Introduction
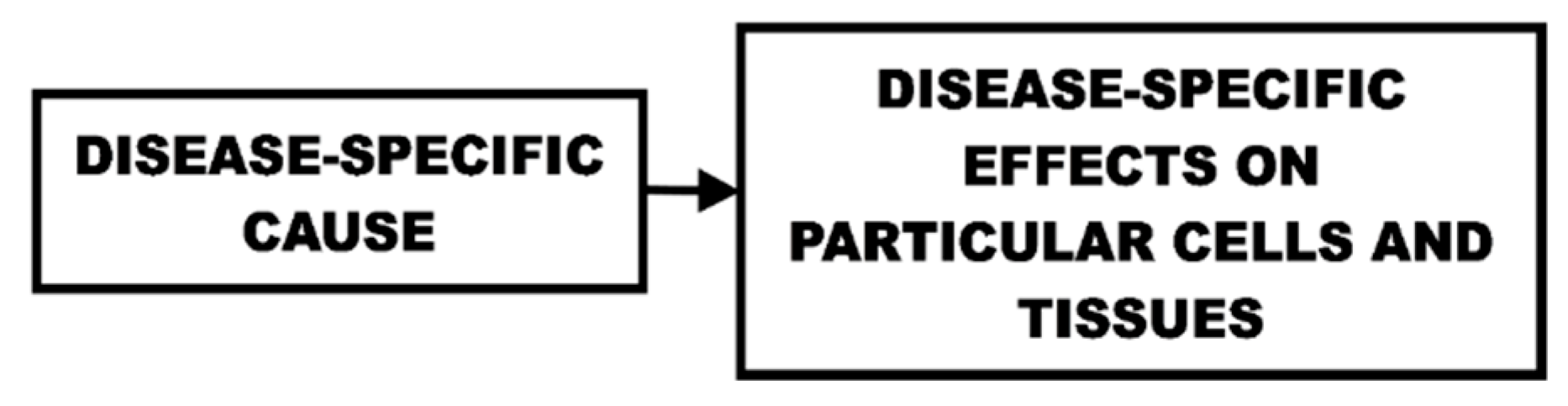
Medical and pharmaceutical research efforts have historically been substantially based on finding the most direct and immediate cause of a particular disease and then finding ways to treat that cause. The causes of different diseases are largely considered to be independent of each other. Because not every individual suffers from any particular disease, escaping that disease is clearly possible and every disease is broadly considered to be potentially treatable and even curable and/or preventable. By studying differences between individuals that acquire a disease and those that do not, we can determine causes and therefore methods for treatment or prevention. This cause-effect approach (
Figure 1) is reasonable and has been very successful. This in turn has resulted in a dramatic increase in the importance of aging and age-related diseases in overall public health.
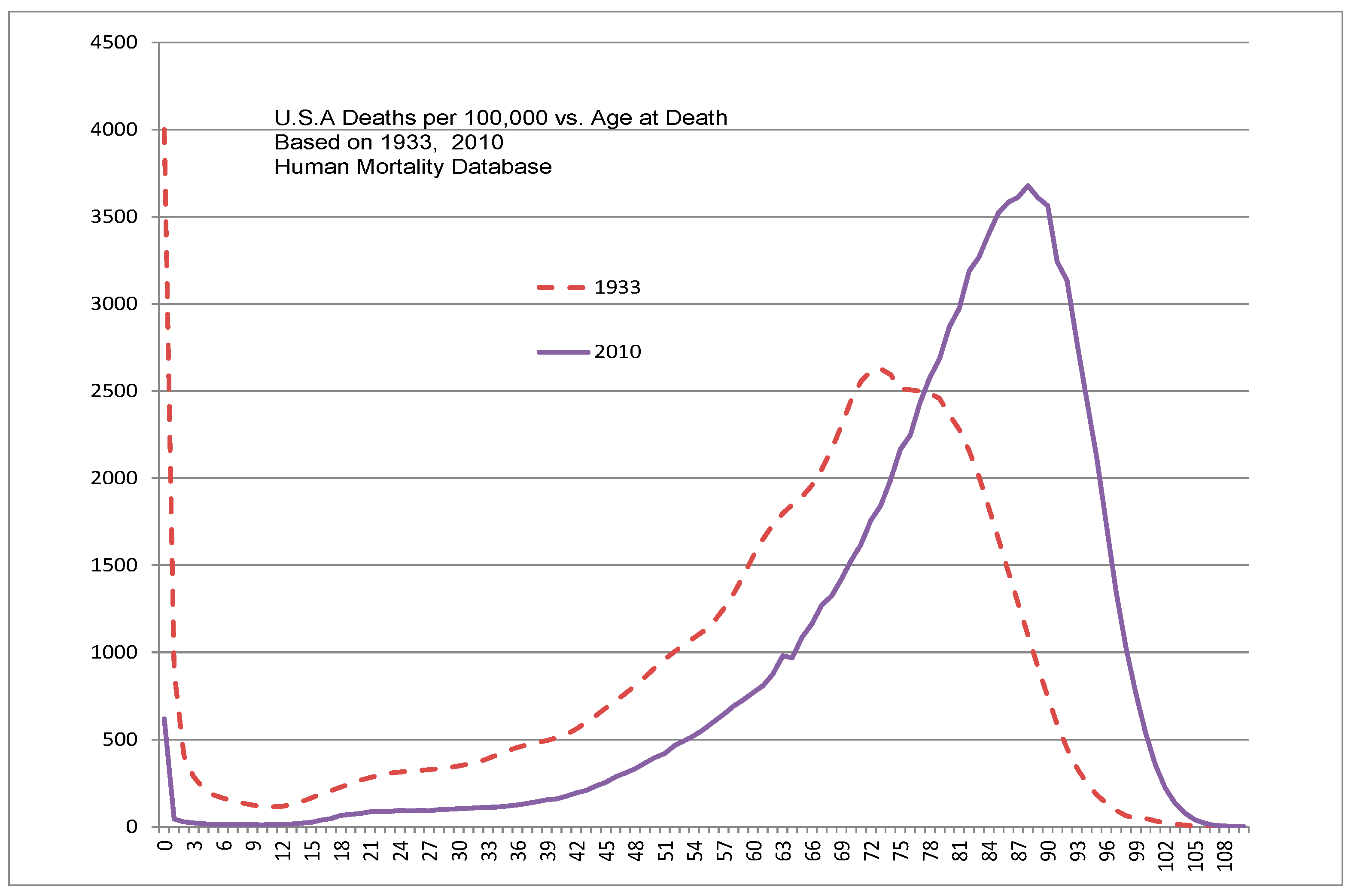
Age related diseases are those that dramatically increase in incidence and severity with age and include cancer, heart disease, stroke, and Alzheimer’s disease. Indicators of aging include death rate that tends to increase exponentially with age starting from a species-specific age (e.g. about 22 years for humans-
Figure 2).
Age related conditions are essentially universal in any particular mammal species and include loss of strength, loss of sensory and immunity capability, mental deterioration, and eventual “death of old age.” Because of their universal nature they are considered less treatable, determining a cause is more difficult, and they are more likely to be considered “normal,” “inescapable” and less appropriate for research and treatment. Because of the very long-term and gradual effects of aging, determining cause and effect is difficult relative to the case with most diseases.
However, highly age-related diseases such as cancer, heart disease, and Alzheimer’s disease are clearly substantially caused by aging and therefore have a common cause although most age-related diseases also occasionally occur in young people and therefore have causes in addition to aging. In addition, aging characteristics of a particular species such as internally determined lifespan are extremely specific to the species and vary enormously between mammal species. This led to the now widely accepted idea that aging is in some way the result of the evolution process that determined the many other species-specific design characteristics of that species. These evolutionary theories of aging provided a much better match to mammal aging observations than earlier theories to the effect that aging was an inevitable result of laws of physics or chemistry.
Today there are two main classes of evolutionary aging theories:
Non-programmed aging theories contend that aging has little negative effect on a wild mammal population and that therefore there was little evolutionary force toward eliminating each of the many different causes of the different age-related diseases and conditions. This concept is based on the observation that under wild conditions few members of a particular species population would survive beyond a species-specific age because of mortality due to
external conditions such as infectious diseases, predators, and limited food supply or habitat. The evolutionary value of further lifetime could be expected to decline with age in a population and species-specific manner. In 1952 Medawar proposed that each of many different age-related diseases and other manifestations was caused by different mutational changes that accumulated in an organism’s genome [
1]. Because these mutations only caused fitness loss in older individuals, there was little evolutionary force toward removing them. This concept fits well with the traditional medical/pharmaceutical cause-effect model. Note that the non-programmed theories suggest that evolved differences in aging are entirely the result of inherited genetic differences.
Programmed aging theories propose that aging,
per se, creates an evolutionary advantage for an aging population causing the evolution of potentially complex biological mechanisms that
purposely cause the many different effects (symptoms) of aging seen in mammals. Programmed implies an evolved mechanism that sequences events as a function of time. Where the non-programmed theories suggest there is evolutionary force toward achieving a species-specific
minimum lifespan, programmed theories suggest there is force toward achieving a minimum lifespan
and limiting lifespan beyond a particular age, that is, achieving a species-unique
optimum lifespan. Although programmed aging was first formally proposed by Weismann in 1882 [
2], as recently as 2002 it was widely thought to be theoretically impossible because of conflicts with traditional (Darwinian) evolutionary mechanics theory (that describes the nature of the evolution process). However, other evolutionary mechanics theories such as group selection [
3], kin selection [
4], and evolvability theory [
5,
6] that support programmed aging concepts (e.g. [
7,
8,
9]) have appeared and it is clear that programmed aging is now better accepted in the gerontology community. Nevertheless, there is still no wide scientific agreement regarding even very basic questions regarding mammal aging such as: What is aging? and Why do we age? Resolution of this issue is critical for medical research and public health. We cannot hope to understand and most effectively treat massively age-related diseases such as Alzheimer’s disease, heart disease, and cancer without understanding aging. This article describes a specific concept for the nature of the aging program:
aging is a life-cycle function.
Life Cycle Functions
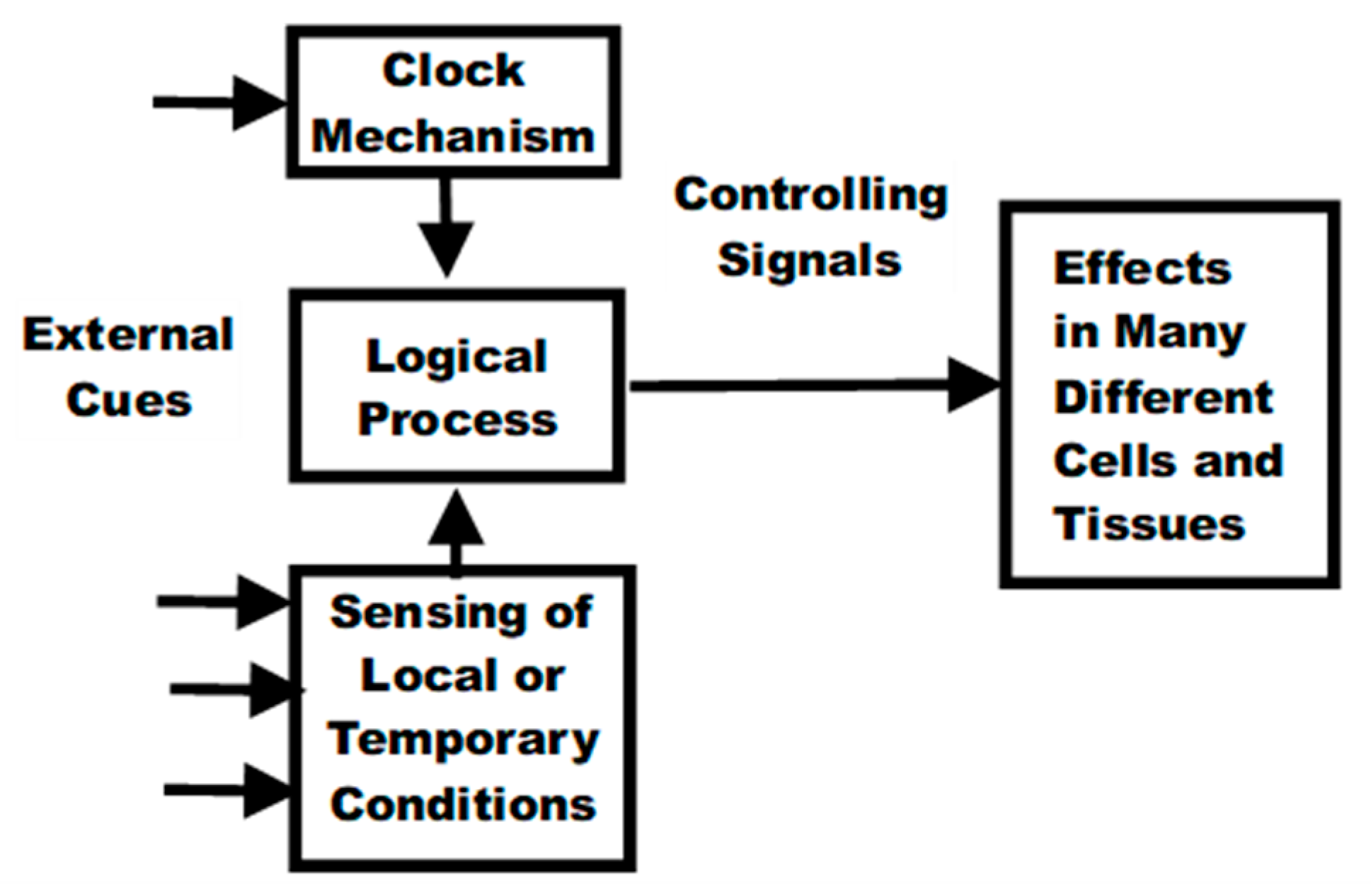
A biological life-cycle function is one that controls and regulates some aspect of life that occurs as a species-specific function of age. These functions include growth, reproduction, menopause, and aging. The life cycle functions have a very different cause-effect situation (
Figure 3) relative to the medical (and non-programmed) model.
All the life-cycle functions involve biological mechanisms for determining elapsed time since birth (or conception), i.e., biological clocks. In some cases, it is obvious that the clock is itself derived from or synchronized to detection of external cues such as the annual cycles seen in mammals that exhibit mating seasons.
Some life-cycle functions (growth and aging) involve controlling the functioning of a very large number of different cells and tissues needed to
implement the observed age-related changes (
effects) in those cells and tissues. However, in order to create the observed synchronization of the diverse effects, their
control must be coordinated (scheduled) by a logically single common mechanism (i.e. the
cause). This implies the existence of a signaling scheme whereby the single control mechanism controls myriad different effects and supports endocrine-based aging theories e.g. [
10]. For example, it is understood that the reproduction function involves hormones (intra-individual signaling) and even pheromones or inter-individual signaling. Reproduction and possibly other life-cycle functions also involve brain and nervous system control.
The various life-cycle functions interact with each other to a large degree. A change to one function would logically require complementary changes to others. Example, evolutionary aging theories generally agree that it would not make sense for an evolved aging trait to cause significant fitness degradation prior to the time (age) that a mammal could complete a first reproduction [
11]. This time would be dependent on other life cycle events that are very specific to particular species such as age at sexual maturity (puberty), length of pregnancy, physical maturity at birth, length of lactation stage, etc.
Rapid Adaptation Mechanisms in Aging and other Life Cycle Functions
Evolution in the genetically controlled design of a complex organism is a slow process. It is common for mammals to possess the ability to adjust a genetically specified design parameter within some range
during their lives in order to respond to detection of local or temporary changes in the organism’s external world that affect the optimum value of that parameter. Examples: some mammals can change their fur density (and therefore insulation properties) in response to detection of seasonal temperature changes [
12]. This allows them to operate over a larger geographic range without annual migration. Mammals can change the size, strength, and associated blood supply of muscles in response to demand on them. This allows the mammal to operate in a mountainous area and also operate on a plain where smaller and lighter muscles would require a smaller food supply and therefore be advantageous.
Rapid adaptation of life cycle functions would also be valuable. Detection of local or temporary population
stress factors that generally
decrease wild mammal lifetime could be used to
increase the genetically specified internally determined lifespan to compensate and continue to deliver an optimum lifespan. Multiple forms of temporary or local population stress include starvation [
13], overcrowding (that increases mass infection risk), predation, and extreme environmental conditions. Starvation and environmental changes cause internal changes that could be detected by an organism, detection of overcrowding might involve pheromones, and detection of predation could include detection of unusual brief intense physical activity or terror.
Multiple life cycle functions could be involved in a coordinated response to population stress. A logical response to famine might involve increasing internally determined lifespan while simultaneously decreasing reproduction because reproduction requires more food than surviving.
It is increasingly accepted that multiple forms of population stress such as starvation and physical stress (exercise) can generally delay aging. Concentrations of multiple human hormones have been observed to vary with age [
14]. These observations fit with the life cycle model.
Objections to the Evolution of Programmed Aging–Individual vs Population Benefit
By far the greatest objection to programmed aging has been that it violates evolution theory regarding the
mechanics of the evolution process. The
facts of evolution, i.e. that current species are descended from earlier, different, species, are not in scientific contention. Traditional (Darwinian) Evolutionary-mechanics Theory (TET [
15]) explains the origin of species, provides plausible explanations for the vast majority of observations regarding organism design characteristics, and eventually became settled science and virtually a law of Biology. TET also plausibly explains suicide mechanisms in many semelparous non-mammals: Salmon die shortly after reproducing from a greatly accelerated aging process [
16], which can be explained as creating a benefit to the adult’s direct descendants by providing food from the adult’s corpse. However, there has always been an apparently relatively minor but annoying academic issue: How to explain the existence of evolved aging in multiparous sexually reproducing organisms such as mammals. TET essentially says that the force of evolution is toward
increasing the probability that an
individual will produce adult descendants, but it was obvious that mammal aging at least somewhat
reduced an individual multiparous organism’s opportunity for producing descendants and this has been confirmed by wild mammal studies [
17].
Shortly after the publication of
Origin critics suggested [
18] that if Darwin’s concept was correct, the force of evolution was toward achieving internal immortality or the absence of any internal (design) limitation on fitness–why has this not occurred? Theories to the effect that aging was not an evolved characteristic, but rather the inevitable result of some laws of physics or chemistry failed to explain the huge differences in lifespan between physically and chemically similar species and more generally the observation that aging and lifespan characteristics were extremely associated with particular species. Darwin did not offer a solution regarding the relationship between aging and the evolution process [
18]. Much later in 2002 the gerontology community issued a position statement to the effect that programmed aging was theoretically impossible because it conflicted with traditional theory [
19]. A widely accepted solution to this problem has not been reached more than 160 years later!
TET is extremely oriented around mutations and around the idea that individual success at reproducing drives the evolution process. We can agree that TET is grossly incompatible with programmed aging, essentially the idea that mammals possess an evolved gradual suicide mechanism that limits individual lifespan in order to increase the probability that a population will avoid extinction, and further supports the idea that aging is inevitable and untreatable. TET and an individual and mutation-based concept also fits well with the evolution of haploid prokaryote species like bacteria. The question now is whether TET is perfectly correct and comprehensive with respect to evolution of diploid sexually reproducing multiparous species like mammals. Of course, “impossible” trumps any amount of direct evidence except perhaps the production of mice with a 100-year lifespan (which would require more than a century to demonstrate)! Genetics discoveries (some quite recent) have added support for most aspects of traditional theory but have also shown that some key assumptions are provably incorrect as follows:
TET assumes that inheritable variation between individuals in a population is essential to the evolution process but contends “natural” variation is an inherent property of life. All organisms are subject to mutations and the propagation of changes caused by mutations could plausibly cause some variation. Darwin could also reasonably assume that biological inheritance was an
analog process that “naturally” produced variation. However, genetics discoveries [
20] proved that inheritance involves the transfer of information defining the organism’s inherited design in
digital form between parent and descendant of any organism. In addition to other important properties [
6], digital information transfer systems inherently produce exact duplicates of the information. It is this feature that has allowed modern species to inherit some aspects of their designs from ancestors that lived billions of years ago. The inheritable mammal variation that we observe (e.g., between siblings) is mainly produced by very complex and obviously evolved biological mechanisms. Identical twins result from a malfunction of these mechanisms.
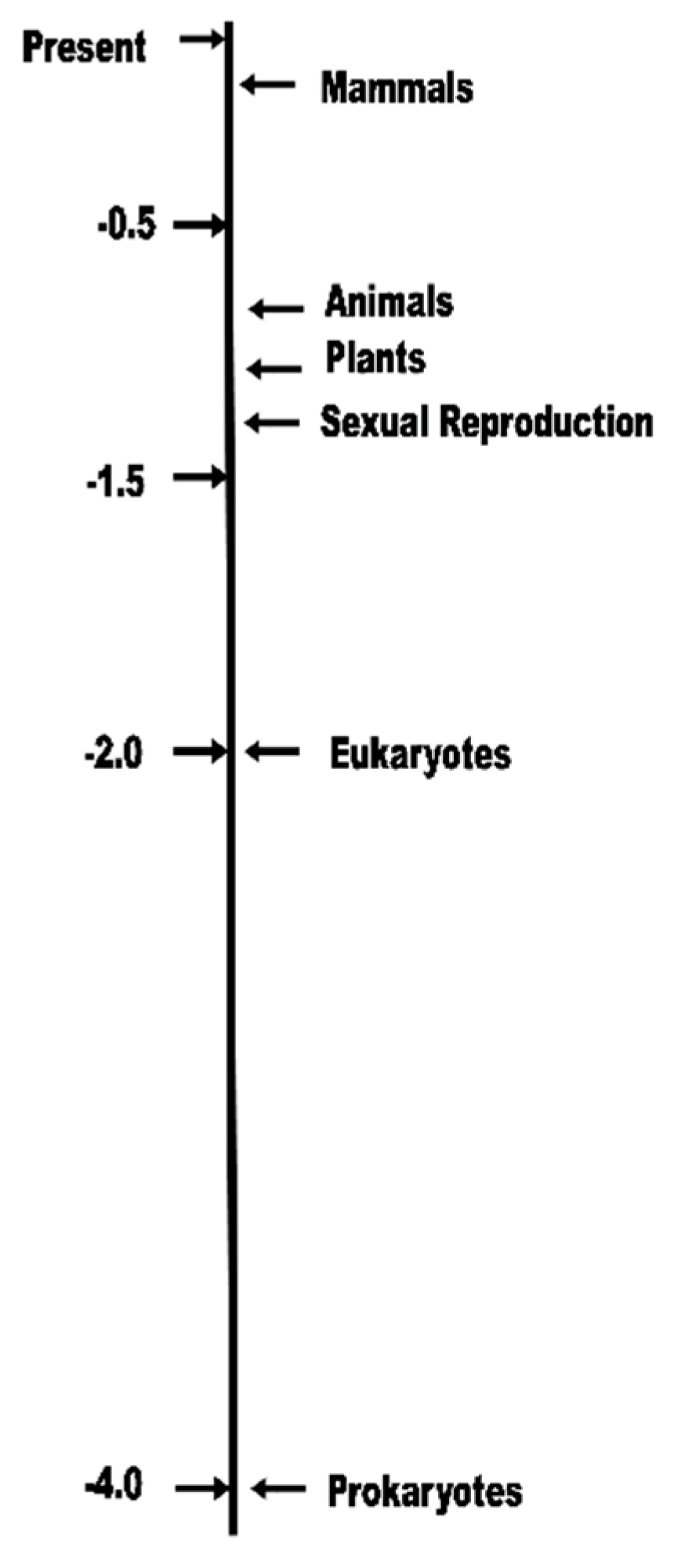
Another critical issue is that TET assumes that the ability to evolve (genetically adapt) is an inherent property of life. All organisms are subject to “natural” selection and “natural” variation. Genetics discoveries show that the ability to evolve in diploid, eukaryotic, sexually reproducing species is actually the result of multiple complex and clearly evolved genomic mechanisms that appeared
after the appearance of haploid prokaryote species (
Figure 4). The evolution process is therefore grossly different and more rapid in mammals as opposed to the prokaryote species. This is the basis of
evolvability theories [
5,
21] that suggest that organisms did evolve design characteristics that increase the rapidity and comprehensiveness of genetic adaptation (evolvability) and therefore allow a species population to adapt more rapidly to changes in its external world.
Evolvability-based theories of aging suggest that an internally limited lifespan increases evolvability in multiple ways [
6]. Because more rapid or comprehensive adaptation would reduce the probability that a population would become extinct, evolution selects design characteristics that increase evolvability. Because speciation eventually blocks crossbreeding between species (even between a species and its parent species), each sexually reproducing species can, substantially independently of the others, evolve a design that is specific to that species’ particular ecological niche, a major evolvability advantage.
Acquisition traits are those that depend for their evolutionary (fitness) benefit on the accumulation of something that accumulates during an organism’s lifetime but
is not genetically passed to descendants. The evolution of traits such as intelligence, immunity, social status, and language capability represents a special need for evolvability and an internally limited lifespan [
11]. TET followers logically reject the whole concept of evolvability, which violates multiple TET tenets.
Darwin’s concept [
15] assumed that evolution occurred in minute increments or “tiny steps.” He also assumed that each tiny increment was processed by natural selection and that evolution was an accumulative process. TET also proposes that evolution is driven by the
performance of an organism in producing descendants. Since latent characteristics (e.g. in juveniles) do not affect performance, the evolution of adult characteristics requires adults. In addition, TET recognized that living organisms were
systems in that each element of their design must be coordinated with the others to result in a performance advantage. For example, longer legs might increase speed enabling gazelles to better escape lions. However, significantly increasing femur length would be adverse unless accompanied by corresponding coordinated changes in other bones, tendons, muscles, etc. This essentially requires the tiny accumulative steps. However, the tiny steps concept also has statistical implications. While a major negative change (such as one causing fetal death) would be immediately “selected out,” selecting or rejecting a tiny positive or negative change would involve comparing a very large number of individuals having the change to those not having the change to produce the necessary statistical basis. This problem was progressively more severe as organisms became more complex. Other statistical problems with traditional theory are that as organisms became larger and their populations became smaller (relative to bacteria and other single-cell organisms) their lifetimes would increase and the process of evolution would slow. Nominally, evolution of a population would proceed at a rate that was proportional to
population size,
and inversely proportional to nominal lifetime,
and inversely proportional to organism complexity!
Figure 4 shows that this has not happened and that the rate of evolution has
increased since the development of diploid sexually reproducing species. This increase has been caused by design changes in the mechanisms of biological inheritance that increase evolvability including internally limited lifespan [
11].
TET assumes that evolution can be completely explained by mutations and natural selection and that new mutations each occur in a single individual and are then processed by natural selection. This logically leads to the idea that evolution occurs on an individual level. [
23] TET says evolution causes an individual possessing an evolved characteristic to have a larger chance of producing descendants than an individual not possessing the characteristic. This idea explicitly prohibits programmed aging because there is wide agreement that aging in mammals
does not increase an individual’s ability to produce descendants. Instead, programmed aging theories are based on population benefit: evolution selects design characteristics that increase the probability that a population of individuals of a particular species will avoid extinction. Multiple programmed aging theories describe multiple population benefits of aging.
Non-programmed evolutionary aging theories (despite fierce protestations of their authors)
also appear to be based on population benefit in that they propose that the force of evolution depends on the
size of a
population age-cohort [
1]. At least one prominent “non-programmed” theory [
24] is arguably a programmed theory!
Genetics discoveries prove that evolution within a mammal species does not necessarily require new mutations and can be accomplished by recombining existing mutations stored in a species’ genome. Example: the huge variations caused by selective breeding and seen in dog breeds could be explained without requiring any new mutations. Selective breeding in dogs has accidentally caused large differences (~2:1) in lifespan between dog breeds. Clearly if we intentionally selectively bred long-lived dogs for increased longevity and bred short-lived dogs for decreased longevity, we could create an even larger lifespan differential. Further it is clear that if a wild mammal population (such as the wolf ancestor of dogs) needed a longer or shorter lifespan, it could similarly adapt (without new mutations). Finally, discoveries support the existence of evolved mechanisms that can change mammal lifespans during their lives as suggested by the life-cycle concept and the stress observations.
This is a summary of some of the conflicts between TET and genetics observations as well as selective breeding that are discussed in more detail elsewhere [
6,
25,
26]. Genetics discoveries have exposed staggering complexity. A typical current genetics textbook is more than 800 pages and requires a new edition every few years [
20]. It is unlikely that we are even close to completely understanding biological inheritance and therefore evolutionary mechanics.