Submitted:
17 July 2023
Posted:
18 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
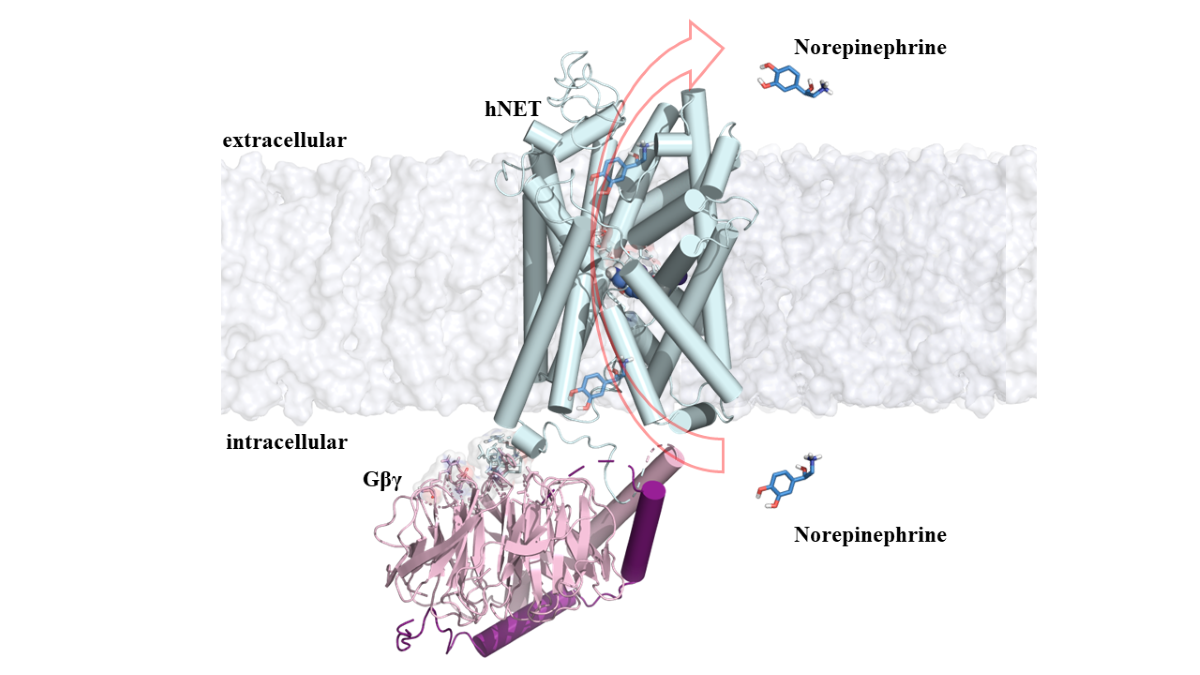
2.1. Homology Modeling and Docking Studies
2.1.1. Complex NET/NE
2.1.2. Protein-Protein Complex
2.1.3. NET/NE/Gβγ System
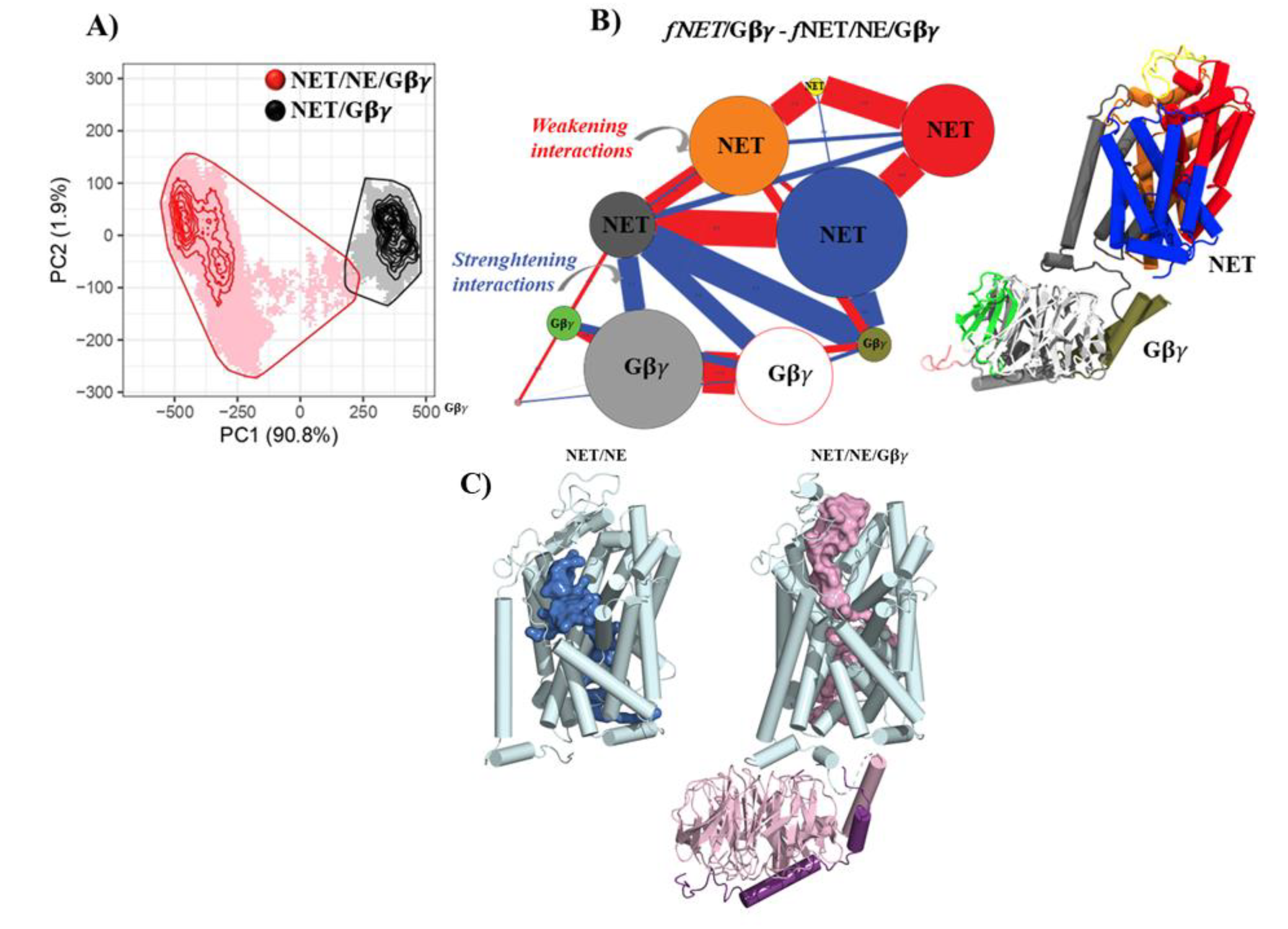
2.2. Molecular Dynamics Simulation
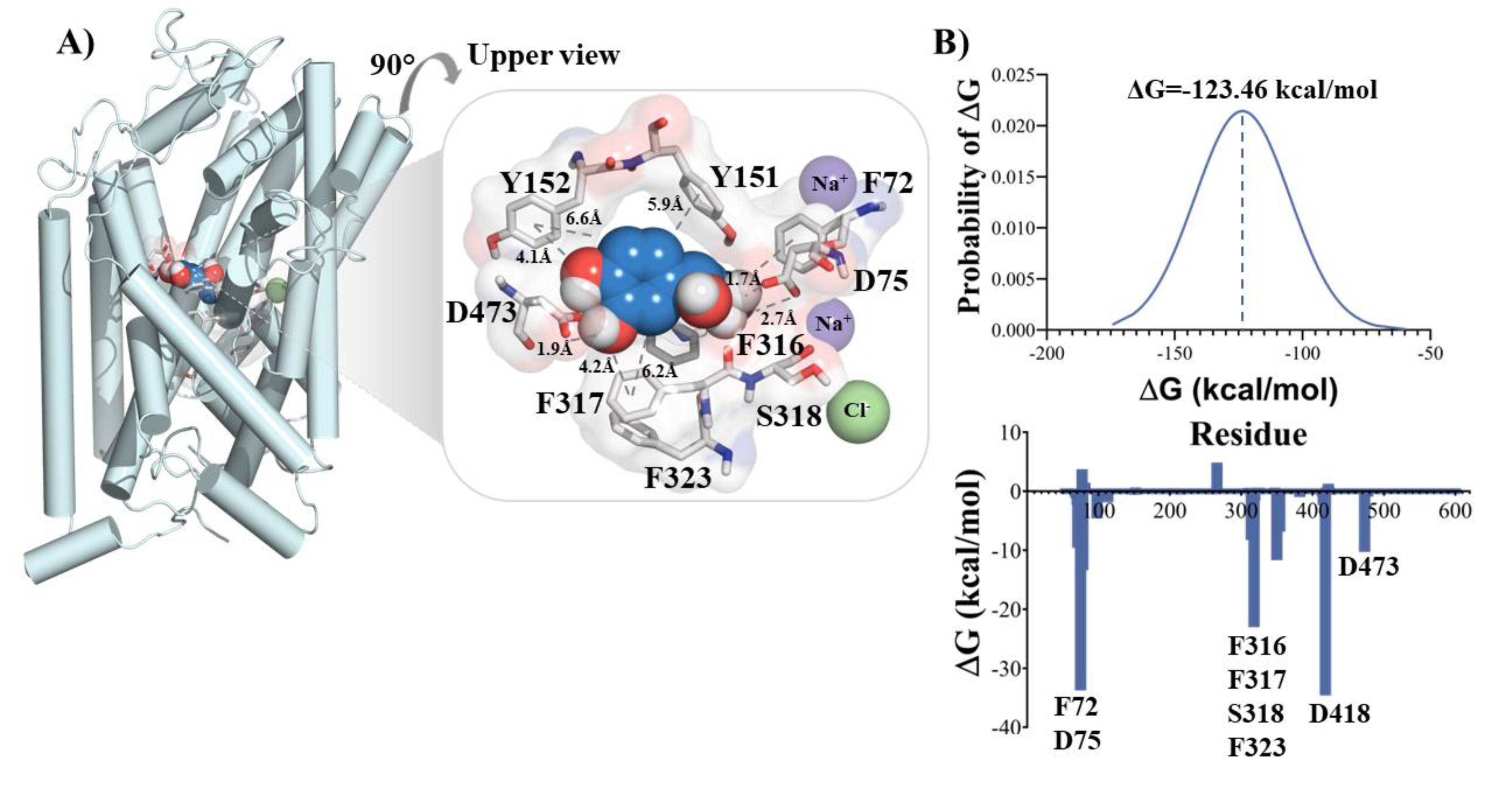
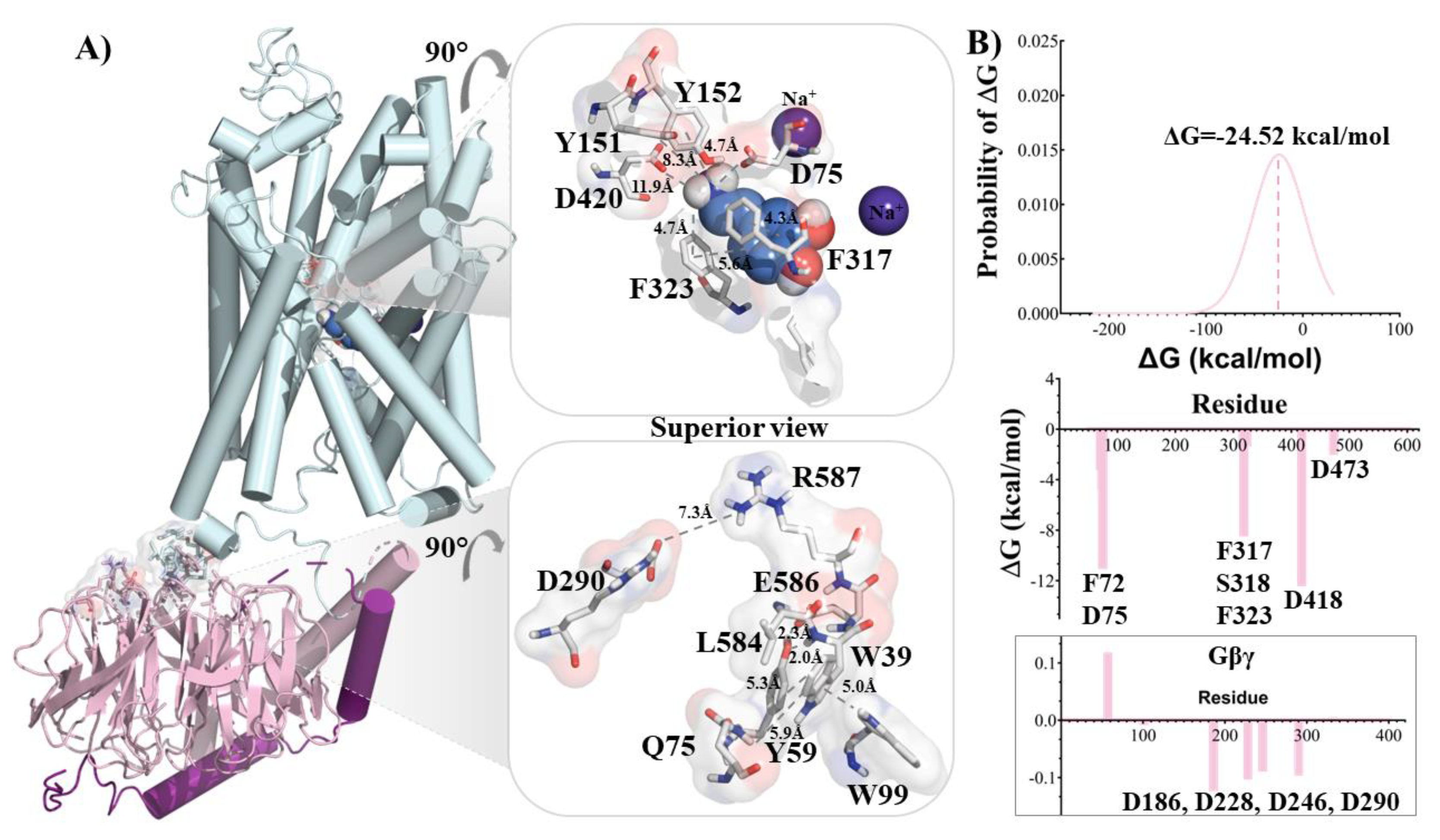
2.3. Energy of Complexes
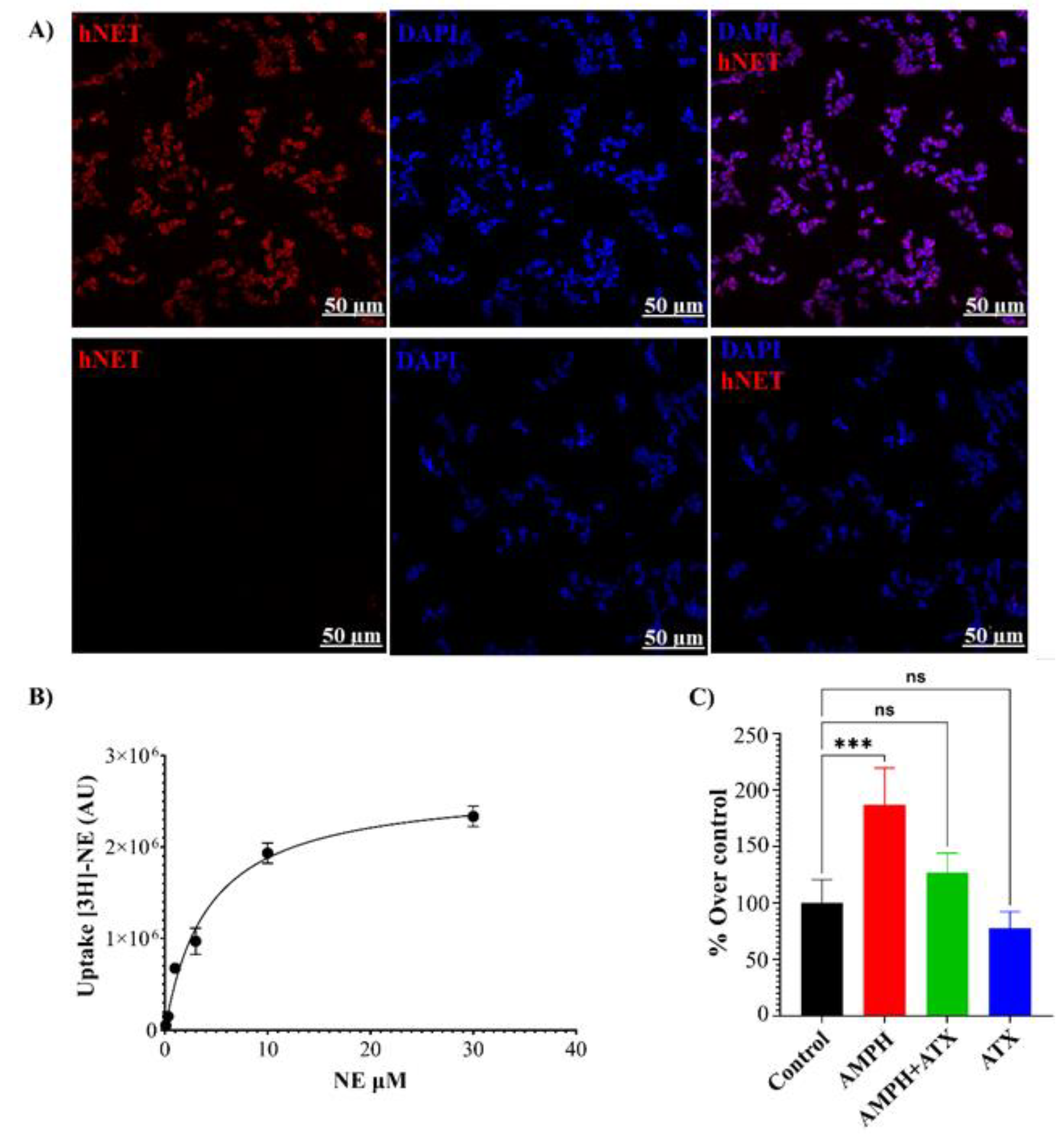
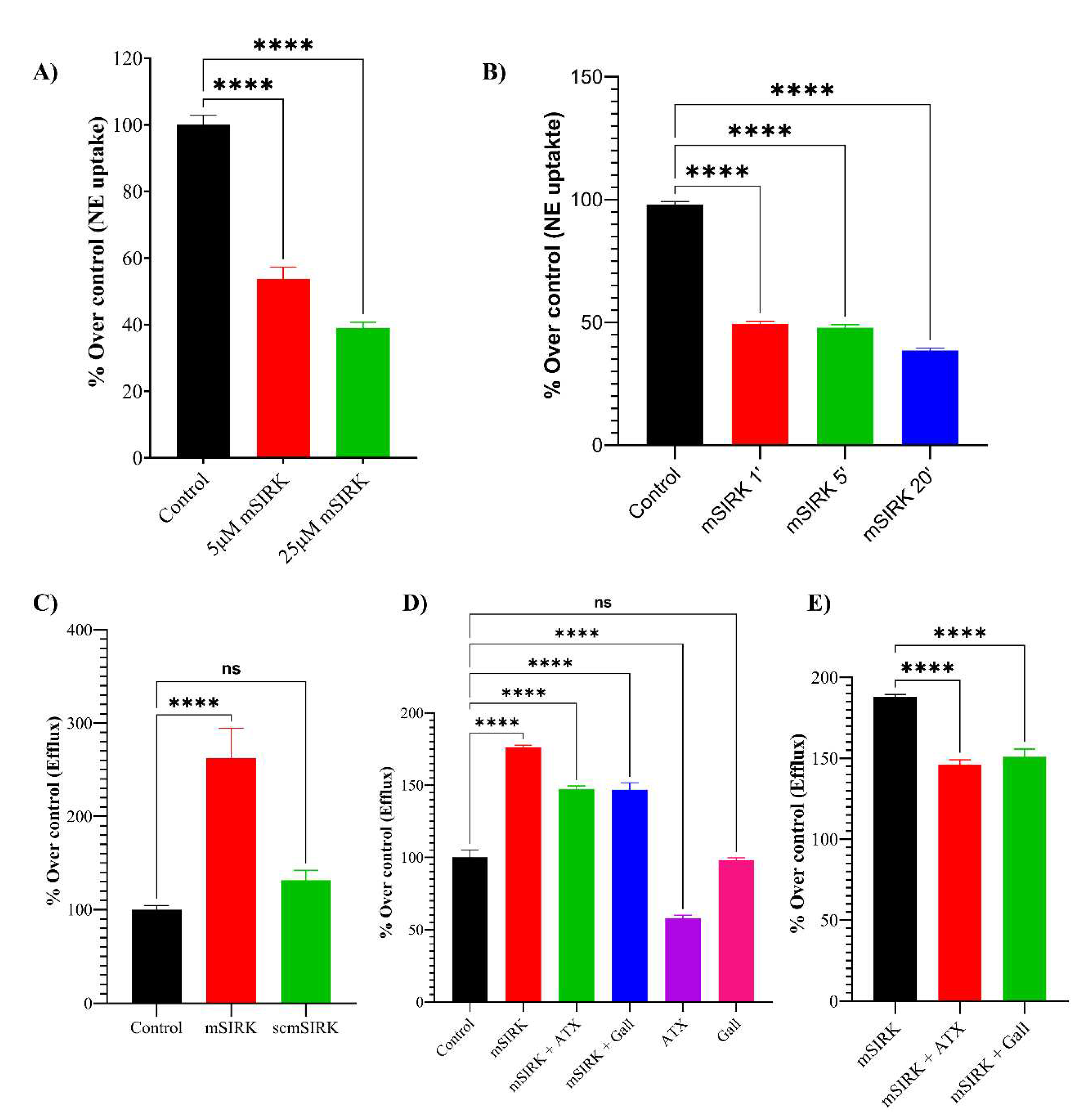
2.4. In Vitro Expression of NET and Functional Analysis
2.5. Immunocytochemistry
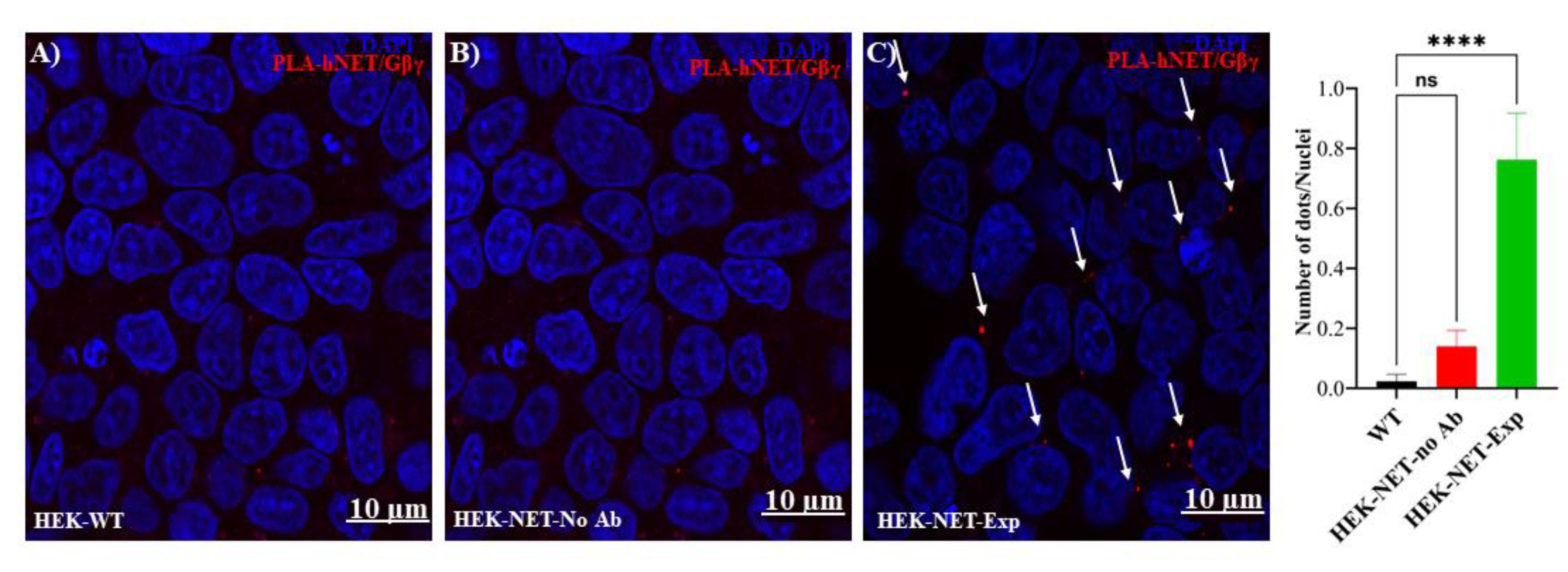
2.6. Proximity Ligation Assay
2.7. Data Analysis
3. Results and Discussion
- NE is stabilized into the NET binding site by a rich electron environment.
- Gβγ binding to NET induces conformational changes that decrease the affinity of NE for the transporter.
- Transfected HEK293-hNET cells show [3H]-NE uptake and efflux in the presence of AMPH or mSIRK
- Interaction between NET and Gβγ revealed by a proximity ligation assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- G. Aston-Jones and B. Waterhouse, “Locus coeruleus: From global projection system to adaptive regulation of behavior,” Brain Res., vol. 1645, no. 12, pp. 75–78, Aug. 2016. [CrossRef]
- Borodovitsyna, M. Flamini, and D. Chandler, “Noradrenergic Modulation of Cognition in Health and Disease,” Neural Plast., vol. 2017, no. Lc, pp. 1–14, 2017. [CrossRef]
- G. E. Torres, R. R. G. E. Torres, R. R. Gainetdinov, and M. G. Caron, “Plasma membrane monoamine transporters: structure, regulation and function,” Nat. Rev. Neurosci., vol. 4, no. 1, pp. 13–25, Jan. 2003. [CrossRef]
- Góral, K. Łątka, and M. Bajda, “Structure Modeling of the Norepinephrine Transporter,” Biomolecules, vol. 10, no. 1, p. 102, Jan. 2020. [CrossRef]
- G. Zheng et al., “Exploring the inhibitory mechanism of approved selective norepinephrine reuptake inhibitors and reboxetine enantiomers by molecular dynamics study,” Sci. Rep., vol. 6, no. December 2015, pp. 1–13, 2016. [CrossRef]
- E. Bogi et al., “Perinatal exposure to venlafaxine leads to lower anxiety and depression-like behavior in the adult rat offspring,” Behav. Pharmacol., vol. 29, no. 5, pp. 445–452, 2018. [CrossRef]
- S. Iyengar, A. A. S. Iyengar, A. A. Webster, S. K. Hemrick-Luecke, J. Y. Xu, and R. M. A. Simmons, “Efficacy of Duloxetine, a Potent and Balanced Serotonin-Norepinephrine Reuptake Inhibitor in Persistent Pain Models in Rats,” J. Pharmacol. Exp. Ther., vol. 311, no. 2, pp. 576–584, Nov. 2004. [CrossRef]
- S. S. Somkuwar, K. M. S. S. Somkuwar, K. M. Kantak, and L. P. Dwoskin, “Effect of methylphenidate treatment during adolescence on norepinephrine transporter function in orbitofrontal cortex in a rat model of attention deficit hyperactivity disorder,” J. Neurosci. Methods, vol. 252, no. 1, pp. 55–63, Aug. 2015. [CrossRef]
- K. M. Kahlig et al., “Amphetamine induces dopamine efflux through a dopamine transporter channel,” Proc. Natl. Acad. Sci., vol. 102, no. 9, pp. 3495–3500, Mar. 2005. [CrossRef]
- G. Rudnick and S. C. Wall, “The molecular mechanism of ‘ecstasy’ [3,4-methylenedioxy-methamphetamine (MDMA)]: serotonin transporters are targets for MDMA-induced serotonin release.,” Proc. Natl. Acad. Sci., vol. 89, no. 5, pp. 1817–1821, Mar. 1992. [CrossRef]
- F. Binda et al., “Syntaxin 1A Interaction with the Dopamine Transporter Promotes Amphetamine-Induced Dopamine Efflux,” Mol. Pharmacol., vol. 74, no. 4, pp. 1101–1108, Oct. 2008. [CrossRef]
- L. A. Johnson, B. L. A. Johnson, B. Guptaroy, D. Lund, S. Shamban, and M. E. Gnegy, “Regulation of Amphetamine-stimulated Dopamine Efflux by Protein Kinase C β,” J. Biol. Chem., vol. 280, no. 12, pp. 10914–10919, Mar. 2005. [CrossRef]
- J. Garcia-Olivares et al., “Gβγ subunit activation promotes dopamine efflux through the dopamine transporter,” Mol. Psychiatry, vol. 22, no. 12, pp. 1673–1679, Dec. 2017. [CrossRef]
- J. A. Pino, G. J. A. Pino, G. Nuñez-Vivanco, G. Hidalgo, M. Reyes Parada, H. Khoshbouei, and G. E. Torres, “Identification of Critical Residues in the Carboxy Terminus of the Dopamine Transporter Involved in the G Protein βγ-Induced Dopamine Efflux,” Front. Pharmacol., vol. 12, no. March, pp. 1–10, Mar. 2021. [CrossRef]
- J. C. Mauna et al., “G protein βγ subunits play a critical role in the actions of amphetamine,” Transl. Psychiatry, vol. 9, no. 1, p. 81, Dec. 2019. [CrossRef]
- N. Eswar et al., “Comparative Protein Structure Modeling Using Modeller,” Curr. Protoc. Bioinforma., vol. 15, no. 1, pp. 27–49, Sep. 2006. [CrossRef]
- M. Wiederstein and M. J. Sippl, “ProSA-web: Interactive web service for the recognition of errors in three-dimensional structures of proteins,” Nucleic Acids Res., vol. 35, no. SUPPL.2, pp. 407–410, 2007. [CrossRef]
- R. A. Laskowski, M. W. R. A. Laskowski, M. W. MacArthur, D. S. Moss, and J. M. Thornton, “PROCHECK: a program to check the stereochemical quality of protein structures,” J. Appl. Crystallogr., vol. 26, no. 2, pp. 283–291, 1993. [CrossRef]
- J. C. Gordon, J. B. J. C. Gordon, J. B. Myers, T. Folta, V. Shoja, L. S. Heath, and A. Onufriev, “H++: A server for estimating pKas and adding missing hydrogens to macromolecules,” Nucleic Acids Res., vol. 33, no. SUPPL. 2, pp. 368–371, 2005. [CrossRef]
- S. Arancibia, M. S. Arancibia, M. Marambio, J. M. Campusano, and A. Fierro, “Modeling of the Binding of Octopamine and Dopamine in Insect Monoamine Transporters Reveals Structural and Electrostatic Differences,” ACS Chem. Neurosci., vol. 10, no. 5, pp. 2310–2317, May 2019. [CrossRef]
- C. Dominguez, R. C. Dominguez, R. Boelens, and A. M. J. J. Bonvin, “HADDOCK: A Protein−Protein Docking Approach Based on Biochemical or Biophysical Information,” J. Am. Chem. Soc., vol. 125, no. 7, pp. 1731–1737, Feb. 2003. [CrossRef]
- G. Rojas, I. G. Rojas, I. Orellana, R. Rosales-Rojas, J. García-Olivares, J. Comer, and A. Vergara-Jaque, “Structural Determinants of the Dopamine Transporter Regulation Mediated by G Proteins,” J. Chem. Inf. Model., vol. 60, no. 7, pp. 3577–3586, Jul. 2020. [CrossRef]
- J. R. Biller, H. J. R. Biller, H. Elajaili, V. Meyer, G. M. Rosen, S. S. Eaton, and G. R. Eaton, “The Amber biomolecular simulation programs.,” J. Magn. Reson., vol. 236, no. 16, pp. 47–56, 2013.
- C. J. Dickson et al., “Lipid14: The Amber Lipid Force Field - Supplementary Data,” J. Chem. Theory Comput., vol. 10, no. 2, pp. 865–879, 2014, [Online]. Available: http://pubs.acs.org/doi/abs/10.1021/ct4010307.
- P. Mark and L. Nilsson, “Structure and dynamics of the TIP3P, SPC, and SPC/E water models at 298 K,” J. Phys. Chem. A, vol. 105, no. 43, pp. 9954–9960, 2001. [CrossRef]
- B. R. Miller, T. D. B. R. Miller, T. D. McGee, J. M. Swails, N. Homeyer, H. Gohlke, and A. E. Roitberg, “MMPBSA.py: An efficient program for end-state free energy calculations,” J. Chem. Theory Comput., vol. 8, no. 9, pp. 3314–3321, 2012. [CrossRef]
- D. Ouedraogo, M. D. Ouedraogo, M. Souffrant, X.-Q. Yao, D. Hamelberg, and G. Gadda, “Non-active Site Residue in Loop L4 Alters Substrate Capture and Product Release in D-Arginine Dehydrogenase,” Biochemistry, vol. 62, no. 5, pp. 1070–1081, Mar. 2023. [CrossRef]
- U. Doshi, M. J. U. Doshi, M. J. Holliday, E. Z. Eisenmesser, and D. Hamelberg, “Dynamical network of residue-residue contacts reveals coupled allosteric effects in recognition, catalysis, and mutation,” Proc. Natl. Acad. Sci. U. S. A., vol. 113, no. 17, pp. 4735–4740, 2016. [CrossRef]
- H. Wickham, Elegant Graphics for Data Analysis: ggplot2. 2008.
- W. Humphrey, A. W. Humphrey, A. Dalke, and K. Schulten, “VMD: Visual molecular dynamics,” J. Mol. Graph., vol. 14, no. 1, pp. 33–38, Feb. 1996. [CrossRef]
- S. Pidathala, A. K. S. Pidathala, A. K. Mallela, D. Joseph, and A. Penmatsa, “Structural basis of norepinephrine recognition and transport inhibition in neurotransmitter transporters,” Nat. Commun., vol. 12, no. 1, pp. 1–12, 2021. [CrossRef]
- A. Schlessinger et al., “Structure-based discovery of prescription drugs that interact with the norepinephrine transporter, NET,” Proc. Natl. Acad. Sci. U. S. A., vol. 108, no. 38, pp. 15810–15815, 2011. [CrossRef]
- S. Manepalli, C. K. S. Manepalli, C. K. Surratt, J. D. Madura, and T. L. Nolan, “Monoamine Transporter Structure, Function, Dynamics, and Drug Discovery: A Computational Perspective,” AAPS J., vol. 14, no. 4, pp. 820–831, Dec. 2012. [CrossRef]
- H. Xhaard, V. H. Xhaard, V. Backström, K. Denessiouk, and M. S. Johnson, “Coordination of Na + by Monoamine Ligands in Dopamine, Norepinephrine, and Serotonin Transporters,” J. Chem. Inf. Model., vol. 48, no. 7, pp. 1423–1437, Jul. 2008. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




