Submitted:
18 July 2023
Posted:
20 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
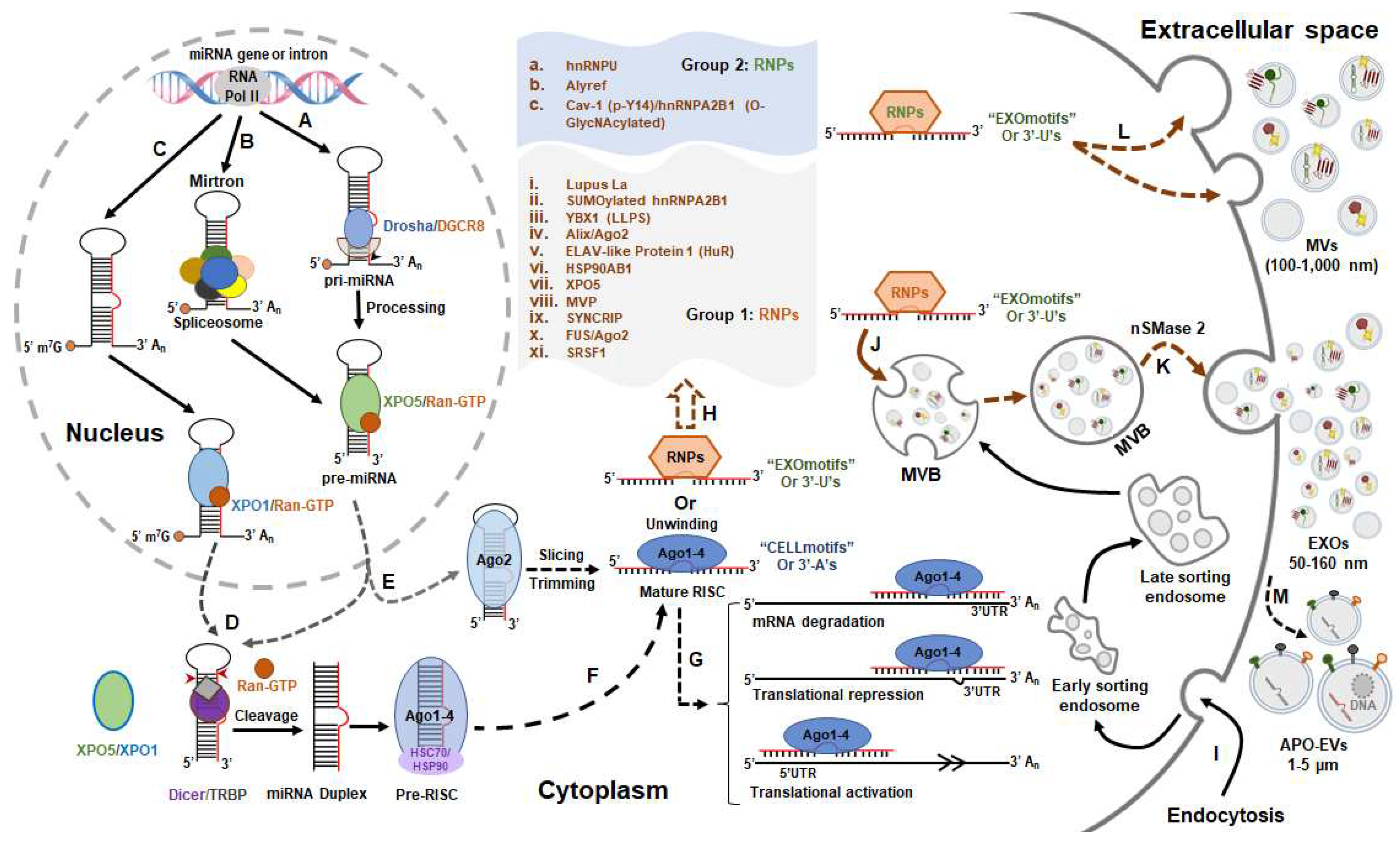
2. miRNA Biogenesis and Extracellular Vesicle Selection
2.1. miRNAs and Extracellular Vesicle Biogenesis
2.2. Mechanisms of miRNA Selection into EVs
3. EV miRNAs in the Pathogenesis of Heart Failure
3.1. EV-miRNA in the Cardiac Hypertrophy
3.2. EV-miRNA in the Cardiac Fibrosis
3.3. EV-miRNA in Cardiac Angiogenesis during Heart Failure
3.3. Cardiac EV miRNA-Mediated Inter-Organ Communication in Heart Failure
4. Extracellular Vesicle miRNA-Based Prognosis, Diagnosis and Therapeutics of Heart Failure
5. Perspectives and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roger, V.L. Epidemiology of Heart Failure: A Contemporary Perspective. Circ Res 2021, 128, 1421–1434. [Google Scholar] [CrossRef]
- Wollert, K.C.; Drexler, H. The renin–angiotensin system and experimental heart failure. Cardiovascular Research 1999, 43, 838–849. [Google Scholar] [CrossRef]
- Verbrugge, F.H.; Tang, W.H.W.; Mullens, W. Renin-Angiotensin-Aldosterone System Activation During Decongestion in Acute Heart Failure. JACC: Heart Failure 2015, 3, 108–111. [Google Scholar] [CrossRef]
- Jia, G.; Aroor, A.R.; Hill, M.A.; Sowers, J.R. Role of Renin-Angiotensin-Aldosterone System Activation in Promoting Cardiovascular Fibrosis and Stiffness. Hypertension 2018, 72, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Florea, V.G.; Cohn, J.N. The autonomic nervous system and heart failure. Circ Res 2014, 114, 1815–1826. [Google Scholar] [CrossRef]
- Kishi, T. Heart failure as an autonomic nervous system dysfunction. Journal of Cardiology 2012, 59, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.Y.; Anderson, A.S. The sympathetic nervous system and heart failure. Cardiol Clin 2014, 32, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Booth, L.C.; May, C.N.; Yao, S.T. The role of the renal afferent and efferent nerve fibers in heart failure. Front Physiol 2015, 6, 270. [Google Scholar] [CrossRef] [PubMed]
- van Rooij, E.; Sutherland, L.B.; Liu, N.; Williams, A.H.; McAnally, J.; Gerard, R.D.; Richardson, J.A.; Olson, E.N. A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc Natl Acad Sci U S A 2006, 103, 18255–18260. [Google Scholar] [CrossRef] [PubMed]
- van Rooij, E.; Sutherland, L.B.; Qi, X.; Richardson, J.A.; Hill, J.; Olson, E.N. Control of stress-dependent cardiac growth and gene expression by a microRNA. Science 2007, 316, 575–579. [Google Scholar] [CrossRef]
- Younger, S.T.; Corey, D.R. Transcriptional gene silencing in mammalian cells by miRNA mimics that target gene promoters. Nucleic Acids Res 2011, 39, 5682–5691. [Google Scholar] [CrossRef]
- Lytle, J.R.; Yario, T.A.; Steitz, J.A. Target mRNAs are repressed as efficiently by microRNA-binding sites in the 5’ UTR as in the 3’ UTR. Proc Natl Acad Sci U S A 2007, 104, 9667–9672. [Google Scholar] [CrossRef] [PubMed]
- Tay, Y.; Zhang, J.; Thomson, A.M.; Lim, B.; Rigoutsos, I. MicroRNAs to Nanog, Oct4 and Sox2 coding regions modulate embryonic stem cell differentiation. Nature 2008, 455, 1124–1128. [Google Scholar] [CrossRef]
- Gu, W.; Xu, Y.; Xie, X.; Wang, T.; Ko, J.H.; Zhou, T. The role of RNA structure at 5’ untranslated region in microRNA-mediated gene regulation. Rna 2014, 20, 1369–1375. [Google Scholar] [CrossRef]
- Kumarswamy, R.; Thum, T. Non-coding RNAs in cardiac remodeling and heart failure. Circ Res 2013, 113, 676–689. [Google Scholar] [CrossRef] [PubMed]
- Melman, Y.F.; Shah, R.; Das, S. MicroRNAs in heart failure: is the picture becoming less miRky? Circ Heart Fail 2014, 7, 203–214. [Google Scholar] [CrossRef]
- Gholaminejad, A.; Zare, N.; Dana, N.; Shafie, D.; Mani, A.; Javanmard, S.H. A meta-analysis of microRNA expression profiling studies in heart failure. Heart Fail Rev 2021, 26, 997–1021. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.N.; Wang, J.L.; Fu, Y.P. The microRNA Expression Profiling in Heart Failure: A Systematic Review and Meta-Analysis. Front Cardiovasc Med 2022, 9, 856358. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Martin, R.; Wang, G.; Brandão, B.B.; Zanotto, T.M.; Shah, S.; Kumar Patel, S.; Schilling, B.; Kahn, C.R. MicroRNA sequence codes for small extracellular vesicle release and cellular retention. Nature 2022, 601, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Koppers-Lalic, D.; Hackenberg, M.; Bijnsdorp, I.V.; van Eijndhoven, M.A.J.; Sadek, P.; Sie, D.; Zini, N.; Middeldorp, J.M.; Ylstra, B.; de Menezes, R.X.; et al. Nontemplated nucleotide additions distinguish the small RNA composition in cells from exosomes. Cell Rep 2014, 8, 1649–1658. [Google Scholar] [CrossRef]
- Kosaka, N.; Iguchi, H.; Yoshioka, Y.; Takeshita, F.; Matsuki, Y.; Ochiya, T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem 2010, 285, 17442–17452. [Google Scholar] [CrossRef] [PubMed]
- Ha, M.; Kim, V.N. Regulation of microRNA biogenesis. Nature Reviews Molecular Cell Biology 2014, 15, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Takagi, M.; Ono, S.; Kumaki, T.; Nishimura, N.; Murakami, H.; Enomoto, Y.; Naruto, T.; Ueda, H.; Kurosawa, K. Complex congenital cardiovascular anomaly in a patient with AGO1-associated disorder. Am J Med Genet A 2023, 191, 882–892. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Ranjan, P.; Suleiman, Z.G.; Goswami, S.K.; Li, J.; Prasad, R.; Verma, S.K. mRNA modifications in cardiovascular biology and disease: with a focus on m6A modification. Cardiovasc Res 2022, 118, 1680–1692. [Google Scholar] [CrossRef]
- Zhu, M.; Gao, J.; Lin, X.J.; Gong, Y.Y.; Qi, Y.C.; Ma, Y.L.; Song, Y.X.; Tan, W.; Li, F.Y.; Ye, M.; et al. Novel roles of an intragenic G-quadruplex in controlling microRNA expression and cardiac function. Nucleic Acids Res 2021, 49, 2522–2536. [Google Scholar] [CrossRef]
- da Costa Martins, P.A.; Bourajjaj, M.; Gladka, M.; Kortland, M.; van Oort, R.J.; Pinto, Y.M.; Molkentin, J.D.; De Windt, L.J. Conditional dicer gene deletion in the postnatal myocardium provokes spontaneous cardiac remodeling. Circulation 2008, 118, 1567–1576. [Google Scholar] [CrossRef]
- Varela, M.A.; Roberts, T.C.; Wood, M.J. Epigenetics and ncRNAs in brain function and disease: mechanisms and prospects for therapy. Neurotherapeutics 2013, 10, 621–631. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, M.; Han, J.; Yeom, K.H.; Lee, S.; Baek, S.H.; Kim, V.N. MicroRNA genes are transcribed by RNA polymerase II. Embo j 2004, 23, 4051–4060. [Google Scholar] [CrossRef]
- Krol, J.; Loedige, I.; Filipowicz, W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet 2010, 11, 597–610. [Google Scholar] [CrossRef]
- Davis-Dusenbery, B.N.; Hata, A. Mechanisms of control of microRNA biogenesis. J Biochem 2010, 148, 381–392. [Google Scholar] [CrossRef]
- Lee, Y.; Jeon, K.; Lee, J.T.; Kim, S.; Kim, V.N. MicroRNA maturation: stepwise processing and subcellular localization. Embo j 2002, 21, 4663–4670. [Google Scholar] [CrossRef] [PubMed]
- Gregory, R.I.; Yan, K.P.; Amuthan, G.; Chendrimada, T.; Doratotaj, B.; Cooch, N.; Shiekhattar, R. The Microprocessor complex mediates the genesis of microRNAs. Nature 2004, 432, 235–240. [Google Scholar] [CrossRef]
- Lund, E.; Güttinger, S.; Calado, A.; Dahlberg, J.E.; Kutay, U. Nuclear export of microRNA precursors. Science 2004, 303, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Heo, I.; Tian, Y.; Simanshu, D.K.; Chang, H.; Jee, D.; Patel, D.J.; Kim, V.N. Dicer recognizes the 5’ end of RNA for efficient and accurate processing. Nature 2011, 475, 201–205. [Google Scholar] [CrossRef]
- Benoit, M.P.; Imbert, L.; Palencia, A.; Pérard, J.; Ebel, C.; Boisbouvier, J.; Plevin, M.J. The RNA-binding region of human TRBP interacts with microRNA precursors through two independent domains. Nucleic Acids Res 2013, 41, 4241–4252. [Google Scholar] [CrossRef]
- Mourelatos, Z.; Dostie, J.; Paushkin, S.; Sharma, A.; Charroux, B.; Abel, L.; Rappsilber, J.; Mann, M.; Dreyfuss, G. miRNPs: a novel class of ribonucleoproteins containing numerous microRNAs. Genes Dev 2002, 16, 720–728. [Google Scholar] [CrossRef] [PubMed]
- Kawamata, T.; Tomari, Y. Making RISC. Trends Biochem Sci 2010, 35, 368–376. [Google Scholar] [CrossRef]
- Ni, W.J.; Leng, X.M. miRNA-Dependent Activation of mRNA Translation. Microrna 2016, 5, 83–86. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Frontiers in Endocrinology 2018, 9. [Google Scholar] [CrossRef]
- Lee, S.; Vasudevan, S. Post-transcriptional stimulation of gene expression by microRNAs. Adv Exp Med Biol 2013, 768, 97–126. [Google Scholar] [CrossRef]
- Bang, C.; Batkai, S.; Dangwal, S.; Gupta, S.K.; Foinquinos, A.; Holzmann, A.; Just, A.; Remke, J.; Zimmer, K.; Zeug, A.; et al. Cardiac fibroblast-derived microRNA passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. J Clin Invest 2014, 124, 2136–2146. [Google Scholar] [CrossRef]
- Tian, C.; Hu, G.; Gao, L.; Hackfort, B.T.; Zucker, I.H. Extracellular vesicular MicroRNA-27a* contributes to cardiac hypertrophy in chronic heart failure. J Mol Cell Cardiol 2020, 143, 120–131. [Google Scholar] [CrossRef]
- Ruby, J.G.; Jan, C.H.; Bartel, D.P. Intronic microRNA precursors that bypass Drosha processing. Nature 2007, 448, 83–86. [Google Scholar] [CrossRef]
- Xie, M.; Li, M.; Vilborg, A.; Lee, N.; Shu, M.D.; Yartseva, V.; Šestan, N.; Steitz, J.A. Mammalian 5’-capped microRNA precursors that generate a single microRNA. Cell 2013, 155, 1568–1580. [Google Scholar] [CrossRef] [PubMed]
- Cheloufi, S.; Dos Santos, C.O.; Chong, M.M.; Hannon, G.J. A dicer-independent miRNA biogenesis pathway that requires Ago catalysis. Nature 2010, 465, 584–589. [Google Scholar] [CrossRef]
- Michel, L.Y.M. Extracellular Vesicles in Adipose Tissue Communication with the Healthy and Pathological Heart. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef]
- Yuan, Y.; Mei, Z.; Qu, Z.; Li, G.; Yu, S.; Liu, Y.; Liu, K.; Shen, Z.; Pu, J.; Wang, Y.; et al. Exosomes secreted from cardiomyocytes suppress the sensitivity of tumor ferroptosis in ischemic heart failure. Signal Transduct Target Ther 2023, 8, 121. [Google Scholar] [CrossRef]
- Akbar, N.; Braithwaite, A.T.; Corr, E.M.; Koelwyn, G.J.; van Solingen, C.; Cochain, C.; Saliba, A.E.; Corbin, A.; Pezzolla, D.; Møller Jørgensen, M.; et al. Rapid neutrophil mobilization by VCAM-1+ endothelial cell-derived extracellular vesicles. Cardiovasc Res 2023, 119, 236–251. [Google Scholar] [CrossRef]
- Tian, C.; Gao, L.; Rudebush, T.L.; Yu, L.; Zucker, I.H. Extracellular Vesicles Regulate Sympatho-Excitation by Nrf2 in Heart Failure. Circ Res 2022, 131, 687–700. [Google Scholar] [CrossRef]
- Rädler, J.; Gupta, D.; Zickler, A.; Andaloussi, S.E. Exploiting the biogenesis of extracellular vesicles for bioengineering and therapeutic cargo loading. Mol Ther 2023, 31, 1231–1250. [Google Scholar] [CrossRef]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367. [Google Scholar] [CrossRef]
- Nabhan, J.F.; Hu, R.; Oh, R.S.; Cohen, S.N.; Lu, Q. Formation and release of arrestin domain-containing protein 1-mediated microvesicles (ARMMs) at plasma membrane by recruitment of TSG101 protein. Proc Natl Acad Sci U S A 2012, 109, 4146–4151. [Google Scholar] [CrossRef]
- Clancy, J.W.; Schmidtmann, M.; D’Souza-Schorey, C. The ins and outs of microvesicles. FASEB Bioadv 2021, 3, 399–406. [Google Scholar] [CrossRef]
- Segawa, K.; Nagata, S. An Apoptotic ’Eat Me’ Signal: Phosphatidylserine Exposure. Trends Cell Biol 2015, 25, 639–650. [Google Scholar] [CrossRef]
- Ozkocak, D.C.; Phan, T.K.; Poon, I.K.H. Translating extracellular vesicle packaging into therapeutic applications. Front Immunol 2022, 13, 946422. [Google Scholar] [CrossRef]
- Atkin-Smith, G.K.; Tixeira, R.; Paone, S.; Mathivanan, S.; Collins, C.; Liem, M.; Goodall, K.J.; Ravichandran, K.S.; Hulett, M.D.; Poon, I.K. A novel mechanism of generating extracellular vesicles during apoptosis via a beads-on-a-string membrane structure. Nat Commun 2015, 6, 7439. [Google Scholar] [CrossRef]
- Holmgren, L.; Szeles, A.; Rajnavölgyi, E.; Folkman, J.; Klein, G.; Ernberg, I.; Falk, K.I. Horizontal transfer of DNA by the uptake of apoptotic bodies. Blood 1999, 93, 3956–3963. [Google Scholar] [CrossRef] [PubMed]
- Bergsmedh, A.; Szeles, A.; Henriksson, M.; Bratt, A.; Folkman, M.J.; Spetz, A.L.; Holmgren, L. Horizontal transfer of oncogenes by uptake of apoptotic bodies. Proc Natl Acad Sci U S A 2001, 98, 6407–6411. [Google Scholar] [CrossRef]
- Vickers, K.C.; Palmisano, B.T.; Shoucri, B.M.; Shamburek, R.D.; Remaley, A.T. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nature Cell Biology 2011, 13, 423–433. [Google Scholar] [CrossRef]
- Linhares-Lacerda, L.; Temerozo, J.R.; Ribeiro-Alves, M.; Azevedo, E.P.; Mojoli, A.; Nascimento, M.T.C.; Silva-Oliveira, G.; Savino, W.; Foguel, D.; Bou-Habib, D.C.; et al. Neutrophil extracellular trap-enriched supernatants carry microRNAs able to modulate TNF-α production by macrophages. Scientific Reports 2020, 10, 2715. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Ansel, K.M.; Bitzer, M.; Breakefield, X.O.; Charest, A.; Galas, D.J.; Gerstein, M.B.; Gupta, M.; Milosavljevic, A.; McManus, M.T.; et al. The Extracellular RNA Communication Consortium: Establishing Foundational Knowledge and Technologies for Extracellular RNA Research. Cell 2019, 177, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Tijsen, A.J.; Creemers, E.E.; Moerland, P.D.; de Windt, L.J.; van der Wal, A.C.; Kok, W.E.; Pinto, Y.M. MiR423-5p as a circulating biomarker for heart failure. Circ Res 2010, 106, 1035–1039. [Google Scholar] [CrossRef]
- Jansen, F.; Yang, X.; Proebsting, S.; Hoelscher, M.; Przybilla, D.; Baumann, K.; Schmitz, T.; Dolf, A.; Endl, E.; Franklin, B.S.; et al. MicroRNA expression in circulating microvesicles predicts cardiovascular events in patients with coronary artery disease. J Am Heart Assoc 2014, 3, e001249. [Google Scholar] [CrossRef]
- Chang, Y.J.; Wang, K.C. Therapeutic perspectives of extracellular vesicles and extracellular microRNAs in atherosclerosis. Curr Top Membr 2021, 87, 255–277. [Google Scholar] [CrossRef]
- Villarroya-Beltri, C.; Gutiérrez-Vázquez, C.; Sánchez-Cabo, F.; Pérez-Hernández, D.; Vázquez, J.; Martin-Cofreces, N.; Martinez-Herrera, D.J.; Pascual-Montano, A.; Mittelbrunn, M.; Sánchez-Madrid, F. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat Commun 2013, 4, 2980. [Google Scholar] [CrossRef] [PubMed]
- Zietzer, A.; Hosen, M.R.; Wang, H.; Goody, P.R.; Sylvester, M.; Latz, E.; Nickenig, G.; Werner, N.; Jansen, F. The RNA-binding protein hnRNPU regulates the sorting of microRNA-30c-5p into large extracellular vesicles. J Extracell Vesicles 2020, 9, 1786967. [Google Scholar] [CrossRef] [PubMed]
- Shurtleff, M.J.; Yao, J.; Qin, Y.; Nottingham, R.M.; Temoche-Diaz, M.M.; Schekman, R.; Lambowitz, A.M. Broad role for YBX1 in defining the small noncoding RNA composition of exosomes. Proc Natl Acad Sci U S A 2017, 114, E8987–e8995. [Google Scholar] [CrossRef]
- Shurtleff, M.J.; Temoche-Diaz, M.M.; Karfilis, K.V.; Ri, S.; Schekman, R. Y-box protein 1 is required to sort microRNAs into exosomes in cells and in a cell-free reaction. Elife 2016, 5. [Google Scholar] [CrossRef]
- Santangelo, L.; Giurato, G.; Cicchini, C.; Montaldo, C.; Mancone, C.; Tarallo, R.; Battistelli, C.; Alonzi, T.; Weisz, A.; Tripodi, M. The RNA-Binding Protein SYNCRIP Is a Component of the Hepatocyte Exosomal Machinery Controlling MicroRNA Sorting. Cell Rep 2016, 17, 799–808. [Google Scholar] [CrossRef]
- Hobor, F.; Dallmann, A.; Ball, N.J.; Cicchini, C.; Battistelli, C.; Ogrodowicz, R.W.; Christodoulou, E.; Martin, S.R.; Castello, A.; Tripodi, M.; et al. A cryptic RNA-binding domain mediates Syncrip recognition and exosomal partitioning of miRNA targets. Nat Commun 2018, 9, 831. [Google Scholar] [CrossRef]
- Statello, L.; Maugeri, M.; Garre, E.; Nawaz, M.; Wahlgren, J.; Papadimitriou, A.; Lundqvist, C.; Lindfors, L.; Collén, A.; Sunnerhagen, P.; et al. Identification of RNA-binding proteins in exosomes capable of interacting with different types of RNA: RBP-facilitated transport of RNAs into exosomes. PLoS One 2018, 13, e0195969. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, K.; Ghoshal, B.; Ghosh, S.; Chakrabarty, Y.; Shwetha, S.; Das, S.; Bhattacharyya, S.N. Reversible HuR-microRNA binding controls extracellular export of miR-122 and augments stress response. EMBO Rep 2016, 17, 1184–1203. [Google Scholar] [CrossRef]
- Teng, Y.; Ren, Y.; Hu, X.; Mu, J.; Samykutty, A.; Zhuang, X.; Deng, Z.; Kumar, A.; Zhang, L.; Merchant, M.L.; et al. MVP-mediated exosomal sorting of miR-193a promotes colon cancer progression. Nat Commun 2017, 8, 14448. [Google Scholar] [CrossRef]
- Xu, Y.F.; Xu, X.; Gin, A.; Nshimiyimana, J.D.; Mooers, B.H.M.; Caputi, M.; Hannafon, B.N.; Ding, W.Q. SRSF1 regulates exosome microRNA enrichment in human cancer cells. Cell Commun Signal 2020, 18, 130. [Google Scholar] [CrossRef] [PubMed]
- Temoche-Diaz, M.M.; Shurtleff, M.J.; Nottingham, R.M.; Yao, J.; Fadadu, R.P.; Lambowitz, A.M.; Schekman, R. Distinct mechanisms of microRNA sorting into cancer cell-derived extracellular vesicle subtypes. Elife 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.M.; Ma, L.; Schekman, R. Selective sorting of microRNAs into exosomes by phase-separated YBX1 condensates. Elife 2021, 10. [Google Scholar] [CrossRef]
- Lee, H.; Li, C.; Zhang, Y.; Zhang, D.; Otterbein, L.E.; Jin, Y. Caveolin-1 selectively regulates microRNA sorting into microvesicles after noxious stimuli. J Exp Med 2019, 216, 2202–2220. [Google Scholar] [CrossRef]
- Squadrito, M.L.; Baer, C.; Burdet, F.; Maderna, C.; Gilfillan, G.D.; Lyle, R.; Ibberson, M.; De Palma, M. Endogenous RNAs modulate microRNA sorting to exosomes and transfer to acceptor cells. Cell Rep 2014, 8, 1432–1446. [Google Scholar] [CrossRef]
- Iavello, A.; Frech, V.S.; Gai, C.; Deregibus, M.C.; Quesenberry, P.J.; Camussi, G. Role of Alix in miRNA packaging during extracellular vesicle biogenesis. Int J Mol Med 2016, 37, 958–966. [Google Scholar] [CrossRef]
- Liu, Y.; Song, J.W.; Lin, J.Y.; Miao, R.; Zhong, J.C. Roles of MicroRNA-122 in Cardiovascular Fibrosis and Related Diseases. Cardiovasc Toxicol 2020, 20, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Hosen, M.R.; Goody, P.R.; Zietzer, A.; Xiang, X.; Niepmann, S.T.; Sedaghat, A.; Tiyerili, V.; Chennupati, R.; Moore, J.B.t.; Boon, R.A.; et al. Circulating MicroRNA-122-5p Is Associated With a Lack of Improvement in Left Ventricular Function After Transcatheter Aortic Valve Replacement and Regulates Viability of Cardiomyocytes Through Extracellular Vesicles. Circulation 2022, 146, 1836–1854. [Google Scholar] [CrossRef]
- Liu, X.; Xu, Y.; Deng, Y.; Li, H. MicroRNA-223 Regulates Cardiac Fibrosis After Myocardial Infarction by Targeting RASA1. Cell Physiol Biochem 2018, 46, 1439–1454. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Long, B.; Liu, F.; Wang, J.X.; Liu, C.Y.; Zhao, B.; Zhou, L.Y.; Sun, T.; Wang, M.; Yu, T.; et al. A circular RNA protects the heart from pathological hypertrophy and heart failure by targeting miR-223. Eur Heart J 2016, 37, 2602–2611. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, Y.; Li, M.; Yuan, J.; Yu, Y.; Bi, X.; Hong, H.; Ye, J.; Liu, P. MicroRNA-34c-5p provokes isoprenaline-induced cardiac hypertrophy by modulating autophagy via targeting ATG4B. Acta Pharm Sin B 2022, 12, 2374–2390. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, Q.; Feng, Y.; Chen, X.; Yang, L.; Xu, M.; Wang, X.; Li, W.; Niu, X.; Gao, D. MicroRNA-26a Protects the Heart Against Hypertension-Induced Myocardial Fibrosis. J Am Heart Assoc 2020, 9, e017970. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Guo, M.; Ma, G.; Zhang, N.; Pan, F.; Fan, X.; Wang, R. MicroRNA-30c-5p protects against myocardial ischemia/reperfusion injury via regulation of Bach1/Nrf2. Toxicol Appl Pharmacol 2021, 426, 115637. [Google Scholar] [CrossRef]
- Danielson, L.S.; Park, D.S.; Rotllan, N.; Chamorro-Jorganes, A.; Guijarro, M.V.; Fernandez-Hernando, C.; Fishman, G.I.; Phoon, C.K.; Hernando, E. Cardiovascular dysregulation of miR-17-92 causes a lethal hypertrophic cardiomyopathy and arrhythmogenesis. Faseb j 2013, 27, 1460–1467. [Google Scholar] [CrossRef]
- Nair, N.; Kumar, S.; Gongora, E.; Gupta, S. Circulating miRNA as novel markers for diastolic dysfunction. Mol Cell Biochem 2013, 376, 33–40. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, L.; Li, Z.; Fu, G. miR-1231 exacerbates arrhythmia by targeting calciumchannel gene CACNA2D2 in myocardial infarction. Am J Transl Res 2017, 9, 1822–1833. [Google Scholar]
- Tucker, N.R.; Chaffin, M.; Fleming, S.J.; Hall, A.W.; Parsons, V.A.; Bedi, K.C., Jr.; Akkad, A.D.; Herndon, C.N.; Arduini, A.; Papangeli, I.; et al. Transcriptional and Cellular Diversity of the Human Heart. Circulation 2020, 142, 466–482. [Google Scholar] [CrossRef] [PubMed]
- Litviňuková, M.; Talavera-López, C.; Maatz, H.; Reichart, D.; Worth, C.L.; Lindberg, E.L.; Kanda, M.; Polanski, K.; Heinig, M.; Lee, M.; et al. Cells of the adult human heart. Nature 2020, 588, 466–472. [Google Scholar] [CrossRef]
- Howard, C.M.; Baudino, T.A. Dynamic cell-cell and cell-ECM interactions in the heart. J Mol Cell Cardiol 2014, 70, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.P.; Zhang, Y.S.; Xu, X.; Zhou, Q.; Li, J.D.; Yan, C. Vinpocetine Attenuates Pathological Cardiac Remodeling by Inhibiting Cardiac Hypertrophy and Fibrosis. Cardiovasc Drugs Ther 2017, 31, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Travers, J.G.; Kamal, F.A.; Robbins, J.; Yutzey, K.E.; Blaxall, B.C. Cardiac Fibrosis: The Fibroblast Awakens. Circ Res 2016, 118, 1021–1040. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.G.; Lee, P.; Gordon, R.E.; Sahoo, S.; Kho, C.; Jeong, D. Analysis of extracellular vesicle miRNA profiles in heart failure. J Cell Mol Med 2020, 24, 7214–7227. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Wang, C.; Mang, G.; Xu, X.; Fu, S.; Chen, J.; Wang, X.; Wang, W.; Li, H.; Zhao, P.; et al. Extracellular vesicle-packaged mitochondrial disturbing miRNA exacerbates cardiac injury during acute myocardial infarction. Clin Transl Med 2022, 12, e779. [Google Scholar] [CrossRef]
- Das, S.; Halushka, M.K. Extracellular vesicle microRNA transfer in cardiovascular disease. Cardiovasc Pathol 2015, 24, 199–206. [Google Scholar] [CrossRef]
- Tian, C.; Gao, L.; Zimmerman, M.C.; Zucker, I.H. Myocardial infarction-induced microRNA-enriched exosomes contribute to cardiac Nrf2 dysregulation in chronic heart failure. Am J Physiol Heart Circ Physiol 2018, 314, H928–h939. [Google Scholar] [CrossRef]
- Fang, X.; Stroud, M.J.; Ouyang, K.; Fang, L.; Zhang, J.; Dalton, N.D.; Gu, Y.; Wu, T.; Peterson, K.L.; Huang, H.D.; et al. Adipocyte-specific loss of PPARγ attenuates cardiac hypertrophy. JCI Insight 2016, 1, e89908. [Google Scholar] [CrossRef]
- Callis, T.E.; Pandya, K.; Seok, H.Y.; Tang, R.H.; Tatsuguchi, M.; Huang, Z.P.; Chen, J.F.; Deng, Z.; Gunn, B.; Shumate, J.; et al. MicroRNA-208a is a regulator of cardiac hypertrophy and conduction in mice. J Clin Invest 2009, 119, 2772–2786. [Google Scholar] [CrossRef] [PubMed]
- Chistiakov, D.A.; Orekhov, A.N.; Bobryshev, Y.V. Cardiac-specific miRNA in cardiogenesis, heart function, and cardiac pathology (with focus on myocardial infarction). J Mol Cell Cardiol 2016, 94, 107–121. [Google Scholar] [CrossRef]
- Nie, X.; Fan, J.; Li, H.; Yin, Z.; Zhao, Y.; Dai, B.; Dong, N.; Chen, C.; Wang, D.W. miR-217 Promotes Cardiac Hypertrophy and Dysfunction by Targeting PTEN. Mol Ther Nucleic Acids 2018, 12, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yu, X.; Xue, F.; Li, Y.; Liu, W.; Zhang, S. Exosomes derived from cardiomyocytes promote cardiac fibrosis via myocyte-fibroblast cross-talk. Am J Transl Res 2018, 10, 4350–4366. [Google Scholar] [PubMed]
- Tang, C.; Hou, Y.X.; Shi, P.X.; Zhu, C.H.; Lu, X.; Wang, X.L.; Que, L.L.; Zhu, G.Q.; Liu, L.; Chen, Q.; et al. Cardiomyocyte-specific Peli1 contributes to the pressure overload-induced cardiac fibrosis through miR-494-3p-dependent exosomal communication. Faseb j 2023, 37, e22699. [Google Scholar] [CrossRef]
- Fu, X.; Mishra, R.; Chen, L.; Arfat, M.Y.; Sharma, S.; Kingsbury, T.; Gunasekaran, M.; Saha, P.; Hong, C.; Yang, P.; et al. Exosomes mediated fibrogenesis in dilated cardiomyopathy through a MicroRNA pathway. iScience 2023, 26, 105963. [Google Scholar] [CrossRef]
- Su, M.; Li, W.; Yuan, Y.U.E.; Liu, S.; Liang, C.; Liu, H.E.; Zhang, R.; Liu, Y.; Sun, L.I.; Wei, Y.; et al. Epididymal white adipose tissue promotes angiotensin II-induced cardiac fibrosis in an exosome-dependent manner. Translational Research 2022, 248, 51–67. [Google Scholar] [CrossRef]
- Cai, L.; Chao, G.; Li, W.; Zhu, J.; Li, F.; Qi, B.; Wei, Y.; Chen, S.; Zhou, G.; Lu, X.; et al. Activated CD4(+) T cells-derived exosomal miR-142-3p boosts post-ischemic ventricular remodeling by activating myofibroblast. Aging (Albany NY) 2020, 12, 7380–7396. [Google Scholar] [CrossRef]
- Hinkel, R.; Ramanujam, D.; Kaczmarek, V.; Howe, A.; Klett, K.; Beck, C.; Dueck, A.; Thum, T.; Laugwitz, K.L.; Maegdefessel, L.; et al. AntimiR-21 Prevents Myocardial Dysfunction in a Pig Model of Ischemia/Reperfusion Injury. J Am Coll Cardiol 2020, 75, 1788–1800. [Google Scholar] [CrossRef]
- Ramanujam, D.; Sassi, Y.; Laggerbauer, B.; Engelhardt, S. Viral Vector-Based Targeting of miR-21 in Cardiac Nonmyocyte Cells Reduces Pathologic Remodeling of the Heart. Mol Ther 2016, 24, 1939–1948. [Google Scholar] [CrossRef]
- Thum, T.; Chau, N.; Bhat, B.; Gupta, S.K.; Linsley, P.S.; Bauersachs, J.; Engelhardt, S. Comparison of different miR-21 inhibitor chemistries in a cardiac disease model. J Clin Invest 2011, 121, 461–462. [Google Scholar] [CrossRef]
- Thum, T.; Gross, C.; Fiedler, J.; Fischer, T.; Kissler, S.; Bussen, M.; Galuppo, P.; Just, S.; Rottbauer, W.; Frantz, S.; et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature 2008, 456, 980–984. [Google Scholar] [CrossRef] [PubMed]
- Ramanujam, D.; Schön, A.P.; Beck, C.; Vaccarello, P.; Felician, G.; Dueck, A.; Esfandyari, D.; Meister, G.; Meitinger, T.; Schulz, C.; et al. MicroRNA-21-Dependent Macrophage-to-Fibroblast Signaling Determines the Cardiac Response to Pressure Overload. Circulation 2021, 143, 1513–1525. [Google Scholar] [CrossRef] [PubMed]
- Ottaviani, L.; Juni, R.P.; de Abreu, R.C.; Sansonetti, M.; Sampaio-Pinto, V.; Halkein, J.; Hegenbarth, J.C.; Ring, N.; Knoops, K.; Kocken, J.M.M.; et al. Intercellular transfer of miR-200c-3p impairs the angiogenic capacity of cardiac endothelial cells. Mol Ther 2022, 30, 2257–2273. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Qiu, Z.; Li, C.; Zhao, R.; Zhang, Y.; Shen, C.; Liu, W.; Long, X.; Zhuang, S.; Wang, Y.; et al. Exosomal MiR-29a in Cardiomyocytes Induced by Angiotensin II Regulates Cardiac Microvascular Endothelial Cell Proliferation, Migration and Angiogenesis by Targeting VEGFA. Current Gene Therapy 2022, 22, 331–341. [Google Scholar] [CrossRef]
- Ranjan, P.; Kumari, R.; Goswami, S.K.; Li, J.; Pal, H.; Suleiman, Z.; Cheng, Z.; Krishnamurthy, P.; Kishore, R.; Verma, S.K. Myofibroblast-Derived Exosome Induce Cardiac Endothelial Cell Dysfunction. Front Cardiovasc Med 2021, 8, 676267. [Google Scholar] [CrossRef]
- Sun, L.L.; Duan, M.J.; Ma, J.C.; Xu, L.; Mao, M.; Biddyut, D.; Wang, Q.; Yang, C.; Zhang, S.; Xu, Y.; et al. Myocardial infarction-induced hippocampal microtubule damage by cardiac originating microRNA-1 in mice. J Mol Cell Cardiol 2018, 120, 12–27. [Google Scholar] [CrossRef]
- Akbar, N.; Digby, J.E.; Cahill, T.J.; Tavare, A.N.; Corbin, A.L.; Saluja, S.; Dawkins, S.; Edgar, L.; Rawlings, N.; Ziberna, K.; et al. Endothelium-derived extracellular vesicles promote splenic monocyte mobilization in myocardial infarction. JCI Insight 2017, 2. [Google Scholar] [CrossRef]
- Panda, R.; Kubes, P. Extracellular vesicles selectively mobilize splenic neutrophils. Cardiovasc Res 2023, 119, 1–2. [Google Scholar] [CrossRef]
- Lyu, L.; Wang, H.; Li, B.; Qin, Q.; Qi, L.; Nagarkatti, M.; Nagarkatti, P.; Janicki, J.S.; Wang, X.L.; Cui, T. A critical role of cardiac fibroblast-derived exosomes in activating renin angiotensin system in cardiomyocytes. J Mol Cell Cardiol 2015, 89, 268–279. [Google Scholar] [CrossRef]
- Savvatis, K.; Pappritz, K.; Becher, P.M.; Lindner, D.; Zietsch, C.; Volk, H.D.; Westermann, D.; Schultheiss, H.P.; Tschöpe, C. Interleukin-23 deficiency leads to impaired wound healing and adverse prognosis after myocardial infarction. Circ Heart Fail 2014, 7, 161–171. [Google Scholar] [CrossRef]
- Kuwabara, Y.; Ono, K.; Horie, T.; Nishi, H.; Nagao, K.; Kinoshita, M.; Watanabe, S.; Baba, O.; Kojima, Y.; Shizuta, S.; et al. Increased microRNA-1 and microRNA-133a levels in serum of patients with cardiovascular disease indicate myocardial damage. Circ Cardiovasc Genet 2011, 4, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Carbone, F.; Nencioni, A.; Mach, F.; Vuilleumier, N.; Montecucco, F. Pathophysiological role of neutrophils in acute myocardial infarction. Thromb Haemost 2013, 110, 501–514. [Google Scholar] [CrossRef]
- Helseth, R.; Shetelig, C.; Andersen, G.; Langseth, M.S.; Limalanathan, S.; Opstad, T.B.; Arnesen, H.; Hoffmann, P.; Eritsland, J.; Seljeflot, I. Neutrophil Extracellular Trap Components Associate with Infarct Size, Ventricular Function, and Clinical Outcome in STEMI. Mediators Inflamm 2019, 2019, 7816491. [Google Scholar] [CrossRef] [PubMed]
- Arruda-Olson, A.M.; Reeder, G.S.; Bell, M.R.; Weston, S.A.; Roger, V.L. Neutrophilia predicts death and heart failure after myocardial infarction: a community-based study. Circ Cardiovasc Qual Outcomes 2009, 2, 656–662. [Google Scholar] [CrossRef]
- Dogan, I.; Karaman, K.; Sonmez, B.; Celik, S.; Turker, O. Relationship between serum neutrophil count and infarct size in patients with acute myocardial infarction. Nucl Med Commun 2009, 30, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Magnussen, C.; Blankenberg, S. Biomarkers for heart failure: small molecules with high clinical relevance. J Intern Med 2018, 283, 530–543. [Google Scholar] [CrossRef] [PubMed]
- Siranart, N.; Laohasurayotin, K.; Phanthong, T.; Sowalertrat, W.; Ariyachaipanich, A.; Chokesuwattanaskul, R. Proenkephalin as a Novel Prognostic Marker in Heart Failure Patients: A Systematic Review and Meta-Analysis. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Creemers, E.E.; Tijsen, A.J.; Pinto, Y.M. Circulating microRNAs: novel biomarkers and extracellular communicators in cardiovascular disease? Circ Res 2012, 110, 483–495. [Google Scholar] [CrossRef]
- Zhou, R.; Wang, L.; Zhao, G.; Chen, D.; Song, X.; Momtazi-Borojeni, A.A.; Yuan, H. Circulating exosomal microRNAs as emerging non-invasive clinical biomarkers in heart failure: Mega bio-roles of a nano bio-particle. IUBMB Life 2020, 72, 2546–2562. [Google Scholar] [CrossRef]
- Huang, J.P.; Chang, C.C.; Kuo, C.Y.; Huang, K.J.; Sokal, E.M.; Chen, K.H.; Hung, L.M. Exosomal microRNAs miR-30d-5p and miR-126a-5p Are Associated with Heart Failure with Preserved Ejection Fraction in STZ-Induced Type 1 Diabetic Rats. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef] [PubMed]
- Melman, Y.F.; Shah, R.; Danielson, K.; Xiao, J.; Simonson, B.; Barth, A.; Chakir, K.; Lewis, G.D.; Lavender, Z.; Truong, Q.A.; et al. Circulating MicroRNA-30d Is Associated With Response to Cardiac Resynchronization Therapy in Heart Failure and Regulates Cardiomyocyte Apoptosis: A Translational Pilot Study. Circulation 2015, 131, 2202–2216. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Salvador, A.M.; Li, G.; Valkov, N.; Ziegler, O.; Yeri, A.; Yang Xiao, C.; Meechoovet, B.; Alsop, E.; Rodosthenous, R.S.; et al. Mir-30d Regulates Cardiac Remodeling by Intracellular and Paracrine Signaling. Circ Res 2021, 128, e1–e23. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.P.; Tian, T.; Wang, J.Y.; He, J.N.; Chen, T.; Pan, M.; Xu, L.; Zhang, H.X.; Qiu, X.T.; Li, C.C.; et al. Hypoxia-elicited mesenchymal stem cell-derived exosomes facilitates cardiac repair through miR-125b-mediated prevention of cell death in myocardial infarction. Theranostics 2018, 8, 6163–6177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wei, Q.; Liu, X.; Zhang, T.; Wang, S.; Zhou, L.; Zou, L.; Fan, F.; Chi, H.; Sun, J.; et al. Exosomal microRNA-98-5p from hypoxic bone marrow mesenchymal stem cells inhibits myocardial ischemia-reperfusion injury by reducing TLR4 and activating the PI3K/Akt signaling pathway. Int Immunopharmacol 2021, 101, 107592. [Google Scholar] [CrossRef]
- Yuan, J.; Yang, H.; Liu, C.; Shao, L.; Zhang, H.; Lu, K.; Wang, J.; Wang, Y.; Yu, Q.; Zhang, Y.; et al. Microneedle Patch Loaded with Exosomes Containing MicroRNA-29b Prevents Cardiac Fibrosis after Myocardial Infarction. Adv Healthc Mater 2023, e2202959. [Google Scholar] [CrossRef]
- Pu, L.; Kong, X.; Li, H.; He, X. Exosomes released from mesenchymal stem cells overexpressing microRNA-30e ameliorate heart failure in rats with myocardial infarction. Am J Transl Res 2021, 13, 4007–4025. [Google Scholar]
- Yan, F.; Cui, W.; Chen, Z. Mesenchymal Stem Cell-Derived Exosome-Loaded microRNA-129-5p Inhibits TRAF3 Expression to Alleviate Apoptosis and Oxidative Stress in Heart Failure. Cardiovasc Toxicol 2022, 22, 631–645. [Google Scholar] [CrossRef]
- Shafei, S.; Khanmohammadi, M.; Ghanbari, H.; Nooshabadi, V.T.; Tafti, S.H.A.; Rabbani, S.; Kasaiyan, M.; Basiri, M.; Tavoosidana, G. Effectiveness of exosome mediated miR-126 and miR-146a delivery on cardiac tissue regeneration. Cell Tissue Res 2022, 390, 71–92. [Google Scholar] [CrossRef]
- Gao, L.; Qiu, F.; Cao, H.; Li, H.; Dai, G.; Ma, T.; Gong, Y.; Luo, W.; Zhu, D.; Qiu, Z.; et al. Therapeutic delivery of microRNA-125a-5p oligonucleotides improves recovery from myocardial ischemia/reperfusion injury in mice and swine. Theranostics 2023, 13, 685–703. [Google Scholar] [CrossRef]
- Wang, T.; Li, T.; Niu, X.; Hu, L.; Cheng, J.; Guo, D.; Ren, H.; Zhao, R.; Ji, Z.; Liu, P.; et al. ADSC-derived exosomes attenuate myocardial infarction injury by promoting miR-205-mediated cardiac angiogenesis. Biol Direct 2023, 18, 6. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhang, C.; Zhang, J.; Jiao, Z.; Dong, N.; Wang, G.; Wang, Z.; Wang, L. Localized injection of miRNA-21-enriched extracellular vesicles effectively restores cardiac function after myocardial infarction. Theranostics 2019, 9, 2346–2360. [Google Scholar] [CrossRef] [PubMed]
- Vandergriff, A.; Huang, K.; Shen, D.; Hu, S.; Hensley, M.T.; Caranasos, T.G.; Qian, L.; Cheng, K. Targeting regenerative exosomes to myocardial infarction using cardiac homing peptide. Theranostics 2018, 8, 1869–1878. [Google Scholar] [CrossRef] [PubMed]
- Won, Y.W.; McGinn, A.N.; Lee, M.; Bull, D.A.; Kim, S.W. Targeted gene delivery to ischemic myocardium by homing peptide-guided polymeric carrier. Mol Pharm 2013, 10, 378–385. [Google Scholar] [CrossRef]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol 2011, 29, 341–345. [Google Scholar] [CrossRef]
- Moholkar, D.N.; Kandimalla, R.; Gupta, R.C.; Aqil, F. Advances in lipid-based carriers for cancer therapeutics: Liposomes, exosomes and hybrid exosomes. Cancer Lett 2023, 565, 216220. [Google Scholar] [CrossRef]
| miRNAs | Sorting mechanism | Functions in HF | Ref. |
|---|---|---|---|
| miR-122 | The binding of Lupus La protein, hnRNPU and/or HuR to miR122 controls extracellular export | Promote apoptosis, inflammation, fibrosis, pathological hypertrophy and remodeling | [73,76,81,82] |
| miR-223 | Selective sorting of miR-223 into EXOs by phase-separated YBX1 condensates | Promote cardiac fibrosis and hypertrophy | [69,77,83,84] |
| miR-34c-5p | The binding of Alyref and/or Fus to the CGGGAG motif at the 3’ end of miR-34c | Cardiac hypertrophy | [19,85] |
| miR-26a | The binding of Alyref and/or Fus to the CGGGAG motif at the 3’ end of miR-34c; alternatively, 3’-end uridylation of miR-26a | Protects the Heart Against Hypertension-Induced Myocardial Fibrosis | [19,20,86] |
| miR-30c-5p | The binding of hnRNPU to the AAMRUGCU motif of miR-30c-5p | Protects against myocardial ischemia/reperfusion injury | [67,87] |
| miR-17/92 | The binding of cav-1/hnRNPA2B1 complex to miR-17/92 regulates its MV sorting | Hypertrophic and arrhythmogenic cardiomyopathy | [78,88] |
| miR-1246 | The binding of SRSF1 to miR-1246 regulates its exosomal enrichment | Upregulated in diastolic dysfunction | [75,89] |
| miR-1231 | The binding hnRNPA2B1 to the GGAG EXOmotif at the 3’ end of miR-1231 | Induction of arrhythmias in ischemic hearts | [70,90] |
| Pathological phenotype | miRNA | Cell source | Target cell | Potential functional mechanism | Ref. |
|---|---|---|---|---|---|
| Cardiac hypertrophy | miRNA-21-3p | CF | CM | Translational inhibition of both SORBS2 and PDLIM5 | [41] |
| miRNA-27a-5p | CF | CM | Translational inhibition of PDLIM5 | [42] | |
| miRNA-27a-3p, miRNA-28-3p miRNA-34a | CF | CM | Dysregulation of Nrf2/ARE signaling and oxidative stress | [99] | |
| miR-200a | Adipocyte | CM | Selective activation of PPARγ signaling, and decreased TSC1 and subsequent mTOR activation | [100] | |
| miRNA-208a | CM | CM | Repression of Thrap1 and myostatin expression | [101,102] | |
| miRNA-217 | CF | CM | Targeting PTEN | [103] | |
| Cardiac fibrosis | miRNA-208a | CM | CF | Targeting Dyrk2 to promote NFAT dephosphorylation and nuclear translocation | [104] |
| miRNA-217 | CM | CF | Targeting PTEN | [103] | |
| miRNA-494-3p | CM | CF | Targeting PETN to enhance the phosphorylation of AKT, ERK and SMAD2/3 | [105] | |
| miRNA-218-5p | CM | CF | Targeting TNFAIP3 to activate TGF-β signaling | [106] | |
| miRNA-23a-3p | Adipocyte | CF | Targeting RAP1 | [107] | |
| miR-142-3p | Activated CD4+ T cell | CF | Targeting APC to activate the WNT signaling pathway | [108] | |
| miRNA-21 | MP and/or CM | CF | Targeting Spry1 to augment ERK-MAP kinase activity | [109-113] | |
| Angiogenesis | miRNA-200c-3p | CM | EC | Impaired endothelial migration and tube formation as well as a lower proliferation capacity. | [114] |
| miRNA-29a | CM | EC | Inhibiting the proliferation, migration and angiogenic ability of cardiac microvascular ECs | [115] | |
| miRNA-200a-3p | Activated CF | EC | Targeting ETS1/VEGF-A signaling axis | [116] | |
| Inter-organ communications | miRNA-1 | CM | Neuron | Targeting TPPP/p25 to disturb the stability of neuronal microtubules | [102,117] |
| miRNA-27a-3p, miRNA-28-3p and miRNA-34a | CM and/or CF | Neuron | Targeting Nrf2/ARE signaling to induce oxidative stress and subsequently elicit sympathetic excitation | [49] | |
| miRNA-126 | EC | NEUT | Transcriptional activation of NEUTs, and contribution to cardiac inflammation and chemokine production | [48,118,119] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





