Introduction
Ultrasonography (US) in medicine allows real time assessment of structure as "panoramic" and “dynamic” view where the procedure takes place. Panoramic view is the digital stitching of multiple ultrasound images into a broader one. It can display a structure being investigated and show its relationship to nearby structures based on dynamic behavior over time.
This feature and design could be more advantageous than radiological “static” view imaging. Radiological static imaging is the acquisition of a single image as a “snapshot” of the radiopharmaceutical distribution of a particular structure of the body.
The absence of ionizing radiation, availability and low cost are advantages of US.
It is the convincing imaging tool for many structural cardiovascular and gastrointestinal interventions [
1].
However, sonographic devices have limitations and perform very poorly image quality when there is bone, fat or gas between the transducer and the organ of interest. The extreme differences in acoustic impedance and the depth penetration of ultrasound could attenuate the sound beam. Thus, the accuracy of diagnosis may be limited depending on the frequency of imaging [
1].
These limitations could be minimized in transesophageal echocardiography (TOE), which could provide better image resolution due to the absence of intervening air or bone. However, transthoracic echocardiography (TTE) can assess certain cardiovascular structures better than that of TOE such as estimation of left ventricular function. Thus, TOE complement and cannot replace TTE.
Introduction of three-dimension innovation in transesophageal echocardiography (TOE) allows real time assessment of cardiovascular structure where the investigation takes place. It helps for understanding the complicated multiform morphology, and for evaluating its function. It has become an indispensable companion of two dimensional (2D) -TOE during structural cardiovascular diagnostic and interventions [
1].
On the other hand, endosonographic ultrasound (EUS) is recommended in current guidelines for cancer management, as it provides images with highest resolution for the local staging of gastrointestinal tumors [
2]. In addition, the precision of EUS in targeting adjacent organs and then inserting a needle into them, which is a well-established procedure in the daily gastrointestinal interventions, could facilitate the possibility of performing transesophageal extra- and intracardiac based interventions under direct EUS guidance. The benefits of investigating the cardiovascular structures using this diagnostic and therapeutic tool are unknown.
Materials and Methods
To understand the difference between echocardiographic and endosonographic ultrasound we summarized the main features of transesophageal and endosonographic ultrasound as a diagnostic modality and how do they work in this article.
Transesophageal Echocardiography in Focus
Images produced by echocardiography are created by analyzing reflected ultrasonic waves [
3]. The intensity and frequency of sound are its defining characteristics. Echocardiography uses ultrasounds with frequencies between 1.5 and 7.5 Megahertz (MHz) [
4]. In general, high frequency waves generally produce images with higher quality [
5]. Cardiac examination through gastroenterological tract is called transesophageal echocardiography (TOE) [
6].
Lateral, and longitudinal resolution—affect an ultrasound's resolution of one single image. Lateral resolution is the shortest distance between two adjacent structures that can be separated while still producing two different echoes. Longitudinal (axial) resolution is the ability to discern between two structures that are near to one another front to back. On the other hand, temporal resolution is the capacity to precisely determine the location of an anatomical structure at a specific moment in time. It is determined by the frame rate and affect an ultrasound's resolution of a series of images [
7]. The eye can only see 25 frames per second (fps), which corresponds to a 40 ms temporal resolution. On the other hand, Sector size (width and depth) and line density affect the frame rate (which affects lateral resolution) [
7]. However, 2D-TOE Analysis has technical limitations. It is a semiquantitative technique depending on the quality of manual adjustments of the different regions of interest, requires good image quality and optimization of frame rates. In addition, the temporal stability of tracking patterns, due to physiological changes in interrogation angles between moving tissue and the ultrasonic beam during each cardiac cycle, and the intervendor variability, are associated with technical differences among post-processing algorithms. Moreover, reductions in myocardial deformation indices are age-related, more commonly detected in males than in females. Of note, myocardial deformation depends not only on contractile properties of the myocardial fibers (“contractility”), but also on their loading conditions (pre- and after-load), chamber geometry, desynchrony, and segment interactions [
8]. All these factors could partially affect ultrasonographic quality.
Endosonographic Ultrasound in Focus
EUS allows a simultaneous endoscopic and ultrasound imaging. It consists of an ultrasound processor, connected to either radial or linear transducer design coupled at the tip of an endoscope. Scopes are also connected to a standard video processor [
2]. Three major manufacturers, Fujifilm Endoscopy (Fujifilm Europe GmbH, Germany), Olympus (Olympus Europa SE and Co., KG), and Hitachi (Hitachi Medical Systems Europe, Zug, Switzerland and now is belong to Fujifilm), produce ultrasound processors, compatible with different types of EUS [
5].
An endoscope is made up of 1) an image transmission system to send images outside the body, 2) a bending mechanism to deflect the endoscope tip, 3) channels for air to inflate the body cavity and for suction to remove fluids, 4) water to wash the objective lens and to introduce biopsy forceps, 5) and a lighting system from an external light source [
2,
5].
As in TOE, the frequency used directly correlates with the quality of the image produced. So, a higher frequency results in a higher spatial resolution. However, the evaluation of the adjacent organs may be more challenging because high frequency ultrasonography may not penetrate as well as lower frequency ultrasound [
5].
In addition, endoscopes are fitted with charge-coupled devices (CCD) chips and produce signal images with high-resolution, or high definition (HD) [
5].
Moreover, the endoscopic camera has a 400x400 camera sensor with attributes such as 1.75mm x 1.75mm pixel size and 714mm x 714mm picture area [
5].
Furthermore, the EUS differ in US tip design, flexibility, and balloon insufflation control design around its tip. The possibility of Balloon inflation prior to insertion may reduce contact injury and severe discomfort pharyngeal pain.
There are 2 fundamental echoendoscope designs: interventional linear array and diagnostic radial array.
Radial EUS is used only for diagnostic purposes and has the capacity to capture Doppler, color flow, elastosonography tool and contrast-enhanced endosonographic imaging. It is mainly used for luminal imaging and evaluation of the wall layers of the GI tract, pancreas, and the biliary system.
Linear array EUS probe (used for both diagnostic and therapeutic purposes) provides several advantages, including improved maneuverability. In addition, the presence of bend points at the distal end of the of the endoscope allow high control of the angle of exit of EUS needles or other devices from the working channel with simultaneous visualization and intervention of the target lesion [
2]. Thus, a linear array is preferred in most interventional gastrointestinal operations due to these benefits [
9] (
Supplementary Figure S7).
A new generation of the electronic linear EUS scopes allowed a significant improvement in image quality, through improvement of resolution, delineation, and depth of imaging, while reducing noise. Thus, facilitate the development of more diagnostic and therapeutic EUS-based interventions [
10].
Endosonographic Standard Views and the Way to Look at Cardiovascular Structures
In general, the adjustable ultrasound frequency is of 5-13 MHz, local resolution is very good to a depth up to 100 mm depending on tissue impedance and manufacture [
11,
12].
In addition, transgastric image quality can be optimized by gastric fluid instillation to further lower impedance [
2].
After pharyngeal local anesthesia, advancing smooth and without great resistance is of utmost importance to prevent iatrogenic tissue injures and complications. The EUS probe (viewing angle 120°) is advanced transesophageal according to sonographic anatomic landmarks by the experienced operator. Of note, all radial EUS scopes have 360 degrees viewing angle. Linear echoendoscopes of Olympus have 180 degrees, and the new "J" generation of Hitachi (now Fujifilm) has 150 degrees [
11,
12].
The start maneuver from inside the oral cavity through the hypopharynx and the upper esophageal sphincter can be challenging, because of the lateral view optics of the endosonographic probe.
After passing the oral cavity the first anatomic landmarks are the right carotid artery and the adjacent thyroid lobe (C4 level). Then, the probe is advanced under moderate pressure (insufflation of carbon dioxide can be helpful) to pass the upper esophageal sphincter (C6 level). After that, it can be further advanced along the superior vena cava, the ascending aorta and the aortic arch (T4 level) [
13]. At the T4-T8 level from the esophagus, cardiac structures can be examined as follow:
Around T5/6 you can see dorsal part of the right pulmonary artery and the left atrium.
If the transducer is turned to the right at the level of the T4/5 aorta, you can also see the azygos vein, which can be traced to its continuity with the vena cava. In addition, due to the proximity of the posterior mediastinum to esophagus and EUS design, cardiovascular structures can be technically accessed by EUS. However, the endosonographic probe does not allow for plane rotation, thus limits the assessment of different valvular planes.
High Level Disinfection of the Endoscope
The endoscope and all its parts should be cleaned in a disinfectant-detergent cleaning solution, and thoroughly triple-rinsed with tap water to remove the disinfectant-detergent. The rinsed endoscope should then be soaked in a high-level disinfectant at the labeled exposure time and temperature (10.0 min to 20.0 min at temperatures of about 20 °C to about 25 °C.) for any particular high-level disinfectant to kill all Gram-Positive and Gram-Negative vegetative bacteria, all fungi, all mycobacteria (TB), and all types of viruses, hydrophylic and lipophylic, within the labeled exposure time and temperature.
In addition, automatic endoscope reprocessing (AER) machines are claimed to be able to clean the endoscopes as well as to disinfect them by force rather than by brush. However, the endoscope should be manually brushed before it is placed into the machine [
14].
Table 1.
The main differences between transesophageal echocardiography and endosonographic ultrasound.
Table 1.
The main differences between transesophageal echocardiography and endosonographic ultrasound.
| |
Transesophageal Echocardiography |
Endosonographic
Ultrasound |
| Ultrasonic funktions |
|
|
| Frequency |
Between 2 and 7 MHz [7] |
5, 6.5, 7.5, 9, 10 and up to 13 MHz [15] |
| Frame rate |
30 fps [3] |
22 fps [16] |
| Scanning angle |
180° [17] |
120-180° [15] |
| Depth of field |
2-30 mm [17] |
5-100 mm [15] |
| 3D function |
Yes [17] |
A 3D-based model on registered preoperative data is underway [18] |
| Endosonographic imaging |
Depends on
temporal resolution lateral resolution longitudinal resolution axial resolution different frequencies
|
Depends on
|
Endoscopic image
resolution
|
| |
Not available |
|
| Probe design |
|
|
| Phased array [17] |
Convex radial and linear array [21] |
| Ultrasound |
Ultrasound and endoscope |
| Working channels |
Only an image transmission system [17]. |
1) A lighting system,
2) A channel for suction and for air,
3) A channel for water to clean the lens and to insert biopsy forceps, or needles in case of longuitudinal EUS
4) A channel with a bending mechanism to deflect the endoscope tip, and
5) An image transmission system [5] |
| Image rotation property |
Yes [17] |
No |
| Navigating of probe under direct visualization |
No |
Under direct sonographic and endoscopic visualization [15] |
| Procedure |
|
|
| Purposes |
Diagnostic |
Diagnostic and intervention in case of longuitudinal EUS |
| Visible anatomical structures |
Almost all cardiac structures [22] |
Cardiac structures with limitations regarding right-sided cardiac structures and different valvular planes assessement [23] |
| Duration of investigation |
More examinations can be performed per time unit |
More time required for preparation and investigation [24] |
| Complication rate |
Range from 0.2% to 0.5% [25] |
Lower, with rate of 0.15% [26] |
| Sterilization |
Requires about 20 min in sterilizing fluid [17] |
Has to go through a sterilization machine, which takes longer time
(AER) machines takes shorter time [14] |
| Cost |
Lower [17] |
Higher [15] |
Table 2 and
Figure 1 and
Figure 2: summarize the main features of the previous generation of endosonographic ultrasound EG-3870TK and EG-3270UK of Hitachi respectively. Both endoscopes used to investigate the cardiovascular structures in this article. It’s important to take into consideration the new generations and feature differences between the three major manufacturers, Fujifilm, Olympus, and the new "J" generation of Hitachi (now Fujifilm) [
11].
On the other hand, manufacturer Fujifilm has radial and linear EUS scopes with different features.
Table 3 summarize the main features of EG-740UT curved linear EUS scope.
Fujifilm EG-580UT curved linear EUS Scope main features are the powerful bending capability combined with a short rigid section supports manipulation in duodenum and stomach, 40° forward oblique viewing angle designed to enhance tissue visualization and improve therapeutic flow and elevator locking assist designed to enable flexible and subtle endoscopic operations during therapeutic procedures and supports stable puncture trajectory [
11].
Fujifilm EG-580UR radial EUS scope enhance patient care by performing complete diagnostic and staging exams with the same scope, mitigating the need for scope exchange and second scope reprocessing. Its main features are 0° forward viewing angle for ease of advancement and instrument deployment, 190° bending capability enables retroflexion in the stomach and duodenum, 11.4mm distal tip outer diameter facilitates ease of scope insertion and 2.8mm working channel with exceptional suction power [
11].
The third EUS manufacturer Olympus has radial and linear EUS scopes with different features.
Table 4.
Summarize the main features of TGF-UC180J linear ultrasound endoscope.
Table 4.
Summarize the main features of TGF-UC180J linear ultrasound endoscope.
| Main Features of TGF-UC180J Linear Ultrasound Endoscope [12] |
|---|
| Optical system |
|
| Field of view |
100° |
| Direction of view |
Forward oblique viewing 50° |
| Depth of field |
3-100 mm |
| Insertion section |
|
| Distal end outer diameter |
ø 13.4 mm |
| Distal end enlarged |
1 Light guide lens
2 Air/water nozzle
3 Objective lens
4 Instrument channel outlet
5 Ultrasound transducer
6 Balloon aspiration port
7 Balloon water feeding Port |
| Insertion tube outer diameter |
ø 10.9 mm |
| Insertion section working length |
1,250 mm |
| Instrument channel |
|
| Channel inner diameter |
ø 2.2 mm |
| Minimum visible distance |
5 mm |
| Airflow Rate |
20 cm3/s |
| Bending range capability U/D/R/L |
130°/90°/90°/90° |
| Total length |
1,563 mm |
| Ultrasound function |
|
| Transducer surface maximum temperature |
<43°C |
| Ultrasound Method |
Electronic radial array |
| Ultrasound Direction |
Perpendicular to the insertion direction |
| Frequency |
5/6/7.5/10/12 MHz |
| Scanning Range |
360° |
| Contact Method |
Balloon method, sterile deaerated water immersion method |
| Ultrasound function with EU-ME2 Premier Plus operation mode |
B-mode, THE mode, H-Flow mode, color flow mode, Power flow mode, PW mode, CH-EUS mode, and ELST mode (Elastography) |
GF-UE190 radial ultrasound endoscope is a 360° radial array scanning endoscope with advanced features that offer impressive performance for improved diagnostic and staging accuracy, such as high image quality and improved penetration depth, excellent insertability and maneuverability, improved endoscopic image with oblique view and easy handling and reprocessing [
12].
Figure 3.
The main features of Olymbus
TGF-UC180J linear ultrasound endoscope for interventional EUS procedures with extremely short distal end, wide angulation, straight channel port and auxiliary water channel. The EZ Shot 3 plus FNB needle offers access to lesions in difficult locations, enhanced echogenicity, and reduced puncture force in 19 G, 22 G, and 25 G configurations to suit preferences in a variety of clinical settings [
12].
Figure 3.
The main features of Olymbus
TGF-UC180J linear ultrasound endoscope for interventional EUS procedures with extremely short distal end, wide angulation, straight channel port and auxiliary water channel. The EZ Shot 3 plus FNB needle offers access to lesions in difficult locations, enhanced echogenicity, and reduced puncture force in 19 G, 22 G, and 25 G configurations to suit preferences in a variety of clinical settings [
12].
The benefits of investigating the cardiovascular structures, during routine gastrointestinal procedure, using this diagnostic tool are unknown.
In this article, we discuss cases, in which a cardiovascular anatomy or pathology was investigated using EUS as a diagnostic tool during routine gastrointestinal investigations and in some cases influenced a change in management strategies [
13]. The EUS used in all cases was Pentax EG-3870 UTK endosonography and EG-3270UK endosonography [
15], while the transesophageal echocardiography used was Philips Epic 7 [
17].
Results and Clinical Applications of EUS in Cardiovascular Medicine
The following are applications of the endosonography in the field of cardiovascular medicine.
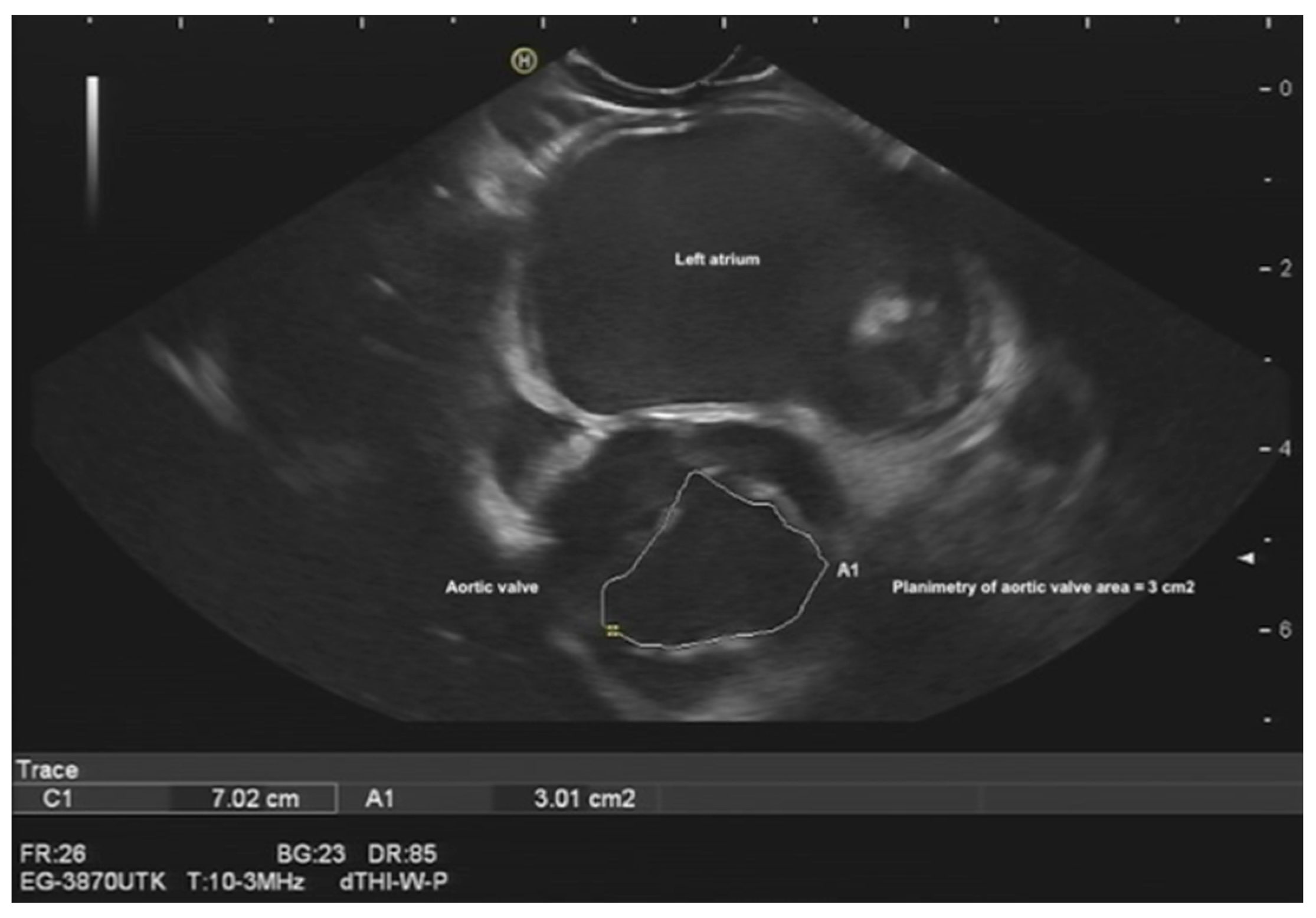
Assessment of aortic valve morphology, function, and vegetation (
Figure 4, Videos S1 and S2) [
13].
- 2.
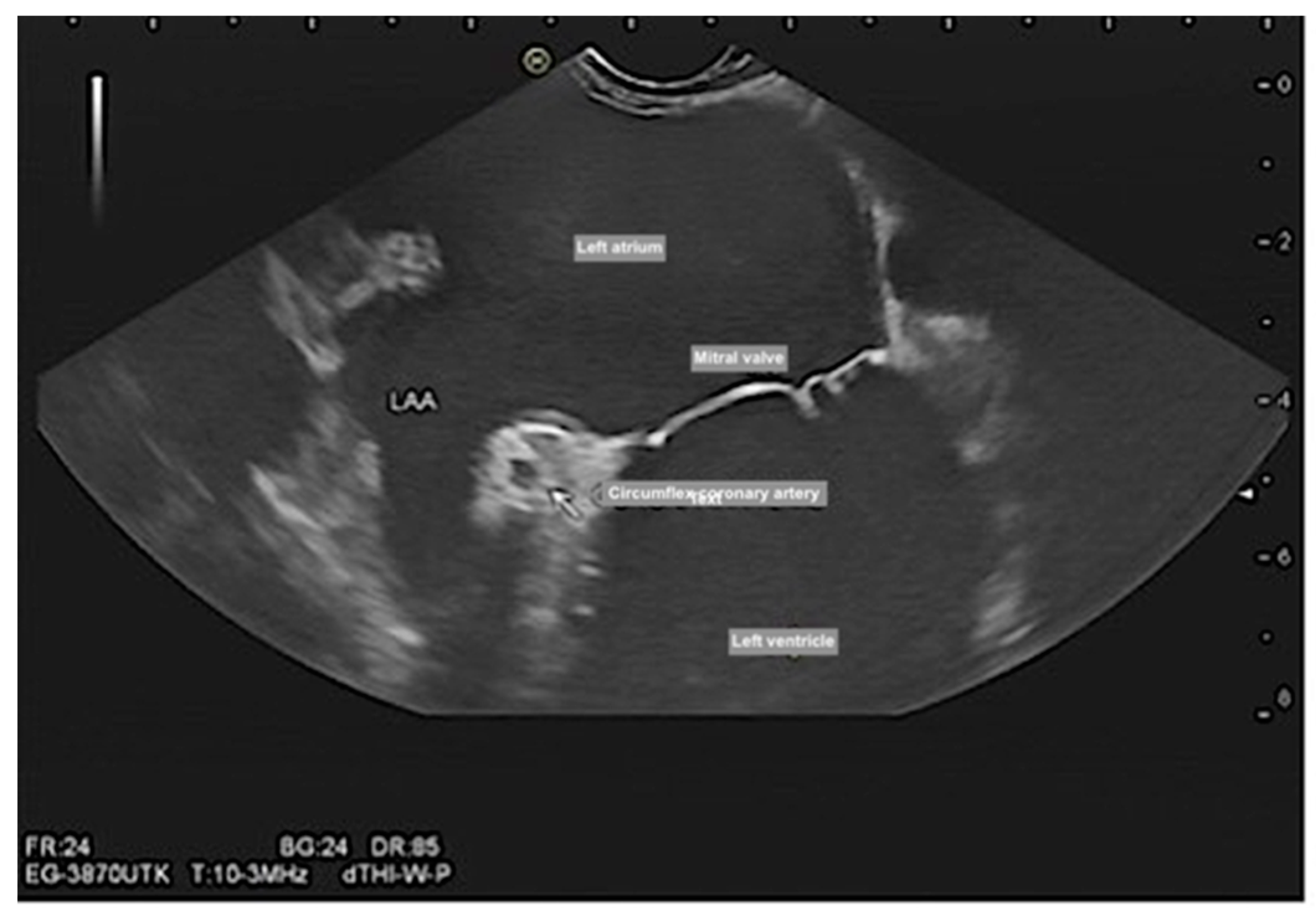
Assessment of mitral valve morphology, function, vegetation [
13] and Mitral clip (
Figure 5, and Videos S3–S5). Mitral clip is a catheter-based edge-to-edge mitral valve repair for treating symptomatic functional mitral regurgitation (MR) in patients who are at high/prohibitive surgical risk.
- 3.
Evaluation of tricuspid valve morphology and function (Video S1).
- 4.
Assessment of main stem, right and left pulmonary artery morphology/diameter, structure, embolism, and pulmonary valve function and pulmonary artery embolism (Video S6).
- 5.
- 6.
Evaluation of atrial septal defect and patent foramen ovale with right/left shunt, thrombus or vegetation.
- 7.
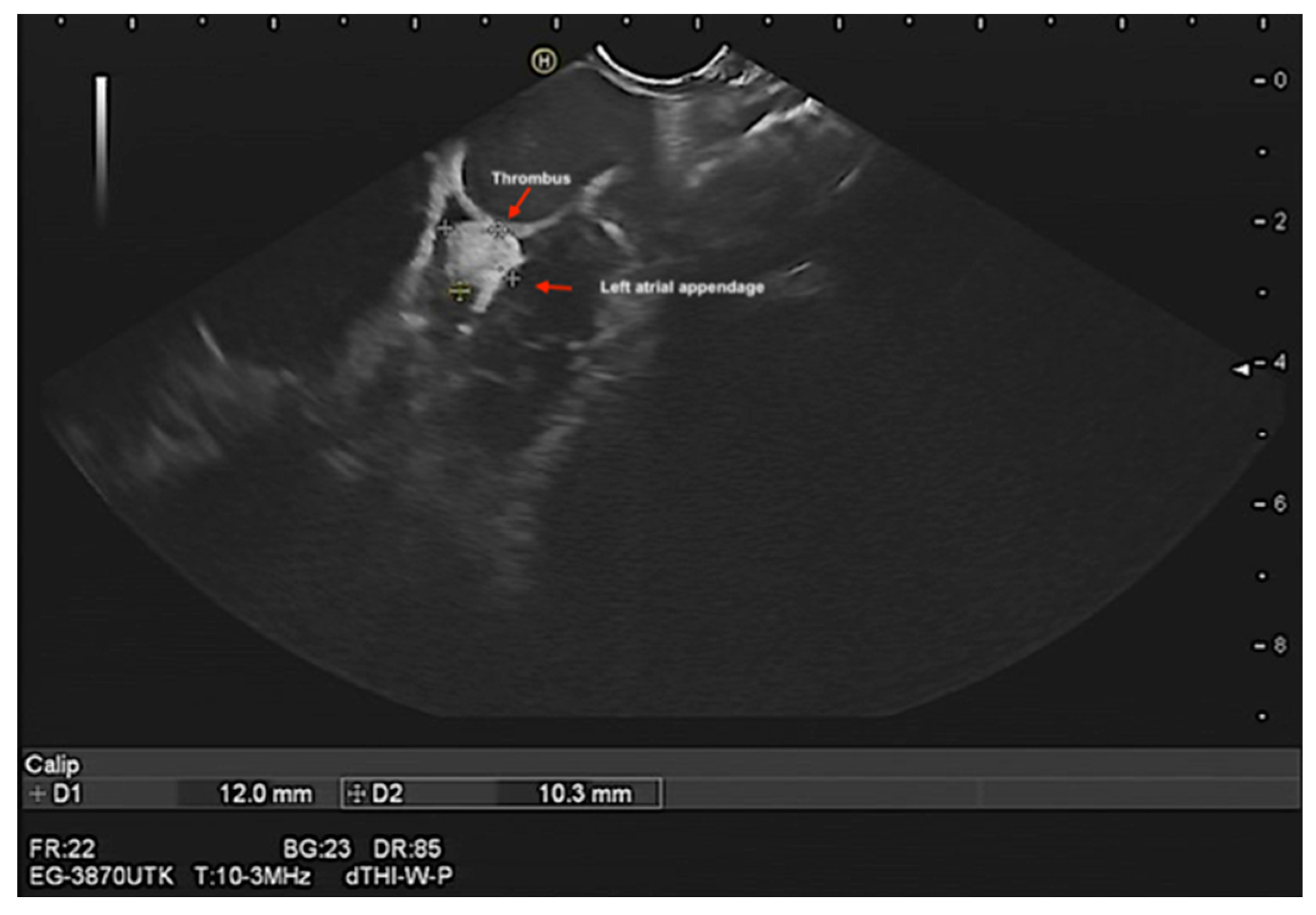
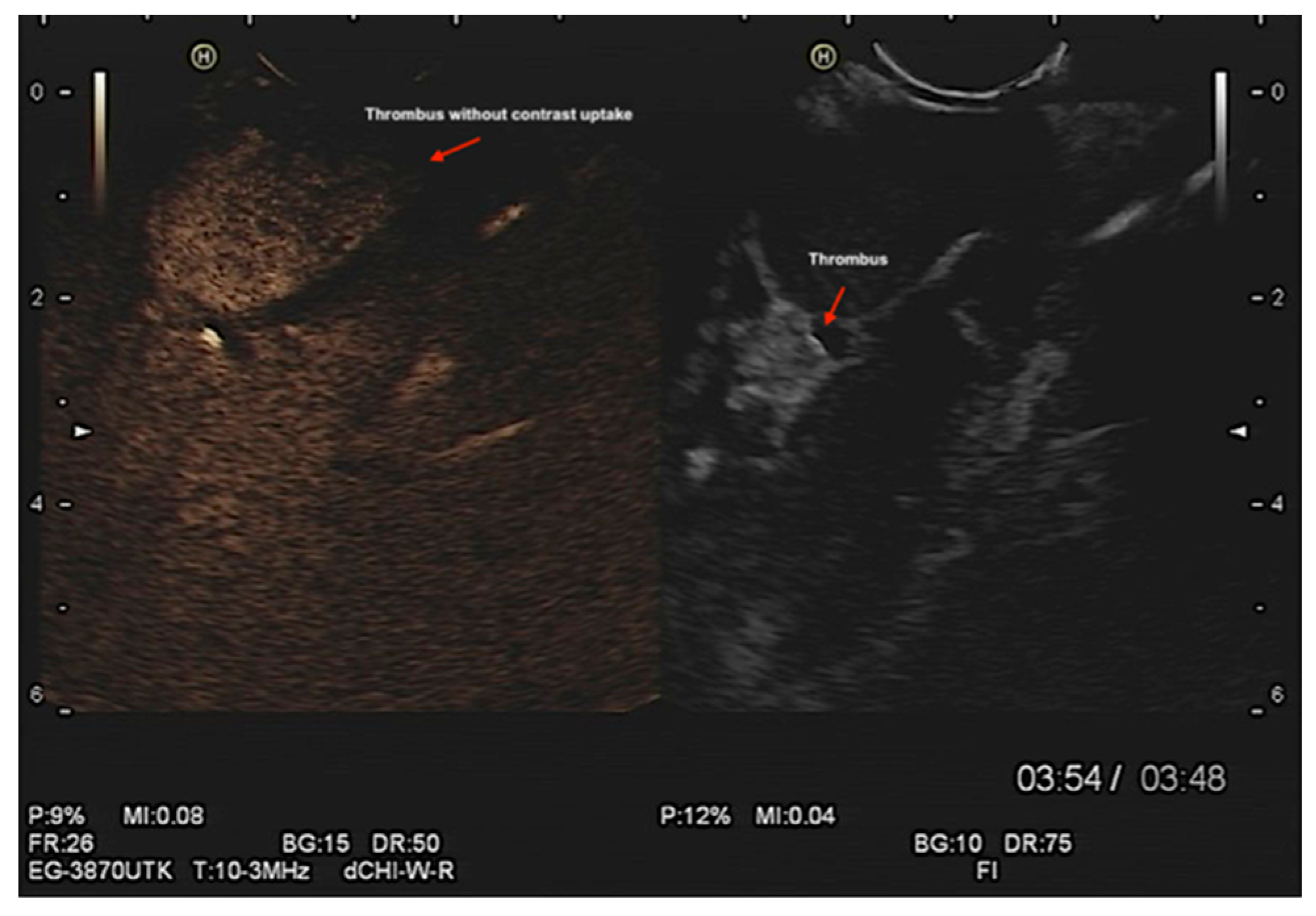
Assessment of left atrial appendage (LAA) morphology, thrombus/ myxoma, fibroelastoma, etc. (
Figure 6 and Videos S7–S9). In addition, contrast endosonographic ultrasound can be used to image vascularity and vessel patterns in an organ of interest, especially for small volume and slow velocity blood flow. Consequently, EUS can differentiate both thrombus and myxoma not only by their distinguishing features of size, origin, shape, mobility, and prolapse but also by contrast enhancement. Compared with the adjacent myocardium, malignant and vascular tumors are hyper-enhanced, whereas stromal tumors are hypo-enhanced and thrombi are non-enhancing [
27] (
Figure 7 and Suppementary
Figure S4, and Videos S9 and S10).
- 8.
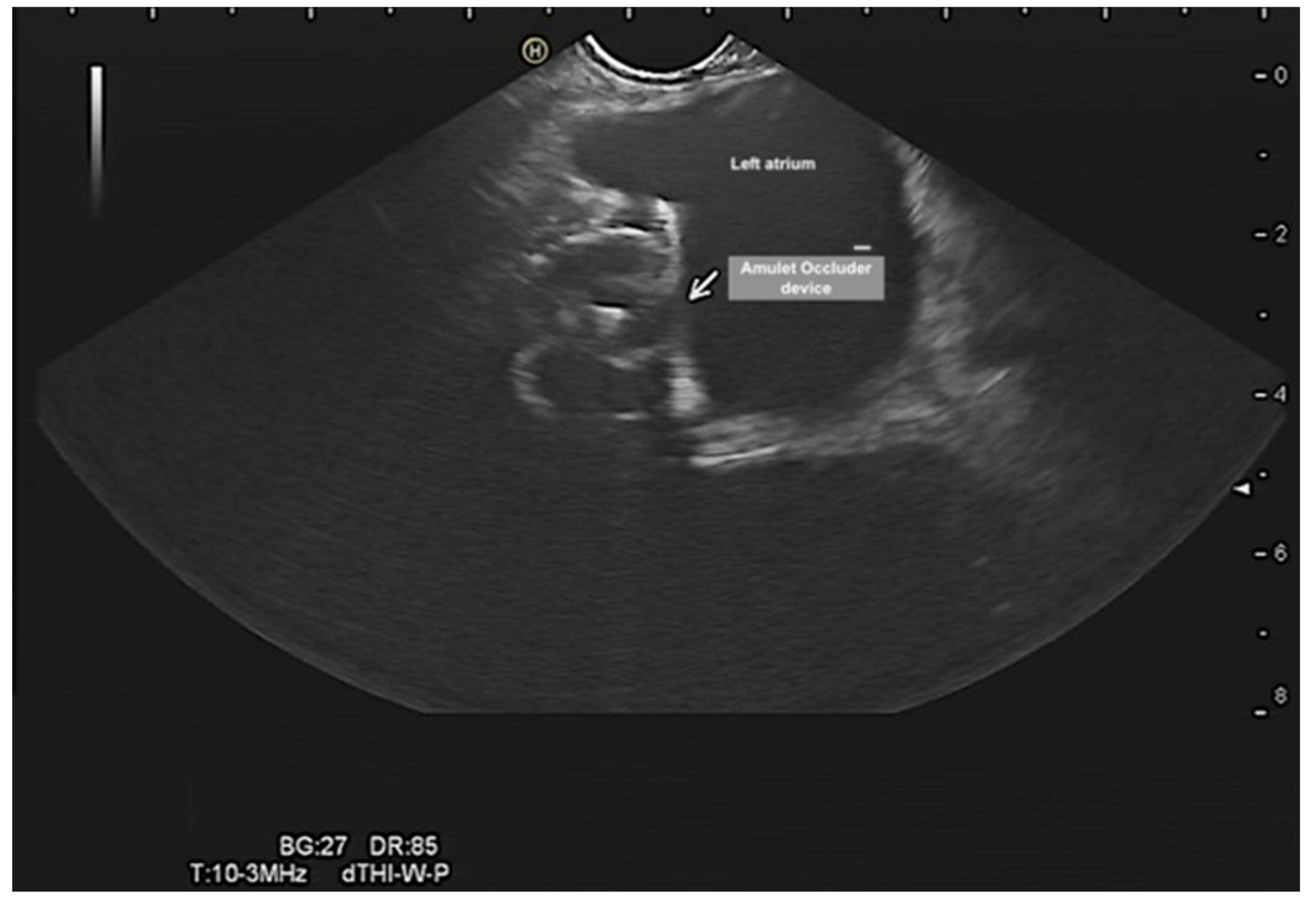
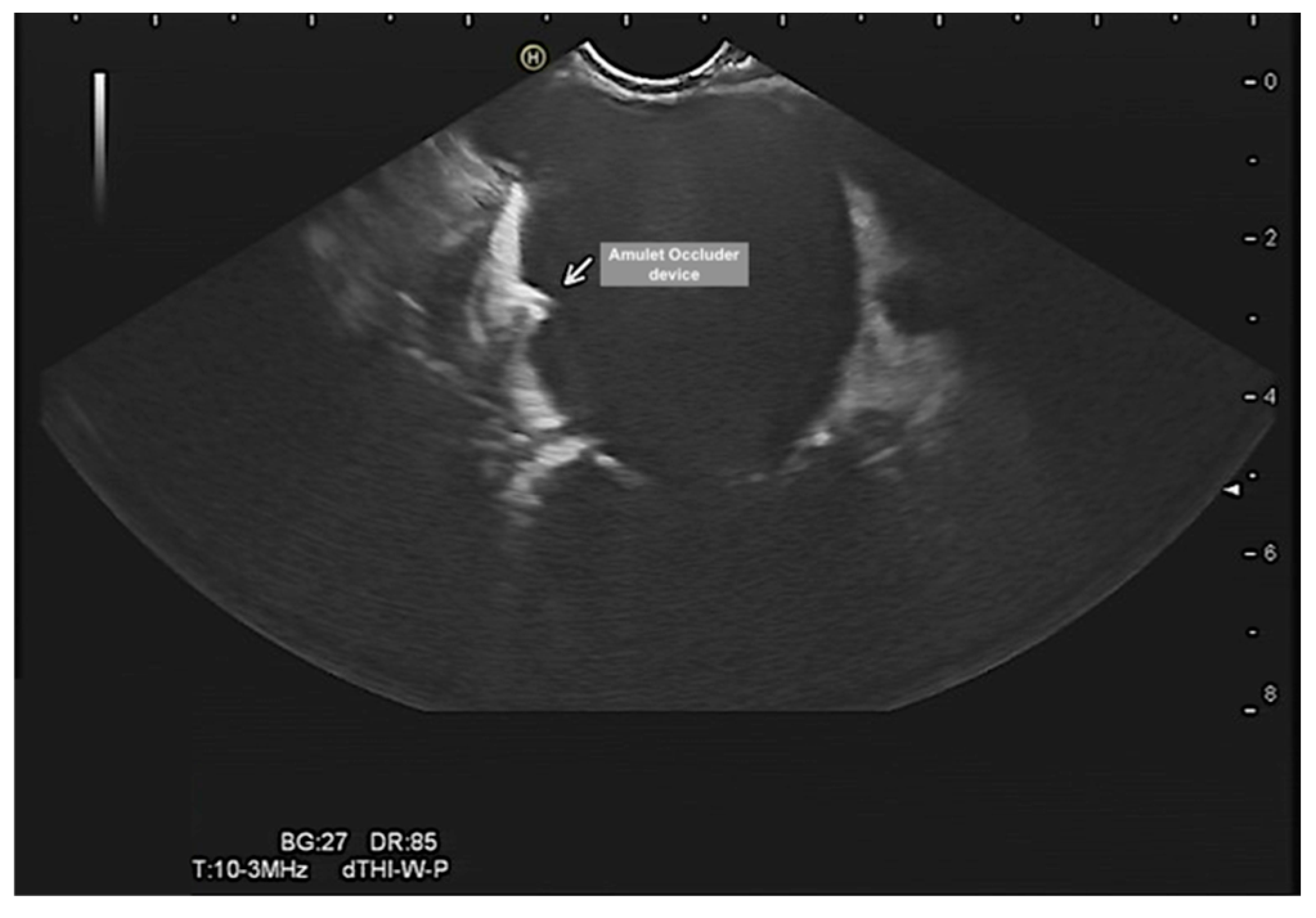
Evalutation of the left atrial appendage occluder, device dislocation, device-related leak, vegetation, or thrombus (
Figure 8 and
Figure 9). Of note, percutaneous occlusion device of the LAA is a minimally invasive catheter-based intervention that keeps any blood clots in the left atrial appendage from entering the bloodstream. Thus, reduces the risk of thromboembolic complications associated with non-valvular atrial fibrillation. The EUS could provide adequate and precise visualization of the LAA and could complement TOE in LAA evaluation. EUS in this context can (1) rule out LAA thrombus, (2) confirm device stability after release, (3) check for device-related leaks, (4) rule in/out device related thrombus and vegetation (4) monitor complications such as cardiac tamponade. However, more studies are needed to evaluate this diagnostic modality.
- 9.
- 10.
- 11.
Assessment of pericardial effusion (
Figure 10).
- 12.
Evaluation of left ventriculer function and thrombus (Video S11).
- 13.
EUS-Guided Cardiovascular Interventions
In the last years, endoscopic EUS has been evolving in gastroenterology from a diagnostic to interventional based procedure [
28].
On the other side, to our information, EUS in cardiology as diagnostic or therapeutic tool is still poorly discussed or rarely used in daily clinical practice. Due to the proximity of the posterior mediastinum to esophagus and EUS design, cardiovascular structures can be technically accessed by EUS. It could provide real-time sampling to confirm diagnosis of rare primary cardiac tumors instead of open-heart surgery in high-risk patients. In animal models, samples of the coronaries, atria, ventricles, and valvular apparatus with no major adverse events are reported in isolated cases [
29].
In daily clinical practice, there are little case reports in the literature of EUS usage as diagnostic tool to assess cardiovascular structures in high risk non operable patients.
A case report of a 65-year-old female cardiac CT revealed a right-sided cardiac tumor with signs of invasion of the interatrial septum, superior and inferior caval veins, and liver parenchyma. An attempt of radiological guided puncture and biopsy was unsuccessful.
Thus, a second attempt of EUS-guided transesophageal puncture was performed without any adverse events as follow:
A linear echoendoscope was introduced in the middle thoracic esophagus, and a solid hypoechoic mass depending on the atrial right wall was observed. The lesion was punctured with a 22-gauge needle (Expect, Boston Scientific, Natick, Mass) with smooth to-and-fro movements. On-site cytopathology results confirmed the diagnosis of cardiac angiosarcoma [
30].
Another case of 26-year-old male presented with recent-onset shortness of breath. Cardiac magnetic resonance imaging showed a large, infiltrative mass with post-contrast enhancement in the right atrium with pericardial extension.
Linear EUS showed a large mass (8×8cm) within the right atrium along with diffusely thickened inferior and posterior.
25G core needle (Acquire, Boston Scientific Co, Massachusetts, United States) from the posterior pericardium with two smooth to-and-fro movements. On-site cytopathology results confirmed the diagnosis of cardiac angiosarcoma [
31].
Furthermore, a case of successful EUS-guided transesophageal pericardial cyst drainage has been reported [
32].
Moreover, two case reports of Endoscopic ultrasound with bronchoscope-guided fine-needle aspiration (EUS-B-FNA) of left atrial masses has been successfully performed in which cardiac surgery was hazardous due to the comorbidity or previous surgical interventions. The cytopathology confirmed the diagnosis of a Burkitt lymphoma and a synovial sarcoma [
33].
These findings suggest the possibility that in selected cases, linear endosonography can be used as a minimally invasive technique for intracardiac tumor diagnostics. While the reports are exciting, these are anecdotal cases, and more studies are required to assess the safety and efficacy of such interventions.
Discussion
We report clinical applications of EUS in cardiovascular medicine, which could be investigated during routine endosonographic gastrointestinal procedures. An early detection of cardiovasculer structures and pathologies can help in early differentiation between possible causes, and prompt management stratiges can be taken to minimize adverse consequences.
Thanks to the collaboration in the last decade between Hitachi and Pentax in HI-VISION Preirus Ultrasound system [
34], the electronic radial and linear EUS scopes allowed a significant improvement in image quality, through improvement of resolution, delineation, blending the outstanding contrast, and depth of imaging, while reducing noise. Thereby, these innovations give the potential of more diagnostic and therapeutic EUS-based applications [
10].
Moreover, a new innovation in compounding high resolution imaging with mapping of the elastic properties and stiffness of soft tissue is the capabilities of sonoelastography. It can identify whether the tissue is hard or soft. Thus, will give more diagnostic information about the status of disease.
Qualitative assessment is based on superimposing a colored image over the conventional grayscale EUS image in a region of interest. The strain level of the hard tissue is colored in blue, the intermediate hard tissue colored in green and the soft tissue is colored in red. In addition, semi-qualitative assessment using strain ratio and strain histogram facilitate identification of tissue properities.
However, it is important to obtain a still image, and for this reason, multiple measurements are performed in each patient.
In this context, sonoelastography could be applied in cardiology to differentiate hard mass (organized old thrombus or tumor) and soft mass (soft thrombus). More studies are required to assess and understand the efficacy of this diagnostic tools. Furthermore, this technique allows the selection of the most probable lymph nodes to be malignant, thus helps for the selection and guidance of EUS-guided fine needle aspiration (FNA) for differentiation of the lymph nodes and for staging purposes in lung, cardiac and mediastinal cancers. The main limitations are motion artifacts, the size and depth of the region of interest should be similar when strain rate is calculated and the negative predictive value remains low, at 60%-70% [35].
The main strengths of EUS over TOE and its role in cardiology can be summarized as follows:
The higher resolution provides more precise information about the investigated pathology, which is the key in management to improve outcomes [
36].
Early
detection of asymptomatic cardiac pathologies could influence changes in the management strategy, save time, reduce procedural complications [
25,
26], allow for the efficient use of resources and infrastructure, and improve economic outcomes. In addition, it prevents double investigations for the patient and the operator.
Introducing the endoscope under direct visualization of gastroesophageal anatomy using an endoscopic camera, an advantage of EUS over TOE, could reduce procedural complications. Thus, situation with difficult initial blind intubation with the TOE probe can be better performed using EUS. Particularly, in patients with contraindication for transesophageal echocardiography due to high risk of esophageal bleeding or complications that may require immediate intervention.
Contrast-enhanced EUS imaging is used to image vascularity and vessel patterns in an organ of interest, especially for small volume and slow velocity blood flow, and for the differential diagnosis of focal masses not only by their distinguishing features of size, origin, shape, mobility, and prolapse but also by contrast enhancement. The recent development of low mechanical index contrast harmonic EUS imaging helps for improved diagnosis, staging and monitoring of anti-angiogenic treatment [
27].
Sonoelastography helps for mapping of the elastic properties and stiffness of soft tissue, differentiation of the lymph nodes (LN); for example: benign lymph nodes appear homogenous and are colored red, whereas malignant LNs are colored blue, thus, can be targeted by the needle to confirm the presence of focal hard malignant infiltration (blue) area up to 3-5mm within in an otherwise normal homogenous soft LN (red) area [
37].
Feasibility of EUS-guided cardiovascular intervention. The parallel development of improved sonographic imaging modalities nowadays, capable of providing accurate anatomic and real time assessment during the procedures is the game changer in both percutaneous and minimal invasive gastrointestinal interventions. It could facilitate the possibility of performing transesophageal extra and intracardiac based intervention under direct EUS guidance.
These applications highlights the importance of the interdisciplinary team work concept in diagnosis and treatment strategies especialy in accompaining symptoms and diseases. For example:
Patients with gastrointestinal tumor are immunocompromised and have a higher risk of pulmonary embolisms, ischemic events, and infective endocarditis. Routine cardiovascular investigation, particularry high risk patients, during gastrointestinal EUS investigation could early dedect thease pathologies. Thus, it could help for effective management strategy without delay, prevent double investigation and could improve outcomes.
The secondary pain in tumor patient may mask and overshadow the symptoms of other pathologies, such as pulmonary embolism.
Upper gastrointestinal pain may be due to angina pectoris/aortic dissection or a peptic ulcer.
Patients with gastrointestinal bleeding under oral anticoagulation are admitted primarly at the internal medicine and not the cardiology department. A left atrial appendage closure device helps in this context to reduce the rate of repeated hospital admissions, the rates of gastrointestinal bleeding events, and drug–drug interactions. It can improve the patient's quality of life, helps for effective resources usage, prevent double investigations, and improve outcomes.
Fevers of unknown origin could be due to infective endocarditis or abscess formation.
Gastrointestinal ischemic or thromboembolic events could be of primary cardiac origin.
Dyspnea of unknown cause could be a pulmonary embolism or pulmonary artery sarcoma. An EUS or endobronchial ultrasound (EBUS) could differentiate and identify both structures.
Intracardiac structures can be identified and evaluated for further differential diagnoses, such as intracardiac thrombus, myxoma, and tumors, using EUS with contrast.
Heyde syndrome is a multisystem disorder characterized by the triad of aortic stenosis (AS), gastrointestinal bleeding, and acquired von Willebrand syndrome [
38].
As explained, the introduction of endosonography in cardiology is feasible and allows a real-time assessment of cardiovascular anatomy and pathology during gastrointestinal procedures.
It could complement transesophageal echocardiography as ideal high resolution imaging modality for accurate definition of cardiovascular structures. In addition, feasibility of EUS-based cardiovascular intervention, by providing real-time sampling to confirm diagnosis, is an expanding new exciting field in both percutaneous and minimal invasive interventions und could help for avoidance of unnecessary explorative surgical interventions.
However, EUS requires more time for sterilization, preparation, and investigation. In addition, its main disadvantage is its higher cost. Moreover, three dimension -based technology to enhance the spatial understanding of EUS anatomy and to facilitate assessment of the relationship with major surrounding vessels is still underway in endosonographic Ultrasound [
10].
We hope to raise awareness among cardiologists and gastroentrologists of this diagonstic tool for both cardiovasculer and gastrointestinal diseases and highlights interdisciplinary teamwork benefits.
Finally, further studies are required to compare this procedure with other cardiac imaging modalities and to understand the exact applications and benefits of EUS in cardiovascular medicine as a diagnostic and therapeutic tool and could help in the future cardiovascular-based interventions.
Conclusions
The introduction of endosonography in cardiology allows for a high-resolution real-time assessment of cardiovascular anatomy and pathology during routine gastrointestinal procedures. Thus, it could help for optimal management strategy without delay, helps for effective resources usage, prevent double investigation and could improve outcomes.
The endosonographic ultrasound differs in the US tip design, possess an endoscope, has additional diagnostic tools such as contrast-enhanced EUS imaging and mapping of the elastic properties and stiffness of soft tissue. Thus, it could facilitate diagnosis, reduce sample error, and minimize unnecessary explorative surgical interventions.
It could complement TOE in the evaluation of cardiovascular structures.
In contrast to TOE, EUS could reduce the risk of esophageal trauma due to the direct visualization of the anatomy during introduction of the probe using a high-resolution camera. It Future Perspectives.
Feasibility of performing transesophageal extra and intracardiac based intervention under direct EUS guidance is a new exciting field in both percutaneous and minimal invasive interventions. Many serias cases are still required to evaluate the safty and efficacy of this investigation in cardiovascular medicine.
Future challenges include an integration of 3D technology, development of micromachined ultrasonic transducers (CMUTs) which are amenable to be integrated in several linear and ring arrays with different configurations and mounted on a catheter [
37], a higher image resolution, and a reduction of probe size. These will translate into more accuracy in diagnosis, a further expansion of clinical diagnostic applications and minimal invasive interventions.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org, Figure S1: Ultrasonographic ultrasound demonstrated the morphology of left atrium in one view and the relation to pulmonary artery; Figure S2: Ultrasonographic ultrasound demonstrated the morphology of left atrium in another views and the relation to aortic valve; Figure S3: Ultrasonographic ultrasound demonstrated the anatomy of ascending, and aortic arch respectively; Figure S4: Ultrasonographic ultrasound demonstrated the anatomy of abdominal aorta; Figure S5: Endosonographic ultrasound demonstrated a moderate left pleural effusion; Figure S6: Endosonographic ultrasound-guided punction and aspiration of lymph nodes; Video S1: Aortic valve in short axis view and tricuspid valve in endosonographic ultrasound; Video S2: Aortic valve and ascending aorta in long axis view using endosonographic ultrasound; Video S3: Mitral valve in two chamber view in endosonographic ultrasound; Video S4: left atrial appendage, circumflex coronary artery, left atrium and mitral valve in endosonographic ultrasound; Video S5: mitral valve with mitral clip in endosonographic ultrasound; Video S6: pulmonary valve and main pulmonary artery in endosonographic ultrasound; Video S7: left atrium in endosonographic ultrasound; Video S8: left atrium and left atrial appendage in another view in endosonographic ultrasound; Video S9: Left atrial appendage with thrombus in endosonographic ultrasound; Video S10: Left atrial appendage with thrombus without contrast uptake in endosonographic ultrasound; Video S11: Left ventricle und longitudinal and short axis view in endosonographic ultrasound.
Author Contributions
Conceptualization, A.E. and P.R.; methodology, A.E., P.S. and M.R.; M.E.; validation, A.E., P.R. and M.S.; formal analysis, A.E.; investigation, P.S. and M.R.; resources, A.E. and K.K.; data curation, M.S., A.E. and K.K.; writing—original draft preparation, A.E., K.K., M.E. and M.S.; writing—review and editing, M.S. and P.R.; visualization, M.E.; supervision, M.S.; project administration, A.E.; funding acquisition, A.E. All authors have read and agreed to the published version of the manuscript.
Funding
The authors received no financial support for the publication of this article.
Institutional Review Board Statement
Ethical review and approval were waived for this study as all procedures were during routine gastrointestinal endosonographic investigations.
Informed Consent Statement
Informed consent was obtained from all individual participants involved in the study.
Data Availability Statement
The datasets used and all clinical and investigation images and laboratory results during the current study are available from the corresponding author on reasonable request.
Acknowledgments
This work was supported by my wife Aline Wenner and my son Yousef. Great thank to Boris Baetge for his support and Kadir Aybar for his efforts and comments during review of the article.
Conflicts of Interest
The authors A.E., P.R., M.S., K.K., M.R., M.E. and P.S. declare that they have no competing interests. The authors whose names are listed in this manuscript certify that they have NO affiliations with or involvement in any organization or entity with a financial or non-financial interest in the subject matter or materials discussed in this manuscript. All authors confirmed that there are no relationships with any industry.
References
- Faletra, F.; Berrebi, A.; Pedrazzini, G.; et at. 3D transesophageal echocardiography: A new imaging tool for assessment of mitral regurgitation and for guiding percutaneous edge-to-edge mitral valve repair. Prog Cardiovasc. Dis. 2017, 60, 305–32. [CrossRef]
- Yamaguchi, T.; Rinsho, B. The function of the endoscope. Jpn. J. Clin. Pathol. 1990, 38, 399–402.
- Freeman, R.; Otto, C. Diagnostic Echocardiography (Ultrasound Imaging in Cardiovascular Diagnosis). Thoracic Key, Fastest Thoracic Insight Engine, 2016. Available online: https://thoracickey.com/diagnostic-echocardiography-ultrasound-imaging-in-cardiovascular-diagnosis/ (accessed on Dez. 2022).
- Heydarian, H.; Kimball, T. Echocardiography: Basic Principles and Imaging, Thoracic key, Fastest Thoracic Insight Engine, 2016. Available online: https://thoracickey.com/echocardiography-basic-principles-and-imaging/.
- Murad, F.; Komanduri, S.; Dayyeh, B.; et al. Gastrointestinal endoscopy. Echoendoscopes 2015, 82, 189–202.
- Currie, P. Transesophageal Echocardiography New Window to the Heart. AHA J. 1989, 80, 215–217.
- Mohamed, A.; Arifi, A.; Omran, A. The basics of echocardiography, journal of the Saudi Heart Association. 2010, 22, 71–76. [CrossRef]
- Sonaglioni, A.; Nicolosi, G.; Rigamonti, E.; et al. Molecular Approaches and Echocardiographic Deformation Imaging in Detecting Myocardial Fibrosis. Int. J. Mol. Sci. 2022, 23, 10944. [CrossRef]
- DiMagno, E.; DiMagno, M. Endoscopic ultrasonography: from the origins to routine EUS. Dig. Dis. Sci. 2016, 61, 342–353. [CrossRef]
- Săftoiu, A. State-of-the-art imaging techniques in endoscopic ultrasound. World J. Gastroenterol. 2011, 17, 691–696. [CrossRef]
- Fujifilm. Available online: https://healthcaresolutions-us.fujifilm.com/products/endoscopy/interventional-gastroenterology/eus-scopes accessed on 17 9 2023).
- Olympus. Available online: https://www.olympus.de/medical/media/local_content/EUS_Brochure_EN_E0428739_52885.pdf (accessed on 9 2023).
- Elhakim, A.; Karkour, K.; Sauter und, P.; et al. The Role of Endosonography in Cardiology: Case Series and Literature Review. Eur. Heart J. Imaging Methods Pract. 2023, qyad002. [CrossRef]
- Miner, N. Cleaning, Disinfection and Sterilization of Heat-Sensitive Endoscopes. Endoscopy. InTech, Apr. 2012, 30. [CrossRef]
- Medical, P. Endoscopic Ultrasound EG-3870UTK. Available online: https://www.pentaxmedical.com/pentax/download/fstore/uploadFiles/Pdfs/Product%20Datasheets/EMEA_PROD_ENDO_EG_3870URK_08.08.17.pdf.
- Malay, S.; Fusaroli, P.; Löwe, A.; et al. General principles of image optimization in EUS. Endosc. Ultrasound 2021, 10, 168–184. [CrossRef]
- Epiq-7-Ultrasound-System-for-Cardiology#Specifications, Philips. Available online: https://www.usa.philips.com/healthcare/product/HC795200C/ (accessed on 12 2022).
- Valencia, L. First-in-Human Navigation Endoscopic Ultrasound (EUS) System Clinical Study (APEUS-Nav1). Available online: https://clinicaltrials.gov/ct2/show/study/NCT05515705 (accessed on 12 12 2022).
- Ng, A.; Wanevelder, J. Resolution in ultrasound imaging. Contin. Educ. Anesth. Crit. Care Pain 2011, 11, 186–192. [CrossRef]
- Jang, J. The past, present, and future of image-enhanced endoscopy. Clin. Endosc. 2015, 48, 466–475. [CrossRef]
- EG 3870UTK Ultrasound Video Endoscope, Pentax Medical. Available online: https://www.pentaxmedical.com/pentax/en/95/1/EG-3870UTK-Ultrasound-Video-Endoscope (accessed on 8 2022).
- Anderson, M. Endoscope Camera: Medical Imaging Camera Quality, ATL Technology, 2021. Available online: https://atltechnology.com/blog/endoscope-camera-imaging-camera-quality/.
- Kinza, S.; Sawhney, M.; Pleskow, D.; et al. The Use of Standard Gastrointestinal Endoscopic Ultrasound to Assess Cardiac Anatomy. Cardiovasc. Anesthesiol. Orig. Clin. Res. Rep. 2016, 123, 547–550. [CrossRef]
- Jenssen, C.; Hocke, M.; Fusaroli, P.; et al. EFSUMB guidelines on interventional ultrasound (INVUS) part IV: EUSguided interventions: general aspects and EUS-guided sampling (short version). Ultraschall Med. 2016, 37, 157–169. [CrossRef]
- Krishnan, S.; Ngai und, J.; Kanchuge, M. Complications of Transesophageal Echocardiography, Thoracic KeyFastest Thoracic Insight Engine, 2016. Available online: https://thoracickey.com/complications-of-transesophageal-echocardiography/.
- Von Bartheld, M.; Van Breda und, A.; Annema, J. Complication rate of endosonography (endobronchial and endoscopic ultrasound): A systematic review. Respiration 2014, 87, 343–351. [CrossRef]
- Kirkpatrick, J.N.; Wong, T.; Bednarz, J.E.; et al. Differential diagnosis of cardiac masses using contrast echocardiographic perfusion imaging. J. Am. Coll. Cardiol. 2004, 43, 1412–1419. [CrossRef]
- Dhar, J.; Samanta, J. Endoscopic ultrasound-guided vascular interventions: An expanding paradigm. World J. Gastrointest. Endosc. 2023, 15, 216–239. [CrossRef]
- Fritscher-Ravens, A.; Ganbari, A.; Mosse, C.A.; et al. Transesophageal endoscopic ultrasound guided access to the heart. Endoscopy 2007, 39, 385–389. [CrossRef]
- Gornals, J.B.; de la Hera, M.; de Albert, M.; et al. EUS cardiac puncture-guided right atrial tumor. Gastrointest Endosc. 2015, 82, 165. [CrossRef]
- Haja, A.; Lakhtakia, S.; Sekaran, A.; et al. Cardiac sarcoma diagnosed with EUS-FNB. Endosc. Int. Open. 2021, 9, E152–E153. [CrossRef]
- Larghi, A.; Stobinski, M.; Galasso, D. EUS-guided drainage of a pericardial cyst: closer to the heart (with video). Gastrointest. Endosc. 2009, 70, 1273–1274. [CrossRef]
- Martinez, H.A.; Kuijvenhoven, J.C.; Annema, J.T. Intracardiac EUS-B-Guided FNA for Diagnosing Cardiac Tumors. Respiration 2021, 100, 918–922. [CrossRef]
- Medgadget, Hitachi and PENTAX Collaborate on HI VISION Preirus Ultrasound, Fujifilm Endoscopy, 2013. Available online: https://www.medgadget.com/2013/05/hi-vision-preirus.html (accessed on 08 12 2022).
- Seicean, A.; Mosteanu, O.; Seicean, R. Maximizing the endosonography: The role of contrast harmonics, elastography and confocal endomicroscopy. World J. Gastroenterol. 2017, 23, 25–41. [CrossRef]
- Jeong, S.H.; Yoon, H.H.; Kim, E.J.; et al. High-resolution endoscopic ultrasound imaging and the number of needle passages are significant factors predicting high yield of endoscopic ultrasound-guided fine needle aspiration for pancreatic solid masses without an on-site cytopathologist. Medicine 2017, 96, e5782. [CrossRef]
- Wells, P.; Liang, H. Medical ultrasound: imaging of soft tissue strain and elasticity. J. R. Soc. Interface 2011, 8, 1521–1549. [CrossRef]
- Theis, S.; Turner, S. Heyde Syndrome, StatPearls Publishing, Treasure Island (FL), 11 Jul. 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK551625/.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).