1. Introduction
Metronidazole belongs to the nitroimidazole class of antibiotics and is extensively used to treat obligate anaerobic bacterial infections as well as protozoa infections including Entamoeba histolytica, Giardia lamblia, and Trichomonas vaginalis. It is rapidly absorbed after oral intake, and the highest concentration is found in the kidneys, liver, urinary bladder, gastrointestinal tract, and vagina (Ings et al., 1975; Manthei et al., 1969; Placidi et al., 1970). The elimination half-life of metronidazole in healthy adults is approximately eight hours, with metabolization into active compounds, 2-hydroxy-metronidazole, and L-acetic acid metronidazole. It also exhibits a post-antibiotic effect that lasts over 3 hours, and its duration depends on the concentration (Lamp et al., 1999). Although mainly prescribed at an 8-hour dosage interval, the combination of long elimination half-life, the favorable ratio of steady-state serum levels to minimum inhibitory concentration, and the presence of active metabolites leads to consideration of metronidazole at a 12-hour dosing interval (Bunz et al., 1990). There is a variation in practice due to a need for more substantial consensus on the optimal dosing regimen for metronidazole. Since optimizing the dosing regimen of antimicrobial therapy is essential to minimize the side effects while keeping the efficacy, cost-effectiveness, and drug tolerability in focus, we conducted this systematic review to compare twice-daily doses versus thrice-daily dosing of metronidazole.
2. Methods
Two reviewers (TJ and PF) conducted a systematic search beginning May 15, 2023, using eligibility criteria established by the patient, intervention, comparison, and outcome (PICO) model
(Richardson et al., 1995) (see
Table 1). Reviewers completed the evaluation of all relevant studies by June 16, 2023. When we needed further information was needed, we contacted the study’s authors. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist guided our data collection and reporting
(Page et al., 2019). This review protocol's registration number on the PROSPERO international prospective register of systematic reviews is CRD42023424926 (
https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=424926).
Inclusion and Exclusion Criteria
We searched PubMed, Scopus, Science Direct, the Cochrane Database of Systematic Reviews, and the Cochrane Central Register of Controlled Trials without restriction on the publication language, country, or year. We included studies of patients with a culture-confirmed or clinically suspected infection who received oral or intravenous metronidazole on an 8-hour or 12-hour dosing schedule with subsequent reporting of the patient’s clinical outcomes. Title, abstract and keyword search terms included medical subject headings (MeSH) terms for metronidazole AND dose* (with truncation when possible) OR “extended dose” OR “12-hour” OR “12 hour” OR “twice daily”. We excluded studies involving the use of metronidazole in animal models, metronidazole use in the prophylactic setting, studies that evaluated the pharmacodynamics of metronidazole without reporting clinical outcomes, metronidazole use in combination with other antimicrobials for the treatment of Helicobacter pylori or Clostridioides difficile, metronidazole administered by a route other than oral (PO) or intravenous (IV), and studies that did not compare metronidazole dosing schedules.
Two reviewers (TJ and PF) independently accessed the titles and abstracts of articles and then screened full-text articles based on eligibility criteria to finalize articles for data extraction. They listed studies in separate Excel files, utilizing a column to explain the reason for the exclusion or inclusion of articles after reading titles and abstracts. Reviewers checked the concordance of their findings and consulted with a third reviewer (MV) to resolve any disagreements.
Study Quality
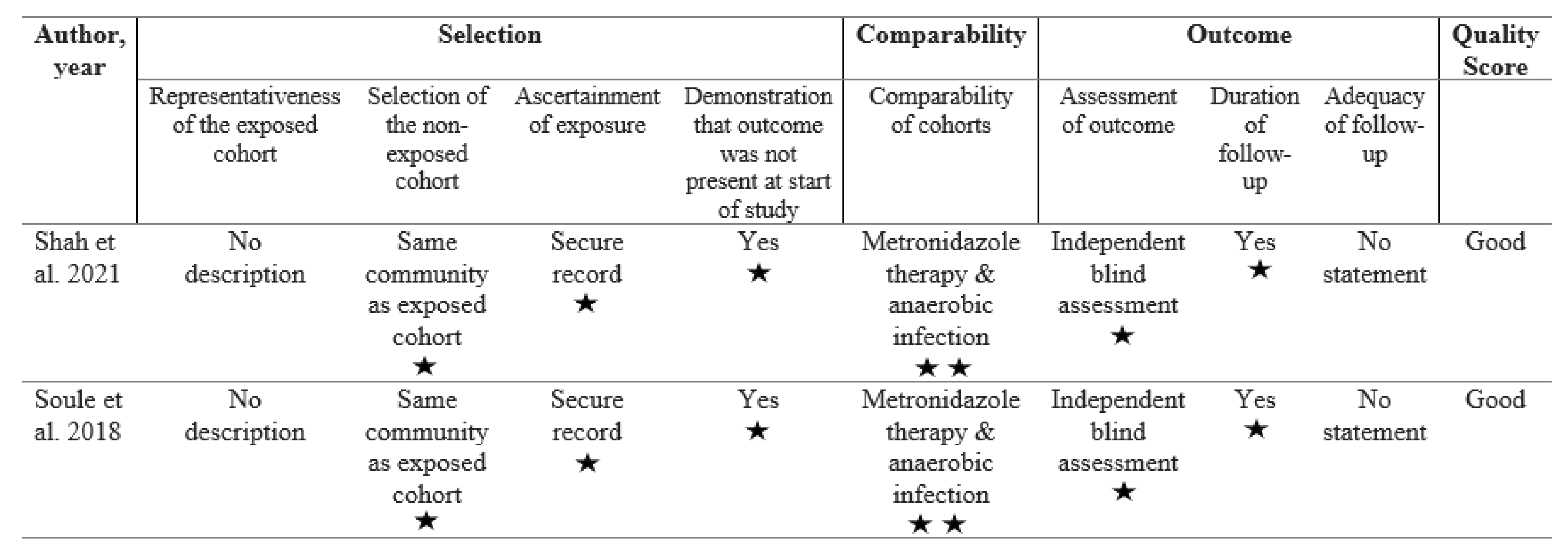
Two investigators (TJ and MV) independently assessed the risk of bias using the Newcastle-Ottawa quality assessment scale (NOS) for observational studies (Wells et al., 2021). The Newcastle-Ottawa scale evaluates three domains of non-randomized cohort studies: (a) selection, encompassing representativeness of the exposed cohort, selection of the non-exposed cohort, ascertainment of exposure, and demonstration that the outcome of interest was not present at the start of the study; (b) comparability, evaluating the comparability of cohorts on the basis of the design or analysis; and (c) outcome, assessing the outcome method, duration of follow-up, and adequacy of follow-up (Wells et al., 2021). We appraised the quality of each study on a nine-star scale by adding stars to each domain with a maximum of four stars for selection, two stars for comparability, and three stars for the outcome domain (Wells et al., 2021)A good quality study scores three or four stars in the selection domain, one or two stars in the comparability domain, and two or three stars in the exposure domain (Agency for Healthcare Research and Quality, 2019).
Statistical Analysis
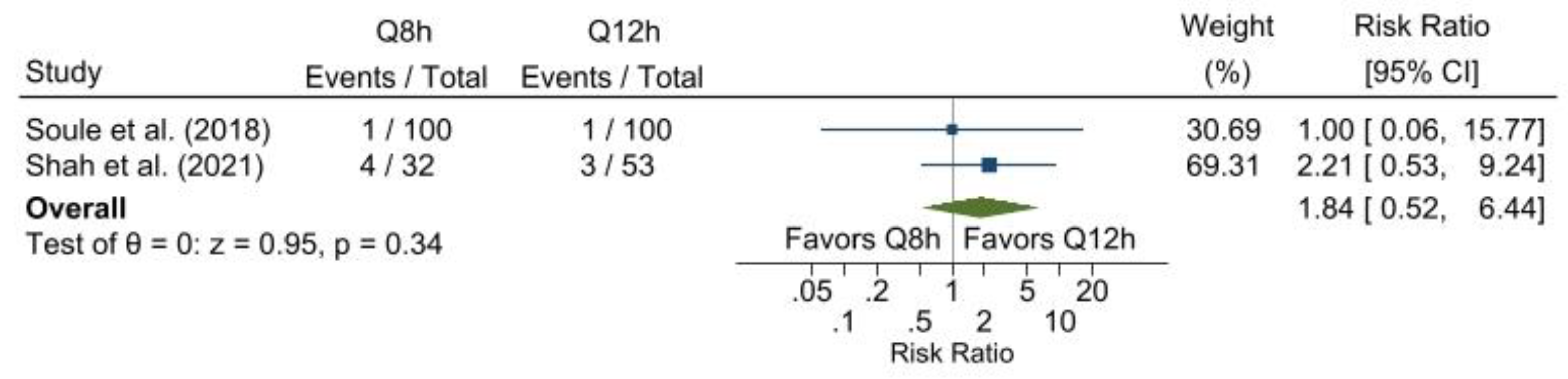
Study-specific descriptive and outcome statistics for continuous variables are presented as mean and standard deviation or median and interquartile range, whereas categorical variables are presented as count and percentage. The only outcome eligible for inclusion in the meta-analysis was the escalation of antibiotic therapy. Both were prospective cohort studies, and escalation was quantified using the risk ratio presented alongside its 95% confidence interval. Further, because variance estimation requires a minimum of three observations, the random-effects model was inappropriate; as such, the pooled estimate was based on a fixed-effects model using the Mantel-Haenszel method. As a sensitivity analysis, the results of a fixed-effects model are presented using the inverse variance method. All analyses were conducted using the meta package within Stata v. 17.0 (StataCorp, 2021).
3. Results
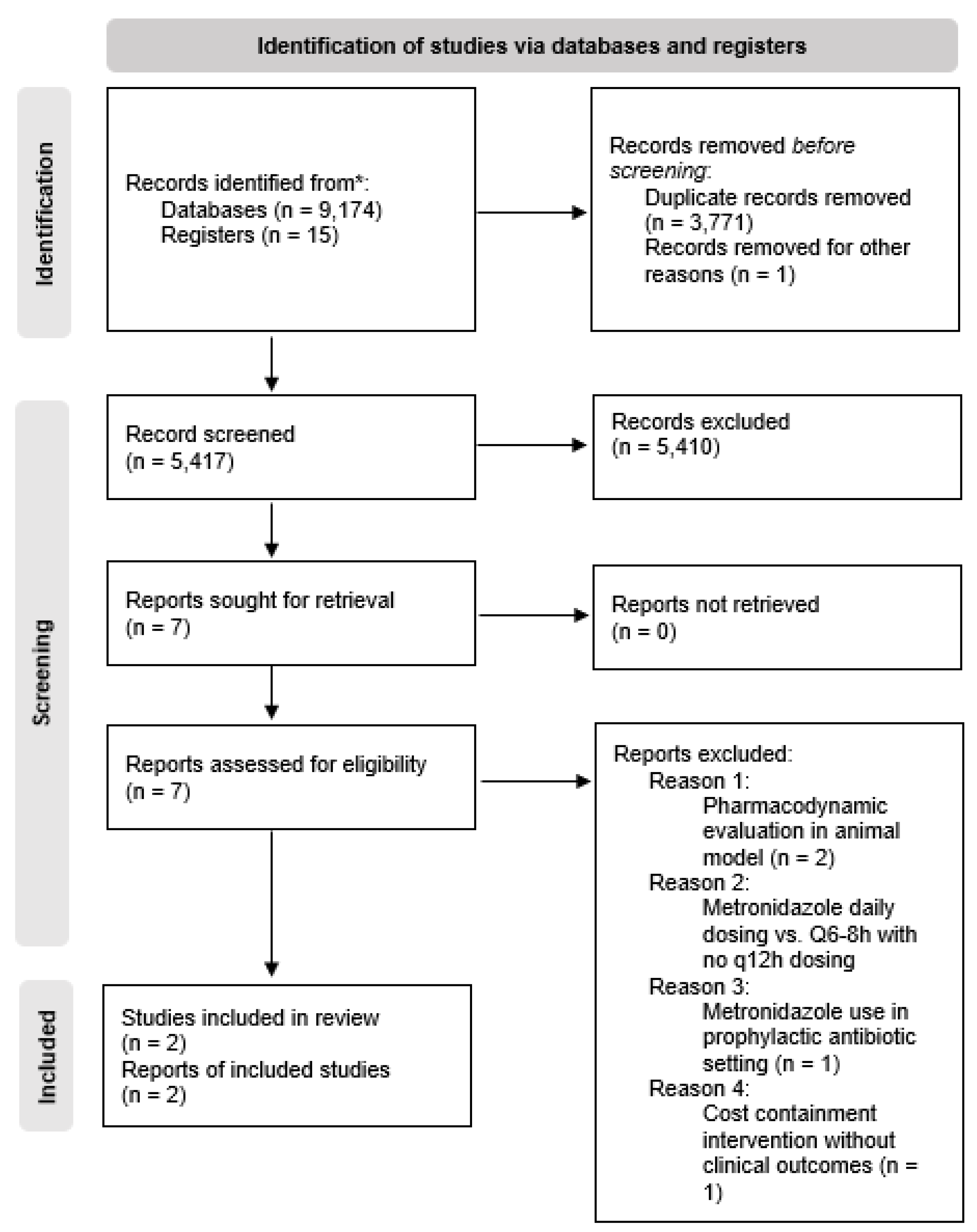
The initial database searches identified 9,174 records based on the defined search terms (see
Table 2). After removing duplicates and a poster abstract without the authors' contact information, 5,417 articles remained. The reviewers performed an initial screening by checking all titles and abstracts, retrieving the full manuscript for review if necessary to determine the record's relevance to the clinical question. The PRISMA diagram in
Figure 1 outlines the selection process of potentially relevant articles.
Excluded Studies with Reasons
The initial screening process excluded 5,410 records, leaving seven abstracts for full manuscript review. The reviewers excluded five of those seven studies for the following reasons: (a) one study's methodology focused on a cost-savings intervention with no clinical outcomes reported (Bunz et al., 1990), (b) two studies reported metronidazole serum drug concentrations only and lacked clinical outcomes (Earl et al., 1989; Ibrahim et al., 2004), (c) one study evaluated metronidazole in the prophylactic setting following dental surgery (Lloyd and Earl, 1994), and (d) one study's dosing methods included metronidazole daily versus dosing every six or eight hours without every 12-hour or twice daily dosing schedule (Wang et al., 2007).
Included Studies and Quality Assessment
The final analysis included two retrospective cohort studies which are of ‘good’ quality, as measured by the Newcastle-Ottawa scale (see
Table 3). Both studies were conducted in the United States and were published. Descriptions of the study and patient characteristics are summarized in
Table 4 and
Table 5.
Single-Site Study
Researchers at a 531-bed tertiary care hospital in the Southeast United States conducted a retrospective analysis of a two-year pre- and post-intervention antibiotic dosing protocol (Soule et al., 2018). During the first year, all patients within the facility received metronidazole on an eight-hour dosing frequency. During the second year, the hospital implemented a 12-hour metronidazole dosing program for all indications except Clostridioides difficile, central nervous system (CNS), or parasitic/amoebic infections. The researchers retrospectively collected all data, including patients over age 18 who received metronidazole for at least three days for a presumed anaerobic or mixed anaerobic infection. The authors excluded analysis of data from subjects who received another antibiotic with anaerobic coverage for greater than 24 hours prior to initiating metronidazole or received concurrent anaerobic coverage with another agent.
Reported primary outcomes included the following: (a) clinical cure, as defined by improvement or resolution of the principle sign/symptom of infection with normalization of white blood cells (>4000 and <12,000 cells/μl) and temperature (>96.8°F and <100.4°F) at the end of therapy or discharge, whichever occurred first, (b) escalation of antibiotic therapy, as defined by an additional antibiotic agent added for coverage, and (c) mortality, a defined by death during hospitalization (Soule et al., 2018). Secondary outcomes were the duration of all antibiotic therapy, duration of metronidazole therapy, and hospital length of stay (LOS). The study reported outcome data on 200 patients, with 100 patients in the 8-hour dosing group and 100 patients in the 12-hour dosing group.
The researchers reported no statistically significant differences in the baseline characteristics of patients in the two dosing groups, except for mean age for which older patients were observed in the 8-hour dosing group (62.5 ± 18.7 vs. 53.9 ± 19.6 years, for 8- and 12-hour dosing groups, respectively; p = 0.002). The researchers acknowledged no statistically significant differences between the two cohorts in regarding to male gender (8-hour: n = 44, 44% vs. 12-hour: n = 40, 40%, p = 0.567), intensive care unit admission (8-hour: n = 5, 5% vs. 12-hour: n = 10, 10%, p = 0.179), and liver dysfunction (8-hour: n = 6, 6% vs. 12-hour: n = 9, 9%, p = 0.421). The researchers stratified body mass index (BMI) as underweight, normal, overweight, obese, and morbidly obese, but the differences between the two groups were not statistically significant (p = 0.804). There were also no significant differences in infection characteristics, including infection type (p = 0.187; abdominal was most prevalent in both groups; 89% vs. 79% in the 8-hour and 12-hour dosing groups, respectively), source control (8-hour: n = 26, 26% vs. 12-hour: n = 31, 31%, p = 0.434), concurrent antibiotic therapy (8-hour: n = 93, 93% vs. 12-hour: n = 95, 95%, p = 0.552), presence of abdominal pain (8-hour: n = 73, 73% vs. 12-hour: n = 64, 64%, p = 0.171), elevated temperature (8-hour: n = 18, 18% vs. 12-hour: n = 23, 23%, p = 0.381), elevated white blood cells (8-hour: n = 51, 51% vs. 12-hour: n = 53, 53%, p = 0.777), and radiographic confirmation (8-hour: n = 77, 77% vs. 12-hour: n = 74, 74%, p = 0.622).
No statistically significant between-group differences were indicated for any primary outcome that included clinical cure (8-hour: n = 83, 83% vs. 12-hour: n = 83, 83%, p = 1.00) and mortality (8-hour: n = 2, 2% vs. 12-hour: n = 3, 3%, p = 0.651) (Soule et al., 2018). Additionally, there were no statistically significant differences in secondary clinical outcomes that included days of antibiotic therapy (8-hour: 5.8 ± 4.0 vs. 12-hour: 5.9 ± 4.3, p = 0.891), days of metronidazole (8-hour: 4.8 ± 2.8 vs. 12-hour: 4.9 ± 2.4, p = 0.827), or hospital LOS in days (8-hour: 6.7 ± 5.2 vs. 12-hour: 8.1 ± 7.0, p = 0.110). In the manuscript’s discussion section, Soule et al. reported that of the 17 patients from 8-hour dosing group who failed metronidazole therapy, seven (41.2%) had leukopenia. The authors did not provide leukopenia rates for the 12-hour dosing group to determine whether this leukopenia incidence was statistically significant.
Multi-Site Study
A retrospective chart review of patients admitted within a five-hospital health system in the Northeast United States included patients in the analysis with a culture-confirmed anaerobic bacteremia and received 500 milligrams of intravenous or oral metronidazole on every eight-hour or every 12-hour interval based upon provider discretion (Shah et al., 2021). In their analysis, the authors excluded subjects with a suspected metronidazole-resistant anaerobe, those who initially received more than 72-hours of an anaerobic agent other than metronidazole, or an antimicrobial agent suspected of being susceptible to the primary pathogen. Patients who received less than three days of metronidazole, those with Clostridioides difficile, and those with CNS infections were also excluded. This study included 85 patients in the final analysis, 37.6% (n = 32) of patients received metronidazole every 8-hours and 62.4% (n = 53) were dosed every 12-hours.
Reported clinical outcomes included the following: (a) all-cause 30-day mortality, (b) post-infection days of hospitalization, as defined by the duration between a positive blood culture and hospital discharge, (c) escalation of antimicrobial therapy, as defined by broadening antimicrobial therapy or increasing the dosing frequency from every 12-hours to every eight hours, (e) 30-day readmission due to infection, and (f) positive repeat blood cultures following initiation of metronidazole (Shah et al., 2021).
The authors reported no significant difference in the baseline demographics for the two cohorts, including median age (71 vs. 65 years, for 8- and 12-hour dosing groups, respectively; p = 0.08), male gender (8-hour: n = 13, 40.6% vs. 12-hour: n = 25, 47.2%, p = 0.557), median BMI (25 vs. 27.8, for 8- and 12-hour dosing groups, respectively; p = 0.246), median Charleston comorbidity index (8-hour: n = 2, 6.3% vs. 12-hour: n = 3, 5.6%, p = 0.0.21), median PITT bacteremia score (0 vs. 1, for 8- and 12-hour dosing groups, respectively; p = 0.162), elevated temperature (8-hour: n = 20, 62.5% vs. 12-hour: n = 35, 66%, p = 0.741), elevated white blood cells (8-hour: n = 18, 56.3% vs. 12-hour: n = 28, 52.8%, p = 0.759) patient need for mechanical ventilation (8-hour: n = 0, 0% vs. 12-hour: n = 2, 3.7%, p = 0.525), and vasopressor requirements (8-hour: n = 1, 3.1% vs. 12-hour: n = 4, 7.5%, p = 0.646.
The primary outcome analyses indicated that patients who received metronidazole every 8 hours, as compared to those who received metronidazole every 12 hours, had no statistically significant difference in all-cause 30-day mortality (8-hour: n = 5, 15.6% vs 12-hour: n = 5, 9.4%, p = 0.492), median post-infection days of hospitalization (8-hour: 9 days, interquartile range [IQR]: 6 to12.8 days vs. 12-hour: 8 days, IQR: 4 to 10 days, p = 0.271), or 30-day readmission due to infection (8-hour: n = 1, 3.1% vs. 12-hour: n = 1, 1.9%, p = 0.999) (Shah et al., 2021)Further, for patients who had repeat blood cultures (8-hour: n = 18, 56% vs. 12-hour: n = 47, 88.7%, p = 0.999), all samples were sterile.
4. Discussion
Metronidazole has remained a drug of choice for treating anaerobic bacterial infections. This systematic review primarily focused on the impact of the dosing frequency of metronidazole on clinical outcomes. For specific diseases like pelvic inflammatory disease and trichomoniasis, the standard of care already includes administering metronidazole every 12 hours (Centers for Disease Control and Prevention, 2021). Conversely, for treating Helicobacter pylori, multiple viable options are available, including dosing regimens every 12 hours (Chey et al., 2017).
Shah et al. studied the clinical outcomes of 85 patients with Bacteroides species bacteremia, with most cases originating from intra-abdominal sources (2021). The researchers divided patients into two groups based on their antibiotic treatment dosage frequency: one group received the antibiotic every 8 hours, while the other group received it every 12 hours. The study evaluated clinical failure rates, 30-day mortality, escalation of antimicrobial therapy, and post-infection length of stay between the two groups. In this study, the dosage frequency of antibiotics in treating Bacteroides species bacteremia did not significantly impact clinical failure rates, 30-day mortality, or the need for escalation of antimicrobial therapy. However, patients receiving antibiotics every 8 hours had a slightly longer post-infection LOS than those receiving antibiotics every 12 hours. These findings indicate that an every 12-hour dosing regimen may be equally effective in treating Bacteroides species bacteremia while potentially reducing the length of hospital stay (Shah et al., 2021)
Although the studies in our review excluded Clostridioides difficile infections and CNS infections, it still gives insight into the most common clinical conditions requiring metronidazole, including intra-abdominal infections and anaerobic bacteremia. Both studies have manifested no statistically significant differences in primary outcomes due to changes in metronidazole dosing frequency from every 8-hour to every 12-hour dosing. Both these studies had no significant differences in the baseline characteristics of the patients while including comparable infection types. Interestingly, Soule et al. reported a higher frequency of leukopenia in an 8-hour dosing group. Considering that leukopenia is a known side-effect of metronidazole use, less frequent dosing with comparable outcomes can be a reasonable way of avoiding such side effects. Based on two clinical studies, it is challenging to recommend changing to every 12-hour dosing. However, a thoughtful review of the clinical experience of using every 12-hour dosing could be reasonable to assess the efficacy and adverse events. A possible clinical evaluation method would be a review of the patient data and outcomes six months from the implementation of the change to every 12-hour dosing.
The forest plot of the results of the antibiotic escalation meta-analysis demonstrates that every 12-hour dosing is just as efficacious as every 8-hour dosing. Additionally, a change in metronidazole dosing frequency from every 8 to every 12 hours for the infection types included in this review affords cost-saving benefits (Bunz et al., 1990). By reducing the number of infusion supplies required and decreasing the frequency of administration per day, there is potential to enhance the cost savings further and provide the nursing staff with more available time.
Implementation of every 12-hour dosing reduces the risk of antimicrobial resistance primarily due to the lower overall dose of the medication (Dingsdag and Hunter, 2018). By spacing out the dosing intervals, the body has more time to metabolize and eliminate the drug, reducing the likelihood of accumulating toxic levels. Additionally, lower doses mitigate the emergence of resistance by minimizing the selective pressure on bacteria. This change in dosing strategy can improve patient safety and more effective treatment outcomes (Dingsdag and Hunter, 2018). Health systems contemplating a system-wide initiative to change every 12-hour metronidazole dosing may re-evaluate outcomes after six months to determine this change's overall efficacy and toxicity.
Limitations
All systematic reviews carry the risk of the search strategy's failure to uncover all studies on the target topic. However, we utilized the PRISMA guidelines, and multiple authors performed independent searches to limit this risk. This systematic review excluded studies of patients with Clostridoides difficile, Helicobacter pylori, CNS, and amoebiasis infections, so the findings may not apply to these infection types.
Additional limitations of this systemic review include several known disadvantages of retrospective cohort studies. First, studies captured by this review included a larger population of patients in the 12-hour dosing group compared to the 8-hour dosing, which may reflect an inherent acceptance of the 12-hour dosing schedule within those institutions. The prescribing practices of providers within the institutions who participated in these research studies may differ from most providers' prescribing practices. Next, there is a potential for unrecognized confounding variables. Although the authors of the observational studies used in this systematic review made efforts to ensure an equitable comparison of the two antibiotic dosing groups, there are potentially unrecognized differences in the cohorts that could have impacted recorded outcomes. Finally, the small number of studies on this topic may indicate a risk of publication bias.
5. Conclusions
This systematic review showed no differences in the outcomes of patients who received metronidazole dosed every 12 hours versus every 8 hours. A meta-analysis of the need for antibiotic escalation did not show a statistically significant difference between dosing groups. Based on the data, it is justifiable to consider implementing an every 12-hour dosing schedule for metronidazole in most clinical situations. Electronic prescribing systems should offer 500 mg IV or PO every 12 hours to facilitate this change while retaining every 6-hour and every 8-hour dosing options specifically for CNS, Helicobacter pylori, or Clostridioides difficile infections. Including a note indicating that these alternative dosing options are intended only for the mentioned exception indications would be beneficial. If a healthcare system attempts to adopt this dosing, carefully reviewing patient outcomes would be appropriate to minimize patient harm.
Author Contributions
All authors have read and agreed to the published version of the manuscript. Conceptualization MV; Methodology TAJ; RWW; PAF, FA; Formal Analysis RWW; Data Curation TAJ, RWW, FA; Writing – Original Draft Preparation TAJ; Writing – Review & Editing FA, RWW, PAF, CJD, MV;
Conflicts of Interest
The authors declare no conflict of interest.
References
- Placidi, G. F.; Masouka, D.; Alcaraz, A.; Taylor, J. A.; Earle, R. Distribution and metabolism of 14C-metronidazole in mice. Archives Internationales de Pharmacodynamie et de Therapie 1970, 188. [Google Scholar]
- Ings, R.M.J.; McFadzean, J.A.; Ormerod, W.E. The Fate of Metronidazole and its Implications in Chemotherapy. Xenobiotica 1975, 5, 223–235. [Google Scholar] [CrossRef]
- Manthei, R.W.; Feo, L.G.; E Stambaugh, J. Identification of the metabolites of metronidazole in the human vagina. Wiadomosci Parazytol. 1969, 15. [Google Scholar]
- Lamp, K.C.; Freeman, C.D.; Klutman, N.E.; Lacy, M.K. Pharmacokinetics and Pharmacodynamics of the Nitroimidazole Antimicrobials. Clin. Pharmacokinet. 1999, 36, 353–373. [Google Scholar] [CrossRef] [PubMed]
- Bunz, D.; Gupta, S.; Jewesson, P. Metronidazole cost containment: a two-stage intervention. Hosp. Formul. 1990, 25. [Google Scholar]
- Richardson, W.S.; Wilson, M.C.; Nishikawa, J.; Hayward, R.S. The well-built clinical question: a key to evidence-based decisions. ACP J. club 1995, 123, A12–A13. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef] [PubMed]
- Wells, G. A.; Shea, B.; O'Connell, D. Wells, G. A.; Shea, B.; O'Connell, D., et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. (accessed May 9, 2023). 9 May.
- Agency for Healthcare Research and Quality Assessing the risk of bias of individual studies in systematic review of health care interventions. (accessed , 2023). 15 May.
- StataCorp Stata Statistical Software. 2021, 17. StataCorp Stata Statistical Software. 2021, 17.
- Earl, P.; Sisson, P.R.; Ingham, H.R. Twelve-hourly dosage schedule for oral and intravenous metronidazole. J. Antimicrob. Chemother. 1989, 23, 619–621. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, K.H.; Gunderson, B.W.; Hermsen, E.D.; Hovde, L.B.; Rotschafer, J.C. Pharmacodynamics of Pulse Dosing versus Standard Dosing: In Vitro Metronidazole Activity against Bacteroides fragilis and Bacteroides thetaiotaomicron. Antimicrob. Agents Chemother. 2004, 48, 4195–4199. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, C.; Earl, P. Metronidazole: two or three times daily—a comparative controlled clinical trial of the efficacy of two different dosing schedules of metronidazole for chemoprophylaxis following third molar surgery. Br. J. Oral Maxillofac. Surg. 1994, 32, 165–167. [Google Scholar] [CrossRef]
- Wang, S.; Cunha, B.; Hamid, N.; Amato, B.; Feuerman, M.; Malone, B. Metronidazole Single versus Multiple Daily Dosing in Serious Intraabdominal/Pelvic and Diabetic Foot Infections. J. Chemother. 2007, 19, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Soule, A.F.; Green, S.B.; Blanchette, L.M. Clinical efficacy of 12-h metronidazole dosing regimens in patients with anaerobic or mixed anaerobic infections. Ther. Adv. Infect. Dis. 2018, 5, 57–62. [Google Scholar] [CrossRef]
- Shah, S.; Adams, K.; Merwede, J.; McManus, D.; Topal, J. Three is a crowd: Clinical outcomes of a twice daily versus a thrice daily metronidazole dosing strategy from a multicenter study. Anaerobe 2021, 71, 102378. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention Pelvic inflammatory disease. https://www.cdc.gov/std/treatment-guidelines/pid.htm.
- Chey, W.D.; Leontiadis, G.I.; Howden, C.W.; Moss, S.F. ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am. J. Gastroenterol. 2017, 112, 212–239. [Google Scholar] [CrossRef] [PubMed]
- A Dingsdag, S.; Hunter, N. Metronidazole: an update on metabolism, structure–cytotoxicity and resistance mechanisms. J. Antimicrob. Chemother. 2017, 73, 265–279. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).