Submitted:
17 July 2023
Posted:
19 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis
2.2.1. Direct Alumination of SBA-15
2.3. Characterizations
2.4. Catalytic performance assessment
2.4.1. Esterification
3. Results and Discussion
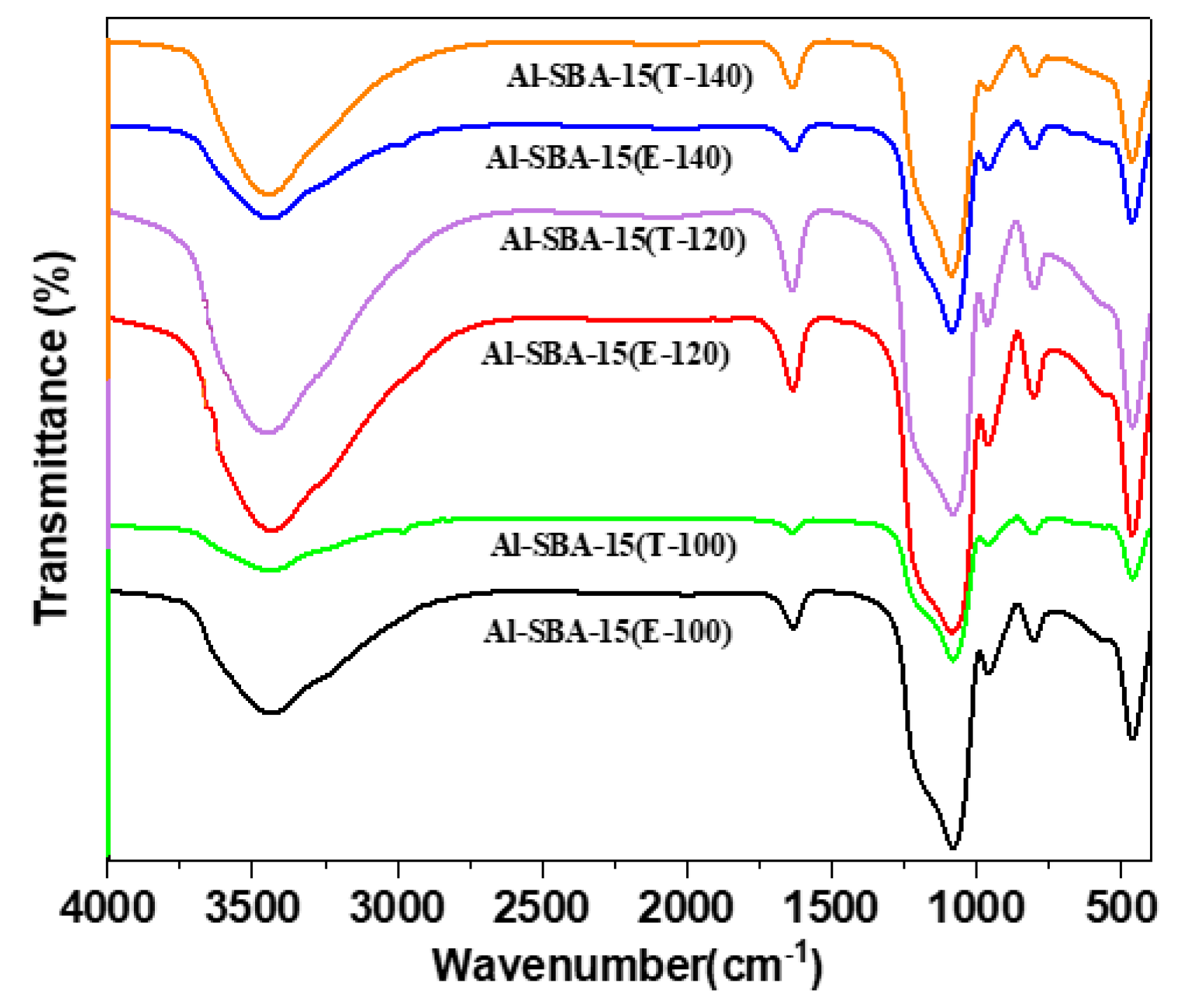
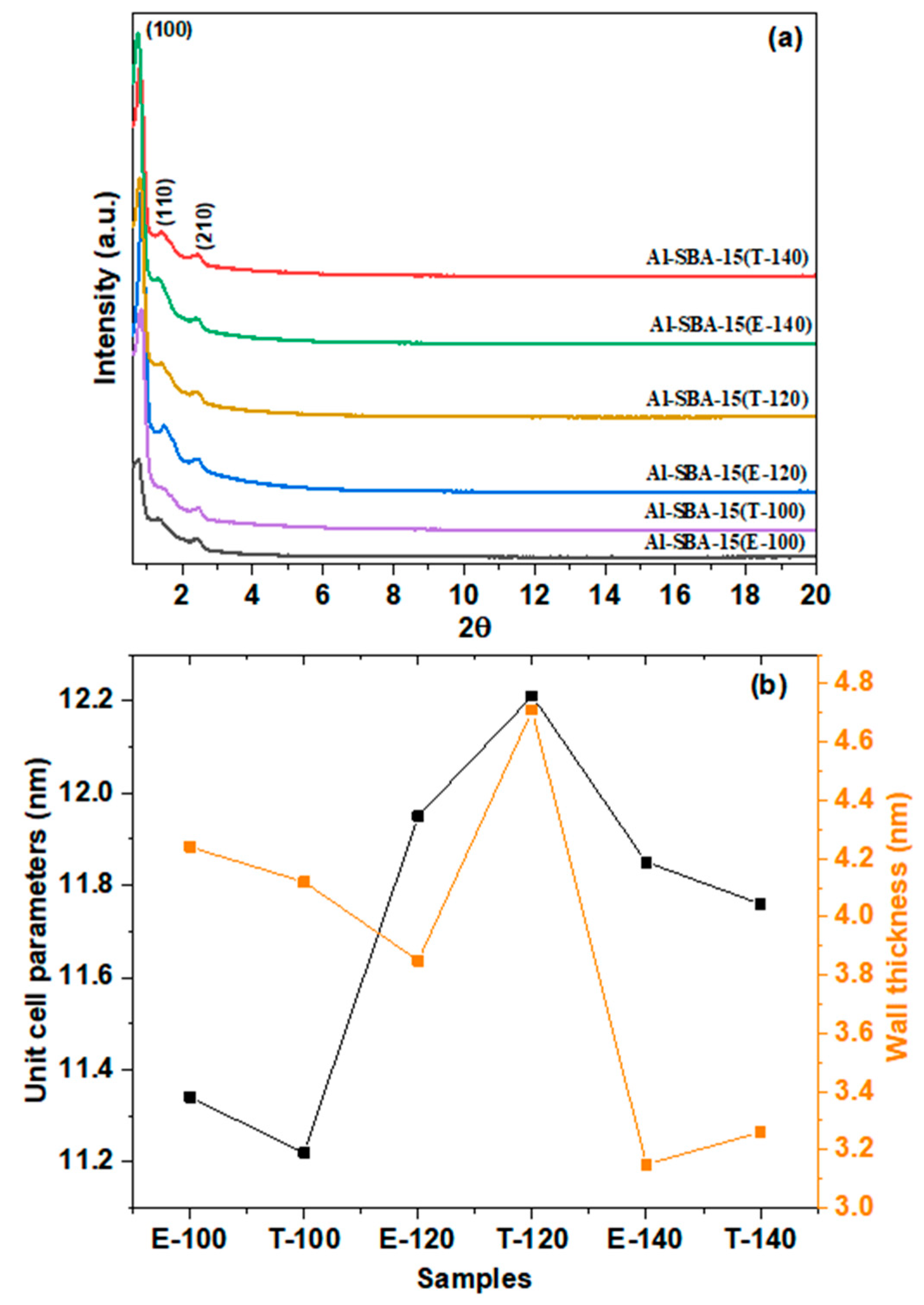
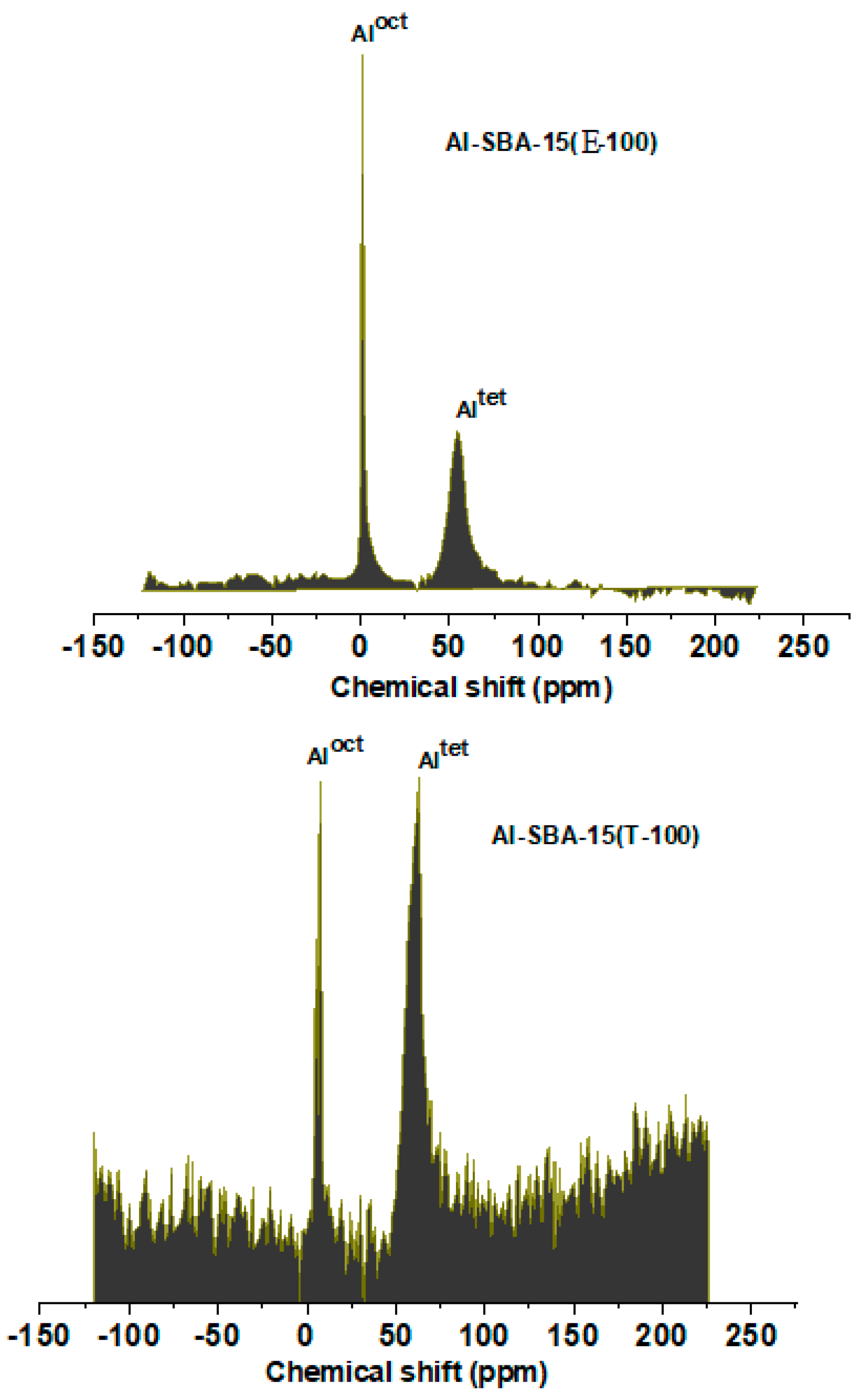
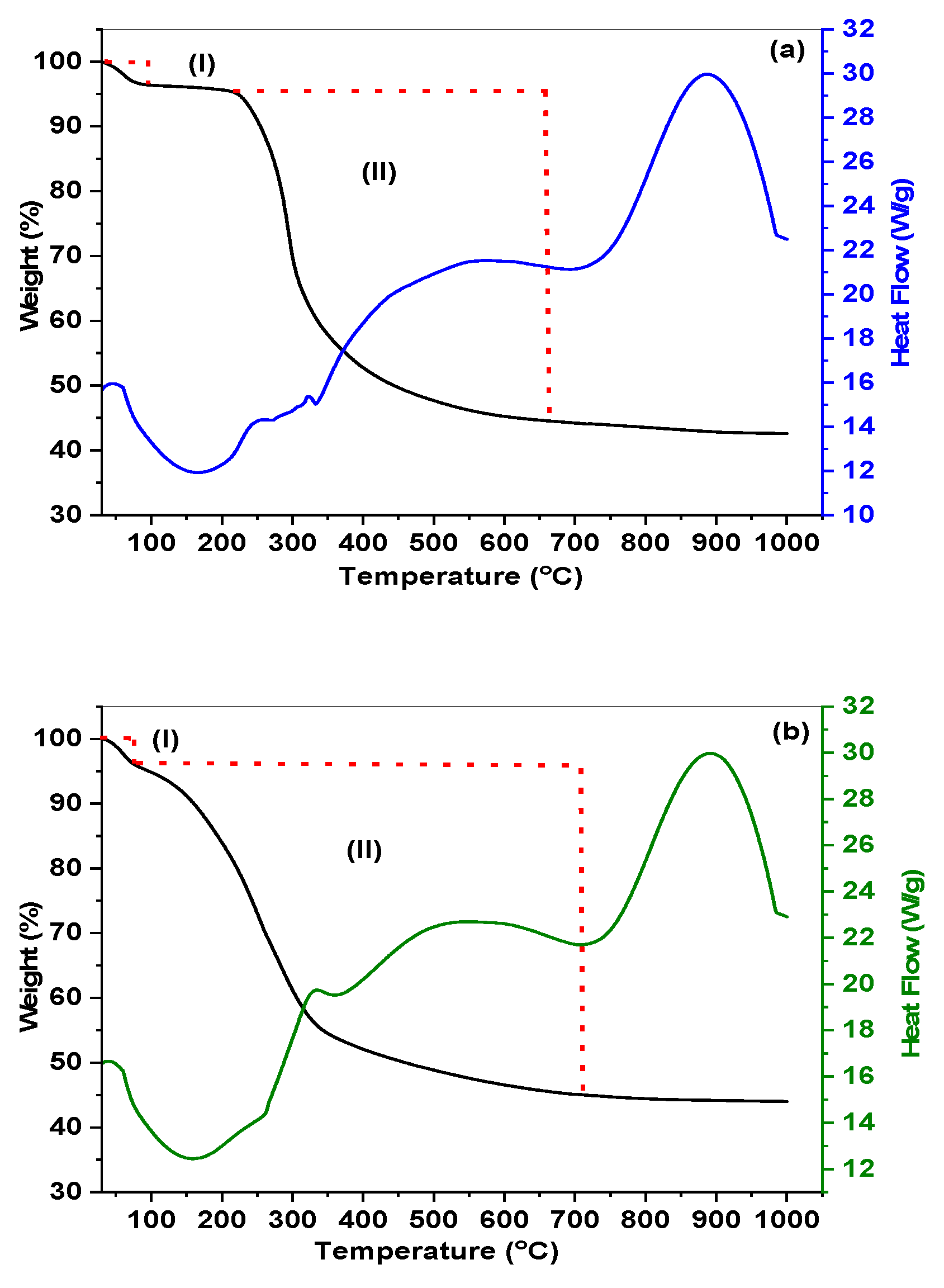
3.1. Materials characterization
3.1.2. The characteristics of acid sites
3.2. Catalytic performance
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Awual, M.R. Novel nanocomposite materials for efficient and selective mercury ions capturing from wastewater. Chem. Eng. J. 2017, 307, 456–465. [Google Scholar] [CrossRef]
- Mota, F.M.; Eliášová, P.; Jung, J.; Ryoo, R. Mesoporous EU-1 zeolite as a highly active catalyst for ethylbenzene hydroisomerization. Catal. Sci. Technol. 2016, 6, 2735–2741. [Google Scholar] [CrossRef]
- El Rahman, S.K.A.; Hassan, H.M.A.; El-Shall, M.S. Metal-organic frameworks with high tungstophosphoric acid loading as heterogeneous acid catalysts. Appl. Catal. A Gen. 2014, 487, 110–118. [Google Scholar]
- Guisnet, M.; Gilson, J.-P. Zeolites for cleaner technologies; Imperial College Press: London, UK, 2002. [Google Scholar]
- Corma, A. Solid State Mat. Sci. 1997, 2, 63. [Google Scholar]
- Gallo, J.M.R.; Bisio, C.; Gatti, G.; Marchese, L.; Pastore, H.O. Physicochemical characterization and surface acid properties of mesoporous [Al]-SBA-15 obtained by direct synthesis. Langmuir. 2010, 26, 5791–5800. [Google Scholar] [CrossRef]
- Chytil, S.; Glomm, W.R.; Blekkan, E.A. Characterization of Pt/SBA-15 prepared by the deposition–precipitation method. Catal. Today. 2009, 147, 217–223. [Google Scholar] [CrossRef]
- Kang, Y.; Rao, X.; Yuan, P.; Wang, C.; Wang, T.; Yue, Y. Al-functionalized mesoporous SBA-15 with enhanced acidity for hydroisomerization of n-octane. Fuel Process. Technol. 2021, 215, 106765. [Google Scholar] [CrossRef]
- Hassan, H.M.A.; Betiha, M.A.; Elshaarawy, R.F.M.; Ahmed, E.A. Facile tailoring of hierarchical mesoporous AlSBA-15 by ionic liquid and their applications in heterogeneous catalysis. J. Porous Mater. 2018, 25, 63–73. [Google Scholar] [CrossRef]
- Betiha, M.A.; Hassan, H.M.A.; Al-Sabagh, A.M.; El Rahman, S.K.A.; Ahmed, E.A. Direct synthesis and the morphological control of highly ordered mesoporous AlSBA-15 using urea-tetrachloroaluminate as a novel aluminum source. J. Mater. Chem. 2012, 22, 17551–17559. [Google Scholar] [CrossRef]
- Dragoi, B.; Dumitriu, E.; Guimon, C.; Auroux, A. Acidic and adsorptive properties of SBA-15 modified by aluminum incorporation. Microporous Mesoporous Mater. 2009, 121, 7–17. [Google Scholar] [CrossRef]
- Wu, P.; Tatsumi, T.; Komatsu, T.; Yashima, T. Postsynthesis, characterization, and catalytic properties in alkene epoxidation of hydrothermally stable mesoporous Ti-SBA-15. Chem. Mater. 2002, 14, 1657–1664. [Google Scholar] [CrossRef]
- Luan, Z.; Hartmann, M.; Zhao, D.; Zhou, W.; Kevan, L. Alumination and ion exchange of mesoporous SBA-15 molecular sieves. Chem. Mater. 1999, 11, 1621–1627. [Google Scholar] [CrossRef]
- Lapisardi, G.; Chiker, F.; Launay, F.; Nogier, J.-P.; Bonardet, J.-L. A “one-pot” synthesis of adipic acid from cyclohexene under mild conditions with new bifunctional Ti-AlSBA mesostructured catalysts. Catal. Commun. 2004, 5, 277–281. [Google Scholar] [CrossRef]
- Yue, Y.; Gédéon, A.; Bonardet, J.-L.; D’Espinose, J.-B.; Fraissard, J.; Melosh, N. Direct synthesis of AlSBA mesoporous molecular sieves: Characterization and catalytic activities. Chem. Commun. 1999, 19, 1967–1968. [Google Scholar] [CrossRef]
- Xiong, L.; Shi, J.; Zhang, L.; Nogami, M. Facile one-step synthesis of highly ordered bimodal mesoporous phosphosilicate monoliths. J. Am. Chem. Soc. 2007, 129, 11878–11879. [Google Scholar] [CrossRef]
- Kemache, N.; Hamoudi, S.; Arul, J.; Belkacemi, K. Activity and selectivity of nanostructured sulfur-doped Pd/SBA-15 catalyst for vegetable oil hardening. Ind. Eng. Chem. Res. 2010, 49, 971–979. [Google Scholar] [CrossRef]
- Rivera-Jiménez, S.M.; Méndez-González, S.; Hernández-Maldonado, A. Metal (M= Co2+, Ni2+, and Cu2+) grafted mesoporous SBA-15: Effect of transition metal incorporation and pH conditions on the adsorption of Naproxen from water. Microporous Mesoporous Mater. 2010, 132, 470–479. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Q. A simple method to directly synthesize Al-SBA-15 mesoporous materials with different Al contents. Solid State Commun. 2008, 148, 529–533. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Q.; Liu, Q. Synthesis and characterization of Sn or Al-containing SBA-15 mesoporous materials without mineral acids added. Microporous Mesoporous Mater. 2007, 102, 51–57. [Google Scholar] [CrossRef]
- Garrett, C.E.; Prasad, K. The art of meeting palladium specifications in active pharmaceutical ingredients produced by Pd-catalyzed reactions. Adv. Synth. Catal. 2004, 346, 889–900. [Google Scholar] [CrossRef]
- Wu, S.; Han, Y.; Zou, Y.-C.; Song, J.-W.; Zhao, L.; Di, Y.; Liu, S.-Z.; Xiao, F.-S. Synthesis of heteroatom substituted SBA-15 by the “pH-adjusting” method. Chem. Mater. 2004, 16, 486–492. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Wang, H.J.; Zhuang, T.T.; Sun, L.B.; Wang, Y.M.; Zhu, J.H. Multiple Functionalization of Mesoporous Silica in One-Pot: Direct Synthesis of Aluminum-Containing Plugged SBA-15 from Aqueous Nitrate Solutions. Adv. Funct. Mater. 2008, 18, 82–94. [Google Scholar] [CrossRef]
- Vinu, A.; Murugesan, V.; Böhlmann, W.; Hartmann, M. An optimized procedure for the synthesis of AlSBA-15 with large pore diameter and high aluminum content. J. Phys. Chem. B. 2004, 108, 11496–11505. [Google Scholar] [CrossRef]
- Zecchina, A.; Bordiga, S.; Spoto, G.; Marchese, L.; Petrini, G.; Leofanti, G.; Padovan, M. Silicalite characterization. 1. Structure, adsorptive capacity, and IR spectroscopy of the framework and hydroxyl modes. J. Phys. Chem. 1992, 96, 4985–4990. [Google Scholar] [CrossRef]
- Góra-Marek, K.; Derewiński, M.; Sarv, P.; Datka, J. IR and NMR studies of mesoporous alumina and related aluminosilicates. Catal. Today. 2005, 101, 131–138. [Google Scholar] [CrossRef]
- Kleitz, F.; Berube, F.; Guillet-Nicolas, R.; Yang, C.-M.; Thommes, M. Probing adsorption, pore condensation, and hysteresis behavior of pure fluids in three-dimensional cubic mesoporous KIT-6 silica. J. Phys. Chem. C. 2010, 114, 9344–9355. [Google Scholar] [CrossRef]
- Liu, Y.-M.; Cao, Y.; Yi, N.; Feng, W.-L.; Dai, W.-L.; Yan, S.-R.; He, H.-Y.; Fan, K.-N. Vanadium oxide supported on mesoporous SBA-15 as highly selective catalysts in the oxidative dehydrogenation of propane. J. Catal. 2004, 224, 417–428. [Google Scholar] [CrossRef]
- Selvaraj, M.; Park, D.-W.; Ha, C.S. Well ordered two-dimensional mesoporous CeSBA-15 synthesized with improved hydrothermal stability and catalytic activity. Microporous Mesoporous Mater. 2011, 138, 94–101. [Google Scholar] [CrossRef]
- Kudo, T.; Hisamitsu, Y.; Kihara, K.; Mohamedi, M.; Uchida, I. Electrochemical behaviour of Ni+ Al alloy as an alternative material for molten carbonate fuel cell cathodes. J. Appl. Electrochem. 2002, 32, 179–184. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Imperor-Clerc, M.; Davidson, P.; Davidson, A. Existence of a microporous corona around the mesopores of silica-based SBA-15 materials templated by triblock copolymers. J. Am. Chem. Soc. 2000, 122, 11925–11933. [Google Scholar] [CrossRef]
- Ravikovitch, P.I.; Neimark, A.V. Characterization of micro-and mesoporosity in SBA-15 materials from adsorption data by the NLDFT method. J. Phys. Chem. B. 2001, 105, 6817–6823. [Google Scholar] [CrossRef]
- Khder, A.S.; El-Sharkawy, E.A.; El-Hakam, S.A.; Ahmed, A.I. Surface characterization and catalytic activity of sulfated tin oxide catalyst. Catal. Commun. 2008, 9, 769–777. [Google Scholar] [CrossRef]
- Barbosa, S.L.; Dabdoub, M.J.; Hurtado, G.R.; Klein, S.I.; Baroni, A.C.M.; Cunha, C. Solvent free esterification reactions using Lewis acids in solid phase catalysis. Appl. Catal. A Gen. 2006, 313, 146–150. [Google Scholar] [CrossRef]
- Bordwell, F.G. Equilibrium acidities in dimethyl sulfoxide solution, Acc. Chem. Res. 1988, 21, 456–463. [Google Scholar] [CrossRef]
- Han, J.; Xu, G.; Ding, B.; Pan, J.; Dou, H.; MacFarlane, D.R. Porous nitrogen-doped hollow carbon spheres derived from polyaniline for high performance supercapacitors. J. Mater. Chem. A. 2014, 2, 5352–5357. [Google Scholar] [CrossRef]
- Kubota, Y.; Nishizaki, Y.; Ikeya, H.; Saeki, M.; Hida, T.; Kawazu, S.; Yoshida, M.; Fujii, H.; Sugi, Y. Organic–silicate hybrid catalysts based on various defined structures for Knoevenagel condensation. Microporous Mesoporous Mater. 2004, 70, 135–149. [Google Scholar] [CrossRef]
- Chen, X.; Arruebo, M.; Yeung, K.L. Flow-synthesis of mesoporous silicas and their use in the preparation of magnetic catalysts for Knoevenagel condensation reactions. Catal. Today. 2013, 204, 140–147. [Google Scholar] [CrossRef]
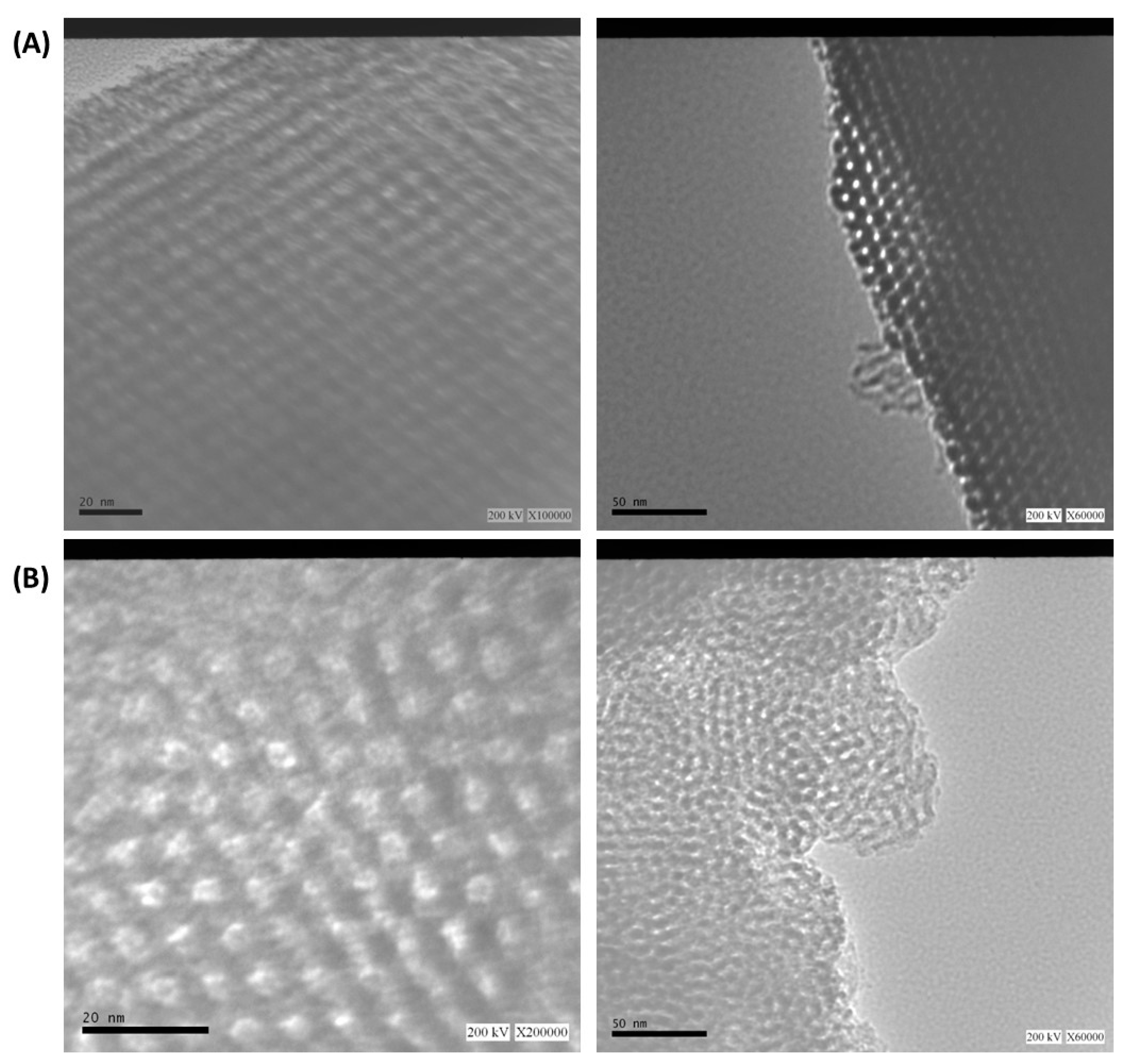
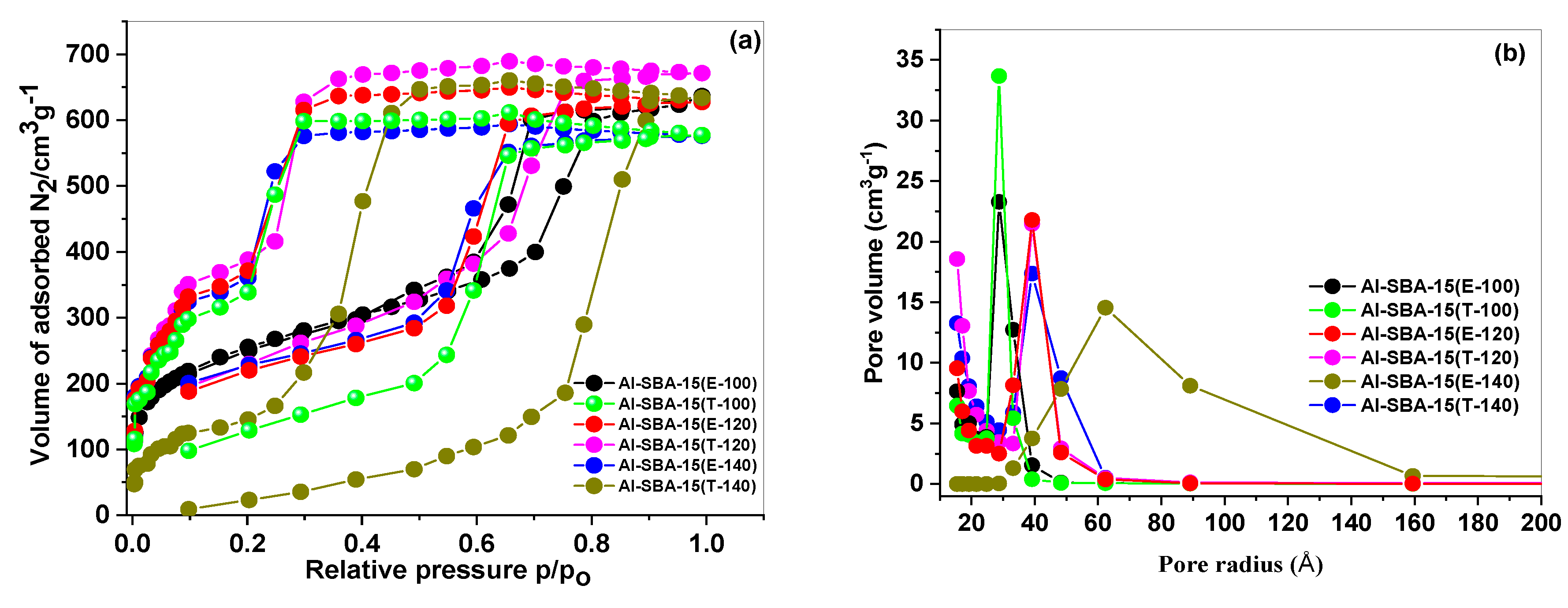
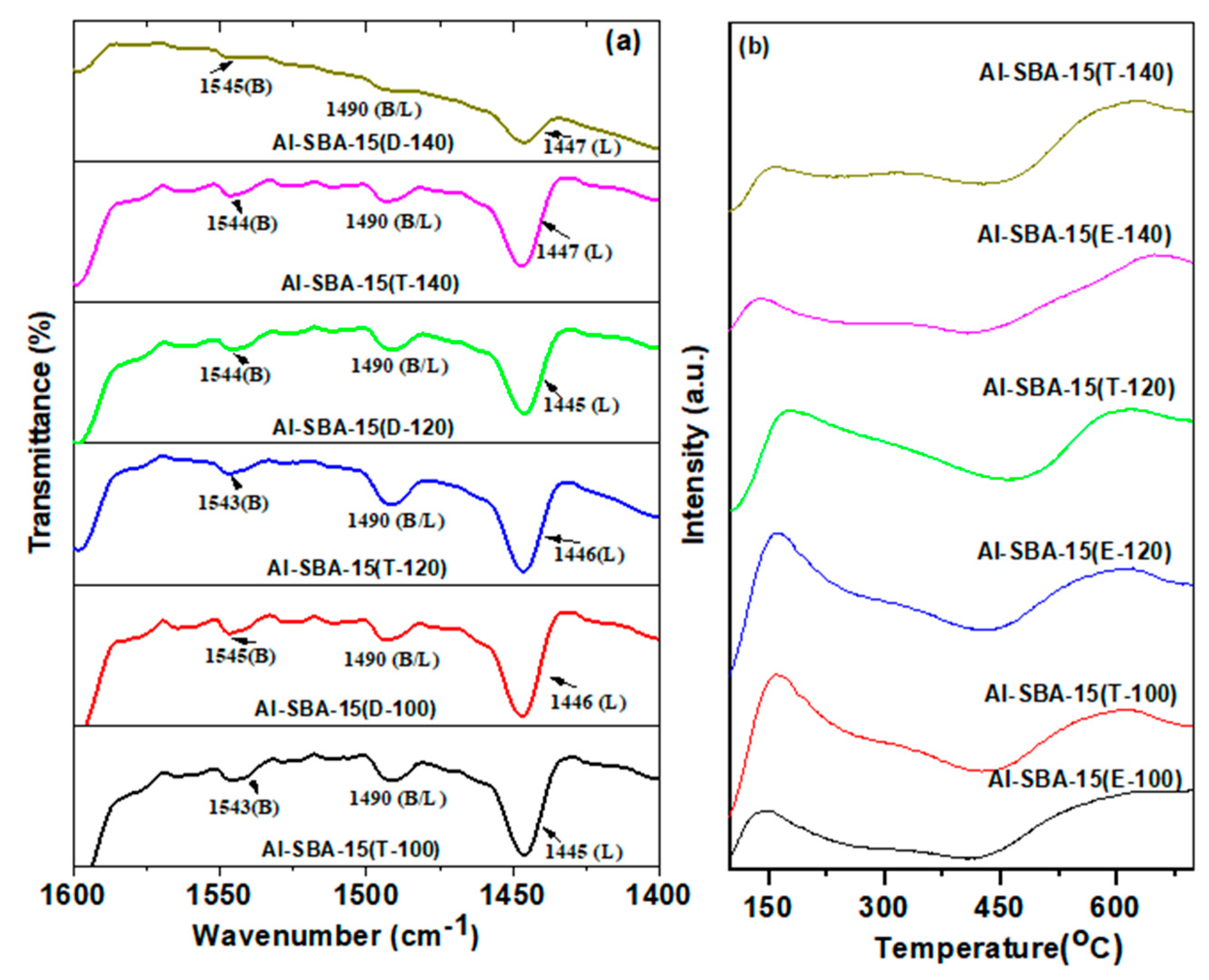
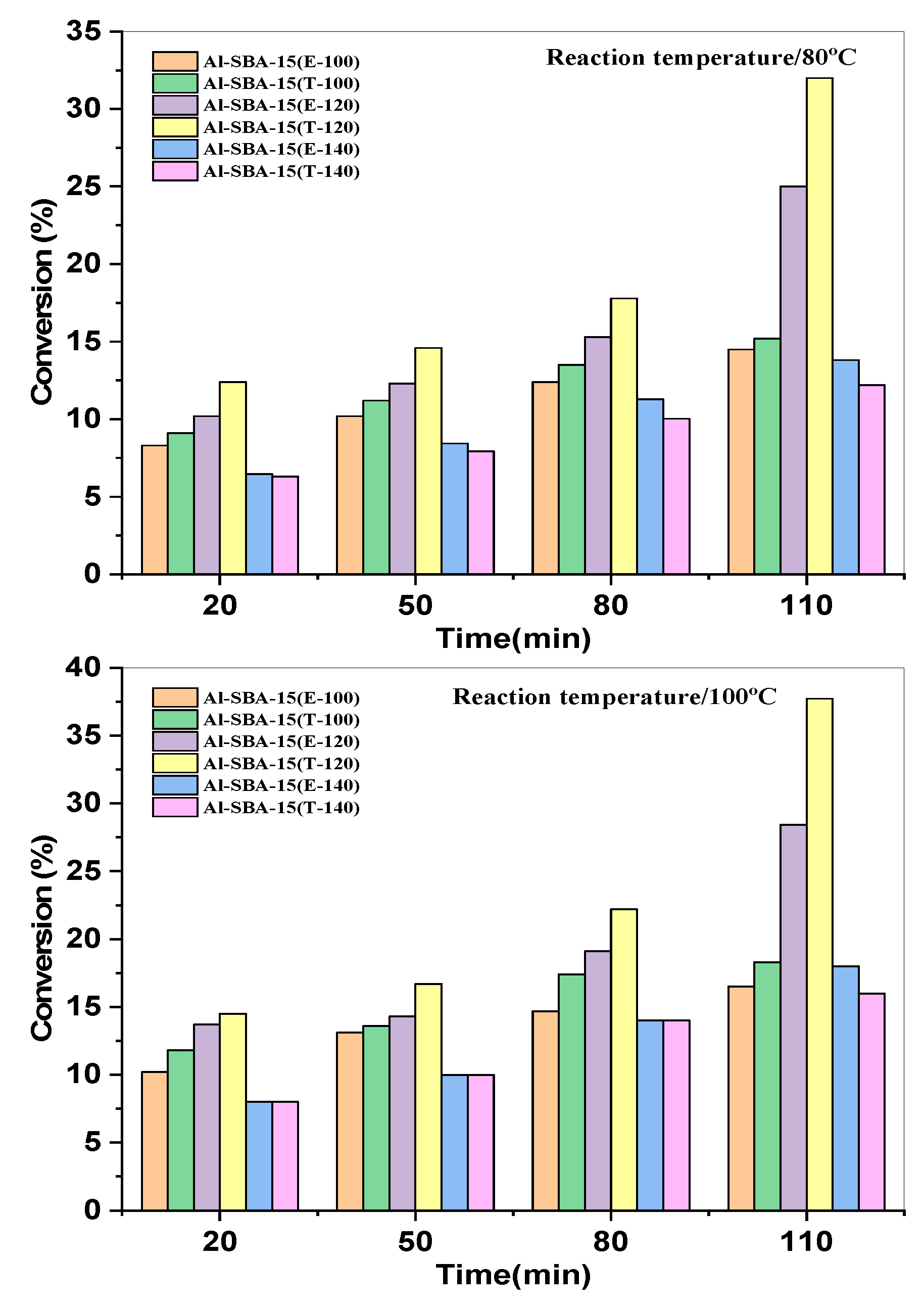
| Sample | nSi/nAl |
SBET/ (m2g-1) |
VP/ (cm3/g) |
DP, ads/nm | W (nm)a |
| Al-SBA-15(E-100) | 13 | 822 | 1.17 | 7.1 | 4.24 |
| Al-SBA-15(T-100) | 10 | 869 | 1.04 | 7.1 | 4.12 |
| Al-SBA-15(E-120) | 11 | 844 | 1.14 | 8.1 | 3.85 |
| Al-SBA-15(T-120) | 9 | 890 | 1.21 | 8.5 | 3.71 |
| Al-SBA-15(E-140 | 31 | 319 | 1.12 | 8.7 | 3.15 |
| Al-SBA-15(T-140) | 36 | 333 | 1.01 | 8.5 | 3.26 |
| Catalyst | Ei (mV)a |
Acid amount/ (mmol n-butylamine)a |
Total number of acid sites (mmol g−1)b | Number of acid sites (μmol g−1)c | B/L Ratio c |
|
| Brönsted (B) | Lewis (L) | |||||
| Al-SBA-15(E-100) | 99 | 0.22 | 0.897 | 18 | 11 | 1.64 |
| Al-SBA-15(T-100) | 111 | 0.31 | 0.934 | 21 | 14 | 1.50 |
| Al-SBA-15(E-120) | 120 | 0.43 | 1.012 | 33 | 18 | 1.83 |
| Al-SBA-15(T-120) | 130 | 0.55 | 1.134 | 43 | 21 | 2.05 |
| Al-SBA-15(E-140 | 100 | 0.38 | 1.064 | 31 | 16 | 1.93 |
| Al-SBA-15(T-140) | 105 | 0.46 | 1.032 | 34 | 22 | 1.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





