1. Introduction
Many countries around the world largely depend on the socioeconomic contribution of the citrus industry. In Morocco, approximately 2.62 million tons of citrus are produced, of which 715,000 tons are destined for export, representing a value of more than 432 million dollars. Among these citrus fruits, clementines represent about 25% of the total planted area [
1].
Citrus fruit production is intended for either direct consumption (fresh) or processing (drinks, jams, etc.) [2, 3]. Citrus juice manufacturing generates a significant amount of waste or byproducts. These by-products are underexploited in the agri-food sector and generally consumed as animal feed. However, these by-products are valuable sources of nutritional biomolecules, such as essential oils, phenolic compounds, flavonoids, and polysaccharides, like cellulose, or more specifically, pectins [4, 5].
Pectins are a class of complex polysaccharides that are found in plant cell walls. They are classified as food additives (registration number E440). The pectin chains are largely made up of α-(1→4)-linked D-galacturonic acid units, connected by glycosidic linkages and a small amount of branched -L-rhamnose [
6].
In the food industry, pectins are widely applied as thickeners, gelling agents, and emulsifiers in jams, jellies, fruit juices, desserts, and dairy products [
7]. They are also used as reinforcement in biomaterials [8, 9] and as an encapsulation agent for active substances in various fields (pharmaceutical, agri-food, cosmetics, etc.), thanks to their ability to envelop compounds of interest such as flavors and vitamins [10, 11, 12, 13]. The global demand for pectins exceeds 30,000 tons annually and is growing at about 4-5% per year [
14].
Commercial pectin may be obtained by extractions from various plant sources, mainly from apple pomace and citrus peels [15, 16]. The extraction process includes hydrolyzing protopectin using acids, such as sulfuric, phosphoric, nitric, hydro-chloric, or citric acid, at high temperatures [
17]. Many reports have demonstrated that the factors affecting pectin extraction are pH, temperature, extraction time, particle size, type of acid and solvent-to-sample ratio (SSR) [18, 2, 19]. Furthermore, researchers have optimized the yield of these extraction conditions using response surface methodology (RSM) [18, 2, 20]. RSM can be an effective tool for optimizing experimental conditions for a specific response while using a minimum number of experiments. [21, 22, 23, 24, 25].
The RSM helps reduce experimental trials and perform multiple factors analysis to optimize conditions for pectin extraction [26, 20]. However, to our knowledge, no previous data has been published on extracting pectin from Moroccan clementine peel. Therefore, the current study aims to (i) identify the optimal acidic conditions for the extraction of pectin from clementine by-products to obtain the maximum yield using a three-variable Box-Behnken response surface design, (ii) investigate the chemical and functional properties of the extracted pectin.
2. Results and discussion
2.1. Experimental design
Substituting the variables coded as -1, +1, and 0 by their real values, the experimental conditions, and the corresponding experimental responses (pectin yield) were obtained and presented in
Table 1. Fourteenth experiments with three independent variables (pH, temperature, and particle size) were performed to evaluate the corresponding pectin yield.
A similar result reported by Aina [
27] shows that the maximum yield from
Citrus sinensis was found to be 29.41% at pH 3.2 and a temperature of 70°C. The optimized extraction from
C. Limon resulted in a maximum yield of 36.71% at pH 3.2 and a temperature of 60°C [
28].
Thus, a pH of 3.2 appears to be optimal for the extraction of pectin from all the citrus peels studied. The optimal temperature for pectin extraction was observed to be 70°C for
Citrus sinensis and
C. limetta, but not for
C. Limon, in which case a lower temperature of 60°C was preferred [
28].
2.2. Estimated model
The observed responses were used to compute the model coefficients using the least square method. This allowed us to write the following estimated model:
2.3. Statistical analysis and validation of the model
The statistical ‘model fit summary’ confirms the suitability of the linear model compared with the quadratic or 2FI model (
Table 2). Also, the predicted R² of 0.8556 and the insignificant lack-of-fit imply that the linear model was the best model to represent the parametric effects on the yield (
Table 3).
The results illustrated in
Table 4 indicate that the model F-value of 46.45 implies that the model is significant. Model terms are significant when the P-value is less than 0.0500. In this case, X1, X2, and X3 are significant model terms, and values greater than 0.1000 reveal that the model terms are not significant. The lack-of-fit F-value of 818.41 implies that the lack-of-fit is significant.
The predicted R² of 0.8556 is in reasonable agreement with the adjusted R² of 0.9130, i.e., the difference is less than 0.2. A ratio greater than 4 is desirable. The ratio of 19.301 indicates an adequate signal (
Table 5). Overall, this model can be used to navigate the design space.
The coefficient estimate represented in the
Table 6 reveals the expected change in a factor’s value per unit of response when the other factors are maintained constant. The average response of all the runs is the intercept in an orthogonal design. Based on the factor settings, the coefficients are changed to approximate the average. The variance inflation factor (VIF) is one if the factors are orthogonal. Multi-linearity is indicated by VIFs more than 1, and we have a stronger correlation with higher VIF. VIFs ranging less than 10 are generally acceptable.
2.4. Interpretation of the response surface model
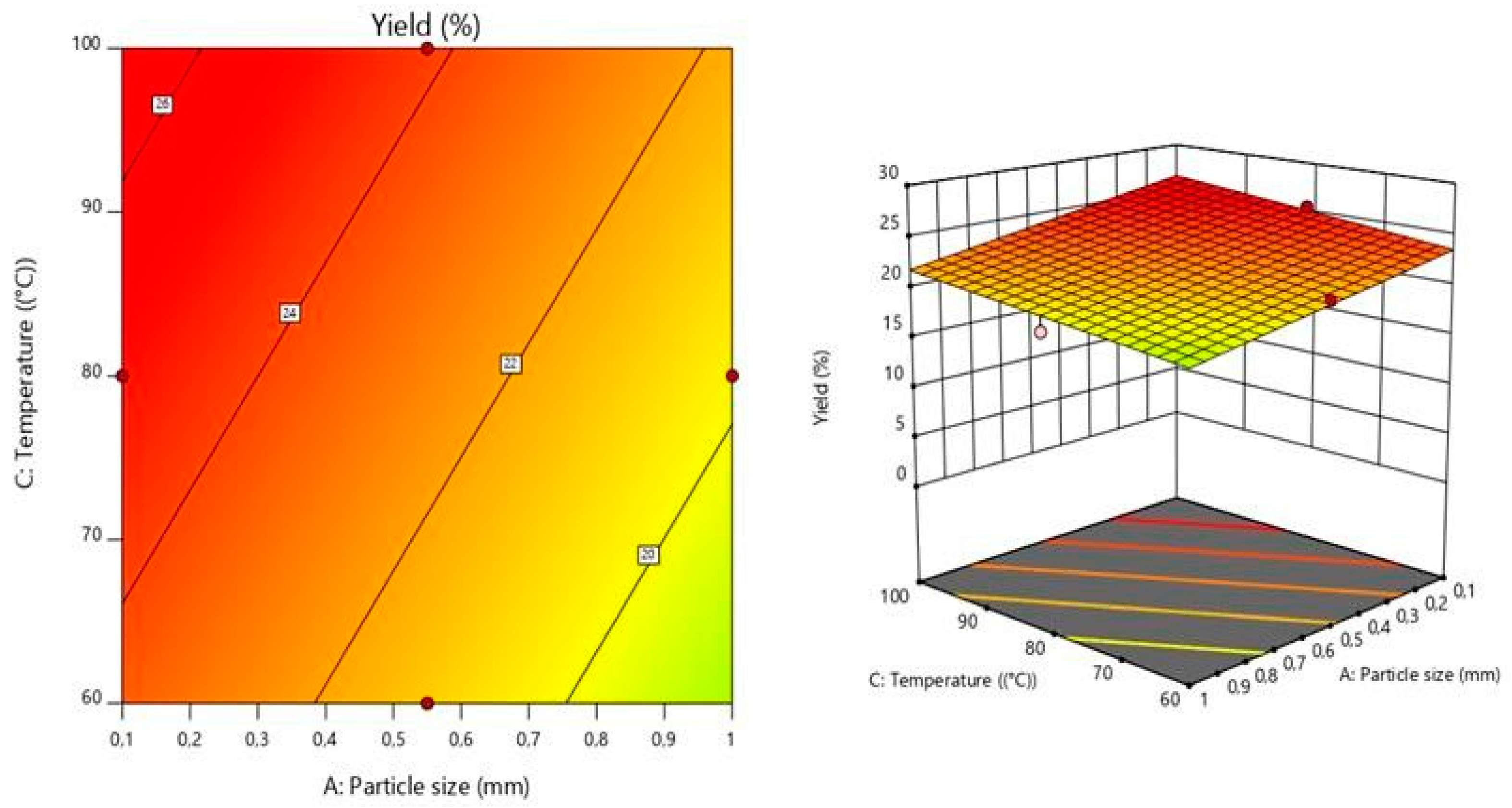
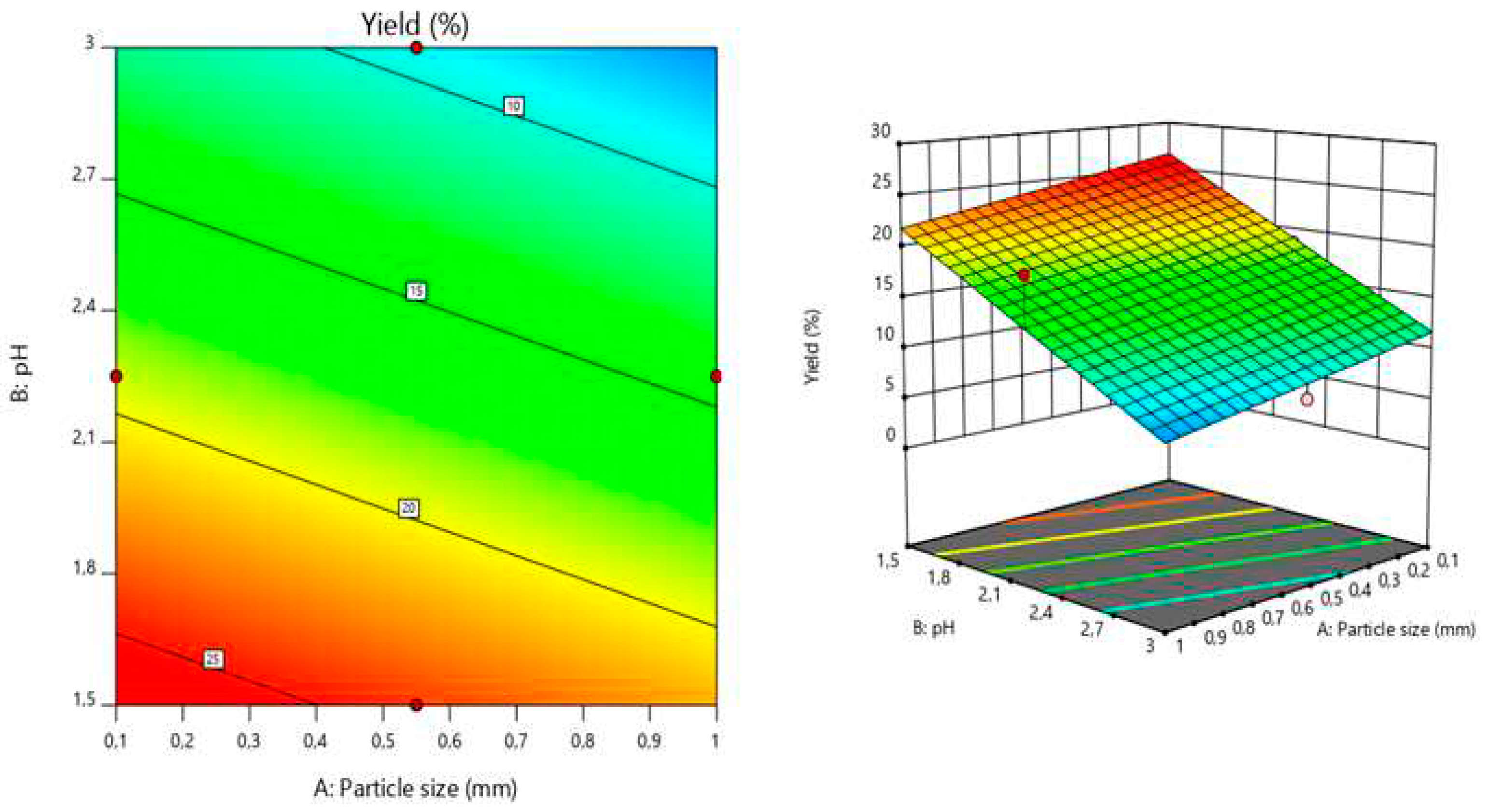
The response surface model graphically illustrates the relationship between the response and the experimental variables by plotting three-dimensional plots [
2]. The graphics presenting the relationship between the response (extraction yield) and the experimental variables (pH, temperature, particle size) are shown in the Figures 1 and 2. Each of the two horizontal axes represents one of the three independent variables, while the vertical axes reflect the pectin extraction yield (%). By fixing a factor either in its lower or upper level, or at its average value, a graphical representation of the response as a function of the other two factors can be displayed.
The pectin yield variation as a function of pH variation (1.5 to 3) indicates that the pectin yield increased when decreasing the pH. This could be attributed to the fact that, at low pH, citric acid, can hydrolyze insoluble pectin and transform it into its soluble form, thus increasing the yield of pectin extraction [
29]. Furthermore, low pH may decrease the molecular weight of pectin, and thus, increase its release from the plant tissue without degradation [30, 31, 32]. Similar results were published on apple pomace, sugar beet pulp, mango peels, and pomegranate peels by Canteri-Schemin [
33], Yapo [
34], Prakash Maran [
7], and Moorthy [
31], respectively.
Temperature is considered to be one of the most crucial parameters affecting the pectin extraction yield. The effect of temperature is evaluated in
Figure 1. The results show that when the pH was fixed at 1.5, the extraction yield increased relatively with increasing temperature.
According to Yang [
35], the extraction yield of pectin increases when temperature increases due to the solvent’s enhanced solubility and diffusivity in the plant tissue. These results are similar to those observed by Pagan [
36] and Raji [
18], who reported a significant improvement in the yield of pectin extraction from peach pomace and melon rind, respectively, with increasing temperature [
32].
The results shown in
Figure 2 reveal that particle size has a substantial impact on pectin yield. At a fixed temperature of 100°C, the production of pectin was greater for the 0.1 mm size raw material (26.64%). This could be because protopectin is more commonly available in small particles than in large ones. In other words, when the particle size is reduced, the surface area increases, which increases the exposure to the extracting agent [
33].
2.5. Determination of optimum conditions
To select the optimal conditions, the particle size value was fixed at 0.1 mm and pH was plotted versus temperature. The conditions that obtained the highest yield (Y1 = 26.64%) were: a temperature of 100°C, a pH of 1.5, a grain size of 0.1 mm, a solid/liquid ratio of 1:50 (m/v), and an extraction time of 30 min.
The validation experiments that were performed under the selected conditions obtained an experimental yield of pectin (21.36%) that was lower than that calculated mathematically by the optimization model (26.64%). This difference of about 5% between the theoretical and experimental values may be due to the difficulty of controlling the stability and precision of the experimental conditions.
2.6. Chemical characteristics
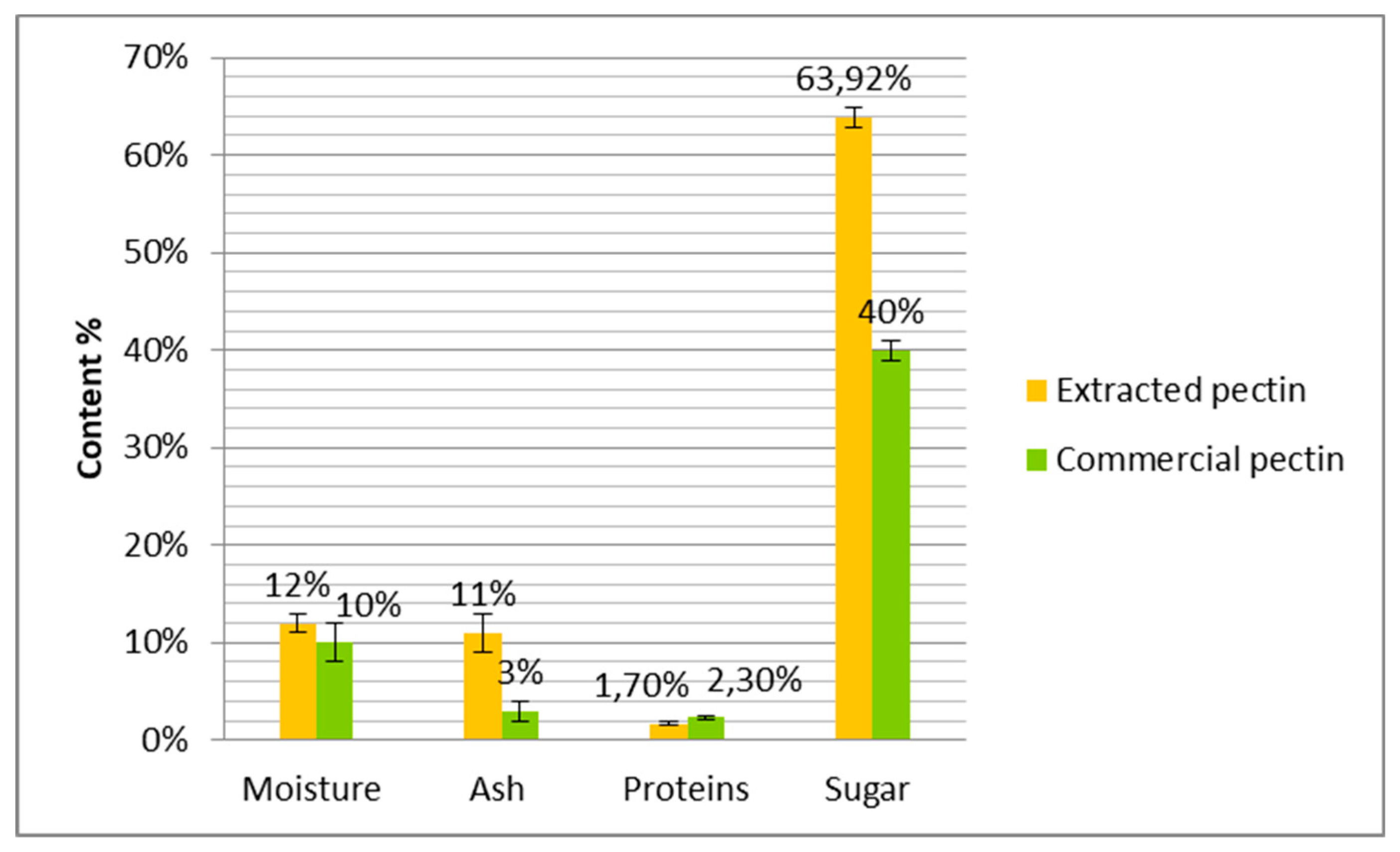
The comparison between extracted and commercial pectin based on moisture, ash, protein, and sugar contents is reported in
Figure 3.
The moisture content of extracted pectin was about 12%. This value is comparable to this reported by Baississe (2009) [
37] who used aluminum chloride and aluminum sulfate to extract pectin from oranges and found moisture values of 8 and 13%. These values are still acceptable for good storage stability [
37]. When compared to commercial pectin, the moisture content of extracted pectin is slightly higher with 2%. This difference may be attributed mainly to extraction conditions, species of used citrus, etc.
The ash content of the pectin obtained from clementine is 10.6%. This value is significantly higher than the commercial pectin (3%), it can be explained by the fact that in our extraction we used citric acid as extraction solvent, therefore in commercial pectin, the industrials use powerful acidic agents to extract and purify pectin. This result is similar to those obtained by Baississe [
37] and Deluca and Joslyn [
38] who showed that the ash content of pectins precipitated by mineral salts, especially aluminum sulfate and aluminum chloride, varied between 8 to 25%. Extraction conditions can also affect the precipitation of impurities with pectins and impact ash content. Thus, Yapo et al [
34] concluded that pectins extracted at pH 1.5 are purer than those obtained by solubilization at pH 2.0 from beet pulp. Khotimchenko et al, [
39] show that the sorption activity of pectin towards heavy metals is closely related to pH, where it varies in the range of 4-8.
The maximum protein content in pectins is 2.5%, according to LEU and FAO/WHO, which are cited by Herbstreith and Fox [
40]. Regarding the recorded value of 1.7% obtained in this study, it indicates that the pectin extracted from clementines is still within the permitted range of 2.5%. Pectins extracted from apple pomace by Massiot and Renard [
41] show protein content ranging from 2.1 to 7.5%, while those obtained by Schieber et al. (year) are about 4%. Yapo et al [
34] obtained a protein content of 3.7% in pectins extracted from beet pulp by hydrochloric acid.
Moreover, the percentage of total sugars in the extracted pectin is 63.9% which is higher than that obtained for commercial pectin (40%). These results are comparable with those described by Baississe [
37] who reported values of 70.8% and 74.1% in total sugars of pectins extracted by aluminum chloride and aluminum sulfate respectively. The study of Lekbir, [
42] showed lower values than those of our study with a total sugar content that varies between 24.2 and 18.9% for orange pectins precipitated with aluminum chloride and aluminum sulfate respectively. Several studies have shown that parietal polysaccharides, especially pectins, are subject to qualitative and quantitative variations depending on: variety, maturity stage, geographical origin and storage [43, 44]. According to Seymour and Konx [
45] and Thang et al. [
46], the difference in total sugar levels can be explained by fundamental changes in fruit parietal polysaccharides during maturation and storage of raw materials, in addition to extraction conditions.
2.7. functional properties
According to
Table 7, the analytical results showed that the pectin extracted from clementine peels had a significant gelling power (164°SAG) than the commercial one (150°SAG). This result is comparable to those of Benchabane [
47], who reported that a good apple pectin has a gelling power of 177 to 220 ° SAG, while that of an orange pectin varies between 170 to 200 ° SAG. This difference can be explained by the structural difference between apple and orange pectins (molecular weight, content of neutral oses, and presence or not of acetyl groups). Several studies have shown that the sugar content, the distribution of non-esterified carboxyl groups and the charge of pectins, together with the content of neutral oses and the presence of acetyl groups, have a strong influence on the structural and textural properties of pectic gels [
48,
49].
The emulsifying activity of the extracted pectin (38.46%) is significantly lower than that of the commercial pectin which has a value of (51%). Our results are comparable with those obtained by Leroux et al, [
50] and Yapo et al, [
34] which are respectively 43.2 and 47.1% for beet pectin and are higher than that found by Huang et al, [
51] which is 30.3%. According to Yapo et al [
34], this remarkable emulsifying activity of citric acid extracted pectins may be due to the fact that they are endowed with a tensioactive activity that increases the viscosity of the aqueous phase and reduces the tendency for the emergence of dispersed oil globules. Also, it is mainly time and temperature that have a major influence on surface activity. The most surface active pectins are those extracted at a temperature of 80°C, at a pH of 1.5 and for one hour.
3. Materials and Methods
3.1. Plant material
The clementine fruits (Citrus clementina) were harvested from the experimental station of the Regional Center of Agricultural Research of Tadla in Béni Mellal, Morocco (Latitude: 32.26, longitude: -6.52). The fruit peels were manually removed from the pulp, washed, and blanched at 90°C for 10 min to inactivate enzymes. Then, the peels were dried in a greenhouse at a controlled temperature (45°C) for 8 days. The dried peels were ground with an electric grinder (Retsch SR300, Germany) to obtain a fine and a homogeneous powder (0.1-1 mm). The obtained powder was stored in hermetically-sealed vacuum bags and protected from the light until experimental analysis.
3.2. Pectin extraction
Hot acid extraction of the pectin was carried out according to Canteri-Schemin [
33] with the following modifications. The dried and ground peel powder (1:50 w/v) was soaked in distilled water and the pH was adjusted (pH 1.5-3) using an aqueous citric acid solution (25% w/v) and a pH meter (HANNA, hi 98161, France), under agitation. The solution was extracted at 60-100°C for 30 min. The obtained pectic extract was kept at 4°C for 24 hours. Pectins were precipitated by mixing the extracted pectic juice with two volume of 96% ethanol; the obtained pectic gel was washed with acetone (100%). Finally, the precipitated pectins were dried at 60°C to a constant weight in a vacuum oven (Memmert, GmbH, VO29, Germany).
Commercial pectin (pastry pectin, Louis Francois-France) was analyzed for comparison.
3.3. Experimental methodology
The pectin extraction parameters were optimized using RSM. A Box-Behnken design, an element of RSM, was used to identify the best experimental conditions.
Particle size (X1) (0.1-1 mm), pH (X2) (1.5-3), extraction temperature (X3) (60-100°C) were chosen as the independent variables. The extraction time was 30 min. For each factor, the experimental range was chosen based on the results from the literature and preliminary experiments.
In this study, to model the pectin extraction process, a linear model without interactions was used to approximate the relationship between the yield extraction and the three selected variables (X1, X2, and X3) as represented in equation 2:
Where: Y is the calculated response function; b1, b2, b3 are successively the coefficients of the parameters X1, X2, X3.
In order to validate the mathematical optimization model, the influencing factors and their variation ranges chosen for this study are illustrated in
Table 8.
It is important to note that all computation and graphics in this study were performed using Design Expert Statistical Software 10 by Stat Ease, Inc.
3.4. Validation of the model
Validation of the mathematical model is an important step. Thus, the validation was carried out by an appropriate analysis of variance (ANOVA), as described for the case of a composite design by Kamoun [
52] and Masmoudi [
2].
3.5. Chemical characterisation and pectin yield
3.5.1. Moisture content
To determine the moisture content (H %), one gram (1g) of the sample is weighed (P1) in a tared porcelain crucible, then placed in an oven set at 105 °C. After 5h, the sample is transferred to a desiccator for 30 minutes and weighed; the operation is repeated until obtaining a constant weight [
53].
The moisture content is calculated as follow:
Where:
P1: Weight of the sample before drying,
P2: Weight after drying in the oven.
3.5.2. Ash content
Ash content was determined using the AOAC method [
54]. Pectin weighing 1g is placed in a previously tared porcelain capsule and placed in a muffle oven (NAHITA SERIE 642, France) for 24 hours at 550 °C. Then, the capsule is cooled in a desiccator and weighed. The ash content was calculated as the percentage of sample weights before and after muffle oven-drying.
3.5.3. Protein content
The protein content was assessed using Micro-Kjeldahl apparatus (Kel plus, Pelican, India), in accordance with AOAC method [
55]. A total of 0.2 g of pectin powder was digested in a solution of catalyst (1 g) and 5 mL of H2O2 and conc. H2SO4. The digested sample was allowed to boil before collecting the distillate of ammonia liberated in boric acid. The distillate was titrated with hydrochloric acid until the blue color was completely removed. The protein content was estimated using the formula below.
Where:
S = mL of HCl required for sample titration
B = mL of HCl required for blank titration,
N = Normality of HCl (0.02 N)
3.5.4. Carbohydrate content
The procedure described by Dubois in 1956 [
56] was used to determine the amount of carbohydrates in the sample. After 0.5ml of a pectin extract and 0.5ml of a phenol solution at 5% for 15–30 minutes in darkness and ambient cooling, 3ml of concentrated sulfuric acid was added. Galactose is used to generate the standard range, and a UV-Visible spectrophotometer is performed to measure the optical density (OD) at 485 nm.3.5.5. Pectin yield
The pectin extraction yield, subject of this study, was calculated as follows:
3.6. Functional properties
3.6.1. Determination of gelling power
The traditional SAG method was used to measure the strength of gels formed under the following conditions: 65.0% soluble solids (sucrose), 0.70 wt% pectin, and a pH of 2.3 to determine the gelling capacity (or power) of pectins [
57] .In order to prevent evaporation, the jelly mixture was placed entirely within a Ridgelimeter glass. It was then left undisturbed at ambient temperature for 2 hours before aging for a further 22 hours in a water bath at 30 °C. The gel was then delicately demolded onto a Ridgelimeter glass plate without causing any damage. The pointer of the device (Ridgelimeter) was gently lowered until it contacted the gel surface after exactly 2 minutes of standing, and the percentage of sagging under its specific gravity was measured. The following equation was used to determine the gelling power:
Where A, B, and F are the amounts of sugar and pectin in gel and the factor of sagging percentage, respectively.
3.6.2. Emulsifying activity analysis
The extracted pectin’s emulsion activity (EA) was determined using the method reported by Hosseini et al. [
58]. In a brief, 5 mL sunflower oil, 5 mL of extracted pectin solution (0.5% w/v), and 0.02% sodium azide (NaN3) were combined and subjected to 4000 g centrifugation for 5 min. Finally, EA was determined as follows:
Where VE was the volume of the emulsion layer and VT was the total volume.
3.7. Statistical analysis
All experiments were performed in triplicate, and results were expressed as the means ± standard deviation (SD). Analysis of variance was performed using (SPSS Corporation, Northampton, MA, USA). The significant level was set as p < 0.05 throughout the study.
4. Conclusions
In this work, the optimal yield of pectin extraction from Citrus clementina peel with citric acid solvent was investigated using a Box-Behnken design for three independent variables, consisting of the temperature (60-100°C), particle size (0.1-1 mm), and pH (1.5-3). The results revealed that increasing temperature and reducing particle size and pH considerably improved the extraction yield. The optimal conditions determined using the response surface designs were : pH = 1.5, T = 100°C and particle size = 0.1 mm for a solid/liquid ratio of 1:50 (m/v) and an extraction time of 30 min. Under these conditions, the response value (extraction yield) that the model predicts is 26.64%. The chemical composition of the extracted pectin was statically similar to the commercial pectin. In terms of functional properties, the extracted pectins had significantly higher gelling power (164°SAG) than commercial pectin (150°SAG). Inversely, the extracted pectins’ emulsifying activity (38.46%) was lower than commercial pectin (51%).
Further studies must be focused on determining the effect of extracted pectin from the clementine peel on the preparation of food products, such as jams and jellies, to confirm their effectiveness as gelling and emulsifying agents, as well as their environmental and chemical stability.
Author Contributions
Conceptualization, Hanane Azzouzi, Souad Salmaoui and Elfazazi Kaoutar; Data curation, Hanane Azzouzi, Loubna Elhajji and Mouad Achchoub; Formal analysis, Hanane Azzouzi, Loubna Elhajji, Mouad Achchoub and Hasnaa Harrak; Methodology, Hanane Azzouzi, Loubna Elhajji, Abdelilah Ammadi and Elfazazi Kaoutar; Resources, Rachid Touzani and Younes Noutfia; Software, Abdelilah Ammadi; Supervision, Elfazazi Kaoutar; Validation, Elfazazi Kaoutar; Visualization, Rachid Touzani and Younes Noutfia; Writing – original draft, Hanane Azzouzi and Elfazazi Kaoutar; Writing – review & editing, Hasnaa Harrak and Rachid Touzani.
Funding
This study was fully funded by the National Institute of Agricultural Research of Morocco (INRA) under the Medium-Term Research Program (MTRP 2021-2025) - Citrus Megaproject.
Data Availability Statement
Data other than presented in this paper are available upon request. Please send all communications to Dr. Elfazazi Kaoutar at Kaoutar.elfazazi@inra.ma or Mrs Azzouzi Hanane hananeazzouzi94@gmail.com.
Acknowledgments
The authors would like to thank the staff of Afourer experimental station of CRRAT- INRA for providing samples and their support during this study.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- MAMPDREF. Ministry of Agriculture, Maritime Fishing, Rural Development and Water and Forests. (2020), Morocco https://www.agriculture.gov.ma/fr/filiere/agrumicole.
- Masmoudi, M.a; Souhail, b.; Moncef, C.; Christelle, R.; Michel, P.; Christophe, B.; Hamadi, A. Optimization of pectin extraction from lemon by-product with acidified date juice using response surface methodology. Carbohydrate Polymers 2008, 74, 185–192. [Google Scholar] [CrossRef]
- Azzouzi, H.; Elfazazi, K.; Achchoub, M.; Chafik, L.; Jbilou, M.; Salmaoui, S. Effect of thermal pasteurization on the physicochemical stability and nutritional quality of Moroccan Valencia late orange juice. International journal of engineering sciences & research technology 2018, 7, 277–283. [Google Scholar]
- Bicu, I.; Mustata, F. Cellulose extraction from orange peel using sulfite digestion reagents. Bioresource Technology 2011, 102, 10013–10019. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.J.; Rao, V.N.M.; Smit, C.J.B. Demethylation of pectin using acid and ammonia. Journal of Food Science. 1978, 43, 74–78. [Google Scholar] [CrossRef]
- Fishman, M.L.; Jen, J.J. Chemistry and Function of Pectins. American Chemical Society. Washington 1986.
- Prakash Maran, J.; Sivakumar, V.; Thirugnanasambandham, K.; Sridhar, R. Microwave assisted extraction of pectin from waste Citrullus lanatus fruit rinds. Carbohydrate Polymers 2014, 101, 786–791. [Google Scholar] [CrossRef]
- Munarin, F.; Guerreiro, S.G.; Grellier, M.A.; Tanzi, M.C.; Barbosa, M.A.; Petrini, P.; Granja, P.L. Pectin - Based Injectable Biomaterials for Bone Tissue Engineering. Biomacromolecules 2011, 12, 568–577. [Google Scholar] [CrossRef]
- Sumathraa, M.; Govindaraja, D.; Jeyarajb, M.; Al Arfajc, A.; Munusamyc, M.A.; Selvaraj Suresh, K.S.; Rajana, M. Sustainable pectin fascinating hydroxyapatite nanocomposite scaffolds to enhance tissue regeneration. Sustainable Chemistry and Pharmacy 2017, 5, 46–53. [Google Scholar] [CrossRef]
- Liu, L.S.; Fishman, M.L.; Kost, J.; Hicks, K.B. Pectin - based systems for colonspecific drugdelivery via oral route. Biomaterials 2003, 24, 3333–3343. [Google Scholar] [CrossRef]
- Zam, W.; Bashour, G.; Abdelwahed, W.; Khayata, W. Formulation and in-vitro release of pomegranate peels’ polyphénols microbeads. International Journal of Pharmaceutical Sciences and Research 2013, 4, 3536–3540. [Google Scholar]
- Martins, A.; Barros, L.; Carvalho, A.M.; Santos-Buelga, C.; Fernandes, I.P.; Barreiro, F.; Ferreira, I.C.F.R. Phenolic extracts of Rubus ulmifolius Schott flowers: Characterization, microencapsulation and incorporation into yogurts as nutraceutical sources. Food and Function 2014, 5, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.F.; Yusoff, M.M.; Gimbun, J. Assessment of phenolic compounds stability and retention during spray drying of Orthosiphon stamineusextracts. Food Hydrocolloids 2014, 37, 159–165. [Google Scholar] [CrossRef]
- Yeoh, S.; Shi, J.; Langrish, T.A.G. Comparisons between different techniques for water-based extraction of pectin from orange peels. Desalination 2008, 218, 229–237. [Google Scholar] [CrossRef]
- Kurita, T.; Fujiwara, E. ; Yamazaki. Characterization of the pectin extracted from citrus peel in the presence of citric acid, Carbohydrate Polymers 2008, 74, 725–730. [Google Scholar]
- Maran, V.; Sivakumar, K.; Thirugnanasambandham, R. ; Sridhar.Optimization of microwave assisted extraction of pectin from orange peel. Carbohydrate Polymers 2013, 97, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Minkov, S.; Minchev, A.; Paev, K. . Modelling of the hydrolysis and extraction of apple pectin. Journal of Food Engineering 1996, 29, 107–113. [Google Scholar] [CrossRef]
- Raji, Z.; Khodaiyan, F.; Rezaei, K.; Kiani, H.; Hosseini, SS. Extraction optimization and physicochemical properties of pectin from melon peel. Int. J. Biol. Macromolecules 2017, 98, 709–716. [Google Scholar] [CrossRef]
- Levigne, S.; Thomas, M.; Ralet, M. C.; Quéméner, B.; Thibault, J. F. Determination of the degrees of methylation and acetylation of pectins using a C18 column and internal standards. Food Hydrocolloids 2002, 16, 547–550. [Google Scholar] [CrossRef]
- Wu, S.; W Cui, J. ; Tang, X Gu. Optimization of extraction process of crude polysaccharides from boat-fruited sterculia seeds by response surface methodology, Food Chemistry 2007, 105, 1599–1605. [Google Scholar]
- Box, E. P.; Hunter, W. G.; Hunter, J. S.. Statistics of experimenters. New York: Wiley. (1978).
- Carlson, R. Design and optimization in organic synthesis. Amsterdam: Elsevier (1992).
- Montgomery, D. C. Design and analysis of experiments. New York: John Wiley. (1991).
- Goupy, J. Goupy, J. Plans d’Expériences Pour Surfaces de Réponse. Paris:Dunod. (1999).
- Box, George.E. P.; Hunter William, G.; Hunter, J Stuart. «Statistics for Experimenters» deuxième édition. John Wiley and Sons. New-York. (2005) 633 pages.
- Bitaraf, F.; Khodaiyan, M.A.; Mohammadifar, S.M.; Mousavi. Application of response surface methodology to improve fermentation time and rheological properties of probiotic yogurt containing lactobacillus reuteri. Food Bioprocess Technology 2012, 5, 1394–1401. [Google Scholar] [CrossRef]
- Aina, V.O.; M. B. Mustapha.; Mamman, O.A.; Zakari, H.; Haruna, M.S.; Umar, B.A.; Yagan, R.C.; Sanjay. Extraction and Characterization of Pectin from Peels of Grape Fruit (Citrus paradisi), Sweet Orange (Citrus limetta) and Lemon (Citrus limon). British Journal of Pharmacology and Toxicology 2012, 3, 259–262. [Google Scholar]
- Kanmani, E.; Dhivya, J.; Aravind, K.; Kumaresan. Extraction and Analysis of Pectin from Citrus Peels: Augmenting the Yield from Citrus limon Using Statistical Experimental Design. Iranica Journal of Energy and Environment 2014, 5, 303–312. [Google Scholar] [CrossRef]
- El-Nawawi, S.A.; Shehata, F.R. Extraction of pectin from egyptian orange peel. Factors affecting the extraction. Biological Wastes 1987, 20, 281–290. [Google Scholar] [CrossRef]
- Faravash, RS. ; Ashtiani, FZ. The effect of pH, ethanol volume and acid washing time on the yield of pectin extraction from peach pomace. International Journal of Food Science and Technology 2007, 42, 1177–1187. [Google Scholar] [CrossRef]
- Moorthy, IG.; Maran, JP.; Surya, SM.; Naganyashree, S.; Shivamathi, CS. Response surface optimization of ultrasound assisted extraction of pectin from pomegranate peel. International Journal of Biological Macromolecules 2015, 72, 1323–1328. [Google Scholar] [CrossRef]
- Pasandide, B.; Khodaiyan, F.; Mousavi, Z.; Hosseini, S. S. Pectin extraction from citron peel: optimization by Box–Behnken response surface design. Food Science and Biotechnology 2018, 27, 997–1005. [Google Scholar] [CrossRef]
- Canteri-Schemin, MH.; Fertonani, HCR. ; Waszczynskyj, N.; Wosiacki, G. Extraction of pectin from apple pomace. Brazilian Archives of Biology and Technology 2005, 48, 259–266. [Google Scholar] [CrossRef]
- Yapo, BM.; Robert, C.; Etienne, I.; Wathelet, B.; Paquot, M. Effect of extraction conditions on the yield, purity and surface properties of sugar beet pulp pectin extracts. Food Chemistry 2007, 100, 1356–1364. [Google Scholar] [CrossRef]
- Yang, Z. ; Zhai, W. Optimization of microwave-assisted extraction of anthocyanins from purple corn (Zea mays L.) cob and identification with HPLC-MS. Innovative Food Science & Emerging Technologies 2010, 11, 470–476. [Google Scholar]
- Pagan, J.; Ibarz, A.; Llorca, M.; Pagan, A.; Barbosa-Canovas, GV. Extraction and characterization of pectin from stored peach pomace. Food Research International 2001, 34, 605–612. [Google Scholar] [CrossRef]
- Baississe, Salima. Extraction et appréciation des pectines à partir d’écorces d’oranges, de pulpes d’abricots et de pommes. Diss. Batna, Université El Hadj Lakhdar. Faculté des sciences, 2009.
- Delluca, A.; Joslyn, M.A. The recovery of pectin from orange peel extracts as aluminum pectin ate. Food Technology 1957, 3, 137–141. [Google Scholar]
- Khotimchenko, M.; Kovalev, V.; Khotimchenko, Y. Equilibrium studies of sorption of lead (II) ions by different pectin compounds. Journal of Hazardous Materials 2007, 149, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Herbstrieth ; Fox (a). Chances and limits for the use of pectin as emulsifier. Corporate Group. Turnstraße 37. 75305 Neuenbürg/Württ. (1998) Germany,1-14.
- Massiot, L.; Renard, C.M.G.C. Composition physicochemical properties and enzymatic degradation of fibres prepared from different tissues of apple. Lebensm.-Wiss.U.- Technol. 1997, 30, 800–806. [Google Scholar] [CrossRef]
- Lekbir, Adel. Extraction et appréciation des pectines à partir des écorces d’oranges et de dattes. Diss. Batna, Université El Hadj Lakhdar. Faculté des sciences, 2008.
- Senter, S. D. ; J. A. Robertson; F. I. Meredith. “Phenolic compounds of the mesocarp of cresthaven peaches during storage and ripening. Journal of Food Science 1989, 54.5, 1259–1268. [Google Scholar] [CrossRef]
- Kurz, C.; Carle, R.; Schieber, A. Characterisation of cell wall polysaccharide profiles of apricots (Prunus armeniaca L.), Peaches (Prunus persica L.), and pumpkins (Cucurbita sp) for the evaluation of fruit product authenticity. Food Chem. 2008, 106, 421–430. [Google Scholar] [CrossRef]
- Seymour, G.; Knox, J.P. Pectins and their manipulation. Blackwell Publishing press (2002) 250 p.
- Thang P.T.N. Ripening behaviour of capsicum (Capsicum annum L.) fruit. These de doctora, . (2007). South Australia.
- Benchabane, A. Rapport de synthèse de l’atelier “technologie et qualité de la datte”.Ciheam –Options Mediterranèennes. Institut National Agronomique ElHarrach16200 Alger. (1994). Algerie.
- Constenla, D.; Lozano, J.E. Kinetic model of pectin demethylation. Latin American Applied Research 2003, 33, 91–96. [Google Scholar]
- Sahari, M. A.; Akbarian, A.M.; Hamedi, M. Effect of variety and acid washing method on extraction yield and quality of sunflower head pectin. Food Chemi 2003, 83, 43–47. [Google Scholar] [CrossRef]
- Leroux, J.; Langendorff, V.; Schick, G.; Vaishnav, V.; Mazyer, J. Emulsion stabilising properties of pectin. Food Hydrocolloids 2003, 1 7, 455–462. [Google Scholar] [CrossRef]
- Huang, Y.S.; Ho, S.C. Polmethoxy flavones are responsible or the anti-inflammatory activity of citrus fruit peel. Food Chem 2010, 119, 868–873. [Google Scholar] [CrossRef]
- Kamoun, A.; Samet, B.; Bouaziz, J.; Chaabouni, M. Application of a rotatable orthogonal central composite design to the optimization of the formulation and utilization of an useful plasticizer for cement. Analysis 1999, 27, 91–96. [Google Scholar] [CrossRef]
- Mc Ceady, R.M. Pectin, methods in food analysis. Joslyn M.A. (2 Ed.). Academic Press .New-York and London (1970). pp565-599.
- AOAC. Official methods of analysis (14th ed).Washington, DC: Association of Official Analytical Chemists (1984).
- AOAC (ed). Official methods of analysis. Association of official analytical chemists, 12th edn. AOAC, Washington D.C. (1995).
- Dubois, M. K. “Use of phenol reagent for the determination of total sugar. ” Analytic Chemistry. 1956, 28, 350. [Google Scholar] [CrossRef]
- Yapo, B. M. Biochemical Characteristics and Gelling Capacity of Pectin from Yellow Passion Fruit Rind as Affected by Acid Extractant Nature. Journal of Agricultural and Food Chemistry 2009, 57, 1572–1578. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.S. Khodaiyan, F. Yarmand, M.S. Optimization of microwave assisted extraction of pectin from sour orange peel and its physicochemical properties, Carbohydr. Polym. 2016, 140, 59–65. [Google Scholar] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).