Submitted:
17 July 2023
Posted:
19 July 2023
You are already at the latest version
Abstract
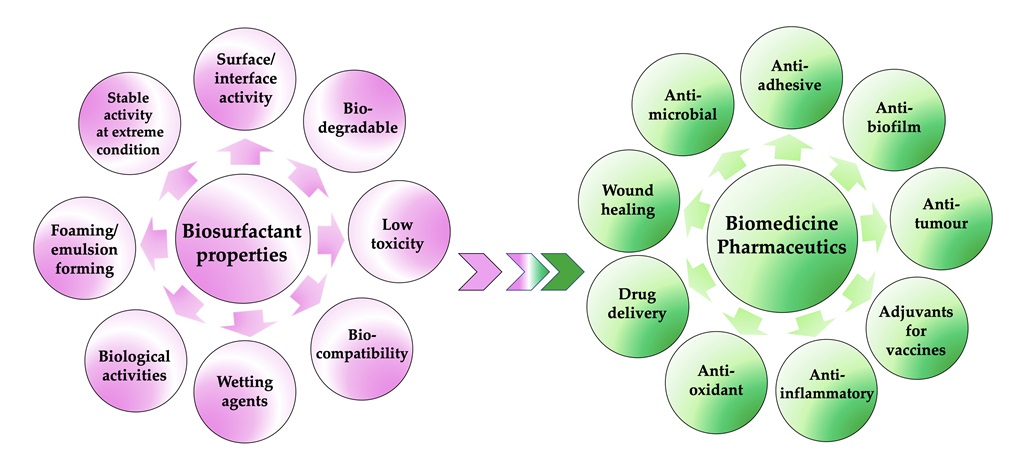
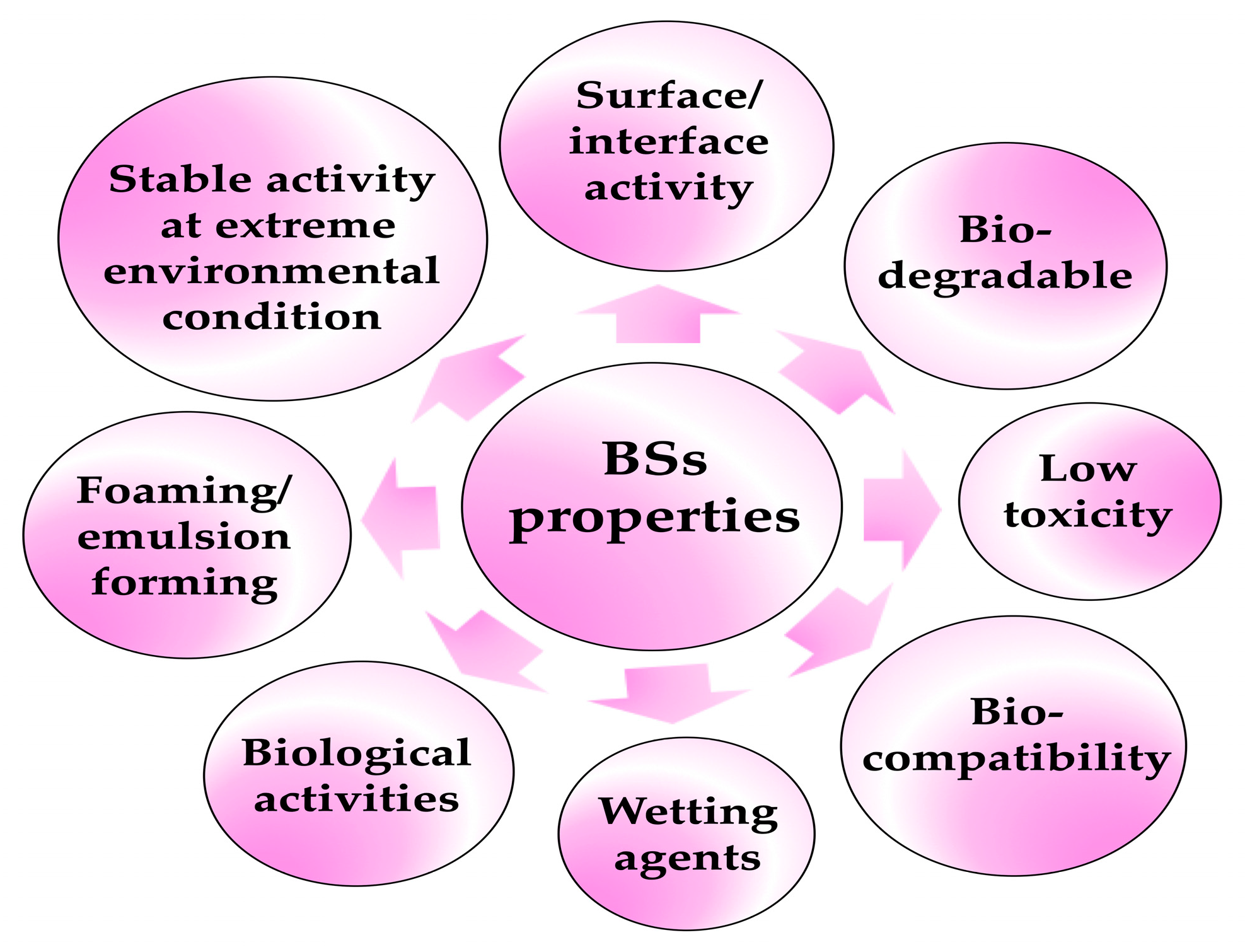
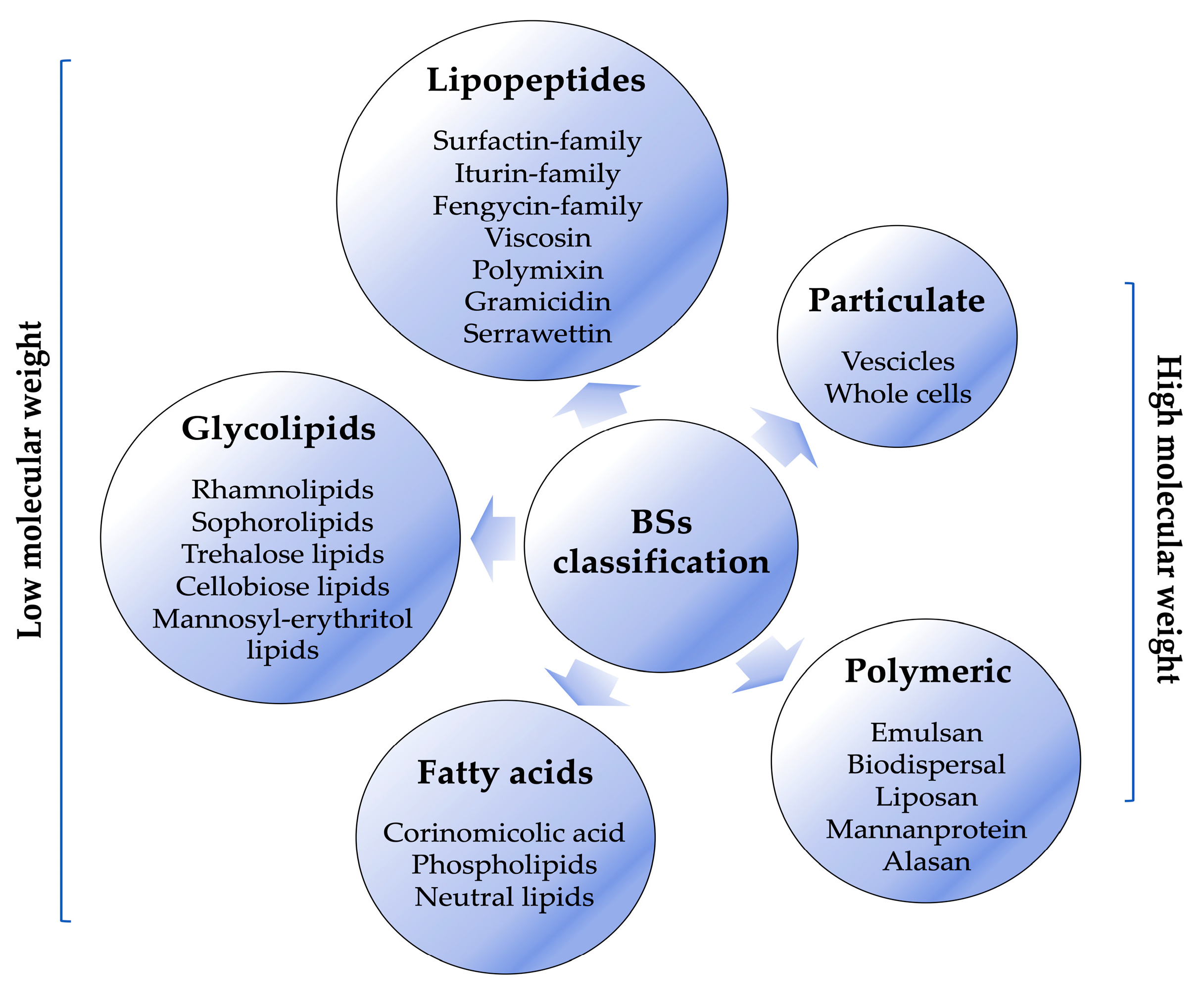
Keywords:
1. Introduction
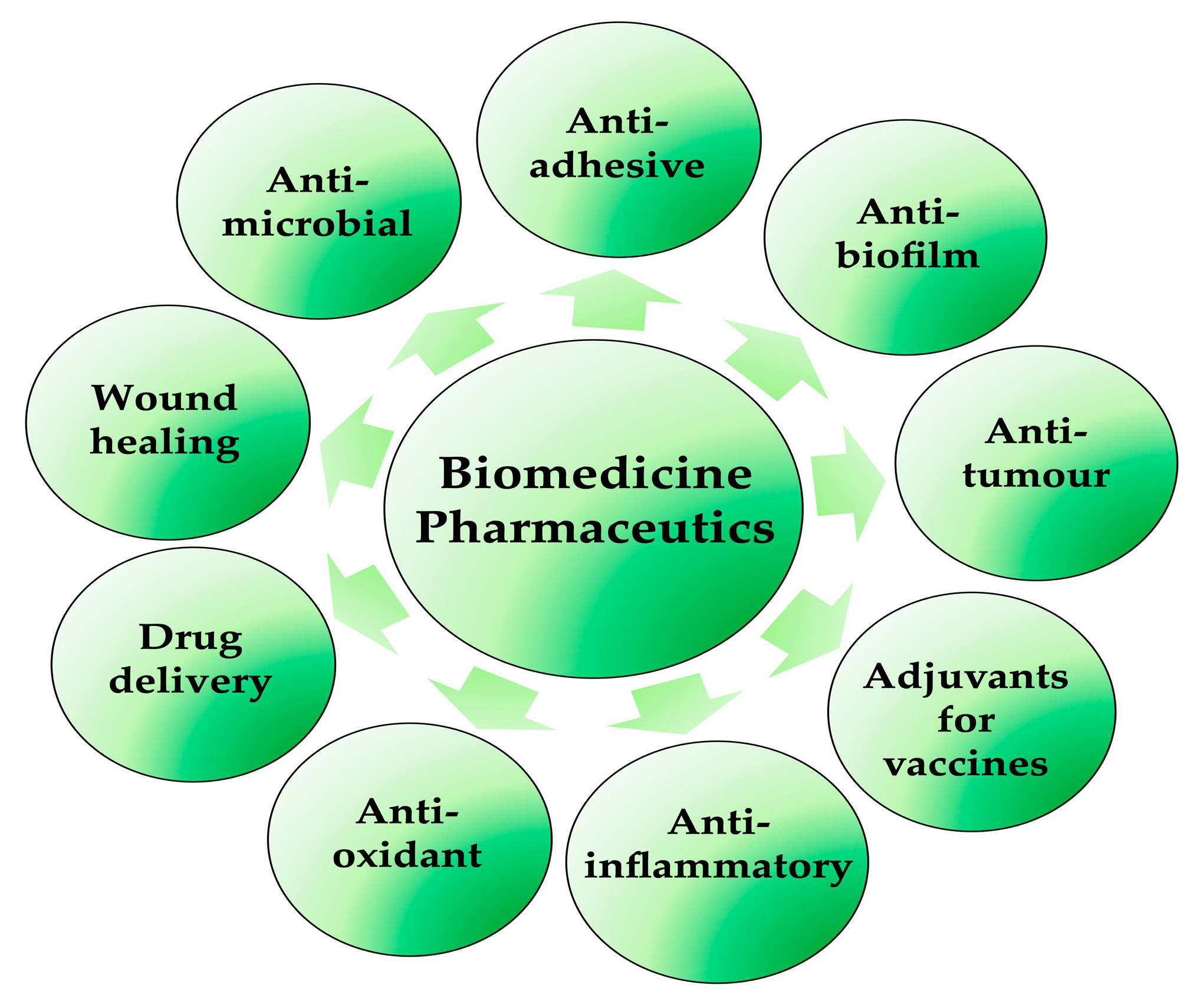
2. Biosurfactants: A Real Prospect for biomedical and pharmaceutical use?
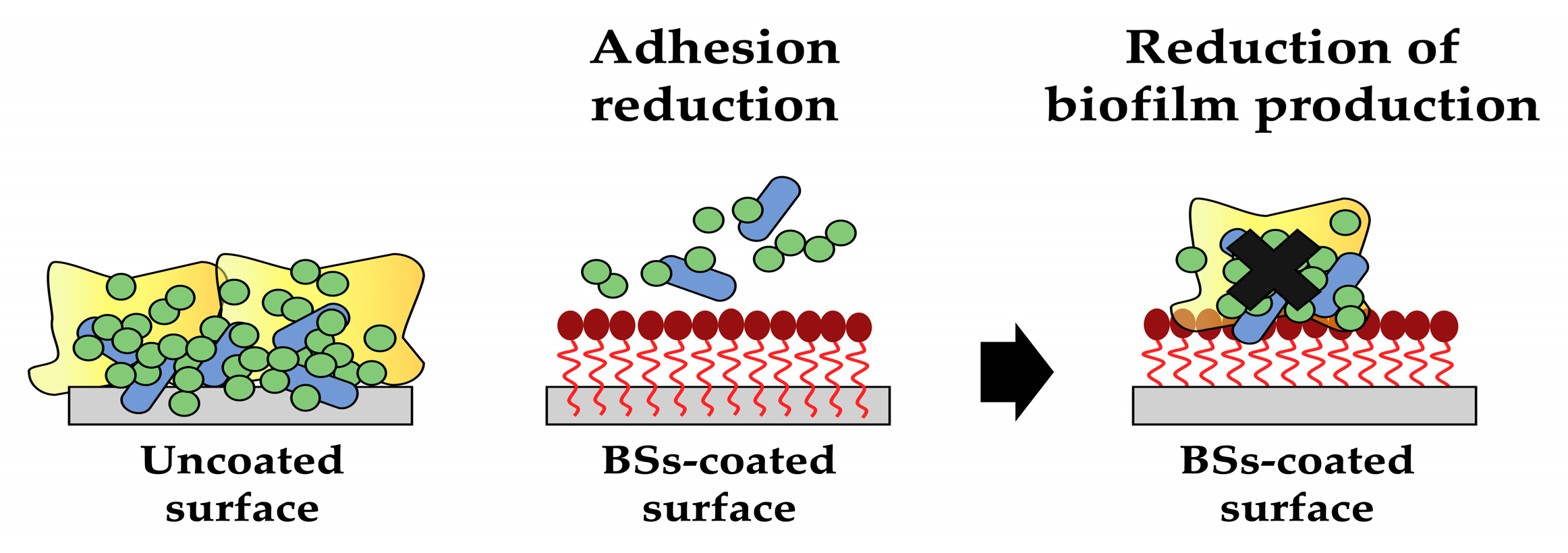
3. Biosurfactants for innovative coatings
4. Biosurfactants as Biological Control Agents
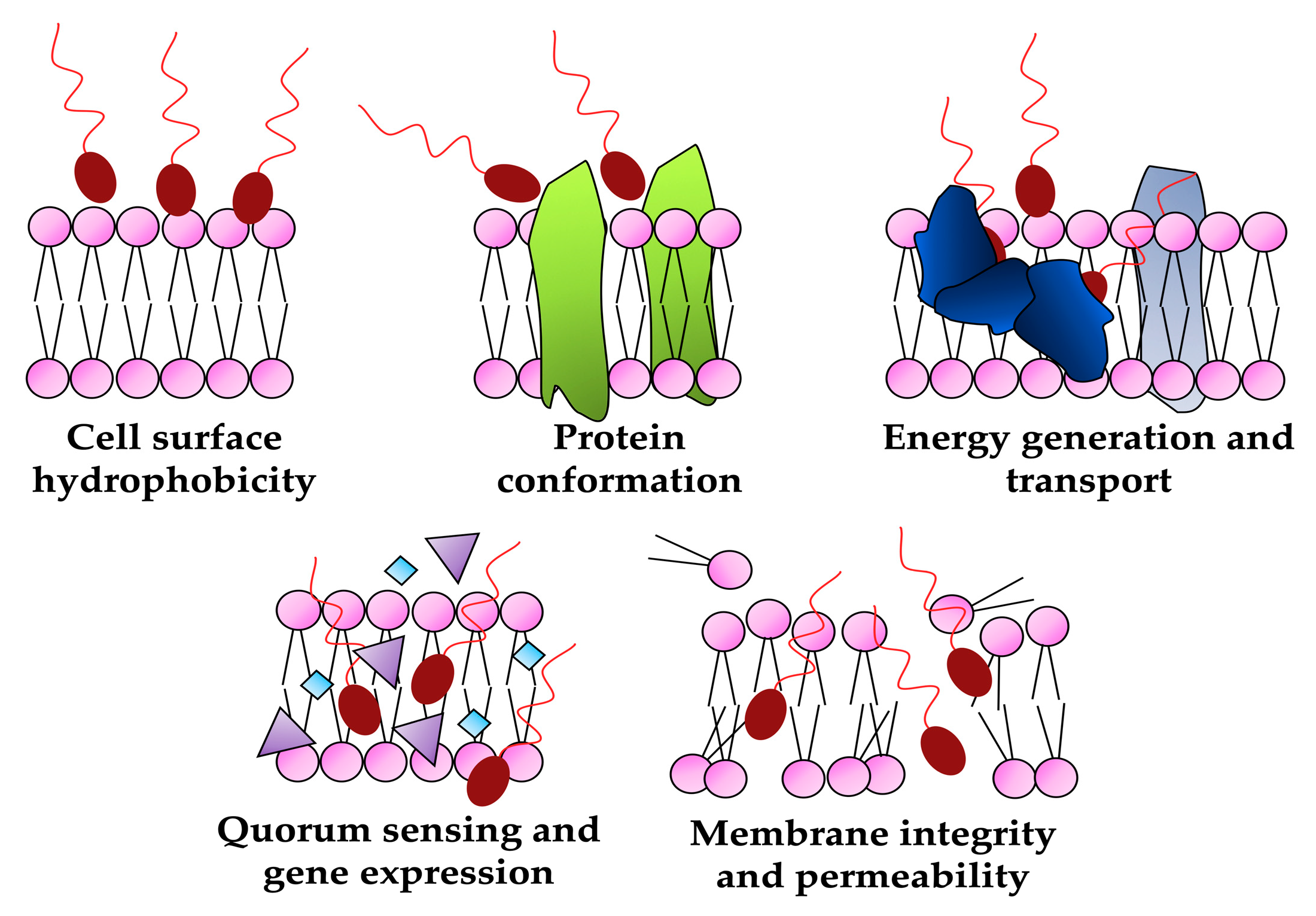
4.1. Biosurfactants as antimicrobials
4.1.1. Marine microorganisms
4.1.2. Probiotic Lactic Acid Bacteria
4.1.3. Other BSs producers
4.2. Antiviral
SARS-CoV-2 and biosurfactants
5. Perspectives of biosurfactants for wound healing, anticancer and immuno-modulatory applications
5.1. Wound healing
5.2. Anticancer agents
5.3. Immuno-modulatory agents
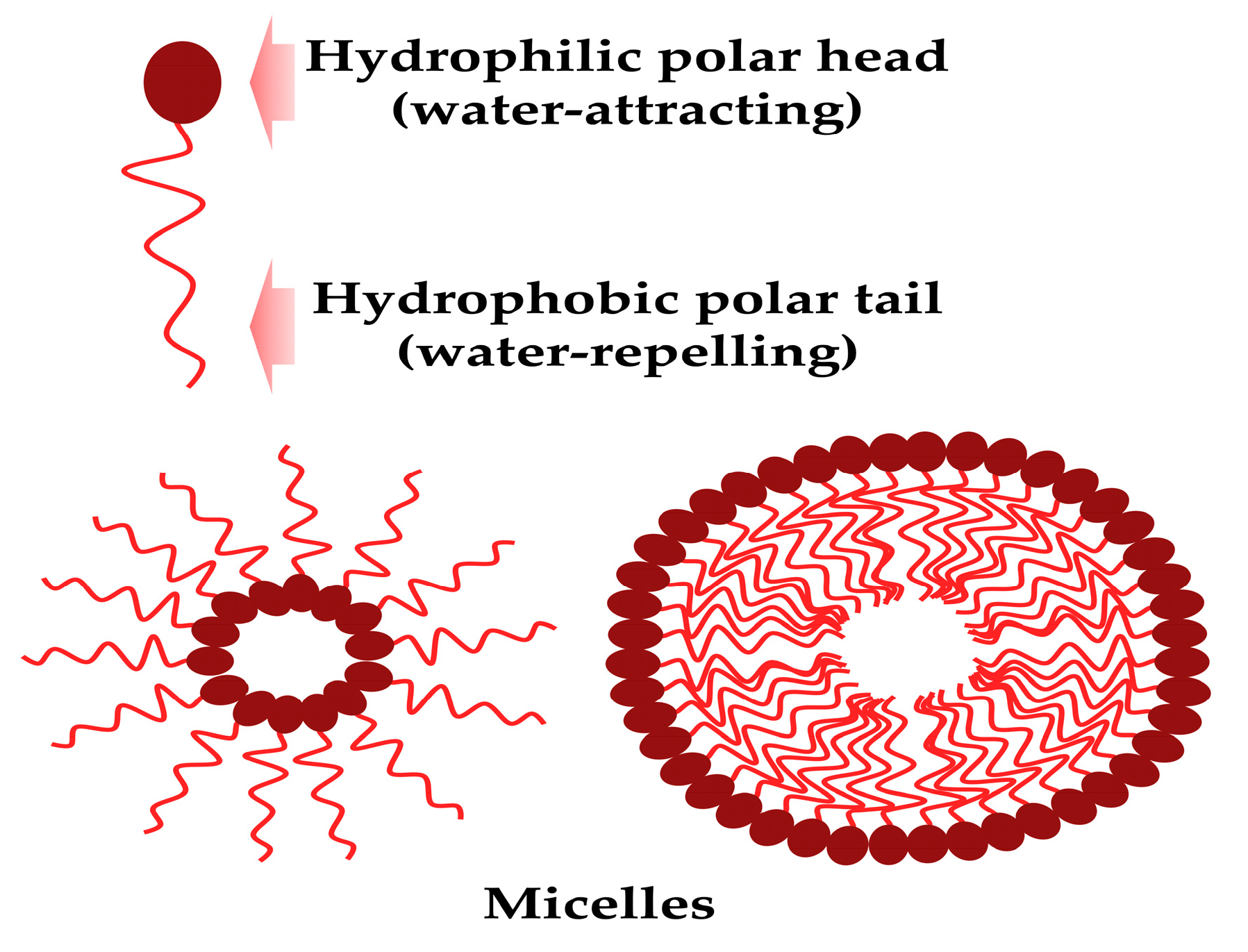
6. Utilizing biosurfactants as adjuvants in medicine: drug delivery systems
6.1. Nanoparticles
6.1.1. Nanoparticles with antibacterial activity
6.1.2. Nanoparticles for drug delivery
6.2. Microemulsions and nanoemulsions
6.3. Liposomes
7. Patents in the biomedical and pharmaceutical fields incorporating biosurfactants
8. Conclusions and future perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Monciardini, P.; Iorio, M.; Maffioli, S.; Sosio, M.; Donadio, S. Discovering new bioactive molecules from microbial sources. Microb Biotechnol. 2014, 7, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, Z.; Wang, Y.; Li, Y. Natural Bioactive Molecules as Potential Agents Against SARS-CoV-2. Front Pharmacol. 2021, 12, 702472. [Google Scholar] [CrossRef]
- Karthikeyan, A.; Joseph, A.; Nair, B.G. Promising bioactive compounds from the marine environment and their potential effects on various diseases. J Genet Eng Biotechnol. 2022, 20, 14. [Google Scholar] [CrossRef]
- Theodoridis, S.D; rakou, E.G.; Hickler, T.; Thines, M.; Nogues-Bravo, D. Evaluating natural medicinal resources and their exposure to global change. Lancet Planet Health. 2023, 7, e155–e163. [Google Scholar] [CrossRef]
- Shekhar, S.; Sundaramanickam, A.; Balasubramanian, T. Biosurfactant producing microbes and their potential applications: A review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1522–1554. [Google Scholar] [CrossRef]
- Pradhan, A.; Bhattacharyya, A. Quest for an eco-friendly alternative surfactant: Surface and foam characteristics of natural surfactants. J Clean Prod. 2017, 150, 127–134. [Google Scholar] [CrossRef]
- Geetha, S.J.; Banat, I.M.; Joshi, S.J. Biosurfactants: production and potential applications in microbial enhanced oil recovery (MEOR). Biocatal Agric Biotechnol. 2018, 14, 23–32. [Google Scholar] [CrossRef]
- Sena, H.H.; Sanches, M.A.; Rocha, D.F.S.; Segundo, W.O.P.F.; de Souza, É.S.; de Souza, J.V.B. Production of Biosurfactants by Soil Fungi Isolated from the Amazon Forest. Int J Microbiol. 2018, 2018, 5684261. [CrossRef]
- Fenibo, E.O.; Ijoma, G.N.; Selvarajan, R.; Chikere, C.B. Microbial surfactants: the next generation multifunctional biomolecules for applications in the petroleum industry and its associated environmental remediation. Microorganisms. 2019, 7, 581. [Google Scholar] [CrossRef]
- Santos, D.K.F.; Rufino, R.D.; Luna, J.M.; Santos, V.A.; Sarubbo, L.A. Biosurfactants: Multifunctional biomolecules of the 21st century. Int. J. Mol. Sci. 2016, 17, 401. [Google Scholar] [CrossRef]
- Sharma, J.; Sundar, D.; Srivastava, P. Biosurfactants: Potential Agents for Controlling Cellular Communication, Motility, and Antagonism. Front Mol Biosci. 2021, 8, 727070. [Google Scholar] [CrossRef]
- Ceresa, C.; Fracchia, L.; Fedeli, E.; Porta, C.; Banat, I.M. Recent Advances in Biomedical, Therapeutic and Pharmaceutical Applications of Microbial Surfactants. Pharmaceutics. 2021, 13, 466. [Google Scholar] [CrossRef] [PubMed]
- Holmberg, K. Natural surfactants. Curr Opin Colloid Interface Sci. 2001, 6, 148–159. [Google Scholar] [CrossRef]
- Banat, I.M.; Franzetti, A.; Gandolfi, I.; Bestetti, G.; Martinotti, M.G.; Fracchia, L.; Smyth, T.J.; Marchant, R. Microbial biosurfactants production, applications and future potential. Appl Microbiol Biotechnol. 2010, 87, 427–444. [Google Scholar] [CrossRef] [PubMed]
- Naughton, P.J.; Marchant, R.; Naughton, V.; Banat, I.M. Microbial biosurfactants: current trends and applications in agricultural and biomedical industries. J Appl Microbiol. 2019, 127, 12–28. [Google Scholar] [CrossRef]
- Kamalakannan, S.; Gopalakrishnan, A.V.; Thangarasu, R.; Kumar, N.S.; Vellingiri, B. Biosurfactants and anti-inflammatory activity: A potential new approach towards COVID-19. Curr Opin Environ Sci Health. 2020, 17, 72–81. [Google Scholar] [CrossRef]
- Marchant, R.; Banat, I.M. Biosurfactants: A sustainable replacement for chemical surfactants? Biotechnol. Lett. 2012, 34, 1597–1605. [Google Scholar] [CrossRef]
- Akbari, S.; Abdurahman, N.H.; Yunus, R.M.; Fayaz, F.; Alara, O.R. Biosurfactants—a new frontier for social and environmental safety: a mini review. Biotechnol Res Innov. 2018, 2, 81–90. [Google Scholar] [CrossRef]
- Tripathy, D.B.; Mishra, A. Sustainable Biosurfactants. In Encyclopedia of Inorganic and Bioinorganic Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2011; pp. 1–11. [Google Scholar]
- Vecino, X.; Barbosa-Pereira, L.; Devesa-Rey, R.; Cruz, J.M.; Moldes, A.B. Study of the surfactant properties of aqueous stream from the corn milling industry. J Agric Food Chem. 2014, 62, 5451–5457. [Google Scholar] [CrossRef]
- Vecino, X.; Barbosa-Pereira, L.; Devesa-Rey, R.; Cruz, J.M.; Moldes, A.B. Optimization of liquid-liquid extraction of biosurfactants from corn steep liquor. Bioprocess Biosyst Eng. 2015, 38, 1629–1637. [Google Scholar] [CrossRef]
- Najmi, Z.; Ebrahimipour, G.; Franzetti, A.; Banat, I.M. In situ downstream strategies for cost-effective bio/surfactant recovery. Biotechnol Appl Biochem. 2018, 65, 523–532. [Google Scholar] [CrossRef]
- Helmy, Q.; Kardena, E.; Funamizu, N.; Wisjnuprapto. Strategies toward commercial scale of biosurfactant production as potential substitute for its chemically counterparts. Int J Biotechnol. 2011, 12, 66–86. [CrossRef]
- Banat, I.M.; Satpute, S.K.; Cameotra, S.S.; Patil, R.; Nyayanit, N.V. Cost effective technologies and renewable substrates for biosurfactants’ production. Front Microbiol. 2014, 5, 697. [Google Scholar] [CrossRef] [PubMed]
- Biosurfactant market https://www.marketsandmarkets.com/Market-Reports/biosurfactant-market-163644922.html.
- Vijayakumar, S.; Saravanan, V. Biosurfactants-types, sources and applications. Res J Microbiol. 2015, 10, 181–192. [Google Scholar] [CrossRef]
- Fracchia, L.; Ceresa, C.; Franzetti, A.; Cavallo, M.; Gandolfi, I.; Van Hamme, J.; Gkorezis, P.; Marchant, R.; Banat, I.M. Industrial Applications of Biosurfactants. In Biosurfactants: Production and Utilization-Processes, Technologies and Economics; Kosaric, N., Sukan, F.V., Eds.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2014; pp. 245–267. [Google Scholar]
- Swarnalatha, M.S.; Rani, J.C. Biosurfactants: Unique properties and their versatile applications. Pharma Innovat. J. 2019, 8, 684–687. [Google Scholar]
- Sandeep, L.; Rajasree, S. Biosurfactant: Pharmaceutical Perspective. J. Anal. Pharm. Res. 2017, 4, 00105. [Google Scholar] [CrossRef]
- Mondal, M.H.; Malik, S.; Roy, A.; Saha, R.; Saha, B. Modernization of surfactant chemistry in the age of gemini and bio-surfactants: a review. RSC Adv. 2015, 5, 92707–92718. [Google Scholar] [CrossRef]
- Patel, S.; Ahmed, S.; Eswari, J.S. Therapeutic cyclic lipopeptides mining from microbes: Latest strides and hurdles. World J. Microbiol. Biotechnol. 2015, 31, 1177–1193. [Google Scholar] [CrossRef]
- Lukic, M.; Pantelic, I.; Savic, S. An overview of novel surfactants for formulation of cosmetics with certain emphasis on acidic active substances. Tenside Surfactants Deterg. 2016, 53, 7–19. [Google Scholar] [CrossRef]
- Fracchia, L.; Ceresa, C.; Banat, I.M. Biosurfactants in Cosmetic, Biomedical and Pharmaceutical Industry. In Microbial Biosurfactants and Their Environmental and Industrial Applications; Banat, I.M., Thavasi, R., Eds.; CRS Press: Boca Raton, FL, USA, 2019; pp. 258–288. [Google Scholar]
- Markande, A.R.; Patel, D.; Varjani, S. A review on biosurfactants: properties, applications and current developments. Bioresour Technol. 2021, 330, 124963. [Google Scholar] [CrossRef]
- Ambaye, T.G.; Vaccari, M.; Prasad, S.; Rtimi, S. Preparation, characterization and application of biosurfactant in various industries: A critical review on progress, challenges and perspectives. Environmental Technology & Innovation. 2021, 24, 102090. 0209. [CrossRef]
- Hemen Sarma, Majeti Narasimha Vara Prasad. 2021 Biosurfactants for a Sustainable Future: Production and Applications in the Environment and Biomedicine. John Wiley & Sons Ltd. [CrossRef]
- Inamuddin, Mohd Imran Ahamed, Ram Prasad. 2021 Microbial Biosurfactants Preparation, Properties and Applications. Springer Singapore. [CrossRef]
- Inamuddin, Charles Oluwaseun Adetunji, Mohd Imran Ahamed 2022 Green Sustainable Process for Chemical and Environmental Engineering and Science Biomedical Application of Biosurfactant in the Medical Sector, Academic Press - Elsevier.
- Ismail, R.; Baaity, Z.; Csóka, I. Regulatory status quo and prospects for biosurfactants in pharmaceutical applications. Drug Discov Today. 2021, 26, 1929–1935. [Google Scholar] [CrossRef]
- Smith, M.L.; Gandolfi, S.; Coshall, P.M.; Rahman, P.K.S.M. Biosurfactants: A Covid-19 Perspective. Front Microbiol. 2020, 11, 1341. [Google Scholar] [CrossRef]
- Rana, S.; Singh, J.; Wadhawan, A.; Khanna, A.; Singh, G.; Chatterjee, M. Evaluation of In Vivo toxicity of Novel Biosurfactant from Candida parapsilosis loaded in PLA-PEG Polymeric Nanoparticles. J Pharm Sci. 2021, 110, 1727–1738. [Google Scholar] [CrossRef] [PubMed]
- Voulgaridou, G.P.; Mantso, T.; Anestopoulos, I.; Klavaris, A.; Katzastra, C.; Kiousi, D.E.; Mantela, M.; Galanis, A.; Gardikis, K.; Banat, I.M.; Gutierrez, T.; Sałek, K.; Euston, S.; Panayiotidis, M.I.; Pappa, A. Toxicity Profiling of Biosurfactants Produced by Novel Marine Bacterial Strains. Int J Mol Sci. 2021, 22, 2383. [Google Scholar] [CrossRef] [PubMed]
- Adu, S.A.; Twigg, M.S.; Naughton, P.J.; Marchant, R.; Banat, I.M. Characterisation of cytotoxicity and immunomodulatory effects of glycolipid biosurfactants on human keratinocytes. Appl Microbiol Biotechnol. 2023, 107, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Caldara, M.; Belgiovine, C.; Secchi, E.; Rusconi, R. Environmental, Microbiological, and Immunological Features of Bacterial Biofilms Associated with Implanted Medical Devices. Clin Microbiol Rev. 2022, 35, e0022120. [Google Scholar] [CrossRef]
- Singh, S.; Singh, S.K.; Chowdhury, I.; Singh, R. Understanding the Mechanism of Bacterial Biofilms Resistance to Antimicrobial Agents. Open Microbiol J. 2017, 11, 53–62. [Google Scholar] [CrossRef]
- Tambone, E.; Marchetti, A.; Ceresa, C.; Piccoli, F.; Anesi, A.; Nollo, G.; Caola, I.; Bosetti, M.; Fracchia, L.; Ghensi, P.; Tessarolo, F. Counter-Acting Candida albicans-Staphylococcus aureus Mixed Biofilm on Titanium Implants Using Microbial Biosurfactants. Polymers (Basel) 2021, 13, 2420. [Google Scholar] [CrossRef]
- Dardouri, M.; Bettencourt, A.; Martin, V.; Carvalho, F.A.; Santos, C.; Monge, N.; Santos, N.C.; Fernandes, M.H.; Gomes, P.S.; Ribeiro, I.A.C. Using plasma-mediated covalent functionalization of rhamnolipids on polydimethylsiloxane towards the antimicrobial improvement of catheter surfaces. Biomater Adv. 2022, 134, 112563. [Google Scholar] [CrossRef]
- Ali, S.A.M.; Sayyed, R.Z.; Mir, M.I.; Khan, M.Y.; Hameeda, B.; Alkhanani, M.F.; Haque, S.; Mohammad Al Tawaha, A.R.; Poczai, P. Induction of Systemic Resistance in Maize and Antibiofilm Activity of Surfactin From Bacillus velezensis MS20. Front Microbiol. 2022, 13, 879739. [Google Scholar] [CrossRef]
- Kannan, S.; Solomon, A.; Krishnamoorthy, G.; Marudhamuthu, M. Liposome encapsulated surfactant abetted copper nanoparticles alleviates biofilm mediated virulence in pathogenic Pseudomonas aeruginosa and MRSA. Sci Rep. 2021, 11, 1102. [Google Scholar] [CrossRef]
- Ceresa, C.; Rinaldi, M.; Tessarolo, F.; Maniglio, D.; Fedeli, E.; Tambone, E.; Caciagli, P.; Banat, I.M.; Diaz De Rienzo, M.A.; Fracchia, L. Inhibitory Effects of Lipopeptides and Glycolipids on C. albicans-Staphylococcus spp. Dual-Species Biofilms. Front Microbiol. 2021, 11, 545654. [CrossRef]
- Cheffi, M.; Maalej, A.; Mahmoudi, A.; Hentati, D.; Marques, A.M.; Sayadi, S.; Chamkha, M. Lipopeptides production by a newly Halomonas venusta strain: Characterization and biotechnological properties. Bioorg Chem. 2021, 109, 104724. [Google Scholar] [CrossRef]
- Sharaf, M.; Sewid, A.H.; Hamouda, H.I.; Elharrif, M.G.; El-Demerdash, A.S.; Alharthi, A.; Hashim, N.; Hamad, A.A.; Selim, S.; Alkhalifah, D.H.M.; Hozzein, W.N.; Abdalla, M.; Saber, T. Rhamnolipid-Coated Iron Oxide Nanoparticles as a Novel Multitarget Candidate against Major Foodborne E. coli Serotypes and Methicillin-Resistant S. aureus. Microbiol Spectr. 2022, 10, e0025022. [CrossRef]
- Goyal, S.; Singh, J. Bioprocess optimization for glycopeptide biosurfactant production by means of Lactobacillus delbrueckii: Design expert laden approach. J Food Process Preserv. 2022, 46, e17195. [Google Scholar] [CrossRef]
- Firdose, A., Chong, N.H.H.; Ramli, R.; Aqma, W.S. Antimicrobial, antiadhesive, and antibiofilm actions of rhamnolipids on ESKAPE pathogens. Lett Appl Microbiol. 2023, 76, ovad013. [CrossRef]
- van Duin, D.; Paterson, D.L. Multidrug-Resistant Bacteria in the Community: An Update. Infect Dis Clin North Am. 2020, 34, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Marquez, L.; Quave, C.L. Prevalence and Therapeutic Challenges of Fungal Drug Resistance: Role for Plants in Drug Discovery. Antibiotics (Basel) 2020, 9, 150. [Google Scholar] [CrossRef]
- Satpute, S.K.; Banpurkar, A.G.; Banat, I.M.; Sangshetti, J.N.; Patil, R.H.; Gade, W.N. Multiple Roles of Biosurfactants in Biofilms. Curr Pharm Des. 2016, 22, 1429–1448. [Google Scholar] [CrossRef]
- Paraszkiewicz, K.; Moryl, M.; Płaza, G.; Bhagat, D.; K, Satpute, S.; Bernat, P. Surfactants of microbial origin as antibiofilm agents. Int J Environ Health Res. 2021, 31, 401–420. [CrossRef]
- Satpute, S.K.; Banat, I.M.; Dhakephalkar, P.K.; Banpurkar, A.G.; Chopade, B.A. Biosurfactants, bioemulsifiers and exopolysaccharides from marine microorganisms. Biotechnol Adv. 2010, 28, 436–450. [Google Scholar] [CrossRef]
- Gudiña, E.J.; Teixeira, J.A.; Rodrigues, L.R. Biosurfactants Produced by Marine Microorganisms with Therapeutic Applications. Mar Drugs. 2016, 14, 38. [Google Scholar] [CrossRef]
- Kubicki, S.; Bollinger, A.; Katzke, N.; Jaeger, K.E.; Loeschcke, A.; Thies, S. Marine Biosurfactants: Biosynthesis, Structural Diversity and Biotechnological Applications. Mar Drugs 2019, 408. [Google Scholar] [CrossRef] [PubMed]
- Englerová, K.; Bedlovičová, Z.; Nemcová, R.; Király, J.; Maďar, M.; Hajdučková, V.; Styková, E.; Mucha, R.; Reiffová, K. Bacillus amyloliquefaciens-Derived Lipopeptide Biosurfactants Inhibit Biofilm Formation and Expression of Biofilm-Related Genes of Staphylococcus aureus. Antibiotics (Basel) 2021, 10, 1252. [Google Scholar] [CrossRef]
- Gharaei, S.; Ohadi, M.; Hassanshahian, M.; Porsheikhali, S.; Forootanfar, H. Isolation, Optimization, and Structural Characterization of Glycolipid Biosurfactant Produced by Marine Isolate Shewanella algae B12 and Evaluation of Its Antimicrobial and Anti-biofilm Activity. Appl Biochem Biotechnol. 2022, 194, 1755–1774. [Google Scholar] [CrossRef]
- Amirinejad, N.; Shahriary, P.; Hassanshahian, M. Investigation of the synergistic effect of glycolipid biosurfactant produced by Shewanella algae with some antibiotics against planktonic and biofilm forms of MRSA and antibiotic resistant Acinetobacter baumannii. World J Microbiol Biotechnol. 2022, 39, 45. [Google Scholar] [CrossRef]
- Buonocore, C.; Giugliano, R.; Della Sala, G.; Palma Esposito, F.; Tedesco, P.; Folliero, V.; Galdiero, M.; Franci, G.; de Pascale, D. Evaluation of Antimicrobial Properties and Potential Applications of Pseudomonas gessardii M15 Rhamnolipids towards Multiresistant Staphylococcus aureus. Pharmaceutics. 2023, 15, 700. [Google Scholar] [CrossRef] [PubMed]
- De Giani, A.; Zampolli, J.; Di Gennaro, P. Recent Trends on Biosurfactants with Antimicrobial Activity Produced by Bacteria Associated With Human Health: Different Perspectives on Their Properties, Challenges, and Potential Applications. Front Microbiol. 2021, 12, 655150. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Dai, X.; Jin, H.; Lin, G.; Wang, Z.; Song, Y.; Zhang, W.; Man, C.; Jiang, Y. Physicochemical and transcriptomic responses of Lactobacillus brevis JLD715 to sodium selenite. J Sci Food Agric. 2021, 101, 4332–4341. [Google Scholar] [CrossRef]
- Haddaji, N.; Ncib, K.; Bahia. W.; Ghorbel. M.; Leban, N.; Bouali, N.; Bechambi, O.; Mzoughi, R.; Mahdhi, A. Control of Multidrug-Resistant Pathogenic Staphylococci Associated with Vaginal Infection Using Biosurfactants Derived from Potential Probiotic Bacillus Strain. Fermentation. 2022, 8, 19. [CrossRef]
- Patel M, Siddiqui AJ, Ashraf SA, Surti M, Awadelkareem AM, Snoussi M, Hamadou WS, Bardakci F, Jamal A, Jahan S, Sachidanandan M, Adnan M. Lactiplantibacillus plantarum-Derived Biosurfactant Attenuates Quorum Sensing-Mediated Virulence and Biofilm Formation in Pseudomonas aeruginosa and Chromobacterium violaceum. Microorganisms. 2022, 10, 1026. [CrossRef]
- Abruzzo, A.; Giordani, B.; Parolin, C.; De Gregorio, P.R.; Foschi, C.; Cerchiara, T.; Bigucci, F.; Vitali, B.; Luppi, B. Lactobacillus crispatus BC1 Biosurfactant Delivered by Hyalurosomes: An Advanced Strategy to Counteract Candida Biofilm. Antibiotics (Basel) 2021, 10, 33. [Google Scholar] [CrossRef]
- Alfian, A.R.; Watchaputi, K.; Sooklim, C.; Soontorngun, N. Production of new antimicrobial palm oil-derived sophorolipids by the yeast Starmerella riodocensis sp. nov. against Candida albicans hyphal and biofilm formation. Microb Cell Fact. 2022, 21, 163. [Google Scholar] [CrossRef]
- Manikkasundaram, V.; Baskaran, A.; Kaari, M.; Angamuthu, V.; Venugopal, G.; Manikkam, R. Production and characterization of glycolipid biosurfactant from Streptomyces enissocaesilis HRB1 and its evaluation for biomedical and bioremediation applications. J Surfact Deterg. 2022, 2022, 1–13. [Google Scholar] [CrossRef]
- Pradhan, D.; Biswasroy, P.; Kar, B.; Bhuyan, S.K.; Ghosh, G.; Rath, G. Clinical Interventions and Budding Applications of Probiotics in the Treatment and Prevention of Viral Infections. Arch Med Res. 2022, 53, 122–130. [Google Scholar] [CrossRef]
- Hegazy, G.E.; Abu-Serie, M.M.; Abou-Elela, G.M.; Ghozlan, H.; Sabry, S.A.; Soliman, N.A.; Teleb, M.; Abdel-Fattah, Y.R. Bioprocess development for biosurfactant production by Natrialba sp. M6 with effective direct virucidal and anti-replicative potential against HCV and HSV. Sci Rep. 2022, 12, 16577. [CrossRef]
- Rodrigues, L.; Banat, I.M.; Teixeira, J.; Oliveira, R. Biosurfactants: potential applications in medicine. J Antimicrob Chemother. 2006, 57, 609–618. [Google Scholar] [CrossRef]
- Khemili-Talbi, S.; Kebbouche-Gana, S.; Akmoussi-Toumi, S.; Angar, Y.; Gana, M.L. Isolation of an extremely halophilic arhaeon Natrialba sp. C21 able to degrade aromatic compounds and to produce stable biosurfactant at high salinity. Extremophiles. 2015, 19, 1109–1120. [Google Scholar] [CrossRef]
- Torregrosa-Crespo, J.; Galiana, C.P.; Martínez-Espinosa, R.M. Biocompounds from Haloarchaea and Their Uses in Biotechnology. Archaea - New Biocatalysts, Novel Pharmaceuticals and Various Biotechnological Applications. InTech, 2017. [CrossRef]
- Kebbouche-Gana, S.; Gana, M.L.; Ferrioune, I.; Khemili, S.; Lenchi, N.; Akmouci-Toumi, S.; Bouanane-Darenfed, N.A.; Djelali, N.E. Production of biosurfactant on crude date syrup under saline conditions by entrapped cells of Natrialba sp. strain E21, an extremely halophilic bacterium isolated from a solar saltern (Ain Salah, Algeria). Extremophiles. 2013, 17, 981–993. [CrossRef]
- Mabrouk, M.E.M.; Youssif, E.M.; Sabry, S.A. Biosurfactant production by a newly isolated soft coral-associated marine Bacillus sp. E34: Statistical optimization and characterization. Life Sci. J. 2014, 11, 756–768.
- Yeh, M.L.; Huang, C.I.; Huang, C.F.; Hsieh, M.H.; Liu, T.W.; Lin, Y.H.; Liang, P.C.; Hsieh, M.Y.; Lin, Z.Y.; Chen, S.C.; Huang, J.F.; Kuo, P.L.; Dai, C.Y.; Yu, M.L.; Chuang, W.L. Pretreatment Hepatitis B Viral Load Predicts Long-Term Hepatitis B Response After Anti-Hepatitis C Therapy in Hepatitis B/C Dual-Infected Patients. J Infect Dis. 2019, 219, 1224–1233. [Google Scholar] [CrossRef]
- Silverman, A.I.; Boehm, A.B. Systematic Review and Meta-Analysis of the Persistence of Enveloped Viruses in Environmental Waters and Wastewater in the Absence of Disinfectants. Environmental Science and Technology. 2021, 55, 14480–14493. [Google Scholar] [CrossRef] [PubMed]
- Manoj ,S.; Jogger, C.R.; Myscofski, D.; Yoon, M.; Spear, P.G. Mutations in herpes simplex virus glycoprotein D that prevent cell entry via nectins and alter cell tropism. Proc Natl Acad Sci U S A. 2004, 101, 12414–12421. [CrossRef]
- Behzadnia, A.; Moosavi-Nasab, M.; Mohammadi, A.; Babajafari, S.; Tiwari, B.K. Production of an ultrasound-assisted biosurfactant postbiotic from agro-industrial wastes and its activity against Newcastle virus. Front Nutr. 2022, 9, 966338. [Google Scholar] [CrossRef] [PubMed]
- Coronavirus WHO. dashboard-Available from: https://covid19. who. int. Geneva World Heal Organ 2021.
- Kumari, K.; Nandi, A.; Sinha, A.; Ghosh, A.; Sengupta, S.; Saha, U.; Singh, P.K.; Panda, P.K.; Raina, V.; Verma, S.K. The paradigm of prophylactic viral outbreaks measures by microbial biosurfactants. J Infect Public Health. 2023, 16, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, P.; Raichur, A.M. Synthesis of spherical NiO nanoparticles through a novel biosurfactant mediated emulsion technique. Materials Science and Engineering: C. 2009, 29, 199–204. [CrossRef]
- Xie, Y.; Ye, R.; Liu, H. Synthesis of silver nanoparticles in reverse micelles stabilized by natural biosurfactant. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2006, 279, 175–178. [CrossRef]
- Panda, P.K.; P. Chavda, V.; Neve, K.; Mishra, S.; Verma, S.K.; Ahuja, R. (2022). COVID-19: Lesson Learnt from Diagnostics to Therapeutics. In: Suar, M., Misra, N., Dash, C. (eds) Microbial Engineering for Therapeutics. Springer, Singapore. [CrossRef]
- Celik, P.A.; Manga, E.B.; Cabuk, A.; Banat, I.M. Biosurfactants Potential Role in Combating COVID 19 and Similar Future Microbial Threats. Applied Sciences. 2021, 11, 334. [Google Scholar] [CrossRef]
- Ohadi, M.; Forootanfar, H.; Dehghannoudeh, N.; Banat, I.M.; Dehghannoudeh, G. The role of surfactants and biosurfactants in the wound healing process: a review. J Wound Care. 2023, 32, xxxix–xlvi. [Google Scholar] [CrossRef]
- Sajid, M.; Ahmad Khan, M.S.; Singh Cameotra, S.; Safar Al-Thubiani, A. Biosurfactants: Potential applications as immunomodulator drugs. Immunol Lett. 2020, 223, 71–77. [Google Scholar] [CrossRef]
- Guo, S.; Dipietro, L.A. Factors affecting wound healing. J Dent Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation in Chronic Wounds. Int J Mol Sci. 2016, 17, 2085. [Google Scholar] [CrossRef]
- Mehrabani, M.; Esmaeili-Tarzi, M.; Forootanfar, H.; Nematollahi, M.H., Banat, I.M.; Ohadi, M.; Dehghannoudeh, G. Lipopeptide Biosurfactant from Acinetobacter junii B6: A Promising Natural Surfactant for Promoting Angiogenesis. Int J Pept Res Ther. 2021, 27, 1197–1203. [CrossRef]
- Cheffi, M.; Maalej, A.; Mahmoudi, A.; Hentati, D.; Marques, A.M.; Sayadi, S.; Chamkha, M. Lipopeptides production by a newly Halomonas venusta strain: Characterization and biotechnological properties. Bioorg Chem. 2021, 109, 104724. [Google Scholar] [CrossRef]
- Ohadi, M.; Forootanfar, H.; Dehghannoudeh, G.; Eslaminejad, T.; Ameri, A.; Shakibaie, M.; Adeli-Sardou, M. Antimicrobial, anti-biofilm, and anti-proliferative activities of lipopeptide biosurfactant produced by Acinetobacter junii B6. Microb Pathog. 2020, 138, 103806. [Google Scholar] [CrossRef]
- Ohadi, M.; Forootanfar, H.; Rahimi, H.R.; Jafari, E.; Shakibaie, M.; Eslaminejad, T.; Dehghannoudeh, G. Antioxidant Potential and Wound Healing Activity of Biosurfactant Produced by Acinetobacter junii B6. Curr Pharm Biotechnol. 2017, 18, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, J.; Kirsner, R. Pathophysiology of acute wound healing. Clin Dermatol. 2007, 25, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Afsharipour, S., Asadi, A., Ohadi, M.; Ranjbar, M.; Forootanfar, H.; Jafari E.; Dehghannoudeh, G. Preparation and Characterization of Nano-Lipopeptide Biosurfactant Hydrogel and Evaluation of Wound-Healing Properties. BioNanoSci. 2021, 11, 1061–1069. [CrossRef]
- Yan, L.; Liu, G.; Zhao, B.; Pang, B.; Wu, W.; Ai, C.; Zhao, X.; Wang, X.; Jiang, C.; Shao, D.; Liu, Q.; Li, M.; Wang, L.; Shi, L. Novel Biomedical Functions of Surfactin A from Bacillus subtilis in Wound Healing Promotion and Scar Inhibition. Agric. Food Chem. 2020, 68, 6987–6997. [Google Scholar] [CrossRef] [PubMed]
- Diaz De Rienzo, M.A.; Stevenson, P.S.; Marchant, R.; Banat, I.M. Effect of biosurfactants on Pseudomonas aeruginosa and Staphylococcus aureus biofilms in a BioFlux channel. Appl Microbiol Biotechnol. 2016, 100, 5773–5779. [Google Scholar] [CrossRef] [PubMed]
- Hentati, D.; Chebbi, A.; Mahmoudi, A.; Hadrich, F.; Cheffi, M.; Frikha, I.; Sayadi, S.; Chamkha, M. Biodegradation of hydrocarbons and biosurfactants production by a newly halotolerant Pseudomonas sp. strain isolated from contaminated seawater. Biochemical Engineering Journal 2021, 166, 107861. [Google Scholar] [CrossRef]
- Sekhon Randhawa, K.K.; Rahman, P.K. Rhamnolipid biosurfactants-past, present, and future scenario of global market. Front Microbiol. 2014, 5, 454. [Google Scholar] [CrossRef]
- Adu, S.A.; Naughton, P.J.; Marchant, R.; Banat, I.M. Microbial Biosurfactants in Cosmetic and Personal Skincare Pharmaceutical Formulations. Pharmaceutics 2020, 12, 1099. [Google Scholar] [CrossRef]
- Sil, J.; Dandapat, P.; Das, S. Health care applications of different biosurfactants: review. Int. J. Sci. Res. 2015, 6, 41. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Feinberg, B.; Kish, J.; Dokubo, I.; Wojtynek, J.; Lord, K. Reports of the demise of chemotherapy have been greatly exaggerated. Am J Manag Care. 2019, 25, 270–272. [Google Scholar]
- Pucci, C.; Martinelli, C.; Ciofani, G. Innovative approaches for cancer treatment: current perspectives and new challenges. Ecancermedicalscience 2019, 13, 961. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Shin, H.R.; Bray, F.; Forman, D.; Mathers, C.; Parkin, D.M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer J Int du Cancer. 2010, 127, 2893–2917. [Google Scholar] [CrossRef] [PubMed]
- Mansoori, B.; Mohammadi, A.; Davudian, S.; Shirjang, S.; Baradaran, B. The different mechanisms of cancer drug resistance: a brief review. Adv Pharm Bull. 2017, 7, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Arruebo, M.; Vilaboa, N.; Sáez-Gutierrez, B.; Lambea, J.; Tres, A.; Valladares, M.; González-Fernández, A. Assessment of the evolution of cancer treatment therapies. Cancers (Basel) 2011, 3, 3279–3330. [Google Scholar] [CrossRef]
- Dan, A.K.; Manna, A.; Ghosh, S.; Sikdar, S.; Sahu, R.; Parhi, P.K.; Parida, S. Molecular mechanisms of the lipopeptides from Bacillus subtilis in the apoptosis of cancer cells-a review on its current status in diferent cancer cell lines. Adv Cancer Biol-Metastasis. 2021, 3, 100019. [CrossRef]
- Meena, K.R.; Kanwar, S.S. Lipopeptides as the antifungal and antibacterial agents: applications in food safety and therapeutics. Biomed Res Int. 2015, 2015, 473050. [Google Scholar] [CrossRef]
- Gudiña, E.J.; Rangarajan, V.; Sen, R.; Rodrigues, L.R. Potential therapeutic applications of biosurfactants. Trends Pharmacol Sci. 2013, 34, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Adu, S.A.; Twigg, M.S.; Naughton, P.J.; Marchant, R.; Banat, I.M. Biosurfactants as Anticancer Agents: Glycolipids Affect Skin Cells in a Differential Manner Dependent on Chemical Structure. Pharmaceutics. 2022, 14, 360. [Google Scholar] [CrossRef]
- Haque, F.; Khan, M.S.A.; AlQurashi, N. ROS-Mediated Necrosis by Glycolipid Biosurfactants on Lung, Breast, and Skin Melanoma Cells. Front Oncol. 2021, 11, 622470. [Google Scholar] [CrossRef]
- Etemadzadeh, S.S.; Emtiazi, G.; Soltanian, S. Production of biosurfactant by salt-resistant Bacillus in lead-supplemented media: application and toxicity. Int Microbiol. 2023. [Google Scholar] [CrossRef]
- Wu, Y.S.; Ngai, S.C.; Goh, B.H.; Chan, K.G.; Lee, L.H.; Chuah, L.H. Anticancer Activities of Surfactin and Potential Application of Nanotechnology Assisted Surfactin Delivery. Front Pharmacol. 2017, 8, 761. [Google Scholar] [CrossRef]
- Sarangi, M.K.; Padhi, S.; Patel, L.D.; Rath, G.; Nanda, S.S.; Yi, D.K. Theranostic efficiency of biosurfactants against COVID-19 and similar viruses - A review. J Drug Deliv Sci Technol. 2022, 76, 103764. [Google Scholar] [CrossRef]
- Thakur, P.; Saini, N.K.; Thakur, V.K.; Gupta, V.K.; Saini, R.V.; Saini, A.K. Rhamnolipid the Glycolipid Biosurfactant: Emerging trends and promising strategies in the field of biotechnology and biomedicine. Microb Cell Fact. 2021, 20, 1. [Google Scholar] [CrossRef]
- Kwak, M.J.; Ha, D.J.; Choi, Y.S.; Lee, H.; Whang, K.Y. Protective and restorative effects of sophorolipid on intestinal dystrophy in dextran sulfate sodium-induced colitis mouse model. Food Funct. 2022, 13, 161–169. [Google Scholar] [CrossRef]
- Daverey, A.; Dutta, K.; Joshi, S.; Daverey, A. Sophorolipid: a glycolipid biosurfactant as a potential therapeutic agent against COVID-19. Bioengineered 2021, 12, 9550–9560. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, F.; Sharifi, I.; Ohadi, M.; Mohamadi, N.; Salarkia, E.; Banat, I.M., Dehghannoudeh, G. The Potential Role of Lipopeptide Biosurfactant Generated by Acinetobacter junii B6 on Leishmania Tropica: The Synergy of Lipopeptide Biosurfactant and Glucantime. Int J Pept Res Ther. 2023, 29, 57. [CrossRef]
- Khodavirdipour, A.; Chamanrokh, P.; Alikhani, M.Y.; Alikhani, M.S. Potential of Bacillus subtilis Against SARS-CoV-2 - A Sustainable Drug Development Perspective. Front Microbiol. 2022, 13, 718786. [Google Scholar] [CrossRef] [PubMed]
- K.K. Jain, Drug delivery systems-an overview, Drug Delivery Systems, 437, Springer, 2008, pp. 150. [CrossRef]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; Santini, A.; Souto, E,B. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules. 2020, 25, 3731. [CrossRef]
- McClements, D.J. Nanoemulsions versus microemulsions: terminology, differences, and similarities. Soft matter. 2012, 8, 1719–1729. [Google Scholar] [CrossRef]
- Sriwidodo, Umar, A.K.; Wathoni, N.; Zothantluanga, J.H.; Das, S.; Luckanagul, J.A. Liposome-polymer complex for drug delivery system and vaccine stabilization. Heliyon. 2022, 8, e08934. [CrossRef]
- Abruzzo, A.; Parolin, C.; Corazza, E.; Giordani, B.; di Cagno, M.P.; Cerchiara, T.; Bigucci, F.; Vitali, B.; Luppi, B. Influence of Lactobacillus Biosurfactants on Skin Permeation of Hydrocortisone. Pharmaceutics 2021, 13, 820. [Google Scholar] [CrossRef]
- Corazza, E.; Abruzzo, A.; Giordani, B.; Cerchiara, T.; Bigucci, F.; Vitali, B.; di Cagno, M.P.; Luppi, B. Human Lactobacillus Biosurfactants as Natural Excipients for Nasal Drug Delivery of Hydrocortisone. Pharmaceutics 2022, 14, 524. [Google Scholar] [CrossRef]
- Ferreira, W.T.; Hong, H.A.; Hess, M.; Adams, J.R.G.; Wood, H.; Bakun, K.; Tan, S.; Baccigalupi, L.; Ferrari, E.; Brisson, A.; Ricca, E.; Teresa Rejas, M.; Meijer, W.J.J.; Soloviev, M.; Cutting, S.M. Micellar Antibiotics of Bacillus. Pharmaceutics 2021, 13, 1296. [Google Scholar] [CrossRef]
- Lassenberger, A.; Martel, A.; Porcar, L.; Baccile, N. Interpenetrated biosurfactant-silk fibroin networks - a SANS study. Soft Matter. 2021, 17, 2302–2314. [Google Scholar] [CrossRef]
- Ma, E.; Chen, K.; Sun, L.; Fu, Z.; Guo, J.; Liu, J.; Zhao, J.; Liu, Z.; Lei, Z.; Li, L.; Hu, X.; Guo, X. Rapid Construction of Green Nanopesticide Delivery Systems Using Sophorolipids as Surfactants by Flash Nanoprecipitation. J Agric Food Chem. 2022, 70, 4912–4920. [Google Scholar] [CrossRef] [PubMed]
- Worakitsiri, P.; Pornsunthorntawee, O.; Thanpitcha, T.; Chavadej, S.; Weder, C.; Rujiravanit, R. Synthesis of polyaniline nanofbers and nanotubes via rhamnolipid biosurfactant templating. Synth Meth. 2011, 161, 298–306. [Google Scholar] [CrossRef]
- Saikia, J.P.; Bharali, P.; Konwar, B.K. Possible protection of silver nanoparticles against salt by using rhamnolipid. Colloids Surf B Biointerfaces 2013, 104, 330–332. [Google Scholar] [CrossRef] [PubMed]
- Hazra, C.; Kundu, D.; Chaudhari, A.; Jana, T. Biogenic synthesis, characterization, toxicity and photocatalysis of zinc sulphide nanoparticles using rhamnolipids from Pseudomonas aeruginosa BS01 as capping and stabilizing agent. J Chem Technol Biotechnol. 2013, 88, 1039–1048. [Google Scholar] [CrossRef]
- Sharma, R.K.; Dey, G.; Banerjee, P.; Maity, J.P.; Lu, C.M.; Wang, S.C.; Huang, Y.H.; Lin, P.Y.; Chen, Y.P.; Chen, C.Y. Influence of chemical and bio-surfactants on physiochemical properties in mesoporous silica nanoparticles synthesis. Journal of Materials Research and Technology 2023, 24, 2629–2639. [Google Scholar] [CrossRef]
- Sharma, R.K.; Wang, S.C.; Maity, J.P.; Banerjee, P.; Dey, G.; Huang, Y.H.; Bundschuh, J.; Hsiao, P.G.; Chen, T.H.; Chen, C.Y. A novel BMSN (biologically synthesised mesoporous silica nanoparticles) material: synthesis using a bacteria-mediated biosurfactant and characterization. RSC Adv. 2021, 11, 32906–32916. [Google Scholar] [CrossRef]
- Chauhan, V.; Dhiman, V.K.; Mahajan, G.; Pandey, A.; Kanwar, S.S. Synthesis and characterization of silver nanoparticles developed using a novel lipopeptide(s) biosurfactant and evaluating its antimicrobial and cytotoxic efficacy. Process Biochemistry 2023, 124, 51–62. [Google Scholar] [CrossRef]
- Shikha, S.; Chaudhuri, S.R.; Bhattacharyya, M.S. Facile One Pot Greener Synthesis of Sophorolipid Capped Gold Nanoparticles and its Antimicrobial Activity having Special Efficacy Against Gram Negative Vibrio cholerae. Sci Rep. 2020, 10, 1463. [Google Scholar] [CrossRef]
- Athira, K.; Gurrala, L.; Kumar, D.V.R. Biosurfactant-mediated biosynthesis of CuO nanoparticles and their antimicrobial activity. Appl Nanosci. 2021, 11, 1447–1457. [Google Scholar] [CrossRef]
- Mahta Falakaflaki, Jaleh Varshosaz, Mina Mirian, Local delivery of usnic acid loaded Rhamnolipid vesicles by gelatin / tragacanth gum / montmorillonite/ vanillin cryogel scaffold for expression of osteogenic biomarkers and antimicrobial activity. Journal of Drug Delivery Science and Technology. 2022, 69, 103147. [CrossRef]
- Müller, F.; Hönzke, S.; Luthardt, W.O.; Wong, E.L.; Unbehauen, M.; Bauer, J.; Haag, R.; Hedtrich, S.; Rühl, E.; Rademann, J. Rhamnolipids form drug-loaded nanoparticles for dermal drug delivery. Eur J Pharm Biopharm. 2017, 116, 31–37. [Google Scholar] [CrossRef]
- Lewińska, A.; Domżał-Kędzia, M.; Wójtowicz, K.; Bazylińska, U. Surfactin-stabilised poly(D,L-lactide) nanoparticles for potential skin application. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2022, 648, 129216. [CrossRef]
- Yi, G.; Son, J.; Yoo, J.; Park, C.; Koo, H. Rhamnolipid nanoparticles for in vivo drug delivery and photodynamic therapy. Nanomedicine 2019, 19, 12–21. [Google Scholar] [CrossRef]
- Ohadi, M.; Forootanfar, H.; Dehghannoudeh, G. Eslaminejad, T.; Ameri, A.; Shakibaie, M.; Najafi, A. Biosynthesis of Gold Nanoparticles Assisted by Lipopeptide Biosurfactant Derived from Acinetobacter junii B6 and Evaluation of Its Antibacterial and Cytotoxic Activities. BioNanoSci. 2020, 10, 899–908. [CrossRef]
- Lee, Y.; Lee, D.; Park, E.; Jang, S.Y.; Cheon, S.Y.; Han, S.; Koo, H. Rhamnolipid-coated W/O/W double emulsion nanoparticles for efficient delivery of doxorubicin/erlotinib and combination chemotherapy. J Nanobiotechnology 2021, 19, 411. [Google Scholar] [CrossRef] [PubMed]
- Wadhawan, A.; Singh, J.; Sharma, H.; Handa, S.; Singh, G.; Kumar, R.; Barnwal, R.P.; Pal Kaur, I.; Chatterjee, M. Anticancer Biosurfactant-Loaded PLA-PEG Nanoparticles Induce Apoptosis in Human MDA-MB-231 Breast Cancer Cells. ACS Omega. 2022, 7, 5231–5241. [Google Scholar] [CrossRef]
- Ascenso, A., Simões, S., Marto, J., Ribeiro, H.M., Almeida, A.J. (2021). Colloidal Disperse Systems: Microemulsions and Nanoemulsions. In: Eloy, J.O., Abriata, J.P., Marchetti, J.M. (eds) Nanocarriers for Drug Delivery. Nanomedicine and Nanotoxicology. Springer, Cham. [CrossRef]
- Luo, J.; Yang, B.; Yang, X.; Ji, S.; Guo, Z.; Liu, Y.; Chen, Q.; Zhao, T.; Wang, Y.; Lu, B. Sophorolipid-based microemulsion delivery system: Multifaceted enhancement of physicochemical properties of xanthohumol. Food Chem. 2023, 413, 135631. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wang, T.; Yu, Z.; Shao, J.; Chu, J.; Zhu, H.; Yao, R. Formulation and Physicochemical and Biological Characterization of Etoposide-Loaded Submicron Emulsions with Biosurfactant of Sophorolipids. AAPS PharmSciTech. 2022, 23, 181. [Google Scholar] [CrossRef]
- Ganesan, N.G.; Singh, R.D.; Dwivedi, D.; Rangarajan, V. Synergy evaluation between diverse biosurfactants toward the formulation of green oil-in-water nanoemulsions by ultrasonication method. Journal of Cleaner Production 2023, 400, 136735. [Google Scholar] [CrossRef]
- El-Moslemany, R.M.; El-Kamel, A.H.; Allam, E.A.; Khalifa, H.M.; Hussein, A.; Ashour, A.A. Tanshinone IIA loaded bioactive nanoemulsion for alleviation of lipopolysaccharide induced acute lung injury via inhibition of endothelial glycocalyx shedding. Biomed Pharmacother. 2022, 155, 113666. [Google Scholar] [CrossRef]
- Kubendiran, L.; Theerthagiri, S.; Al-Dhabi, N.A.; Palaninaicker, S.; Subramanian, S.M.; Srinivasan, V.; Karuppiah, P. In vitro preparation of biosurfactant based herbal-nano topical ointment from Tridax procumbens infused oil using gelatin stabi- lized silver nanoparticle and its efficacy on fibroblastic cell lines. Applied Nanoscience 2023, 13, 719–734. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Q. Surface Functionalization of Piperine-Loaded Liposomes with Sophorolipids Improves Drug Loading and Stability. J Pharm Innov. 2022. [Google Scholar] [CrossRef]
- Cheng, C.; Wu, Z.; McClements, D.J.; Zou, L.; Peng, S.; Zhou, W., Liu, W. Improvement on stability, loading capacity and sustained release of rhamnolipids modified curcumin liposomes. Colloids Surf, B. 2019, 183, 110460. [CrossRef]
- Khan, W.; Ndlovu, T.; Clements, T.L.; Mutta, N.B. Coating of materials with biosurfactant compounds. World Patent WO2022067358A1, 31 March 2022.
- Granjeiro, P.A.; Gonçalves, D.B.; Da Silva, J.A.; Segura Cortés, M.E.; Galdino, A.S.; Guimarães, P.P.G.; Carvalho, F.S.; Parreira, A.G.; De Almeida, D. Isolados de bacillus subtilis ATCC 19659 e seu uso para prevenir aderência bacteriana em titânio e cateteres. Brazil Patent BR102016020677A2, 20 March 2018.
- Qian, P.; She, W.; Cheng, A.; Ye, W.; Wang, R.; Cheng, J.; Ma, C. Potent antifouling agents albofungins target multiple fouling organisms. U.S. Patent US20230086634A1, 23 March 2023.
- Allegrone, G.; Carmagnola, I.; Ceresa, C.; Chiono, V.; Ciardelli, G.; Fracchia, L. Rhamnolipid coating of medical devices. World Patent WO2022225444A1, 27 October 2022.
- Farmer, S.; Alibek, K. Materials and methods for enhanced treatment and prevention of biofilms. World Patent WO2020190699A1, 24 September 2020.
- Parreira, A.G.; Bastos, Gonçalves, D.B.; Magalhães, J.T., Pires, M.E.E; Granjeiro, P.A. Processo de produção de surfactina por bacillus subtilis ATCC 19659 e uso para disruptura de biofilme. Brazil Patent BR102014014185A2, 19 April 2016.
- Jabaji, S. Method of using biosurfactant-producing bacteria against fungal and bacterial pathogens. U.S. Patent US2023106836A1, 6 April 2023.
- Monsul, N.T.; Berkes, E.A.; Boehm, F.T. Protective barrier compositions, and uses thereof. U.S. Patent US20220378049A1, 1 December 2022.
- Junqueira, J.C.; Fuchs, H.B.; Mylonakis, E. Probiotic bacteria-directed prevention or treatment of fungal infection. U.S. Patent US11478516, 25 October 2022.
- Simmons, S.; Parkar, S.; Miller, E.A.; Kovarik, J.E. Topical Application of Lactobacillus Crispatus to Ameliorate Barrier Damage and Inflammation. U.S. Patent US20230131201A1, 27 April 2017.
- Ochrombel, I.; Speckmann, B.; Pelzer, S.; Schwarm, M.; Pfefferle W. Synbiotic compositions. U.S. Patent US20220088091A1, 24 March 2023.
- Kovarik, J.E. Method and System to Improve the Health of a Person's Skin Microbiome. U.S. Patent US20220118031A1, 21 April 2022.
- Kazmi, I.; Beg, S.; Rahman, M.; Al-Abbasi, F.A.; Afzal, M.; Altayeb, H.N. Chemotherapeutic self-nanoemulsifying drug delivery systems and uses thereof. U.S. Patent US11547690B1, 1 October 2023.
- Apadopoulou, V.; Dayton, P.A.; Conlon, S.E.; Conlon B.P.; Durham, P.G.; Borden, M.A. Methods and systems for enhancing delivery of therapeutic agents to biofilms using low boiling point phase change contrast agents. U.S. Patent US2023173070A1, 6 August 2023.
- Leighton, A. Combination treatment of rhamnolipid and niclosamide. World Patent WO2021222589A1, 4 November 2021.
- Farmer, S. Biosurfactant formulations for use in skincare and wound treatment. World Patent WO2023076663A1, 4 May 2023.
- Dell’Acqua, G.; Scoca, P.; Peralta, R. Nano- or micro-emulsion compositions and methods of use thereof. World Patent WO2022164804A1, 4 August 2022.
- Hässler, T. Cosmetic composition comprising cellodextrins. World Patent WO2021224463A1, 11 November 2021.
- Grainger, D.S.; Westwood, N.J., Mcardle-Ismaguilov, T.A. Detergent composition. World Patent WO2023041694A1, 23 March 2023.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




