Submitted:
17 July 2023
Posted:
19 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Morphological Identification
2.2. DNA Extraction, Amplification, and Sequencing
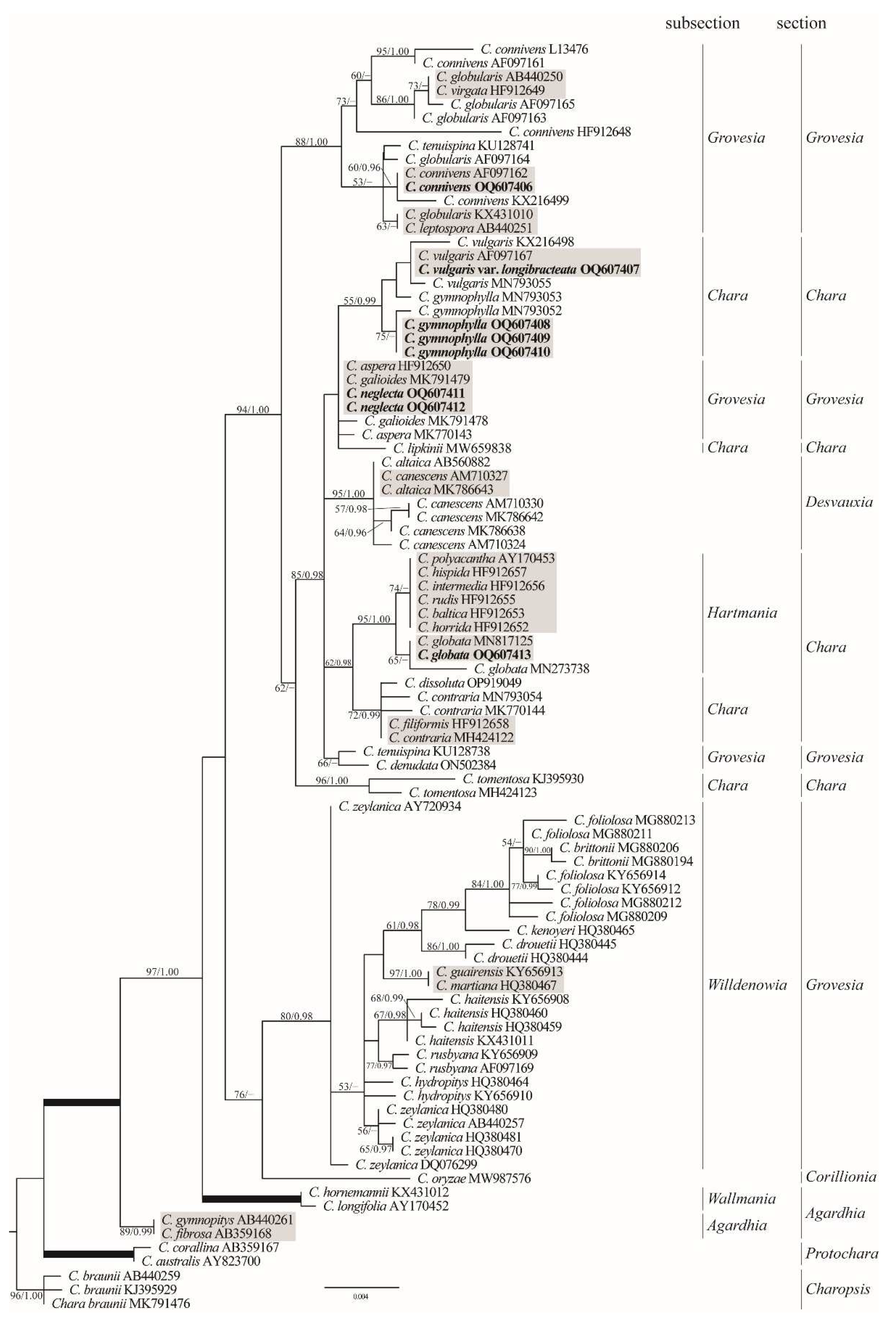
2.3. Phylogenetic Analyses
3. Results
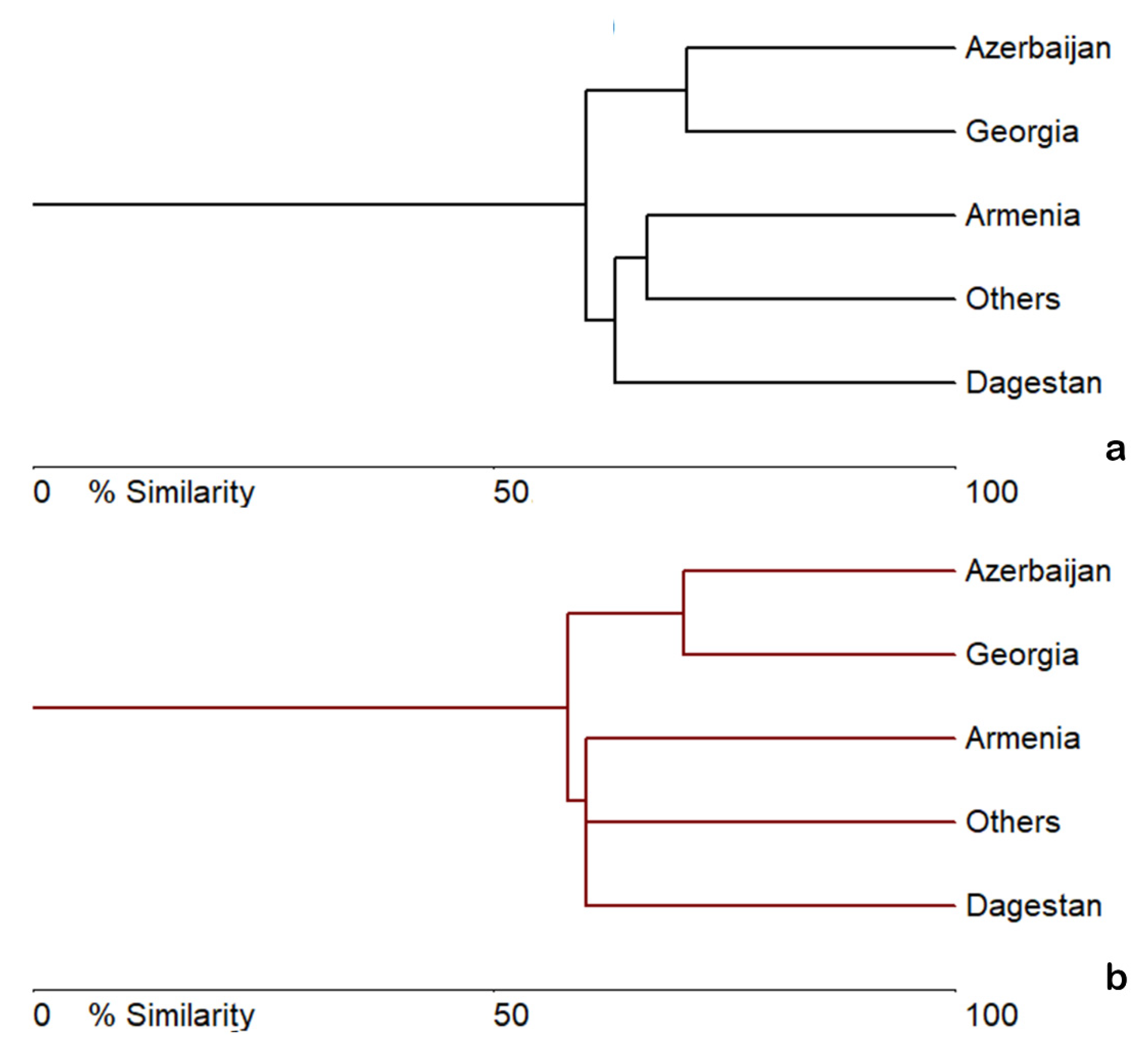
3.1. Charophytes Diversity and Distribution
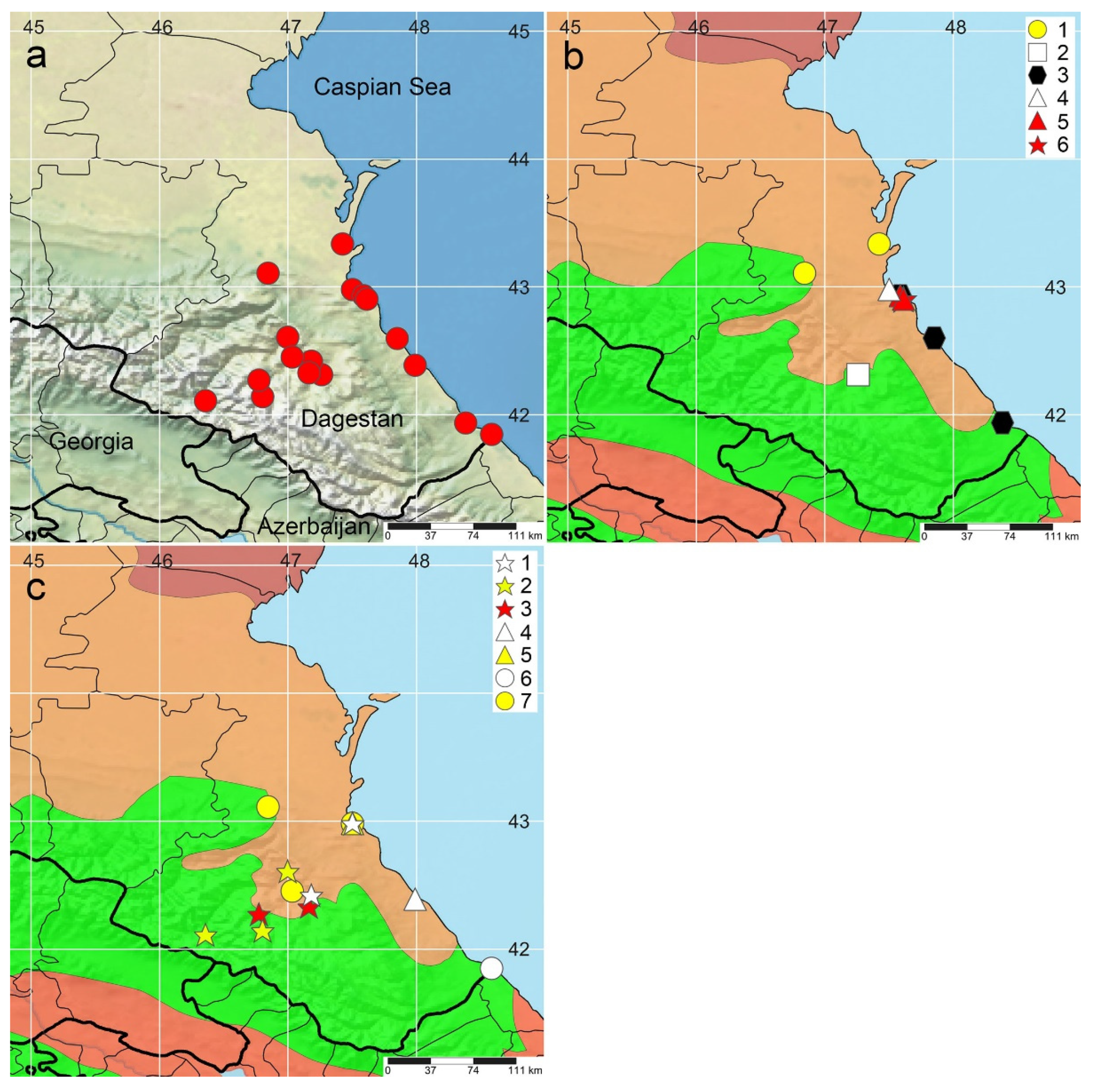

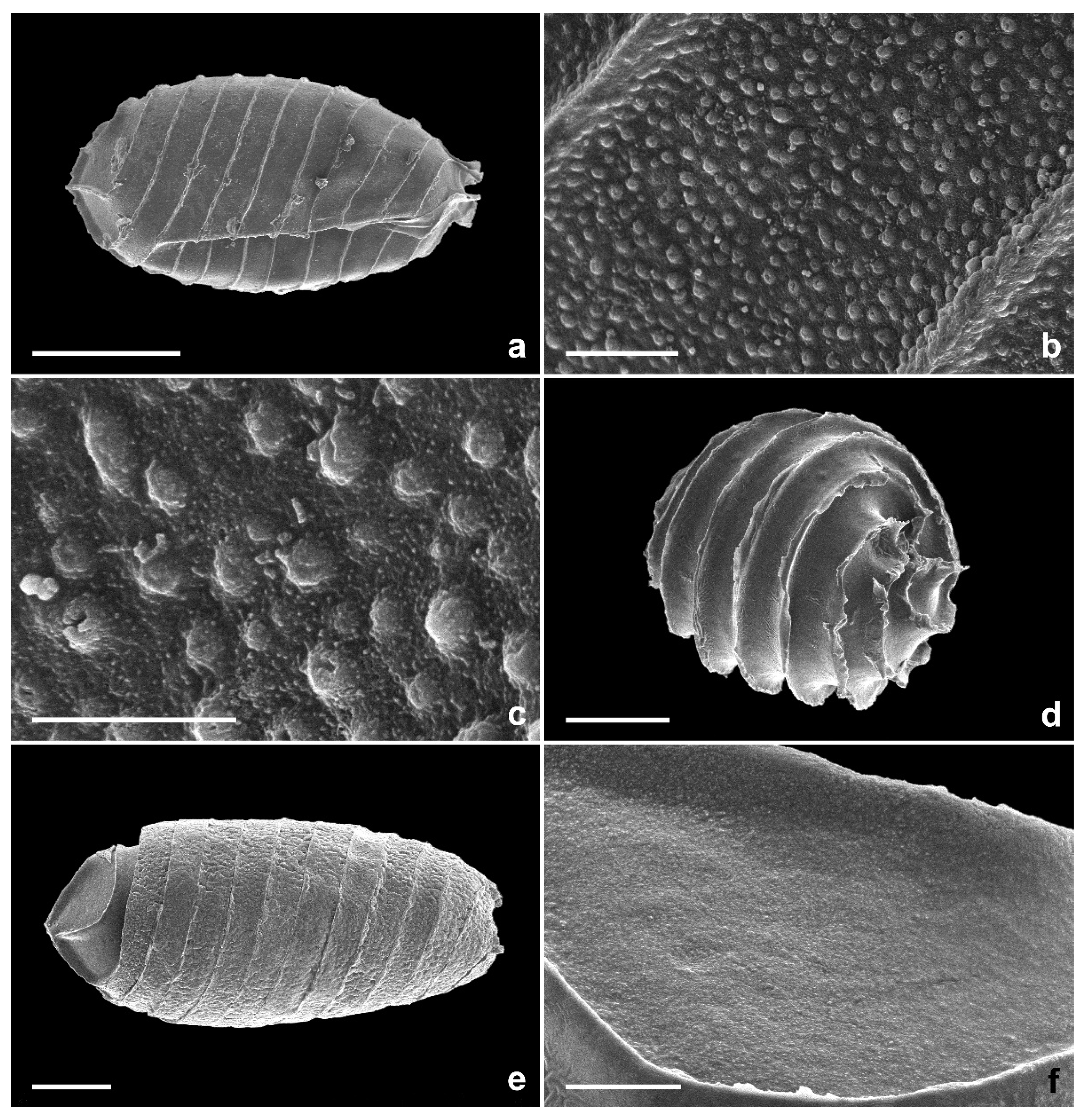

3.2. Species Identification and Phylogenetic Analysis
4. Discussion

| Species | Dagestan1 | Others2 | Georgia3 | Armenia4 | Azerbaijan5 |
|---|---|---|---|---|---|
| Chara baltica (Hartm.) Bruzelius | – | + | – | – | (+) |
| C. braunii C.C.Gmel. | – | – | + | – | + |
| C. canescens Desv. & Loisel. in Loisel. | – | + | + | + | + |
| C. connivens Salzm. ex A.Braun | + | + | + | – | + |
| C. contraria A.Braun ex Kütz. | + | + | + | + | + |
| C. denudata A.Braun | – | – | – | – | + |
| C. globata Migula | + | + | + | + | – |
| C. globularis Thuill. | – | + | + | + | + |
| C. gymnophylla A.Braun | + | + | + | + | + |
| C. hispida L. | – | – | + | – | (+) |
| C. neglecta Hollerbach | + | – | – | – | + |
| C. papillosa Kütz. | – | – | + | (+) | + |
| C. squamosa Desf. | – | + | – | – | – |
| C. strigosa A.Braun | – | – | – | – | (+) |
| C. tomentosa L. | – | (+) | – | – | – |
| C. vulgaris L. | + | + | + | + | + |
| Lamprothamnium papulosum (Wallr.) J.Groves | – | + | + | – | + |
| Nitella capillaris Krock. | – | – | + | – | + |
| N. flexilis (L.) C.Agardh | – | + | – | – | – |
| N. mucronata (A.Braun) Miq. | – | + | – | – | – |
| N. opaca (C.Agardh ex Bruzelius) C.Agardh | – | – | + | – | – |
| Nitellopsis obtusa (Desvaux) J.Groves | – | – | + | – | – |
| Sphaerochara prolifera (Ziz ex A.Braun) Soulié-Märsche | – | – | + | – | – |
| Tolypella glomerata (Desv. in Loisel.) Leonh. | – | – | + | – | + |
| T. nidifica (O.F.Müll.) A.Braun | + | + | + | – | – |
| Number of species | 7 | 13(14) | 17 | 6(7) | 13(16) |
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schubert, H.; Blindow, I.; Bueno, N.C.; Casanova, M.T.; Pełechaty, M.; Pukacz, A. Ecology of charophytes – permanent pioneers and ecosystem engineers. Perspectives in Phycology 2018, 5, 61–74. [Google Scholar] [CrossRef]
- Casanova, M.T.; Brock, M.A. Life histories of charophytes from permanent and temporary wetlands in Eastern Australia. Australian Journal of Botany 1999, 47, 383–397. [Google Scholar] [CrossRef]
- Calero, S.; Rodrigo, M.A. The life cycle of a parthenogenetic population of Chara canescens from an interdunal Mediterranean pond. Botany Letters 2018, 165, 1–55. [Google Scholar] [CrossRef]
- Blindow, I.; Carlsson, M.; van de Weyer, K. Re-Establishment techniques and transplantations of charophytes to support threatened species. Plants 2021, 10, 1830. [Google Scholar] [CrossRef] [PubMed]
- Vilhelm, J. Characeae Europae orientalis et Asiae ex herbario instituti cryptogamici horti botanici reipublicae rossicae (ante Petropolitani). Spisy vydávané Přírodovědeckou Fakultou Karlovy University 1928, 80, 1–24. [Google Scholar]
- Vilhelm, J. Ad Characearum Europae orientalis et Asiae cognitionem additamentum. Izv. Bot. Sada Akad. Nauk S.S.S.R. [Bulletin du Jardin Botanique Principal de l’URSS] 1930, 29, 582–596. [Google Scholar]
- Hollerbach, M.M. The systematic list of charophytes, found on USSR territory till 1935 inclusively. Trudy Botanicheskogo Instituta im. V.L. Komarova AN SSSR. Ser. 2. Sporovye rasteniya 1950, 5, 20–94. [Google Scholar]
- iNaturalist.org web. [continuously updated]. Available online: http://www.inaturalist.org (accessed on 10 June 2023).
- Thiers, B. Index herbariorum: A global directory of public herbaria and associated staff. New York Botanical Garden’s Virtual Herbarium. 2022. [continuously updated]. Available online: http://sweetgum.nybg.org/ih/.
- Romanov, R.E.; Gontcharov, A.A.; Barinova, S.S. Chara globata Mig. (Streptophyta: Charales): rare species revised. Fottea 2015, 15, 39–50. [Google Scholar] [CrossRef]
- Schubert, H.; Blindow, I.; Nat, E.; Korsch, H.; Gregor, T.; Denys, L.; Stewart, N.; van der Weyer, K.; Romanov, R.; Casanova, M.T. (Eds.) Characeae of Europe; Springer Nature: Berlin, Germany, 2023. [Google Scholar]
- Shorthouse, D.P. SimpleMappr, an online tool to produce publication-quality point maps; 2010. Available online: https://www.simplemappr.net (accessed on 6 December 2022).
- McAleece, N., Gage, J.D.G., Lambshead, P.J.D., Paterson, G.L.J. BioDiversity Professional statistics analysis software. Jointly developed by the Scottish Association for Marine Science and the Natural History Museum, London, 1997.
- Echt, C.S.; Erdahl, L.A.; McCoy, T.J. Genetic segregation of random amplified polymorphic DNA in diploid cultivated alfalfa. Genome 1992, 35, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Kiselev, K.V.; Dubrovina, A.S.; Tyunin, A.P. The methylation status of plant genomic DNA influences PCR efficiency. Journal of Plant Physiology 2015, 175, 59–67. [Google Scholar] [CrossRef]
- Romanov, R.E.; Barinova, S.S.; Nikulin, Y.V.; Gontcharov, A.A. Chara lipkinii (Charales, Charophyceae): A new dioecious Mediterranean species under risk of extinction in the wild and some implications for the taxonomy of the genus Chara. Fottea 2022, 22, 1–12. [Google Scholar] [CrossRef]
- Bonfield, J.K.; Smith, K.F.; Staden, R. A new DNA sequence assembly program. Nucleic Acids Research 1995, 23, 4992–4999. [Google Scholar] [CrossRef] [PubMed]
- Galtier, N.; Gouy, M.; Gautier, C. Seaview and phylo-win: Two graphic tools for sequence alignment and molecular phylogeny. Computer Applications in the Biosciences 1996, 12, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 2 March 2023).
- Romanov, R.E.; Nikulin, A.Yu.; Nikulin, V.Yu.; Gontcharov, A.A. New species Chara oryzae and a new section Corillionia of Chara (Charales, Charophyceae) from European Mediterranean rice fields. European Journal of Phycology 2022, 57, 328–342. [Google Scholar] [CrossRef]
- Swofford, D.L. PAUP*. Phylogenetic Analysis using Parsimony (*and Other Methods). Version 4.0b10; Sinauer Associates: Sunder-land, MA, USA, 2002. [Google Scholar]
- Huelsenbeck, J.P.; Ronquist, F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef] [PubMed]
- Akaike, H. A new look at the statistical model identification. IEEE Transactions on Automatic Control 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nature Methods 2012, 9, 772. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, A.; Hoover, P.; Rougemont, J. A rapid bootstrap algorithm for the RAxML web servers. Systematic Biology 2008, 57, 758–771. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, A.M.; Darriba, D.; Flouri, T.; Morel, B.; Stamatakis, A. RAxML-NG: A fast, scalable and user-friendly tool for maximum likelihood phylogenetic inference. Bioinformatics 2019, 35, 4453–4455. [Google Scholar] [CrossRef] [PubMed]
- Wood, R.D. A revision of the Characeae. In Monograph of the Characeae; Wood, R.D., Imahori, K., Eds.; Verlag von J. Cramer: Weinheim, Germany, 1965. [Google Scholar]
- Casanova, M.T.; Karol, K.G. A revision of Chara sect. Protochara, comb. et stat. nov. (Characeae: Charophyceae). Australian Systematic Botany 2014, 27, 23–37. [Google Scholar] [CrossRef]
- Holzhausen, A.; Nowak. P.; Ballot, A.; Becker, R.; Gebert, J., Gregor, T., Karol, K.G.; Lambert, E.; Pérez, W.; Raabe, U.; Schneider, S.C.; Stewart, N.; van de Weyer, K.; Wilde, V.; Schubert, H. Plastid DNA sequences and oospore characters of some European taxa of Tolypella section Tolypella (Characeae) identify five clusters, including one new cryptic Tolypella taxon from Sardinia, but they do not coincide with current morphological descriptions. Frontiers in Plant Science 2023, 14, 1096181. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.C.; Nowak, P.; Ammon, U.V.; Ballot, A. Species differentiation in the genus Chara (Charophyceae): considerable phenotypic plasticity occurs within homogenous genetic groups. European Journal of Phycology 2016, 51, 282–293. [Google Scholar] [CrossRef]
- Schneider, S.C.; Rodrigues, A.; Moe, T.F.; Ballot, A.; Clerck, O., de. DNAbarcoding the genus Chara: Molecular evidence recovers fewer taxa than the classical morphological approach. Journal of Phycology 2015, 51, 367–380. [Google Scholar] [CrossRef] [PubMed]
- Romanov, R.E.; Barinova, S.S.; Nikulin, V.Y.; Gontcharov, A.A. ‘Unfinished’ morphogenesis hides different speciation pathways in charophytes: Evidence from the 190-year-old original material of Chara denudata (Charales, Charophyceae). Diversity 2023, 15, 249. [Google Scholar] [CrossRef]
- Urbaniak, J.; Sakayama, H. Taxonomical analysis of closely related species of Chara L. section Hartmania (Streptophyta: Charales) based on morphological and molecular data. Fottea 2017, 17, 222–239. [Google Scholar] [CrossRef]
- Urbaniak, J.; Kwiatkowski, P. Taxonomic studies on the Chara section Hartmania in Poland based on morphological and molecular data. PhytoKeys 2019, 135, 71–90. [Google Scholar] [CrossRef] [PubMed]
- Saber, A.A.; Gontcharov, A.A.; Nikulin, A.Yu.; Nikulin, V.Yu.; Rayan, W.A.; Cantonati, M. Integrative taxonomic, ecological and genotyping study of charophyte populations from the Egyptian Western-Desert Oases and Sinai Peninsula. Plants 2021, 10, 1157. [Google Scholar] [CrossRef] [PubMed]
- Romanov, R.E. Typification of Chara connivens Salzmann ex A.Braun f. brevifolia Vilhelm, C. crinita Wallroth and C. glomerulifera Ruprecht and clarification of conflicting species concepts for C. arcuatifolia Vilhelm (Charales, Charophyceae). Notulae algarum 2022, 223, 1–4. [Google Scholar]
- Petunnikow, A. Die kaukasischen Characeen. Vestnik Tiflisskago Botaniceskago Sada. Moniteur du Jardin Botanique de Tiflis 1919, XIII–XIV–1917–18, 8–15. [In Russian].
- Romanov, R.E.; Barinova, S.S. Species of Nitella (Charophyceae, Charales) from Israel: low species richness and rare occurrence. Botanica Serbica 2016, 40, 1–11. [Google Scholar] [CrossRef]
- Romanov, R.E.; Barinova, S.S.; Vishnyakov, V.S. Species of Tolypella from Western, Central and Northern Asia. In Program & abstracts of the 21st Meeting of the Group of European Charophytologists, València, Spain, 18-22 September 2017; Valencia, Spain, 2017; 23.
- Romanov, R.E.; (Komarov Botanical Institute of the Russian Academy of Sciences, Saint-Petersburg, Russia). Unpublished Data; personal communication, 2023.
- Ruprecht, F.J. Distributio cryptogamarum vascularium in Imperio Rossico. Beiträge zur Pflanzenkunde des Russischen Reiches 1845, 3, 1–56. [Google Scholar]
- Iwanoff, L. Bericht über die algologische Exkursion im Sommer des Jahres 1901 in Kaukasus. Travaux de la Société Impériale de naturalistes de St. Petérsbourg 1902, 33, 1–9. [Google Scholar]
- Petrov, K.M. Macrophytes of Black Sea nearshore of the Taman Peninsula and the North Caucasus. In Proceedings of the Novorossiysk Biological Station; Rostov University Publishing: Rostov-na-Donu, Russia, 1961; 41–67. [In Russian]. [Google Scholar]
- Nikolaeva, E.V. Towards freshwater algal flora of artificial and natural water bodies of Big Sochi Area. In Contemporary problems of algology: proceedings of the International Scientific Conference and VII School for Marine Biology; SSC Publishing: Rostov-na-Donu, Russia, 2008. [Google Scholar]
- Slonov, T.L.; Slonov, L.Kh.; Chipova, D.A.; Zhemukhov, D.A.; Adzhieva, D.Kh. The charophytes (Charophyta) of some water ecosystems of the Republic of Kabardino-Balkaria (Central Caucasus). In Materials of the XX Annivessary International Scientific Conference “Biological diversity of Caucasus and south of Russia”; IPE RD Publishing: Makhachkala, Russia, 2018. [Google Scholar]
- GBIF.org (10 June 2023) GBIF Occurrence Download. Available online: https://doi.org/10.15468/dl.hvrzbv.
- Hollerbach, M.M.; Krasavina, L.K. Determination key of freshwater algae of USSR. Vol. 14. Charophyta; Nauka: Leningrad, Russia, 1983. [Google Scholar]
- Nahucrishvili, I.G. (Ed.) Flora of cryptogamous plants of Georgia: Conspectus; Mecniereba: Tbilisi, Georgia, 1986. [Google Scholar]
- GBIF.org (10 June 2023) GBIF Occurrence Download. Available online: https://doi.org/10.15468/dl.htwked.
- Gambaryan, P.P. Distribution of macrophytes of Lake Sevan. Proceedings of Sevan Hydrobiological Station of the Academy of Sciences of Armenian SSR 1979, 17, 122–129. [Google Scholar]
- Barsegyan, A.M. Aquatic and swamp vegetation of the Armenian SSR; Yerevan, Armenia, 1990; 1–353 [In Russian].
- Krilov, A.V. (Ed.) Lake Sevan. Ecological state during the period of water level change; Filigran: Yaroslavl, Russia, 2016. [Google Scholar]
- GBIF.org (10 June 2023) GBIF Occurrence Download. Available online: https://doi.org/10.15468/dl.uhgc79.
- Aliev, D.A. Charophytes occurring in water bodies of Talysh and Apsheron peninsula. Scientific proceedings of the Kirov Azerbaijan State University 1961, 4, 45–46. [Google Scholar]
- Hollerbach, M.M. De specie nova Chara neglecta Hollerb. (Charophyta). Novitates Systematicae Plantarum non Vascularium 1981, 18, 3–9. [Google Scholar]
- Musaev, N.; Ismailov, E. Bioelectrical properties of Chara gymnophylla plasmomembrane during interaction with Cobalt, (Co2+). Ekoloji 2007, 16, 1–6. [Google Scholar]
- GBIF.org (10 June 2023) GBIF Occurrence Download. Available online: https://doi.org/10.15468/dl.bw5swp.
- Klinkova, G.Yu.; Zhakova, L.V. New and rare species of Charales in the flora of the Lower Volga Region. Byulleten MOIP. Otdel biologicheskiy [Bulletin of Moscow Society of Naturalists. Biological series] 2014, 119, 61–66. [Google Scholar]
- Kolada, A. Charophyte variation in sensitivity to eutrophication affects their potential for the trophic and ecological status indication. Knowledge & Management of Aquatic Ecosystems 2021, 422, 1–12. [Google Scholar] [CrossRef]
- Krause, W. Charales (Charophyceae) Süßwasserflora von Mitteleuropa. In Ettl, H.; Gärtner, G., Heynig, H., Eds.; Mollenhauer, D., Eds., Süßwasserflora von Mitteleuropa 18; Fischer: Jena, Germany, 1997. [Google Scholar]
- Noedoost, F.; Riahi, H.; Sheidai, M.; Ahmadi, A. Distribution of charophytes from Iran with three new records of Characeae (Charales, Chlorophyta). Cryptogamie Algologie 2015, 36, 1–17. [Google Scholar] [CrossRef]
- Romanov, R.E.; Barinova, S.S. The charophytes of Israel: historical and contemporary species richness, distribution, and ecology. Biodiversity Research and Conservation 2012, 25, 57–64. [Google Scholar] [CrossRef]
- Romanov, R. New interesting records of charophytes (Charales, Charophyceae) from Eurasia and Africa. Webbia 2019, 74, 159–166. [Google Scholar] [CrossRef]
- Nurashov, S.; Jumakhanova, G.; Barinova, S.; Romanov, R.; Sametova, E.; Jiyenbekov, A.; Shalgimbayeva, S.; Smith, T.E. Diversity and distribution of charophytes (Charophyceae, Charales) in south and southeast Kazakhstan. Plants 2023, 12, 368. [Google Scholar] [CrossRef] [PubMed]
- Morozova-Vodyanitskaya, N. Phytobenthos of the Karkinitzky Gulf. Proceedings of the Sevastopol Biological Station of the Academy of Sciences of USSR 1936, 5, 219–232. [Google Scholar]
- Borysova, O.V.; Palamar-Mordvintseva, G.M.; Tsarenko, P.M. Flora algarum Ucrainicae. Charophyta, 12(2); Kholodny Institute of Botany of the National Academy of Science of Ukraine: Kyiv, Ukraine, 2016. [Google Scholar]
- Palamar-Mordvintseva, G.M. Сharophyta of the Crimean Peninsula (Ukraine). Algologia 1998, 8, 14–22. [Google Scholar]
- Crain, C.M.; Halpern, B.S.; Beck, M.W.; Kappel, C.V. Understanding and managing human threats to the coastal marine environment. The Year in Ecology and Conservation Biology, 2009: Annals of the New York Academy of Sciences 2009, 116, 39–62. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bellerby, R.; Craft, C.; Widney, S.E. Coastal wetland loss, consequences, and challenges for restoration. Anthropocene Coasts 2018, 1, 1–15. [Google Scholar] [CrossRef]
- Korolesova, D.D. The biocoenoses of charophytes as an important element of coastal ecosystems (by the example of Tendrovsky and Yagorlytsky Bays of the Black Sea). Odesa National University Herald Geography and Geology 2015, 20(1), 134–148. [in Russian].
- Korolesova, D.D. Current state of the macrophytobenthos in Tendrivska and Yagorlycka Bays of the Black Sea Biosphere Reserve. Chornomorski Botanical Journal 2017, 13(4), 457–467. [in Ukrainian].
- Berezenko, N.S.; Milchakova, N.A. Long-term changes of macrophytobenthos of “Sudzhuk Lagoon” Natural Monument (Black Sea). Nature Conservation Research 2018, 3(4), 59–67. [Google Scholar] [CrossRef]
- Romanov, R.; Korolesova, D.; Afanasyev, D.; Zhakova, L. Chara baltica (Charophyceae, Charales) from the Black Sea region and taxonomic implications of extrastipulodes. Botanica 2020, 26(2), 126–139. [Google Scholar] [CrossRef]
- Scapini, F.; Ciampi, G. (Eds.) Coastal water bodies nature and culture conflicts in the Mediterranean; Springer: Dordrecht Heidelberg London New York, 2010. [Google Scholar]
- Murtazaliev, R.A. The conspectus of the flora of Dagestan. Vol. 1-4; Epokha Press: Makhachkala, Russia, 2009. [Google Scholar]
- Grossheim, A.A. Analysis of the flora of the Caucasus; Baku, Azerbaijan, 1936: 1–259. [in Russian].
- Takhtajan, A.L. (Ed.) Caucasian flora conspectus. Vol. 1-3(1,2); Saint-Petersburg University Press, KMK Press: Saint Petersburg, Moscow, Russia, 2003–2013. [Google Scholar]
- Solomon, J.C.; Schatz, G.E.; Shulkina, T. Red list of the endemic plants of the Caucasus: Armenia, Azerbaijan, Georgia, Iran, Russia, and Turkey; Missouri Botanical Garden Press: St. Louis, USA, 2013. [Google Scholar]
- Fayvush, G.; Aleksanyan, A. Plant diversity in riverine wetlands of Armenia. Bocconea 2021, 29, 77–89. [Google Scholar] [CrossRef]
- Tedoradze, G.; Akobia, I.; Janiashvili, Z.; Jugeli, T.; Lachashvili, N.; Megvinetukhutsesi, N.; Metreveli, V.; Mikeladze, G.; Kikvidze, Z. The annotated checklist of plant species that occur in the wetland habitats of Georgia (the Caucasus). Caucasiana 2023, 2, 97–107. [Google Scholar] [CrossRef]
- Romanov, R.E.; Boboev, M.T. Charophytes (Streptophyta, Charales) from the South-Tajik Depression (Tajikistan). Botanicheskii zhurnal 2016, 101, 275–286, [in Russian]. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





