistent with the other studies indicating that restoration of antigen presentation renders virus infected cells more susceptible to host immune response [
25,
26]. Moreover, Antigen processing: Ubiquitination & Proteasome degradation pathway (p-value: 1.12E-06) was also enriched significantly. This pathway plays a critical role in antigen processing and presentation, influencing immune recognition and response. Several key genes involved in ubiquitination and proteasome degradation were found to be upregulated, including RPS27A, RNF111, CUL5, FBXO4, FBXL4. These genes contribute to the regulation of protein degradation, ensuring the proper turnover of antigens for presentation by MHC molecules [
27,
28]. In the context of HPV and cancer, HPV infection can dysregulate antigen processing and presentation, allowing infected cells to evade immune recognition [
29,
30]. The identification of upregulated genes within the Antigen processing: Ubiquitination & Proteasome degradation pathway suggests that fig latex treatment may counteract the HPV-induced disruption of antigen processing and presentation.
In conclusion, our findings provide valuable insights into the molecular mechanisms underlying fig latex's action and its immunomodulatory potential in combating HPV-positive cervical cancer. By targeting immune evasion mechanisms and enhancing immune recognition, fig latex or its active components hold promise for the development of novel therapeutic strategies against cervical cancer.
4. Materials and Methods
4.1. Chemicals and Reagents
Cell Culture medium, Dulbecco’s modified Eagle medium (DMEM), Roswell Park Memorial institute (RPMI)1640 medium, Keratinocyte serum-free medium (SFM) with supplements including EGF (Epidermal Growth Factor) and Bovine Pituitary Extract (BPE, penicillin-streptomycin, trypsin, Dulbecco’s Phosphate Buffered Saline (DPBS) and Sodium pyruvate were purchased from Gibco (ThermoFisher, UK). Y-27632, Rho kinase inhibitor and Sulforhodamine B (SRB) assay kit were purchased from Abcam, UK. Dimethyl sulfoxide (DMSO) and fetal bovine serum (FBS) were purchased from Sigma, UK. GenElute RNA/DNA/Protein Purification Plus kit was purchased from Sigma-Aldrich, UK.
4.2. Collection and Purification of Ficus Carica Latex
Ficus Carica latex was collected drop by drop without squeezing over summer months from unripe fruits of fig trees in the suburb of Antalya, Turkey. We performed the purification of fig latex, as described in our previous study [
17]. Briefly, the latex was initially filtered using a Whatman No. 1 filter from Fisher Scientific, UK. After filtration, it was then centrifuged at 13000 rpm and a temperature of 4°C to separate the polymeric gum from the liquid filtrate. The aqueous part was further purified by filtration using a disposable filter membrane with a pore size of 5 µm from Sigma, UK. It was stored at -20
oC for further analysis.
4.3. Cell Lines and Cell Culture Conditions
Human cervical cancer cell lines, namely HPV type 16 positive CaSki and HPV type 18 positive HeLa were obtained from American Type Culture Collection (Manassas, VA, USA). HPV-free human cervical keratinocytes, HCKT1 was kindly gifted by Prof. Tohru Kiyono, Japan National Cancer Center. HeLa and CaSki cells were maintained in DMEM supplemented with 10% heat-inactivated FBS and 100 μg/mL of penicillin-streptomycin. HCKT1 cells, on the other hand, were cultured in serum free medium supplemented with 20 μg/mL BPE, 0.2 ng/mL and 10 uM Y-27632. All cell lines were grown in a humidified atmosphere with 5% CO2 at 37oC.
4.4. SRB Cell Viability Assay
In order to investigate the effect of fig latex on cell growth, Sulforodamine B (SRB) assay was performed. For cell viability analysis, the aqueous part of the plant extract was subjected to freeze-drying to obtain a powder form. The freeze-dried powder was then dissolved in DMSO to prepare a 1 mg/ml stock solution. Several concentrations were prepared by diluting the stock solution with cell culture medium. Human cervical cancer cells (HeLa and CaSki) and normal HCKT1 cells were cultured at concentration of 5 x 104 in 0.1 mL of medium, in a 96 well plate. The following day, cells were treated with various concentrations (5,10,50,100,200) of fig latex. After 72h of treatment, cells were fixed by fixation solution for 1h. After 3 washes with distilled water, cells were stained with SRB solution for 15 mins and rinsed with washing solution 3 times. Protein-bound dye was solubilized, and the op-tical density was determined at 545 based on manufactures recommendations. For all the experiments, the percentage of cytotoxicity was calculated as: [(O.D. vehicle) × (O.D. sample)/O.D. vehicle] × 100. Background correction was carried out by subtracting the O.D. of culture media. The percent of proliferation in each treated cell line was normalised based on their control wells. All experiments were performed at least in triplicate. All treatments were adjusted to equal concentrations of DMSO between 0.1~0.2%.
4.5. RNA Preparation
Total RNA extraction from fig latex treated and untreated cell lines was performed using Gen Elute kit according to the manufacturer’s instructions. The quality of total RNA was assessed using The Agilent 2100 bioanalyzer (Agilent, Palo Alto, CA, USA) with RNA 6000 Nano LabChip kit. All RNA samples selected for sequencing had a RIN value of greater than 7.5.
4.6. RNA Sequencing (RNA Seq)
RNA samples were sent to CeGaT GmbH, Germany for library preparation, sequencing and bioinformatic analysis. Libraries were prepared using the SMART-Seq Stranded Kit (Takara). Multiplexed libraries were sequenced on the Illumina NovaSeq 6000 platform, at 100bp paired end reads. The sequencing depth for each sample was >20 million reads. All samples were passed quality control based on manufacturer’s standards.
4.7. Bioinformatic Analysis
The sequence reads were analyzed further by using diverse bioinformatic tools. Demultiplexing of the sequencing reads was performed with Illumina bcl2fastq (vs 2.20). Adapters were trimmed with Skewer (vs 0.2.2) [
31]. Trimmed raw reads were aligned to hg19-cegat using STAR (version 2.7.3) [
32]. Pseudo autosomal regions (PAR) were masked on chromosome Y (chrY:10001-2649520, chrY:59034050-59363566). Reads originating from these regions can be found at the respective location on chromosome X. Normalized counts have been calculated with DESeq2 (version 1.24.0) in R (version 3.6.1) [
33]. DESeq2 uses a negative binomial generalized linear model to test for differential expression based on gene counts.
For functional enrichment analysis, the RNA sequencing (RNA-Seq) data obtained from drug-treated and untreated cells was used. Gene Set Enrichment Analysis (GSEA) was performed using the GSEA software [
34,
35]. The RNA-Seq data sets were preprocessed and normalized, and the resulting gene expression profiles were analyzed against a comprehensive collection of gene sets derived from public databases, such as MSigDB [
34]. The GSEA algorithm computed an enrichment score for each gene set, indicating the extent to which the gene set was overrepresented among the differentially expressed genes.
Moreover, EnrichR, an online platform for comprehensive gene set enrichment analysis, was utilized [
36,
37,
38]. The preprocessed RNA-Seq data sets were uploaded to EnrichR, and the analysis was conducted by following the pro-vided instructions. EnrichR integrates multiple pathway and gene set databases, such as KEGG and Reactome, to identify enriched pathways associated with the differentially expressed genes. The analysis generated enriched pathway results with corresponding statistical significance. The results obtained from both GSEA and EnrichR were used to gain insights into the biological processes and pathways affected by the drug treatment in the cells [
34,
35,
36,
37,
38].
4.8. Statistical Analysis
The data were collected from at least three independent experiments and presented as the mean ± standard deviation for each group. Statistical analyses, including one-way analysis of variance (ANOVA) followed by post hoc Tukey's test, were conducted using R Studio software with the 'stats' package for ANOVA and the 'agricolae' package for post hoc testing. A significance level of P < 0.05 was considered to indicate a statistically significant difference.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org.
Author Contributions
Conceptualization, G.H.A.; Methodology, M.O.C. and U.B., G.H.A.; software, U.B.; validation, G.H.A. and M.O.C.; formal analysis, G.H.A. and M.O.C.; data curation, M.O.C. and U.B.; writing – original draft preparation, M.O.C.; writing – review and editing, G.H.A., D.P.N. and M.O.C.; visualization, M.O.C.; supervision, G.H.A.; project administration, G.H.A.; funding acquisition, G.H.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by Kingston University London Innovation Seedcorn Grant.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data is not publicly available due to confidentiality.
Acknowledgments
Authors would like to acknowledge Kingston University London for providing laboratory facilities.
Conflicts of Interest
Declare conflicts of interest or state “The authors declare no conflict of interests.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021, 71, 209–249. [CrossRef]
- zur Hausen, H. Papillomaviruses and Cancer: From Basic Studies to Clinical Application. Nat Rev Cancer 2002, 2, 342–350. [CrossRef]
- Senba, M.; Mori, N. Mechanisms of Virus Immune Evasion Lead to Development from Chronic Inflammation to Cancer Formation Associated with Human Papillomavirus Infection. Oncol Rev 2012, 6, 17. [CrossRef]
- Ashrafi, G.H.; Haghshenas, M.R.; Marchetti, B.; O’Brien, P.M.; Campo, M.S. E5 Protein of Human Papillomavirus Type 16 Selectively Downregulates Surface HLA Class I. Int J Cancer 2005, 113, 276–283. [CrossRef]
- Ashrafi, G.H.; Haghshenas, M.; Marchetti, B.; Campo, M.S. E5 Protein of Human Papillomavirus 16 Downregulates HLA Class I and Interacts with the Heavy Chain via Its First Hydrophobic Domain. Int J Cancer 2006, 119, 2105–2112. [CrossRef]
- Campo, M.S.; Graham, S.V.; Cortese, M.S.; Ashrafi, G.H.; Araibi, E.H.; Dornan, E.S.; Miners, K.; Nunes, C.; Man, S. HPV-16 E5 down-Regulates Expression of Surface HLA Class I and Reduces Recognition by CD8 T Cells. Virology 2010, 407, 137–142. [CrossRef]
- Smola, S.; Trimble, C.; Stern, P.L. Human Papillomavirus-Driven Immune Deviation: Challenge and Novel Opportunity for Immunotherapy. Ther Adv Vaccines 2017, 5, 69–82. [CrossRef]
- Trimble, C.L.; Morrow, M.P.; Kraynyak, K.A.; Shen, X.; Dallas, M.; Yan, J.; Edwards, L.; Parker, R.L.; Denny, L.; Giffear, M.; et al. Safety, Efficacy, and Immunogenicity of VGX-3100, a Therapeutic Synthetic DNA Vaccine Targeting Human Papillomavirus 16 and 18 E6 and E7 Proteins for Cervical Intraepithelial Neoplasia 2/3: A Randomised, Double-Blind, Placebo-Controlled Phase 2b Trial. The Lancet 2015, 386, 2078–2088. [CrossRef]
- Maldonado, L.; Teague, J.E.; Morrow, M.P.; Jotova, I.; Wu, T.C.; Wang, C.; Desmarais, C.; Boyer, J.D.; Tycko, B.; Robins, H.S.; et al. Intramuscular Therapeutic Vaccination Targeting HPV16 Induces T Cell Responses That Localize in Mucosal Lesions. Sci Transl Med 2014, 6. [CrossRef]
- Davies-Oliveira, J.C.; Smith, M.A.; Grover, S.; Canfell, K.; Crosbie, E.J. Eliminating Cervical Cancer: Progress and Challenges for High-Income Countries. Clin Oncol 2021, 33, 550–559. [CrossRef]
- George, I.A.; Chauhan, R.; Dhawale, R.E.; Iyer, R.; Limaye, S.; Sankaranarayanan, R.; Venkataramanan, R.; Kumar, P. Insights into Therapy Resistance in Cervical Cancer. Advances in Cancer Biology - Metastasis 2022, 6, 100074. [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Last 25 Years. J Nat Prod 2007, 70, 461–477. [CrossRef]
- Cragg, G.M.; Grothaus, P.G.; Newman, D.J. New Horizons for Old Drugs and Drug Leads. J Nat Prod 2014, 77, 703–723. [CrossRef]
- Kubczak, M.; Szustka, A.; Rogalińska, M. Molecular Targets of Natural Compounds with Anti-Cancer Properties. Int J Mol Sci 2021, 22, 13659. [CrossRef]
- Soltana, H.; Pinon, A.; Limami, Y.; Zaid, Y.; Khalki, L.; Zaid, N.; Salah, D.; Sabitaliyevich, U.Y.; Simon, A.; Liagre, B.; et al. Antitumoral Activity of Ficus Carica L. on Colorectal Cancer Cell Lines. Cell Mol Biol 2019, 65, 6–11. [CrossRef]
- Hashemi SA, A.S.G.M.A.M.Y.Y.D.AA. The Effect of Fig Tree Latex (Ficus Carica) on Stomach Cancer Line. Iran Red Crescent Medical Journal 2011, 13, 272–275.
- Ghanbari, A.; Le Gresley, A.; Naughton, D.; Kuhnert, N.; Sirbu, D.; Ashrafi, G.H. Biological Activities of Ficus Carica Latex for Potential Therapeutics in Human Papillomavirus (HPV) Related Cervical Cancers. Sci Rep 2019, 9, 1013. [CrossRef]
- Burmeister, C.A.; Khan, S.F.; Schäfer, G.; Mbatani, N.; Adams, T.; Moodley, J.; Prince, S. Cervical Cancer Therapies: Current Challenges and Future Perspectives. Tumour Virus Res 2022, 13, 200238. [CrossRef]
- Antoniou, A.N.; Powis, S.J.; Elliott, T. Assembly and Export of MHC Class I Peptide Ligands. Curr Opin Immunol 2003, 15, 75–81. [CrossRef]
- Elliott, T.; Neefjes, J. The Complex Route to MHC Class I-Peptide Complexes. Cell 2006, 127, 249–251. [CrossRef]
- Purcell, A.W.; Elliott, T. Molecular Machinations of the MHC-I Peptide Loading Complex. Curr Opin Immunol 2008, 20, 75–81. [CrossRef]
- Wei, J.; Kishton, R.J.; Angel, M.; Conn, C.S.; Dalla-Venezia, N.; Marcel, V.; Vincent, A.; Catez, F.; Ferré, S.; Ayadi, L.; et al. Ribosomal Proteins Regulate MHC Class I Peptide Generation for Immunosurveillance. Mol Cell 2019, 73, 1162-1173.e5. [CrossRef]
- Wearsch, P.A.; Cresswell, P. The Quality Control of MHC Class I Peptide Loading. Curr Opin Cell Biol 2008, 20, 624–631. [CrossRef]
- York IA, R.K. Antigen Processing and Presentation by the Class I Major Histocompatibility Complex. . Annu Rev Immunol 1996, 14, 369–396. [CrossRef]
- Davis, D.A.; Shrestha, P.; Aisabor, A.I.; Stream, A.; Galli, V.; Pise-Masison, C.A.; Tagawa, T.; Ziegelbauer, J.M.; Franchini, G.; Yarchoan, R. Pomalidomide Increases Immune Surface Marker Expression and Immune Recognition of Oncovirus-Infected Cells. Oncoimmunology 2019, 8, e1546544. [CrossRef]
- Davis, D.A.; Mishra, S.; Anagho, H.A.; Aisabor, A.I.; Shrestha, P.; Wang, V.; Takamatsu, Y.; Maeda, K.; Mitsuya, H.; Zeldis, J.B.; et al. Restoration of Immune Surface Molecules in Kaposi Sarcoma-Associated Herpes Virus Infected Cells by Lenalidomide and Pomalidomide. Oncotarget 2017, 8, 50342–50358. [CrossRef]
- van Endert, P. Post-Proteasomal and Proteasome-Independent Generation of MHC Class I Ligands. Cellular and Molecular Life Sciences 2011, 68, 1553–1567. [CrossRef]
- Hearn, A.; York, I.A.; Bishop, C.; Rock, K.L. Characterizing the Specificity and Cooperation of Aminopeptidases in the Cytosol and Endoplasmic Reticulum during MHC Class I Antigen Presentation. The Journal of Immunology 2010, 184, 4725–4732. [CrossRef]
- Đukić, A.; Lulić, L.; Thomas, M.; Skelin, J.; Bennett Saidu, N.E.; Grce, M.; Banks, L.; Tomaić, V. HPV Oncoproteins and the Ubiquitin Proteasome System: A Signature of Malignancy? Pathogens 2020, 9, 133. [CrossRef]
- Wang, Y.; Xie, Y.; Sun, B.; Guo, Y.; Song, L.; Mohammednur, D.E.; Zhao, C. The Degradation of Rap1GAP via E6AP-Mediated Ubiquitin-Proteasome Pathway Is Associated with HPV16/18-Infection in Cervical Cancer Cells. Infect Agent Cancer 2021, 16, 71. [CrossRef]
- Jiang, H.; Lei, R.; Ding, S.-W.; Zhu, S. Skewer: A Fast and Accurate Adapter Trimmer for next-Generation Sequencing Paired-End Reads. BMC Bioinformatics 2014, 15, 182. [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast Universal RNA-Seq Aligner. Bioinformatics 2013, 29, 15–21. [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol 2014, 15, 550. [CrossRef]
- Liberzon, A.; Birger, C.; Thorvaldsdóttir, H.; Ghandi, M.; Mesirov, J.P.; Tamayo, P. The Molecular Signatures Database Hallmark Gene Set Collection. Cell Syst 2015, 1, 417–425. [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene Set Enrichment Analysis: A Knowledge-Based Approach for Interpreting Genome-Wide Expression Profiles. Proceedings of the National Academy of Sciences 2005, 102, 15545–15550. [CrossRef]
- Kuleshov, M. V.; Jones, M.R.; Rouillard, A.D.; Fernandez, N.F.; Duan, Q.; Wang, Z.; Koplev, S.; Jenkins, S.L.; Jagodnik, K.M.; Lachmann, A.; et al. Enrichr: A Comprehensive Gene Set Enrichment Analysis Web Server 2016 Update. Nucleic Acids Res 2016, 44, W90–W97. [CrossRef]
- Chen, E.Y.; Tan, C.M.; Kou, Y.; Duan, Q.; Wang, Z.; Meirelles, G.V.; Clark, N.R.; Ma’ayan, A. Enrichr: Interactive and Collaborative HTML5 Gene List Enrichment Analysis Tool. BMC Bioinformatics 2013, 14, 128. [CrossRef]
- Xie, Z.; Bailey, A.; Kuleshov, M. V.; Clarke, D.J.B.; Evangelista, J.E.; Jenkins, S.L.; Lachmann, A.; Wojciechowicz, M.L.; Kropiwnicki, E.; Jagodnik, K.M.; et al. Gene Set Knowledge Discovery with Enrichr. Curr Protoc 2021, 1. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).