Submitted:
17 July 2023
Posted:
19 July 2023
You are already at the latest version
Abstract
Keywords:
Introduction
Material and Method
Patients
Data acquisition and image reconstruction
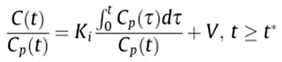
Delineation of VOIs
Efficacy evaluation
Data analysis
Result
Patients
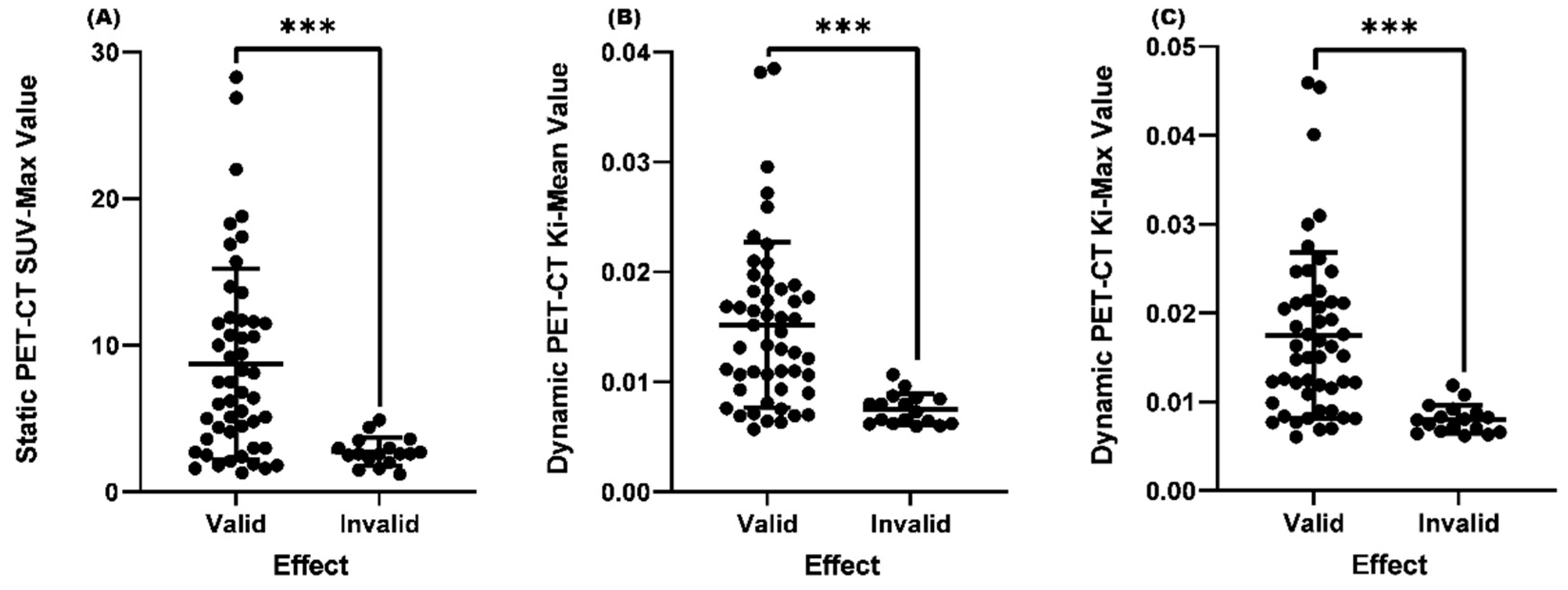
Comparison of SUVmax, Ki-Mean and Ki-Max values between the valid and invalid groups
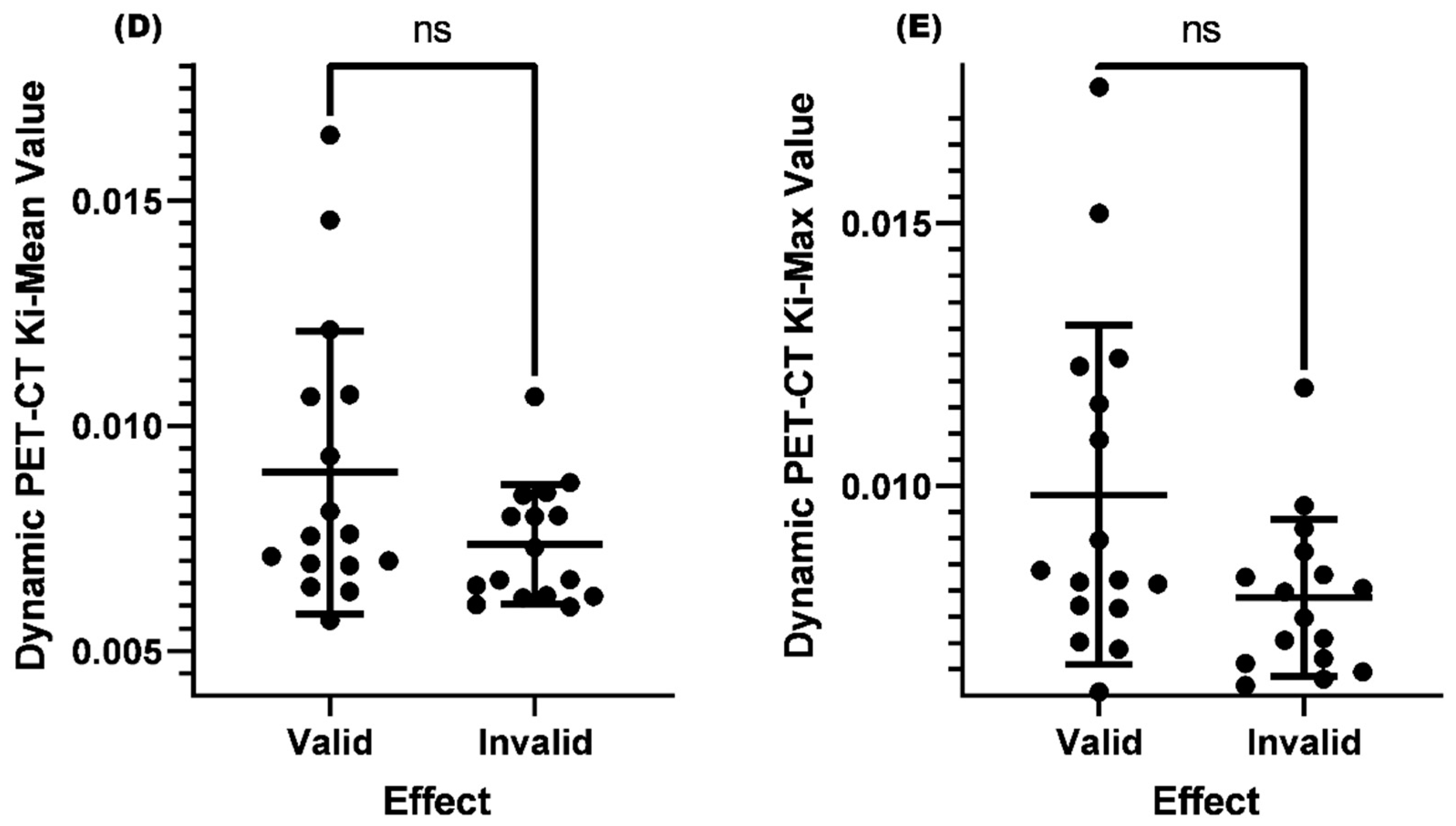
Comparison of Ki-Mean and Ki-Max values between valid and invalid groups when SUV-Max≤4.5
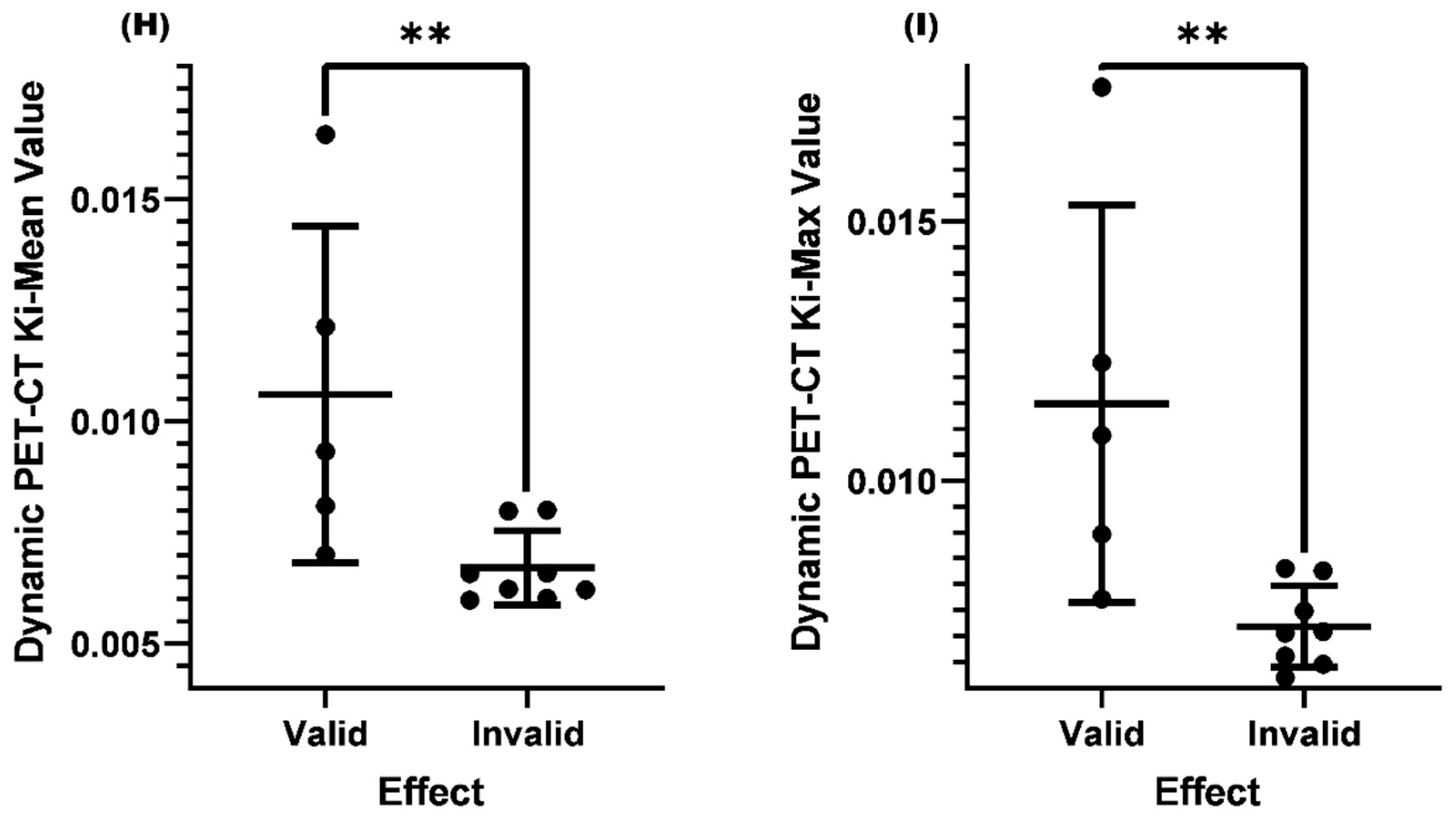
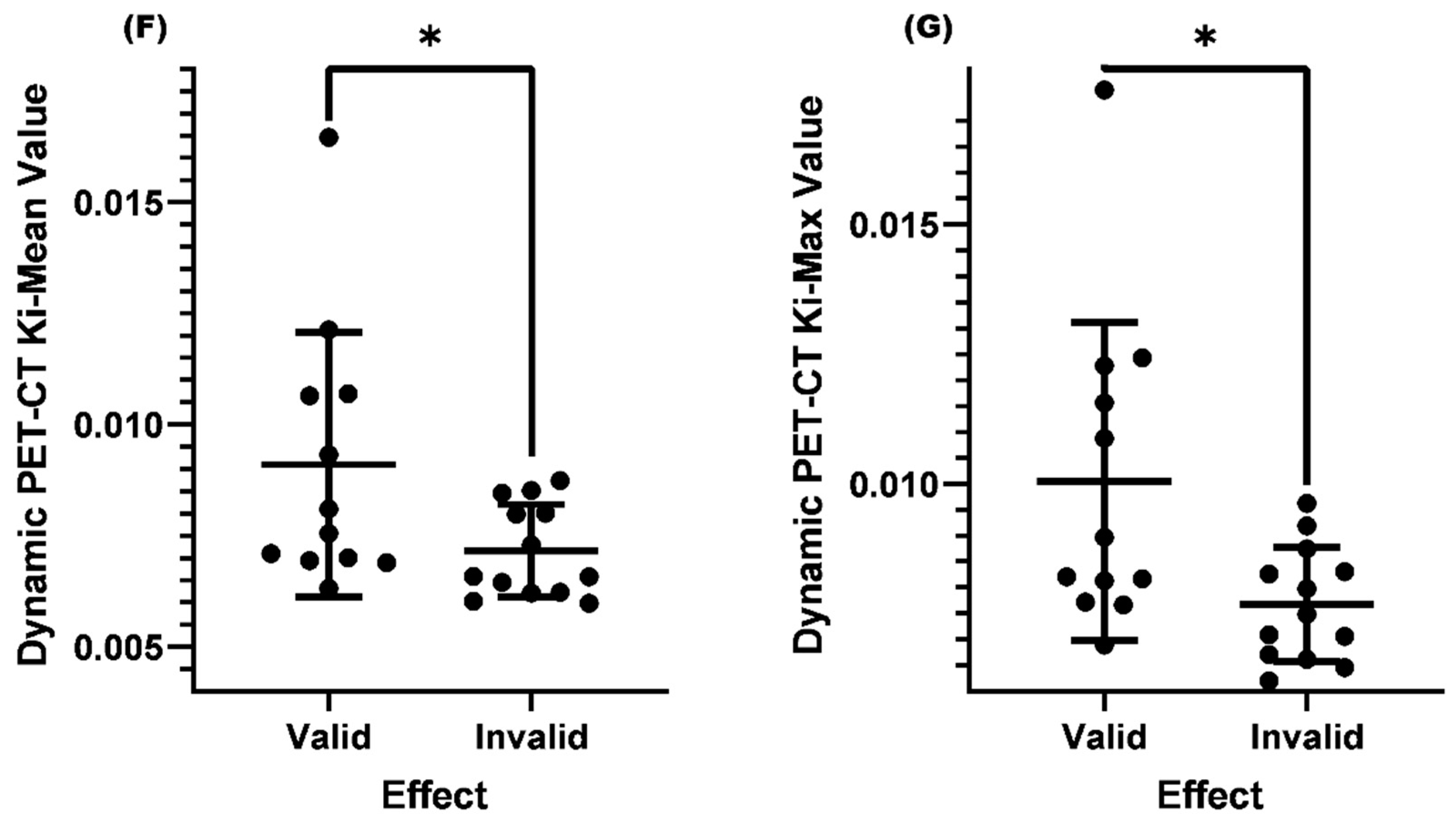
Comparison of Ki-Mean and Ki-Max between the valid and invalid groups with SUV-Max≤4.5 and lymph nodes<1.0cm before treatment
Comparison of Ki-Mean and Ki-Max between the valid and invalid groups with SUV-Max≤4.5 and lymph node<1.0cm and normal EBV-DNA replication before treatment
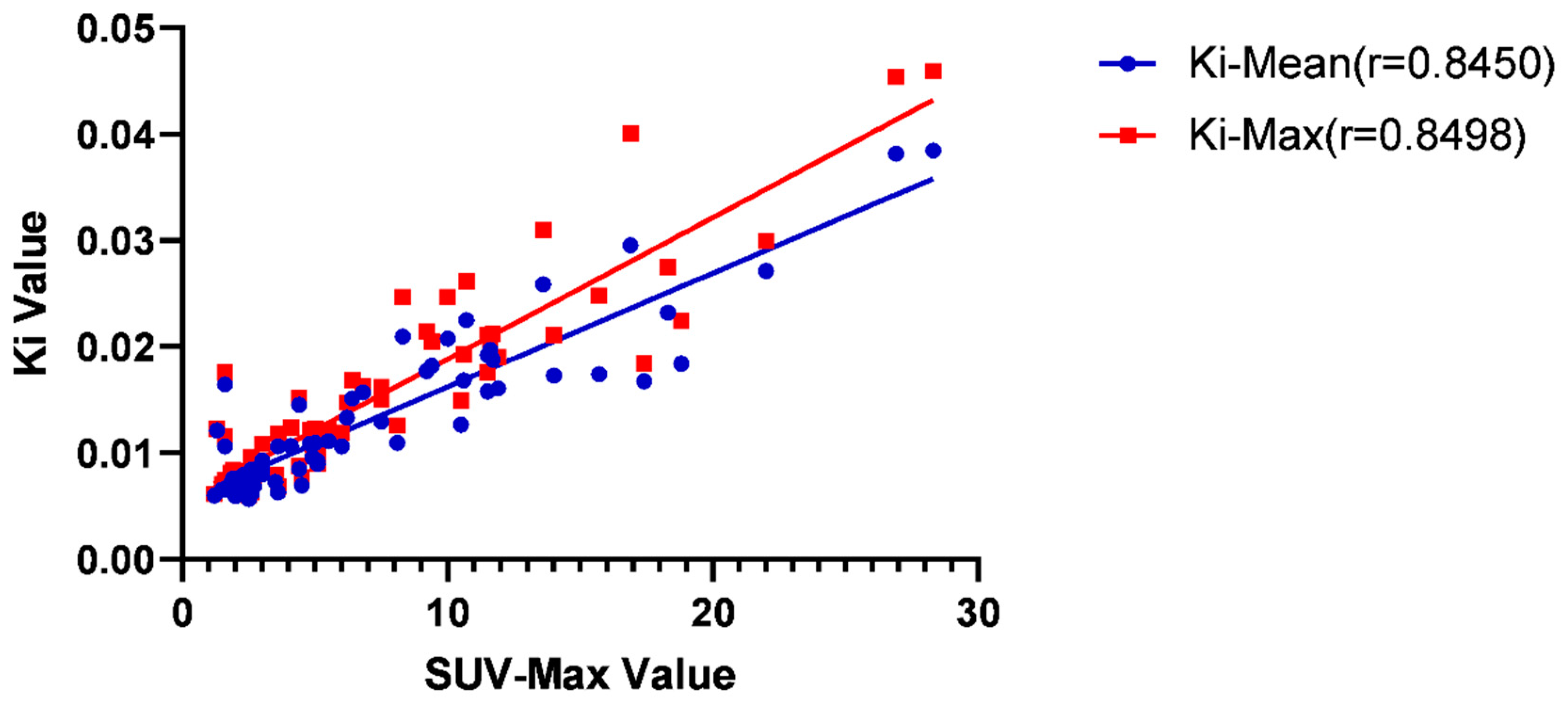
The correlation between different factors and Ki-Mean and Ki-Max
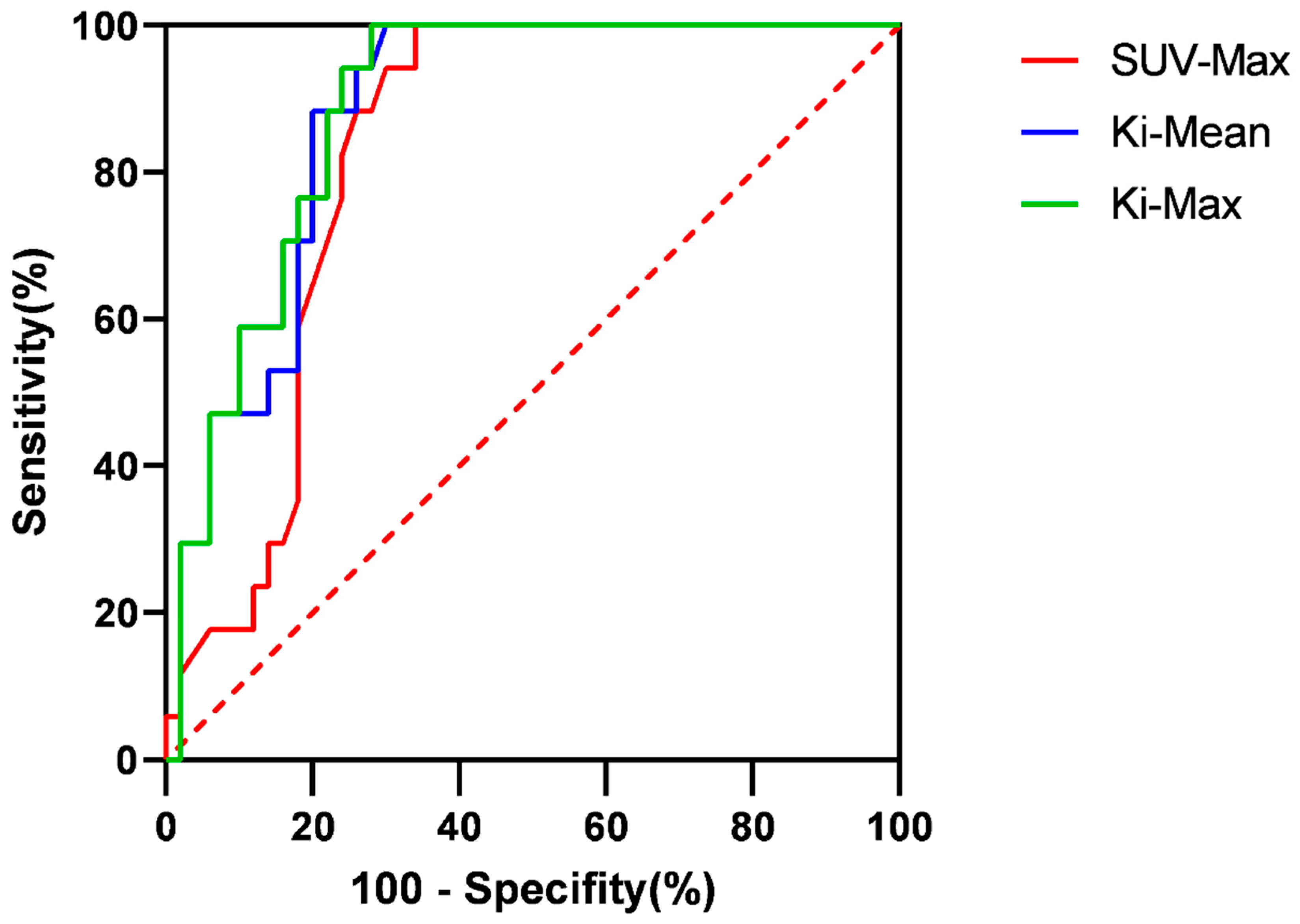
Diagnostic accuracy of SUV-Max and Ki for cervical lymph node metastasis in nasopharyngeal cancer
Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen YP, Chan ATC, Le QT, et al. Nasopharyngeal carcinoma. Lancet. 2019, 394, 64–80.
- Ho FC, Tham IW, Earnest A, et al. Patterns of regional lymph node metastasis of nasopharyngeal carcinoma: A meta-analysis of clinical evidence. BMC Cancer, 2012, 12, 1–13.
- Wei WI, Chan JY, Ng RW, et al. Surgical salvage of persistent or recurrent nasopharyngeal carcinoma with maxillary swing approach - Critical appraisal after 2 decades. Head Neck 2011, 33, 969–975. [CrossRef]
- Liu FY, Lin CY, Chang JT, et al. 18F-FDG PET Can Replace Conventional Work-up in Primary M Staging of Nonkeratinizing Nasopharyngeal Carcinoma. Journal of Nuclear Medicine Official Publication Society of Nuclear Medicine 2007, 48, 1614.
- Tang LQ, Chen QY, Fan W, et al. Prospective study of tailoring whole-body dual-modality [18F]fluorodeoxyglucose positron emission tomography/computed tomography with plasma Epstein-Barr virus DNA for detecting distant metastasis in endemic nasopharyngeal carcinoma at initial staging. Journal of Clinical Oncology Official Journal of the American Society of Clinical Oncology, 2013, 31, 2861.
- De Jaeghere EA, Laloo F, Lippens L, et al. Splenic 18F-FDG uptake on baseline PET/CT is associated with oncological outcomes and tumor immune state in uterine cervical cancer. Gynecologic Oncology 159, 335–343. [CrossRef] [PubMed]
- Yang M, Lin Z, Xu ZQ, et al. Influx rate constant of 18F-FDG increases in metastatic lymph nodes of non-small cell lung cancer patients. Eur J Nucl Med Mol Imaging, 2020, 47, 1198–1208. [CrossRef]
- Yang M, Lin Z, Xu ZQ, et al. Influx rate constant of 18F-FDG increases in metastatic lymph nodes of non-small cell lung cancer patients. Eur J Nucl Med Mol Imaging. 2020, 47, 1198–1208. [CrossRef]
- Zhuang M, Karakatsanis NA, Dierckx R, et al. Impact of Tissue Classification in MRI-Guided Attenuation Correction on Whole-Body Patlak PET/MRI. Mol Imaging Biol. 2019, 21, 1147–1156. [CrossRef]
- Zhuang M, Karakatsanis NA, Dierckx R, et al. Quantitative analysis of heterogeneous [(18)F]FDG static (SUV) vs. Patlak (Ki) whole-body PET imaging using different segmentation methods: a simulation study. Mol Imaging Biol. 2019, 21, 317–327. [CrossRef]
- Patlak CS, Blasberg RG, Fenstermacher JD. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab. 1983, 3, 1–7. [CrossRef] [PubMed]
- Karakatsanis NA, Lodge MA, Zhou Y, et al. Dynamic whole-body PET parametric imaging: II. task-oriented statistical estimation. Phys Med Biol. 2013, 58, 7419–7445. [CrossRef] [PubMed]
- Karakatsanis NA, Lodge MA, Tahari AK, et al. Dynamic whole-body PET parametric imaging: I. Concept, acquisition protocol optimization and clinical application. Phys Med Biol. 2013, 58, 7391–7418. [CrossRef] [PubMed]
- Patlak CS, Blasberg RG. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. Generalizations. J Cereb Blood Flow Metab. 1985, 5, 584–90. [CrossRef]
- Minocha J, Lewandowski RJ. Assessing Imaging Response to Therapy. Radiol Clin North Am. 2015, 53, 1077–1088. [CrossRef]
- Matsubara R, Kawano S, Chikui T, et al. Clinical significance of combined assessment of the maximum standardized uptake value of F-18 FDG PET with nodal size in the diagnosis of cervical lymph node metastasis of oral squamous cell carcinoma. Acad Radiol. 2012, 19, 708–717. [CrossRef]
- van den Brekel, MW. Lymph node metastases: CT and MRI. Eur J Radiol. 2000, 33, 230–8. [Google Scholar] [CrossRef]
- Tsao SW, Tsang CM, Lo KW. Epstein-Barr virus infection and nasopharyngeal carcinoma. Philos Trans R Soc Lond B Biol Sci. 2017, 372, 20160270. [CrossRef]
- Li Z, Tsai MH, Shumilov A, et al. Epstein-Barr virus ncRNA from a nasopharyngeal carcinoma induces an inflammatory response that promotes virus production. Nat Microbiol. 2019, 4, 2475–2486. [CrossRef]
- Lo Y, Chan A, Chan L, et al. Molecular prognostication of nasopharyngeal carcinoma by quantitative analysis of circulating Epstein-Barr virus DNA. Cancer Res. 2000, 60, 6878–81.
- Yuan H, Yang BB, Xu ZF, et al. The clinical value of quantitative analysis of plasma Epstein - Barr virus DNA in patients with nasopharyngeal carcinoma. Zhonghua Er Bi Yan Hou Ke Za Zhi. 2004, 39, 162–5.
- Fleming I, Copper J, Henson D, et a1. American joint committee on cancer: AJCC cancer staging manual. 5th ed. Philadelphia: Lippincott-Raven, 1997.
- Fang FM, Tsai WL, Chen HC, et al. Intensity-modulated or conformal radiotherapy improves the quality of life of patients with nasopharyngeal carcinoma: comparisons of four radiotherapy techniques. Cancer 2007, 109, 313–321. [CrossRef] [PubMed]
- Lu H, Peng L, Yuan X, et al. Concurrent chemoradiotherapy in locally advanced nasopharyngeal carcinoma: a treatment paradigm also applicable to patients in Southeast Asia. Cancer Treat Rev. 2009, 35, 345–353. [CrossRef] [PubMed]
- Bedwinek JM, Perez CA, Keys DJ. Analysis of failures after definitive irradiation for epidermoid carcinoma of the nasopharynx. Cancer. 1980, 45, 2725–2729. [CrossRef]
- Hsu MM, Tu SM. Nasopharyngeal carcinoma in Taiwan clinical manifestations and results of therapy. Cancer 1983, 52, 362–368. [CrossRef]
- Muzi M, O"Sullivan F, Mankoff DA, et al.Quantitative Assessment of Dynamic PET Imaging Data in Cancer Imaging. Magn Reson Imaging. 2012, 30, 1203–1215. [CrossRef]
- Laffon E, de Clermont H, Begueret H, et al. Assessment of dual-time-point 18F-FDG-PET imaging for pulmonary lesions. Nucl Med Commun. 2009, 30, 455–461. [CrossRef]
- Huang XT, Zhuang MZ, Yang S, et al. The valuable role of dynamic 18F FDG PET/CT-derived kinetic parameter Ki in patients with nasopharyngeal carcinoma prior to radiotherapy: A prospective study. Radiother Oncol. 2023, 179, 109440. [CrossRef]
- Luzuriaga K, Sullivan JL. Infectious mononucleosis. N Engl J Med. 2010, 362, 1993–2000. [CrossRef]
- Kim KY, LE QT, Yom SS, et al. Clinical utility of epstein-barr virus DNA testing in the treatment of nasopharyngeal carcinoma patients. Int J Radiat Oncol Biol Phys. 2017, 98, 996–1001. [CrossRef]
- Hsu CL, Chang KP, Lin CY, et al. Plasma Epstein-Barr virus DNA concentration and clearance rate as novel prognostic factors for metastatic nasopharyngeal carcinoma. Head Neck. 2012, 34, 1064–70. [CrossRef] [PubMed]
- Zhao CX, Zhu W, Ba ZQ, et al. The regulatory network of nasopharyngeal carcinoma metastasis with a focus on EBV,lncRNAs and miRNAs. Am J Cancer Res. 2018, 8, 2185–2209.
- Duffy MJ, Sturgeon C, Lamerz R, et al. Tumor markers in pancreatic cancer: a European Group on Tumor Markers (EGTM) status report. Ann Oncol. 2010, 21, 441–447. [CrossRef] [PubMed]
| Variable | Number(%) |
| Median Age(years) | 48 |
| Sex | |
| Male | 36(71) |
| Female | 15(29) |
| T Stage | |
| T1 | 7(14) |
| T2 | 9(17) |
| T3 | 28(55) |
| T4 | 7(14) |
| N Stage | |
| N1 | 21(41) |
| N2 | 27(53) |
| N3 | 3(6) |
| M Stgae | |
| Mx | 3(6) |
| M0 | 43(86) |
| M1 | 3(8) |
| Clinical Stage | |
| I | 0(0) |
| II | 8(16) |
| III | 27(53) |
| IV | 15(29) |
| Not Identified | 1(2) |
| EBV-DNA Status | |
| High Level | 23(45) |
| Normal Level | 28(55) |
| Clinical Efficacy | Number(%) | Mean SUV-Max | P Value | Mean Ki-Mean | P Value | Mean Ki-Max | P Value |
| Valid | 50(75) | 7.2 | <0.001 | 0.01323 | <0.001 | 0.01510 | <0.001 |
| Invalid | 17(25) | 4.3 | 0.00978 | 0.01077 |
| Clinical Efficacy | Mean Ki-Mean | P Value | Mean Ki-Max | P Value |
| Valid | 0.00897 | >0.05 | 0.00982 | >0.05 |
| Invalid | 0.00737 | 0.00787 |
| Clinical Efficacy | Mean Ki-Mean Value | P Value | Mean Ki-Max Value | P Value |
| Valid | 0.00910 | 0.0457 | 0.01004 | 0.0298 |
| Invalid | 0.00716 | 0.00767 |
| Clinical Efficacy | Mean Ki-Mean Value | P Value | Mean Ki-Max Value | P Value |
| Valid | 0.01060 | 0.0062 | 0.01149 | 0.0062 |
| Invalid | 0.00670 | 0.00719 |
| Factors | Ki-Mean | Ki-Max | ||
| r | P value | r | P value | |
| SUV-Max | 0.8450 | <0.0001 | 0.8498 | <0.0001 |
| T-Stage | 0.0659 | 0.5965 | 0.0517 | 0.6780 |
| Normal EBV-DNA level | 0.2171 | 0.0776 | 0.2318 | 0.0591 |
| Age | 0.0558 | 0.6539 | 0.0739 | 0.5523 |
| Lymph node <1.0cm | 0.6369 | <0.0001 | 0.6416 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





