Submitted:
15 July 2023
Posted:
18 July 2023
You are already at the latest version
Abstract
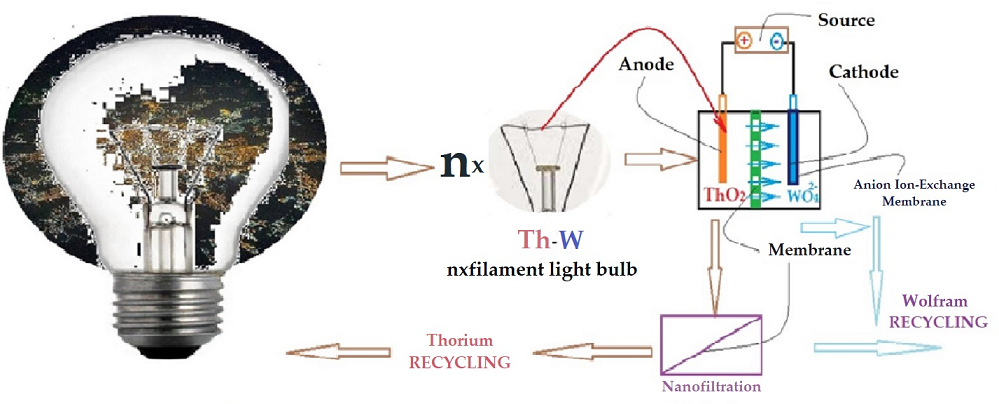
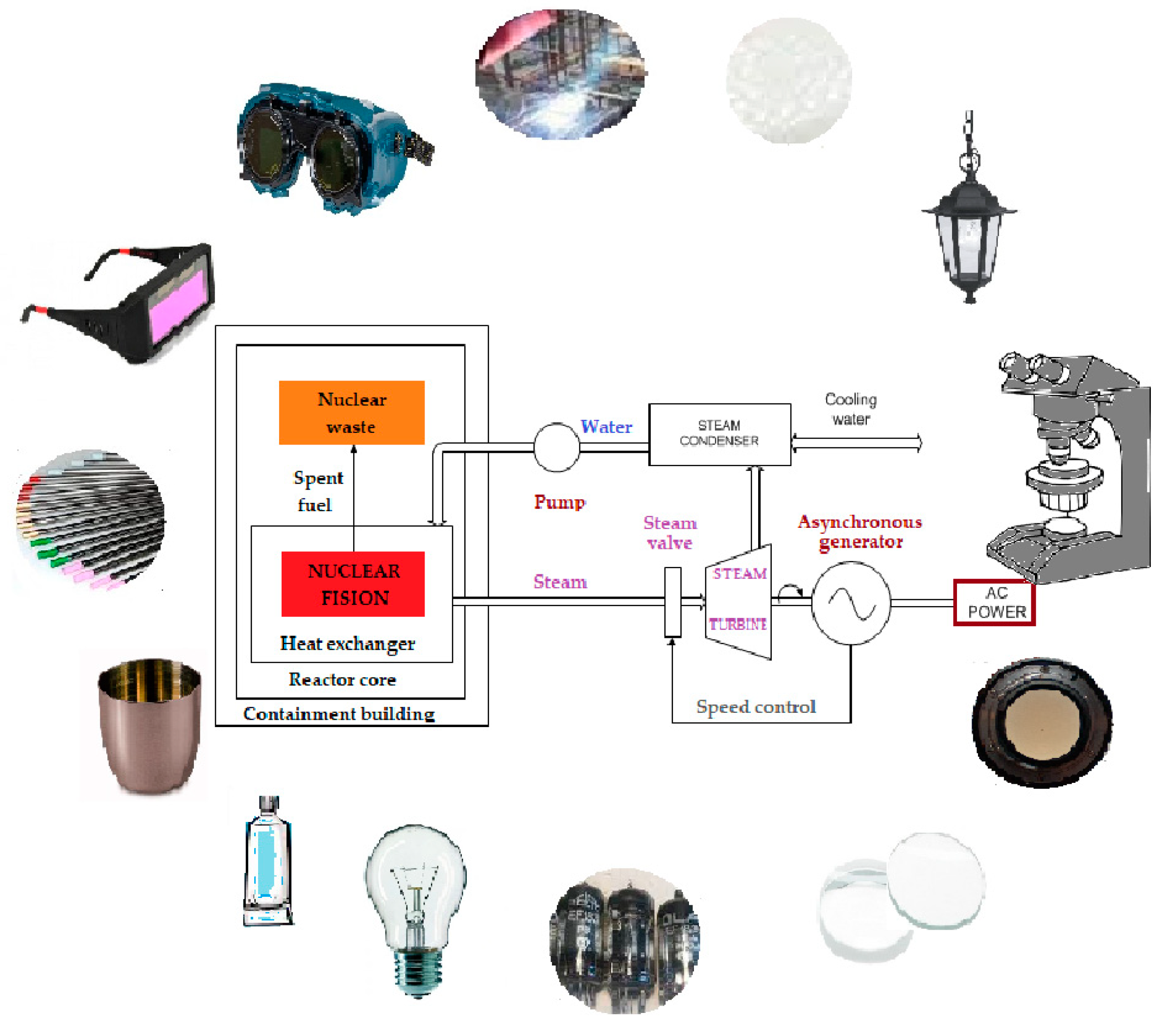
Keywords:
1. Introduction
2. Applications of thorium
3. Toxicity and bio-medical implications
- the amounts of thorium in the environment can be accidentally increased during processing;
- humans absorb thorium through food or drinking water (in areas adjacent to mining operations);
- the quantities in the air are very small (insignificant and generally neglected);
- near hazardous waste storage or processing sites;
- industrial laboratories or mining laboratories, milling minerals containing thorium.
- the chances of developing lung diseases;
- occurrence of lung and pancreas cancer;
- changes in the genetic material;
- blood cancer;
- develop liver diseases (when injecting thorium for x-rays);
- stored in bones (long-term exposure) can lead to the generation of bone cancer.
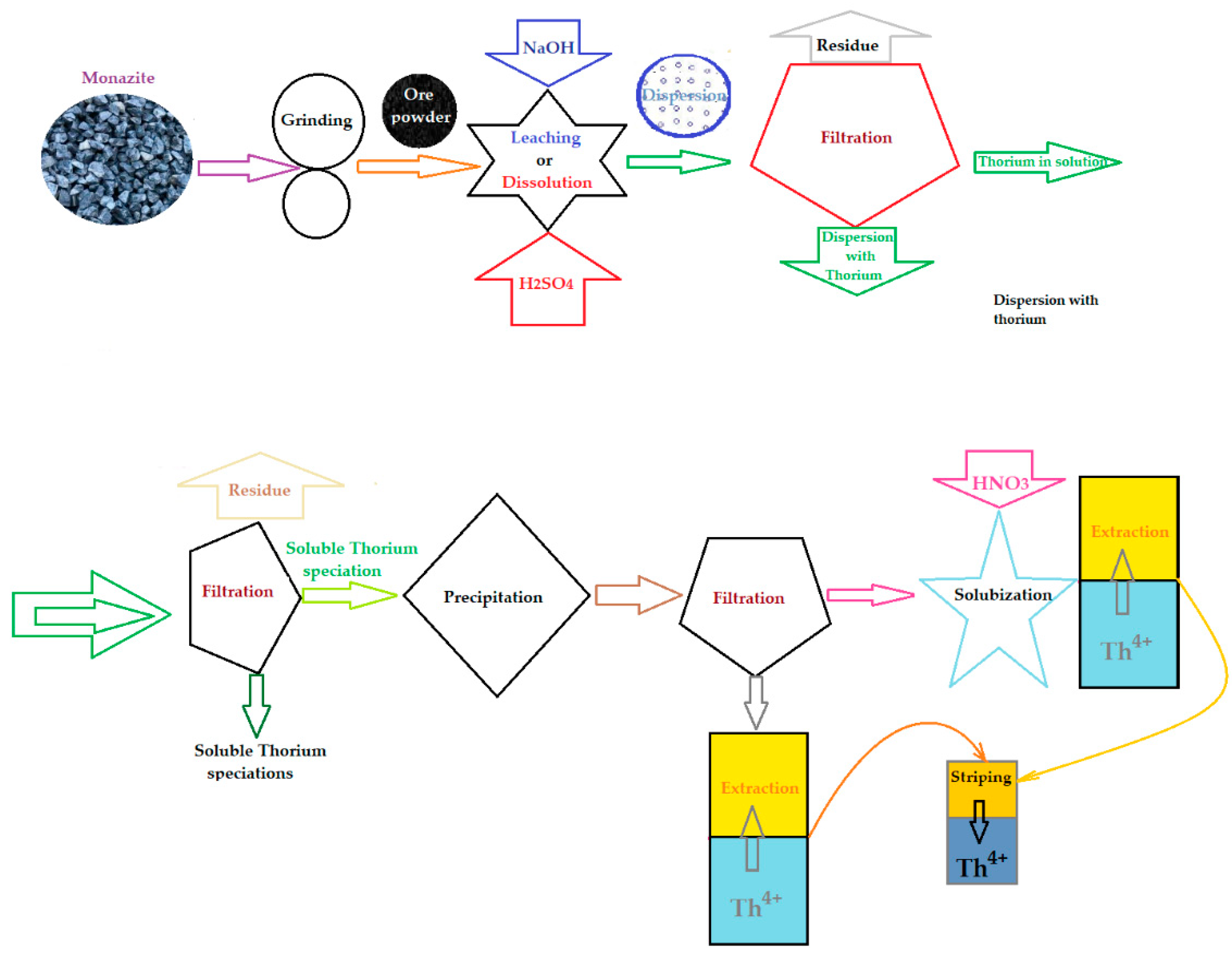
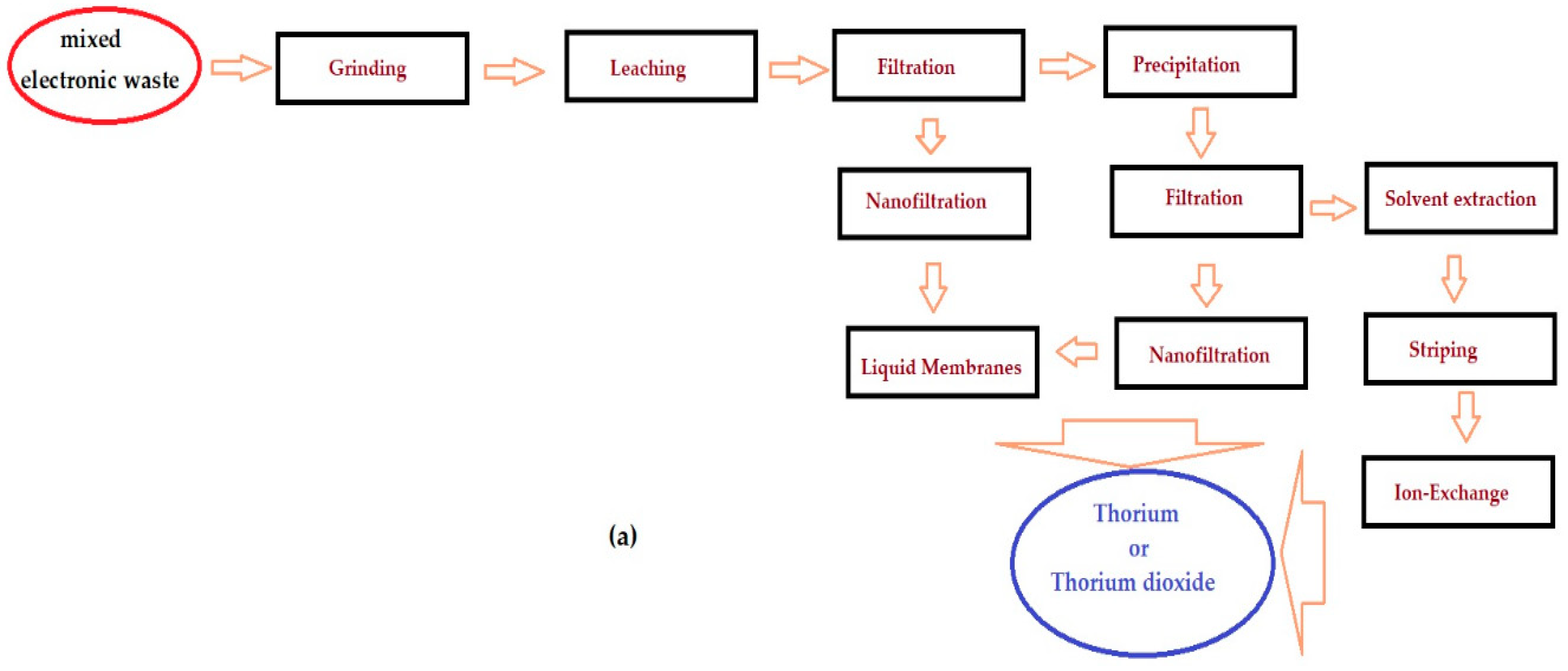
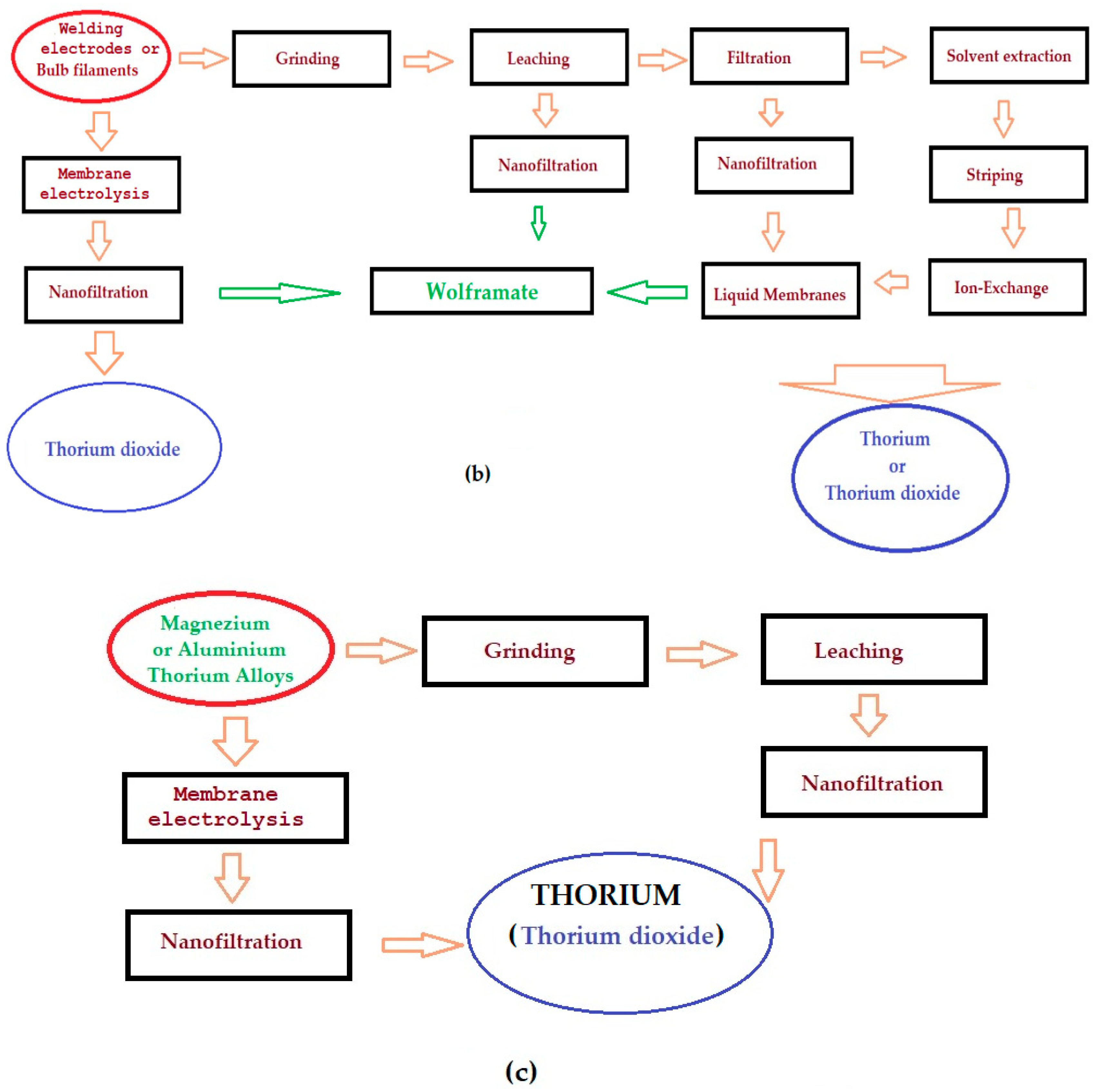
4. Classical technology
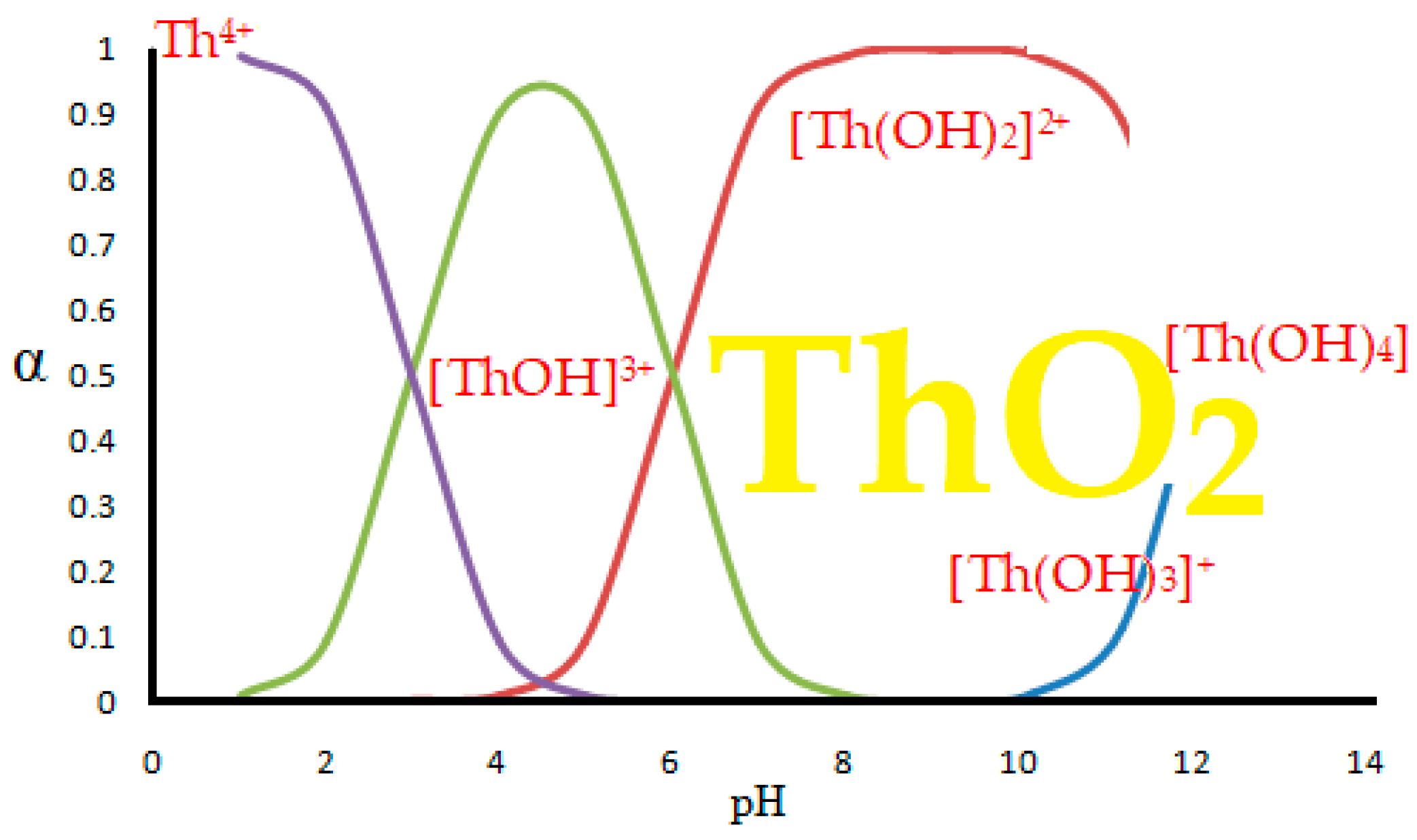
5. Thorium speciation
6. Thorium determination
| Analytical Methods | Samples and/or applications | Refs. |
|---|---|---|
| Inductively Coupled Plasma Mass Spectrometry | Microwave Digestion Technique for the Analysis of Rare Earth Elements, Thorium and Uranium in Geochemical Certified Reference Materials and Soils | [97] |
| Quadrupole-ICP-MS | Determination of trace element concentrations and stable lead, uranium and thorium isotope ratios by in NORM and NORM-polluted sample leachates | [98] |
| Inductively coupled plasma–atomic emission spectrometric | Chemical separation and determination of seventeen trace metals in thorium oxide matrix using a novel extractant – Cyanex-923 | [99] |
| ICP-AES with MSF | Determination of Th and U | [100] |
| Inductively coupled plasma-atomic emission spectrometry | Determination of trace thorium in uranium dioxide | [101] |
| ICP-AES after matrix solvent extraction | Determination of REE, U, Th, Ba, and Zr in simulated hydrogeological leachates | [102] |
| Inductively coupled plasma mass spectrometry and on-line-coupled size-exclusion chromatography. | Determination of thorium and light rare-earth elements in soil water and its high molecular mass organic fractions | [103] |
| ICP-OES | Determination of Trace Thorium and Uranium Impurities in Scandium with High Matrix | [104] |
| Electrothermal atomization atomic absorption spectrometry | Thorium, zirconium, and vanadium as chemical modifiers in the determination of arsenic | [105] |
| Cyclic voltametric | Uranyl ion in sulfuric acid solutions. Application to some nuclear materials characterization. |
[106] |
| Chemically modified electrode | Determination of thorium by adsorptive type with a poly-complex system |
[107] |
| Fluorogenic thorium sensors | Based on 2,6-pyridinedicarboxylic acid-substituted tetraphenylethenes with aggregation-induced emission characteristics | [108] |
| Selective optode | Design and evaluation of a thorium (IV) | [109] |
| Electrothermal vaporization — inductively coupled plasma-atomic emission spectrometry | Trace metal determination in uranium and thorium compounds without prior matrix separation | [110] |
| Micellar electrokinetic chromatographic | Analysis of thorium, uranium, copper, nickel, cobalt and iron in ore and fish samples | [111] |
| laser induced breakdown spectrometry | Determination of trace constituents in thoria | [112] |
| preconcentration and inductively coupled plasma-mass spectrometric (ICP-MS) | Determination of thorium(IV), titanium(IV), iron(III), lead(II) and chromium(III) on 2-nitroso-1-naphthol impregnated MCI GEL CHP20P resin | [113] |
| laser induced breakdown spectrometry | Determination of trace constituents in thoria | [114] |
| Electrochemical and spectro-electro-chemical | Studies of bis(diketonate) thorium(IV) and uranium(IV) porphyrins | [115] |
| Electrochemical detector based on a modified graphite electrode | With phthalocyanine for the elemental analysis of actinides | [116] |
| selective extraction and trace determination of thorium | Synthesis of UiO-66-OH zirconium metal-organic framework and its application in water samples by spectrophotometry | [117] |
| Anodic polarization of thorium and electrochemical impedance spectroscopy | Study at tungsten, cadmium and thorium electrodes | [118] |
| High performance liquid chromatographic | Studies on lanthanides, uranium and thorium on amide modified reversed phase supports | [119] |
| Extraction of thorium on resin | Using a commercially available extraction chromatographic resin | [120] |
| ion exchange and extraction chromatography | Separation of actinium from proton-irradiated thorium metal | [121] |
7. Thorium separation and/or pre-concentration [122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140]
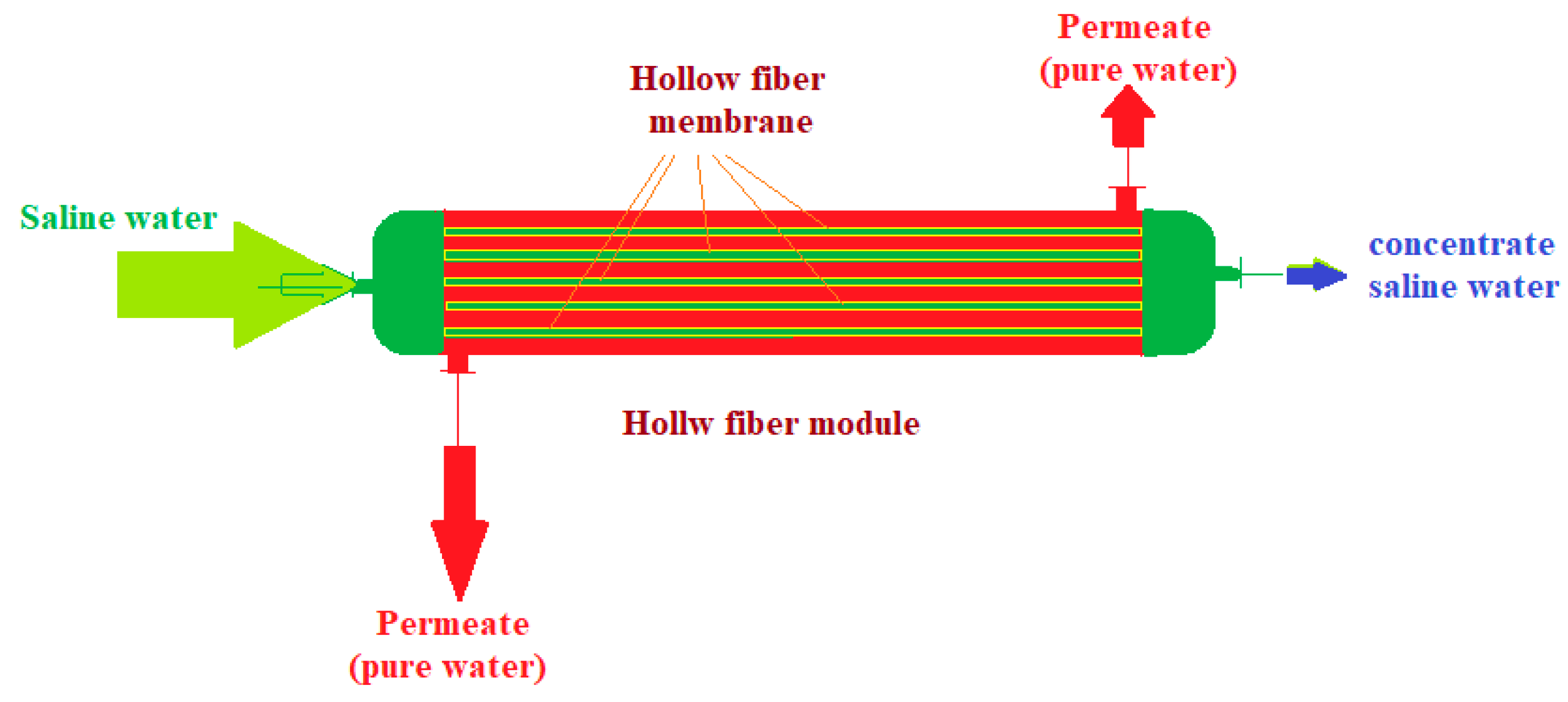
8. Membrane and membrane processes
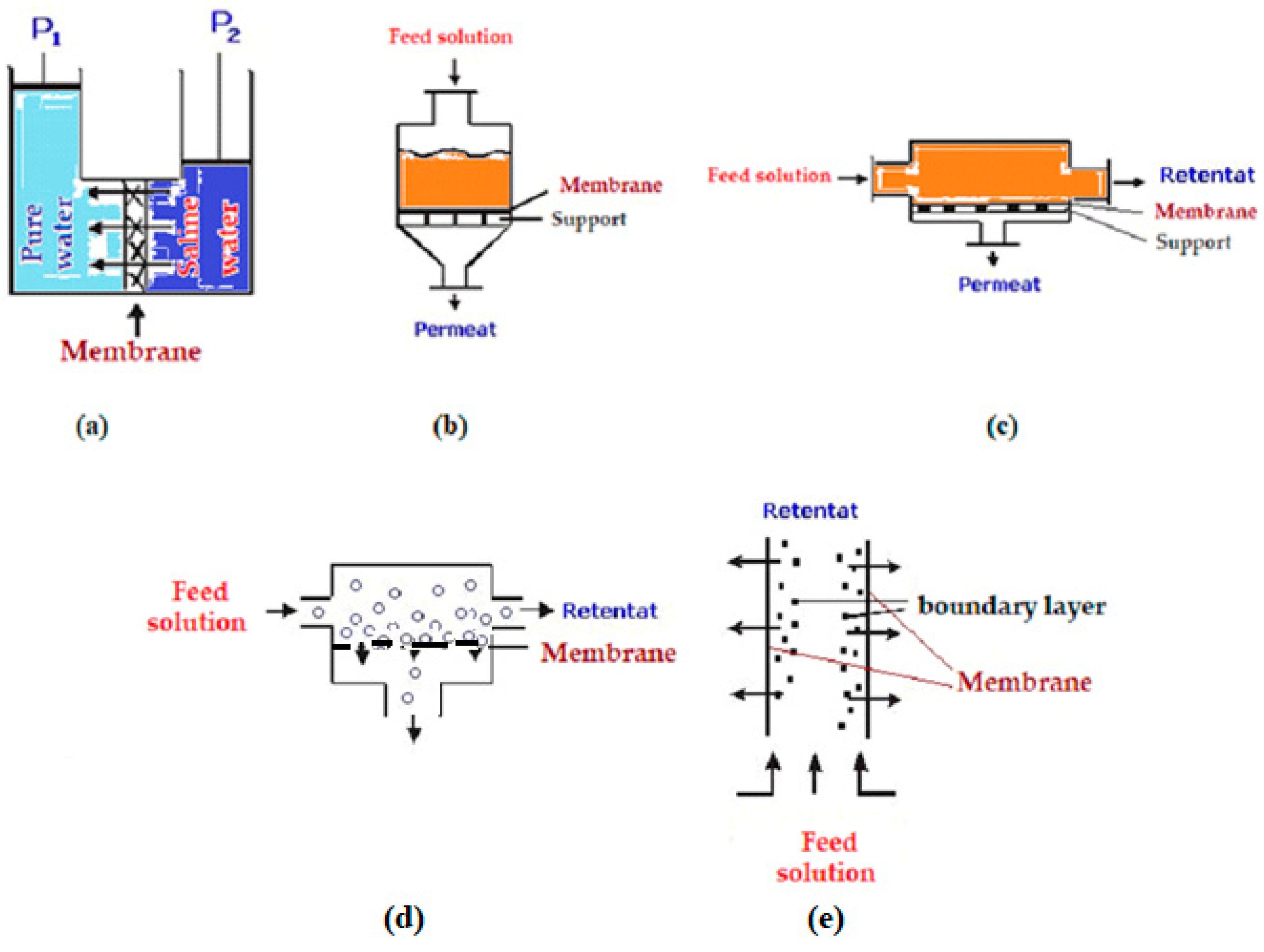
8.1. Introduction in membrane and membrane processes
- P = transmembrane pressure difference;
- Δc = concentration difference between the two compartments separated by a membrane;
- ΔE = potential difference.
8.2. Barro membrane processes
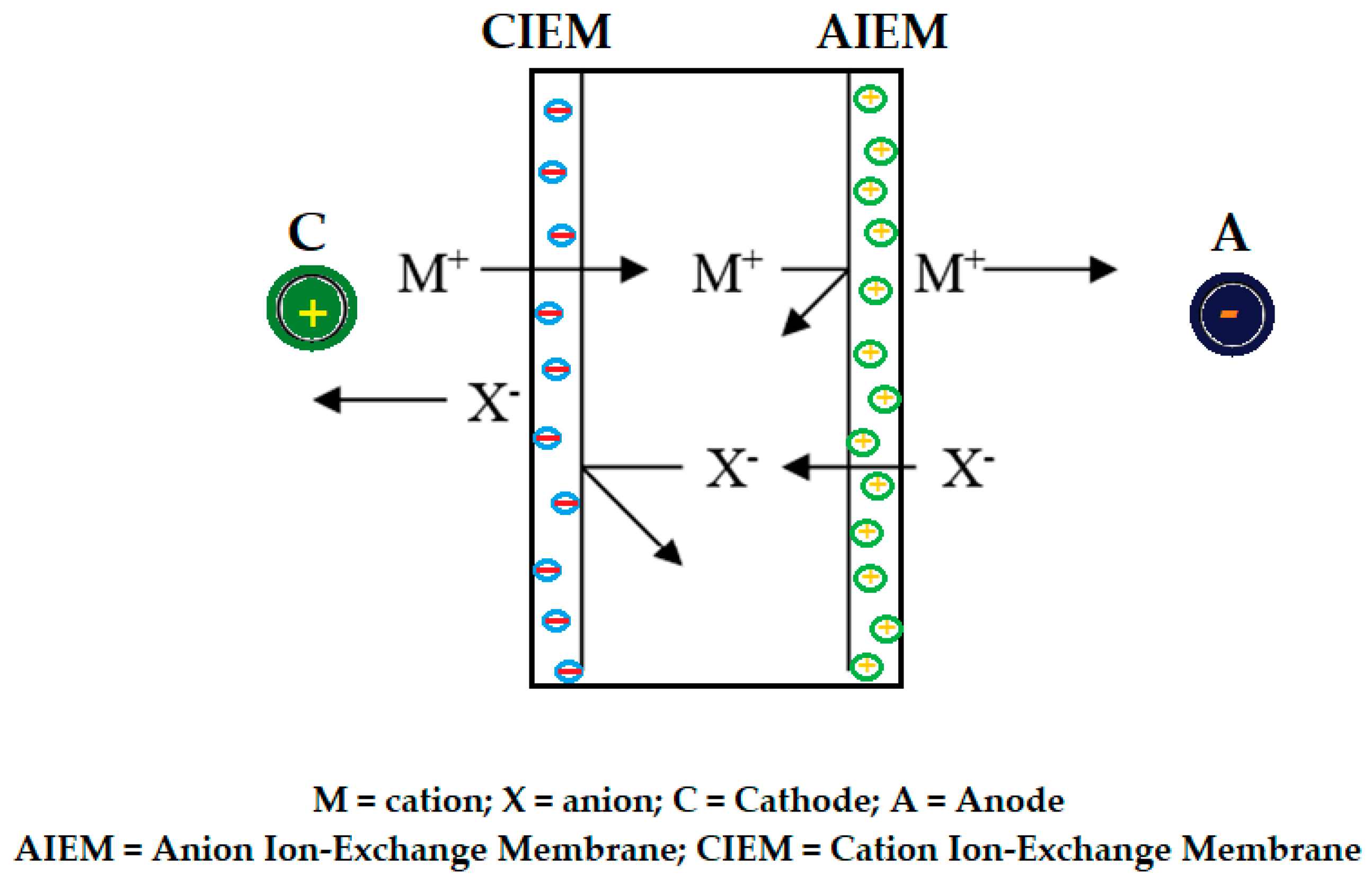
8.3. Electro-membrane processes
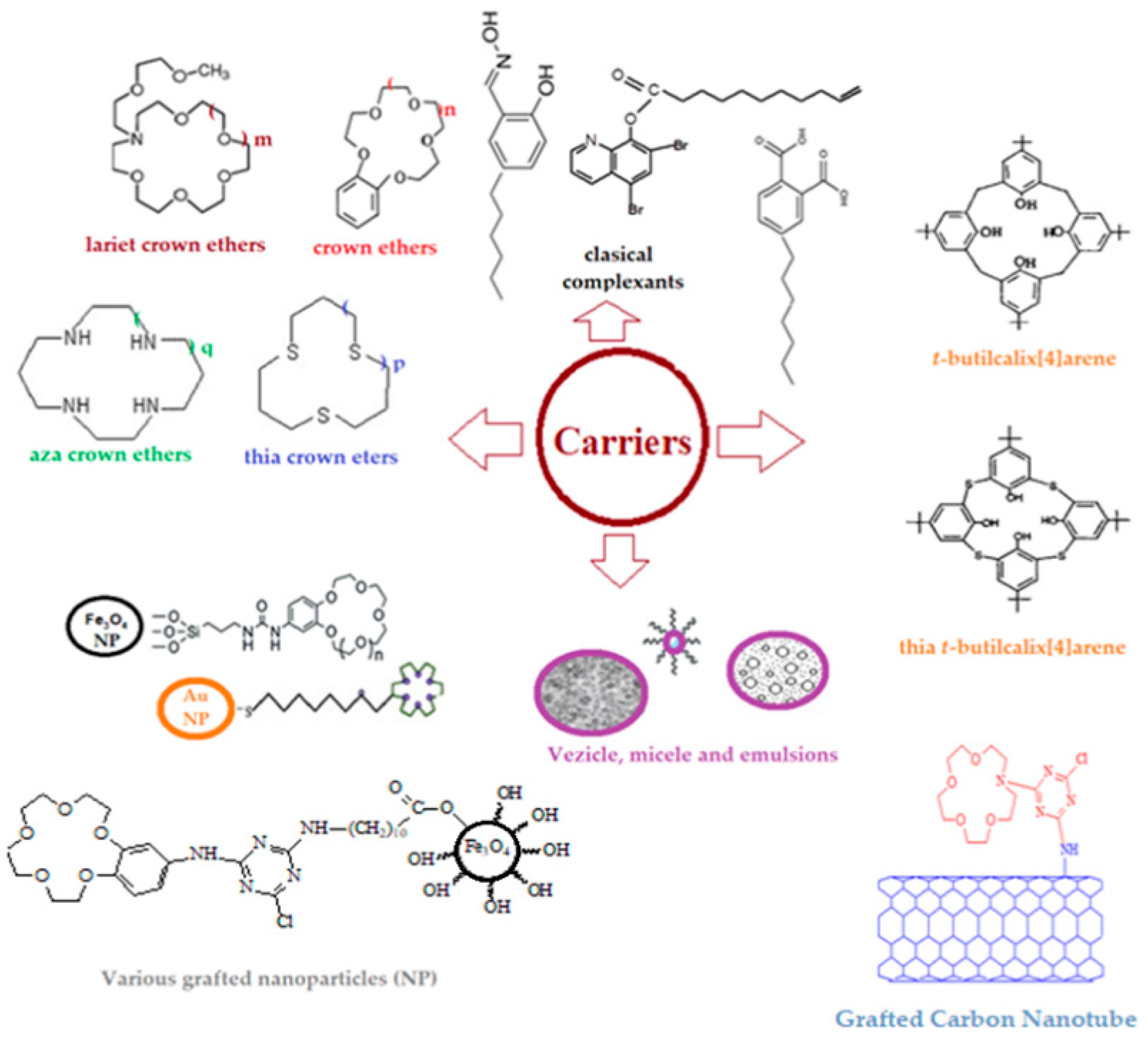
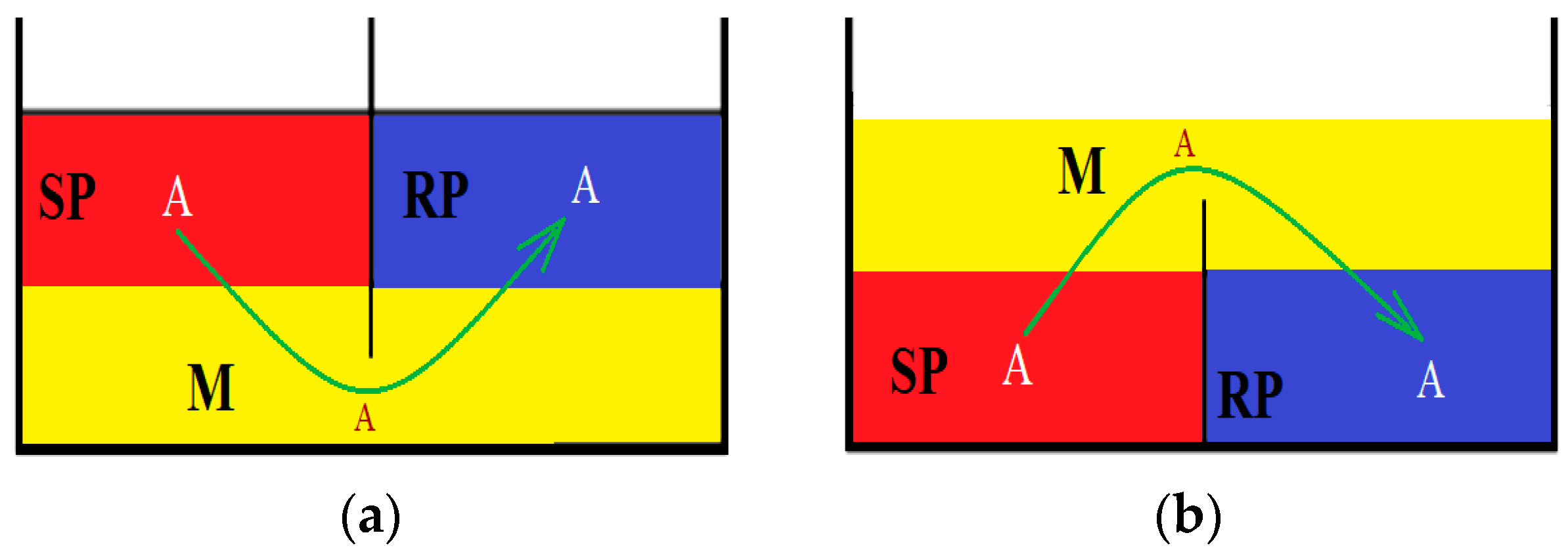
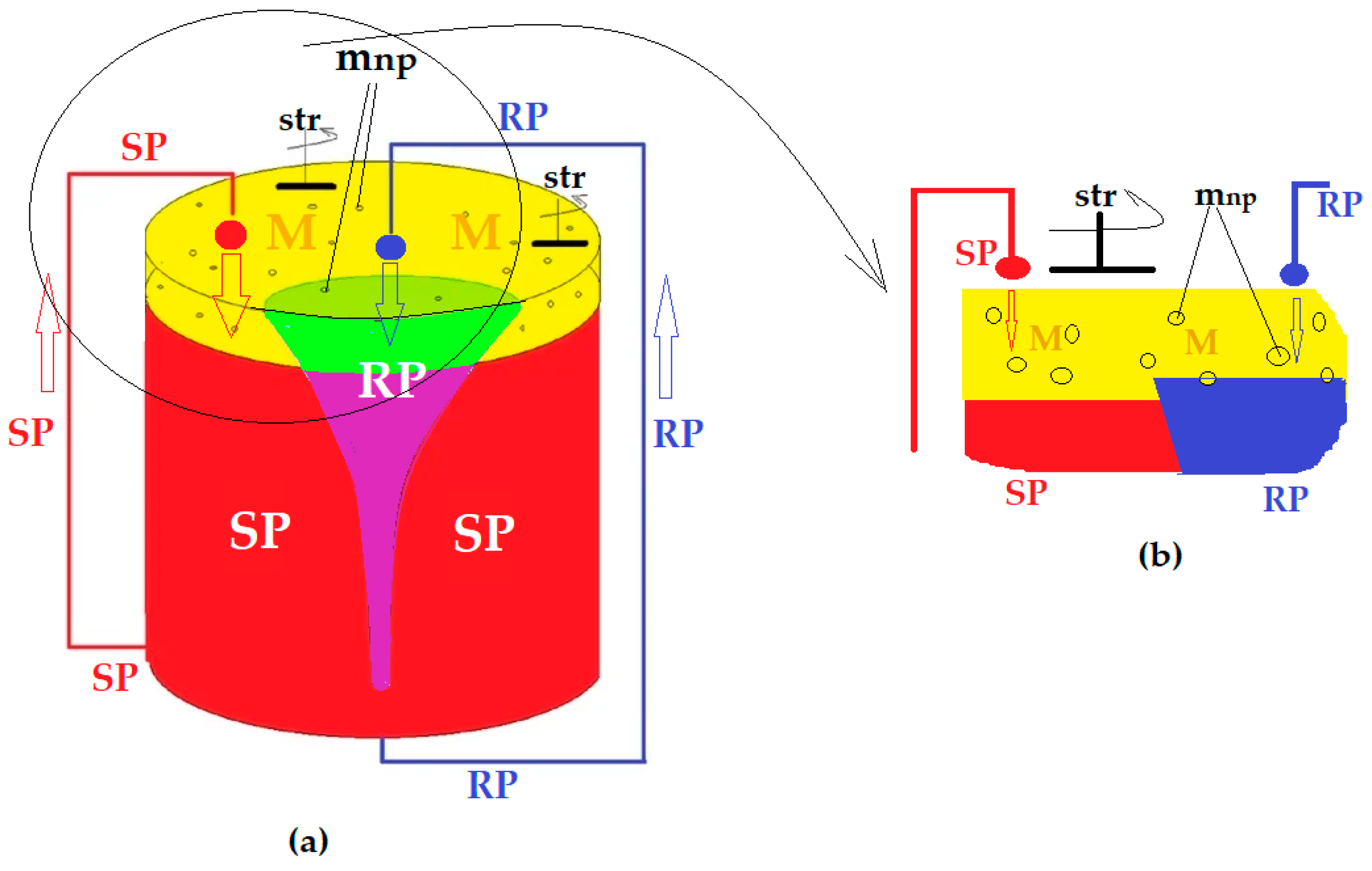
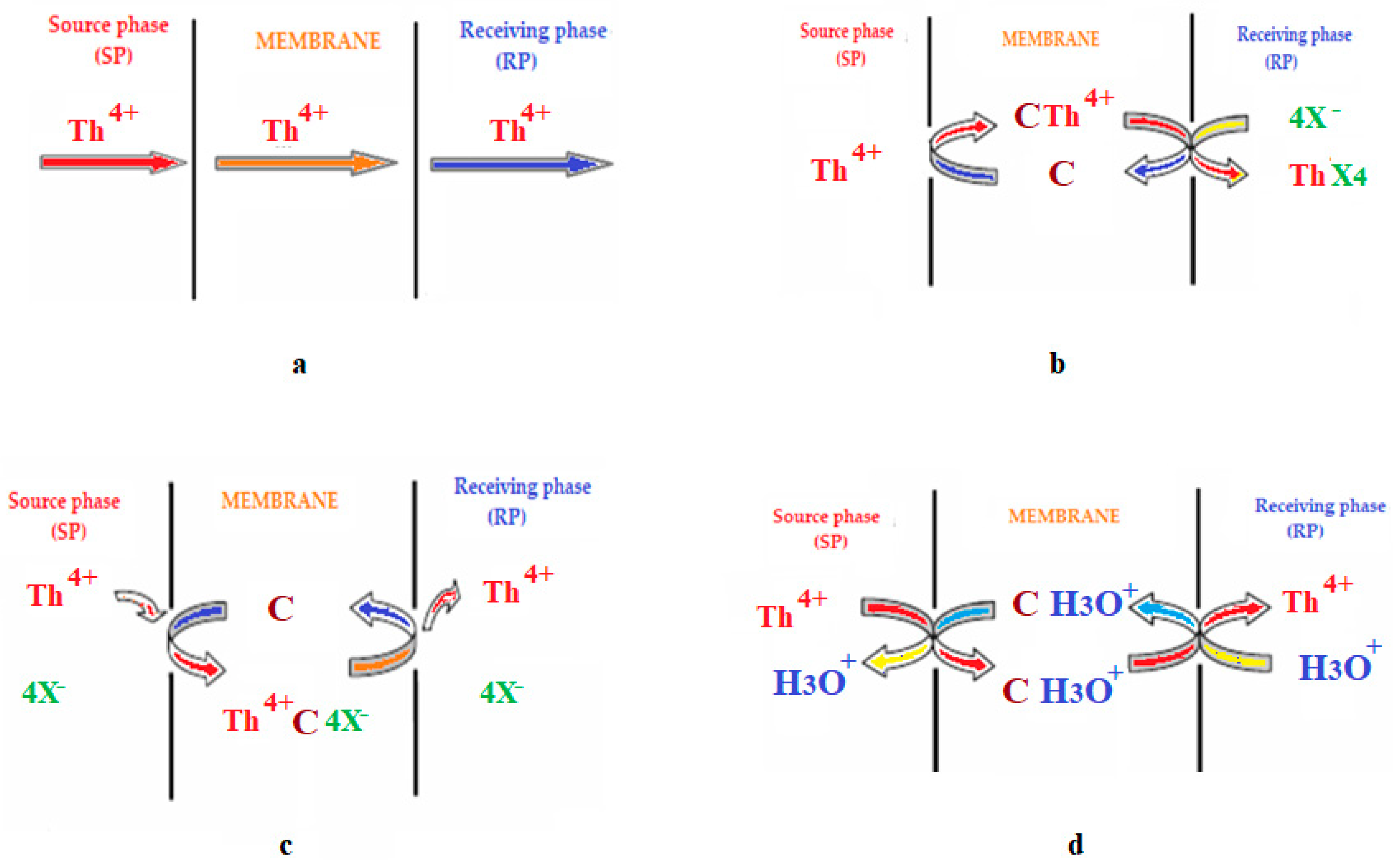
8.4. Membrane processes carried out under a concentration gradient (liquid membrane)
8.5. Transport in liquid membranes
8.5.1. Physical “simply” shipping
8.5.2. Facilitated transport or carrier-mediated transport
8.5.3. Coupled co- or counter-transport
8.6. Hybrid membrane processes
9. Problems in application, achievements and development perspectives of an urban thorium mining
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| D | dialysis. |
| ED | electrodialysis. |
| EDI | reverse electrodialysis. |
| MF | microfiltration. |
| UF | ultrafiltration. |
| NF | nanofiltration |
| RO | reverse osmosis. |
| DM | membrane distillation. |
| SG | gas separation. |
| PV | pervaporation. |
| ELM | liquid membrane extraction. |
| TC | facilitated transport. |
| BLM (MLV) | Bulk liquid membranes |
| HLM | hybrid liquid membranes |
| HFCLM | liquid membranes thin capillary or tubular fibers |
| HFLM | hollow fiber liquid membranes |
| HMS | hybrid multi-membrane systems |
| P | precipitation |
| F | filtration |
| M | milling |
| E | extraction |
| S | striping |
| RE | re-extraction |
| N | neutralization |
| TBP | tri-butyl phosphate |
| REE | rare earth elements |
References
- Jyothi, R.K.; De Melo, L.G.T.C.; Santos, R.M.; Yoon, H.S. An overview of thorium as a prospective natural resource for future energy. Frontiers in Energy Research, 2023, 11, 1132611. [CrossRef]
- Takaki, N.; Mardiansah, D. Core Design and Deployment Strategy of Heavy Water Cooled Sustainable Thorium Reactor. Sustainability 2012, 4, 1933-1945. [CrossRef]
- Takaki, N.; Sidik, P.; Sekimoto, H. Feasibility of Water Cooled Thorium Breeder Reactor Based on LWR Technology; American Nuclear Society: La Grange Park, IL, USA, 2007; 1733–1738.
- Sidik, P.; Takaki, N.; Sekimoto, H. Feasible region of design parameters for water cooled thorium breeder reactor. J. Nucl. Sci. Technol. 2007, 44, 946–957.
- Sidik, P.; Takaki, N.; Sekimoto, H. Breeding capability and void reactivity analysis of heavy-water-cooled thorium reactor. J. Nucl. Sci. Technol. 2008, 45, 589–600. [CrossRef]
- Ault, T.; Krahn, S.; Croff, A. Comparing the environmental impacts of uranium- and thorium-based fuel cycles with different recycle options, Progress in Nuclear Energy, Volume 2017, 100, 114-134. [CrossRef]
- Perron, R.; Gendron, D.; Causey, P.W. Construction of a thorium/actinium generator at the Canadian Nuclear Laboratories, Applied Radiation and Isotopes, Volume 164, 2020, 109262, ISSN 0969-8043. [CrossRef]
- https://scholar.google.com/scholar?q=Thorium+reserve&hl=en&as_sdt=0,5 (accessed on June 24th, 2023).
- Cazalaà, J.B. Radium and thorium applications for the general public: unexpected consequences of the discovery from Pierre and Marie Curie. The Journal of the American Society of Anesthesiologists, 2012, 117(6), pp.1202–1202. [CrossRef]
- Pohjalainen, I.; Moore, I.D.; Geldhof, S.; Rosecker, V.; Sterba, J.; Schumm, T. Gas cell studies of thorium using filament dispensers at IGISOL, Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms, 2020, 484, 59-70. [CrossRef]
- Lavi, N.; Alfassi, Z.B.; Development of Marinelli beaker standards containing thorium oxide and application for measurements of radioactive environmental samples. Radiation Measurements, 2005, 39(1), 15-19. [CrossRef]
- Mas, J.L.; Villa, M.; Hurtado, S.; García-Tenorio, R. Determination of trace element concentrations and stable lead, uranium and thorium isotope ratios by quadrupole-ICP-MS in NORM and NORM-polluted sample leachates, Journal of Hazardous Materials, 2012, 205–206, 198-207. [CrossRef]
- Hassan, H.J.; Hashim, S.; Sanusi, M.S.M.; Bradley, D.A.; Alsubaie, A.; Tenorio, R.G.; Bakri, N.F.; Tahar, R.M. The Radioactivity of Thorium Incandescent Gas Lantern Mantles. Appl. Sci. 2021, 11, 1311. [CrossRef]
- Das, S.; Kaity, S.; Kumar, R.; Banerjee, J.; Roy, S.B.; Chaudhari, G.P.; Daniel, B.S.S., Determination of Room Temperature Thermal Conductivity of Thorium—Uranium Alloys. In Thorium—Energy for the Future: Select Papers from ThEC15, 2019. pp. 277-286. Springer Singapore.
- Dabare, P.R., Mahakumara, P.D. and Mahawatte, P., Method Validation of In-Situ Gamma Spectroscopy for Quantification of Naturally Occurring Radioactive Materials (NORM) K-40, TH-232 and U-238 in Soil. In Management of Naturally Occurring Radioactive Material (NORM) in Industry. Proceedings of an International Conference. Supplementary Files. 2022.
- Vasiliev, A.N.; Severin, A.; Lapshina, E.; Chernykh, E.; Ermolaev, S.; Kalmykov, S. Hydroxyapatite particles as carriers for 223 Ra. J. Radioanal. Nucl. Chem. 311, 1503–1509 (2017). [CrossRef]
- Peterson, S.; Adams, R.E; Douglas Jr, D.A. Properties of thorium, its alloys, and its compounds 1965, (No. ORNL-TM-1144), Oak Ridge National Lab., Tenn. (accessed on June, 24, 2023).
- Zhang, Y.; Shao, X.; Yin, L.; Ji, Y. Estimation of Inhaled Effective Doses of Uranium and Thorium for Workers in Bayan Obo Ore and the Surrounding Public, Inner Mongolia, China. Int. J. Environ. Res. Public Health 2021, 18, 987. [CrossRef]
- Stojsavljević, A.; Borković-Mitić, S.; Vujotić, L.; Grujičić, D.; Gavrović-Jankulović, M.; Manojlović, D., The human biomonitoring study in Serbia: background levels for arsenic, cadmium, lead, thorium and uranium in the whole blood of adult Serbian population. Ecotoxicology and environmental safety 2019, 169, 402-409. [CrossRef]
- International Commission on Radiological Protection (ICRP). ICRP Publication 142: Radiological protection from naturally occurring radioactive material (NORM) in industrial processes. Ann. ICRP 2019, 48, 5–67.
- Guo, P.; Duan, T.; Song, X.; Xu, J.; Chen, H. Effects of soil pH and organic matter on distribution of thorium fractions in soil contaminated by rare-earth industries. Talanta 2008, 77(2) 624-627. [CrossRef]
- Hayes, C.T.; Fitzsimmons, J.N.; Boyle, E.A.; McGee, D.; Anderson, R.F.; Weisend, R.; Morton, P.L. Thorium isotopes tracing the iron cycle at the Hawaii Ocean Time-series Station ALOHA. Geochimica et Cosmochimica Acta, 2015,169, 1-16. [CrossRef]
- Suarez-Navarro, J.A.; Pujol, Ll.; Suarez-Navarro M.J. Determination of specific alpha-emitting radionuclides (uranium, plutonium, thorium and polonium) in water using [Ba+Fe]-coprecipitation method,Applied Radiation and Isotopes, 2017,130, 162-171. [CrossRef]
- Chaudhury, D.; Sen, U.; Sahoo, B.K.; Bhat, N.N.; Kumara, S.; Karunakara, N.; Biswas, S.; Shenoy, S.; Bose, B., Thorium promotes lung, liver and kidney damage in BALB/c mouse via alterations in antioxidant systems. Chemico-Biological Interactions, 2022. 363, 109977. [CrossRef]
- Wickstroem, K.; Hagemann, U.B.; Kristian, A.; Ellingsen, Ch.; Sommer, A.; Ellinger-Ziegelbauer, H.; Wirnitzer, U.; Hagelin, E-M.; Larsen, A.; Smeets, R.; Bjerke, R.M.; Karlsson, J.; Ryan, O.B.; Wengner, A.M.; Linden, L.; Mumberg, D.; Cuthbertson, A.S. Preclinical Combination Studies of an FGFR2 Targeted Thorium-227 Conjugate and the ATR Inhibitor BAY 1895344, International Journal of Radiation Oncology*Biology*Physics, 2019, 105(2), 410-422. [CrossRef]
- Yantasee, W.; Sangvanich, T.; Creim, J.A.; Pattamakomsan, K.; Wiacek, R.J.; Fryxell, G.E.; Addleman, R.S.; Timchalk, C. Functional sorbents for selective capture of plutonium, americium, uranium, and thorium in blood. Health physics, 2010, 99(3), 413-419. [CrossRef]
- Kumar, A.; Mishra, P.; Ghosh, S.; Sharma, P.; Ali, M.; Pandey, B.N.; Mishra, K.P. Thorium-induced oxidative stress mediated toxicity in mice and its abrogation by diethylenetriamine pentaacetate. International Journal of Radiation Biology, 2008, 84(4), 337-349. [CrossRef]
- Chen, X.A., Cheng, Y.E. and Rong, Z., Recent results from a study of thorium lung burdens and health effects among miners in China. J. Radiol. Prot. 2005, 25, 451. [CrossRef]
- Chaudhury, D.; Sen, U.; Sahoo, B.K., Bhat, N.N.; Kumara, S.; Karunakara, N.; Biswas, S.; Shenoy, S.; Bose, B. Thorium promotes lung, liver and kidney damage in BALB/c mouse via alterations in antioxidant systems. Chemico-Biological Interactions, 2022, 363, 109977. [CrossRef]
- Zapadinskaia, E.E.; Gasteva, G.N.; Titiova, I.N. Analysis of health state in individuals exposed to thorium and chemical hazards in occupational environment. Med. Tr. Promyshlennaia Ekol. 2005, 11, 14–19.
- Polednak, A.P.; Stehney, A.F.; Lucas, H.F. Mortality among male workers at a thorium-processing plant. Health Phys. 1983, 44, 239–251. [CrossRef]
- Yin, L.L.; Tian, Q.; Shao, X.Z.; Shen, B.M.; Su, X.; Ji, Y.Q. ICP-MS measurement of uranium and thorium contents in minerals in China. Nucl. Sci. Tech. 2016, 27, 10. [CrossRef]
- Shao, X.Z.; Xu, Y.; Zhang, Y.; Yin, L.L.; Kong, X.Y.; Ji, Y.Q. Monitoring of ultra-trace uranium and thorium in six-grade particles. Chemosphere 2019, 233, 76–80. [CrossRef]
- Rump, A.; Hermann, C.; Lamkowski, A.; Popp, T.; Port, M. A comparison of the chemo-and radiotoxicity of thorium and uranium at different enrichment grades. Arch Toxicol 2023, 97, 1577–1598. [CrossRef]
- Gao, L.; Kano, N.; Sato, Y.; Li, C.; Zhang, S.; Imaizumi, H. Behavior and distribution of heavy metals including rare earth elements, thorium, and uranium in sludge from industry water treatment plant and recovery method of metals by biosurfactants application. Bioinorganic Chemistry and Applications, 2012, SN 1565-3633. [CrossRef]
- von Gunten, H.R.; Roessler, E.; Lowson, R.T.; Reid, P.D.; Short, S.A. Distribution of uranium- and thorium series radionuclides in mineral phases of a weathered lateritic transect of a uranium ore body, Chemical Geology, 1999, 160(3), 225-240. [CrossRef]
- Eyal, Y.; Olander, D.R. Leaching of uranium and thorium from monazite: I. Initial leaching. Geochim. Et Cosmochim. Acta 1990, 54, 1867–1877. [CrossRef]
- Abdel-Rehim, A.M. An innovative method for processing Egyptian monazite. Hydrometallurgy 2002, 67, 9–17.
- Vijayalakshmi, R.; Mishra, S.L.; Singh, H.; Gupta, C.K. Processing of xenotime concentrate by sulphuric acid digestion and selective thorium precipitation for separation of rare earths. Hydrometallurgy 2001, 61, 75–80. [CrossRef]
- Lapidus, G.T.; Doyle, F.M. Selective thorium and uranium extraction from monazite: I. Single-stage oxalate leaching. Hydrometallurgy 2015, 154, 102–110. [CrossRef]
- Lapidus, G.T.; Doyle, F.M. Selective thorium and uranium extraction from monazite: II. Approaches to enhance the removal of radioactive contaminants. Hydrometallurgy 2015, 155, 161–167. [CrossRef]
- El-Nadi, Y.A.; Daoud, J.A.; Aly, H.F. Modified leaching and extraction of uranium from hydrous oxide cake of Egyptian monazite. Int. J. Miner. Process. 2005, 76, 101–110. [CrossRef]
- Amer, T.E.; Abdella, W.M.; Wahab, G.M.A.; El-Sheikh, E.M. A suggested alternative procedure for processing of monazite mineral concentrate. Int. J. Miner. Process. 2013, 125, 106–111. [CrossRef]
- Borai, E.H.; Abd El-Ghany, M.S.; Ahmed, I.M.; Hamed, M.M.; Shahr El-Din, A.M.; Aly, H.F. Modified acidic leaching for selective separation of thorium, phosphate and rare earth concentrates from Egyptian crude monazite. Int. J. Miner. Process. 2016, 10, 34–41. [CrossRef]
- Parzentny, H.R.; Róg, L. The Role of Mineral Matter in Concentrating Uranium and Thorium in Coal and Combustion Residues from Power Plants in Poland. Minerals 2019, 9, 312. [CrossRef]
- Evseeva, T.; Geras’kin, S.; Majstrenko, T.; Brown, J.; Belykh, E. Comparative estimation of 232 th and stable Ce (III) toxicity and detoxification pathways in freshwater alga chlorella vulgaris. Chemosphere 2010, 81, 1320–1327. [CrossRef]
- Selvig, L.K.; Inn, K.G.W.; Outola, I.M.J.; Kurosaki, H.; Lee, K.A. Dissolution of resistate minerals containing uranium and thorium: Environmental implications. J. Radioanal. Nucl. Chem. 2005, 263, 341–348. [CrossRef]
- Borai, E.H.; Abd El-Ghany, M.S.; Ahmed, I.M.; Hamed, M.M.; Shahr El-Din, A.M.; Aly, H.F. Modi fi ed acidic leaching for selective separation of thorium, phosphate and rare earth concentrates from Egyptian crude monazite. Int. J. Miner. Process. 2016, 149. [CrossRef]
- Shaeri, M.; Torab-Mostaedi, M.; Rahbar Kelishami, A. Solvent extraction of thorium from nitrate medium by TBP, Cyanex272 and their mixture. J Radioanal Nucl Chem 303, 2093–2099 (2015). [CrossRef]
- Nasab, M.E., 2014. Solvent extraction separation of uranium (VI) and thorium (IV) with neutral organophosphorus and amine ligands. Fuel, 2015, 116, 595-600. [CrossRef]
- Alex, P.; Hubli, R.C.; Suri, A.K. Processing of rare earth concentrates. Rare Met. 2005, 24, 210–215.
- Panda, R.; Kumari, A.; Jha, M.K.; Hait, J.; Kumar, V.; Kumar, J.R.; Lee, J.Y. Leaching of rare earth metals (REMs) from Korean monazite concentrate. J. Ind. Eng. Chem. 2014, 20, 2035–2042. [CrossRef]
- Kul, M.; Topkaya, Y.; Karakaya, I. Rare earth double sulfates from pre-concentrated bastnasite. Hydrometallurgy 2008, 93, 129–135. [CrossRef]
- Fourest, B.; Lagarde, G.; Perrone, J.; Brandel, V.; Dacheux, N.; Genet, M. Solubility of thorium phosphate-diphosphate. New J. Chem. 1999, 23, 645–649.
- Chi, R.; Xu, Z. A solution chemistry approach to the study of rare earth element precipitation by oxalic acid. Met. Mater Trans B 1999, 30, 189–195. [CrossRef]
- Tomazic, B.; Branica, M. Separation of uranium(VI) from rare earths(III) by hydrolytic precipitation. Inorg. Nucl. Chem. Lett. 1968, 4, 377–380. [CrossRef]
- Kang, M.J.; Han, B.E.; Hahn, P.S. Precipitation and adsorption of uranium (VI) under various aqueous conditions. Environ. Eng. Res. 2002, 7, 149–157. [CrossRef]
- Bearse, A.E.; Calkins, G.D.; Clegg, J.W.; Filbert, J.R.B. Thorium and rare earths from monazite. Chem. Eng. Prog. 1954, 50, 235–239.
- Crouse, D.J.; Brown, K.B. The Amex Process for Extracting Thorium Ores with Alkyl Amines. Ind. Eng. Chem. 1959, 51, 1461–1464. [CrossRef]
- Wang, X.; Huang, W.; Gong, Y.; Jiang, F.; Zheng, H.; Zhu, T.; Long, D.; and Li, Q. Electrochemical behavior of Th (IV) and its electrodeposition from ThF4-LiCl-KCl melt. Electrochimica Acta, 2016, 196, 286-293. [CrossRef]
- Kumar, R.; Gupta, S.; Wajhal, S.; Satpati, S.K.; Sahu, M.L. Effect of process parameters on the recovery of thorium tetrafluoride prepared by hydrofluorination of thorium oxide, and their optimization. Nuclear Engineering and Technology, 2022, 54(5), 1560-1569. [CrossRef]
- Manchanda, V.K. Thorium as an abundant source of nuclear energy and challenges in separation science. Radiochimica Acta, 2023, 111(4), 243-263. [CrossRef]
- Rump, A.; Hermann, C.; Lamkowski, A.; Popp, T.; Port, M. A comparison of the chemo-and radiotoxicity of thorium and uranium at different enrichment grades. Arch Toxicol 97, 1577–1598 (2023). [CrossRef]
- Schmidt, M.; Lee, S.S.; Wilson, R.E.; Soderholm, L.; Fenter, P. Sorption of tetravalent thorium on muscovite. Geochimica et Cosmochimica Acta, 2012, 88, pp.66-76. [CrossRef]
- Chadirji-Martinez, K.; Grosvenor, A.P.; Crawford, A.; Chernikov, R.; Heredia, E.; Feng, R.; Pan, Y. Thorium speciation in synthetic anhydrite: Implications for remediation and recovery of thorium from rare-earth mine tailings. Hydrometallurgy, 2022, 214, 105965. [CrossRef]
- Khan, A.A.; Zada, Z.; Reshak, A.H.; Ishaq, M.; Zada, S.; Saqib, M.; Ismail, M.; Fazal-ur-Rehman, M.; Murtaza, G.; Zada, S.; et al. GGA and GGA + U Study of ThMn2Si2 and ThMn2Ge2 Compounds in a Body-Centered Tetragonal Ferromagnetic Phase. Molecules 2022, 27, 7070. [CrossRef]
- Nisbet, H.; Migdisov, A.; Xu, H.; Guo, X., van Hinsberg, V.; Williams-Jones, A.E.; Boukhalfa, H.; Roback, R. An experimental study of the solubility and speciation of thorium in chloride-bearing aqueous solutions at temperatures up to 250 C. Geochimica et Cosmochimica Acta, 2018, 239, pp.363-373. [CrossRef]
- Oliveira, M.S.; Duarte, I.M.; Paiva, A.V.; Yunes, S.N.; Almeida, C.E. The role of chemical interactions between thorium, cerium, and lanthanum in lymphocyte toxicity. Arch. Environ. Occup. Health 2014, 69, 40–45. [CrossRef]
- Ma, Y.; Wang, J.; Peng, C.; Ding, Y.; He, X.; Zhang, P.; Li, N.; Lan, T.; Wang, D.; Zhang, Z. Toxicity of cerium and thorium on daphnia magna. Ecotoxicol. Environ. Saf. 2016, 134, 226–232. [CrossRef]
- Peng, C.; Ma, Y.; Ding, Y.; He, X.; Zhang, P.; Lan, T.; Wang, D.; Zhang, Z.; Zhang, Z. Influence of Speciation of Thorium on Toxic Effects to Green Algae Chlorella pyrenoidosa. Int. J. Mol. Sci. 2017, 18, 795. [CrossRef]
- Hirose, K. Chemical speciation of trace metals in seawater: a review. Analytical Sciences, 2006, 22(8), pp.1055-1063. [CrossRef]
- Huang, W.; Hongwei, L.; Xin, X.; Guocheng, W. Development status and research progress in rare earth hydrometallurgy in China. J.-Chin. Rare Earth Soc.-Chin. 2006, 24, 129.
- Santschi, P.H.; Murray, J.W.; Baskaran, M.; Benitez-Nelson, C.R.; Guo, L.D.; Hung, C.C.; Lamborg, C.; Moran, S.B.; Passow, U.; Roy-Barman, M. Thorium speciation in seawater. Marine Chemistry, 2006, 100(3-4), 250-268. [CrossRef]
- Chapter 58 Thorium, Comprehensive Analytical Chemistry, Elsevier, 1996, 30, 721-728, ISSN 0166-526X, ISBN 9780444823687. [CrossRef]
- Rao, T.P.; Metilda, P. Gladis, J. M. Preconcentration techniques for uranium(VI) and thorium(IV) prior to analytical determination—an overview. Talanta, 2006, 68(4). [CrossRef]
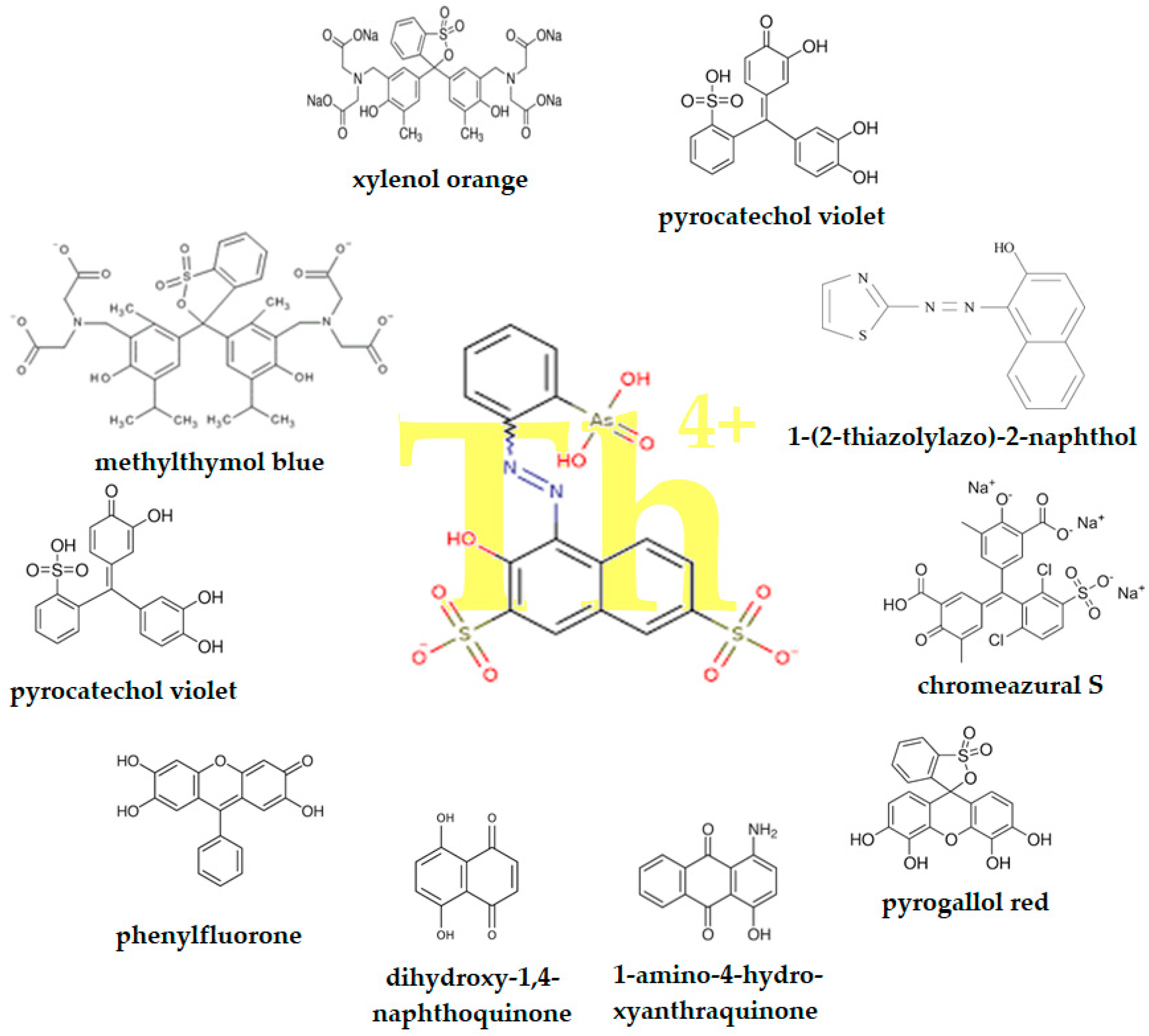
- Niazi, A.; Ghasemi, N.; Goodarzi, M.; Ebadi, A., Simultaneous spectrophotometric determination of uranium and thorium using arsenazo III by H-point standard addition method and partial least squares regression. Journal of the Chinese Chemical Society, 2007, 54(2), 411-418. [CrossRef]
- Sai, Y.; Zhang, J. Spectrophotometric Determination of Microamounts of Thorium with 3,5-Dibromosalicylfluorone by Ion-exchange Separation. Spectrosc. Spectr. Anal. 2001, 21, 843–845.
- Xia, C.B. Spectrophotometric Determination of Trace Thorium in Coal Gangue. Chin. J. Rare Met. 2003, 27, 416–417. [CrossRef]
- Pavón, J.L.P.; Cordero, B.M., Micellar systems in flow injection: determination of gadolinium with 1-(2-pyridylazo)-2-naphthol in the presence of Triton X-100. Analyst, 1992, 117(2), 215-217. [CrossRef]
- Khan, M.H.; Hafeez, M.; Bukhari, S.M.H.; Ali, A. Spectrophotometric determination of microamounts of thorium with thorin in the presence of cetylpyridinium chloride as surfactant in perchloric acid. Journal of Radioanalytical and Nuclear Chemistry, 2014, 301, 703-709. [CrossRef]
- Agnihotri, N.K.; Singh, V.K.; Singh, H.B. Simultaneous derivative spectrophotometric determination of thorium and uranium in a micellar medium. Talanta, 1993, 40(12), pp.1851-1859. [CrossRef]
- Abu-Bakr, M.S.; Sedaira, H.; Hashem, E.Y. Complexation equilibria and spectrophotometric determination of iron (III) with 1-amino-4-hydroxyanthraquinone. Talanta, 1994, 41(10), pp.1669-1674. [CrossRef]
- Ramesh, A.; Krishnamacharyulu, J.; Ravindranath, L.K.; Brahmaji Rao, S. Simultaneous determination of uranium and thorium using 4-(2′-thiazolylazo) resacetophenone oxime as analytical reagent. A derivative spectrophotometric method. Journal of radioanalytical and nuclear chemistry, 1993, 170, pp. 181-187. [CrossRef]
- Ramesh, A.; Krishnamacharyulu, J.; Ravindranath, L.K.; Brahmaji Rao, S. Simultaneous determination of uranium and thorium using 4-(2′-thiazolylazo) resacetophenone oxime as analytical reagent. A derivative spectrophotometric method. Journal of radioanalytical and nuclear chemistry, 1993, 170, pp. 181-187. [CrossRef]
- Sharma, C.; Eshwar, M. Rapid spectrophotometric determination of thorium (IV) with 1-(2′-thiazolylazo)-2-naphthol. Journal of radioanalytical and nuclear chemistry, 1985, 91(2), pp.323-328. [CrossRef]
- Mori, I.; Fujita, Y.; Fujita, K.; Kitano, S.; Ogawa, I.; Kawabe, H.; Koshiyama, Y.; Tanaka, T. Color reaction among pyrogallol red, thorium (IV) and samarium (III), and its application to the determination of these metals. Bulletin of the Chemical Society of Japan, 1986, 59(3), pp.955-957. [CrossRef]
- Jarosz, M. Study of ternary thorium complexes with some triphenylmethane reagents and cationic surfactants. Analyst, 1986, 111(6), pp.681-683. [CrossRef]
- Mori, I.; Fujita, Y.; Sakaguchi, K. Solvent extraction and spectrophotometry of Th (IV) with 3, 4, 5, 6-tetrachlorogallein in the presence of La (III) and cetyltrimethylammonium chloride. Bunseki Kagaku; (Japan), 1982, 31(2).
- Zaki, M.T.M.; El-Sayed, A.Y., Determination of thorium using phenylfluorone and quercetin in the presence of surfactants and protective colloids. Anal. Lett.1995, 28(8), pp.1525-1539. [CrossRef]
- Dacheux, N.; Aupiais, J. Determination of uranium, thorium, plutonium, americium, and curium ultratraces by photon electron rejecting α liquid scintillation. Anal. Chem. 1997, 69(13), 2275–82. [CrossRef]
- Amrane.; M. Oufni, L. Determination for levels of uranium and thorium in water along Oum Er-Rabia River using alpha track detectors, Journal of Radiation Research and Applied Sciences, 2017, 10(3), 246-251. [CrossRef]
- De Carvalho, M.S.; Domingues, M.D.L.F.; Mantovano, J.L.; Da Cunha, J.W.S.D. Preconcentration method for the determination of thorium in natural water by wavelength dispersive X-ray fluorescence spectrometry. J. Radioanal. Nucl. Chem. 2002, 253(2), 253–6. [CrossRef]
- Misra, N.L.; Dhara, S.; Adya, V.C.; Godbole, S.V.; Mudher, K.S.; Aggarwal, S.K. Trace element determination in thorium oxide using total reflection X-ray fluorescence spectrometry, Spectrochimica Acta Part B: Atomic Spectroscopy. 2008, 63(1), 81-85. [CrossRef]
- Pantelica, A.I.; Georgesecu, I.; Murariu-magureanu, D. M.; Margaritescu, I.; Cincu, E. Thorium determination in intercomparison samples and in some Romanian building materials by gamma ray spectrometry. Radiat. Protect. Dosim. 2001, 97(2), 187-191. [CrossRef]
- Comprehensive Analytical Chemistry, Chapter 58. Thorium, Elsevier, 1996, 30, 721-728, ISSN 0166-526X, ISBN 9780444823687. [CrossRef]
- Prasada Rao, T.; Metilda, P.; Mary Gladis, J. Preconcentration techniques for uranium(VI) and thorium(IV) prior to analytical determination—an overview, Talanta, 2006, 68(4), 1047-1064. [CrossRef]
- Kasar, S.; Murugan, R.; Arae, H.; Aono, T.; Sahoo, S.K. A Microwave Digestion Technique for the Analysis of Rare Earth Elements, Thorium and Uranium in Geochemical Certified Reference Materials and Soils by Inductively Coupled Plasma Mass Spectrometry. Molecules 2020, 25, 5178. [CrossRef]
- Mas, J.L.; Villa, M.; Hurtado, S.; García-Tenorio, R. Determination of trace element concentrations and stable lead, uranium and thorium isotope ratios by quadrupole-ICP-MS in NORM and NORM-polluted sample leachates, Journal of Hazardous Materials, 2012, 205–206, 198-207. [CrossRef]
- Kulkarni, M.J.; Argekar, A.A.; Mathur, J.N.; Page, A.G. Chemical separation and inductively coupled plasma–atomic emission spectrometric determination of seventeen trace metals in thorium oxide matrix using a novel extractant—Cyanex-923, Analytica Chimica Acta, 1998, 370(2–3), 163-171. [CrossRef]
- Luo, Y.; Cong, H.X.; Zhao, Z.Q.; Hu, W.Q.; Zhou, W.; He, S.H. ICP-AES with MSF for Determination of Th and U. J. Nucl. Radiochem. 2015, 37, 37–40. [CrossRef]
- Hou, L.Q.; Luo, S.H.; Wang, S.A.; Sheng, H.W.; Xi, Y.F. Determination of trace thorium in uranium dioxide by inductively coupled plasma-atomic emission spectrometry. Metall. Anal. 2006, 26, 50–52.
- Ayranov, M.; Cobos, J.; Popa, K.; Rondinella, V.V. Determination of REE, U, Th, Ba, and Zr in simulated hydrogeological leachates by ICP-AES after matrix solvent extraction. J. Rare Earths 2009, 27, 123–127. [CrossRef]
- Casartelli, E.A.; Miekeley, N. Determination of thorium and light rare-earth elements in soil water and its high molecular mass organic fractions by inductively coupled plasma mass spectrometry and on-line-coupled size-exclusion chromatography. Anal. Bioanal. Chem. 2003, 377, 58–64. [CrossRef]
- She, Z.; Li, M.; Feng, Z.; Xu, Y.; Wang, M.; Pan, X.; Yang, Z. Determination of Trace Thorium and Uranium Impurities in Scandium with High Matrix by ICP-OES. Materials 2023, 16, 3023. [CrossRef]
- Castro, M.A.; Garcı́a-Olalla, C.; Robles, L.C.; Aller, A.J. Behavior of thorium, zirconium, and vanadium as chemical modifiers in the determination of arsenic by electrothermal atomization atomic absorption spectrometry, Spectrochimica Acta Part B: Atomic Spectroscopy, 2002, 57(1), 1-14. [CrossRef]
- Casadio, S.; Lorenzinl, L. Cyclic voltammetric behavior of uranyl ion in sulfuric acid solutions. Application to some nuclear materials characterization. Anal. Lett. 1973, 6(9), 809–20. [CrossRef]
- Jin, L.T.; Shan, Y.; Tong, W.; Fang, YZ. Determination of thorium by adsorptive type chemically modified electrode with a polycomplex system. Microchimica Acta 1989, 97(1), 97–104. [CrossRef]
- Wen, J.; Dong, L.; Hu, S.; Li, W.; Li, S.; Wang, X. Fluorogenic thorium sensors based on 2,6-pyridinedicarboxylic acid-substituted tetraphenylethenes with aggregation-induced emission characteristics. Chem. Asian. J. 2016, 11(1), 49–53. [CrossRef]
- Safavi, A.; Sadeghi, M. Design and evaluation of a thorium (IV) selective optode. Anal. Chim. Acta 2006, 567(2), 184–8. [CrossRef]
- Purohit, P.J.; Goyal, N.; Thulasidas, S.K.; Page, A.G.; Sastry, M.D. Electrothermal vaporization — inductively coupled plasma-atomic emission spectrometry for trace metal determination in uranium and thorium compounds without prior matrix separation, Spectrochimica Acta Part B: Atomic Spectroscopy, 2000, 55(8), 1257-1270. [CrossRef]
- Mirza, M.A.; Khuhawar, M.Y.; Arain, R.; Ch, M.A. Micellar electrokinetic chromatographic analysis of thorium, uranium, copper, nickel, cobalt and iron in ore and fish samples. Arab. J. Chem. 2018, 11(3), 305-312. [CrossRef]
- Sarkar, A.; Alamelu, D.; Aggarwal, S.K. Determination of trace constituents in thoria by laser induced breakdown spectrometry. Journal of nuclear materials 2009, 384(2), 158-162. [CrossRef]
- Aydin, F.A.; Soylak, M. Separation, preconcentration and inductively coupled plasma-mass spectrometric (ICP-MS) determination of thorium(IV), titanium(IV), iron(III), lead(II) and chromium(III) on 2-nitroso-1-naphthol impregnated MCI GEL CHP20P resin, Journal of Hazardous Materials, 2010, 173(1–3), 669-674. [CrossRef]
- Sarkar, A., Alamelu, D. and Aggarwal, S.K., 2008. Determination of thorium and uranium in solution by laser-induced breakdown spectrometry. Applied Optics, 47(31), pp. G58-G64. [CrossRef]
- Kadish, K.M.; Liu, Y.H.; Anderson, J.E. Dormond, A.; Belkalem, M.; Guilard R., Electrochemical and spectroelectrochemical studies of bis(diketonate) thorium(IV) and uranium(IV) porphyrins, Inorganica Chimica Acta 1989, 163(2), 201-205. [CrossRef]
- de Diego Almeida, R.H.; Monroy-Guzmán, F.; Juárez, C.R.A., Rocha, J.M.; Bustos, E.B. Electrochemical detector based on a modified graphite electrode with phthalocyanine for the elemental analysis of actinides. Chemosphere 2021, 276, 130114. [CrossRef]
- Moghaddam, Z.S.; Kaykhaii, M.; Khajeh, M.; Oveisi, A.R. Synthesis of UiO-66-OH zirconium metal-organic framework and its application for selective extraction and trace determination of thorium in water samples by spectrophotometry. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2018, 194, 76-82. [CrossRef]
- Akhila Maheswari, M.; Prabhakaran, D.; Subramanian M.S.; Sivaraman, N.; Srinivasan, T.G.; Vasudeva Rao, P.R. High performance liquid chromatographic studies on lanthanides, uranium and thorium on amide modified reversed phase supports. Talanta, 2007, 72(2), 730-740. [CrossRef]
- Shimada-Fujiwara, A.; Hoshi, A.; Kameo, Y.; Nakashima, M. Influence of hydrofluoric acid on extraction of thorium using a commercially available extraction chromatographic resin. Journal of Chromatography A 2009, 1216(18), 4125-4127. [CrossRef]
- Pakhui, G.; Ghosh, S.; Reddy, B.P. Th4+|Th couple in LiCl-KCl eutectic: Anodic polarization of thorium and electrochemical impedance spectroscopy study at tungsten, cadmium and thorium electrodes. Electrochimica Acta 2019, 295, 354-366. [CrossRef]
- Radchenko, V.; Engle, J.W.; Wilson, J.J.; Maassen, J.R.; Nortier, F.M.; Taylor, W.A.; Birnbaum, E.R.; Hudston, L.A.; John, K.D.; Fassbender, M.E. Application of ion exchange and extraction chromatography to the separation of actinium from proton-irradiated thorium metal for analytical purposes. Journal of Chromatography A, 2015, 1380, 55-63. [CrossRef]
- Singh, D.; Basu, S.; Mishra, B.; Prusty, S.; Kundu, T.; Rao, R. Textural and Chemical Characters of Lean Grade Placer Monazite of Bramhagiri Coast, Odisha, India. Minerals 2023, 13, 742. [CrossRef]
- Lazar, M.M.; Ghiorghita, C.-A.; Dragan, E.S.; Humelnicu, D.; Dinu, M.V. Ion-Imprinted Polymeric Materials for Selective Adsorption of Heavy Metal Ions from Aqueous Solution. Molecules 2023, 28, 2798. [CrossRef]
- Lee, J.; Yi, S.C. Accurate measurement of uranium and thorium in naturally occurring radioactive materials to overcome complex matrix interference. Applied Radiation and Isotopes 2023, 193, 110649. [CrossRef]
- Dumpala, R.M.R.; Sahu, M.; Nagar, B.K., Raut, V.V.; Raje, N.H.; Rawat, N.; Subbiah, J.; Saxena, M.K.; Tomar, B.S. Accountancy for intrinsic colloids on thorium solubility: The fractionation of soluble species and the characterization of solubility limiting phase. Chemosphere, 2021, 269, 129327. [CrossRef]
- Metaxas, M.; Kasselouri-Rigopoulou, V.; Galiatsatou, P.; Konstantopoulou, C.; Oikonomou, D. Thorium Removal by Different Adsorbents. J. Hazard. Mater. 2003, 97, 71–82. [CrossRef]
- Alotaibi, A.M.; Ismail, A.F. Modification of Clinoptilolite as a Robust Adsorbent for Highly-Efficient Removal of Thorium (IV) from Aqueous Solutions. Int. J. Environ. Res. Public Health 2022, 19, 13774. [CrossRef]
- Felix, C.S.A.; Chagas, A.V.B.; de Jesus, R.F.; Barbosa, W.T.; Barbosa, J.D.V.; Ferreira, S.L.C.; Cerdà, V. Synthesis and Application of a New Polymer with Imprinted Ions for the Preconcentration of Uranium in Natural Water Samples and Determination by Digital Imaging. Molecules 2023, 28, 4065. [CrossRef]
- Liu, H.; Qi, C.; Feng, Z.; Lei, L.; Deng, S. Adsorption of Trace Thorium (IV) from Aqueous Solution by Mono-Modified β-Cyclodextrin Polyrotaxane Using Response Surface Methodology (RSM). J. Radioanal. Nucl. Chem. 2017, 314, 1607–1618. [CrossRef]
- Ansari, S.A.; Mohapatra, P.K.; Manchanda, V.K. A Novel Malonamide Grafted Polystyrene-Divinyl Benzene Resin for Extraction, Pre-Concentration and Separation of Actinides. J. Hazard. Mater. 2009, 161, 1323–1329. [CrossRef]
- Karmakar, R.; Singh, P.; Sen, K. Selectivity of Th (IV) Adsorption as Compared to U (VI), La (III), Ce (III), Sm (III) and Gd (III) Using Mesoporous Al2O3. Sep. Sci. Technol. 2021, 56, 2369–2384.
- Kukkonen, E.; Virtanen, E.J.; Moilanen, J.O. α-Aminophosphonates, -Phosphinates, and -Phosphine Oxides as Extraction and Precipitation Agents for Rare Earth Metals, Thorium, and Uranium: A Review. Molecules 2022, 27, 3465. [CrossRef]
- Georgiou, E.; Pashalidis, I.; Raptopoulos, G.; Paraskevopoulou, P. Efficient Removal of Polyvalent Metal Ions (Eu(III) and Th(IV)) from Aqueous Solutions by Polyurea-Crosslinked Alginate Aerogels. Gels 2022, 8, 478. [CrossRef]
- Liao, W.; Zhang, Z.; Li, Y.; Wu, G.; Lu, Y. CN105734286A—Method for Separating Cerium-Fluoride and Thorium. China Patent CN105734286A, 11 December 2014.
- Lu, Y.; Zhang, Z.; Li, Y.; Liao, W. Extraction and recovery of cerium(IV) and thorium(IV) from sulphate medium by an α-aminophosphonate extractant, Journal of Rare Earths, Vol. 35, 2017, 1, pp. 34-40, ISSN 1002-0721. [CrossRef]
- Wei, H.; Li, Y.; Zhang, Z.; Xue, T.; Kuang, S.; Liao, W. Selective Extraction and Separation of Ce (IV) and Th (IV) from RE(III) in Sulfate Medium Using Di(2-Ethylhexyl)-N-Heptylaminomethylphosphonate. Solvent Extr. Ion Exch. 2017, 35, 117–129. [CrossRef]
- Kuang, S.; Zhang, Z.; Li, Y.; Wu, G.; Wei, H.; Liao, W. Selective Extraction and Separation of Ce(IV) from Thorium and Trivalent Rare Earths in Sulfate Medium by an α-Aminophosphonate Extractant. Hydrometallurgy 2017, 167, 107–114. [CrossRef]
- Kuang, S.; Zhang, Z.; Li, Y.; Wei, H.; Liao, W. Extraction and Separation of Heavy Rare Earths from Chloride Medium by α-Aminophosphonic Acid HEHAPP. J. Rare Earths 2018, 36, 304–310. [CrossRef]
- Zhao, Q.; Zhang, Z.; Li, Y.; Bian, X.; Liao, W. Solvent Extraction and Separation of Rare Earths from Chloride Media Using α-Aminophosphonic Acid Extractant HEHAMP. Solvent Extr. Ion Exch. 2018, 36, 136–149. [CrossRef]
- Kaygun, A.K.; Akyil, S. Study of the Behaviour of Thorium Adsorption on PAN/Zeolite Composite Adsorbent. J. Hazard. Mater. 2007, 147, 357–362. [CrossRef]
- Al-shaybe, M.; Khalili, F. Adsorption of Thorium (IV) and Uranium (VI) by Tulul Al- Shabba Zeolitic Tuff, Jordan. Jordan J. Earth Environ. Sci. 2009, 2, 108–119.
- Khazaei, Y.; Faghihian, H.; Kamali, M. Removal of Thorium from Aqueous Solutions by Sodium Clinoptilolite. J. Radioanal. Nucl. Chem. 2011, 289, 529–536. [CrossRef]
- Nurliati, G.; Krisnandi, Y.K.; Sihombing, R.; Salimin, Z. Studies of Modification of Zeolite by Tandem Acid-Base Treatments and Its Adsorptions Performance towards Thorium. At. Indones. 2015, 41, 87. [CrossRef]
- Akl, Z.F.; Hegazy, M.A., Selective cloud point extraction of thorium (IV) using tetraazonium based ionic liquid. Journal of Environmental Chemical Engineering, 2020, 8(5), p.104185. [CrossRef]
- Varala, S.; Kumari, A.; Dharanija, B.; Bhargava, S.K.; Parthasarathy, R.; Satyavathi, B. Removal of thorium (IV) from aqueous solutions by deoiled karanja seed cake: Optimization using Taguchi method, equilibrium, kinetic and thermodynamic studies. Journal of Environmental Chemical Engineering 2016, 4(1), pp. 405-417. [CrossRef]
- Humelnicu, D.; Bulgariu, L.; Macoveanu, M. On the retention of uranyl and thorium ions from radioactive solution on peat moss, Journal of Hazardous Materials, 2010, 174(1–3), 782-787. [CrossRef]
- Scandura, G.; Eid, S.; Alnajjar, A.A.; Paul, T.; Karanikolos, G.N.; Shetty, D.; Omer, K.; Alqerem, R.; Juma, A., Wang, H.; Arafat, H.A. Photo-responsive metal–organic frameworks–design strategies and emerging applications in photocatalysis and adsorption. Materials Advances. 2023, 4, 1258-1285. [CrossRef]
- Wang, X.; Zheng, H.; Xu, Q.; Zhu, T.; Jiang, F.; She, C.; Wang, C.; Cong, H.; Gong, Y.; Huang, W.; Li, Q. Electrochemical behaviors and electrolytic separation of Th (IV) and Ce (III) in ThF4-CeF3-LiCl-KCl quaternary melt. Sep.Purif. Technol.2019, 210, 236-241. [CrossRef]
- Gomaa, H.; Emran, M.Y.; Elsenety, M.M.; Abdel-Rahim, R.D.; Deng, Q.; Gadallah, M.I.; Saad, M.; ALMohiy, H.; Ali, H.R.H.; Faraghally, F.A.; El-Nasr, T.A.S., Selective removal of thorium ions from aqueous solutions using a hybrid mesoporous adsorbent as benzenesulfonamide-derivative@ZrO2. Journal of Water Process Engineering, 2023, 51, 103436. [CrossRef]
- Allam, E.M.; Lashen, T.A.; Abou El-Enein, S.A.; Hassanin, M.A.; Sakr, A.K.; Cheira, M.F.; Almuqrin, A.; Hanfi, M.Y.; Sayyed, M.I. Rare Earth Group Separation after Extraction Using Sodium Diethyldithiocarbamate/Polyvinyl Chloride from Lamprophyre Dykes Leachate. Materials 2022, 15, 1211. [CrossRef]
- Zhao, D.; Yu, S.; Jiang, W.-J.; Cai, Z.-H.; Li, D.-L.; Liu, Y.-L.; Chen, Z.-Z. Recent Progress in Metal-Organic Framework Based Fluorescent Sensors for Hazardous Materials Detection. Molecules 2022, 27, 2226. [CrossRef]
- Talan, D.; Huang, Q. Separation of Radionuclides from a Rare Earth-Containing Solution by Zeolite Adsorption. Minerals 2021, 11, 20. [CrossRef]
- Atanassova, M. Assessment of the Equilibrium Constants of Mixed Complexes of Rare Earth Elements with Acidic (Chelating) and Organophosphorus Ligands. Separations 2022, 9, 371. [CrossRef]
- Atanassova, M. Thenoyltrifluoroacetone: Preferable Molecule for Solvent Extraction of Metals—Ancient Twists to New Approaches. Separations 2022, 9, 154. [CrossRef]
- Salah, B.; S. Gaber, M.; T. Kandil, A.h. The Removal of Uranium and Thorium from Their Aqueous Solutions by 8-Hydroxyquinoline Immobilized Bentonite. Minerals 2019, 9, 626. [CrossRef]
- Felix, C.S.A.; Chagas, A.V.B.; de Jesus, R.F.; Barbosa, W.T.; Barbosa, J.D.V.; Ferreira, S.L.C.; Cerdà, V. Synthesis and Application of a New Polymer with Imprinted Ions for the Preconcentration of Uranium in Natural Water Samples and Determination by Digital Imaging. Molecules 2023, 28, 4065. [CrossRef]
- Stannett, V.T.; Koros, W.J.; Paul, D.R.; Lonsdale, H.K.;Baker, R.W. Recent advances in membrane science and technology. In: Chemistry. Adv. Polym. Sci. 1979, 32. Springer, Berlin, Heidelberg. [CrossRef]
- Kammermeyer, K. Technical Gas Permeation Processes. Chemie Ing. Tech. 1976, 48, 672. [CrossRef]
- Baker, R.W. Membrane Transport Theory. In Membrane Technology and Applications; John Wiley&Sons Ltd, 2012 ISBN 0-470-85445-6.
- Kesting, R. Synthetic polymeric membranes. J. Colloid Interface Sci. 1988. [CrossRef]
- Nechifor, A.C.; Cotorcea, S.; Bungău, C.; Albu, P.C.; Pașcu, D.; Oprea, O.; Grosu, A.R.; Pîrțac, A.; Nechifor, G. Removing of the Sulfur Compounds by Impregnated Polypropylene Fibers with Silver Nanoparticles-Cellulose Derivatives for Air Odor Correction. Membranes 2021, 11, 256. [CrossRef]
- Baker, W. Membrane Technology and Applications, 3rd ed.; John Wiley & SonsLtd., Chichester (UK), ISBN 9780470743720, 2012, 148–149.
- Mulder, M. The Use of Membrane Processes in Environmental Problems. An Introduction. In: Crespo, J.G., Böddeker, K.W. (eds) Membrane Processes in Separation and Purification. NATO ASI Series (Series E: Applied Sciences), 1994, 272. Springer, Dordrecht. [CrossRef]
- Strathmann, H.; Giorno, L.; Drioli, E. Introduction to membrane science and technology. 2011, Institute on Membrane Technology, CNR-ITMat University of Calabria, Via P. Bucci 17/C, 87036 Rende (CS), Italy, 27-58.
- Van Der Bruggen, B.; Vandecasteele, C.; Van Gestel, T.; Doyen, W.; Leysen, R. A review of pressure-driven membrane processes in wastewater treatment and drinking water production. Environ. Prog. 2003, 22(1), 46-56. [CrossRef]
- Drioli, E.; Stankiewicz, A.I.; Macedonio, F. Membrane engineering in process intensification—An overview, J. Membr. Sci., 2011, 380(1–2), 1-8. [CrossRef]
- Iulianelli, A.; Drioli, E. Membrane engineering: Latest advancements in gas separation and pre-treatment processes, petrochemical industry and refinery, and future perspectives in emerging applications. Fuel Processing Technology, 2020, 206, 106464. [CrossRef]
- Li, N.N.; Fane, A.G.; Ho, W.W.; Matsuura, T. eds., 2011. Advanced membrane technology and applications. John Wiley & Sons. https://toc.library.ethz.ch/objects/pdf/e16_978-0-471-73167-2_01.pdf.
- Drioli, E.; Criscuoli, A.; Curcio, E. Membrane Contactors: Fundamentals, Applications and Potentialities, Elsevier, Amsterdam, The Netherlands, 2011.
- Bernardoa, P.; Drioli, E. Membrane Gas Separation Progresses for Process Intensification Strategy in the Petrochemical Industry. Petroleum Chemistry 2010, 50(4), 271–282. [CrossRef]
- Van der Bruggen, B.; Lejon, L.; Vandecasteele, C. Reuse, treatment, and discharge of the concentrate of pressure-driven membrane processes. Environmental science & technology, 2003, 37(17), pp. 3733-3738. [CrossRef]
- Van der Bruggen, B.; Everaert, K.; Wilms, D.; Vandecasteele, C. Application of nanofiltration for the removal of pesticides, nitrate and hardness from ground water: Retention properties and economic evaluation. J. Membr. Sci. 2001, 193, 239–248. [CrossRef]
- Peng, N.; Widjojo, N.; Sukitpaneenit, P.; Teoh, M.M.; Lipscomb, G.G.; Chung, T.S.; Lai, J.Y. Evolution of polymeric hollow fibers as sustainable technologies: Past, present, and future. Progress in Polymer Science, 2012, 37(10), 1401-1424. [CrossRef]
- Kamolov, A.; Turakulov, Z.; Rejabov, S.; Díaz-Sainz, G.; Gómez-Coma, L.; Norkobilov, A.; Fallanza, M.; Irabien, A. Decarbonization of Power and Industrial Sectors: The Role of Membrane Processes. Membranes 2023, 13, 130. [CrossRef]
- Zanco, S.E.; Pérez-Calvo, J.-F.; Gasós, A.; Cordiano, B.; Becattini, V.; Mazzotti, M. Postcombustion CO2 Capture: A Comparative Techno-Economic Assessment of Three Technologies Using a Solvent, an Adsorbent, and a Membrane. ACS Eng. Au 2021, 1, 50–72. [CrossRef]
- Rahman, T.U.; Roy, H.; Islam, M.R.; Tahmid, M.; Fariha, A.; Mazumder, A.; Tasnim, N.; Pervez, M.N.; Cai, Y.; Naddeo, V.; et al. The Advancement in Membrane Bioreactor (MBR) Technology toward Sustainable Industrial Wastewater Management. Membranes 2023, 13, 181. [CrossRef]
- Goh, P.S.; Samavati, Z.; Ismail, A.F.; Ng, B.C.; Abdullah, M.S.; Hilal, N. Modification of Liquid Separation Membranes Using Multidimensional Nanomaterials: Revealing the Roles of Dimension Based on Classical Titanium Dioxide. Nanomaterials 2023, 13, 448. [CrossRef]
- Liu, H.; Qi, C.; Feng, Z.; Lei, L.; Deng, S. Adsorption of trace thorium (IV) from aqueous solution by mono-modified β-cyclodextrin polyrotaxane using response surface methodology (RSM). J. Radioanal. Nucl. Chem. 2017, 314(3):1607–1618. [CrossRef]
- Jakubski, Ł.; Dudek, G.; Turczyn, R. Applicability of Composite Magnetic Membranes in Separation Processes of Gaseous and Liquid Mixtures—A Review. Membranes 2023, 13, 384. [CrossRef]
- Charcosset, C. Classical and Recent Developments of Membrane Processes for Desalination and Natural Water Treatment. Membranes 2022, 12, 267. [CrossRef]
- Shahid, M.K.; Mainali, B.; Rout, P.R.; Lim, J.W.; Aslam, M.; Al-Rawajfeh, A.E.; Choi, Y. A Review of Membrane-Based Desalination Systems Powered by Renewable Energy Sources. Water 2023, 15, 534. [CrossRef]
- Worku, L.A.; Bachheti, A.; Bachheti, R.K.; Rodrigues Reis, C.E.; Chandel, A.K. Agricultural Residues as Raw Materials for Pulp and Paper Production: Overview and Applications on Membrane Fabrication. Membranes 2023, 13, 228. [CrossRef]
- Qasim, M.; Badrelzaman, M.; Darwish, N.N.; Darwish, N.A.; Hilal, N. Reverse osmosis desalination: A state-of-the-art review. Desalination 2019, 459, 59–104. [CrossRef]
- Bundschuh, J.; Kaczmarczyk, M.; Ghaffour, N.; Tomaszewska, B. State-of-the-art of renewable energy sources used in water desalination: Present and future prospects. Desalination 2021, 508, 115035. [CrossRef]
- Wu, W.; Shi, Y.; Liu, G.; Fan, X.; Yu, Y. Recent development of graphene oxide based forward osmosis membrane for water treatment: A critical review. Desalination 2020, 491, 114452. [CrossRef]
- Awad, A.M.; Jalab, R.; Minier-Matar, J.; Adham, S.; Nasser, M.S.; Judd, S.J. The status of forward osmosis technology implementation. Desalination 2019, 461, 10–21. [CrossRef]
- Skuse, C.; Gallego-Schmid, A.; Azapagic, A.; Gorgojo, P. Can emerging membrane-based desalination technologies replace reverse osmosis? Desalination 2021, 500, 114844.
- Zhao, S.; Liao, Z.; Fane, A.; Li, J.; Tang, C.; Zheng, C.; Lin, J.; Kong, L. Engineering antifouling reverse osmosis membranes: A review. Desalination 2021, 499, 114857. [CrossRef]
- Santoro, S.; Timpano, P.; Avci, A.H.; Argurio, P.; Chidichimo, F.; De Biase, M.; Straface, S.; Curcio, E. An integrated membrane distillation, photocatalysis and polyelectrolyte-enhanced ultrafiltration process for arsenic remediation at point-of-use. Desalination 2021, 520, 115378.
- Dunn Jr, R.O., Scamehorn, J.F. and Christian, S.D. Use of micellar-enhanced ultrafiltration to remove dissolved organics from aqueous streams. Sep. Sci. Technol. 1985, 20(4), pp. 257-284. [CrossRef]
- Christian, S.D.; Bhat, S.N.; Tucker, E.E.; Scamehorn, J.F.; El-Sayed, D.A. Micellar-enhanced ultrafiltration of chromate anion from aqueous streams. AIChE journal, 1988, 34(2), pp.189-194. [CrossRef]
- Dunn Jr, R.O.; Scamehorn, J.F.; Christian, S.D. Concentration polarization effects in the use of micellar-enhanced ultrafiltration to remove dissolved organic pollutants from wastewater. Sep. Sci. Technol. 1987, 22(2-3), 763-789. [CrossRef]
- Chen, M.; Jafvert, C.T.; Wu, Y.; Cao, X.; Hankins, N.P. Inorganic anion removal using micellar enhanced ultrafiltration (MEUF), modeling anion distribution and suggested improvements of MEUF: A review. Chemical Engineering Journal, 2020, 398, 125413. [CrossRef]
- Nechifor, G.; Păncescu, F.M.; Albu, P.C.; Grosu, A.R.; Oprea, O.; Tanczos, S.-K.; Bungău, C.; Grosu, V.-A.; Ioan, M.-R.; Nechifor, A.C. Transport and Separation of the Silver Ion with n–decanol Liquid Membranes Based on 10–undecylenic Acid, 10–undecen–1–ol and Magnetic Nanoparticles. Membranes 2021, 11, 936. [CrossRef]
- Burts, K.S.; Plisko, T.V.; Sjölin, M.; Rodrigues, G.; Bildyukevich, A.V.; Lipnizki, F.; Ulbricht, M. Development of Antifouling Polysulfone Membranes by Synergistic Modification with Two Different Additives in Casting Solution and Coagulation Bath: Synperonic F108 and Polyacrylic Acid. Materials 2022, 15, 359. [CrossRef]
- Nagarale, R.K.; Gohil, G.S.; Shahi, V.K. Recent developments on ion-exchange membranes and electro-membrane processes. Advances in colloid and interface science, 2006, 119(2-3), 97-130. [CrossRef]
- Doornbusch, G.; van der Wal, M.; Tedesco, M.; Post, J.; Nijmeijer, K.; Borneman, Z. Multistage electrodialysis for desalination of natural seawater. Desalination 2021, 505, 114973. [CrossRef]
- Tekinalp, Ö.; Zimmermann, P.; Holdcroft, S.; Burheim, O.S.; Deng, L. Cation Exchange Membranes and Process Optimizations in Electrodialysis for Selective Metal Separation: A Review. Membranes 2023, 13, 566. [CrossRef]
- Cournoyer, A.; Bazinet, L. Electrodialysis Processes an Answer to Industrial Sustainability: Toward the Concept of Eco-Circular Economy? —A Review. Membranes 2023, 13, 205. [CrossRef]
- Dammak, L.; Fouilloux, J.; Bdiri, M.; Larchet, C.; Renard, E.; Baklouti, L.; Sarapulova, V.; Kozmai, A.; Pismenskaya, N. A Review on Ion-Exchange Membrane Fouling during the Electrodialysis Process in the Food Industry, Part 1: Types, Effects, Characterization Methods, Fouling Mechanisms and Interactions. Membranes 2021, 11, 789. [CrossRef]
- Solonchenko, K.; Kirichenko, A.; Kirichenko, K. Stability of Ion Exchange Membranes in Electrodialysis. Membranes 2023, 13, 52. [CrossRef]
- Veerman, J.; Gómez-Coma, L.; Ortiz, A.; Ortiz, I. Resistance of Ion Exchange Membranes in Aqueous Mixtures of Monovalent and Divalent Ions and the Effect on Reverse Electrodialysis. Membranes 2023, 13, 322. [CrossRef]
- Gao, W.; Fang, Q.; Yan, H.; Wei, X.; Wu, K. Recovery of Acid and Base from Sodium Sulfate Containing Lithium Carbonate Using Bipolar Membrane Electrodialysis. Membranes 2021, 11, 152. [CrossRef]
- Zhou, H.; Ju, P.; Hu, S.; Shi, L.; Yuan, W.; Chen, D.; Wang, Y.; Shi, S. Separation of Hydrochloric Acid and Oxalic Acid from Rare Earth Oxalic Acid Precipitation Mother Liquor by Electrodialysis. Membranes 2023, 13, 162. [CrossRef]
- Gurreri, L.; Tamburini, A.; Cipollina, A.; Micale, G. Electrodialysis Applications in Wastewater Treatment for Environmental Protection and Resources Recovery: A Systematic Review on Progress and Perspectives. Membranes 2020, 10, 146. [CrossRef]
- V.S. Kislik in Liquid membrane: Principles and Applications in Chemical Separations & Wastewater Treatment, ed. Elsevier (UK), 2010.
- Ho, W.S.W.; Sirka, K.K. Eds. Membrane Handbook. Chapman & Hall: New York, 1992.
- Osada, Y.; Makagawa, T. Eds. Membrane Science and Technology. Marcel Dekker: New York, 1992.
- Noble, R.D.; Stem, S.A. Eds. Membrane Separation Technology. Elsevier: New York, 1995.
- Noble, R.D.; Way, J.D. Eds. Liquid Membranes: Theory and Applications. ACS Symposium Series 347. American Chemical Society: Washington, 1987.
- Araki, T.; Tsukube, H. Eds. Liquid Membranes: Chemical Applications. CRC Press: Boca Raton, FL, 1990.
- Bartsch, R.A.; Way, J.D. Eds. Chemical Separations with Liquid Membranes. ACS Symposium Series 642. American Chemical Society: Washington, 1996.
- Noble, R.D.; Stern, S.A. Membrane Separations Technology: Principles and Applications. Elsevier, 1995.
- Drioli, E.; Romano, M. „Progress and new perspectives on integrated membrane operations for sustainable industrial growth”, Industrial & Engineering Chemistry Research, 2001, 40, 1277-1300. [CrossRef]
- Rosano, H.L.; Schulman, J.H.; Weisbuch, J.B. “Mechanism of the Selective Flux of Salts and Ions through Nonaqueous Liquid Membranes”, Annals of the New York Academy of Sciences, 1961, 92, 457-469. [CrossRef]
- Bacon, E.; Jung, L. „Selective extraction and transport of mercury through a liquid membrane by macrocyclic ligands. Improvement in the transport efficiency and an approach to physiological systems”, J. Membr. Sci. 1985, 24, 185-195. [CrossRef]
- Christensen, J.J. Lamb, J.D.; Brown, P.R.; Oscarson, J.L.; Izatt, R.M. „Liquid Membrane Separations of Metal Cations Using Macrocyclic Carriers”, Sep. Sci. Technol. 1981, 16, 1193-1215. [CrossRef]
- Brown, P.R.; Izatt, R.M.; Christensenand, J.J.; Lamb J.D. “Transport of Eu2+ in a H2O-CHCl3-H2O liquid membrane system containing the macrocyclic polyether 18-crown-6”, J. Membr.Sci. 1983, 13, 85-88. [CrossRef]
- Burgard, M.; Elisoamiadana, P.; Leroy, M.J.F. „Liquid membrane studies: Transport against the concentration gradient of AuCI”, Procs. ISEC’83, Solid-Supported Liquid Membranes, 1983, vol. II, 399-400.
- Kislik, V.S.; Eyal, A.M. „Hybrid liquid membrane (HLM) system in separation technologies”, J. Membr. Sci. 1996, 111, 259 - 272. [CrossRef]
- Majumdar, S.; Sirkar, K.K.; Sengupta, A. (1992). Hollow-Fiber Contained Liquid Membrane. In: Ho, W.S.W., Sirkar, K.K. (eds) Membrane Handbook. Springer, Boston, MA. [CrossRef]
- S. Schlosser, E. Sabol, “Three-phase contactor with distributed U-shaped bundles of hollow-fibers for pertraction”. J. Membr. Sci. 2002, 210(2), 331–347. [CrossRef]
- Wodzki, R.; Nowaczyk, J. „Propionic and acetic acid pertraction through a multimembrane hybrid system containing TOPO or TBP”, Sep.Purif. Technol. 2002, 26, 207-220. [CrossRef]
- Nechifor, G.; Păncescu, F.M.; Albu, P.C.; Grosu, A.R.; Oprea, O.; Tanczos, S.-K.; Bungău, C.; Grosu, V.-A.; Ioan, M.-R.; Nechifor, A.C. Transport and Separation of the Silver Ion with n–decanol Liquid Membranes Based on 10–undecylenic Acid, 10–undecen–1–ol and Magnetic Nanoparticles. Membranes 2021, 11, 936. [CrossRef]
- Nechifor, A.C.; Goran, A.; Grosu, V.-A.; Bungău, C.; Albu, P.C.; Grosu, A.R.; Oprea, O.; Păncescu, F.M.; Nechifor, G. Improving the Performance of Composite Hollow Fiber Membranes with Magnetic Field Generated Convection Application on pH Correction. Membranes 2021, 11, 445. [CrossRef]
- Kubisova, L.; Sabolova, E.; Schlosser, S.; Martak, J.; Kertesz, R.’’Mass-transfer in membrane based solvent extraction and stripping of 5-methyl-2-pyrazinecarboxylic acid and co-transport of sulphuric acid in HF contactors’’, Desalination 2004, 163, 27-38. [CrossRef]
- Eyal, A.; Bressler, E.J.’’Industrial separation of carboxylic and amino acids by liquid membranes: Applicability, process considerations, and potential advantages’’. Biotechnology and Bioengineering 1993, 41, 287-293. [CrossRef]
- Eyal, V. Kislik, ’’Aqueous hybrid liquid membrane a novel system for separation of solutes using water-soluble polymers as carriers’’, J. Membr. Sci. 1999, 161, 207-221. [CrossRef]
- Al-Ani, F.H.; Alsalhy Q.F.; Al-Dahhan, M. H., Enhancing Emulsion Liquid Membrane System (ELM) Stability and Performance for the Extraction of Phenol from Wastewater using Various Nanoparticles, Desalination and Water Treatment, 2021, 210, 180-191. [CrossRef]
- Pavón, S.; Blaesing, L.; Jahn, A.; Aubel, I.; Bertau, M. Liquid Membranes for Efficient Recovery of Phenolic Compounds Such as Vanillin and Catechol. Membranes 2021, 11, 20. [CrossRef]
- Wang, B.-Y.; Zhang, N.; Li, Z.-Y.; Lang, Q.-L.; Yan, B.-H.; Liu, Y.; Zhang, Y. Selective Separation of Acetic and Hexanoic Acids across Polymer Inclusion Membrane with Ionic Liquids as Carrier. Int. J. Mol. Sci. 2019, 20, 3915. [CrossRef]
- Yang, B.; Bai, L.; Li, T.; Deng, L.; Liu, L.; Zeng, S.; Han, J.; Zhang, X. Super selective ammonia separation through multiple-site interaction with ionic liquid-based hybrid membranes. J. Membr. Sci. 2021, 628, 119264. [CrossRef]
- Jean, E.; Villemin, D.; Hlaibi, M.; Lebrun, L. Heavy metal ions extraction using new supported liquid membranes containing ionic liquid as carrier, Sep. Pur. Technol. 2018, 201, 1-9. [CrossRef]
- Craveiro, R.; Neves, L.A.; Duarte, A.R.C.; Paiva, A. Supported liquid membranes based on deep eutectic solvents for gas separation processes. Sep. Pur. Technol. 2021, 254, 117593. [CrossRef]
- Wang, Z.Y.; Sun, Y.; Tang, N.; Miao, C.L.; Wang, Y.T.; Tang, L.H.; Wang, S.X.; Yang, X.J. Simultaneousextraction and recovery of gold(I) from alkaline solutions using an environmentally benign polymer inclusionmembrane with ionic liquid as the carrier. Sep. Purif. Technol. 2019, 222, 136–144.
- Bazhenov, S.D.; Bildyukevich, A.V.; Volkov, A.V. Gas-liquid hollow fiber membrane contactors for different applications, Fibers 2018, 6(4), 76. [CrossRef]
- Diaconu, I.; Nechifor, G.; Nechifor, A.C.; Ruse, E.; Totu, E.E. Membranary techniques used at the separation of some phenolic compounds from aqueous media. UPB Scientific Bulletin, Series B: Chemistry and Materials Science 2009, 71(4), 39-46.
- Diaconu, I.; Gîrdea, R.; Cristea, C.; Nechifor, G.; Ruse, E.; Totu, E.E. Removal and recovery of some phenolic pollutants using liquid membranes, Romanian Biotechnological Letters 2010, 15(6), 5702-5708.
- Koter, S.; Szczepański, P.; Mateescu, M.; Nechifor, G.; Badalau L.; Koter, I. Modeling of the cadmium transport through a bulk liquid membrane. Sep. Purif. Technol. 2013, 107, 135-143. [CrossRef]
- Szczepański, P.; Szidonia, Tanczos, K.; Ghindeanu, D.L.; Wódzki, R. Transport of p-nitrophenol in an agitated bulk liquid membrane system—Experimental and theoretical study by network analysis. Sep. Pur.Technol. 2014, 132, 616-626. [CrossRef]
- Craciun, M.E.; Mihai, M.; Nechifor, G. Characteristics of double jet imobilized membrane. Environmental Engineering and Management Journal 2009, 8(4), 771-776.
- Yoshida, W.; Baba, Y.; Kubota, F.; Kolev, S.D.; Goto, M. Selective transport of scandium(III) across polymerinclusion membranes with improved stability which contain an amic acid carrier. J. Membr. Sci. 2019, 572,291–299. [CrossRef]
- Friess, K.; Izák, P.; Kárászová, M.; Pasichnyk, M.; Lanč, M.; Nikolaeva, D.; Luis, P.; Jansen, J.C. A Review on Ionic Liquid Gas Separation Membranes. Membranes 2021, 11, 97. [CrossRef]
- Rahman, R.O.A.; Ibrahium, H.A.; Hung, Y.-T. Liquid Radioactive Wastes Treatment: A Review. Water 2011, 3, 551-565. [CrossRef]
- Alashkar, A.; Al-Othman, A.; Tawalbeh, M.; Qasim, M. A Critical Review on the Use of Ionic Liquids in Proton Exchange Membrane Fuel Cells. Membranes 2022, 12, 178. [CrossRef]
- Rosli, N.A.H.; Loh, K.S.; Wong, W.Y.; Yunus, R.M.; Lee, T.K.; Ahmad, A.; Chong, S.T. Review of Chitosan-Based Polymers as Proton Exchange Membranes and Roles of Chitosan-Supported Ionic Liquids. Int. J. Mol. Sci. 2020, 21, 632. [CrossRef]
- Barambu, N.U.; Bilad, M.R.; Wibisono, Y.; Jaafar, J.; Mahlia, T.M.I.; Khan, A.L. Membrane Surface Patterning as a Fouling Mitigation Strategy in Liquid Filtration: A Review. Polymers 2019, 11, 1687. [CrossRef]
- Ribas, T.C.F.; Croft, C.F.; Almeida, M.I.G.S.; Mesquita, R.B.R.; Kolev, S.D.; Rangel, A.O.S.S. Use of a Polymer Inclusion Membrane and a Chelating Resin for the Flow-Based Sequential Determination of Copper(II) and Zinc(II) in Natural Waters and Soil Leachates. Molecules 2020, 25, 5062. [CrossRef]
- Dong, X.; Lu, D.; Harris, T.A.L.; Escobar, I.C. Polymers and Solvents Used in Membrane Fabrication: A Review Focusing on Sustainable Membrane Development. Membranes 2021, 11, 309. [CrossRef]
- Nicoletta, F.P.; Cupelli, D.; Formoso, P.; De Filpo, G.; Colella, V.; Gugliuzza, A. Light Responsive Polymer Membranes: A Review. Membranes 2012, 2, 134-197. [CrossRef]
- Bahrami, S.; Dolatyari, L.; Shayani-Jam, H.; Yaftian, M.R.; Kolev, S.D. On the Potential of a Poly(vinylidenefluoride-co-hexafluoropropylene) Polymer Inclusion Membrane Containing Aliquat® 336 and Dibutyl Phthalate for V(V) Extraction from Sulfate Solutions. Membranes 2022, 12, 90. [CrossRef]
- Karuppasamy, K.; Theerthagiri, J.; Vikraman, D.; Yim, C.-J.; Hussain, S.; Sharma, R.; Maiyalagan, T.; Qin, J.; Kim, H.-S. Ionic Liquid-Based Electrolytes for Energy Storage Devices: A Brief Review on Their Limits and Applications. Polymers 2020, 12, 918. [CrossRef]
- Gonçalves, A.R.P.; Paredes, X.; Cristino, A.F.; Santos, F.J.V.; Queirós, C.S.G.P. Ionic Liquids—A Review of Their Toxicity to Living Organisms. Int. J. Mol. Sci. 2021, 22, 5612. [CrossRef]
- Rybak, A.; Rybak, A.; Kolev, S.D. A Modern Computer Application to Model Rare Earth Element Ion Behavior in Adsorptive Membranes and Materials. Membranes 2023, 13, 175. [CrossRef]
- Ferencz, A.; Grosu, A.R.; Al-Ani, H.N.A.; Nechifor, A.C.; Tanczos, S.-K.; Albu, P.C.; Crăciun, M.E.; Ioan, M.-R.; Grosu, V.-A.; Nechifor, G. Operational Limits of the Bulk Hybrid Liquid Membranes Based on Dispersion Systems. Membranes 2022, 12, 190. [CrossRef]
- Sabbatovskii, K.G. The effect of the adsorption of multicharge cations on the selectivity of a nanofiltration membrane. Colloid Journal, 2003, 65, 237-243. [CrossRef]
- Kaptakov, V.O.; Milyutin, V.V.; Nekrasova, N.A.; Zelenin, P.G.; Kozlitin, E.A. Nanofiltration Extraction of Uranium and Thorium from Aqueous Solutions. Radiochemistry, 2021, 63(2), 169-172. [CrossRef]
- Radzyminska-Lenarcik, E.; Ulewicz, M. Polymer Inclusion Membranes (PIMs) Doped with Alkylimidazole and their Application in the Separation of Non-Ferrous Metal Ions. Polymers 2019, 11, 1780. [CrossRef]
- Xu, L.; Gao, X.; Li, Z.; Gao, C. Removal of fluoride by nature diatomite from high-fluorine water: an appropriate pretreatment for nanofiltration process. Desalination, 2015, 369, 97-104. [CrossRef]
- Khedr, M.G. Radioactive contamination of groundwater, special aspects and advantages of removal by reverse osmosis and nanofiltration. Desalination, 2013.321, 47-54. [CrossRef]
- Favre-Reguillon, A.; Lebuzit, G.; Foos, J.; Guy, A.; Draye, M.; Lemaire, M. Selective concentration of uranium from seawater by nanofiltration. Industrial & engineering chemistry research, 2003, 42(23), 5900-5904. [CrossRef]
- Sharma, M.; Sharma, P.; Yadav, L.; Janu, V.C.; Gupta, R. Sequestration and recovery of thorium ions using a recyclable, low-cost, glutathione-based magnetic nanocomposite: experimental study and statistical modeling. Sep.Pur.Technol.2023, 124264. [CrossRef]
- Zahakifar, F.; Keshtkar, A.R.; Talebi, M. Performance evaluation of sodium alginate/polyvinyl alcohol/polyethylene oxide/ZSM5 zeolite hybrid adsorbent for ion uptake from aqueous solutions: a case study of thorium (IV). J. Radioanal. Nucl. Chem. 2021, 327, 65–72 (2021). [CrossRef]
- Zahakifar, F.; Keshtkar, A.; Souderjani.; E.Z.; Moosavian, M. Use of response surface methodology for optimization of thorium (IV) removal from aqueous solutions by electrodeionization (EDI). Prog. Nucl. Energy. 2020, 124, 103335. [CrossRef]
- Zahakifar, F.; Alamdar Milani, S.; Charkhi, A. Continuous bulk liquid membrane technique for thorium transport: modeling and experimental validation. J. Iran. Chem. Soc. 2018, 16, pp.455-464. https://doi. org/10.1007/s13738-018-1516-7.
- Hamza, M.F.; Guibal, E.; Wei, Y.; Ning, S. Synthesis, characterization, and evaluation of thiocarbazide-functionalized maleic-based polymer for thorium (IV) removal from aqueous solutions. Chem. Eng. J. 2023, 464, 142638. [CrossRef]
- Cheira, M.F.; Orabi, A.S.; Atia, B.M.; Hassan, S.M. Solvent extraction and separation of thorium (IV) from chloride media by a Schiff base. J. Solution. Chem. 47, 2018, 611–633. [CrossRef]
- Yousef, L.; Saad, M.; Afifi, S.; Ismail, A. Leaching and precipitation of thorium ions from Cataclastic rocks. Abu Rusheid Area, Southeastern Desert, Egypt Arab Journal of Nuclear Sciences and Applications, 2019, 51(2), 10-19. [CrossRef]
- Moon H-C. Equilibrium ultrafiltration of hydrolyzed thorium (IV) solutions. Bull. Kor. Chem. Soc. 1989 10(3), 270–272.
- Fitzsimmons, J.; Abraham, A.; Catalano, D.;Younes, A.; Cutler, C.S.; Medvedev, D. Evaluation of inorganic ion exchange materials for purification of 225Ac from thorium and radium radioisotopes. Journal of Medical Imaging and Radiation Sciences, 2019, 50(1), p. S11. [CrossRef]
- Xiu, T.; Liu, Z.; Wang, Y.; Wu, P.; Du, Y.; Cai, Z. Thorium adsorption on graphene oxide nanoribbons/manganese dioxide composite material. J. Radioanal. Nucl. Chem. 2019, 319(3), 1059–1067. [CrossRef]
- Liatsou, I.; Christodoulou, E.; Pashalidis, I. Thorium adsorption by oxidized biochar fibres derived from Luffa cylindrica sponges. J. Radioanal. Nucl. Chem. 2018, 317(2), 1065–1070. [CrossRef]
- Yin, Z.; Pan, D.; Liu, P.; Wu, H.; Li, Z.; Wu, W. Sorption behavior of thorium (IV) onto activated bentonite. of thorium (IV) onto activated bentonite. J. Radioanal. Nucl. Chem. 2018, 316(1), 301–312. [CrossRef]
- Kaynar, U.H.; Şabikoğlu, İ. Adsorption of thorium (IV) by amorphous silica; response surface modelling and optimization. J. Radioanal. Nucl. Chem. 2018, 318(2):823–834. [CrossRef]
- Xiong, X.H.; Yuan, Y.H.; Huang, B.; He, M.; Chen, H.; Luo, Y.C.; Zhu, Y.A.; Luo, T.A.; Chen, Q.S. Th (IV) adsorption onto titanium tetrachloride modified sodium bentonite. J. Radioanal. Nucl. Chem. 2019, 319(3), 805–815. [CrossRef]
- Talebi, M.; Abbasizadeh, S.; Keshtkar, A.R. Evaluation of single and simultaneous thorium and uranium sorption from water systems by an electrospun PVA/SA/PEO/HZSM5 nanofiber. Process. Saf. Environ. Prot. 2017, 109, 340–356. [CrossRef]
- Nilchi, A.; Dehaghan, T.S.; Garmarodi, S.R. Kinetics, isotherm and thermodynamics for uranium and thorium ions adsorption from aqueous solutions by crystalline tin oxide nanoparticles. Desalination 2013, 321, 67–71. [CrossRef]
- Abbasizadeh, S.; Keshtkar, A.R.; Mousavian, M.A. Preparation of a novel electrospun polyvinyl alcohol/titanium oxide nanofiber adsorbent modified with mercapto groups for uranium (VI) and thorium (IV) removal from aqueous solution. Chem. Eng. J. 2013, 220,161–171. [CrossRef]
- Tsezos, M.; Volesky, B. Biosorption of uranium and thorium. Biotechnol. Bioeng. 1981, 23(3), 583–604. [CrossRef]
- Wang, Y.; Chen, X.; Hu, X.; Wu, P.; Lan, T.; Li, Y.; Tu, H.; Liu, Y.; Yuan, D.; Wu, Z.; Liu, Z.; Chew, J.W. Synthesis and characterization of poly (TRIM/VPA) functionalized graphene oxide nanoribbons aerogel for highly efficient capture of thorium (IV) from aqueous solutions, Appl. Surf. Sci. 2021, 536, 147829. [CrossRef]
- Zhang, H.; Yang, F.; Bai, R.; Zhao, Z.;Cai, C.; Li, J.; Ma Y., Facile preparation of Ce enhanced vinyl-functionalized silica aerogel-like monoliths for selective separation of radioactive thorium from monazite. Mater. Des. 2020, 186, 108333. [CrossRef]
- Sharma, M.; Laddha, H.; Yadav, P.; Jain, Y.; Sachdev, K.; Janu, V.C.; Gupta, R. Selective removal of uranium from an aqueous solution of mixed radionuclides of uranium, cesium, and strontium via a viable recyclable GO@chitosan based magnetic nanocomposite, Mater. Today Commun. 2022, 32, 104020. [CrossRef]
- Alamdarlo, F.V.; Solookinejad, G.; Zahakifar, F.; Jalal, M.R.; Jabbari, M. Study of kinetic, thermodynamic, and isotherm of Sr adsorption from aqueous solutions on graphene oxide (GO) and (aminomethyl) phosphonic acid–graphene oxide (AMPA–GO), J. Radioanal. Nucl. Chem. 2021, 329, 1033–1043. [CrossRef]
- Zahakifar, F.; Charkhi, A.; Torab-Mostaedi, M.; Davarkhah, R. Kinetic study of uranium transport via a bulk liquid membrane containing Alamine 336 as a carrier, J. Radioanal. Nucl. Chem. 2018, 316, 247–255. [CrossRef]
- Alamdar, S.; Milani, Zahakifar, F.; Charkhi, A. Continuous bulk liquid membrane technique for thorium transport: modeling and experimental validation. J. Iran. Chem. Soc. 2019,16, 455–464. [CrossRef]
- Zahakifar, F.; Keshtkar, A.; Souderjani, E.Z.; Moosavian M., Use of response surface methodology for optimization of thorium (IV) removal from aqueous solutions by electrodeionization (EDI), Prog. Nucl. Energy 124 (2020), 103335. [CrossRef]
- D. Hritcu, D. Humelnicu, G. Dodi, M.I. Popa, Magnetic chitosan composite particles: evaluation of thorium and uranyl ion adsorption from aqueous solutions. Carbohydr. Polym. 2012, 87, 1185–1191. [CrossRef]
- Zolfonoun, E.; Yousefi, S.R. Sorption and preconcentration of uranium and thorium from aqueous solutions using multi-walled carbon nanotubes decorated with magnetic nanoparticles. Radiochim. Acta 2015, 103, 835–841. [CrossRef]
- Karimi, M.; Milani, S.A.; Abolgashemi H., Kinetic and isotherm analyses for thorium (IV) adsorptive removal from aqueous solutions by modified magnetite nanoparticle using response surface methodology (RSM). J. Nucl. Mater. 2016, 479, 174–183. [CrossRef]
- García, A.C.; Latifi, M.; Amini, A.; Chaouki, J. Separation of Radioactive Elements from Rare Earth Element-Bearing Minerals. Metals 2020, 10, 1524. [CrossRef]
- Bejanidze, I.; Petrov, O.; Kharebava, T.; Pohrebennyk, V.; Davitadze, N.; Didmanidze, N. Study of the Healing Properties of Natural Sources of Georgia and Modeling of Their Purification Processes. Appl. Sci. 2020, 10, 6529. [CrossRef]
- Phillip, E.; Choo, T.F.; Khairuddin, N.W.A.; Abdel Rahman, R.O. On the Sustainable Utilization of Geopolymers for Safe Management of Radioactive Waste: A Review. Sustainability 2023, 15, 1117. [CrossRef]
- Kusumkar, V.V.; Galamboš, M.; Viglašová, E.; Daňo, M.; Šmelková, J. Ion-Imprinted Polymers: Synthesis, Characterization, and Adsorption of Radionuclides. Materials 2021, 14, 1083. [CrossRef]
- Inman, G.; Nlebedim, I.C.; Prodius, D. Application of Ionic Liquids for the Recycling and Recovery of Technologically Critical and Valuable Metals. Energies 2022, 15, 628. [CrossRef]
- Brewer, A.; Florek, J.; Kleitz, F. A perspective on developing solid-phase extraction technologies for industrial-scale critical materials recovery. Green Chemistry, 2022, 24(7), 2752-2765. [CrossRef]
- Atanassova, M. Assessment of the Equilibrium Constants of Mixed Complexes of Rare Earth Elements with Acidic (Chelating) and Organophosphorus Ligands. Separations 2022, 9, 371. [CrossRef]
- Allam, E.M.; Lashen, T.A.; Abou El-Enein, S.A.; Hassanin, M.A.; Sakr, A.K.; Cheira, M.F.; Almuqrin, A.; Hanfi, M.Y.; Sayyed, M.I. Rare Earth Group Separation after Extraction Using Sodium Diethyldithiocarbamate/Polyvinyl Chloride from Lamprophyre Dykes Leachate. Materials 2022, 15, 1211. [CrossRef]
- Alotaibi, A.M.; Ismail, A.F. Modification of Clinoptilolite as a Robust Adsorbent for Highly-Efficient Removal of Thorium (IV) from Aqueous Solutions. Int. J. Environ. Res. Public Health 2022, 19, 13774. [CrossRef]
- Santiago-Aliste, A.; Sánchez-Hernández, E.; Langa-Lomba, N.; González-García, V.; Casanova-Gascón, J.; Martín-Gil, J.; Martín-Ramos, P. Multifunctional Nanocarriers Based on Chitosan Oligomers and Graphitic Carbon Nitride Assembly. Materials 2022, 15, 8981. [CrossRef]
- Chen, Y.; Chen, Y.; Lu, D.; Qiu, Y. Synthesis of a Novel Water-Soluble Polymer Complexant Phosphorylated Chitosan for Rare Earth Complexation. Polymers 2022, 14, 419. [CrossRef]
- Yudaev, P.; Chistyakov, E. Chelating Extractants for Metals. Metals 2022, 12, 1275. [CrossRef]
- Zhijun, G.; Wangsuo, W.; Dadong, S.; Minyu, T. Liquid-liquid extraction of uranium (VI) and thorium (IV) by two open-chain crown ethers with terminal quinolyl groups in chloroform. Journal of radioanalytical and nuclear chemistry 2003, 258(1), 199-203. [CrossRef]
- Agrawal, Y.K.; Vora, S.B. Selective extraction and separation of thorium from monazite using N-phenylbenzo-18-crown-6-hydroxamic acid. Microchimica Acta, 2003, 142, 255-261. [CrossRef]
- Xiong, X.H.; Tao, Y.; Yu, Z.W.; Yang, L.X.; Sun, L.J.; Fan, Y.L.; Luo, F. Selective extraction of thorium from uranium and rare earth elements using sulfonated covalent organic framework and its membrane derivate. Chemical Engineering Journal. 2020 15(384), 123240. [CrossRef]
- Alharbi, H.F.; Haddad, M.Y.; Aijaz, M.O.; Assaifan, A.K.; Karim, M.R. Electrospun Bilayer PAN/Chitosan Nanofiber Membranes Incorporated with Metal Oxide Nanoparticles for Heavy Metal Ion Adsorption. Coatings 2020, 10, 285. [CrossRef]

| Keywords *) | Publications number on periods | ||
|---|---|---|---|
| Any time | 2014-23 | 2021-23 | |
| Thorium separation | 162,000 | 82,000 | 12,900 |
| Thorium concentration | 199,000 | 12,200 | 6,200 |
| Thorium recovery | 79,000 | 17,900 | 9,200 |
| Thorium removing | 62,000 | 17,500 | 13,800 |
| Membrane thorium separation | 21,900 | 10,600 | 3,730 |
| Membrane thorium concentration | 27,600 | 19,000 | 4,610 |
| Membrane thorium recovery | 18,000 | 8,600 | 3,850 |
| Membrane thorium removing | 21,600 | 12,000 | 4,500 |
| “Thorium separation” | 883 | 244 | 79 |
| “Thorium recovery” | 611 | 204 | 87 |
| “Thorium recycling” | 50 | 18 | 4 |
| “Thorium membrane” | 7 | 2 | - |
| Application | Materials or techniques | Refs. |
|---|---|---|
| Thorium Removal | Different Adsorbents | [126] |
| Removal of Thorium (IV) from Aqueous Solutions. | Modification of Clinoptilolite as a Robust Adsorbent for Highly-Efficient | [127] |
| Preconcentration of Uranium in Natural Water Samples | New Polymer with Imprinted Ions | [128] |
| Adsorption of Trace Thorium (IV) from Aqueous Solution | Mono-Modified β-Cyclodextrin Polyrotaxane Using Response Surface Methodology (RSM) | [129] |
| Novel Malonamide Grafted Polystyrene-Divinyl Benzene Resin for Extraction | Pre-Concentration and Separation of Actinides | [130] |
| Using Mesoporous | Selectivity of Th (IV) Adsorption as Compared to U (VI), La (III), Ce (III), Sm (III) and Gd (III) | [131] |
| α-Aminophosphonates, -Phosphinates, and -Phosphine Oxides | Extraction and Precipitation Agents for Rare Earth Metals, Thorium, and Uranium | [132] |
| Removal of Polyvalent Metal Ions (Eu(III) and Th(IV)) from Aqueous Solutions by. | Polyurea-Crosslinked Alginate Aerogels | [133] |
| Patented Chinese method | Method for Separating Cerium-Fluoride and Thorium | [134] |
| α-Aminophosphonate Extractant. | Extraction and Recovery of Cerium(IV) and Thorium(IV) from Sulphate Medium | [135] |
| Selective Extraction and Separation of Ce(IV) and Th(IV) from RE(III) | Sulfate Medium Using Di(2-Ethylhexyl)-N-Heptylaminomethylphosphonate | [136] |
| Selective Extraction and Separation of Ce(IV) from Thorium and Trivalent Rare Earths | Sulfate Medium by an α-Aminophosphonate Extractant | [137] |
| Extraction and Separation of Heavy Rare Earths from Chloride Medium | α-Aminophosphonic Acid HEHAPP. | [138] |
| Solvent Extraction and Separation of Rare Earths from Chloride Media Using | α-Aminophosphonic Acid Extractant HEHAMP. | [139] |
| on PAN/Zeolite Composite Adsorbent. | Study of the Behavior of Thorium Adsorption | [140] |
| Tulul Al-Shabba Zeolitic Tuff, Jordan | Adsorption of Thorium (IV) and Uranium (VI) | [141] |
| Sodium Clinoptilolite | Removal of Thorium from Aqueous Solutions | [142] |
| Adsorptions Performance towards Thorium. | Studies of Modification of Zeolite by Tandem Acid-Base Treatments | [143] |
| Tetraazonium based ionic liquid | Selective cloud point extraction of thorium (IV) | [144] |
| Deoiled karanja seed cake | Removal of thorium (IV) from aqueous solutions. Optimization using Taguchi method, equilibrium, kinetic and thermodynamic studies | [145] |
| Peat moss | Retention of uranyl and thorium ions from radioactive solution | [146] |
| To obtain photo-responsive metal-organic frameworks (MOFs) | Photocatalysis and adsorption | [147] |
| Th(IV) and Ce(III) in ThF4-CeF3-LiCl-KCl quaternary melt | Electrochemical behaviors and electrolytic separation | [148] |
| Hybrid mesoporous adsorbent as benzenesulfonamide-derivative@ZrO2 | Selective removal of thorium ions from aqueous solutions | [149] |
| Extraction Using Sodium Diethyldithiocarbamate/Polyvinyl Chloride | Rare Earth Group Separation from Lamprophyre Dykes Leachate. | [150] |
| Metal-Organic Framework Based Fluorescent Sensors | for Hazardous Materials Detection. | [151] |
| Zeolite Adsorption. | Separation of Radionuclides from a Rare Earth-Containing Solution by | [152] |
| Acidic (Chelating) and Organophosphorus Ligands. | Equilibrium Constants of Mixed Complexes of Rare Earth Elements | [153] |
| Thenoyltrifluoroacetone: | Molecule for Solvent Extraction of Metals | [154] |
| 8-Hydroxyquinoline Immobilized Bentonite. | Removal of Uranium and Thorium from Their Aqueous Solutions | [155] |
| New Polymer with Imprinted Ions Samples and Determination by Digital Imaging. | Preconcentration of Uranium in Natural Water | [156] |
| Type of membrane process | Pore diameter (nm) |
Pressure (bar) |
The obtained water content |
|---|---|---|---|
| Reverse osmosis | < 0.6 | 15–60 | Pure water (poorly ionized) |
| Nanofiltration | 0.6–10 | 6–20 | Pure water (traces of molecular substances) |
| Ultrafiltration | 7–70 | 4–15 | Pure water, molecular substances and macromolecules |
| Microfiltration | 50–800 | 0.5–2.5 | Pure water, molecular substances and colloids |
| Technological operation | Losses of thorium or of thorium-contaminated materials | Means of remedial or reduction of losses |
|---|---|---|
| Crushing, grinding | Dust removal Mill shutdown losses Losses when cleaning the machine |
Microfilter installation Micro and ultrafiltration of colloidal washing solutions |
| Solubilization or leaching | Incomplete solubilization with the chosen reagent Complete solubilization Too little concentration of thorium |
Solubilization with a complementary reagent Selective reprecipitation and solubilization Concentration by precipitation and microfiltration |
| Filtration | Thorium retention in the precipitate Reduced concentration of thorium in the filtrate |
Washing with solubilizing reagents Reprecipitation and micro or ultra filtration |
| Precipitation | Incomplete precipitation Precipitation of nanometric particles |
Nanofiltration or reverse osmosis of the filtrate Colloidal ultrafiltration or nanofiltration |
| Extraction | Solvent losses Incomplete extraction |
Solvent recovery Use of selective extractants |
| Ion exchange | Blockage of thorium in the ion exchanger (elution inefficiency) Incomplete retention |
Change eluent Recovery of ion exchangers for destruction (burning) |
| Topics | Application | Refs. |
|---|---|---|
| Ionic Liquid | Gas Separation Membranes | [243] |
| Waste treatment | Liquid Radioactive Wastes Treatment | [244] |
| Ionic Liquids | Proton Exchange Membrane in Fuel Cells | [245] |
| Roles of Chitosan-Supported Ionic Liquids | Chitosan-Based Polymers as Proton Exchange | [246] |
| Strategy in Liquid Filtration | Membrane Surface Patterning as a Fouling Mitigation | [247] |
| Polymer Inclusion Membrane and a Chelating Resin | Sequential Determination of Copper(II) and Zinc(II) in Natural Waters and Soil Leachates | [248] |
| Polymers and Solvents Used in Membrane Fabrication | Sustainable Membrane Development. | [249] |
| Light Responsive Polymer Membranes | Miscellaneous application | [250] |
| Poly(vinylidene-fluoride-co-hexafluoropropylene) Polymer Inclusion Membrane Containing Aliquat® 336 and Dibutyl Phthalate | Extraction from Sulfate Solutions | [251] |
| Ionic Liquid | Based Electrolytes for Energy Storage Devices | [252] |
| Ionic Liquids | Their Toxicity to Living Organisms. | [253] |
| Modern Computer Application | Model Rare Earth Element Ion Behavior in Adsorptive Membranes and Materials | [254] |
| Bulk Hybrid Liquid Membranes Based on Dispersion Systems | Operational Limits | [255] |
| nanofiltration membrane | effect of the adsorption of multicharge cations on the selectivity of a | [256] |
| Nanofiltration | Extraction of Uranium and Thorium from Aqueous Solutions | [257] |
| Polymer Inclusion Membranes (PIMs) Doped with Alkylimidazole | Application in the Separation of Non-Ferrous Metal Ions. | [258] |
| nanofiltration process | Removal of fluoride by nature diatomite from high-fluorine water | [259] |
| reverse osmosis and nanofiltration | removal radioactive contamination of groundwater, special aspects and advantage | [260] |
| Selective concentration | uranium from seawater by nanofiltration | [261] |
| glutathione-based magnetic nanocomposite | Sequestration and recovery of thorium ions using a recyclable, low-cost | [262] |
| evaluation of sodium alginate/polyvinyl alcohol/polyethylene oxide/ZSM5 zeolite hybrid adsorbent | a case study of thorium (IV). | [263] |
| Use of response surface methodology for optimization of thorium (IV) | removal from aqueous solutions by electro-deionization (EDI) | [264] |
| Continuous bulk liquid membrane technique | thorium transport: modeling and experimental validation | [265] |
| Synthesis, characterization, and evaluation of thiocarbazide-functionalized maleic-based polymer | thorium (IV) removal from aqueous solutions | [266] |
| Materials or processes | Application | Refs. |
|---|---|---|
| Solvent extraction and separation of thorium (IV) | from chloride media by a Schiff base. | [267] |
| Leaching and precipitation of thorium ions | from Cataclastic rocks (Abu Rusheid Area, South Eastern Desert, Egypt) |
[268] |
| Equilibrium ultrafiltration of hydrolyzed thorium (IV) solutions. | Solubility of thorium salts | [269] |
| Evaluation of inorganic ion exchange materials. | for purification of 225Ac from thorium and radium radioisotopes | [270] |
| graphene oxide nanoribbons/manganese dioxide composite material. | Thorium adsorption on | [271] |
| oxidized biochar fibers derived from Luffa cylindrica sponges |
Thorium adsorption | [272] |
| onto activated bentonite. | Sorption behavior of thorium(IV) | [273] |
| amorphous silica | Adsorption of thorium(IV) response surface modelling and optimization. | [274] |
| titanium tetrachloride modified sodium bentonite. | Th(IV) adsorption | [275] |
| electrospun PVA/SA/PEO/HZSM5 nanofiber. | Evaluation of single and simultaneous thorium and uranium sorption from water systems | [276] |
| crystalline tin oxide nanoparticles. | Kinetics, isotherm and thermodynamics for uranium and thorium ions adsorption from aqueous solutions by | [277] |
| novel electrospun polyvinyl alcohol/titanium oxide nanofiber adsorbent modified with mercapto groups. | for uranium(VI) and thorium(IV) removal from aqueous solution | [278] |
| Biosorption | uranium and thorium | [279] |
| Synthesis and characterization of poly(TRIM/VPA) functionalized graphene oxide nanoribbons aerogel, | for highly efficient capture of thorium(IV) from aqueous solutions | [280] |
| vinyl-functionalized silica aerogel-like monoliths, | for selective separation of radioactive thorium from monazite | [281] |
| recyclable GO@chitosan based magnetic nanocomposite | Selective removal of uranium from an aqueous solution of mixed radionuclides of uranium, cesium, and strontium via a viable | [282] |
| graphene oxide (GO) and (aminomethyl) phosphonic acid–graphene oxide (AMPA–GO) | Study of kinetic, thermodynamic, and isotherm of Sr adsorption | [283] |
| bulk liquid membrane containing Alamine 336 as a carrier | Kinetic study of uranium transport | [284] |
| Continuous bulk liquid membrane technique | thorium transport: modeling and experimental validation | [285] |
| electrodeionization (EDI) | Use of response surface methodology for optimization of thorium (IV) removal from aqueous solutions | [286] |
| Magnetic chitosan composite particles | evaluation of thorium and uranyl ion adsorption from aqueous solutions | [287] |
| multi-walled carbon nanotubes decorated with magnetic nanoparticles | Sorption and preconcentration of uranium and thorium from aqueous solutions | [288] |
| Kinetic and isotherm analyses using response surface methodology (RSM) | for thorium (IV) adsorptive removal from aqueous solutions by modified magnetite nanoparticle | [289] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





