1. Introduction
In recent years, the over-exploitation of the seafood populations to meet global market demands resulted in detrimental environmental impacts, including biodiversity depletion, water eutrophication, coral reduction, and pathogen invasions, among others [
1]. Furthermore, growing concerns about population health have driven the development of plant-based meat alternatives [
2]. In recent years, the market for plant-based foods experienced expressive growth, such as in Brazil, where vegetable substitutes for meat and seafood witnessed a 30% increase in sales, reaching US
$ 107 million in 2021 compared to the previous year [
3]. In response to this trend, new processing technologies, including spherification and 3D food printing, have been adopted to produce innovative plant-based food.
Spherification, a technique widely used in molecular gastronomy, enables the creation of products with high added value [
4]. It involves the formation of edible spherical elements by extruding liquid through dripping. In this method, a highly viscous polymeric liquid (e.g.: alginate, agar, among others) is extruded through a nozzle, forming droplets when applying an external or gravitational force and submerging into a bath solution [
4]. Concurrently, more complex 3D printing techniques, primarily extrusion-based, are rapidly advancing. This process involves extruding a solution (food ink) with the desired characteristics (e.g.: nutritional composition, sensory attributes, among others) layer by layer from a pre-designed digital model to form a 3D structure [
5].
Despite the utilization of innovative processing technologies, developing fish and seafood analogs with nutritional profiles similar to animal-based counterparts remains challenging, primarily due to the limited nutritional value of vegetable proteins. Consequently, pulses emerged as intriguing sources for producing alternative meats, given their notable nutritional characteristics such as s protein content (~24%) and carbohydrate content (~63%) [
6]. Pulses are also rich in essential amino acids including lysine, cysteine, and methionine [
6]. Additionally, literature reports highlight the presence of phenolic compounds in pulses, such as phenolic acids, flavonoids, isoflavones, and tannins [
6,
7]. The antioxidant capacity of phenolic compounds, attributed to their reducing properties and chemical structure (benzene ring or cyclic structure with one or more hydroxyl substituents that interact with free radicals), contributes to their role in reducing the risk of chronic diseases, including coronary heart disease, diabetes, and cancer [
7].
Extensive research has been conducted on phenolic compounds and their health benefits over the years [
8,
9]. Polyphenols are plant secondary metabolites commonly found in various fruits, vegetables, and plant-based foods. The development of analytical methods for their separation and characterization is of relevant interest due to their bioactive properties, health benefits, and potential use as natural antioxidants in the food, pharmaceutical, and cosmetic industries [
8]. Spectrophotometry (UV-Vis), chromatography, and mass spectrometry are commonly employed analytical tools for polyphenolic analyses [
10,
11,
12]. Spectrometric techniques, in particular, offer advantages such as high sensitivity, selectivity, speed, and the ability to identify and quantify multiple molecules simultaneously. These techniques are widely used to detect and quantify different classes of phenolics by utilizing compounds that react with antioxidants, leading to color changes. They are also valuable for evaluating the antioxidant capacity of matrices [
13]. Electroanalytical techniques, including voltammetric methods, present an alternative approach for detecting and quantifying total phenolics or specific phenolic classes. They offer advantages over conventional methods, such as high sensitivity with low limits of detection and quantification, reduced solvent consumption, lower production of chemical residues, cost-effectiveness, portability, and the ability to assess antioxidant capacity [
14,
15,
16].
Given the established health benefits associated with plant-based antioxidants and the growing consumption of pulse-based meat analogs, it is crucial to enhance the quality control of these foods by providing precise and comprehensive information about their antioxidant content. This research aims to propose a methodology based on differential pulse voltammetry and a glassy carbon electrode for determining the total phenolic compounds in fish (salmon) and seafood (caviar) analogs and evaluating the antioxidant capacity of these food products. Furthermore, this study seeks to contribute to the ongoing discussion on the use of electroanalytical techniques for quality control of plant-based foods by analyzing additional functional compounds with antioxidant characteristics.
2. Materials and Methods
2.1. Materials
Analytical-reagent grade chemicals and ultrapure water (Millipore, United States) were used to prepare solutions. Sodium alginate (ALG) was purchased from CRQ Química (Brazil), calcium chloride (CaCl2) was purchased from Dinâmica Contemporânea LTDA (Brazil), soy oil was purchased from Bunge (Brazil), white bean flour and isolated soy protein were purchased from Zona Cerealista (Brazil), annatto and saffron dyes were purchased from a local supermarket. Genistein (>99% purity) was purchased from L. C. Laboratories (Canada). Phosphate buffers with a pH of 3.0-7.0 were prepared using dibasic sodium phosphate, monobasic potassium phosphate, and phosphoric acid (Sigma-Aldrich, United States) and used as supporting electrolytes. Ethanol and methanol (Sigma-Aldrich, Germany) was used for phenolics compound extraction in caviar and salmon analogs. Alumina oxide powder < 10 micrometers (Sigma-Aldrich, United States), ethanol (Sigma-Aldrich, Netherlands), ultrapure water, and nitric acid (Quimex, Brazil) were used for glassy carbon electrode cleaning.
2.2. Preparation of caviar food ink
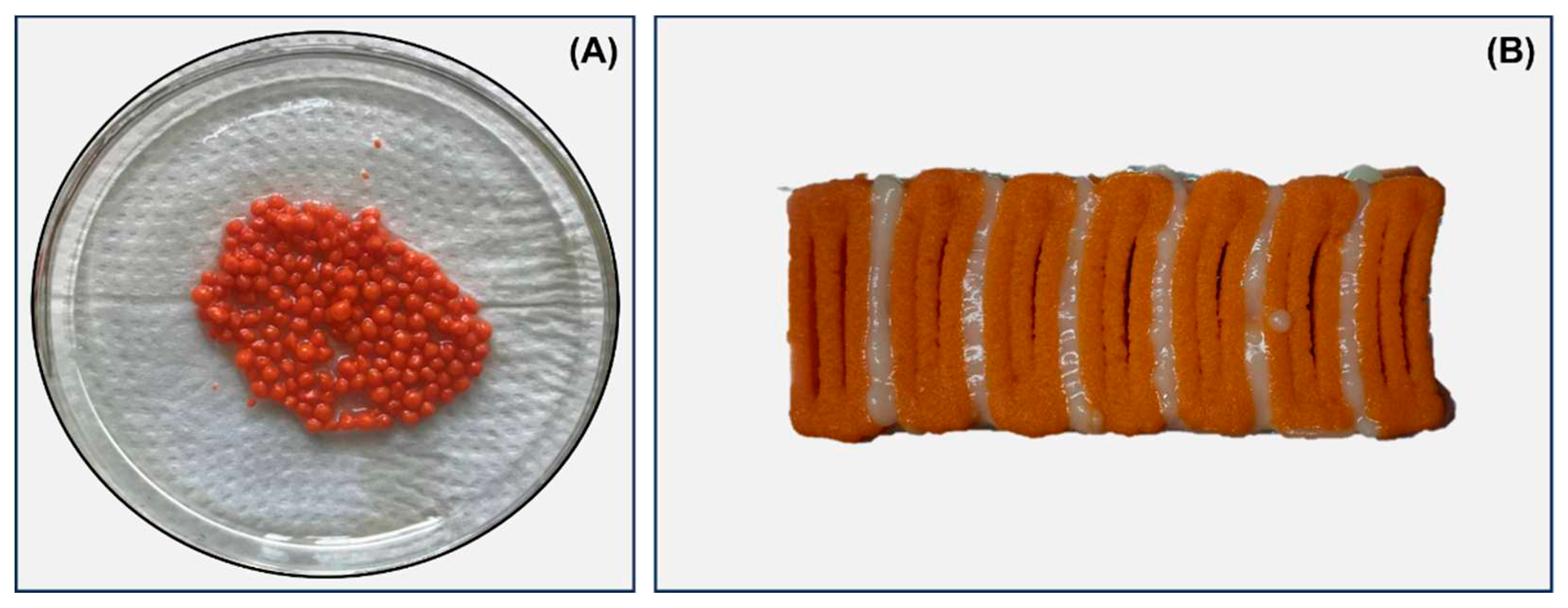
Caviar analog (
Figure 1A) was produced by the ionotropic gelation method using Ca
2+ íons. ALG (1.25% w/v) was dissolved by stirring in filtered water for 30 min at room temperature. Simultaneously, an aqueous solution containing 22.5% w/v white bean flour and an oil solution composed of 45% isolated soy protein and annatto dye were produced. The dissolved substances were added to the alginate solution and then stirred for 10 min.
2.3. Preparation of salmon analog food inks
Two food inks (white and orange) were formulated to produce the salmon analog (
Figure 1B). For the development of the orange ink, isolated soy protein (1.92 g) was solubilized for 10 min at room temperature in a nanoemulsion containing natural annatto and saffron dyes (solution 1). Then, white bean pulse flour (1.92 g) was solubilized in water following the same process and added to solution 1, remaining under stirring for 10 min. To develop white food ink, vegetable oil, and water were used to solubilize the isolated soy protein (0.96 g), and white bean pulse (0.96 g), respectively, following the same methodology described above. Then, the solutions were mixed and stirred for 10 min. Finally, the food inks were added to 5 mL Luer-lock syringes for the 3D printing process.
2.4. Digital manufacturing: Spherification and 3D printing
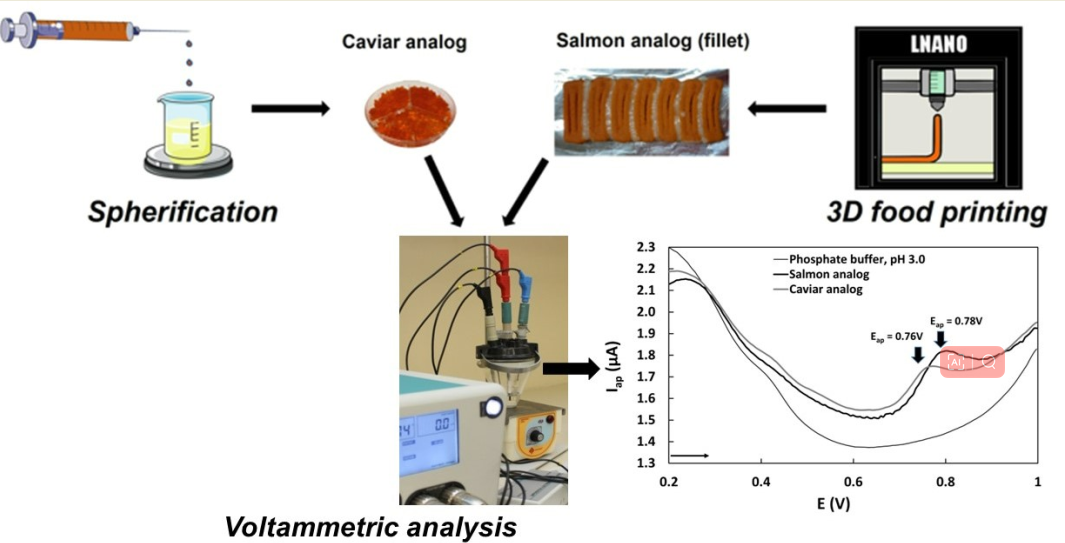
For the development of caviar analog, the food ink was added to syringes with a 3 mL Luer slip nozzle and a hypodermic needle with a caliber of 21 G. The syringe was positioned on an automatic injector at a speed of 200 µL/min (water equivalent) and a height of 8 cm. Below the needle, a beaker containing 20 mL of a 1.5% w/v CaCl2 solution was placed to collect the ink, which formed structures that were left for 5 min, and then washed in distilled water.
For the salmon analog, a computer-aided design (CAD) model was developed in order to contemplate the use of two different food inks. The CAD model considered the two most distinct visual components of the salmon: a white-streaked part and a mostly orange part. The modeling was done using the Tinkercad platform, and the gcode was generated from Slic3r. The bioprinter control was performed using Pronterface. For the 3D food printing process, 14G needles without bevels were attached to 5 mL Luer Lock syringes, which were previously filled with the appropriate food ink. These syringes were then placed one after the other in the STARTER bioprinter (3D Biotechnology Solutions - 3DBS).
2.5. Samples extraction and voltammetric analysis
Phenolic compounds were extracted in quintuplicate from 0.1 g sample of fresh caviar and salmon analogs by Kline shaking (1 h, room temperature) in 2.0 mL of 50% aqueous ethanol. The resulting extract was decanted and the supernatant was transferred to 2.0 mL microtubes and centrifuged for 10 min at 12,100 g. To optimize the experimental conditions and achieve the highest sensitivity for the Differential Pulse Voltammetry (DPV) method, the influence of the solvent used for phenolics extraction (methanol and ethanol) and the solvent concentration (25, 50, 70%) on the total phenolics oxidation peak currents (Ipa) were studied.
The voltammetric measurements were conducted on a PGSTAT 128 N Autolab (Metrohm, Switzerland) with electrochemical software (NOVA 2.16, Metrohm, Switzerland). Voltammetric measurements were carried out in an electrochemical cell composed of a glassy carbon electrode (GC) (Φ = 2.0 mm) as the working electrode, Ag/AgCl (3 mol L-1 KCl) electrode as the reference electrode, and a platinum wire as the auxiliary electrode. Before each measurement and between sample measurements, the surface of the glassy carbon electrode was cleaned and electrochemically activated following this procedure: the electrode surface was manually polished with alumina suspension on a polishing cloth for 2 min and rinsed with distilled water; the electrode was immersed in ethanol for 5 min and in HNO3 1:1 v/v for 1 min, then rinsed with ultrapure water; the electrode was immersed in phosphate buffer (0.2 mol L-1, pH 7.0) and subjected to ten potential cycling by cyclic voltammetry in the potential range between -1.5 to 1.5 V at a scan rate of 50 mV s−1.
The voltammetric profile of the samples was obtained in two different pHs by adding 500 and 800 µL of the extract of salmon and caviar analogs respectively to the electrochemical cell containing 10 mL of 0.2 mol L-1 phosphate buffer, pH 3.0 and 6.0. The extracts were analyzed by DPV with potentials ranging from 0 to 1.0 V at a pulse amplitude of 50 mV and a seep rate of 50 Vs-1. The concentrations of total phenolic compounds in caviar and salmon analog samples were obtained from the standard additions of 5 µL of genistein 1 ✕ 10-3 mol L-1 to the samples and expressed as mg of genistein equivalents per 100 grams of samples (mg GEN 100 g-1). Stock solutions of genistein with concentrations of 1.0 × 10-3 mol L-1 were prepared using a mixture of water and ethanol (1:2) and stored in dark vials under freezing. Calibration curves of genistein were obtained by using DPV by successive six additions of 5 μL of 1.0 × 10-3 mol L-1 genistein to the electrochemical cell containing 10 mL of 0.2 mol L-1 phosphate buffer pH 3.0. The voltammetric measurements for each concentration level were performed in triplicate.
The intra-day precision (repeatability) of the DPV method was evaluated by measuring the relative standard deviation (RSD) of seven independent measurements of genistein oxidation current peaks at fixed concentrations of 0.5, 1.5, and 3.0 µmol L
-1. The inter-day precision (intermediate precision) of the DPV method was evaluated by measuring the (RSD) value of the genistein oxidation current peaks at fixed concentrations of 0.5, 1.5, and 3.0 µmol L
-1 on three different days. Linearity, the limit of detection (LOD), and the limit of quantitation (LOQ) for the DPV method were obtained from the genistein calibration curve [
17]. The LOD was calculated using the expression LOD = 0+ t
(n−1.1−α) × s, where: t is the student distribution for n=10 independent sample blanks; α is the 95% confidence level, and s is the standard deviation of the sample blank values fortified at the lowest acceptable concentration measured for genistein. The LOQ was calculated from the lower end of the working range [
17]. The trueness of the DPV method was assessed by recovery assays in which known amounts of genistein (0.5, 1.5, and 3.0 µmol L
-1) were added to caviar and salmon analog extracts.
2.6. Antioxidant capacity evaluation by the voltammetric method
The antioxidant capacity evaluation of the samples was carried out using the electrochemical index (EI) value, calculated according to Lino et al. (2014) [
18]. The EI was proposed considering the direct information that peak potentials (Eap) can provide about the sample reduction potential, and the peak current (Iap) can provide information about the concentration of antioxidants in the analytical sample. The quotient between Iap and Eap resulted in the EI value. The voltammetric parameters Iap and Eap were obtained from differential pulse voltammograms of 1 mL of caviar and salmon analog extracts in 10 mL of 0.2 mol L
-1 phosphate buffer, pH 3.0, using the following electrochemical parameters: initial potential (Ei) = 0.0 V, final potential (Ef) = 1.0 V, pulse amplitude= 50 mV, scan rate = 50 mV s
-1, working electrode: glassy carbon; reference electrode: Ag/AgCl (KCl 3 mol L
-1); auxiliary electrode: platinum wire (Figure 6).
2.7. Statistical analysis
The data from the optimization and validation experiments of the voltammetric method were summarized using Microsoft Office Excel (version 365). The voltammograms were constructed using the NOVA 2.16 software, suitable for operating the voltammetric analyzer. Specific statistical tests, such as the Grubbs test (used to remove outliers), and the Crochan test (used to test linearity), were performed following the bibliographic references indicated in the INMETRO document, 2020 [
18]. The calculations of the mean, standard deviation (SD), and relative standard deviation (RSD) of the data sets were performed using Microsoft Office Excel software (version 365). Linear regression and the linear curve equation of the analytical curves were also obtained using Microsoft Office Excel software (version 365).
3. Results
3.1. Influence of solvent on sample extraction
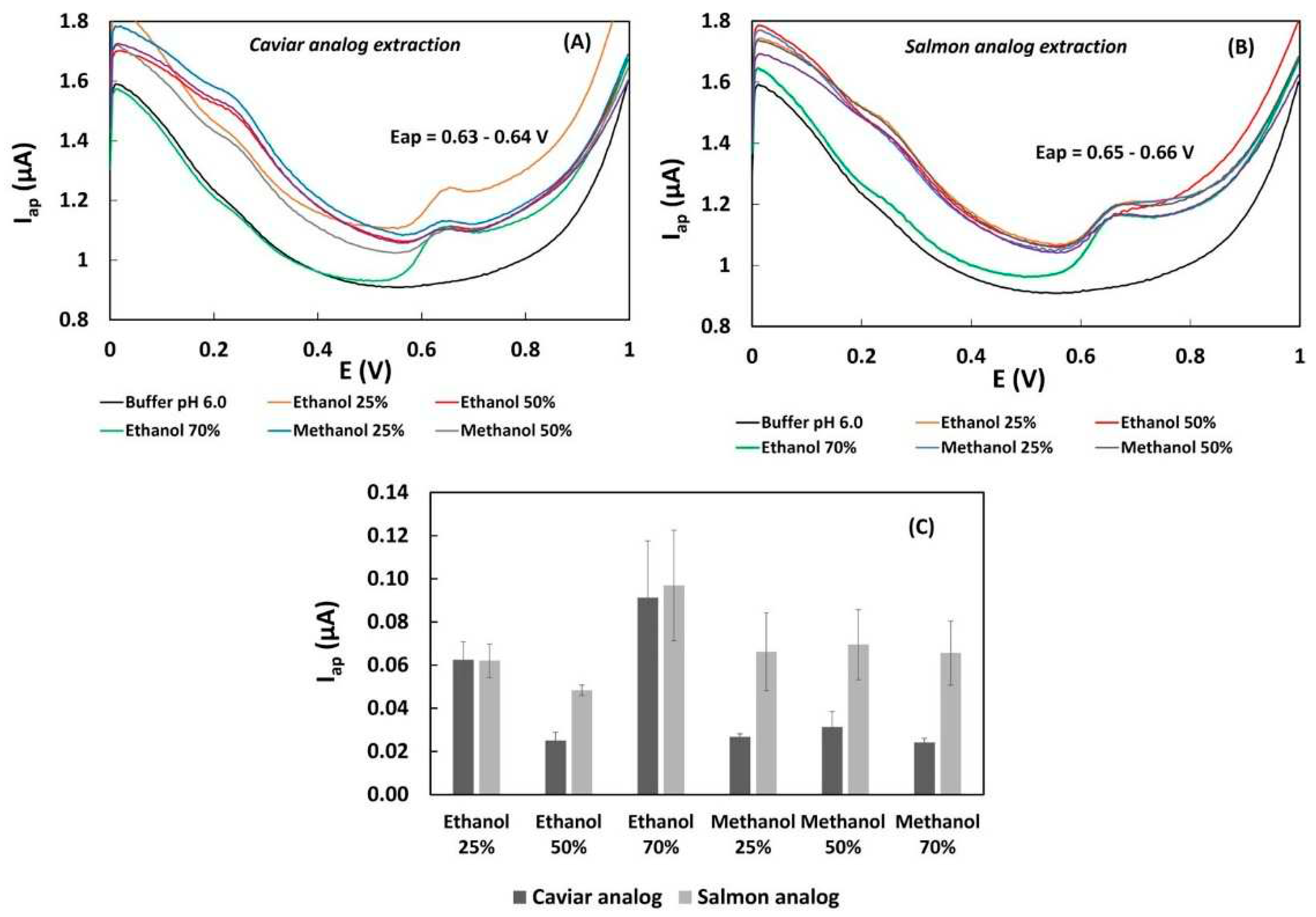
In order to identify the optimal solvent for extracting phenolic compounds from caviar and salmon analogs samples with soy protein and white bean pulse constitution, aqueous methanol and ethanol were used for sample extraction at different concentrations (25%, 50%, 70%). The selection of the suitable extraction solvent was based on the voltammetric profiles and anodic currents of the samples. Favorable results were characterized by well-formed voltammetric curves without overlapped peaks and higher anodic currents. The DPV profiles of the samples extracted with ethanol and methanol exhibited oxidation current peaks at potentials around 0.2 V and 0.6 V in phosphate buffer pH 6.0 (
Figure 2A,B), indicating a similar polyphenolic composition).
Figure 3C demonstrates that ethanol and methanol at various concentrations significantly influenced the extraction of total phenolic content in 0.1 g of fresh caviar and salmon analog samples. The highest intensity of the anodic current peak (Iap) at 0.6 V was observed in samples extracted with 2 mL of 70% ethanol (
Figure 2C). Under these conditions, the extraction method showed a precision evaluated for 0.1 g of the salmon analog sample over three days, with a RSD of 25% (
Figure 2C). Previous research on the voltammetric determination of isoflavones in soybean samples also reported promising results in using 70% ethanol [
19].
3.2. Differential pulse voltammetry measurements of samples with isoflavone standard additions at different pHs
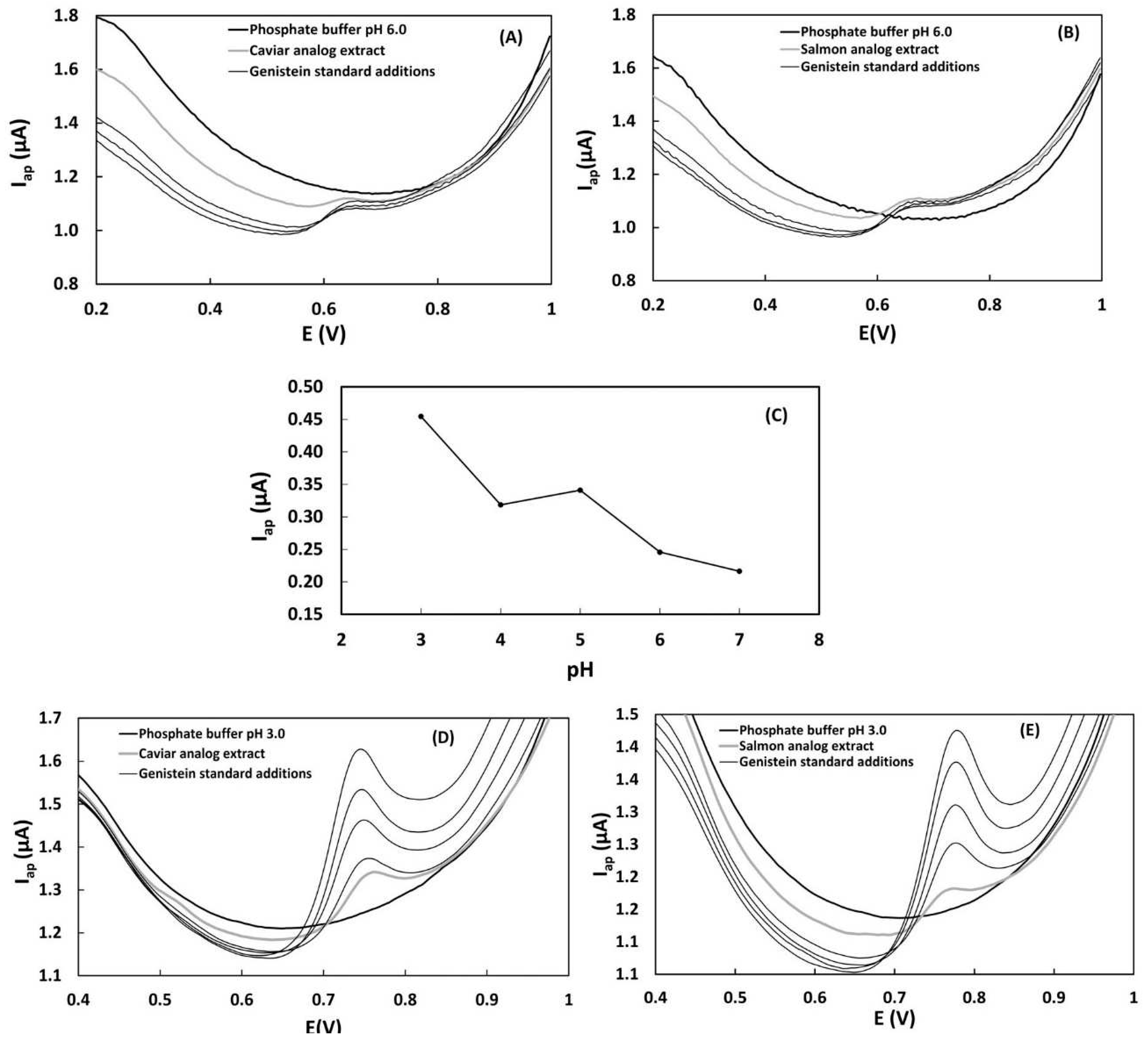
The differential pulse voltammograms obtained for caviar and salmon analog samples with standard additions of genistein in 10 mL of 0.2 mol L
-1 phosphate buffer, pH 3.0 and 6.0 are presented in
Figure 3A,B,D and E. Under the established electrochemical conditions using the glassy carbon electrode, a well-defined anodic current peak was measured at 0.64-0.65 V in phosphate buffer at pH 6.0 (
Figure 3A,B). In the voltammograms obtained in phosphate buffer pH 3.0, the anodic current peak was observed at potentials of 0.76-0.78 V (
Figure 3D,E). This voltammetric profile can be attributed to the oxidation of total isoflavones, a class of polyphenolic compounds commonly found in soy-based foods [
20]. Previous studies have reported similar voltammetric profiles with anodic current peaks at potentials ranging from 0.5 to 0.6 V for isoflavones such as genistein, glycitein, daidzein, daidzin, genistin, and glycitin [
14,
19]. This electrochemical behavior with the oxidation of a specific hydroxyl group located in the benzene B-ring of the isoflavones at the glassy carbon electrode surface, as discussed in our previous work [
14,
19]. No voltammetric signal was observed in the analysis of the samples of dyes used in the food ink of caviar and salmon analogs under the adopted experimental conditions. This observation suggests that the dye molecules may not be electroactive or detectable by the glassy carbon sensor. Additionally, the non-solubility of the dye in 70% ethanol could be a contributing factor.
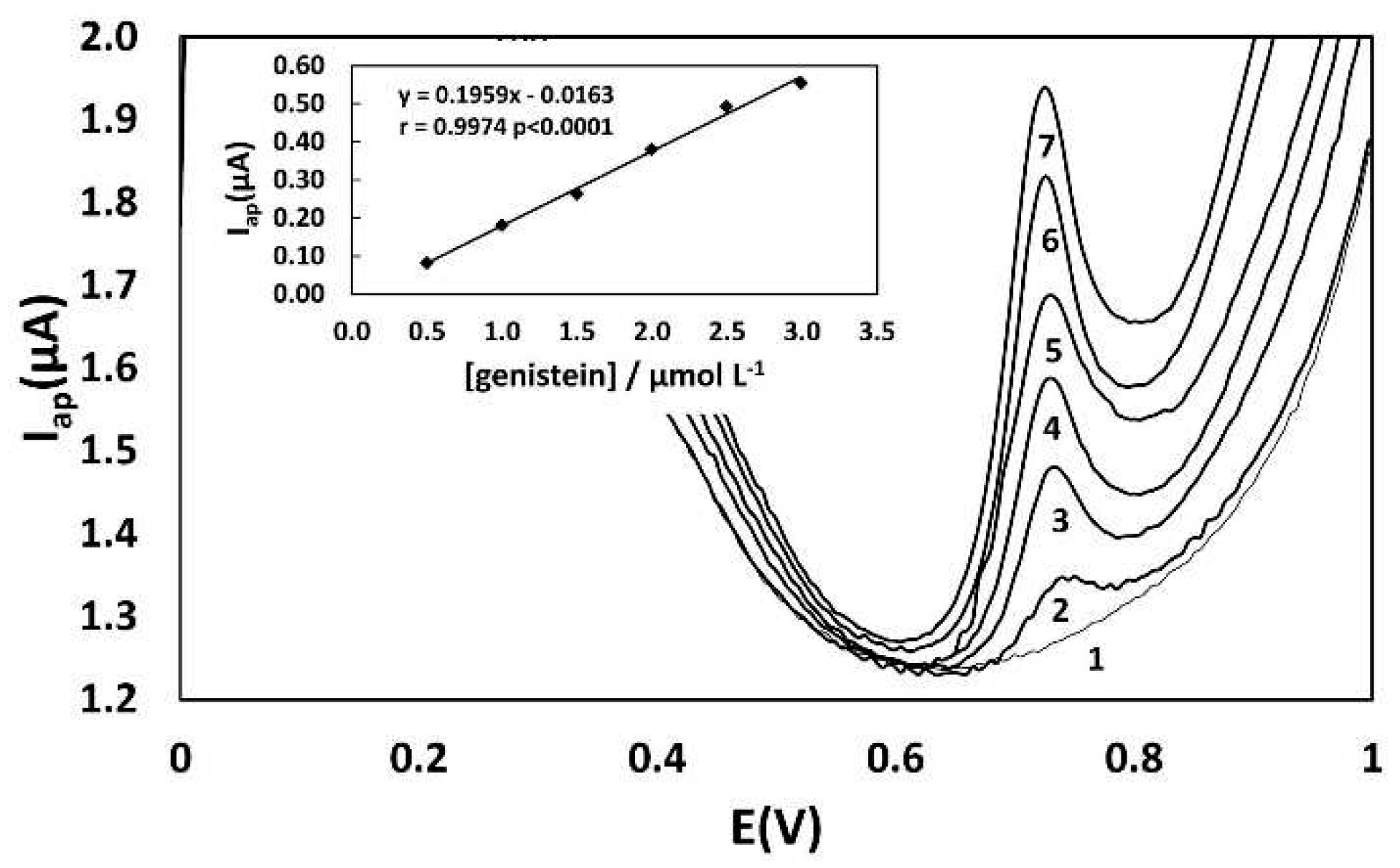
Genistein was chosen as the standard for determining total isoflavones due to its good performance as a quantitation standard for total isoflavones in soy-based food. To obtain more accurate results for the quantification of total phenolic compounds through the construction of a standard addition curve, it is important that the current increments during the addition of the standard are increasing and sensitive, resulting in a linear standard addition curve. The analysis conditions that resulted in more linear curves were obtained in phosphate buffer at pH 3.0. These results support the influence of pH on the determination of genistein. Differential pulse voltammograms of genistein (9.9 µmol L
-1) showed more intense oxidation currents at pH = 3.0 (
Figure 3C). Under these conditions, genistein proved to be a more suitable standard for the quantification of total isoflavones, as evidenced by well-defined peaks of oxidation currents (Eap = 0.64-0.65 V) that perfectly overlapped the signals of the sample without any interference signals such as shoulders and double peaks (
Figure 3D,E).
3.3. Quantification of total phenolic compounds in caviar and salmon analogs – validation parameters
Table 1 summarizes the results of linearity, LOD, LOQ, and precision. The analytical curve for the successive addition of a genistein solution, prepared at 1.0 x 10
-3 mol L
-1 in 0.2 mol L
-1 phosphate buffer at pH 3.0, was obtained using differential pulse voltammetry with a glassy carbon electrode. Differential pulse voltammograms exhibited an increase in the anodic current signal at 0.76-0.78 V with the addition of genistein (
Figure 4). A linear curve of anodic current Iap (µA) as a function of genistein concentration (μmol L
-1) was obtained in the range from 0.5 to 3.0 μmol L
-1(Insert of
Figure 4), with a limit of detection of 0.1 µmol L
-1 and a limit of quantitation of 0.5 µmol L
-1. The linearity of the method within the working range was confirmed by a correlation coefficient (r) greater than 0.99 and the homoscedasticity of the analytical curve, as demonstrated by the calculated Cochran value (α = 95% confidence level) being lower than the theoretical value.
The intra-day precision (repeatability) of the DPV method exhibited an oxidation current with a RSD ranging from 4.8 to 5.6% based on seven independent measurements at three concentration levels of genistein. The inter-day precision (intermediate precision) of the DPV method showed an oxidation current with RSD of 7.4 to 9.6%, considering one independent measurement at three levels of genistein concentration in three different days. These results indicate that the method provides satisfactory intra-day and inter-day precisions in the voltammetric determination of total phenolics using genistein as an equivalent standard compound.
3.4. Concentrations of total phenolic compounds in caviar and salmon analogs and the electrochemical index (EI)
The concentration of total isoflavone compounds in caviar and salmon analogs, expressed as mg of genistein equivalents per 100 grams of samples (mg GEN 100g
−1), were determined. The samples were prepared using extrusion and 3D food printing methods. The mean values and standard deviations are presented in
Table 2. Standard addition curves were constructed by plotting the oxidation current peak against the added concentrations of genistein (0.5, 1.5, 3.0 µmol L
-1) in the caviar and salmon analogs. These curves exhibited good sensitivity (slope = 0.098- 0.12) and a linear correlation (r > 0.99). The recovery range of added genistein in the caviar samples was 72-112%, while for the salmon analog, it ranged from 75-117%.
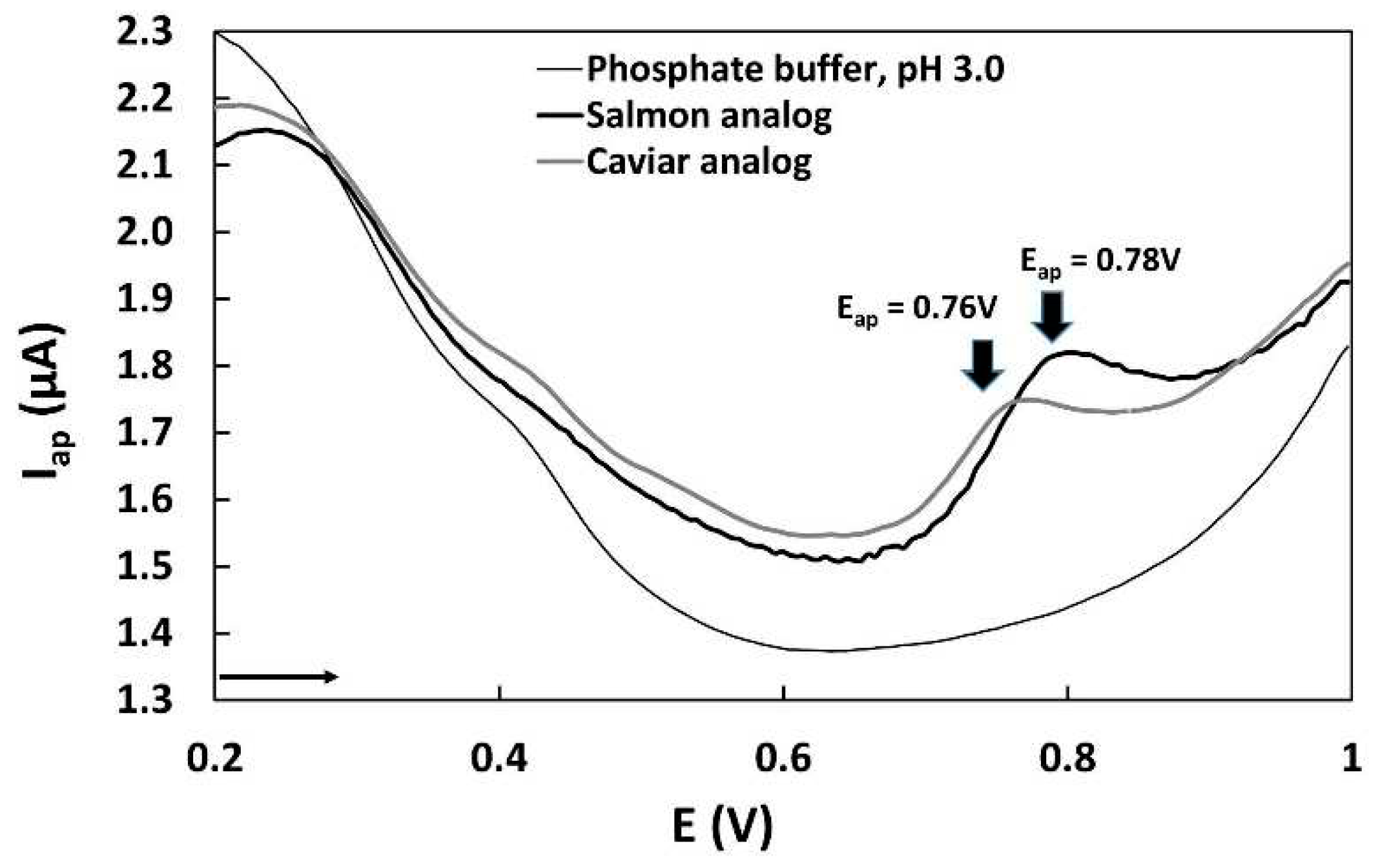
The EI was calculated based on the voltammograms obtained from 1 mL of sample extracts (
Figure 5). The averages of the anodic current peaks and anodic peak potentials (n= 3) were used in the calculation of the EI, which are listed in
Table 2.
4. Discussion
The quality control of plant-based foods, such as fish and seafood analogs, primarily focuses on nutritional analysis, including protein, carbohydrate, and lipid content, which should be similar to their animal-based counterparts. However, it is equally important to consider the concentration of functional compounds in these foods. In our study, we expected to find isoflavones in caviar and salmon analogs based on the extensive literature highlighting the functional and health benefits of isoflavones in legumes such as soybeans and various types of beans [
21,
22,
23,
24,
25,
26]. Interestingly, this antioxidant class of substances is still underexplored as a quality control parameter in commercial plant-based foods.
Isoflavones belong to the flavonoid family and are an important subclass of phytoestrogens commonly found in legumes such as soybeans, beans, chickpeas, and sunflower seeds. They possess antioxidant properties and have the potential to benefit human health by reducing the risk of cancer, preventing obesity and diabetes, mitigating cardiovascular diseases, addressing osteoporosis, and alleviating menopausal symptoms [
27]. In our study, isoflavones in the caviar beads and in the salmon fillet analog primarily originated from isolated soy protein in the food inks. The concentration of isolated soy protein in the salmon analog ink was higher than that in the caviar analog ink, contributing to its higher isoflavone content. However, it is worth noting that the concentration of isoflavones in soybean seeds is generally higher compared to soy protein isolates. Silva et al reported total isoflavone content ranging from 165-336 mg/ 100 g in soybeans with different varieties from Brazil [
28]. Rizzo and Baroni also reported isoflavone concentrations ranging from 99.82 mg 100 g
-1 in soybeans from Brazil [
21]. The variation in isoflavone content is attributed to different climatic conditions and cultivation practices.
During the processing of soy protein isolate, approximately 52% of the isoflavones are lost [
23]. Liu et al found isoflavones in samples of isolated soy proteins with a concentration of 62.5 mg 100 g
-1 (acid leach) [
23]. In their analyses by liquid chromatography, the acid leach processing to soy protein isolates improved the extraction of β-glycosides and aglycones isoflavones. However, even after processing, the concentration of isoflavones found in our study for caviar analog (31.5 ± 1.8 mg Genisteína 100g
-1) and salmon analog (64.3 ± 2.0 mg Genisteína 100g
-1) is higher compared to other reported soy foods. For example, soy burgers contain 0.1–26 mg 100 g
-1 of total aglycone equivalents, soy cheeses 2.3–33 mg 100 g
-1, and soy milk formulas can have up to 31 mg 100 g
-1 [
21].
Regarding the potential adverse effects of soy consumption, such as disruption of the sex hormone network, infertility, and allergies, the studies have yielded conflicting results with limited clinically confirmed investigations [
29]. In 2015, the European Food Safety Authority (EFSA) panel concluded that the intake of 35–150 mg per day of isoflavones from supplements or foods does not have adverse effects. As a precautionary measure, it is recommended not to introduce soy foods beyond 6 months of age to prevent allergies [
21].
In common beans, phenolic acids, flavonoids, and proanthocyanidins are the primary polyphenols, with pigmented seed coats containing higher polyphenol levels. However, white beans, which are present in caviar and salmon analog samples, have a deficient presence of isoflavones. Ombra et al reported total flavonoid content of approximately 5-6 mg 100 g
-1 in white bean varieties [
30]. Gharachorloo et al found a concentration of total phenolics of 10.1 mg 100g
-1 in white bean seeds [
26].
Our results from the DPV demonstrate that a bare glassy carbon sensor can effectively determine polyphenols with good performance. The LOD, linearity, precision, and recovery features of the method confirm its usability. However, it is important to note that the strong adsorption of organic compounds on the electrode surface required a longer cleaning process before each analysis, increasing the time required for electrochemical analysis. It is worth mentioning that voltammetric methods have limitations in selectively detecting phenolic compounds. In the voltammograms of the samples, sensitive oxidation current peaks were observed at the same potential. Hence, for quantitative determination, we adopted genistein, resulting in sensitive current peaks free from interferences. Drawing from our previous experience in determining isoflavones in soybeans, we were able to streamline the optimization process, considering parameters such as sweep speed, amplitude, electrolyte, and standards for quantification. All these optimized parameters were employed in the electrochemical analysis of isoflavone determination in caviar and salmon analogs. The evaluation of sample's antioxidant capacity using the EI revealed that the salmon analog exhibited better antioxidant capacity, primarily due to its slightly higher polyphenolic content, as the anodic peak potentials (0.76 V for caviar analog and 0.78 V for salmon analog). The use of EI is suitable for comparing the antioxidant capacity of a group of samples analyzed using the same electroanalytical method. Additionally, good correlations have been observed between the antioxidant capacity values determined by EI using voltammetry and EC50 determined by spectrophotometry [
18].
Looking ahead, future perspectives involve the utilization of lab-made portable sensors modified with nanostructures, such as green synthesized monometallic or bimetallic nanoparticles. These advancements will enhance the application of electrochemical sensors and address the issue of molecule adsorption from the extracts.
Furthermore, researchers are exploring various legume proteins derived from sources like fava beans, lentils, chickpeas, and peas as alternatives to soy protein. These studies aim to improve the nutritional value and provide advanced fish and seafood analogs.
5. Conclusions
The growing recognition of the health benefits associated with antioxidants from plants and the increasing consumption of plant-based meat analogs underscores the need to enhance the functional quality control of these foods. In this study, we successfully analyzed the antioxidant content of caviar and salmon analogs using a voltammetric technique with a bare glassy carbon electrode. The method exhibited high sensitivity, satisfactory precision, and low limits of detection and quantification, making it suitable for determining antioxidant compounds, such as isoflavones, in the samples. The ethanolic extraction method employed was simple, rapid, and effective in extracting isoflavones, predominantly in the aglycone form. The concentration of isoflavones in the pulse-based ingredients used in the samples, namely isolated soy protein and white bean, aligned with the literature data for total isoflavone content in processed soy-based foods, such as protein isolates. Based on international guidelines, the detected isoflavone levels in the sample found in the samples were deemed safe for daily consumption, considering the limits established to prevent allergies in children and hormone-related issues in adults. Another relevant aspect is that the voltammetric technique did not detect the natural dyes added to the food inks, either due to the non-electroactive nature of the molecules under the specified analysis conditions or their low solubility in 70% ethanol. The assessment of antioxidant activity through the calculation of the electrochemical index proved useful and straightforward for comparing samples subjected to the same conditions. However, for comparative purposes with literature data, this method may not be adequate due to the robustness of data obtained using other techniques. Nevertheless, despite limited literature on the subject, a correlation between the electrochemical index and the EC50 has been observed. The voltammetric method utilizing a bare glassy carbon electrode proved effective in achieving the objectives of this study. Nonetheless, future investigations should focus on enhancing the efficiency of the presented voltammetric method by reducing the analysis time and streamlining the electrode cleaning process. To this end, the use of laboratory-made portable electrodes with modified working surfaces incorporating metallic nanoparticles holds promise. In conclusion, our study contributes to the advancement of quality control methods for plant-based foods, specifically fish and seafood analogs. By employing voltammetry with a bare glassy carbon electrode, we successfully quantified antioxidant compounds in the samples. This research opens avenues for further exploration and optimization of electrochemical analysis techniques, paving the way for improved functional quality assessment in the rapidly growing field of plant-based meat analogs.
Supplementary Materials
Not applicable.
Author Contributions
Conceptualization, Cínthia Caetano Bonatto, Gabriela Mendes da Rocha Vaz, Gabriella Magarelli, Victoria Baggi de Mendonça Lauria, and Luciano Paulino Silva; methodology, Cínthia Caetano Bonatto, Gabriela Mendes da Rocha Vaz, Gabriella Magarelli, and Victoria Baggi de Mendonça Lauria; formal analysis, Cínthia Caetano Bonatto, Gabriela Mendes da Rocha Vaz, Gabriella Magarelli, Victoria Baggi de Mendonça Lauria and Luciano Paulino Silva; investigation, Cínthia Caetano Bonatto, Gabriela Mendes da Rocha Vaz, Gabriella Magarelli, Victoria Baggi de Mendonça Lauria and Luciano Paulino Silva; resources, Luciano Paulino Silva; data curation, Luciano Paulino Silva; writing—original draft preparation, Cínthia Caetano Bonatto, Gabriela Mendes da Rocha Vaz, Gabriella Magarelli and Victoria Baggi de Mendonça Lauria.; writing—review and editing, Luciano Paulino Silva; visualization, Luciano Paulino Silva; supervision, Luciano Paulino Silva; project administration, Luciano Paulino Silva; funding acquisition, Luciano Paulino da Silva. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by The Good Food Institute (GFI) and Embrapa, grant number 10.21.00.088.00.00. We also appreciate the financial support of Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq no. 311825/2021-4). “The APC was waived”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is unavailable due to intellectual property subjects.
Acknowledgment
We would like to express our heartfelt gratitude to The Good Food Institute for their support and grant provided for our research project. Their invaluable assistance has greatly contributed to the success and advancement of our work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Naylor, R. L. et al. Effect of aquaculture on world fish supplies. Nature 2000, 450, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Aschemann-Witzel, J. et al. Plant-based food and protein trend from a business perspective: Markets, consumers, and the challenges and opportunities in the future. Critical Reviews in Food Science and Nutrition 2021, 61, 3119–3128. [Google Scholar] [CrossRef] [PubMed]
- The Food Institute. Available online: https://gfi.org.br/databook/ (accessed on 30 June 2023).
- Lee, P.; Rogers, M. A. Effect of calcium source and exposure-time on basic caviar spherification using sodium alginate. Int. J. Gastron 2012, 1, 96–100. [Google Scholar] [CrossRef]
- Kazir, M.; Livney, Y.D. Plant-based seafood analogs. Molecules 2021, 26, 1559. [Google Scholar] [CrossRef] [PubMed]
- Hall, C; Hillen, C. ; Garden Robinson, J. Composition, nutritional value, and health of pulses. Cereal Chemistry. 2017, 94, 11–31. [Google Scholar] [CrossRef]
- García-Lafuente, A. et al. In vitro anti-inflammatory activity of phenolic rich extracts from white and red common beans. Food Chemistry 2014, 161, 216–223. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and antioxidant methods: an updated overview. Archives of Toxicology 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Lorenzo, C.; et al. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]
- Lamarca, R. S.; Matos, R. C.; Matos, M.- C. C. Determination of phenolic acids in palm oil samples by HPLC-UV-AD using homemade flow cell. Analytical Methods, 2018, 10, 4535–4542. [Google Scholar] [CrossRef]
- Khoddami, A.; Wilkes, M. A.; Roberts, T. H. Techniques for Analysis of Plant Phenolic Compounds. Molecules, 2013, 18, 2328–2375. [Google Scholar] [CrossRef]
- Ignat, I.; Volf, I.; Popa, V. I. A. Critical review of methods for characterization of polyphenolic compounds in fruits and vegetables. Food Chemistry 2011, 126, 1821–1835. [Google Scholar] [CrossRef] [PubMed]
- Lawag, I.L.; Nolden, E.S.; Schaper, A.A.M.; Lim, L.Y.; Locher, C. A Modified Folin-Ciocalteu Assay for the Determination of Total Phenolics Content in Honey. Applied Sciences 2023, 13, 2135. [Google Scholar] [CrossRef]
- Pwavodi, P.C.; Ozyurt, V.H.; Asir, S.; Ozsoz, M. Electrochemical Sensor for Determination of Various Phenolic Compounds in Wine Samples Using Fe3O4 Nanoparticles Modified Carbon Paste Electrode. Micromachines 2021, 12, 312. [Google Scholar] [CrossRef] [PubMed]
- Magarelli, G.; Lima, L.- H. C.; Da Silva, J. G.; SouzaDe, J. R.; De Castro, C. S. P. Rutin and total isoflavone determination in soybean at different growth stages by using voltammetric methods. Microchemical Journal 2014, 117, 149–155. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Cimpeanu, C.; Predoi, G. ; Electrochemical methods for total antioxidant capacity and its main contributors determination: a review. Open Chemistry 2015, 13, 824–856. [Google Scholar] [CrossRef]
- INMETRO, “DOQ-CGECRE-008: Orientação sobre validação de métodos analíticos.” Rev., 9, 30p (2020), Available online: (http://inmetro.gov.br/credenciamento/organismos/doc_organismos.asp?torganismo=calibensaios) (accessed on 04/07/2023).
- Lino, F.M.A.; De Sá, L.Z.; Torres, L.M.S.; Rocha, M.L.; Dinis, T.C.P.; Ghedini, P.C. Somerset, V.S.; Gil, E.S. Voltammetric and spectrometric determination of antioxidant capacity of selected wines. Electrochimica Acta 2014, 128, 25–31. [Google Scholar] [CrossRef]
- Da Silva. ; J. G.; Magarelli, G.; Pedroza, T M.; Cavalcante, R. S.; De Souza, J. R.; Da Silva, J. P.; Carrão-Panizzi, M. C.; De Castro, C. S.P. Determination of total isoflavones and rutin in seeds, roots, and leaves of Brazilian soybean cultivars by using voltammetric methods. Journal of Agriculture and Food Research 2021, 3, 100113. [Google Scholar]
- Popa, O.M.; Diculescu, V.C. Electrochemical behaviour of isoflavones genistein and biochanin A at a glassy carbon electrode. Electroanalysis 2013, 25, 1201–1208. [Google Scholar] [CrossRef]
- Rizzo G, Baroni L. Soy, Soy Foods and Their Role in Vegetarian Diets. Nutrients. 2018, 10, 43. [Google Scholar] [CrossRef]
- Křížová, L.; Dadáková, K.; Kašparovská, J.; Kašparovský, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef]
- Lui, M.C.Y.; Aguiar, C.L.; Alencar, S.M.; Scamparini, A.R.P.; Park, Y.K. Isoflavonas em isolados e concentrados protéicos de soja. Ciência e Tecnologia de Alimentos 2003, 23, 206–212. [Google Scholar] [CrossRef]
- Orak, H. H.; Karamać, M.; Adnan, O. , Amarowicz, R. Antioxidant Potential and Phenolic Compounds of Some Widely Consumed Turkish White Bean (Phaseolus vulgaris L.) Varieties. Pol. Journal of Food and Nutrition Sciences, 2016, 66, 0–0. [Google Scholar]
- Wang Q, Ge X, Tian X, Zhang Y, Zhang J, Zhang P. Soy isoflavone: The multipurpose phytochemical (Review). Biomedical Reports 2013, 1, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Gharachorloo, M.; Tarzi, B. G.; Baharinia, M. The Effect of Germination on Phenolic Compounds and Antioxidant Activity of Pulses. Journal of the American Oil Chemists' Society 2013, 90, 407–411. [Google Scholar] [CrossRef]
- Sajid, M.; Stone, R. S.; Kaur, P. Recent Advances in Heterologous Synthesis Paving Way for Future Green-Modular Bioindustries: A Review With Special Reference to Isoflavonoids. Frontiers in Bioengineering and Biotechnology 2021, 9, 673270. [Google Scholar] [CrossRef]
- Silva, C. E. da; Carrão-Panizzi, M. C.; Mandarino; J. M. G.; Leite, R. S.; Mônaco, A. P. do A. Teores de isoflavonas em grãos inteiros e nos componentes dos grãos de diferentes cultivares de soja (Glycine max (L.) Merrill). Brazilian Journal of Food Technolog 2012, 15, 150–156. [Google Scholar] [CrossRef]
- Kim, IS. Current Perspectives on the Beneficial Effects of Soybean Isoflavones and Their Metabolites for Humans. Antioxidants (Basel). 2021, 10, 1064. [Google Scholar] [CrossRef]
- Ombra MN, d'Acierno A, Nazzaro F, Riccardi R, Spigno P, Zaccardelli M, Pane C, Maione M, Fratianni F. Phenolic Composition and Antioxidant and Antiproliferative Activities of the Extracts of Twelve Common Bean (Phaseolus vulgaris L.) Endemic Ecotypes of Southern Italy before and after Cooking. Oxidative Medicine and Cellular Longevity 2016, 2016, 1398298. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).