1. Introduction
Drought is a prominent abiotic stress factor that poses a threat to the growth and development of plants, resulting in a significant decrease in crop yield [
1]. Such stressful conditions lead to the accumulation of osmotic and oxidative regulators, which in turn induce the expression of stress-related genes [
2].The efficiency of photosynthesis determines a plant's ability to withstand natural drought stress. Chlorophyll fluorescence parameters are key to the study of photosynthesis [
3]. Under drought stress conditions, the process of light absorption and electron transport in photosynthesis can result in the accumulation of ROS not only in chloroplasts [
4], but also in plants. This accumulation can damage the cell membrane system and cause oxidative stress. Fortunately, plants can not only utilize various mechanisms to reduce ROS production but also acquire ROS scavenging systems to protect themselves [
5]. Plants transfer stress signals through signal transduction pathways in vivo and regulate the enzymatic antioxidant system, which includes SOD, POD (peroxidase,POD), CAT (catalase,CAT), and other antioxidant enzymes. This system helps remove excessive ROS components in cells [
6]. In addition, for ROS accumulated in chloroplasts, non-photochemical quenching, photorespiration, and the CBB (Calvin-Benson-Bassham cycle,CBB) can dissipate the energy of excess electrons in chloroplasts and decrease the production of ROS [
7,
8]. In addition, drought stress triggers the accumulation of the plant hormone ABA through hyperosmotic signaling. This accumulation leads to the development of multiple ABA signaling pathways in plants. These pathways can promote adaptive responses to drought stress. Even in the absence of water stress, ABA inhibits plant stem and root growth [
9].
Thus plants have developed various molecular mechanisms, including signal transduction and gene expression, to adapt to abiotic stresses. Evolved transcription factors (TFs), such as the CBF[
10], ERF[
11], BHLH[
12], bZip[
13], ZFP[
14], MYB[
15], NAC[
16], C2H2[
17], Dof[
18], HSF[
19] and WRKY[
20] families, can regulate gene network expression to combat environmental stresses. The WRKY gene family is a group of plant-specific transcription factors (TFs) that play important roles in various aspects, including plant defense response [
21], plant growth and development [
22] and regulation of leaf senescence [
23]. WRKY TFs are characterized by a conserved domain, which includes a WRKYGQK motif at the N-terminus and a zinc finger motif at the C-terminus [
24]. According to the number of conserved WRKY domains and the structural characteristics of zinc finger motifs, they can be divided into three groups: Group Ⅰ, Group Ⅱ and Group Ⅲ. Group Ⅱ can be further divided into five subgroups: Ⅱa, Ⅱb, Ⅱc, Ⅱd and Ⅱe[
25]. When plants are exposed to external stimuli, WRKY TFs are regulated by a cascade of defense signaling networks. They can recognize and bind to the W-box sequence [TTGAC(C/T)] present in the promoter region of the target gene, thereby participating in the regulatory network and enhancing the defense ability of plants [
26].
WRKY TFs also play key roles in the transcriptional regulation and signal transduction processes in plants. They extensively regulate the expression changes of target genes in various physiological programs and are involved in various stress pathways [
27]. Certain
Arabidopsis WRKY TFs function as positive regulators in the pathway of ABA-mediated stomatal closure [
28]. In addition, the
WRKY20 gene in soybean is sensitive to ABA in terms of regulating stomatal closure. This sensitivity can enhance the plant's tolerance to drought stress [
29]. In addition, WRKY TFs can also positively regulate drought resistance by improving ABA biosynthesis, such as the WRKY TF ZmWRKY79 in maize has been demonstrated to have this capability [
30]. In the signal transduction pathways mediated by JA (jasmonate,JA) and SA (salicylic acid,SA), the WRKY70 TF is activated by SA, while its expression is suppressed by JA [
31]. In terms of regulating plant growth and development, VvWRKY2 is specifically expressed in lignified cells of young grape stems. This expression affects the lignin biosynthesis pathway, which in turn impacts xylem development [
32].
There is still a need to further explore the potential of WRKY TFs with different domains in various species. Recent transcriptome studies have shown that herbaceous plants, such as wheat (
Triticum aestivum L.) [
33], licorice (
Glycyrrhiza glabra L.) [
34], broad bean (
Vicia faba L.) [
35], and millet (
Panicum miliaceum) [
36], which upregulated WRKY TFs in response to drought stress. WRKY TFs in various woody plants, including Xanthoceras sorbifolium [
37], Myrothamnus flabellifolia [
38], and oil palm (
Elaeis guineensis Jacq.) [
39], which also responds to drought stress. However, functional studies of WRKY TFS in the woody plant
A. fruticosa are limited.
A. fruticosa is a perennial leguminous woody plant with strong adaptability. It can survive in adverse conditions, including cold, windy, and saline-alkali environments in Northeast China. It can also be used as a plant for greening, soil improvement, windbreak, and forest stabilization [
40,
41]. In addition, the roots, stems, leaves, and fruits of
A. fruticosa not only possess medicinal properties for reducing dampness and swelling, but also have significant economic and practical value [
42,
43]. More importantly,
A. fruticosa has a high tolerance to drought resistance [
44].
Although studies on the drought resistance of
A. fruticosa WRKY TFs are limited, transcriptome sequencing analysis of
A. fruticosa has shown that the
AfWRKY20 gene is upregulated in response to drought-induced expression [
45]. In this study, the
AfWRKY20 gene was cloned from the transcriptome sequencing of
A. fruticosa under drought stress using RT-PCR technology.Subsequently, bioinformatics analysis, phylogenetic tree construction, and subcellular localization verification were conducted. The binding properties of
AfWRKY20 and W-box were verified both in vivo and in vitro through EMSA and dual-Luciferase activity assays. The expression pattern of
AfWRKY20 in response to abiotic stress was investigated through qRT-PCR analysis. To investigate the resistance of
AfWRKY20 transgenic tobacco lines to drought stress at different growth stages, The study first subjected them to sorbitol stress and ABA stress as a means of simulating drought stress treatment. The study then measured phenotypic data, including germination rate, green leaf rate, fresh weight, and root length of the
AfWRKY20 transgenic tobacco lines during the germination stage. Secondly, in this experiment, one-month-old and two-month-old
AfWRKY20 transgenic tobacco lines were subjected to natural drought stress. The chlorophyll fluorescence parameters and the survival rate of the one-month-old
AfWRKY20 transgenic tobacco lines after rewatering were measured. To investigate the potential of
AfWRKY20 in enhancing the detoxification of ROS in tobacco, the study measured the activity of SOD, MDA content, levels of DAB and NBT staining, as well as the expression levels of oxidation kinase genes (
NbSOD,
NbPOD, and
NbCAT) in
AfWRKY20 transgenic tobacco lines after 2 months of natural drought. This study provides a reference for the role of
AfWRKY20 in regulating reactive oxygen species in the ABA signaling response induced by drought stress. It also provides an experimental and theoretical basis for understanding the drought tolerance function of this TF by elucidating the molecular mechanism of
AfWRKY20 in enhancing drought stress regulation.
3. Discussion and Conclusions
The current drought has a significant impact on plant growth and development [
46]. WRKY TFs are critical in regulating plant responses to abiotic stresses [
47,
48]. Related studies have shown that a new WRKY transcription factor, MuWRKY3 (
Macrotyloma uniflorum Lam. Verdc.), can enhance drought resistance in transgenic peanut (
Arachis hypogaea L.) plants [
49]. In addition to the search for novel WRKY transcription factors, further investigation is needed to understand the resistance function of WRKY transcription factors in various species. Therefore,
AfWRKY20 (c194398, graph_c0) was screened during transcriptome sequencing of
A. fruticosa under drought stress to investigate the molecular mechanisms regulating drought stress. Bioinformatics analysis revealed that
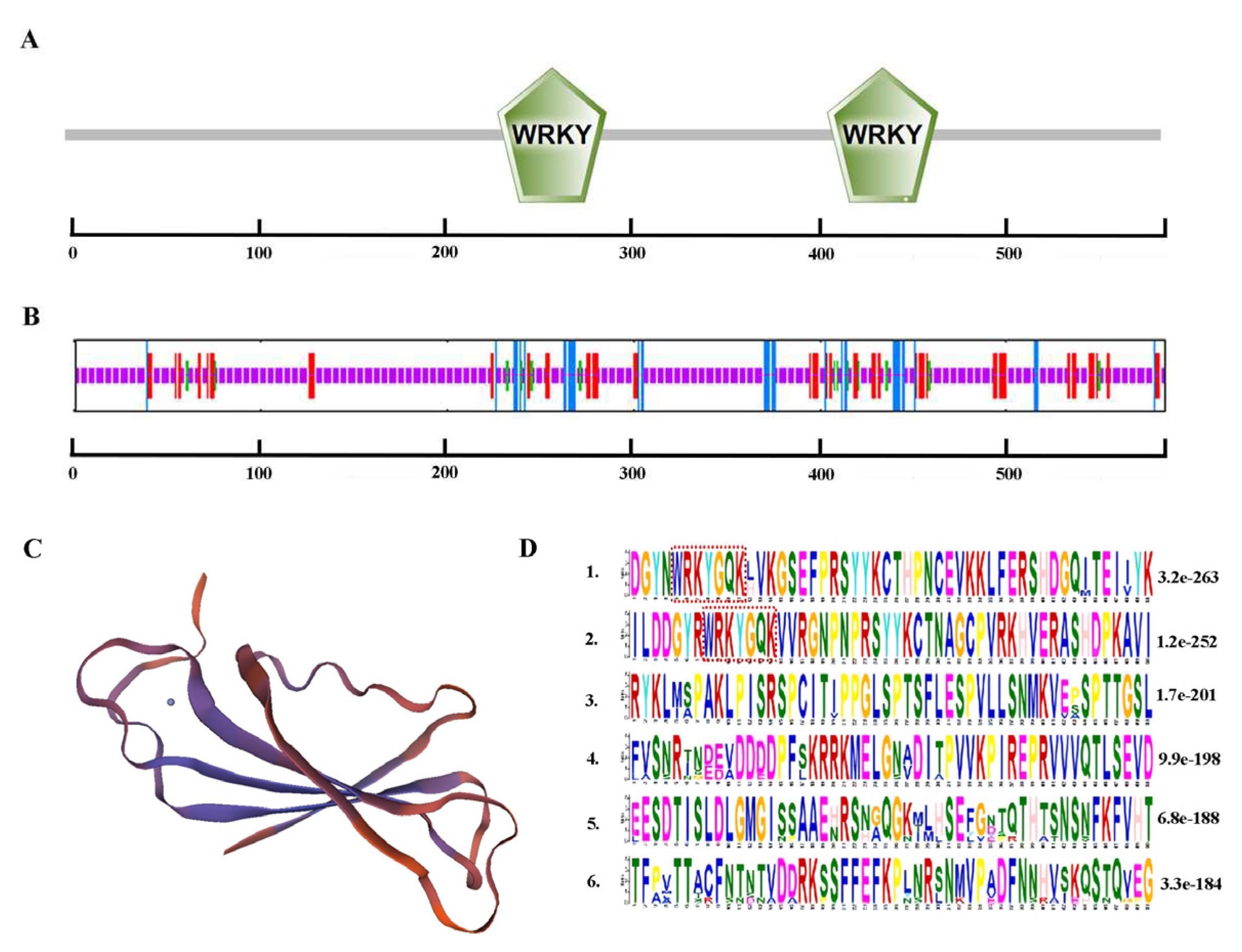
AfWRKY20 contains two WRKY domains (231-289 aa, 407-466 aa) and a C2H2-type zinc finger protein (
Figure 1A). According to the classical classification criteria, this protein was classified into Group I, indicating its close relationship with the growth, development, and stress tolerance of nuclear organisms (
Figure 1A) [
50].
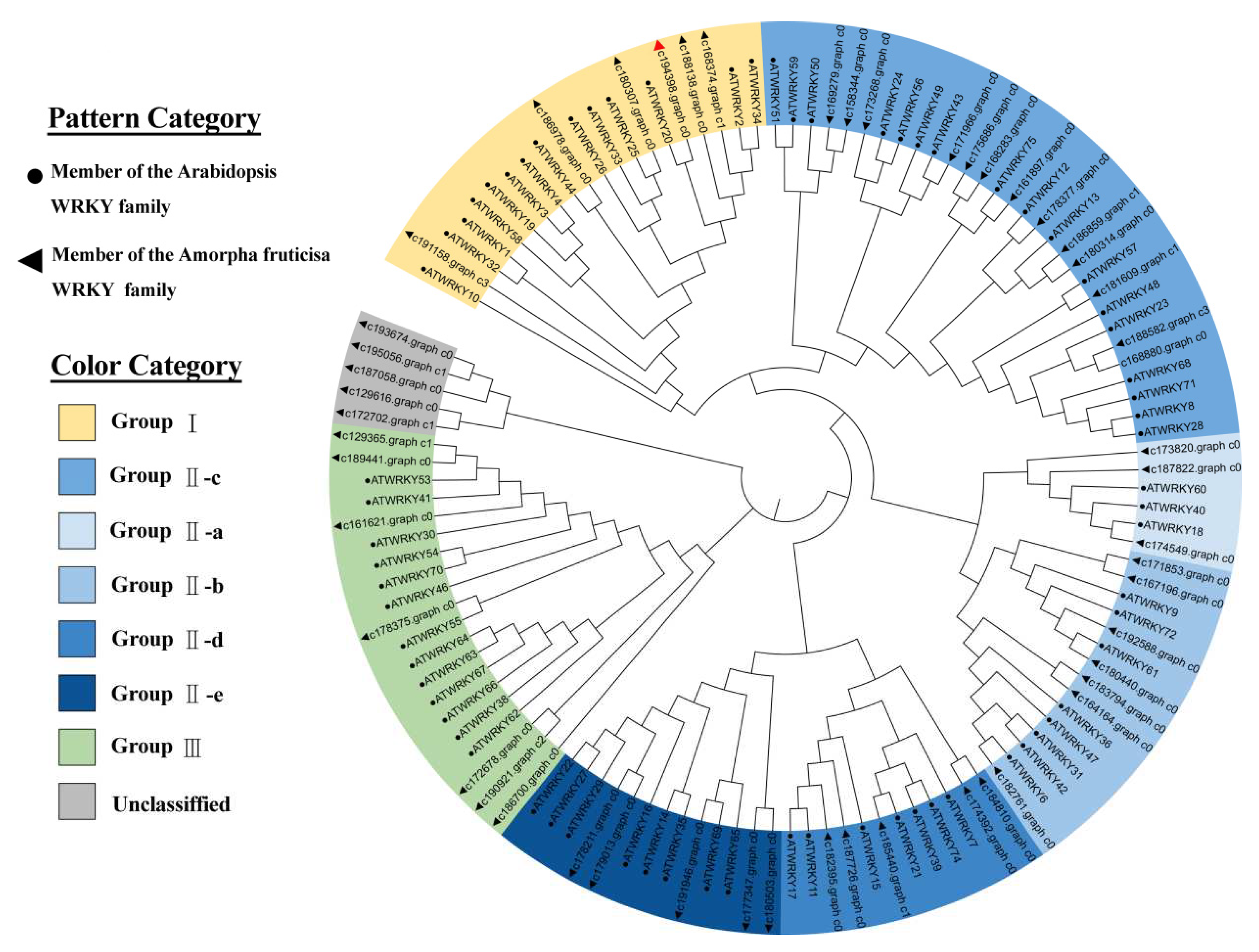
Based on the construction of the phylogenetic tree,
A. fruticosa WRKY20 was found to be most closely related to
Arabidopsis WRKY20 (
Figure 2). Related studies have shown that species with high homology have similar gene functions [
51].
AtWRKY20 coregulates the ABA signaling pathway with ABSCISIC ACID-INSENSITIVE 5 (ABI5). Thus, it plays an important role in biological processes such as seed germination, dormancy, anthocyanin synthesis, and response to stress [
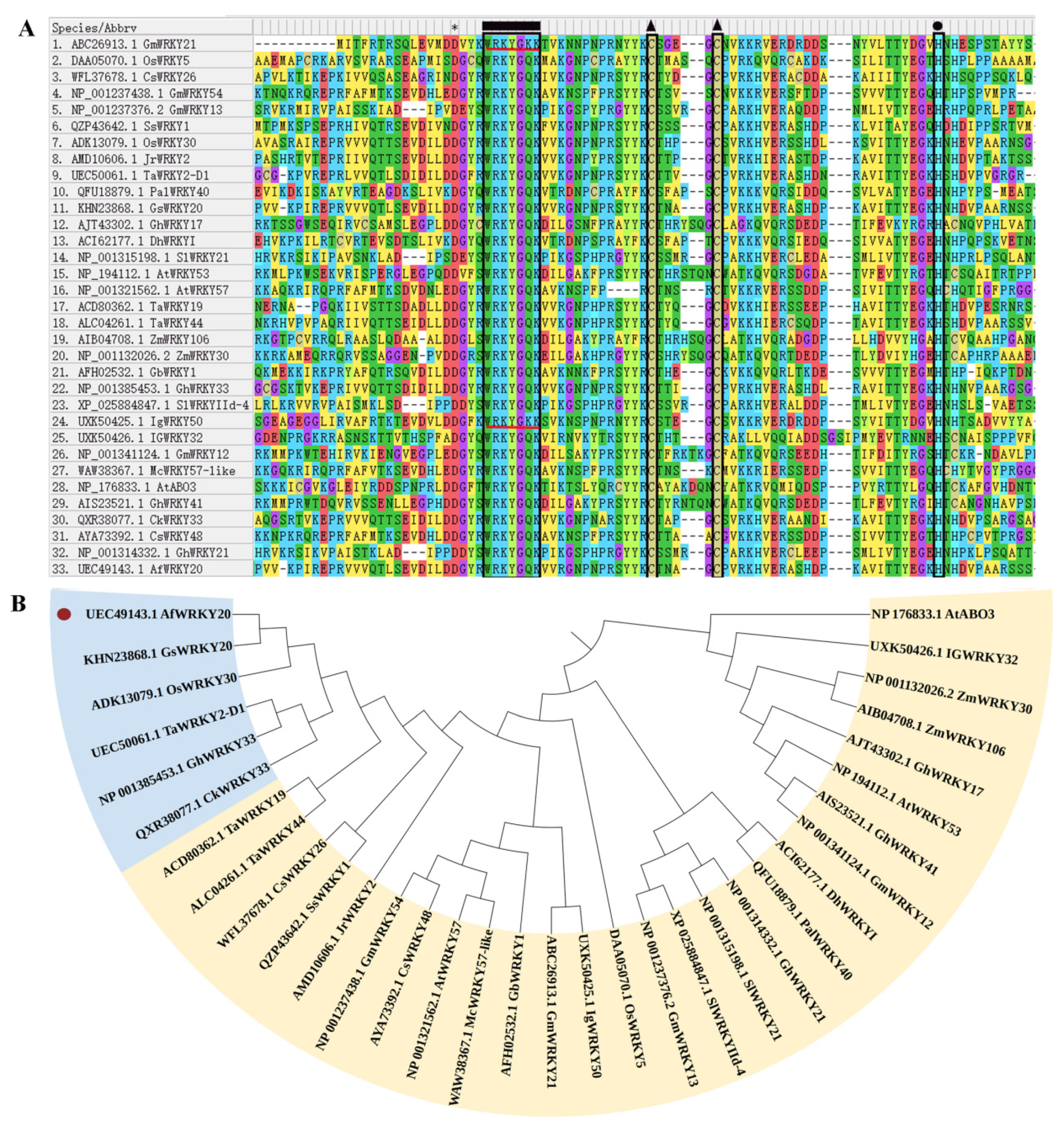
52]. To further explore the correlation between
A. fruticosa WRKY20 and drought-resistant WRKY transcription factors in other species, a phylogenetic tree was constructed. The results revealed a significant similarity between
AfWRKY20 and
GsWRKY20 (
Figure 3).
GsWRKY20 plays an important role in enhancing drought tolerance and regulating ABA signaling [
53]. Therefore, the present study hypothesized that
AfWRKY20 enhances drought stress tolerance in tobacco by regulating the ABA signaling pathway.
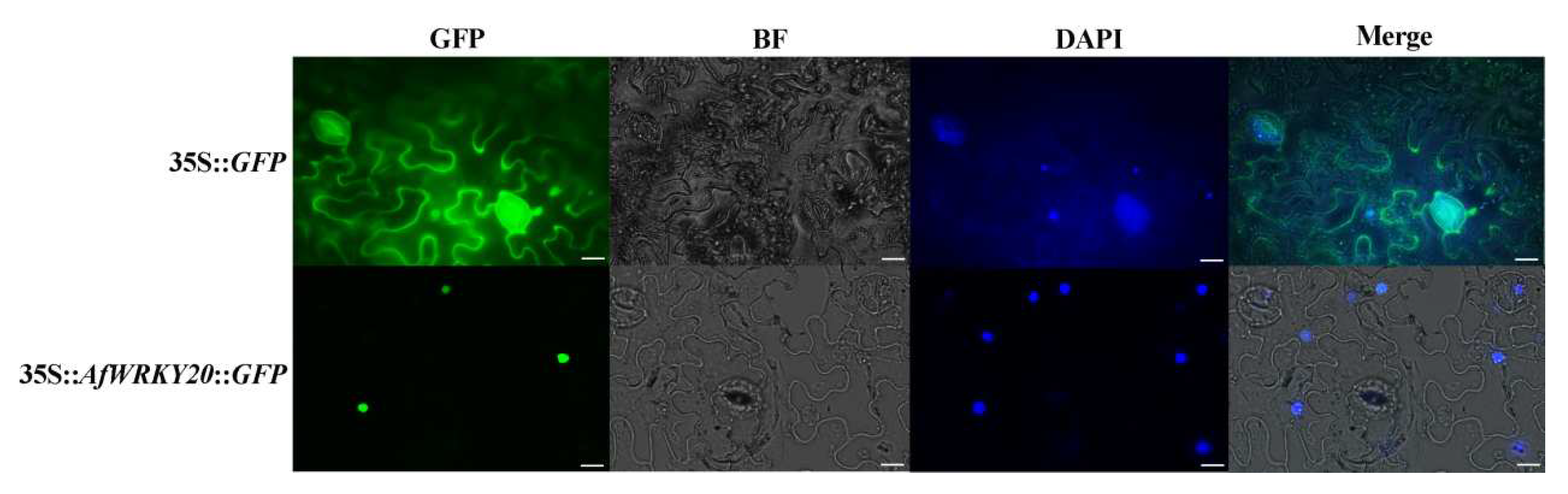
In this study, validation experiments were performed to determine the subcellular localization of the pBI121::
AfWRKY20::
GFP fusion expression vector. The experimental results were consistent with the software's prediction that the protein would be localized in the nucleus. Therefore,
AfWRKY20 may play a role in regulating cell signaling molecules (
Figure 4)[
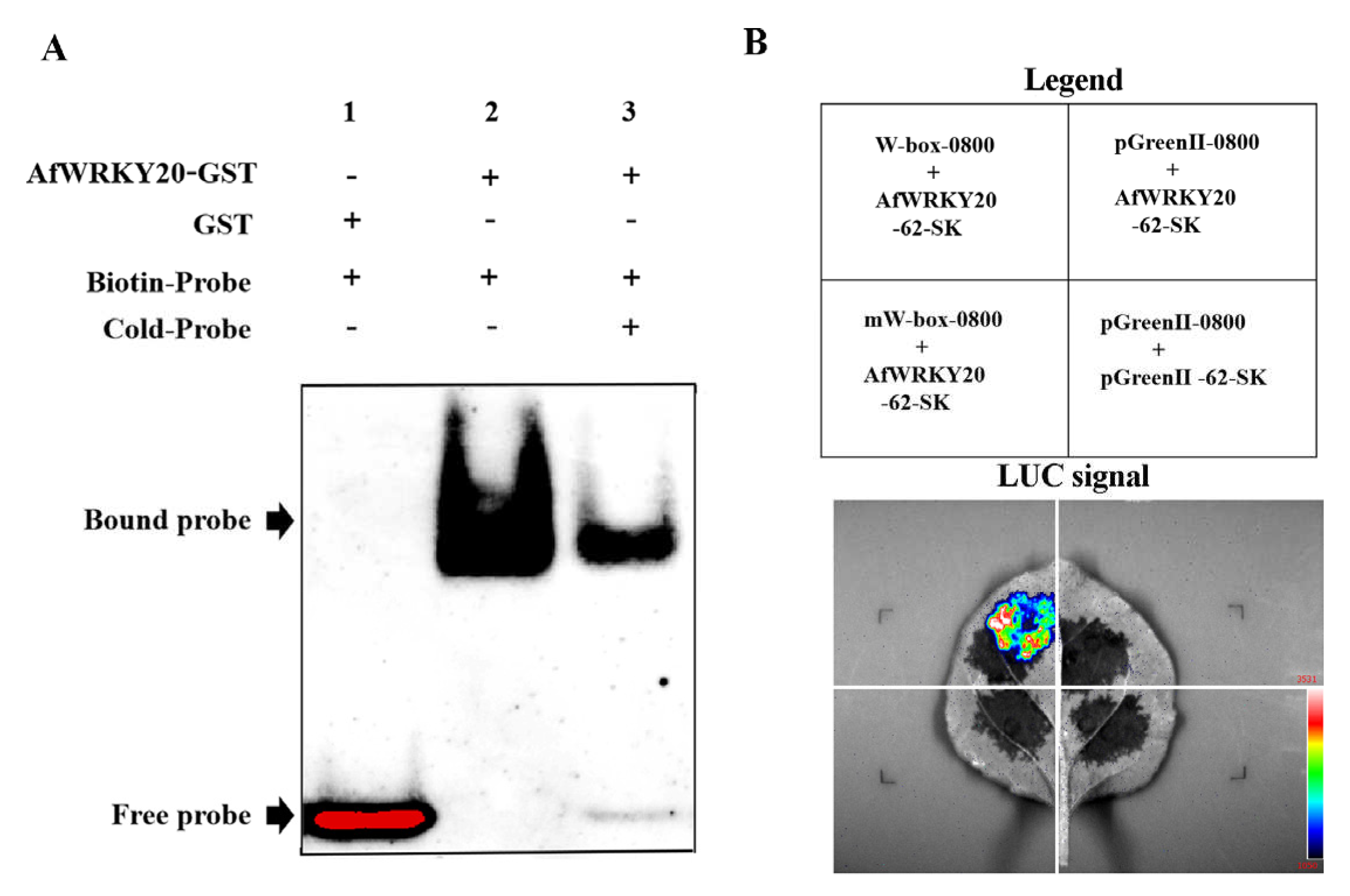
54]. The binding properties of the AfWRKY20 TF and the DNA cis-element W-box were confirmed through EMSA experiments (
Figure 5A) and double LUC experiments (
Figure 5B). The W-box mainly exists in the promoter regions of resistance genes associated with disease and insect resistance, drought, low temperature, saline-alkali, and other factors. It can regulate plant resistance by mediating hormone signal transduction pathways. The molecular mechanism of the AfWRKY20 protein regulating drought stress may be similar to that of SbWRKY30. Both of them induce the expression of drought tolerance genes by binding to W-box elements in the promoters of drought tolerance genes in plants, thereby improving the plant's drought tolerance [
55].
When
A. fruticosa was treated with different concentrations (0%, 10%, 20%, and 30%) of PEG6000 to simulate drought stress, the total expression level of
AfWRKY20 in leaves and roots was 22 times higher in the 30% PEG6000 treatment compared to the untreated group (
Figure 6B). In addition, the effects of three different stress conditions (30% PEG6000, 150 mmol/L NaCl, and 30 mmol/L NaHCO
3) on the expression level of
AfWRKY20 in the roots and leaves of
A. fruticosa were analyzed. Under 30% PEG6000 treatment, the expression of the
AfWRKY20 gene increased most significantly at 12 hours of
A. fruticosa leaf treatment compared to the control. Under the stress of 150 mmol/L NaCl, the expression levels of
AfWRKY20 in the leaves and roots of
A. fruticosa exhibited unstable fluctuations (
Figure 6E-F). Under the treatment of 30 mmol/L NaHCO
3, the expression levels of
AfWRKY20 in the leaves and roots of
A. fruticosa showed an overall trend of initially increasing and then decreasing (
Figure 6G-H).
AfWRKY20 is differentially expressed under these three distinct stress conditions. Therefore, it is speculated that the
AfWRKY20 gene may regulate multiple stress pathways. For example,
AtWRKY53 can respond to both drought stress and salt stress, and
AtWRKY6 can regulate both mechanical damage and oxidative stress [
56].
When tobacco plants overexpressing
AfWRKY20 were exposed to different concentrations of sorbitol stress (
Figure 7-8) and ABA stress (
Figure S5-6), the germination rate, green leaf rate, root length, fresh weight, and other phenotypes were significantly higher compared to those of WT tobacco. Our results suggest that
AfWRKY20 may regulate the growth and development of tobacco by influencing the ABA signaling pathway, thereby enhancing its drought resistance.
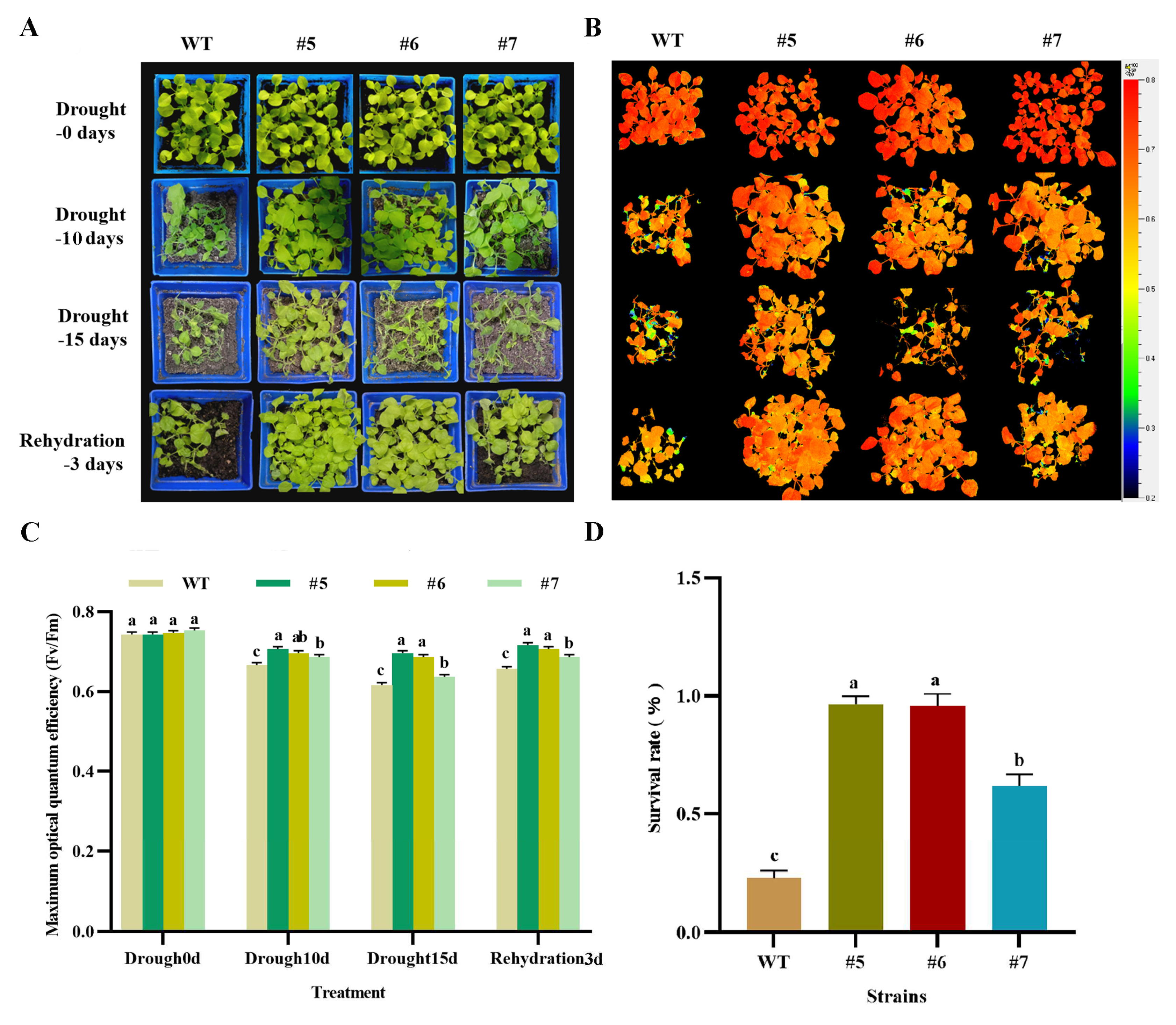
Analysis of the photosynthetic characteristics of transgenic seedlings under drought stress indicated that
AfWRKY20 could reduce the damage caused by stress on photosynthesis and enhance drought stress tolerance. This finding was confirmed by measuring the chlorophyll fluorescence parameters F0, Fm, Fv/Fm, QL, and NPQ of transgenic tobacco plants under natural drought stress (
Figure S4). After rehydration, the Fv/Fm values and survival rate of the transgenic plants were significantly higher than those of the WT plants (
Figure 9). This suggests that
AfWRKY20 plays a crucial role in regulating photosystem damage caused by drought stress [
57].
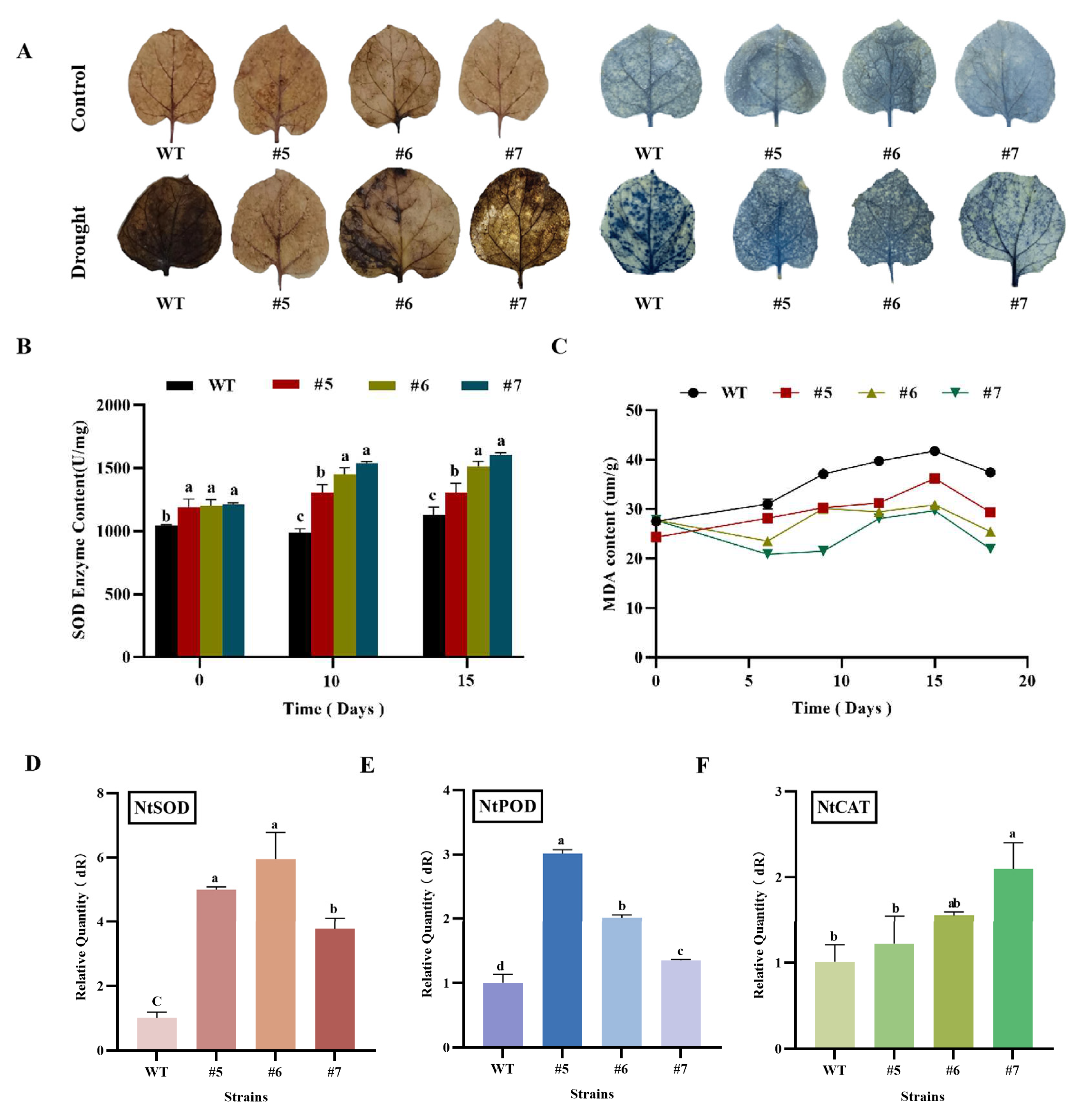
AfWRKY20 overexpression in tobacco under natural drought stress had lower MDA content (
Figure 11-C) and reduced DAB and NBT staining (
Figure 11-A). These findings indicate a decrease in ROS in the transgenic lines. At the same time, it had higher SOD enzyme activity in vivo (
Figure 11-B). The expression levels of
NbSOD,
NbPOD, and
NbCAT in transgenic tobacco were significantly higher than those in the WT tobacco, as determined by real-time fluorescence quantitative PCR (
Figure 11 D-F). This indicates an enhancement in the content of antioxidant enzymes in the plant. It is speculated that the regulatory mechanism of AfWRKY20 may be similar to that of maize ZmWRKY40. This mechanism involves reducing the levels of ROS in transgenic lines during drought stress. This is achieved by enhancing the activities of POD and CAT , which ultimately enhances the drought resistance of the transgenic lines [
58].
The above results suggest that the AfWRKY20 TF can act as a node in the drought stress signaling pathway through ROS balance and ABA signaling pathways, which is similar to the mechanism of XsWRKY20 as a positive regulator [
59]. This study revealed the regulatory mechanism of
AfWRKY20 in response to drought stress-induced ABA signaling, involving ROS. These findings lay the foundation for further studies on the mechanisms through which
AfWRKY20 enhances drought tolerance, providing genetic resources for molecular plant breeding aimed at developing drought-resistant varieties.
4. Materials and Methods
4.1. Plant material
Seeds of A. fruticosa Linn were obtained from Wu Songquan’s research group at Yanbian University, and those of N. benthamiana were acquired from Bu Qingyun's research group at the Northeast Institute of Geography and Agroecology, Chinese Academy of Sciences.
4.2. Strains, vectors, and reagents
Escherichia coli (Top10) competent and Agrobacterium tumefaciens (EHA105) were preserved in the Key Laboratory of Northeast Saline Alkaline Vegetation Restoration and Reconstruction, which is under the Ministry of Education.
PMD18-T vector was acquired from Takara, and the Gateway series entry vector PQBV3, plant expression vector pGWB18, and pBI121-MCS-GFP plant expression vector were obtained from the Key Laboratory of Northeast Salt Vegetation Restoration and Reconstruction Ministry of Education. pGreenⅡ0800-LUC and pGreenⅡ62-SK- LUC carrier were gifted by the Institute of Geography, Chinese Academy of Sciences. T4-DNA ligase, 2×Ex-Taq DNA polymerase, and real-time fluorescent dye 2×SYBR Green qPCR Master Mix were supplied by TaKaRa. Acetosyringone was purchased from Solarbio Biologics, and D-luciferin was obtained from PROMEGA Company. RNA extraction kits, gel recovery kits, and plasmid extraction kits were purchased from Beijing Kangwei Century Company. Domestic analytical reagents were used for all other experiments.
4.3. Cloning and bioinformatics analysis of AfWRKY20 gene
4.3.1. Gene cloning
In the transcriptome data of A. fruticosa under 20% PEG6000 simulated drought stress, after mining of highly differentially expressed genes under drought stress, c194398 and graph_c0 were found to respond to drought stress. Therefore, according to the CDS sequence of c194398, graph_c0, the gene has been designated as AfWRKY20 (accession number: MT859405). Specific primers, AfWRKY20 F1 and AfWRKY20 R1, (AfWRKY20 F1/R1 in table S1)were designed using Primer 5.0 software. The full-length sequence of AfWRKY20 was amplified by RT-PCR and then ligated into the pMD18-T vector. The recombinant vector was transformed into TOP10 through heat shock transformation. The plasmids in the successful bacterial solution were then identified by PCR and extracted using the Beijing Kangwei Century Plasmid Extraction Kit. The recombinant plasmids were then sent to Kumei company for sequencing.
4.3.2. Bioinformatics analysis
The SMART (SMART: Main page (embl-heidelberg.de)) [
60] was used to predict the primary structure of AfWRKY20, while The SOPMA (NPSP@ : SOPMA secondary structure prediction (ibcp.fr)) was used to predict its secondary structure. The SWISS-MODEL (The SWISS-MODEL Interactive Workspace (expasy.org)) [
61] was used to predict the tertiary structure of AfWRKY20. The Plant-mPLoc (Plant-mPLoc server (sjtu.edu.cn)) [
62] was used to predict the subcellular localization of AfWRKY20. The MEME(Introduction-MEME Suite (meme-suite.org)) [
63] was used to predict the conservation of the WRKY domain in AfWRKY20.
4.4. Sequence alignment and construction of phylogenetic trees
Multiple sequence alignments were performed on WRKY amino acid sequences using ClustalW in MEGA 7.0 with default parameters. A phylogenetic tree was constructed using the neighbor-joining (NJ) method with MEGA 7.0 software. These parameters were used in the NJ method: bootstrap (1000 replicates), complete deletion, and amino: p distance[
65]. This setup was employed for constructing all phylogenetic trees in this paper.
In order to compare the phylogenetic relationships of 70 Arabidopsis protein WRKYs and 51 drought-stressed A. fruticosa WRKYs, all 121 WRKY protein sequences were divided into four groups (Ⅰ, Ⅱ, Ⅲ, and unclassified). Group Ⅱ was further divided into five subgroups (Ⅱa, Ⅱb, Ⅱc, Ⅱd, Ⅱe). In order to investigate the phylogenetic relationship between AfWRKY20 and other drought-resistant WRKY transcription factors, we compared the amino acid sequences of 32 reported drought-resistant WRKY transcription factors with AfWRKY20 and constructed a phylogenetic tree. Additionally, a phylogenetic tree was constructed to explore the homology between AfWRKY20 and different species of WRKY.
4.5. Characterisation of gene expression of AfWRKY20
To investigate the differential expression of
AfWRKY20 in various tissues and organs of
A. fruticosa, the seeds of
A. fruticosa were cultured in 96-well plates using the hydroponic method for four weeks. Different tissues (root, stem, leaf, and flower) were selected from the growth and development of
A. fruticosa to extract total RNA. To investigate the gene expression characteristics of
AfWRKY20 under different stress conditions,
A. fruticosa plants with the same growth rate as
A. fruticosa were selected. These plants were then treated with 30 mmol/L NaHCO
3, 150 mmol/L NaCl, and 30% PEG6000. The roots and leaves were collected at 0, 6, 12, 24, and 48 hours. Afterwards,
A. fruticosa seedlings were treated with various concentrations of PEG6000 (0%, 10%, 20%, and 30%) for 48 hours, and then leaves and roots were collected. Expression of
AfWRKY20 gene at 0 h under adversity stress was used as a control.The total RNA was extracted from the samples mentioned above, and the concentration of RNA was examined. One microgram (μg) of total RNA was reverse transcribed into complementary DNA (cDNA) and diluted 100-fold as a template for fluorescence quantitative PCR. After conducting RT-qPCR analysis using primers for the internal reference gene (
AfTubu.F/R in
Table S1) and specific primers for
AfWRKY20 (q
AfWRKY20 F/R in
Table S1), data were collected using the MxPro-Mx3000P system in this study. Each set of experimental data was subjected to three biological replicates, as well as technical replicates.
4.6. Subcellular localization analysis of AfWRKY20
ORF-specific primers (
AfWRKY20-F1/R1 in table S1) were designed using the coding sequence (CDS) of
AfWRKY20 and identified by PCR reaction using the pMD18-T-
AfWRKY20 plasmid as a template. Then, the recovered product of the target band was ligated with the recovered product of the pBI121::
GFP vector, which had been digested with XbaI and SalI. This resulted in the creation of the two-component plant recombinant expression vector pBI121::
AfWRKY20::
GFP, which was placed under the control of the CaMV 35S promoter. In this study, the recombinant vector (35S::
AfWRKY20::
GFP) as an experimental group and the empty vector (35S-
GFP) as a control group were transformed into EHA105 using electroporation. Using the transient transformation method, tobacco plants were successfully transformed by EHA105. The identified strain of EHA105 was injected into the epidermal cells of
N. benthamiana's tobacco. The plants were then incubated in the dark for 12 to 16 hours, followed by incubation under natural light for 3 days. Confocal laser fluorescence microscopy (Olympus) was used to observe the green fluorescence channel, DAPI staining channel, and bright field channel. The superimposed images of the channels were used to confirm the subcellular localization of
AfWRKY20.[
66] Finally, it was verified whether it was consistent with the subcellular localization results predicted by Plant-mPLoc .
4.7. Analysis of binding properties of AfWRKY20 protein and W-box cis-acting elements
In this study, EMSA was used to investigate the binding characteristics between
AfWRKY20 and the W-box element. The prokaryotic expression system was used to express and purify the fusion protein GST-
AfWRKY20. Glutathione Sepharose 4B (GE) GST affinity chromatography was used to obtain purified GST-
AfWRKY20 fusion protein for the subsequent gel blocking assay. Oligonucleotide probes were prepared using synthetic W-box probe primers (W-box F1/R2 in table S1) and synthesized by Doctor Biology Co. The oligonucleotide probes were labeled with biotin, and a reverse complementary DNA was used as a cold competitive probe (Cold-Pr). The preparation was performed according to the instructions of the EMSA kit (Biyuntian Biological Co., Ltd.) [
67,
68].
Four-week-old leaves of
N. benthamiana's tobacco were used for dual-Luciferase activity assays [
69]. The sequences of the W-box (W-box F/R in table S1) and mW-box (mW-box F/R in table S1) elements, which consist of three tandem repeats, were synthesized using oligonucleotide sequencing and then seamlessly cloned into the pGreenII 0800-Luc vector. To construct the recombinant plasmid
AfWRKY20-pGreenII62sk-LUC, specific primers (
Table S1 AfWRKY20-62-SK F/R) were designed to incorporate the homology arm and SalI and BamHI restriction sites. The recombinant plasmid (
Figure S1-D) was successfully identified through double enzyme digestion and then electrotransformed into EHA105. W-box-0800, mW-box-0800, pGreenII 0800-LUC empty vectors, pGreenII 62SK,and
AfWRKY20-62-SK were used to transiently express tobacco leaves.The Agrobacterium hybrid system of pGreenII 0800-LUC vectors and pGreenII 62SK vectors was used as a negative control for transforming
N. benthamiana’s tobacco.Leaves were incubated in the dark for 12 hours and then exposed to normal light for 3 days. After cutting the leaves, the reaction solution containing 1 mmol/Lof the fluorescein substrate D-fluorescein was injected into the wound. The leaves were then left in the dark for 5-7 minutes. Subsequently, the leaves were examined using a fluorescence imaging system, and the exposure time was adjusted based on the experimental results. Finally, the samples were imaged.
4.8. Genetic transformation and drought resistance analysis of AfWRKY20 overexpressed tobacco
4.8.1. Acquisition of AfWRKY20 transgenic tobacco
In this study, tobacco leaves were infected with EHA105 carrying the pBI121::AfWRKY20::GFP recombinant plasmid. The infected tobacco leaves were then soaked and placed on a tobacco co-medium (1/2MS+As). The leaves were cultured under ambient conditions in the dark for three days. Bud differentiation was then induced on tobacco selective differentiation medium containing 50 mg/L of kanamycin. Finally, rooting was induced in a rooting medium (1/2MS + 50 mg/L Kanamycin + 250 mg/L Carbenicillin), resulting in a T0 generation of AfWRKY20 transgenic tobacco lines.
In this study, CTAB was used to extract genomic DNA from T0 generation
AfWRKY20 transgenic tobacco lines (#4, #5, #6, and #7) and the WT.
AfWRKY20 overexpressed plants of the T0 generation were identified using PCR molecular biology techniques with specific primers (
AfWRKY20 F1/R1 in table S1). DNA from the WT was used as the negative control (CK-), the PBI121-
AfWRKY20-
GFP plasmid was used as the positive control (CK+), and DNA from the T0 generation
AfWRKY20 transgenic tobacco lines (#4, #5, #6, and #7) was used as the experimental group. The expression of the
AfWRKY20 gene was identified in different transgenic tobacco lines (#4, #5, #6, and #7) using real-time fluorescence quantitative detection technology. RNA was extracted from the transgenic tobacco lines
AfWRKY20 (#4, #5, #6, and #7) as the experimental group, while the WT was used as the control group. The extracted RNA was then reverse transcribed into cDNA. A 100-fold dilution of cDNA was used as the template. The internal reference primer,
AfTubu.F/R (
AfTubu.F/R in table S1), and the specific primer sequence for
AfWRKY20 real-time fluorescence quantification,
AfWRKY20 F/R (q
AfWRKY20 F/R in table S1), were used. Finally, data were collected using the MxPro-Mx3000P system. Three biological replicates and three technical replicates were performed for each set of experimental data. Finally, the tobacco lines of the experimental group were screened on the 1/2MS medium with kanamycin (50 mg/L) resistance. The aim was to determine if all the seeds of the transgenic tobacco lines germinated and if the T3 generation
AfWRKY20 transgenic seeds were obtained in a pure state [
70].
4.8.2. Tolerance analysis of AfWRKY20 transgenic tobacco at germination stage under sorbitol and ABA simulated drought stress
The seeds of T3 generation AfWRKY20 transgenic tobacco (#5, #6, and #7) and the WT tobacco were sterilized and placed in 1/2MS sorbitol solutions at different concentrations (0, 200, and 300 mM) and 1/2MS ABA stress solutions at different concentrations (0, 2, 2.5, 3, and 5 μM). T3 generation AfWRKY20 transgenic tobacco seeds (#5, #6, and #7) were used as the experimental groups, while wild type tobacco seeds (WT) were used as the control group. The experimental and control groups were seeded with 30 seeds on a 1/2MS plate containing different concentrations of sorbitol stress. The plates were then placed at -4°C and subjected to vernalization at a low temperature for three days. They were then cultured horizontally at 25°C (8 hours light/16 hours dark). Germination rate and green leaf rate were counted at the beginning of seed germination in 1/2MS medium under various stress conditions. Three sets of biological replicates and three sets of technical replicates were performed for each experimental set.
Seeds from both the experimental and control groups were placed in 1/2MS medium and cultured vertically until reaching the trifoliate stage. The tobacco plants from both the experimental and control groups, which had the same growth rate, were exposed to various concentrations of sorbitol stress (0, 200, 250, and 300 mM) and different concentrations of ABA stress (0, 5, 7.5, and 10 μM) in 1/2MS medium. After 15 days of vertical cultivation at 25°C (8/16 h-light/dark), we measured the fresh weight and root length under various stress concentrations using ImageJ software for analysis. Three sets of biological replicates and three sets of technical replicates were performed for each experimental group.
4.8.3. Analysis of photosynthetic characteristics of AfWRKY20 transgenic tobacco seedlings under natural drought stress
The T3 generation
AfWRKY20 transgenic tobacco lines (#5, #6, and #7) mentioned above were vertically cultured until the trifoliate stage. These lines were used as the experimental groups, while the WT tobacco was used as the control group. The tobacco seedlings from both the experimental group and the control group, which had similar growth, were selected for soil cultivation. Sixteen seedlings from each group (4x4) were placed in blue pots and cultured at a temperature of 25℃ (8/16 h-light/dark) for one month. The open chlorophyll fluorescence imaging system was used to investigate the sensitivity of the plant photosystem II response to drought stress in both the experimental and control groups after 0, 10, and 15 days of natural drought treatment. Chlorophyll fluorescence parameters measured by the system include F0 (minimum fluorescence yield in the absence of photosynthetic light), Fm (maximum fluorescence yield in the absence of photosynthetic light), FV/Fm (maximum efficiency of PSII), QL (photochemical quenching coefficient based on the lake model), and NPQ (extent of excess energy dissipation in the form of heat) [
71]. At the same time, photographs of tobacco plants were taken under natural light on days 0, 10, and 15 of the natural drought treatment, as well as on the third day after rewatering. In addition, the tobacco's chlorophyll fluorescence imager (with a cursor range of 0.2-0.8) was used to capture images in Fv/Fm mode. The survival rate of tobacco was then calculated after 3 days of rehydration [
72]. Each experimental group had three biological replicates and three technical replicates.
4.8.4. Determination of physiological indices in AfWRKY20 transgenic tobacco under natural drought treatment
AfWRKY20 transgenic tobacco lines (#5, #6, and #7) and wild-type tobacco lines of the T3 generation, which exhibited consistent growth for two months, were selected. These lines were exposed to natural drought conditions for 0, 10, and 15 days, followed by a period of rewatering for 3 days. Fv/Fm values of tobacco were collected using the FluorCam open chlorophyll fluorescence imaging system on day 0, day 15 of the natural drought treatment, and day 3 of rewatering. The chlorophyll content, MDA content, and SOD activity of tobacco leaves were measured during the natural drought stage and rewatering stage [
73]. The tobacco leaves with the most noticeable phenotypic changes were selected for DAB and NBT chemical staining analysis after 15 days of drought treatment [
74]. RNA was extracted from transgenic tobacco lines expressing
AfWRKY20 (#5, #6, and #7) after 15 days of drought stress as the experimental group. Wild-type RNA tobacco was used as the control group. The extracted RNA was then reverse transcribed into cDNA.
Using a 100-fold diluted cDNA as a template and
NbActin F/R (
NbActin F/R in
Table S1) as the internal reference primer, specific primer sequences
NbSOD F/R,
NbPOD F/R, and
NbCAT F/R (
NbSOD F/R,
NbPOD F/R, and
NbCAT F/R in
Table S1) were used for real-time fluorescence quantification.Finally, data were collected using the MxPro-Mx3000P system. Three sets of biological replicates and three sets of technical replicates were performed for each experimental set.
4.9. Statistical Analysis
All data were processed using the Paired Comparison plot in ORIGIN software. The following parameters were used in the Paired Comparison plot: Error bar - SD, Mean comparison methods - Tukey, and Significance level - 0.05. Lowercase letters (a, b, c, etc.) represent statistical differences with p < 0.05.
Author Contributions
Conceptualization, D.L. and Q.G.; methodology, D.L. and Q.G.; Software, D.L.; validation, D.L., B.G., C.H., J.S., X.W.and S.M.; formal analysis, D.L.; investigation, D.L., J.G., and R.Y.; resources, Q.G.; data curation, D.L.; writing—original draft preparation, D.L.; writing—review and editing, D.L.; visualization, D.L.; supervision, Q.G.; project administration, Q.G.; funding acquisition, Q.G. All authors have read and agreed to the published version of the manuscript.
Figure 1.
Results of bioinformatics analysis of AfWRKY20. (A) Primary structure of the AfWRKY20 protein. (B) The secondary structure of the AfWRKY20 protein is depicted, with the α-helix shown in blue, the β-turn in green, the extended strand in red, and the irregular coil in purple. (C) Tertiary structure of the AfWRKY20 protein. (D) The AfWRKY20 motif consists of a conserved domain, with the two red dashed rectangular regions representing the two WRKY domains.
Figure 1.
Results of bioinformatics analysis of AfWRKY20. (A) Primary structure of the AfWRKY20 protein. (B) The secondary structure of the AfWRKY20 protein is depicted, with the α-helix shown in blue, the β-turn in green, the extended strand in red, and the irregular coil in purple. (C) Tertiary structure of the AfWRKY20 protein. (D) The AfWRKY20 motif consists of a conserved domain, with the two red dashed rectangular regions representing the two WRKY domains.
Figure 2.
Construction of phylogenetic trees for drought stress WRKY transcription factors in A. fruticosa and Arabidopsis WRKY transcription factors. The red triangle represents WRKY20 of A. fruticosa. Triangles indicate WRKY transcription factors in the A. fruticosa drought transcriptome. Circles indicate WRKY transcription factors in Arabidopsis. The classification of WRKY transcription factors is distinguished by different colors.
Figure 2.
Construction of phylogenetic trees for drought stress WRKY transcription factors in A. fruticosa and Arabidopsis WRKY transcription factors. The red triangle represents WRKY20 of A. fruticosa. Triangles indicate WRKY transcription factors in the A. fruticosa drought transcriptome. Circles indicate WRKY transcription factors in Arabidopsis. The classification of WRKY transcription factors is distinguished by different colors.
Figure 3.
(A) The amino acid sequences of the drought-resistant WRKY transcription factors of A. fruticosa WRKY20 and various species were compared using MEGA 7.0 software. The WRKY domain, C, and H residues in the zinc finger motif are represented by rectangles, triangles, and circles, respectively. The region of the red line represents WRKYGKK, which is not the regular WRKY domain motif (WRKYGQK). (B) Phylogenetic trees were constructed for A. fruticosa WRKY20 and drought-resistant WRKYs from various species. The red circles indicate AfWRKY20. The blue regions represent drought-resistant WRKY transcription factors with a very high homology to AfWRKY20. The yellow region represents the drought-resistant WRKY transcription factors that show homology to AfWRKY20.
Figure 3.
(A) The amino acid sequences of the drought-resistant WRKY transcription factors of A. fruticosa WRKY20 and various species were compared using MEGA 7.0 software. The WRKY domain, C, and H residues in the zinc finger motif are represented by rectangles, triangles, and circles, respectively. The region of the red line represents WRKYGKK, which is not the regular WRKY domain motif (WRKYGQK). (B) Phylogenetic trees were constructed for A. fruticosa WRKY20 and drought-resistant WRKYs from various species. The red circles indicate AfWRKY20. The blue regions represent drought-resistant WRKY transcription factors with a very high homology to AfWRKY20. The yellow region represents the drought-resistant WRKY transcription factors that show homology to AfWRKY20.
Figure 4.
Subcellular localization of AfWRKY20 in tobacco cells. 35s-GFP was used as the control group. 35s-AfWRKY20-GFP was used as the experimental group. Ruler: 20 μm.
Figure 4.
Subcellular localization of AfWRKY20 in tobacco cells. 35s-GFP was used as the control group. 35s-AfWRKY20-GFP was used as the experimental group. Ruler: 20 μm.
Figure 5.
(A) Binding of the GST-AfWRKY20 fusion protein to the W-box element. (1) GST+ protein and a biotin-labeled oligonucleotide probe (Biotin-Pr) were added; (2) Adding GST-AfWRKY20 fusion protein and biotin-Pr; (3) Adding GST-AfWRKY20 fusion protein, biotin-Pr and unlabeled oligonucleotide probe (Cold-Pr). (B) Dual-LUC analysis of W-box and mW-box elements.
Figure 5.
(A) Binding of the GST-AfWRKY20 fusion protein to the W-box element. (1) GST+ protein and a biotin-labeled oligonucleotide probe (Biotin-Pr) were added; (2) Adding GST-AfWRKY20 fusion protein and biotin-Pr; (3) Adding GST-AfWRKY20 fusion protein, biotin-Pr and unlabeled oligonucleotide probe (Cold-Pr). (B) Dual-LUC analysis of W-box and mW-box elements.
Figure 6.
Expression characteristics of the AfWRKY20 gene in tissues and organs under PEG6000-induced stress. (A) Tissues and organs: roots, stems, leaves, flowers, and peel. (B) Expression of AfWRKY20 in leaves and roots under 0%, 10%, 20%, and 30% PEG6000 stress. (C) Expression of the AfWRKY20 gene in leaves under 30% PEG6000 stress at 0, 6, 12, 24, and 48 hours. (D) Expression of the AfWRKY20 gene in roots under 30% PEG6000 stress at 0, 6, 12, 24, and 48 hours. (E) Expression of the AfWRKY20 gene in leaves exposed to 150 mmol/L NaCl stress at 0, 6, 12, 24, and 48 hours. (F) Expression of the AfWRKY20 gene in roots under 150 mmol/L NaCl stress at 0, 6, 12, 24, and 48 hours. (G) Expression of the AfWRKY20 gene in leaves exposed to 30 mmol/L NaHCO3 stress at 0, 6, 12, 24, and 48 hours. (H) Expression of the AfWRKY20 gene in roots under 30 mmol/L NaHCO3 stress at 0, 6, 12, 24, and 48 hours. Error bars represent standard errors of three biological replicates, with significant differences at the p<0.05 level.
Figure 6.
Expression characteristics of the AfWRKY20 gene in tissues and organs under PEG6000-induced stress. (A) Tissues and organs: roots, stems, leaves, flowers, and peel. (B) Expression of AfWRKY20 in leaves and roots under 0%, 10%, 20%, and 30% PEG6000 stress. (C) Expression of the AfWRKY20 gene in leaves under 30% PEG6000 stress at 0, 6, 12, 24, and 48 hours. (D) Expression of the AfWRKY20 gene in roots under 30% PEG6000 stress at 0, 6, 12, 24, and 48 hours. (E) Expression of the AfWRKY20 gene in leaves exposed to 150 mmol/L NaCl stress at 0, 6, 12, 24, and 48 hours. (F) Expression of the AfWRKY20 gene in roots under 150 mmol/L NaCl stress at 0, 6, 12, 24, and 48 hours. (G) Expression of the AfWRKY20 gene in leaves exposed to 30 mmol/L NaHCO3 stress at 0, 6, 12, 24, and 48 hours. (H) Expression of the AfWRKY20 gene in roots under 30 mmol/L NaHCO3 stress at 0, 6, 12, 24, and 48 hours. Error bars represent standard errors of three biological replicates, with significant differences at the p<0.05 level.
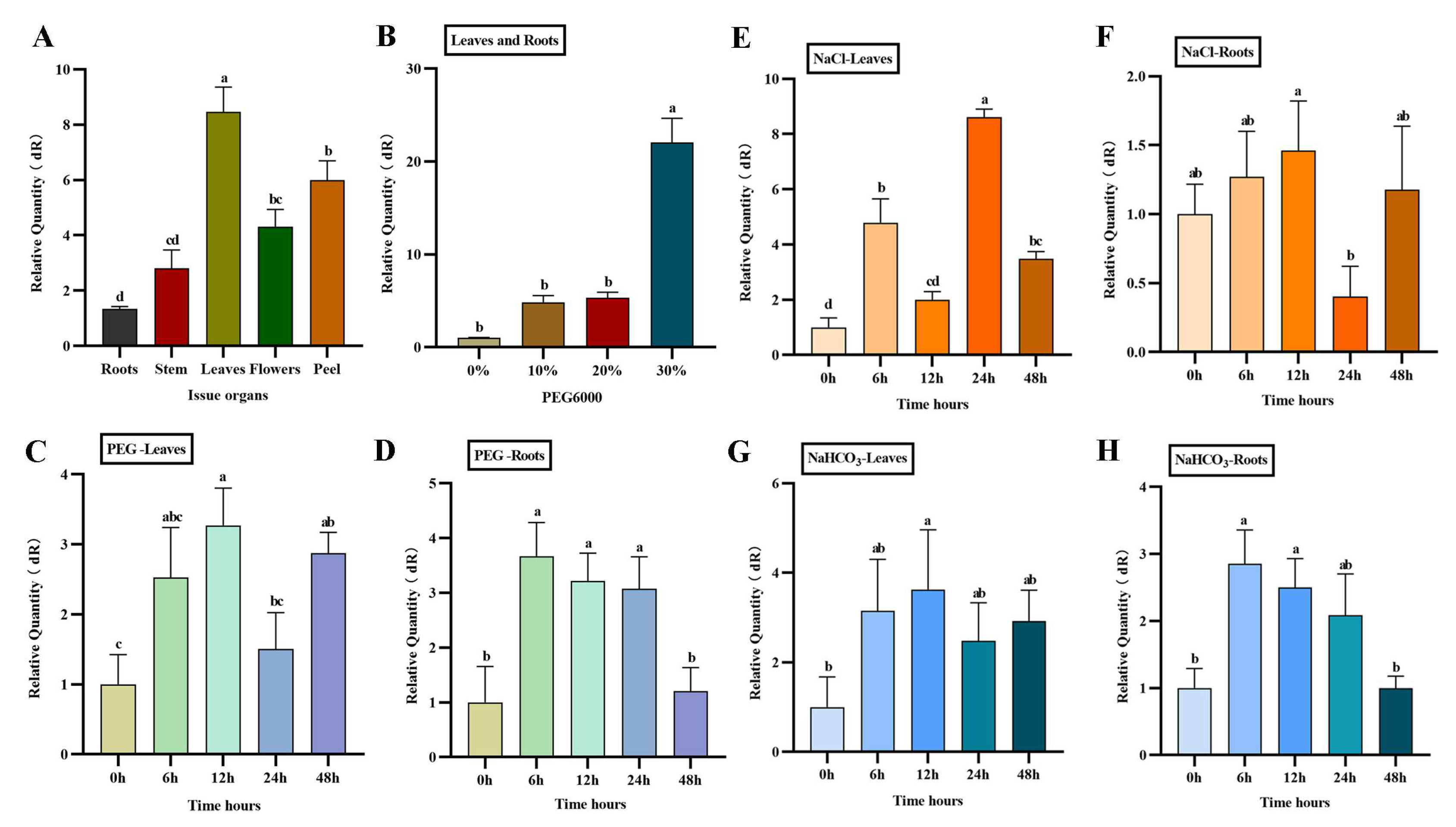
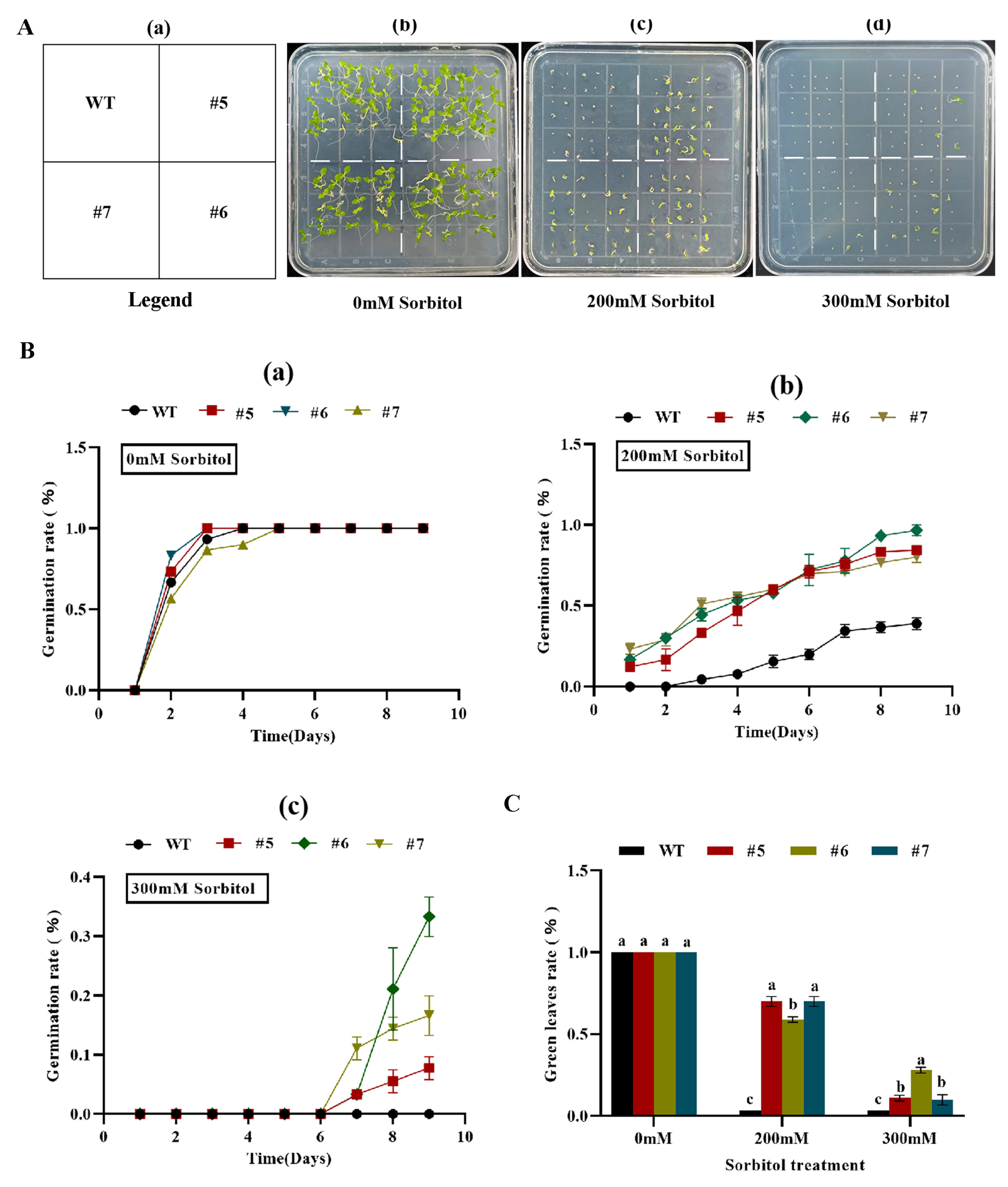
Figure 7.
Analysis of the green leaf rate and germination rate of AfWRKY20 overexpression lines under sorbitol stress at various concentrations. (A) Sorbitol stress phenotypes at different concentrations; (B) Measurement of the percentage of green leaves. (C) Measurement of germination rate. Error bars indicate the standard errors of three biological replicates, which are significantly different at the p < 0.05 level.
Figure 7.
Analysis of the green leaf rate and germination rate of AfWRKY20 overexpression lines under sorbitol stress at various concentrations. (A) Sorbitol stress phenotypes at different concentrations; (B) Measurement of the percentage of green leaves. (C) Measurement of germination rate. Error bars indicate the standard errors of three biological replicates, which are significantly different at the p < 0.05 level.
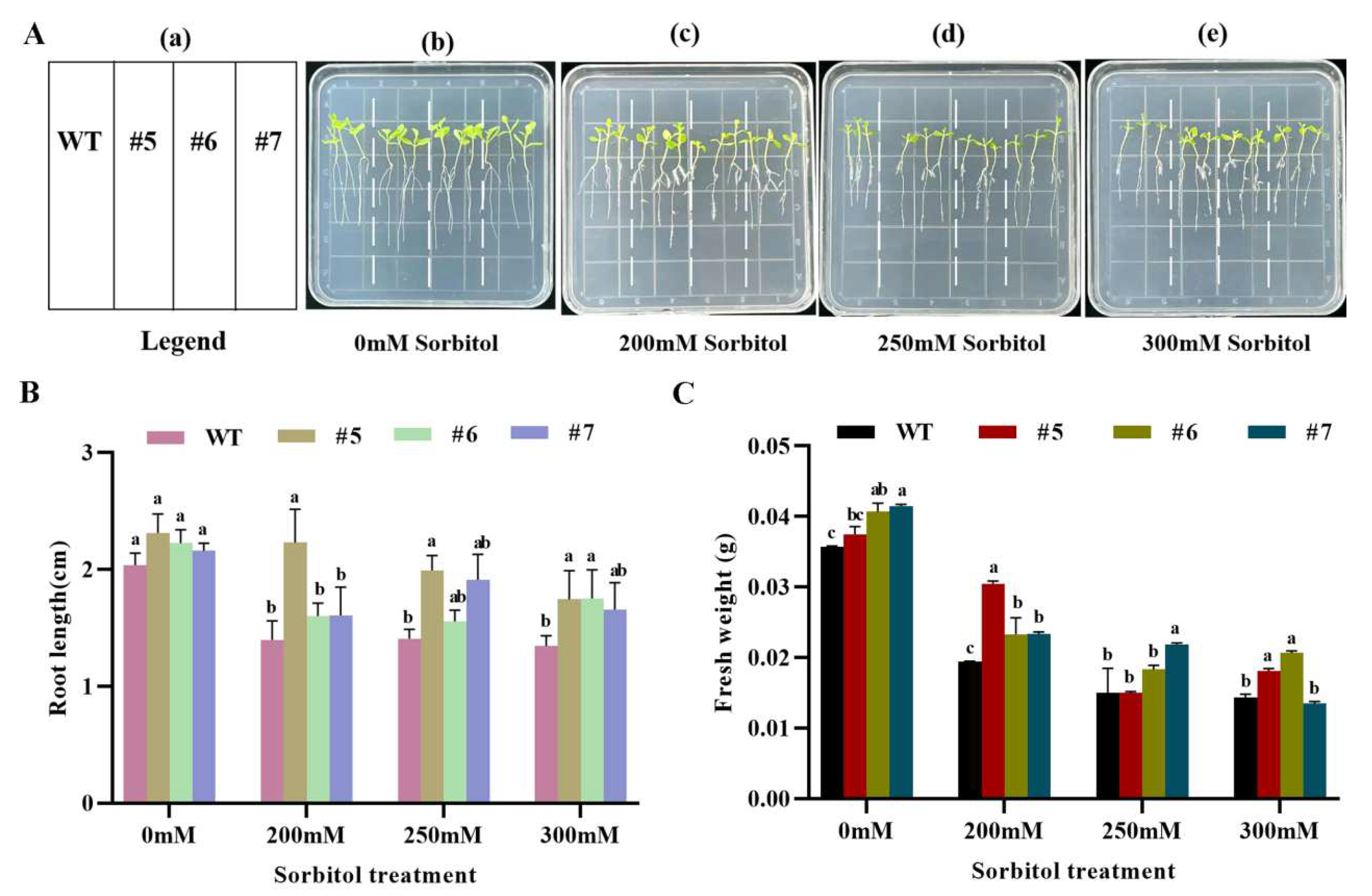
Figure 8.
Analysis of fresh weight and root length of AfWRKY20 overexpression tobacco lines under sorbitol stress at various concentrations.(A) The phenotypes of tobacco under sorbitol stress. (B) Measurement of fresh weight of plants. (C) Measurement of plant root length.
Figure 8.
Analysis of fresh weight and root length of AfWRKY20 overexpression tobacco lines under sorbitol stress at various concentrations.(A) The phenotypes of tobacco under sorbitol stress. (B) Measurement of fresh weight of plants. (C) Measurement of plant root length.
Figure 9.
Analysis of photosynthetic characteristics of AfWRKY20 transgenic tobacco pot seedlings under natural drought stress. (A) Phenotyping of tobacco plants after 0, 10, and 15 days of natural drought treatment, followed by 3 days of rehydration. (B) Images of chlorophyll fluorescence Fv-Fm in transgenic tobacco and the WT tobacco under drought stress treatment, with a cursor range of 0.2-0.8. (C) Fv/Fm values of WT and transgenic tobacco were measured at 0, 10, and 15 days of drought treatment, as well as 3 days after rehydration. (D) Survival rate of tobacco plants after 3 days of rehydration.
Figure 9.
Analysis of photosynthetic characteristics of AfWRKY20 transgenic tobacco pot seedlings under natural drought stress. (A) Phenotyping of tobacco plants after 0, 10, and 15 days of natural drought treatment, followed by 3 days of rehydration. (B) Images of chlorophyll fluorescence Fv-Fm in transgenic tobacco and the WT tobacco under drought stress treatment, with a cursor range of 0.2-0.8. (C) Fv/Fm values of WT and transgenic tobacco were measured at 0, 10, and 15 days of drought treatment, as well as 3 days after rehydration. (D) Survival rate of tobacco plants after 3 days of rehydration.
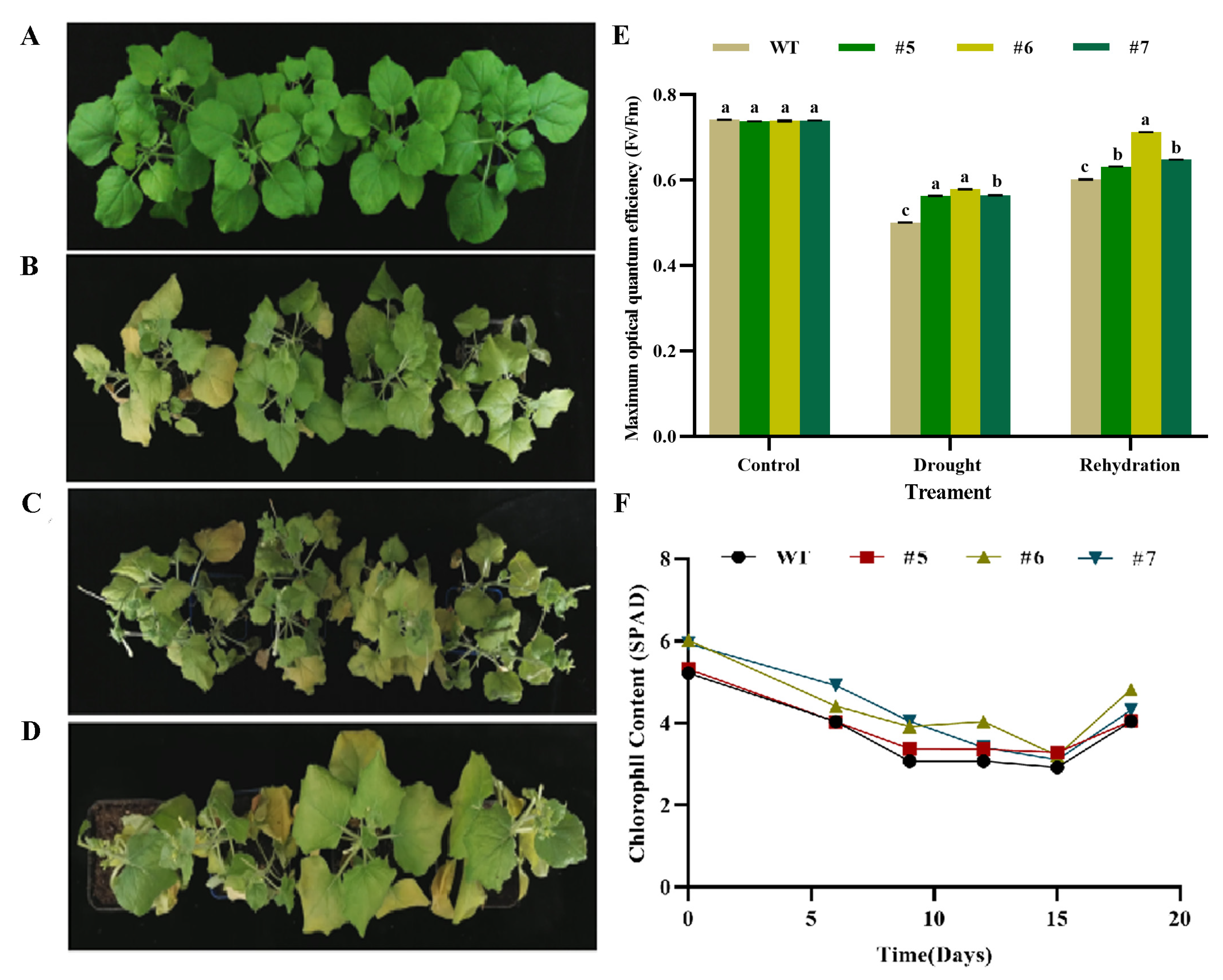
Figure 10.
Phenotypic changes and photosynthetic characteristics of AfWRKY20 transgenic tobacco under natural drought stress. (A) Phenotype of tobacco on 0 day of drought treatment. (B) Phenotypes of tobacco plants after 10 days of drought treatment. (C) Phenotypes of tobacco plants after 15 days of drought treatment. (D) The phenotypes of tobacco after 3 days of rewatering; (E) The Fv/Fm values comparing AfWRKY20 overexpression tobacco with the WT tobacco at 0 day of drought treatment, 15 days of drought treatment, and 3 days after rewatering. (F) Changes in chlorophyll content in tobacco during drought treatment and subsequent rewatering.
Figure 10.
Phenotypic changes and photosynthetic characteristics of AfWRKY20 transgenic tobacco under natural drought stress. (A) Phenotype of tobacco on 0 day of drought treatment. (B) Phenotypes of tobacco plants after 10 days of drought treatment. (C) Phenotypes of tobacco plants after 15 days of drought treatment. (D) The phenotypes of tobacco after 3 days of rewatering; (E) The Fv/Fm values comparing AfWRKY20 overexpression tobacco with the WT tobacco at 0 day of drought treatment, 15 days of drought treatment, and 3 days after rewatering. (F) Changes in chlorophyll content in tobacco during drought treatment and subsequent rewatering.
Figure 11.
Analysis of oxygen free radicals in tobacco plants under natural drought conditions. (A) Histochemical analysis of DAB and NBT in tobacco (B) Determination of the SOD content in tobacco under natural drought conditions. (C) Determination of MDA content in tobacco under natural drought conditions. (D) Real-time quantitative expression of NbSOD during drought stress. (E) Real-time quantitative expression of NbPOD during drought stress. (F) Real-time quantitative expression of NbCAT during drought stress.
Figure 11.
Analysis of oxygen free radicals in tobacco plants under natural drought conditions. (A) Histochemical analysis of DAB and NBT in tobacco (B) Determination of the SOD content in tobacco under natural drought conditions. (C) Determination of MDA content in tobacco under natural drought conditions. (D) Real-time quantitative expression of NbSOD during drought stress. (E) Real-time quantitative expression of NbPOD during drought stress. (F) Real-time quantitative expression of NbCAT during drought stress.