1. Introduction
Acute kidney injury (AKI) is often related to sepsis in critically ill patients, secondary to the dysfunction of other organs or expression of the system-wide endothelial damage caused by hyper-inflammation and positive fluid balance [
1]. Sepsis-induced AKI (sAKI) is a strong risk factor for mortality and adverse outcomes, and achieving a precocious diagnosis for suitable interventions is the major challenge in clinical practice, improving renal recovery and global patient outcomes.
A solution: identify a marker or a panel of markers detecting kidney injury before the glomerular filtration rate (GFR) reduction, highlighting sub-clinical AKI compensated by the renal functional reserve that can be lost after a pathological noxa. The definition of AKI, according to KDIGO criteria, is based on serum creatinine and urinary output, neither sensitive not specific, considering many hours or days occur after the damage before their alterations, not evaluating potential variations depending from several patient’s characteristics. The consequent GFR reduction is not synchronous with the renal damage, and these two parameters vary with delay [
2].
Numerous biomarkers have been investigated to predict AKI, such as neutrophil gelatinase-associated lipocalin (NGAL), tissue inhibitor metal proteinase (TIMP)-2, and insulin growth factor binding protein (IGFBP)-7, improving the diagnosis, and giving prognostic information about the timing to start or stop a renal replacement therapy (RRT) [
3,
4,
5]. In 2014, the American Food and Drug Administration and the European Medicines Agency approved the use of TIMP2*IGFBP7, urinary markers of cell cycle arrest reflecting cellular stress preceding tissue damage, to aid in the early prediction of AKI, with advantages in terms of diagnostic precocity and prognosis, when compared to creatinine arise or urinary output reduction [
6]. A diagnostic superiority in terms of sensitivity and specificity of this test was also revealed when compared to other renal biomarkers, such as urine or plasma NGAL, with a better prediction of moderate to severe AKI (KDIGO stage 2 to 3) in more than one thousand critically ill patients [
7].
In the setting of sAKI, mid-regional pro-adrenomedullin (MR-proADM) is involved in capillary leakage, endothelial dysfunction, and multiple organ failure development [
8]. MR-proADM is the precursor of the active form of adrenomedullin (ADM), a calcitonin peptide like procalcitonin belonging to the calcitonin peptide family. MR-proADM is a stable fragment of 48 amino acids of the proADM molecule, with a short half-life of 22 minutes, proportionally reflecting the ADM blood levels as result of the split off from ADM in a 1:1 ratio [
9]. ADM, produced predominantly by vascular endothelial cells, has a range of systemic biological actions including vasodilatation, increasing the cyclic adenosine monophosphate (cAMP) levels and the formation of nitric oxide, cell growth, regulation of hormone secretion, and antimicrobial effects [
10]. At the renal level, its high expression in the glomeruli and tubules, assessed by immune-histochemical analyses, revealed diuretic and natriuretic actions through the tubules and vasodilatory actions, increasing GFR and the renal blood flow, dilating afferent and efferent arterioles [
11]. Several studies demonstrated the relationship between MR-proADM levels, organ dysfunction, and endothelial damage severity [
12,
13]. High MR-proADM values characterized patients with sepsis, chronic kidney disease (CKD), or cardiac impairment due to myocardial infarction or chronic heart failure, with prognostic implications [
11,
14,
15].
In CKD, ADM levels were markedly increased if compared with the normal controls and related to the disease severity, irrespective of the basal renal disease [
16]. Systemic inflammation and endothelial dysfunction characterized patients undergoing cardiac surgery. The hemodynamic instability, based on intravascular low volume, scarce cardiac preload, and anasarcatic state, promoting hypotension in a vicious circle, leads to shock and organ injury, including AKI [
17].
Starting from these assumptions, this study evaluated a panel of biomarkers, including TIMP2*IGFBP7, and MR-proADM in patients with AKI developed after cardiac surgery. Diagnostic properties have been assessed as a precocious diagnosis of AKI if compared to creatinine and urine output. Prognostic information was also analyzed, evaluating the start of renal replacement therapy and the mortality rate in the intensive care unit.
2. Patients and Methods
2.1. Study Design
This observational prospective cohort single-centre study was conducted in the Intensive Care Unit (ICU) at the Papardo Hospital, Messina, Italy, enrolling two hundred and thirty patients admitted to the ICU between January 2021 and December 2022 and undergoing coronary artery bypass surgery (CABG). Data were collected by the investigators and analyzed by external statisticians. All patients underwent conventional open-heart surgery using aortic cross-clamping, cardioplegic myocardial arrest, and cardiopulmonary bypass (CPB). After surgery, all patients were transferred to the cardiac surgery ICU. Surgery has always been performed by the same experienced surgeons (FP, FC) on normothermic CPB with aortic cross-clamping and intermittent hyperkalemic blood cardioplegia.
The inclusion criteria were: age ≥18 years and urinary catheter in place for at least 48 hours, while exclusion criteria were: age <18 years, patients with anuria or with diuresis less than 30 ml within 24 hours of ICU admission, and a presumed life expectancy of fewer than 48 hours after admission. Patients on hemodialysis or with a GFR < 15 ml/min were not enrolled. Moreover, patients were excluded if they underwent coronarography or radiologic procedures with iodinated contrast agents during the week before the surgery day.
2.2. Measurement of Biomarkers
Sera and urine samples for biomarker profiling evaluation were collected before the cardiac surgery and after 4 hours from the ICU admission.
All blood samples were stored at - 80°C, whereas urinary tests were immediately performed. All the biomarkers were measured at the Papardo Hospital laboratory by standard protocols in a technician-blinded manner. An additional blood sample was collected on the day of the diagnosis of sepsis and then stored at - 80 °C to measure serum MR-proADM retrospectively.
Estimated GFR was calculated by the CKD-Epidemiology Collaboration (CKD EPI) Equation [
18].
2.3. Definitions
Clinical data recorded from the medical records included demographics, comorbidities, laboratories, and biomarker levels.
CKD was classified according to the Kidney Disease: Improving Global Outcomes (KDIGO) 2012 Clinical Practice Guideline for the Evaluation and Management guidelines [
19], whereas AKI was classified using the criteria in kidney disease: Improving Global Outcomes (KDIGO) [
20].
Patients were followed up during the entire ICU stay and the Sequential Organ Failure Assessment (SOFA) score assessed the organ dysfunctions [
21].
Sepsis or septic shock were defined according to the SEPSIS-3 consensus. In particular, sepsis was defined as life-threatening organ dysfunction caused by a dysregulated host response to infection. Organ dysfunction can be identified as an acute change in total SOFA score ≥2 points consequent to the infection. Patients with septic shock can be identified with a clinical construct of sepsis with persisting hypotension requiring vasopressors to maintain MAP ≥65 mm Hg and having a serum lactate level >2 mmol/L (18mg/dL) despite adequate volume resuscitation [
22].
2.4. Outcomes
The primary endpoint was the diagnosis of AKI evaluating the TIMP2*IGBP7 levels and the MR-proADM to identify septic patients. The secondary prognostic endpoint of MR-proADM was the start of renal replacement therapy and the 30-day survival.
2.5. Statistical Analysis
Statistical analyses were performed with NCSS for Windows (version 4.0), the MedCalc (version 20.115; MedCalc Software Acacialaan, Ostend, Belgium) software, and the GraphPad Prism (version 9.4.1; GraphPad Software, Inc., San Diego, CA, USA) package.
Differences between groups were established by unpaired t-test or by ANOVA followed by Bonferroni's test for normally distributed values and by Kruskal-Wallis analysis followed by Dunn's test for nonparametric values. Correlation coefficients were used as appropriate to test correlations between biomarkers and other variables. Before correlations were tested, all non-normally distributed values were log-transformed to better approximate normal distributions.
Receiver-operating characteristics (ROC) analysis was employed to calculate the area under the curve (AUC) for TIMP2*IGBP7 and MR-proADM to find the best cut-off values identifying AKI and septic status, respectively. Kaplan–Meier curves were generated to assess the progression to the endpoint, defined as the start of the renal replacement therapy (RRT) in subjects with serum MR-proADM and urinary TIMP2*IGBP7 values above and below the optimal ROC-derived cut-off levels. Differences were evaluated using the log-rank test.
The local Human Research Ethics Committee approved the study; the study complied with the Declaration of Helsinki.
3. Results
3.1. Baseline Characteristics of the Study Population
The mean age was 65.3±7.9 years, and 63% of patients were male; one hundred fifty-three patients (66%) had diabetes, whereas blood arterial hypertension was detected in one hundred sixty-six patients (72%). Heighty-nine (38%) subjects were active cigarette smokers, while chronic obstructive pulmonary disease (COPD) was revealed in ninety-four patients (41%). CKD was assessed in one hundred-two subjects (44%) with a mean serum creatinine of 1.80±0.9 mg/dl, determining a mean eGFR of 41.8±18 ml/min (IQ range 20 to 55 ml/min). According to the baseline stages of GFR, sixty-height patients belonged to stage III, whereas 34 patients were included in stage IV. Nine-two patients (40%) developed AKI after cardiac surgery and sixty-two required RRT during the ICU stay.
3.2. TIMP2*IGBP7 and Diagnosis of AKI
Baseline TIMP2*IGBP7 levels were in the normal range in all enrolled patients at the pre-surgery evaluation (0.08±0.02). This datum was not influenced by a pre-existing CKD, without differences assessed in patients with (0.10±0.03 mg/l) or without the renal disease (0.07±0.02 mg/l), p > 0.05). After 23.2 (12.7 – 36.5) hours from cardiac surgery, creatinine and urine output alterations occurred, whereas urinary TIMP2*IGBP7 levels were higher at 4 hours in patients who developed AKI when compared to baseline levels (1.1±0.4 mg/l vs. 0.08±0.02 mg/l; p < 0.001). Conversely, in patients without AKI, we did not reveal differences between the two measurements (0.12±0.03 mg/l vs. 0.08±0.02 mg/l; p: 0.12). Furthermore, TIMP2*IGBP7 levels were related to AKI severity, considering that a concentration >2 mg/l assessed after 4 hours from cardiac surgery increases the risk of KDIGO 3 AKI within the next 24 hours, clearly identifying the population at high risk of AKI requiring renal replacement therapy (RRT). Conversely, values of this biomarker below 0.5 ruled out any need for renal replacement in the next 48 hours of observation.
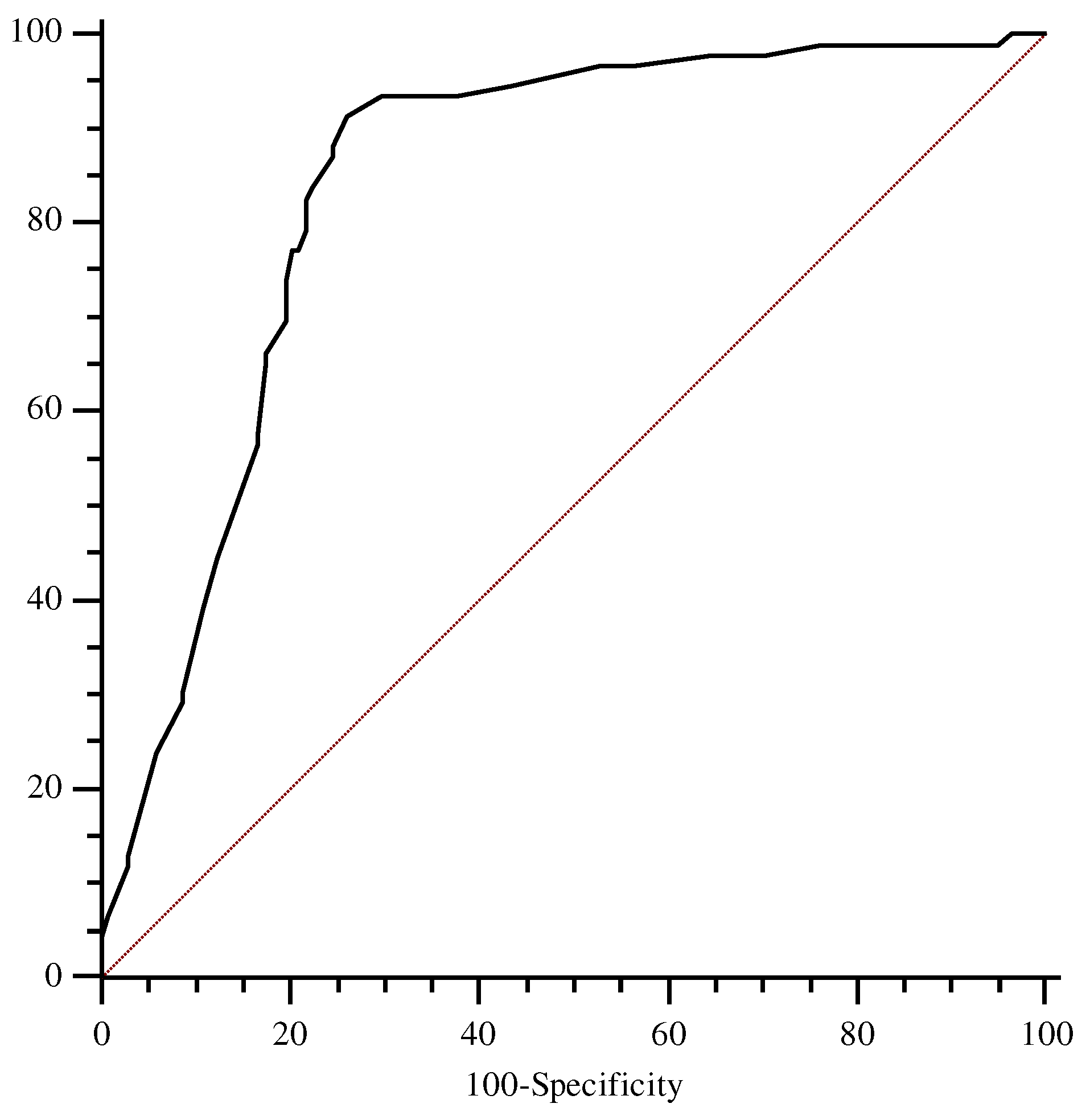
ROC analysis showed an AUC for this marker of 0.78 (95% CI, 0.68 to 0.85), with the best cut-off level found to be 2.0 mg/l (sensitivity 83.9%, specificity 73.8%) (
Figure 1).
3.3. Septic Patients
Sepsis was diagnosed in 83 patients (36%), including 21 subjects with septic shock. In septic patients, median MR-proADM levels were 2.3 nmol/l (0.7–7.8 nmol/l), whereas the highest values characterized patients with septic shock [5.6 nmol/l (3.2–18 nmol/l)]. AKI was observed in 40% of septic patients, with the highest prevalence involving patients with septic shock. In particular, 60 out of 83 patients (72%) were treated with RRT. In these patients, the highest mean values of TIMP2*IGBP7 were recorded after 4 hours from cardiac surgery (3.2±1.1 mg/l) and were associated with the highest values of MR-proADM. MR-proADM positively related with the SOFA score at the ICU admission (r = 0.51; p: 0.001), procalcitonin (r = 0.63; p < 0.0001), C reactive protein (r = 0.38; p: 0.01), and lactate (r = 0.49; p: 0.003), whereas this marker was inversely related with mean blood pressure (r = -0.28; p:0.02) and eGFR (r = - 0.41; p:0.01). After multivariate analysis, the correlation with SOFA score (β: 0.31, p: 0.01), lactate (β: 0.401, p:0.001), and mean blood pressure (β = - 0.33; p:0.01) remained significant.
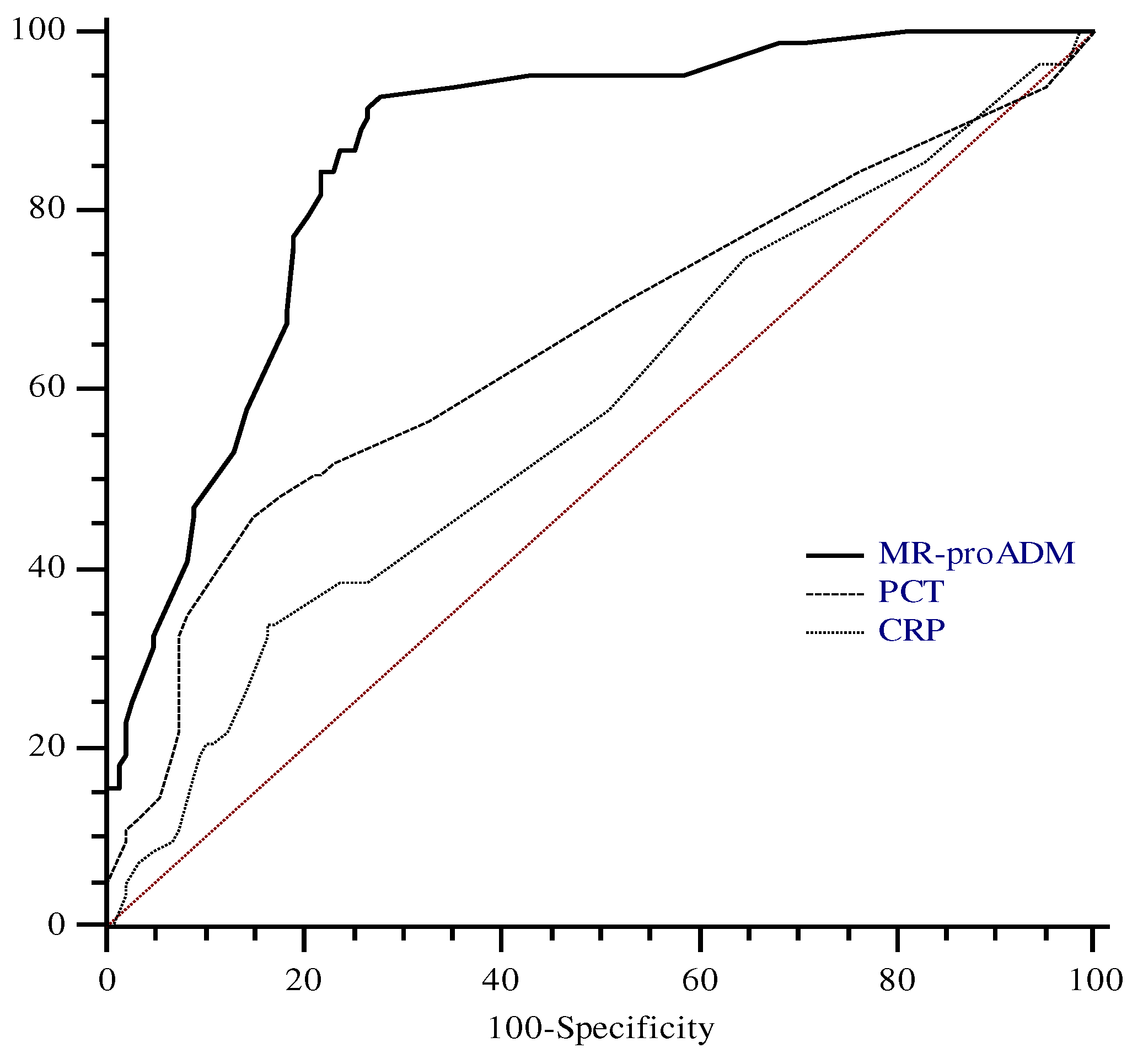
To define the optimal diagnostic cut-off for MR-proADM values to detect sepsis in our cohort, we performed ROC analysis including only data from patients affected by sepsis or septic shock. ROC analysis was performed with CRP and PCT. The AUC for MR-proADM on admission was 0.88 with the best cut-off at 5.1 nmol/l, determining a sensitivity and a specificity of 78.5% and 85%, respectively.
The area under the curve for PCT, and CRP was 0.65 (95% CI, 0.59 to 0.71) and 0.57 (95% CI, 0.50 to 0.64), respectively. Both PCT and CRP areas were significantly different than that of MR-proADM (p: < 0.001). On the contrary, the difference between the PCT and CRP areas was not significant (p: 0.17) (
Figure 2).
3.4. Renal Replacement Therapy in Septic Patients
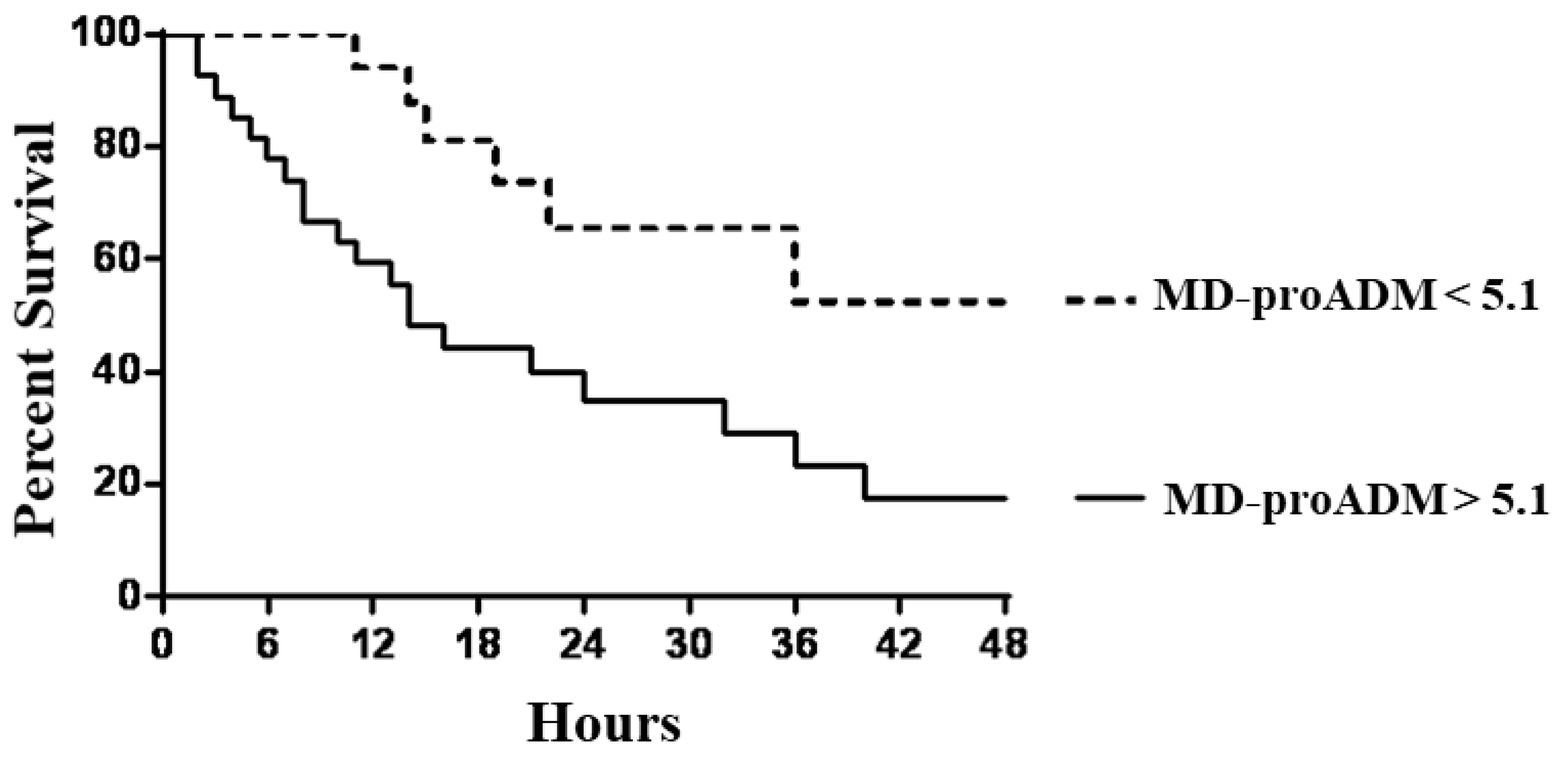
We investigated the potential role of MR-proADM associated with TIMP2*IGBP7 to predict the necessity of RRT in septic patients. ROC curve analysis revealed that MR-proADM at the cut-off > 5.1 nmol/l identified septic patients with a very good diagnostic profile. At the same time, AKI was well defined by TIMP2*IGBP7 cut-off value > 2 mg/l. Subjects with MR-proADM values above 5.1 nmol/l experienced a significantly faster evolution to the endpoint, defined as the start of RRT (p: 0.002), with a mean follow-up time to progression of 2.5.days (95% CI, 1.1 to 4.6) compared with 9.2 days (95% CI, 6.3 to 12.1) for MR-proADM below the cut-off (
Figure 3).
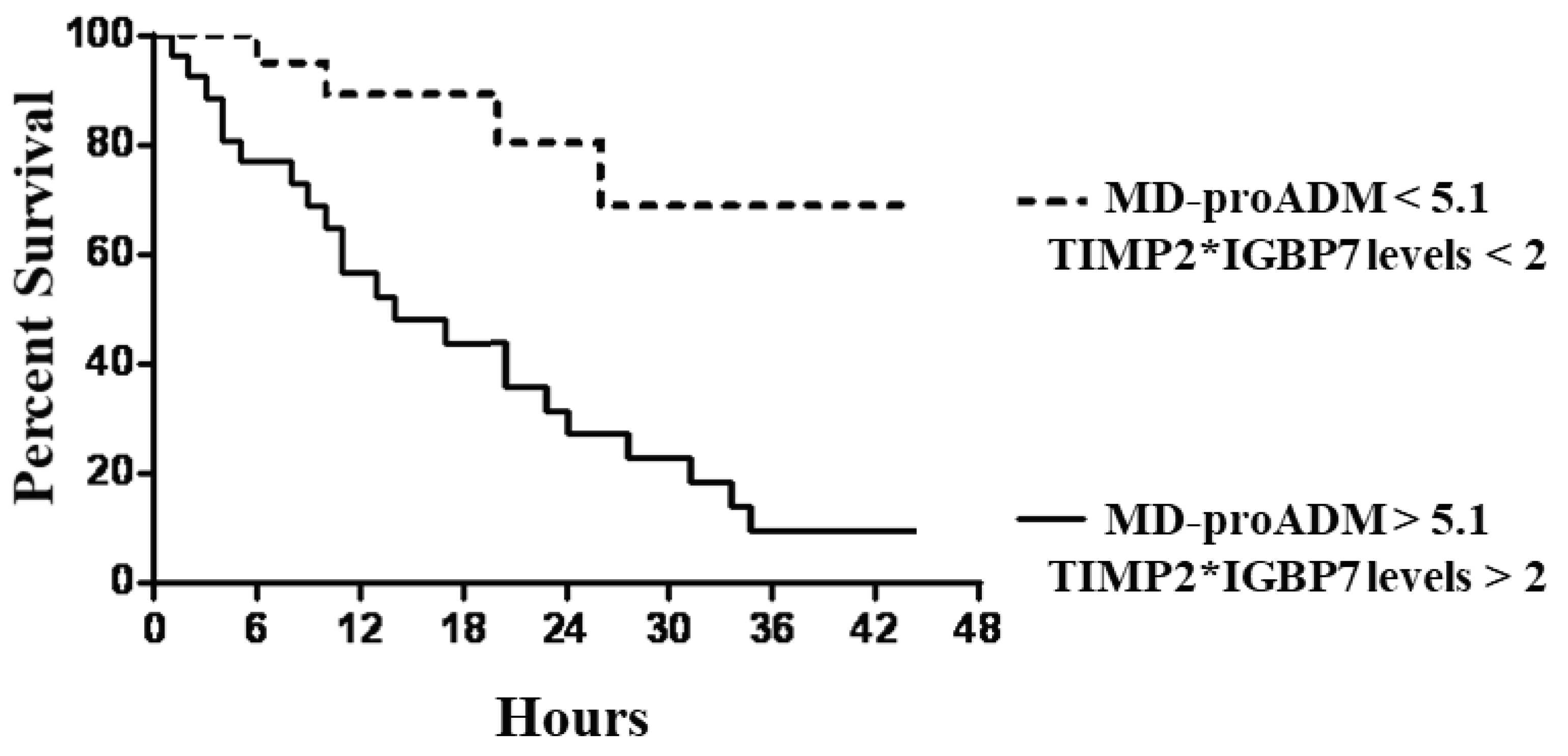
Similar but stronger reports were evidenced if subjects were categorized according to MR-proADM values associated with TIMP2*IGBP7 levels. In particular, subjects with MR-proADM values above 5.1 nmol/l combined with urine TIMP2*IGBP7 levels > 2 mg/l showed a significantly faster progression to the endpoint (p < 0.0001), with a mean follow-up time of 1.1 days (95% CI, 0.7 to 1.9) (
Figure 4).
The 30-day all-cause mortality rate was very high in septic patients, involving 49 out of 83 subjects (59%). The most common causes of mortality included sepsis-induced multiple organ failure (n = 20; 41%), refractory septic shock (n = 18; 36%), and acute respiratory insufficiency (n = 11; 23%). In general, non-surviving patients had significantly higher concentrations of MR-proADM, PCT, and lactate, as well as higher SOFA scores than survivors. This entire cohort experienced AKI events during the ICU stay and was treated by RRT.
4. Discussion
This study demonstrated that urinary TIMP2*IGFBP7 represents a valid biomarker to highlight the high risk for AKI after cardiac surgery. Urine values > 2.0 mg/l were strongly associated with AKI, with good sensitivity and specificity at 4 hours after CABG, predicting the requirement of RRT in critically septic patients and septic shock. These data strengthened the conclusions of a recent meta-analysis, showing a good diagnostic property of this biomarker, characterized by an AUROC of 0.83 for the prediction of AKI within 24 h after cardiac surgery [
23].
In the last two decades, several biomarkers have been tested, but unfortunately, none of them is today used in clinical practice. This failure is related to their costs, availability, and issues related to low sensitivity or specificity. The complexity of the patients negatively influences the research in this field, which should be addressed through a combination of biomarkers. Moreover, different diagnostic criteria were used, and too many methods were applied in various trials, reducing the possibility to obtain uniform and valid conclusions, which could enhance the knowledge of physiopathology, incidence, outcomes, and risk factors for AKI.
This study underlined that TIMP2*IGFBP7 was not influenced by a pre-existing CKD, often characterizing these patients, partially solving the issue observed for NGAL, whose values were higher in urine or sera in CKD patients, independent of AKI [
24]. According to TIMP2*IGFBP7 levels, AKI was diagnosed 23 hours before creatinine or urine output alterations. The precocity of the diagnosis is the key to nephrological management in ICU, evaluating the patient at risk of renal damage from a multidisciplinary point of view, improving hemodynamic and fluid status to preserve an optimal kidney perfusion pressure, and avoiding nephrotoxic agents. The gold standard definition of AKI, based on the KDIGO criteria, refers to kidney function, but not damage, with a consequence: the impossibility to detect structural, subclinical injury in functioning kidneys. A hybrid evaluation, adding a panel of biomarkers, could improve the diagnostic accuracy in these patients.
Moreover, a late AKI diagnosis could be the cause for the failure of several trials to reveal efficacy results after specific treatments, including drugs or RRT, failed to modify the disease course [
25,
26]. If the precocity of AKI diagnosis improves the prognosis of postoperative cardiac surgery patients, this assumption is more suitable for sepsis. Early indicators of organ or immune dysfunctions are essential to guide the most appropriate therapeutic intervention at the earliest opportunity [
27,
28].
The diagnosis of sepsis is based on two cornerstones: the SOFA score, representing the sepsis-related organ dysfunction, and serum lactate, indicating a deterioration in tissue perfusion [
22].
Our study assessed and confirmed that sepsis is a not-negligible clinical problem after cardiac surgery, with high prevalence and a negative impact on patient survival. We found a significant increase in MR-proADM in septic patients, compared with critical patients without infections, with an independent relation with SOFA and lactate levels. Moreover, behind its diagnostic properties, its levels mirrored prognostic information relating to the severity of the septic status. The highest levels were recorded in patients with severe sepsis or septic shock requiring RRT, with better profiles than other classical biomarkers, such as CRP or PCT. Furthermore, as a prognostic marker, MR-proADM levels were significantly higher in septic patients who did not survive than in survivors. Our data suggest the inclusion of MR-proADM evaluation into an early sepsis management protocol, allowing for prognostic classification of septic patients, guiding early diagnostic interventions, and facilitating intensive treatment before any organ dysfunction.
Similar data were described by Elke, revealing that initial use of MRproADM within the first 24 h after sepsis diagnosis resulted in the strongest association with short-term, mid-term, and long-term mortality compared to all other biomarkers or clinical scores [
8].
Our data confirmed the results obtained by the SISPCT trial which enrolling more than one-thousand patients with severe sepsis and septic shock patients, demonstrated that MR-proADM more accurately predicted RRT requirement in the first week after the ICU admission [
29].
Recently, a meta-analysis revealed that high MR-proADM involved Covid-19 patients with a negative outcome, predisposing these subjects to unfavorable prognosis [
30].
The present study has some limitations that should be mentioned. First, it was a single-center study, and the cohort of patients was relatively small. Confirmation in wider cohorts is indispensable to attribute general validity to our reports. Moreover, we did not evaluate MR-proADM as a precocious marker of treatment failure, not evaluating its potential changes during ongoing antimicrobial therapy. Interventional studies to confirm these hypotheses are essential to identify not responder patients who need alternative diagnostic and therapeutic interventions.
5. Conclusions
An associated evaluation of TIMP2*IGBP7 and MR-proADM precociously diagnoses AKI in septic patients after cardiac surgery, giving prognostic information for renal replacement therapy requirement and mortality risk.
Author Contributions
Conceptualization, A.L., F.P., and P.M.; methodology, F.C.; S.C.; validation, D.C., G.B. and E.P.; formal analysis, F.C. and A.L.; investigation, A.L.; P.M.; data curation, A.L.; F.C.; writing—original draft preparation, A.L.; S.C. writing—review and editing, A.L.; S.C.; supervision, A.L.; F.P.; P.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee for studies involving humans .
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
The data associated with the paper are not publicly available but are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Uchino, S.; Kellum, J.A.; Bellomo, R.; Doig, G.S.; Morimatsu, H.; Morgera, S.; Schetz, M.; Tan, I.; Bouman, C.; Macedo, E.; Gibney, N.; Tolwani, A.; Ronco, C.; Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators. Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 2005, 294, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Lacquaniti, A.; Caccamo, C.; Salis, P.; Chirico, V.; Buemi, A.; Cernaro, V.; Noto, A.; Pettinato, G.; Santoro, D.; Bertani, T.; Buemi, M.; David, A. Delayed graft function and chronic allograft nephropathy: diagnostic and prognostic role of neutrophil gelatinase-associated lipocalin. Biomarkers 2016, 21, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Bolignano, D.; Coppolino, G.; Romeo, A.; Lacquaniti, A.; Buemi, M. Neutrophil gelatinase-associated lipocalin levels in chronic haemodialysis patients. Nephrology 2010, 15, 23–26. [Google Scholar] [CrossRef]
- Tao, X.; Chen, C.; Luo, W.; Zhou, J.; Tian, J.; Yang, X.; Hou, F.F. Combining renal cell arrest and damage biomarkers to predict progressive AKI in patient with sepsis. BMC Nephrology 2021, 22, 415. [Google Scholar] [CrossRef]
- Murray, P.T.; Mehta, R.L.; Shaw, A.; Ronco, C.; Endre, Z.; Kellum, J.A.; Chawla, L.S.; Cruz, D.; Ince, C.; Okusa, M.D. ADQI 10 workgroup Potential use of biomarkers in acute kidney injury: report and summary of recommendations from the 10th Acute Dialysis Quality Initiative consensus conference. Kidney Int 2020, 180, 513–521. [Google Scholar]
- Godi, I.; Kashani, K.; Boteanu, R.; Martino, F.; Carta, M.; Giavarina, D.; Ronco, C. Clinical adoption of Nephrocheck® in the early detection of acute kidney injury. Annals of clinical biochemistry 2021, 58, 6–15. [Google Scholar]
- Kashani, K.; Al-Khafaji, A.; Ardiles, T.; Artigas, A.; Bagshaw, S.M.; Bell, M.; Bihorac, A.; Birkhahn, R.; Cely, C.M.; Chawla, L.S.; Davison, D.L.; Feldkamp, T.; Forni, L.G.; Gong, M.N.; Gunnerson, K.J.; Haase, M.; Hackett, J.; Honore, P.M.; Hoste, E.A.; Joannes-Boyau, O.: Kellum, J. A. Discovery and validation of cell cycle arrest biomarkers in human acute kidney injury. Crit care 2013, 17, R25.
- Elke, G.; Bloos, F.; Wilson, D.C.; Brunkhorst, F.M.; Briegel, J.; Reinhart, K.; Loeffler, M.; Kluge, S.; Nierhaus, A.; Jaschinski, U.; Moerer, O.; Weyland, A.; Meybohm, P. SepNet Critical Care Trials Group The use of mid-regional proadrenomedullin to identify disease severity and treatment response to sepsis – a secondary analysis of a large randomised controlled trial. Crit Care 2018, 22, 79. [Google Scholar] [CrossRef]
- Morgenthaler, N.G.; Struck, J.; Alonso, C.; Bergmann, A. Measurement of midregional proadrenomedullin in plasma with an immunoluminometric assay. Clin Chem 2005, 51, 1823–1829. [Google Scholar] [CrossRef]
- Hinson, J.P.; Kapas, S.; Smith, D.M. Adrenomedullin, a multifunctional regulatory peptide. Endocrine reviews 2000, 21, 138–167. [Google Scholar]
- Nishikimi, T. Adrenomedullin in the kidney-renal physiological and pathophysiological roles. Current medicinal chemistry 2007, 14, 1689–1699. [Google Scholar] [CrossRef]
- Temmesfeld-Wollbrück, B.; Brell, B.; Dávid, I.; Dorenberg, M.; Adolphs, J.; Schmeck, B.; Suttorp, N.; Hippenstiel, S. Adrenomedullin reduces vascular hyperpermeability and improves survival in rat septic shock. Intensive Care Med 2007, 33, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Baldirà, J.; Ruiz-Rodríguez, J.C.; Wilson, D.C.; Ruiz-Sanmartin, A.; Cortes, A.; Chiscano, L.; Ferrer-Costa, R.; Comas, I.; Larrosa, N.; Fàbrega, A.; González-López, J.J.; Ferrer, R. Biomarkers and clinical scores to aid the identification of disease severity and intensive care requirement following activation of an in-hospital sepsis code. Annals of intensive care 2020, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- Christ-Crain, M.; Morgenthaler, N.G.; Struck, J.; Harbarth, S.; Bergmann, A.; Müller, B. Mid-regional pro-adrenomedullin as a prognostic marker in sepsis: an observational study. Crit care 2005, 9, R816–R824. [Google Scholar] [CrossRef] [PubMed]
- Maisel, A.; Mueller, C.; Nowak, R.; Peacock, W.F.; Landsberg, J.W.; Ponikowski, P.; Mockel, M.; Hogan, C.; Wu, A.H.; Richards, M.; Clopton, P.; Filippatos, G.S.; Di Somma, S.; Anand, I.; Ng, L.; Daniels, L.B.; Neath, S.X.; Christenson, R.; Potocki, M.; McCord, J.; Anker, S.D. Mid-region pro-hormone markers for diagnosis and prognosis in acute dyspnea: results from the BACH (Biomarkers in Acute Heart Failure) trial. JACC 2010, 55, 2062–2076. [Google Scholar] [CrossRef]
- Nishikimi, T.; Horio, T.; Kohmoto, Y.; Yoshihara, F.; Nagaya, N.; Inenaga, T.; Saito, M.; Teranishi, M.; Nakamura, M.; Ohrui, M.; Kawano, Y.; Matsuo, H.; Ishimitsu, T.; Takishita, S.; Matsuoka, H.; Kangawa, K. Molecular forms of plasma and urinary adrenomedullin in normal, essential hypertension and chronic renal failure. Journal of hypertension 2001, 19, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Hall, R. Identification of inflammatory mediators and their modulation by strategies for the management of the systemic inflammatory response during cardiac surgery. J Cardiothorac Vasc Anesth 2013, 27, 983–1033. [Google Scholar] [CrossRef]
- Levey, S.A.; Stevens, A.L.; Schmid, H.C.; Zhang, Y.P.; Castro, F.A.; Feldman, I.H; et al for the chronic kidney disease epidemiology collaboration (CKD-EPI). A new equation to estimate glomerular filtration rate. Ann Intern Med 2009, 150, 604–612.
- Eknoyan, G.; Lameire, N.; Eckardt, K.; Kasiske, B.; Wheeler, D.; Levin, A. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int 2013, 3, 5–14. [Google Scholar]
- KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl 2012, 2, 1–138.
- Vincent, J. L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonça, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the european society of intensive care medicine. Intensive Care Med 1996, 22, 707–10. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; Hotchkiss, R.S.; Levy, M.M.; Marshall, J.C.; Martin, G.S.; Opal, S.M.; Rubenfeld, G.D.; van der Poll, T.; Vincent, J.L.; Angus, D.C. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–10. [Google Scholar] [CrossRef]
- Su, L.J.; Li, Y.M.; Kellum, J.A.; Peng, Z.Y. Predictive value of cell cycle arrest biomarkers for cardiac surgery-associated acute kidney injury: a meta-analysis. Br J Anaesth 2018, 121, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Bourgonje, A.R.; Abdulle, A.E.; Bourgonje, M.F.; Kieneker, L.M.; la Bastide-van Gemert, S.; Gordijn, S.J.; Hidden, C.; Nilsen, T.; Gansevoort, R.T.; Mulder, D.J.; Dullaart, R.P.F.; de Borst, M.H.; Bakker, S.J.L.; van Goor, H. Biomolecules 2023, 13, 338.
- Ruth, A.; Basu, R.K.; Gillespie, S.; Morgan, C.; Zaritsky, J.; Selewski, D.T.; Arikan, A.A. Early and late acute kidney injury: temporal profile in the critically ill pediatric patient. Clinical kidney journal 2021, 15, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Zarbock, A.; Kellum, J.A; Schmidt, C.; Van Aken, H.; Wempe, C.; Pavenstädt, H.; Boanta, A.; Gerß, J.; Meersch, M. Effect of Early vs. Delayed Initiation of Renal Replacement Therapy on Mortality in Critically Ill Patients With Acute Kidney Injury: The ELAIN Randomized Clinical Trial. JAMA 2016, 315, 2190–2199. [Google Scholar] [CrossRef] [PubMed]
- Bloos, F.; Rüddel, H.; Thomas-Rüddel, D.; Schwarzkopf, D.; Pausch, C.; Harbarth, S.; Schreiber, T.; Gründling, M.; Marshall, J.; Simon, P.; Levy, M.M.; Weiss, M.; Weyland, A.; Gerlach, H.; Schürholz, T.; Engel, C.; Matthäus-Krämer, C.; Scheer, C.; Bach, F.; Riessen, R.; MEDUSA study group. Effect of a multifaceted educational intervention for anti-infectious measures on sepsis mortality: a cluster randomized trial. Intensive Care Med 2017, 43, 1602–1612. [Google Scholar] [CrossRef]
- Andriolo, B.N.; Andriolo, R.B.; Salomao, R.; Atallah, A.N. Effectiveness and safety of procalcitonin evaluation for reducing mortality in adults with sepsis, severe sepsis or septic shock. Cochrane Database Syst Rev 2017, 1, CD010959. [Google Scholar] [CrossRef]
- Bloos, F.; Trips, E.; Nierhaus, A. Effect of sodium selenite administration and procalcitonin-guided therapy on mortality in patients with severe sepsis or septic shock: a randomized clinical trial. JAMA Intern Med 2016, 176, 1266–76. [Google Scholar] [CrossRef]
- Fialek, B.; De Roquetaillade, C.; Pruc, M.; Navolokina, A.; Chirico, F.; Ladny, J. R.; Peacock, F.W.; Szarpak, L. Systematic review with meta-analysis of mid-regional pro-adrenomedullin (MR-proadm) as a prognostic marker in Covid-19-hospitalized patients. Annals of medicine 2023, 55, 379–387. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).