Submitted:
18 July 2023
Posted:
19 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Collection of C. goeringii samples
2.2. DNA Purification
2.3. Multiplex PCR for 12 SSR Markers
2.4. Phylogenetic and Sibling Analysis
2.5. Statistical Analysis
3. Results
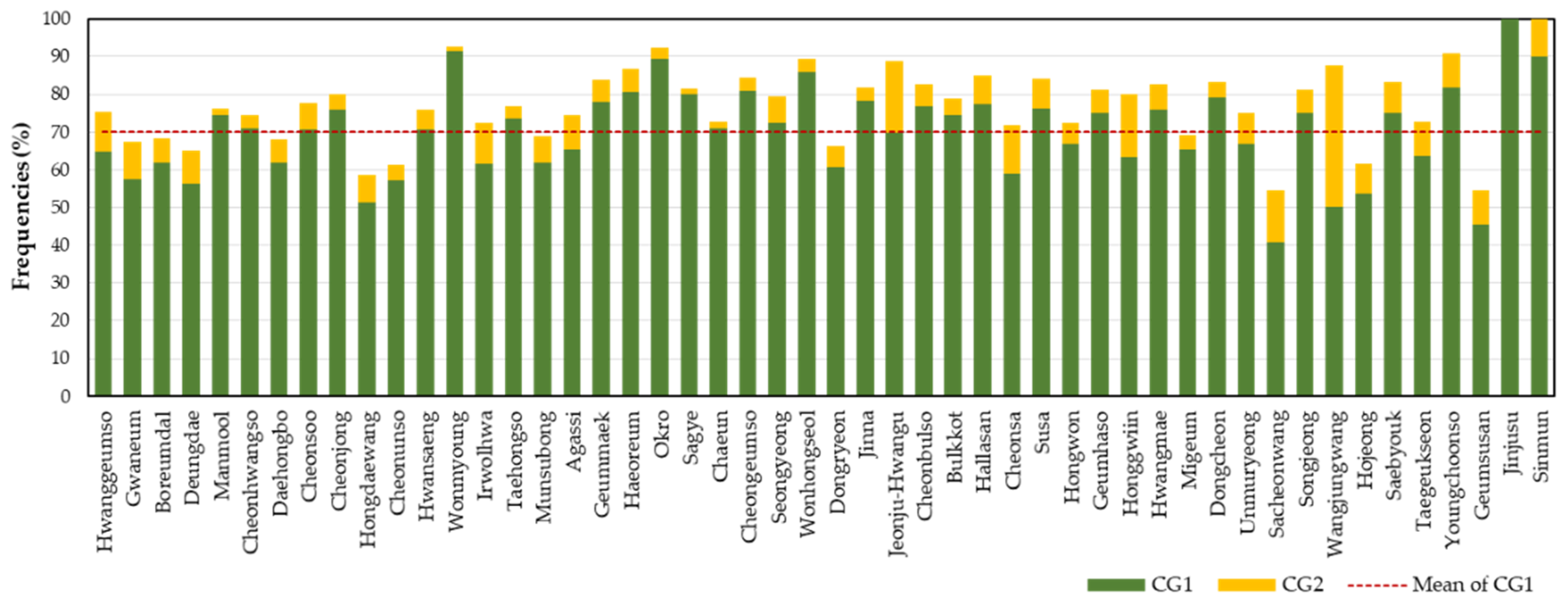
3.1. Determination of Combined Genotypes
3.2. Determination of Cultivar-Specific Combined Genotypes
3.3. Tracing Cultivar Origin for Samples Assumed to Be Non-Genuine
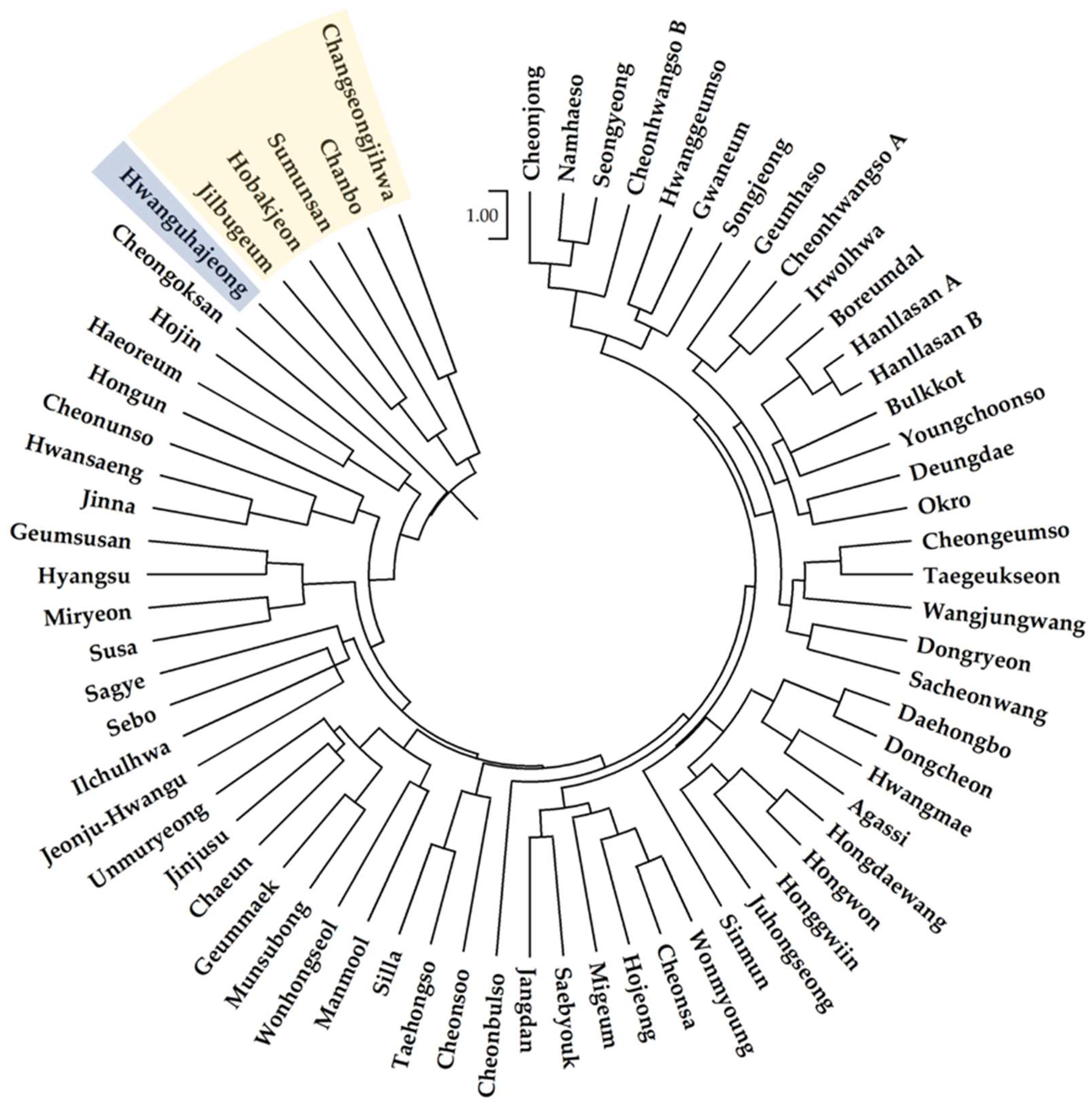
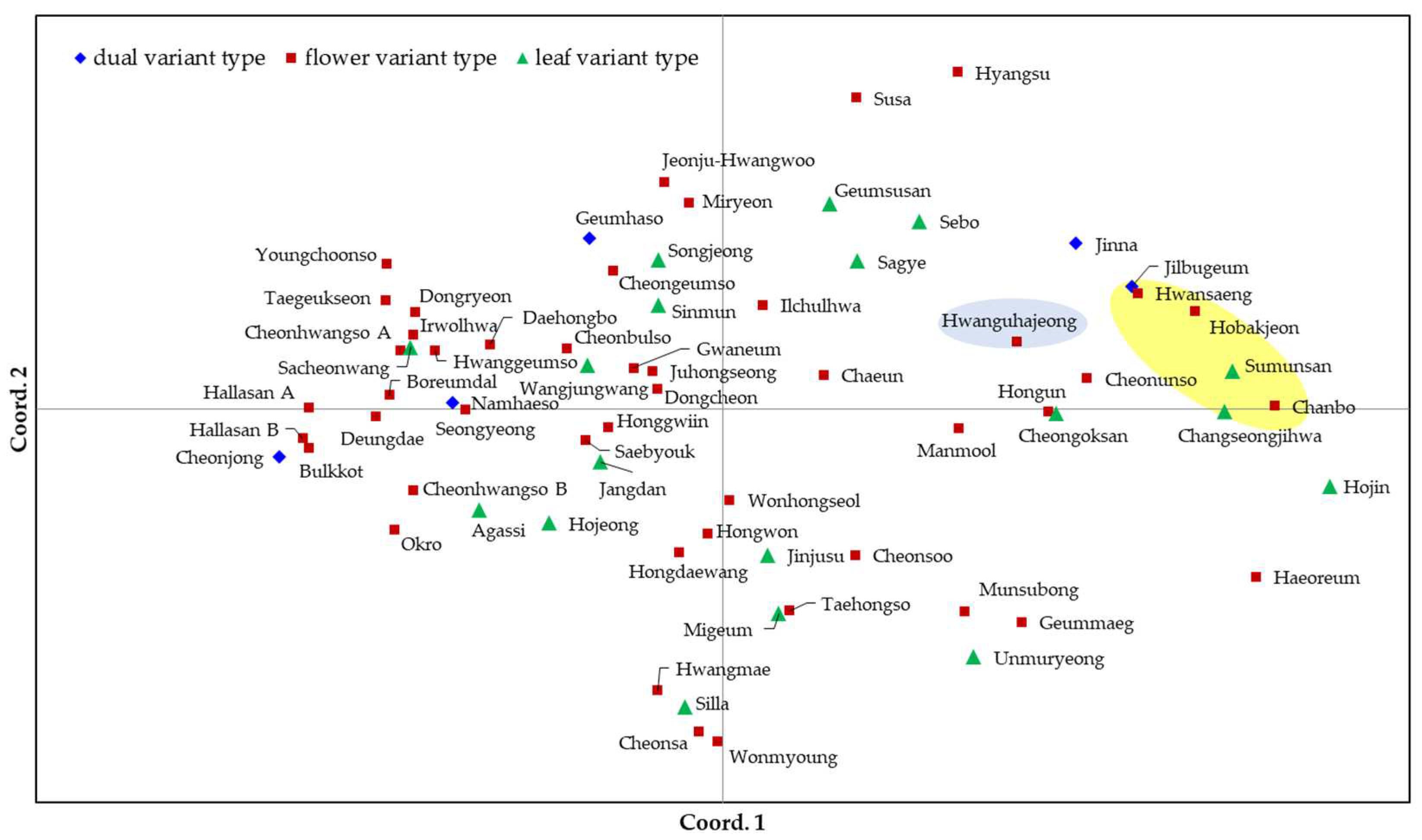
3.4. Phylogenetic Analysis among Cultivars
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DuPuy, D.; Cribb, P.J. The genus Cymbidium. Richmond. Kew Publishing: UK, 2007. [Google Scholar]
- Lawler, L.J. Ethnobotany of Orchidaceae, p. 30-116. In: J. Arditti (ed.). Orchid Bioiogy: Reviews and perspectives. Comstock Publishing Associate. Cornell University Press, Ithaca, NY. 1994; Volume 3. [Google Scholar]
- Lee, H.J.; Park, H.R.; Lee, A.J.; Nam, D.E.; Lee, D.G.; Do, Y.; Chung, K.W. Genetic authentication of cultivars with flower-variant types using SSR markers in spring orchid, Cymbidium goeringii. Hortic Environ Biotechnol 2020, 61, 577–590. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, B.M.; Yu, S.O.; Ryu, K.H. Analysis of phenotypic and genetic polymorphism of self-pollinated seedings of Korean native Cymbidium goeringii. Kor J Hort Sci Technol 2004, 22, 486–490. [Google Scholar]
- Suetsugu, K. Autonomous self-pollination and insect visitors in partially and fully mycoheterotrophic species of Cymbidium (Orchidaceae). J Plant Res 2015, 128, 115–125. [Google Scholar] [CrossRef]
- Nam, D.E.; Yu, J.S.; Noh, S.W.; Lee, K.S.; Hwang, J.H.; Lee, D.G.; Chung, K.W. Genetic kinship and discrimination between the cultivars consisting a sister cultivar group in spring orchid Cymbidium goeringii. Hort Sci Technol 2021, 39, 106–121. [Google Scholar]
- Huang, J.L.; Zeng, C.X.; Li, H.T.; Yang, J.B. Isolation and characterization of 15 microsatellite markers from the spring orchid (Cymbidium goeringii) (Orchidaceae). Am J Bot 2011, 98, e76–e77. [Google Scholar] [CrossRef]
- Hyun, Y.S.; Kim, J.; Chung, K.W. Development of polymorphic microsatellite markers for Cymbidium goeringii (Orchidaeceae). Am J Bot 2012, 99, e193–e198. [Google Scholar] [CrossRef] [PubMed]
- Moe, K.T.; Zhao, W.; Song, H.-S.; Kim, Y.-H.; Chung, J.-W.; Cho, Y.-I.; Park, P.H.; Park, H.-S.; Chae, S.-C.; Park, Y.-J. Development of SSR markers to study diversity in the genus Cymbidium. Biochem Syst Ecol 2010, 38, 585–594. [Google Scholar] [CrossRef]
- Lee, D.G.; Koh, J.C.; Chung, K.W. Determination and application of simple sequence repeats (SSR) DNA ID for cultivars of Cymbidium goeringii. Kor J Hort Sci Technol 2012, 30, 78–285. [Google Scholar]
- Tang, M.; Zeng, C.X.; Bi, Y.F.; Yang, J.B. Microsatellite markers for the Chinese endangered and endemic orchid Cymbidium tortisepalum (Orchidaceae). Am J Bot 2012, 99, e11–e13. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jin, F.; Jin, L.; Jackson, A.; Huang, C.; Li, K.; Shu, X. Development of Cymbidium ensifolium genic-SSR markers and their utility in genetic diversity and population structure analysis in cymbidiums. BMC Genet 2014, 5, 124. [Google Scholar]
- Noh, S.W.; Park, J.-K.; Yu, J.S.; Nam, D.E.; Do, Y.; Chung, K.W. Genetic diversity and population structure of the spring orchid Cymbidium goeringii in Korean distant islands. Diversity 2020, 12, 486. [Google Scholar] [CrossRef]
- Bär, W.; Brinkmann, B.; Budowle, B.; Carracedo, A.; Gill, P.; Lincoln, P.; Mayr, W.; Olaisen, B. DNA recommendations-Further report of the DNA Commission of the ISFH regarding the use of short tandem repeat systems. Int J Legal Med 1997, 110, 175–176. [Google Scholar] [PubMed]
- Evett, I.W.; Weir, B.S. Interpreting DNA evidence: statistical genetics for forensic scientists., Sunderland, MA, Sinauer Associates, USA, 1998, Volume 244.
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: genetic analysis in Excel. Population genetic software for teaching and research-an update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Park, S.Y.; Lee, A.R.; Jang, S.G.; Im, D.E.; Jun, T.H.; Lee, J.; Chung, J.W.; Ham, T.H.; Kwon, S.W. Next-generation sequencing yields the complete chloroplast genome of C. goeringii acc. smg222 and phylogenetic analysis. Mitochondrial DNA B Resour 2018, 3, 215–216. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.I.; Lyu, J.I.; Lee, H.O.; Kim, J.B.; Kim, S.H. Complete chloroplast genome sequence of an orchid hybrid Cymbidium sinense (♀) × C. goeringii (♂). Mitochondrial DNA B Resour 2020, 5, 3802–3803. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhu, G.; Wang, Z.; Liu, H.; Xu, Q.; Huang, D.; Zhao, C. Integrated mRNA and microRNA transcriptome variations in the multi-tepal mutant provide insights into the floral patterning of the orchid Cymbidium goeringii. BMC Genomics 2017, 18, 367. [Google Scholar] [CrossRef] [PubMed]
- Ramya, M.; Park, P.H.; Chuang, Y.C.; Kwon, O.K.; An, H.R.; Park, P.M.; Baek, Y.S.; Kang, B.C.; Tsai, W.C.; Chen, H.H. RNA sequencing analysis of Cymbidium goeringii identifies floral scent biosynthesis related genes. BMC Plant Biol. 2019, 19, 337. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, S.W.; Lim, G.H.; Lyu, J.I.; Choi, H.I.; Jo, Y.D.; Kang, S.Y.; Kang, B.C.; Kim, J.B. Transcriptome analysis to identify candidate genes associated with the yellow-leaf phenotype of a Cymbidium mutant generated by γ-irradiation. PLoS One 2020, 5, e0228078. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Li, H.; Chen, X.; Zhang, Y.; Lu, L.; Li, S.; Tao, X.; Zhu, W.; Wang, J.; Ma, L. Insight into the molecular mechanisms of leaf coloration in Cymbidium ensifolium. Front Genet 2022, 13, 923082. [Google Scholar] [CrossRef] [PubMed]

| Cultivar name | Type1 | Main phenotype | Sample number |
|---|---|---|---|
| Cultivars originated from Korea | |||
| Hwanggeumso | F | Yellow flower with non-anthocyanin lip | 468 |
| Gwaneum | F | Yellow flower with non-anthocyanin lip | 301 |
| Boreumdal | F | Yellow flower with non-anthocyanin lip | 250 |
| Deungdae | F | Orange-yellow flower with non-anthocyanin lip | 194 |
| Manmool | F | Orange-yellow flower | 184 |
| Cheonhwangso | F | Yellow flower with non-anthocyanin lip | 161 |
| Daehongbo | F | Red flower | 144 |
| Cheonsoo | F | Orange-yellow flower with non-anthocyanin lip | 143 |
| Cheonjong | D | Short leaf and round flower both with yellow variegation | 125 |
| Hongdaewang | F | Red flower | 109 |
| Cheonunso | F | Flower with mixed colors and non-anthocyanin lip | 98 |
| Hwansaeng | F | Yellow flower with non-anthocyanin lip | 95 |
| Wonmyoung | F | Round yellow flower | 93 |
| Irwolhwa | F | Small round flower with non-anthocyanin lip | 91 |
| Taehongso | F | Orange-yellow flower with non-anthocyanin lip | 91 |
| Munsubong | F | Flower with mixed colors | 84 |
| Agassi | L | Leaf with narrow yellow stripes | 78 |
| Geummaek | F | Small round yellow flower | 68 |
| Haeoreum | F | Red flower | 67 |
| Okro | F | Flower with mixed colors and orange bordering | 66 |
| Sagye | L | Short leaf with yellow variegation | 65 |
| Chaeun | F | Orange-yellow flower with non-anthocyanin lip | 62 |
| Cheongeumso | F | Yellow flower with non-anthocyanin lip | 58 |
| Seongyeong | F | Leaf with yellow stripes and flower with non-anthocyanin lip | 58 |
| Wonhongseol | F | Purple small round flower | 57 |
| Dongryeon | F | Leaf with short yellow stripes and small round flower | 56 |
| Jinna | D | Leaf and flower with yellow variegation | 55 |
| Jeonju-Hwangu | F | Yellow flower | 53 |
| Cheonbulso | F | Red flower with non-anthocyanin lip | 52 |
| Bulkkot | F | Red flower | 47 |
| Hallasan | F | Small round flower | 40 |
| Cheonsa | F | Orange-yellow flower with non-anthocyanin lip | 39 |
| Susa | F | Red flower | 38 |
| Hongwon | F | Round red flower | 36 |
| Geumhaso | D | Leaf and flower with yellow stripe | 32 |
| Honggwiin | F | Orange-yellow flower with non-anthocyanin lip | 30 |
| Hwangmae | F | Pale yellow round flower | 29 |
| Migeum | L | Leaf with short multiple yellow stripes and spots | 26 |
| Dongcheon | F | Orange-yellow flower with non-anthocyanin lip | 24 |
| Unmuryeong | L | Overall yellowish leaf | 24 |
| Sacheonwang | L | Leaf with yellow stripes | 22 |
| Songjeong | L | Leaf with narrow yellow stripes | 16 |
| Wangjungwang | L | Short leaf with yellow stripes | 16 |
| Hojeong | L | Short leaf with yellow stripes | 13 |
| Saebyeok | F | Red flower with non-anthocyanin lip | 12 |
| Taegeukseon | F | Flower with yellow and green mixed colors | 11 |
| Youngchoonso | F | Yellow flower with non-anthocyanin lip | 11 |
| Geumsusan | L | Overall yellowish leaf | 11 |
| Jinjusu | L | Leaf with yellow stripes | 10 |
| Sinmun | L | Leaf with yellow stripes | 10 |
| Sebo | L | Leaf with yellow stripes | 6 |
| Silla | L | Short leaf with yellow bordering | 5 |
| Ilchulhwa | F | Small round flower | 4 |
| Cheongoksan | L | Leaf with multiple yellow spots | 3 |
| Hongun | F | Red flower | 3 |
| Jangdan | F | Red flower | 3 |
| Miryeon | F | Round flower with pale anthocyanin lip | 3 |
| Hojin | L | Leaf with yellow variegation | 2 |
| Hyangsu | F | Green flower with yellow bordering | 2 |
| Namhaeso | D | Leaf and flower with white bordering | 2 |
| Juhongseong | F | Flower with mixed colors and orange bordering | 1 |
| Cultivars originated from Japan | |||
| Chanbo | F | Yellow flower with non-anthocyanin lip | 40 |
| Jilbugeum | D | Leaf and flower with multiple yellow stripes and spots | 14 |
| Changseongjihwa | L | Leaf showing leopard print | 13 |
| Hobakjeon | F | Orange-yellow flower with non-anthocyanin lip | 11 |
| Sumunsan | L | Leaf with yellowish variegation | 8 |
| Cultivar originated from China | |||
| Hwanguhajeong2 | F | Floral fragrance | 5 |
| Total | - | - | 4048 |
| Cultivars | CG1 % | Genotype of SSR markers1 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CG415 | CG649 | CG709 | CG722 | CG787 | CG1023 | CG1028 | CG1085 | CG1210 | CG1281 | CG1320 | CG1400 | ||
| Hwanggeumso | 64.7 | 11-11 | 15-30.1 | 17-18 | 12-20 | 24-29 | 13-13 | 15-17 | 14-15 | 29-29 | 16-16 | 10-13 | 13.1-20 |
| Gwaneum | 57.4 | 11-13 | 21-30.1 | 18-18 | 12-20 | 24-24 | 13-13 | 15-15 | 14-19 | 15-29 | 16-16 | 10-13 | 13.1-20 |
| Boreumdal | 62.0 | 11-20 | 15-15 | 17-25 | 17-17 | 24-24 | 13-13 | 6-7 | 14-14 | 6-6 | 16-16 | 10-10 | 15-20 |
| Deungdae | 56.2 | 11-15 | 16-16 | 17-17 | 12-12 | 24-24 | 13-13 | 7-19 | 14-38 | 15-29 | 16-16 | 12-12 | 20-21 |
| Manmool | 74.5 | 13-13 | 15-29.1 | 23-23 | 12-17 | 18-18 | 13-20 | 15-17 | 19-19 | 17-17 | 9-9 | 12-12 | 20-20 |
| Cheonhwangso | 41.0 (A) | 11-19 | 14-15 | 17-34 | 12-12 | 24-24 | 13-13 | 17-17 | 15-15 | 12-12 | 16-16 | 12-12 | 20-20 |
| 29.8 (B) | 11-11 | 15-29.1 | 17-34 | 12-20 | 24-24 | 13-25 | 17-17 | 16-16 | 17-17 | 16-16 | 7-12 | 13.1-20 | |
| Daehongbo | 61.8 | 13-22 | 15-15 | 17-18 | 20-20 | 24-24 | 13-13 | 17-17 | Null | 15-15 | 16-16 | 7-7 | 19-19 |
| Cheonsoo | 70.6 | 11-13 | 14-15 | 18-18 | 17-20 | 24-24 | 20-20 | 17-17 | 14-14 | 15-15 | 10-16 | 7-12 | 20-20 |
| Cheonjong | 76.0 | 11-11 | 15-19 | 17-17 | 12-20 | 24-24 | 13-13 | 6-30 | 14-14 | 15-15 | 9-9 | 10-15 | 13.1-20 |
| Hongdaewang | 51.4 | 13-13 | 15-33.1 | 17-22 | 12-20 | 24-24 | 19-19 | 30-30 | 14-14 | 6-15 | 16-16 | 6-7 | 19-20 |
| Cheonunso | 57.1 | 13-13 | 15-15 | 18-18 | 30-30 | Null | 26-26 | 17-17 | 14-14 | 15-15 | 16-16 | 7-7 | 10.1-13.1 |
| Hwansaeng | 70.5 | 13-13 | 26.1-30.1 | 18-18 | 11-11 | Null | 13-13 | 6-12 | 14-15 | 17-29 | 16-16 | 7-9 | 20-20 |
| Wonmyoung | 91.4 | 11-13 | 15-15 | 22-22 | 11-12 | 24-24 | 13-20 | 17-17 | 15-15 | 15-15 | 16-16 | 12-12 | 13.1-13.1 |
| Irwolhwa | 61.5 | 11-19 | 15-15 | 17-34 | 12-17 | 22-24 | 13-13 | 15-15 | 14-14 | 15-33 | 9-9 | 7-7 | 19-19 |
| Taehongso | 73.6 | 11-13 | 15-20 | 17-17 | 12-20 | 22-22 | 20-20 | 6-6 | 14-14 | 15-28 | 9-16 | 12-12 | 15-15 |
| Munsubong | 61.9 | 13-13 | 25.1-29.1 | 17-22 | 12-12 | 22-24 | 20-20 | 15-17 | 14-19 | 12-15 | 16-16 | 7-12 | 13.1-18 |
| Agassi | 65.4 | 13-22 | 15-15 | 17-17 | 12-20 | 24-24 | 13-20 | 15-17 | 16-16 | 6-15 | 10-17 | 7-12 | 19-19 |
| Geummaek | 77.9 | 13-13 | 15-29.1 | 17-22 | 11-12 | 22-25 | 13-20 | 6-12 | 19-19 | 12-15 | 10-16 | 10-12 | 19-20 |
| Haeoreum | 80.6 | 11-13 | 14-14 | 25-25 | 11-11 | 25-25 | 20-20 | 17-17 | 14-14 | 17-17 | 9-9 | 7-7 | 13.1-13.1 |
| Okro | 89.4 | 11-13 | 15-20 | 17-17 | 12-22 | 24-24 | 13-13 | 7-17 | 14-15 | 12-15 | 16-16 | 6-12 | 13.1-13.1 |
| Sagye | 80.0 | 13-15 | 15-26.1 | 18-26 | 12-12 | 16-22 | 13-25 | 17-30 | 14-15 | 6-17 | 9-16 | 7-12 | 15-15 |
| Chaeun | 71.0 | 13-13 | 29.1-29.1 | 16-16 | 12-12 | 22-24 | 13-13 | 15-15 | 14-14 | 17-17 | 9-16 | 7-13 | 13.1-20 |
| Cheongeumso | 81.0 | 13-19 | 15-29.1 | 17-25 | 12-17 | 24-25 | 13-13 | 17-29 | 14-24 | 15-15 | 16-16 | 7-7 | 13.1-19 |
| Seongyeong | 72.4 | 11-11 | 15-29.1 | 13-17 | 12-20 | 17-24 | 13-13 | 17-17 | 14-14 | 15-29 | 9-16 | 6-6 | 20-20 |
| Wonhongseol | 86.0 | 13-13 | 16-34.1 | 13-17 | 12-17 | 24-24 | 13-20 | 7-17 | 14-19 | 11-17 | 9-9 | 7-7 | 13.1-20 |
| Dongryeon | 60.7 | 15-15 | 15-15 | 17-25 | 12-20 | 22-24 | 13-13 | 15-15 | 14-14 | 15-15 | 16-16 | 12-12 | 20-20 |
| Jinna | 78.2 | 10-13 | 14-29.1 | 18-18 | 11-17 | Null | 13-13 | 7-7 | 14-14 | 15-15 | 16-16 | 7-7 | 19-19 |
| Jeonju-Hwangu | 69.8 | 13-15 | 15-15 | 34-34 | 12-20 | Null | 13-13 | 7-24 | 14-14 | 12-12 | 16-16 | 7-7 | 15-19 |
| Cheonbulso | 76.9 | 11-19 | 23-26.1 | 25-34 | 20-20 | 24-24 | 13-13 | 30-30 | 14-14 | 15-15 | 16-16 | 12-12 | 20-20 |
| Bulkkot | 74.5 | 10-11 | 15-15 | 17-17 | 12-12 | 24-24 | 13-13 | 17-17 | Null | 12-13 | 10-16 | 12-12 | 19-20 |
| Hallasan | 47.5 (A) | 11-15 | 15-15 | 17-17 | 17-17 | 24-24 | 13-13 | 12-12 | 14-14 | 17-17 | 10-16 | 10-10 | 13.1-13.1 |
| 30.0 (B) | 11-11 | 15-15 | 17-17 | 17-17 | 24-24 | 13-13 | 17-17 | 14-14 | 15-17 | 16-16 | 10-10 | 13.1-13.1 | |
| Cheonsa | 59.0 | 11-13 | 15-25.1 | 17-18 | 11-12 | 24-24 | 13-20 | 17-17 | 14-14 | 6-15 | 9-16 | 7-12 | 19-20 |
| Susa | 76.3 | 13-22 | 14-20 | 13-22 | 12-17 | 15-22 | 13-13 | 17-17 | 14-15 | 12-15 | 10-16 | 7-7 | 13.1-19 |
| Hongwon | 66.7 | 13-13 | 15-15 | 19-22 | 12-20 | 24-24 | 19-19 | 30-30 | 14-14 | 15-29 | 16-16 | 7-7 | 19-19 |
| Geumhaso | 75.0 | 11-19 | 14-34.1 | 18-26 | 12-17 | 24-24 | 13-13 | 6-17 | 14-19 | 15-17 | 16-16 | 7-13 | 13.1-20 |
| Honggwiin | 63.3 | 13-13 | 15-34.1 | 22-22 | 12-20 | 24-24 | 13-13 | 7-12 | 14-14 | 17-17 | 16-16 | 10-10 | 13.1-13.1 |
| Hwangmae | 75.9 | 13-13 | 15-15 | 17-17 | 11-22 | 24-24 | 13-20 | 17-30 | 13-14 | 15-15 | 16-16 | 7-7 | 20-23.1 |
| Migeum | 65.4 | 11-19 | 15-15 | 22-25 | 11-11 | 19-24 | 13-20 | 6-6 | 14-14 | 17-17 | 10-10 | 7-7 | 19-20 |
| Dongcheon | 79.1 | 13-13 | 15-15 | 18-18 | 20-20 | 24-24 | 13-13 | 12-17 | 14-14 | 15-15 | 16-16 | 7-7 | 13.1-13.1 |
| Unmuryeong | 66.7 | 13-13 | 15-29.1 | 16-17 | 11-11 | 17-24 | 13-20 | 12-12 | 14-14 | 12-12 | 16-16 | 10-10 | 19-19 |
| Sacheonwang | 40.9 | 15-15 | 15-15 | 13-13 | 12-12 | 24-24 | 13-13 | 6-7 | 14-14 | 15-15 | 16-16 | 7-13 | 20-20 |
| Songjeong | 75.0 | 11-12 | 19-30.1 | 17-18 | 12-20 | 17-18 | 13-13 | 17-17 | 24-25 | 29-30 | 16-16 | 13-13 | 13.1-20 |
| Wangjungwang | 56.3 | 13-15 | 15-15 | 18-25 | 12-22 | 24-24 | 13-29 | 7-17 | 15-19 | 15-17 | 17-17 | 10-13 | 13.1-20 |
| Hojeong | 53.8 | 11-11 | 15-23 | 13-22 | 11-12 | 24-24 | 13-13 | 16-16 | 14-22 | 15-15 | 9-9 | 12-13 | 13.1-20 |
| Saebyeok | 75.0 | 11-15 | 15-32.1 | 17-18 | 11-22 | 22-24 | 13-13 | 30-30 | 14-14 | 15-15 | 16-16 | 12-12 | 19-20 |
| Taegeukseon | 63.6 | 13-15 | 15-15 | 17-25 | 12-17 | 24-24 | 13-13 | 17-17 | 14-14 | 15-15 | 16-16 | 6-7 | 19-20 |
| Youngchoonso | 81.8 | 15-15 | 14-15 | 17-17 | 12-17 | 24-24 | 13-13 | 17-17 | 15-15 | 15-17 | 8-8 | 7-12 | 13.1-13.1 |
| Geumsusan | 45.5 | 13-22 | 14-14 | 17-17 | 17-20 | 25-25 | 13-13 | 17-17 | 15-15 | 15-17 | 9-9 | 7-7 | 19-19 |
| Jinjusu | 100.0 | 12-13 | 15-29.1 | 12-17 | 11-12 | 22-24 | 13-13 | 15-17 | 14-16 | 17-27 | 9-16 | 7-10 | 19-20 |
| Sinmun | 90.0 | 13-15 | 15-29.1 | 18-18 | 17-23 | 24-24 | 13-13 | 6-17 | 14-14 | 15-17 | 16-16 | 7-7 | 17-20 |
| Cultivar names mentioned by suppliers | Genetically matching cultivars1 |
|---|---|
| Hwanggeumso (n=468) | Gwaneum (50), Youngchoonso (7), Namhaeso (5), Cheonhwangso (1), Geumhaso (1), Chanbo (3)2, Hobakjeon (1)2 |
| Gwaneum (n=301) | Youngchoonso (30), Cheongeumso (2), Geumhaso (1), Hobakjeon (5)2, Chanbo (1)2 |
| Boreumdal (n=250) | Saebyeok (1) |
| Cheonhwangso (n=161) | Gwaneum (6), Youngchoonso (1) |
| Daehongbo (n=144) | Wonmyoung (1) |
| Cheonsoo (n=143) | Chanbo (10)2 |
| Cheonjong (n=125) | Sebo (3), Hwansaeng (1) |
| Hongdaewang (n=109) | Jangdan (8) |
| Cheonunso (n=98) | Taegeukseon (4) |
| Hwansaeng (n=95) | Deungdae (1) |
| Wonmyoung (n=93) | Hwanguhajeong (1)3 |
| Irwolhwa (n=91) | Miryeon (1), Cheonjong (1) |
| Munsubong (n=84) | Hyangsu (6) |
| Agassi (n=78) | Jinjusu (7), Sinmun (3), Sebo (1) |
| Geummaek(n=68) | Ilchulhwa (4) |
| Chaeun (n=62) | Cheonsa (1) |
| Cheongeumso (n=58) | Wonhongseol (1) |
| Cheonbulso (n=52) | Hwanggeumso (1), Sacheonwang (1) |
| Bulkkot (n=47) | Juhongseong (2) |
| Cheonsa (n=39) | Chaeun (1), Chanbo (5)2 |
| Hongwon (n=36) | Hongun (1) |
| Sacheonwang (n=22) | Sinmun (3) |
| Songjeong (n=16) | Sebo (1) |
| Taegeukseon (n=11) | Songjeong (1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





