Submitted:
18 July 2023
Posted:
18 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
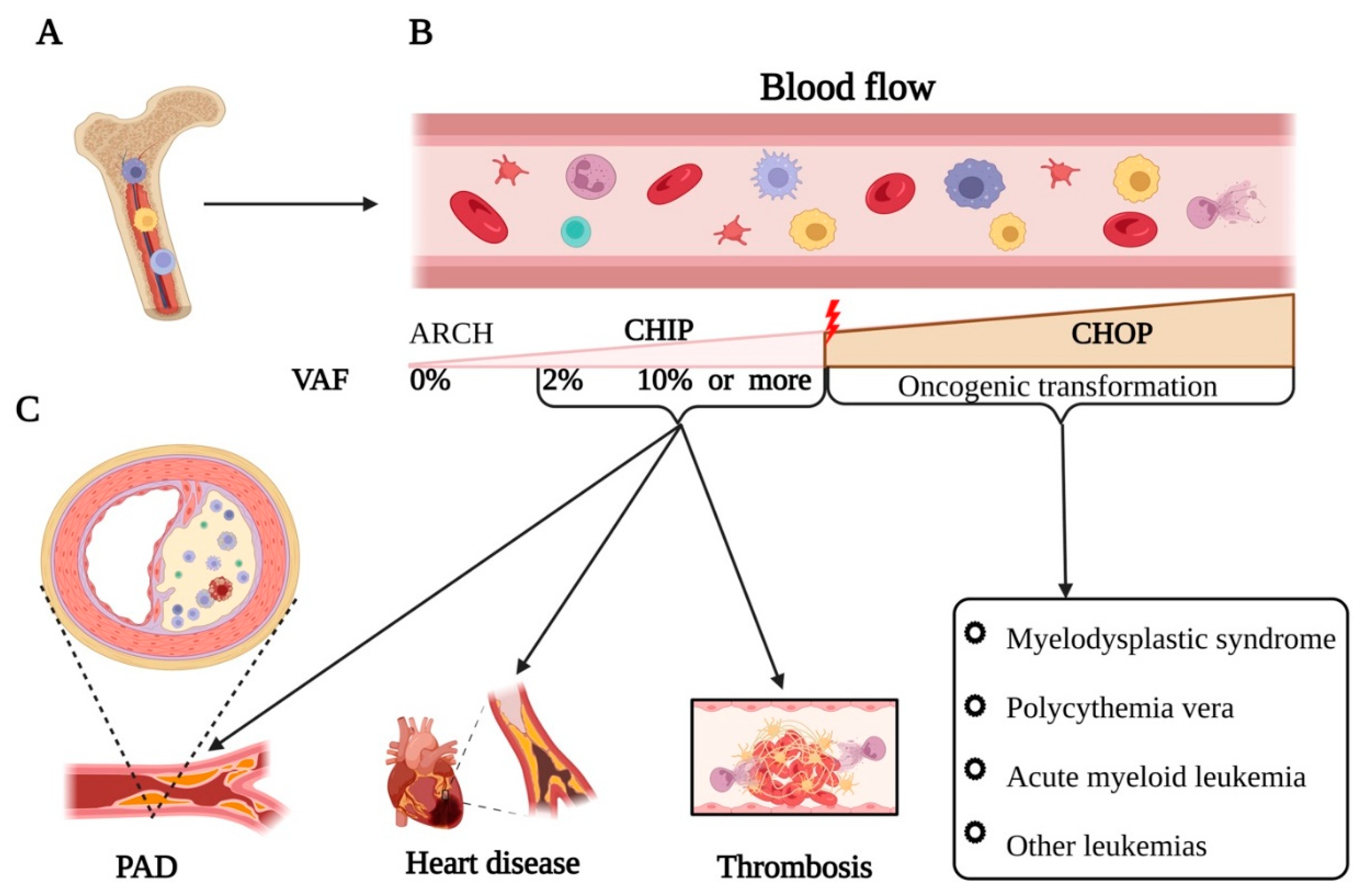
2. Molecular Mechanisms of Somatic Mutations in PAD
3. Genetic Landscape of Peripheral Arterial Diseases
4. CHIP as Prognostic Markers in PAD
4.1. Accumulation of Mutant Clones in Ischemic Tissues of PAD Patients:
5. Diabetes mellitus and atherosclerosis as a one of the main drivers of somatic mutation accumulation in patients PAD
6. Somatic Mutation Search in PAD-related Thrombosis
7. Molecular Aspects of Somatic Mutation and Inflammation
8. Somatic Mutation and Regeneration, Unraveling the Connection
9. Conclusion
Author Contributions
Funding
Acknowledgments
References
- Hinchliffe, R.J.; Forsythe, R.O.; Apelqvist, J.; Boyko, E.J.; Fitridge, R.; Hong, J.P.; Katsanos, K.; Mills, J.L.; Nikol, S.; Reekers, J.; et al. Guidelines on diagnosis, prognosis, and management of peripheral artery disease in patients with foot ulcers and diabetes (IWGDF 2019 update). Diabetes/Metabolism Res. Rev. 2020, 36, e3276. [Google Scholar] [CrossRef] [PubMed]
- Klarin, D.; Tsao, P.S.; Damrauer, S.M. Genetic Determinants of Peripheral Artery Disease. Circ. Res. 2021, 128, 1805–1817. [Google Scholar] [CrossRef] [PubMed]
- Amancherla, K.W.J.; Bick, A.G. Clonal hematopoiesis and vascular disease. Seminars in immunopathology 2022, 44. [Google Scholar] [CrossRef] [PubMed]
- Bonafiglia, Q.A.; Bendeck, M.; Gotlieb, A.I. Vascular Pathobiology: Atherosclerosis and Large Vessel Disease. Cardiovasc. Pathol. 2022, 265–306. [Google Scholar] [CrossRef]
- Salybekov, A.A.; Wolfien, M.; Kobayashi, S.; Steinhoff, G.; Asahara, T. Personalized Cell Therapy for Patients with Peripheral Arterial Diseases in the Context of Genetic Alterations: Artificial Intelligence-Based Responder and Non-Responder Prediction. Cells 2021, 10, 3266. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Qian, M.; Kyler, K.; Xu, J. Endothelial–Vascular Smooth Muscle Cells Interactions in Atherosclerosis. Front. Cardiovasc. Med. 2018, 5, 151. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Du, W.; Ren, L.; Hamblin, M.H.; Becker, R.C.; Chen, Y.E.; Fan, Y. Vascular Smooth Muscle Cells in Aortic Aneurysm: From Genetics to Mechanisms. J. Am. Hear. Assoc. 2021, 10, e023601. [Google Scholar] [CrossRef] [PubMed]
- Steffensen, L.B.; et al. Somatic mutations reveal clonal cell populations in atherosclerotic plaques. medRxiv 2022. [Google Scholar] [CrossRef]
- Bejar, R. CHIP, ICUS, CCUS and other four-letter words. Leukemia 2017, 31, 1869–1871. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.d.M.; et al. Clonal hematopoiesis of indeterminate potential, DNA methylation, and risk for coronary artery disease. Nature Communications 2022, 13, 5350. [Google Scholar] [CrossRef]
- DeZern, A.E.; Malcovati, L.; Ebert, B.L. CHIP, CCUS, and Other Acronyms: Definition, Implications, and Impact on Practice. American Society of Clinical Oncology Educational Book, 2019(39): p. 400-410.
- Marnell, C.S.; Bick, A.; Natarajan, P. Clonal hematopoiesis of indeterminate potential (CHIP): Linking somatic mutations, hematopoiesis, chronic inflammation and cardiovascular disease. J. Mol. Cell. Cardiol. 2021, 161, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Klarin, D.; Program, V.M.V.; Lynch, J.; Aragam, K.; Chaffin, M.; Assimes, T.L.; Huang, J.; Lee, K.M.; Shao, Q.; Huffman, J.E.; et al. Genome-wide association study of peripheral artery disease in the Million Veteran Program. Nat. Med. 2019, 25, 1274–1279. [Google Scholar] [CrossRef] [PubMed]
- Heimlich, J.B.; Bick, A.G. Somatic Mutations in Cardiovascular Disease. Circ. Res. 2022, 130, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Genovese, G.; Kähler, A.K.; Handsaker, R.E.; Lindberg, J.; Rose, S.A.; Bakhoum, S.F.; Chambert, K.; Mick, E.; Neale, B.M.; Fromer, M.; et al. Clonal Hematopoiesis and Blood-Cancer Risk Inferred from Blood DNA Sequence. N. Engl. J. Med. 2014, 371, 2477–2487. [Google Scholar] [CrossRef] [PubMed]
- Visconte, V.; Nakashima, M.O.; Rogers, H.J. Mutations in Splicing Factor Genes in Myeloid Malignancies: Significance and Impact on Clinical Features. Cancers 2019, 11, 1844. [Google Scholar] [CrossRef] [PubMed]
- Haring, B.; Wissel, S.; Manson, J.E. Somatic Mutations and Clonal Hematopoiesis as Drivers of Age-Related Cardiovascular Risk. Curr. Cardiol. Rep. 2022, 24, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Feldkamp, J.D.; Vetter, V.M.; Arends, C.M.; Lang, T.J.L.; Bullinger, L.; Damm, F.; Demuth, I.; Frick, M. Clonal hematopoiesis of indeterminate potential-related epigenetic age acceleration correlates with clonal hematopoiesis of indeterminate potential clone size in patients with high morbidity. Haematologica 2022, 107, 1703–1708. [Google Scholar] [CrossRef]
- Förstermann, U.; Sessa, W.C. Nitric oxide synthases: regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef]
- Shibuya, M. Vascular endothelial growth factor (VEGF) and its receptor (VEGFR) signaling in angiogenesis: A crucial target for anti- and pro-angiogenic therapies. Genes Cancer 2011, 2, 1097–1105. [Google Scholar] [CrossRef]
- Ocaranza, M.P.; Riquelme, J.A.; García, L.; Jalil, J.E.; Chiong, M.; Santos, R.A.S.; Lavandero, S. Counter-regulatory renin–angiotensin system in cardiovascular disease. Nat. Rev. Cardiol. 2020, 17, 116–129. [Google Scholar] [CrossRef]
- Newman, J.D.; Cornwell, M.G.; Zhou, H.; Rockman, C.; Heguy, A.; Suarez, Y.; Cheng, H.S.; Feinberg, M.W.; Hochman, J.S.; Ruggles, K.V.; et al. Gene Expression Signature in Patients With Symptomatic Peripheral Artery Disease. Arter. Thromb. Vasc. Biol. 2021, 41, 1521–1533. [Google Scholar] [CrossRef] [PubMed]
- Koller, A.; Fazzini, F.; Lamina, C.; Rantner, B.; Kollerits, B.; Stadler, M.; Klein-Weigel, P.; Fraedrich, G.; Kronenberg, F. Mitochondrial DNA copy number is associated with all-cause mortality and cardiovascular events in patients with peripheral arterial disease. J. Intern. Med. 2020, 287, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Denny, J.C.; Bastarache, L.; Ritchie, M.D.; Carroll, R.J.; Zink, R.; Mosley, J.D.; Field, J.R.; Pulley, J.M.; Ramirez, A.H.; Bowton, E.; et al. Systematic comparison of phenome-wide association study of electronic medical record data and genome-wide association study data. Nat. Biotechnol. 2013, 31, 1102–1111. [Google Scholar] [CrossRef] [PubMed]
- Zekavat, S.M.; et al. TP53-mediated clonal hematopoiesis confers increased risk for incident peripheral artery disease. medRxiv 2021. [Google Scholar] [CrossRef]
- Fuster, J.J.; MacLauchlan, S.; Zuriaga, M.A.; Polackal, M.N.; Ostriker, A.C.; Chakraborty, R.; Wu, C.-L.; Sano, S.; Muralidharan, S.; Rius, C.; et al. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science 2017, 355, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Jin, Y.; Tang, W.H.; Qin, L.; Zhang, X.; Tellides, G.; Hwa, J.; Yu, J.; Martin, K.A.; Y, X.; et al. Ten-Eleven Translocation-2 (TET2) Is a Master Regulator of Smooth Muscle Cell Plasticity. Circulation 2013, 128, 2047–2057. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Yang, Q.; Li, A.-F.; Li, R.-Q.; Wang, Z.; Liu, L.-S.; Ren, Z.; Zheng, X.-L.; Tang, X.-Q.; Li, G.-H.; et al. Tet methylcytosine dioxygenase 2 inhibits atherosclerosis via upregulation of autophagy in ApoE−/− mice. Oncotarget 2016, 7, 76423–76436. [Google Scholar] [CrossRef]
- Saini, S.K.; McDermott, M.M.; Picca, A.; Li, L.; Wohlgemuth, S.E.; Kosmac, K.; Peterson, C.A.; Tian, L.; Ferrucci, L.; Guralnik, J.M.; et al. Mitochondrial DNA damage in calf skeletal muscle and walking performance in people with peripheral artery disease. Free. Radic. Biol. Med. 2020, 160, 680–689. [Google Scholar] [CrossRef]
- Sirbu, B.M.; Cortez, D. DNA Damage Response: Three Levels of DNA Repair Regulation. Cold Spring Harb. Perspect. Biol. 2013, 5, a012724. [Google Scholar] [CrossRef]
- Daiber, A.; Di Lisa, F.; Oelze, M.; Kröller-Schön, S.; Steven, S.; Schulz, E.; Münzel, T. Crosstalk of mitochondria with NADPH oxidase via reactive oxygen and nitrogen species signalling and its role for vascular function. Br. J. Pharmacol. 2016, 174, 1670–1689. [Google Scholar] [CrossRef]
- Kay, J.; Thadhani, E.; Samson, L.; Engelward, B. Inflammation-induced DNA damage, mutations and cancer. DNA Repair 2019, 83, 102673. [Google Scholar] [CrossRef] [PubMed]
- Bick, A.G.; Pirruccello, J.P.; Griffin, G.K.; Gupta, N.; Gabriel, S.; Saleheen, D.; Libby, P.; Kathiresan, S.; Natarajan, P. Genetic Interleukin 6 Signaling Deficiency Attenuates Cardiovascular Risk in Clonal Hematopoiesis. Circulation 2020, 141, 124–131. [Google Scholar] [CrossRef] [PubMed]
- A comprehensive 1000 Genomes–based genome-wide association meta-analysis of coronary artery disease. Nature genetics 2015, 47, 1121–1130. [CrossRef] [PubMed]
- Sibon, I.; Coupry, I.; Menegon, P.; Bouchet, J.-P.; Gorry, P.; Burgelin, I.; Calvas, P.; Orignac, I.; Dousset, V.; Lacombe, D.; et al. COL4A1 mutation in Axenfeld-Rieger anomaly with leukoencephalopathy and stroke. Ann. Neurol. 2007, 62, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; An, H.; Lim, J.; Park, I.S.; Kim, M.H.; Kim, J.H.; Kim, S.W.; Koh, Y.I.; Lee, E.Y.; Cheon, J.H. Interplay between chronic inflammation and clonal haematopoiesis of indeterminate potential in Behçet’s disease. Thromb. Haemost. 2023, 25, 33. [Google Scholar] [CrossRef]
- Li, J.; Wang, C.; Liu, J.; Yu, Y.; Liu, Y.; Peng, Q.; Liu, H.; Guan, X. A feedback loop: Interactions between Inflammatory Signals and Clonal Hematopoiesis in Cardiovascular Disease. Mol. Biol. Rep. 2021, 48, 3785–3798. [Google Scholar] [CrossRef]
- Golledge, J.; Biros, E.; Bingley, J.; Iyer, V.; Krishna, S.M. Epigenetics and Peripheral Artery Disease. Curr. Atheroscler. Rep. 2016, 18, 15. [Google Scholar] [CrossRef]
- Krishna, S.M.; Trollope, A.F.; Golledge, J. The relevance of epigenetics to occlusive cerebral and peripheral arterial disease. Clin. Sci. 2015, 128, 537–558. [Google Scholar] [CrossRef]
- Zekavat, S.M.; Viana-Huete, V.; Matesanz, N.; Jorshery, S.D.; Zuriaga, M.A.; Uddin, M.; Trinder, M.; Paruchuri, K.; Zorita, V.; Ferrer-Pérez, A.; et al. TP53-mediated clonal hematopoiesis confers increased risk for incident atherosclerotic disease. Nat. Cardiovasc. Res. 2023, 2, 144–158. [Google Scholar] [CrossRef]
- Bick, A.G.; Weinstock, J.S.; Nandakumar, S.K.; Fulco, C.P.; Bao, E.L.; Zekavat, S.M.; Szeto, M.D.; Liao, X.; Leventhal, M.J.; Nasser, J.; et al. Inherited causes of clonal haematopoiesis in 97,691 whole genomes. Nature 2020, 586, 763–768. [Google Scholar] [CrossRef]
- Jaiswal, S.; Fontanillas, P.; Flannick, J.; Manning, A.; Grauman, P.V.; Mar, B.G.; Lindsley, R.C.; Mermel, C.H.; Burtt, N.; Chavez, A.; et al. Age-Related Clonal Hematopoiesis Associated with Adverse Outcomes. N. Engl. J. Med. 2014, 371, 2488–2498. [Google Scholar] [CrossRef] [PubMed]
- AH, C.; et al. Tet2 Restrains Inflammatory Gene Expression in Macrophages. Experimental hematology 2017, 55. [Google Scholar]
- SO, A.; et al. An Inflammatory Environment Containing TNFα Favors Tet2-mutant Clonal Hematopoiesis. Experimental hematology 2018, 59. [Google Scholar]
- Rivero, G.A.; Perli, E.; Moreno, S.; Salemi, J.L. Excess in Atherosclerotic and Inflammametabolic Diseases Are Differentially Expressed in Myelodysplasia and Are Highly Dependent on Age, R-IPSS and Ethnicity. Blood 2018, 132, 4855–4855. [Google Scholar] [CrossRef]
- Büttner, P.; Böttner, J.; Krohn, K.; Baber, R.; Platzbecker, U.; Cross, M.; Desch, S.; Thiele, H.; Steiner, S.; Scheinert, D.; et al. Clonal Hematopoiesis Mutations Are Present in Atherosclerotic Lesions in Peripheral Artery Disease. Int. J. Mol. Sci. 2023, 24, 3962. [Google Scholar] [CrossRef] [PubMed]
- Steffensen, L.B.; et al. Somatic mutations reveal clonal cell populations in atherosclerotic plaques. medRxiv 2022. [Google Scholar] [CrossRef]
- A, B.; et al. Association Between Large Detectable Clonal Mosaicism and Type 2 Diabetes With Vascular Complications. Nature genetics 2013, 45. [Google Scholar]
- Albiero, M.; Poncina, N.; Tjwa, M.; Ciciliot, S.; Menegazzo, L.; Ceolotto, G.; de Kreutzenberg, S.V.; Moura, R.; Giorgio, M.; Pelicci, P.; et al. Diabetes Causes Bone Marrow Autonomic Neuropathy and Impairs Stem Cell Mobilization via Dysregulated p66Shc and Sirt1. Diabetes 2014, 63, 1353–1365. [Google Scholar] [CrossRef]
- Fadini, G.P.; Ciciliot, S.; Albiero, M. Concise Review: Perspectives and Clinical Implications of Bone Marrow and Circulating Stem Cell Defects in Diabetes. STEM CELLS 2016, 35, 106–116. [Google Scholar] [CrossRef]
- Kojima, H.; Kim, J.; Chan, L. Emerging roles of hematopoietic cells in the pathobiology of diabetic complications. Trends Endocrinol. Metab. 2014, 25, 178–187. [Google Scholar] [CrossRef]
- Orlandi, A.; Chavakis, E.; Seeger, F.; Tjwa, M.; Zeiher, A.M.; Dimmeler, S. Long-term diabetes impairs repopulation of hematopoietic progenitor cells and dysregulates the cytokine expression in the bone marrow microenvironment in mice. Basic Res. Cardiol. 2010, 105, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Issa, J.-P.; A, H.; D, R.; CS, M.; S, Z.; Ff, H.; Jm, G.; D, C.; Y, I.; Mj, S.; et al. Faculty Opinions recommendation of Increased stem cell proliferation in atherosclerosis accelerates clonal hematopoiesis. Cell 2021, 184, 1348–1361. [Google Scholar] [CrossRef]
- Dezfulian, C.; N, S.; Cb, R.; Ah, S.; Y, G.; Ma, A.; T, R.; Js, B. Faculty Opinions recommendation of Association between advanced age and vascular disease in different arterial territories: a population database of over 3. 6 million subjects. Journal of the American College of Cardiology 2017, 61. [Google Scholar] [CrossRef]
- Fuster, J.J.; Walsh, K. Somatic mutations and clonal hematopoiesis: unexpected potential new drivers of age-related cardiovascular disease. Circ Res. 2018, 122, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, S.; et al. Age-Related Clonal Hematopoiesis Associated with Adverse Outcomes. N. Engl. J. Med. 2014, 371, 2488–2498. [Google Scholar] [CrossRef] [PubMed]
- Shu, J.; Santulli, G. Update on peripheral artery disease: Epidemiology and evidence-based facts. Atherosclerosis 2018, 275, 379–381. [Google Scholar] [CrossRef]
- Jaiswal, S.; Natarajan, P.; Silver, A.J.; Gibson, C.J.; Bick, A.G.; Shvartz, E.; McConkey, M.; Gupta, N.; Gabriel, S.; Ardissino, D.; et al. Clonal Hematopoiesis and Risk of Atherosclerotic Cardiovascular Disease. N. Engl. J. Med. 2017, 377, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Fuster, J.J.; et al. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science 2017, 355, 842–847. [Google Scholar] [CrossRef]
- Shah, A.D.; Langenberg, C.; Rapsomaniki, E.; Denaxas, S.; Pujades-Rodriguez, M.; Gale, C.P.; Deanfield, J.; Smeeth, L.; Timmis, A.; Hemingway, H. Type 2 diabetes and incidence of cardiovascular diseases: a cohort study in 1·9 million people. Lancet Diabetes Endocrinol. 2015, 3, 105–113. [Google Scholar] [CrossRef]
- Brevetti, G.; Giugliano, G.; Brevetti, L.; Hiatt, W.R.; W, H.; E, A.; C, L.; E, B.; R, R.; F, F.; et al. Inflammation in Peripheral Artery Disease. Circulation 2010, 122, 1862–1875. [Google Scholar] [CrossRef]
- Kay, J.; Thadhani, E.; Samson, L.; Engelward, B. Inflammation-induced DNA damage, mutations and cancer. DNA Repair 2019, 83, 102673. [Google Scholar] [CrossRef] [PubMed]
- Desai, U.; Kharat, A.; Hess, C.N.; Milentijevic, D.; Laliberté, F.; Zuckerman, P.; Benson, J.; Lefebvre, P.; Hiatt, W.R.; Bonaca, M.P. Incidence of Major Atherothrombotic Vascular Events among Patients with Peripheral Artery Disease after Revascularization. Ann. Vasc. Surg. 2021, 75, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Kaizer, H.; Naik, R.P.; Lobner, K.; Moliterno, A.R. JAK2 V617F Prevalence Study: Associations in the General Population and Vascular Disease Populations. Blood 2020, 136, 36–36. [Google Scholar] [CrossRef]
- Michael, H.; Criqui, V.A. Epidemiology of Peripheral Artery Disease | Circulation Research. Circulation Research 2015, 116. [Google Scholar]
- Yoshida, K.; Gowers, K.H.C.; Lee-Six, H.; Chandrasekharan, D.P.; Coorens, T.; Maughan, E.F.; Beal, K.; Menzies, A.; Millar, F.R.; Anderson, E.; et al. Tobacco smoking and somatic mutations in human bronchial epithelium. Nature 2020, 578, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Kourie, H.R.; Ameye, L.; Paesmans, M.; Bron, D. Re: Prognostic value of CALR vs. JAK2V617F mutations on splenomegaly, leukemic transformation, thrombosis, and overall survival in patients with primary fibrosis: a meta-analysis. Ann. Hematol. 2016, 95, 2105–2106. [Google Scholar] [CrossRef] [PubMed]
- A, M.; et al. Occurrence of the JAK2 V617F mutation in patients with peripheral arterial disease. American journal of hematology 2015, 90. [Google Scholar]
- F, P.; et al. Roles of JAK2 in Aging, Inflammation, Hematopoiesis and Malignant Transformation. Cells 2019, 8. [Google Scholar]
- Megan E Cosgrove, R.S.; Harrison, H.J.; Jackson, G.E.; Howard, M.R.; Hitchcock, I.S. Endothelial JAK2V617F Expression Drives Inflammation and Cellular Senescence; New Evidence for the Roles of Endothelial Cells in MPN-Related Clotting Abnormalities? Blood 2016, 128. [Google Scholar]
- Barbui, T.; Finazzi, G.; Falanga, A. Myeloproliferative neoplasms and thrombosis. Blood 2013, 122, 2176–2184. [Google Scholar] [CrossRef]
- Veninga, A.; De Simone, I.; Heemskerk, J.W.; Cate, H.T.; van der Meijden, P.E. Clonal hematopoietic mutations linked to platelet traits and the risk of thrombosis or bleeding. Haematologica 2020, 105, 2020–2031. [Google Scholar] [CrossRef] [PubMed]
- Misaka, T.; Kimishima, Y.; Yokokawa, T.; Ikeda, K.; Takeishi, Y. Clonal hematopoiesis and cardiovascular diseases: role of JAK2V617F. J. Cardiol. 2022, 81, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Cook, E.K.; Izukawa, T.; Young, S.; Rosen, G.; Jamali, M.; Zhang, L.; Johnson, D.; Bain, E.; Hilland, J.; Ferrone, C.K.; et al. Comorbid and inflammatory characteristics of genetic subtypes of clonal hematopoiesis. Blood Adv. 2019, 3, 2482–2486. [Google Scholar] [CrossRef] [PubMed]
- Mas-Peiro, S.; Hoffmann, J.; Fichtlscherer, S.; Dorsheimer, L.; A Rieger, M.; Dimmeler, S.; Vasa-Nicotera, M.; Zeiher, A.M. Clonal haematopoiesis in patients with degenerative aortic valve stenosis undergoing transcatheter aortic valve implantation. Eur. Heart J. 2020, 41, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Sano, S.; Oshima, K.; Wang, Y.; Katanasaka, Y.; Sano, M.; Walsh, K. CRISPR-Mediated Gene Editing to Assess the Roles of Tet2 and Dnmt3a in Clonal Hematopoiesis and Cardiovascular Disease. Circ. Res. 2018, 123, 335–341. [Google Scholar] [CrossRef]
- Abplanalp, W.T.; Mas-Peiro, S.; Cremer, S.; John, D.; Dimmeler, S.; Zeiher, A.M. Association of Clonal Hematopoiesis of Indeterminate Potential with Inflammatory Gene Expression in Patients with Severe Degenerative Aortic Valve Stenosis or Chronic Postischemic Heart Failure. JAMA Cardiol. 2020, 5, 1170–1175. [Google Scholar] [CrossRef]
- Abplanalp, W.T.; Cremer, S.; John, D.; Hoffmann, J.; Schuhmacher, B.; Merten, M.; Rieger, M.A.; Vasa-Nicotera, M.; Zeiher, A.M.; Dimmeler, S. Clonal Hematopoiesis–Driver DNMT3A Mutations Alter Immune Cells in Heart Failure. Circ. Res. 2021, 128, 216–228. [Google Scholar] [CrossRef]
- Ito, S.; Shen, L.; Dai, Q.; Wu, S.C.; Collins, L.B.; Swenberg, J.A.; He, C.; Zhang, Y. Tet Proteins Can Convert 5-Methylcytosine to 5-Formylcytosine and 5-Carboxylcytosine. Science 2011, 333, 1300–1303. [Google Scholar] [CrossRef]
- Cull, A.H.; Snetsinger, B.; Buckstein, R.; Wells, R.A.; Rauh, M.J. Tet2 restrains inflammatory gene expression in macrophages. Exp. Hematol. 2017, 55, 56–70. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, K.; Shen, Q.; Han, Y.; Gu, Y.; Li, X.; Zhao, D.; Liu, Y.; Wang, C.; Zhang, X.; et al. Tet2 is required to resolve inflammation by recruiting Hdac2 to specifically repress IL-6. Nature 2015, 525, 389–393. [Google Scholar] [CrossRef]
- Jaiswal, S.; et al. Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. New England Journal of Medicine 2017, 377, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Leoni, C.; Montagner, S.; Rinaldi, A.; Bertoni, F.; Polletti, S.; Balestrieri, C.; Monticelli, S. Dnmt3a restrains mast cell inflammatory responses. Proc. Natl. Acad. Sci. 2017, 114, E1490–E1499. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhou, S.; Wang, C.; Huang, Y.; Li, H.; Wang, Y.; Zhu, Z.; Tang, J.; Yan, M. Epigenetic modifications of interleukin-6 in synovial fibroblasts from osteoarthritis patients. Sci. Rep. 2017, 7, 43592. [Google Scholar] [CrossRef]
- O'Shea, J.J.; Kontzias, A.; Yamaoka, K.; Tanaka, Y.; Laurence, A. Janus kinase inhibitors in autoimmune diseases. Rheumatol. 2013, 72, ii111–ii115. [Google Scholar] [CrossRef]
- Levine, R.L.; Wadleigh, M.; Cools, J.; Ebert, B.L.; Wernig, G.; Huntly, B.J.; Boggon, T.J.; Wlodarska, I.; Clark, J.J.; Moore, S.; et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell 2005, 7, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Sidon, P.; El Housni, H.; Dessars, B.; Heimann, P. The JAK2V617F mutation is detectable at very low level in peripheral blood of healthy donors. Leukemia 2006, 20, 1622–1622. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, C.; Birgens, H.S.; Nordestgaard, B.G.; Kjaer, L.; Bojesen, S.E. The JAK2 V617F somatic mutation, mortality and cancer risk in the general population. Haematologica 2010, 96, 450–453. [Google Scholar] [CrossRef]
- Dorsheimer, L.; Assmus, B.; Rasper, T.; Ortmann, C.A.; Ecke, A.; Abou-El-Ardat, K.; Schmid, T.; Brüne, B.; Wagner, S.; Serve, H.; et al. Association of Mutations Contributing to Clonal Hematopoiesis With Prognosis in Chronic Ischemic Heart Failure. JAMA Cardiol. 2019, 4, 25–33. [Google Scholar] [CrossRef]
- Mooney, L.; Goodyear, C.S.; Chandra, T.; Kirschner, K.; Copland, M.; Petrie, M.C.; Lang, N.N. Clonal haematopoiesis of indeterminate potential: intersections between inflammation, vascular disease and heart failure. Clin. Sci. 2021, 135, 991–1007. [Google Scholar] [CrossRef]
- Varela, C.; De Haro, J.; Bleda, S.; Ferruelo, A.; Acin, F. Serum of peripheral arterial disease patients with poor flow-mediated-arterial-dilation values triggers a genomic over-expression of toll-like-receptor 4 by endothelial cells. Atherosclerosis 2014, 235, e42. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Chen, Y.; Wang, Y.; Zhang, S.; Liu, P.; Chen, Z.; Song, P.; Luo, L.; Luo, Y.; et al. Corilagin Ameliorates Atherosclerosis in Peripheral Artery Disease via the Toll-Like Receptor-4 Signaling Pathway in vitro and in vivo. Front. Immunol. 2020, 11, 1611. [Google Scholar] [CrossRef]
- Hu, Y.; et al. Correlation of mimecan with nuclear factor kappa B and P53 in peripheral arterial disease and peripheral arterial disease combined with type 2 diabetes in the elderly. Chinese Journal of Geriatrics 2014, 26–28. [Google Scholar]
- Gaetani, E.; Flex, A.; Pola, R.; Papaleo, P.; De Martini, D.; Pola, E.; Aloi, F.; Flore, R.; Serricchio, M.; Gasbarrini, A.; et al. The K469E polymorphism of the ICAM-1 gene is a risk factor for peripheral arterial occlusive disease. Blood Coagul. Fibrinolysis 2002, 13, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Flex, A.; Gaetani, E.; Angelini, F.; Sabusco, A.; Chillà, C.; Straface, G.; Biscetti, F.; Pola, P.; Castellot, J.J.; Pola, R. Pro-inflammatory genetic profiles in subjects with peripheral arterial occlusive disease and critical limb ischemia. J. Intern. Med. 2007, 262, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Alleboina, S.; Singh, M.V.; Wong, T.; Dokun, A. OR14-06 Inhibition of Protein Kinase C-beta2 Phosphorylation Restores Nuclear Factor-Kappa B Activation and Improves Peripheral Arterial Disease in Diabetes. J. Endocr. Soc. 2020, 4, OR14–06. [Google Scholar] [CrossRef]
- Golledge, J. Update on the pathophysiology and medical treatment of peripheral artery disease. Nat. Rev. Cardiol. 2022, 19, 456–474. [Google Scholar] [CrossRef]
- Ott, N.; Faletti, L.; Heeg, M.; Andreani, V.; Grimbacher, B. JAKs and STATs from a Clinical Perspective: Loss-of-Function Mutations, Gain-of-Function Mutations, and Their Multidimensional Consequences. J. Clin. Immunol. 2023, 43, 1326–1359. [Google Scholar] [CrossRef]
- Mathur, S.; Sutton, J. Personalized medicine could transform healthcare. Biomed. Rep. 2017, 7, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Poredoš, P.; Šabovič, M.; Mijovski, M.B.; Nikolajević, J.; Antignani, P.L.; Paraskevas, K.I.; Mikhailidis, D.P.; Blinc, A. Inflammatory and Prothrombotic Biomarkers, DNA Polymorphisms, MicroRNAs and Personalized Medicine for Patients with Peripheral Arterial Disease. Int. J. Mol. Sci. 2022, 23, 12054. [Google Scholar] [CrossRef] [PubMed]
- Smith, F.B.; Connor, J.; Lee, A.J.; Cooke, A.; Lowe, G.D.; Rumley, A.; Fowkes, F. Relationship of the platelet glycoprotein PlA and fibrinogen T/G+1689 polymorphisms with peripheral arterial disease and ischaemic heart disease. Thromb. Res. 2003, 112, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Reny, J.L.; et al. The factor II G20210A gene polymorphism, but not factor V Arg506Gln, is associated with peripheral arterial disease: results of a case–control study. Journal of Thrombosis and Haemostasis 2004, 2, 1334–1340. [Google Scholar] [CrossRef] [PubMed]
- Flex, A.; Gaetani, E.; Pola, R.; Santoliquido, A.; Aloi, F.; Papaleo, P.; Lago, A.D.; Pola, E.; Serricchio, M.; Tondi, P.; et al. The −174 G/C Polymorphism of the Interleukin-6 Gene Promoter is Associated with Peripheral Artery Occlusive Disease. Eur. J. Vasc. Endovasc. Surg. 2002, 24, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Aquila, G.; Fortini, C.; Pannuti, A.; Delbue, S.; Pannella, M.; Morelli, M.B.; Caliceti, C.; Castriota, F.; de Mattei, M.; Ongaro, A.; et al. Distinct gene expression profiles associated with Notch ligands Delta-like 4 and Jagged1 in plaque material from peripheral artery disease patients: a pilot study. J. Transl. Med. 2017, 15, 1–14. [Google Scholar] [CrossRef]
- Wassel, C.L.; Lamina, C.; Nambi, V.; Coassin, S.; Mukamal, K.J.; Ganesh, S.K.; Jacobs, D.R.; Franceschini, N.; Papanicolaou, G.J.; Gibson, Q.; et al. Genetic determinants of the ankle-brachial index: A meta-analysis of a cardiovascular candidate gene 50K SNP panel in the candidate gene association resource (CARe) consortium. Atherosclerosis 2012, 222, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Božič-Mijovski, M.; Bedenčič, M.; Stegnar, M.; Salapura, V.; Ježovnik, M.; Kozak, M.; Blinc, A. Nurr1 Haplotypes are Associated with Femoropopliteal Restenosis/Re-occlusion after Percutaneous Transluminal Angioplasty. Eur. J. Vasc. Endovasc. Surg. 2012, 43, 337–338. [Google Scholar] [CrossRef] [PubMed]
- Boc, V.; Mijovski, M.B.; Perme, M.P.; Blinc, A. Diabetes and smoking are more important for prognosis of patients with peripheral arterial disease than some genetic polymorphisms. Vasa 2019, 48, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Perner, F.; Ernst, T.; Heidel, F.H. Roles of JAK2 in Aging, Inflammation, Hematopoiesis and Malignant Transformation. Cells 2019, 8, 854. [Google Scholar] [CrossRef]
- Cosgrove, M.E.; et al. Endothelial JAK2V617F Expression Drives Inflammation and Cellular Senescence; New Evidence for the Roles of Endothelial Cells in MPN-Related Clotting Abnormalities? Blood 2016, 128, 3134. [Google Scholar] [CrossRef]
- Sano, S.; Oshima, K.; Wang, Y.; MacLauchlan, S.; Katanasaka, Y.; Sano, M.; Zuriaga, M.A.; Yoshiyama, M.; Goukassian, D.; Cooper, M.A.; et al. Tet2-Mediated Clonal Hematopoiesis Accelerates Heart Failure Through a Mechanism Involving the IL-1β/NLRP3 Inflammasome. J. Am. Coll. Cardiol. 2018, 71, 875–886. [Google Scholar] [CrossRef]
- Min, K.; Polizio, A.H.; Kour, A.; Thel, M.C.; Walsh, K. Experimental ASXL1 -Mediated Clonal Hematopoiesis Promotes Inflammation and Accelerates Heart Failure. J. Am. Hear. Assoc. 2022, 11, e026154. [Google Scholar] [CrossRef] [PubMed]
- Svensson, E.C.; et al. TET2-driven clonal hematopoiesis and response to canakinumab: an exploratory analysis of the CANTOS randomized clinical trial. JAMA cardiology 2022, 7, 521–528. [Google Scholar] [CrossRef] [PubMed]
| PAD | Shared points | Somatic mutation |
|---|---|---|
| The risk of PAD markedly increases with age. Prevalence of PAD among individuals aged 80–100 years is 22 to 33% [54] | Age | somatic mutations generally increase by around 2-6 mutations per cell division. Somatic mutation incidence increases in the ageing population by 10 to 20 % at the age of 70 years [55,56]. |
| Atherosclerosis incidence among PAD cases is more than 90% [57] | Atherosclerosis | Atherosclerosis patients is sufficient to produce a 3.5-fold increased risk of clonal hematopoiesis by age 70 [53]. Presence of somatic mutation in TET2 increases atherosclerotic lesions of vessels by 3- to 4-fold [58,59]. |
| Diabetic patients have 2- to 4-fold increased risk of developing PAD, CAD, and ischemic stroke [57] | Diabetes mellitus | Clonal mosaic event carriers with T2DM were associated with 2-fold increased prevalence of vascular complications [48,60]. |
| Approximately 70-80% of PAD patients exhibited elevated levels of inflammatory markers, suggesting a high incidence of inflammation in this population [61]. | Inflammation | Inflammation, by generating reactive oxygen and nitrogen species that damage DNA, contributes to mutagenesis and can result in a 2- to 4-fold higher accumulation of somatic mutations compared to non-inflammatory conditions [62]. |
| 15-20% of PAD patients experienced a thrombotic event over a five-year follow-up period [63]. | Thrombosis | The presence of JAK2 V617F mutation in PAD patients increased the risk of thrombosis by 3.1% [64]. |
| Smoking is the most common risk factor for PAD occurrence with a population attributable fraction of 44% [57,65]. | Tobacco smoking | Tobacco smoking dramatically increase the occurrence of somatic mutation from 1,000 to 10,000 mutations per cell [66]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





