Submitted:
17 July 2023
Posted:
19 July 2023
You are already at the latest version
Abstract
Keywords:
Introduction
Subretinal injection of PEG
Histological Studies
Microscopy and image analysis
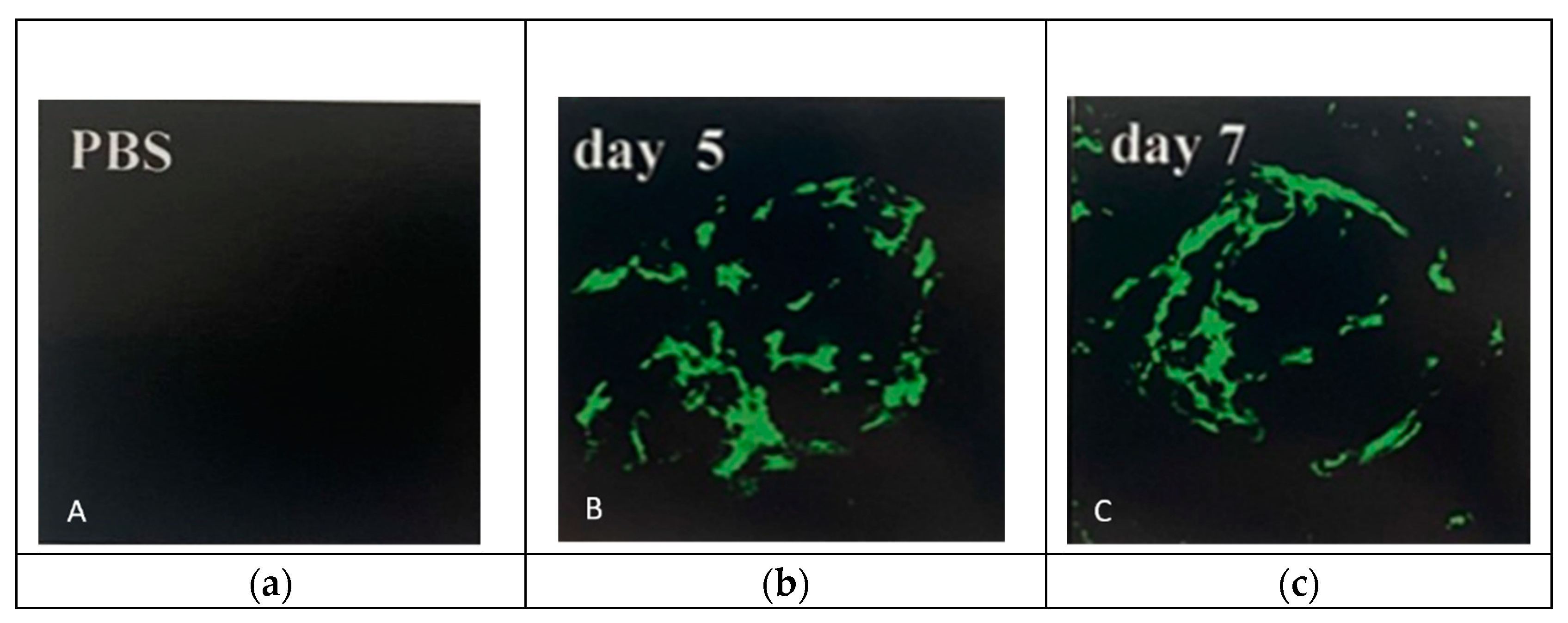
CNV induction after PEG injection
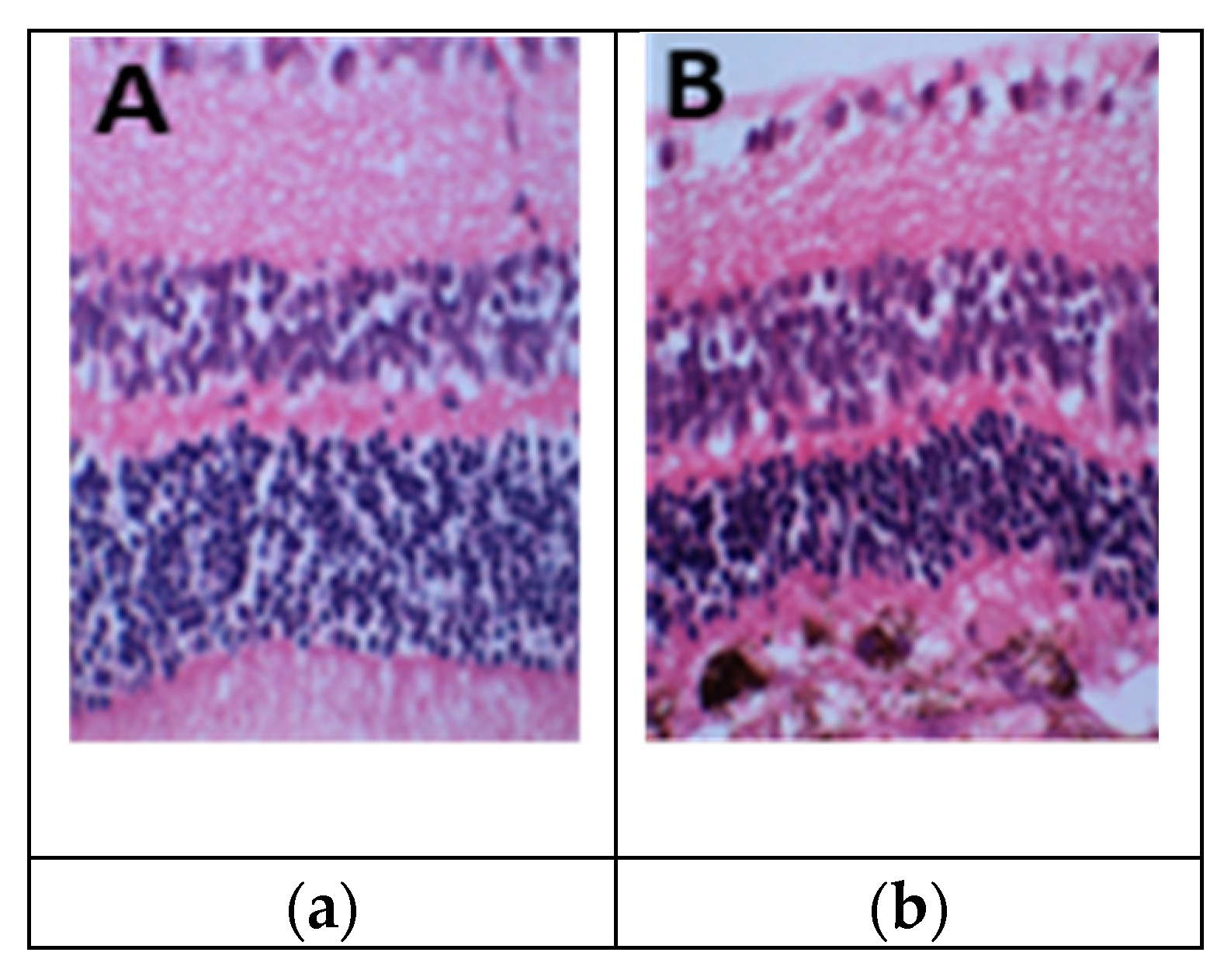
Retinal changes after 1-mg PEG injection
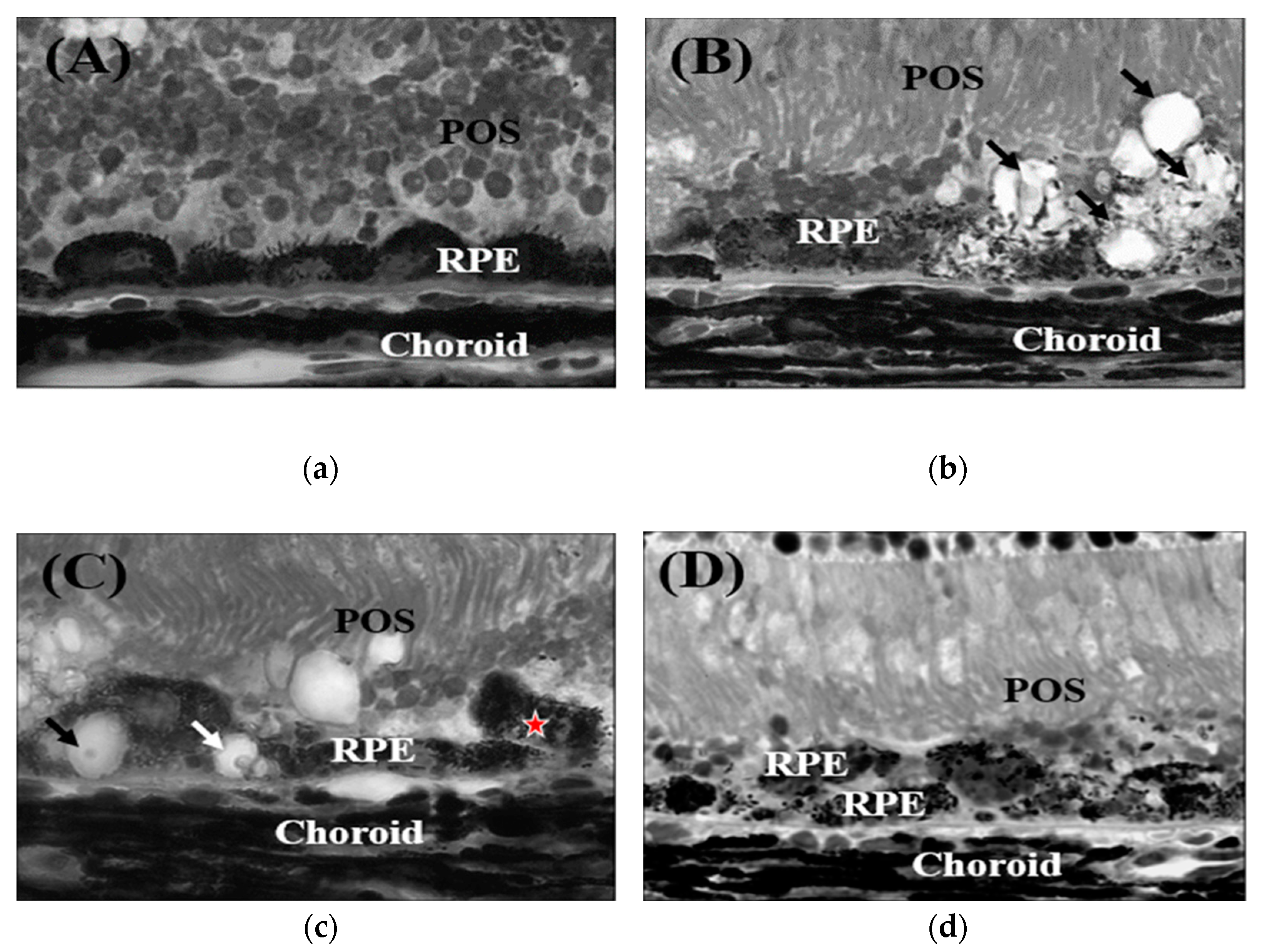
Dry AMD like changes after 0.5-mg PEG injection
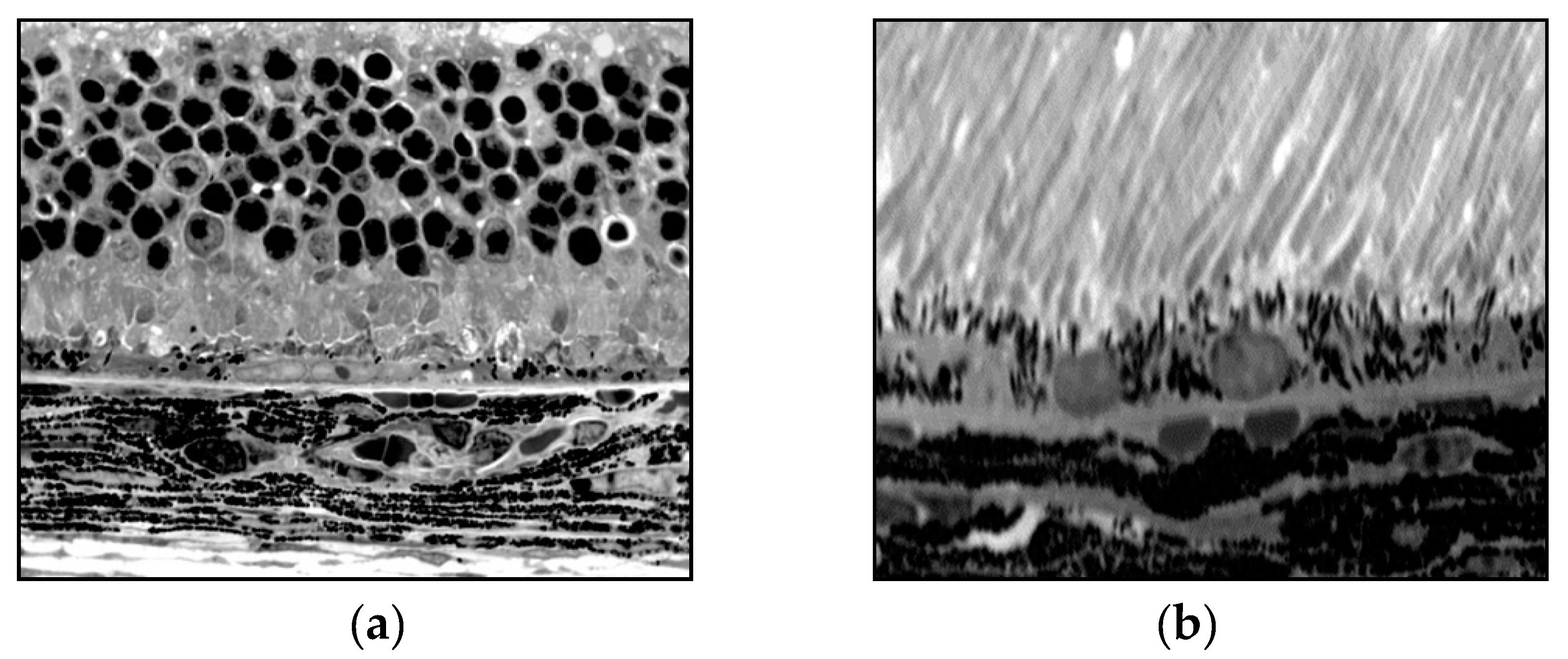
RPE degeneration and activation of autophagy by PEG
Reliability and reproducibility of PEG injection as a basis for AMD
Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgment
Conflicts of Interest
Abbreviations Used
References
- Campochiaro, P. A. Retinal and Choroidal Neovascularizatoin. J. Cell. Physiol. 2000, 184, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Coleman, H. R.; Chan, C. C.; Ferris 3rd, F. L.; and Chew, E. Y. Age-related macular degeneration. Lancet. 2008, 372, 1835–1845. [Google Scholar] [CrossRef]
- Zarbin, M. A. Current concepts in the pathogenesis of age-related macular degeneration. Arch. Ophthalmol. 2004, 122, 598–614. [Google Scholar] [CrossRef]
- Montezuma, S. R.; Vavvas, D.; and Miller, J. W. Review of ocular angiogenesis animal models. Semin. Ophthalmol. 2009, 24, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Elizabeth Rakoczy, P.; Yu, M. J.; Nusinowitz, S.; Chang, B.; and Heckenlively, J.R. Mouse models of age-related macular degeneration. Exp. Eye Res. 2006, 82, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Edwards, A. O.; and Malek, G. Molecular genetics of AMD and current animal models. Angiogenesis. 2007, 10, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Lassota, N. Clinical and histological aspects of CNV formation: studies in an animal model. Acta Ophthalmol. 2008, 86, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Bora, P. S.; Sohn, J. H.; Cruz, J. M.; Jha, P.; Nishihori, H.; Wang, Y.; Kaliappan, S.; Kaplan, H. J.; and Bora, N. S. Role of complement membrane attack complex in laser-induced choroidal neovascularization. J. Immunol. 2005, 174, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Bora, N. S.; Kaliappan, S.; Jha, P.; Xu, Q.; Sohn, J. H.; Dhaulakhandi, D. B.; Kaplan, H. J.; and Bora, P. S. Complement activation via alternative pathway is critical in the development of laser-induced choroidal neovascularization: role of factor B and factor H. J. Immunol. 2006, 177, 1872–1878. [Google Scholar] [CrossRef] [PubMed]
- Lyzogubov, V. V.; Tytarenko, R. G.; Jha, P.; Liu, J.; Bora, N. S.; and Bora, P. S. Role of ocular complements factor H in a murine model of choroidal neovascularization. Am. J. Pathol. 2010, 177, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D. H.; Radeke, M. J.; Gallo, N. B.; Chapin, E. A.; Johnson, P. T.; Curletti, C. R.; Hancox, L. S.; Hu, J.; Ebright, J. N.; Malek, G.; Hauser, M. A.; Rickman, C. B.; Bok, D.; Hageman, G. S.; and Johnson, L. V. The pivotal role of the complement system in aging and age-related macular degeneration: hypothesis re-visited. Prog. Retin. Eye Res. 2010, 29, 95–112. [Google Scholar] [CrossRef]
- Seddon, J. M.; Yu, Y.; Miller, E. C. et al. Rare variants in CFI and C9 are associated with high risk of advanced age-related macular degeneration. Nat. Genet. 2013, 45, 1366–1370. [Google Scholar] [CrossRef] [PubMed]
- Ramo, K.; Cashman, S. M.; and Kumar-Singh, R. Evaluation of adenovirus-delivered human CD59 as a potential therapy for AMD in a model of human membrane attack complex formation on murine RPE. Invest. Ophthalmol. Vis. Sci. 2008, 49, 4126–4136. [Google Scholar] [CrossRef]
- Morgan, B. P. Complement membrane attack on nucleated cells: resistance, recovery and non-lethal effects. Biochem. J. 1989, 264, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Halperin, J. A.; Taratuska, A.; and Nicholson-Weller, A. Terminal complement complex C5b-9 stimulated mitogenesis in 3T3 cells. J. Clin. Invest. 1993, 91, 1974–1978. [Google Scholar] [CrossRef]
- Hamad, I.; Hunter, A. C.; Szebeni, J.; and Moghimi, S. M. Polyethylene glycols generate complement activation produces in human serum through increased alternative pathway turnover and a MASP-2-dependent process. Mol. Immunol. 2008, 46, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Moein, M. S.; Hamad, I.; Bünger, R.; Andresen, T. L.; Jørgensen, K.; Hunter, A.C.; Baranji, L.; Rosivall, L.; and Szebeni, J. Methylation of the phosphate oxygen moiety of phospholipid-methoxy (polyethylene glycol) conjugate prevents PEGylated liposome-mediated complement activation and anaphylatoxin production. J. Liposome Res. 2006, 16, 167–174. [Google Scholar]
- Veronese, F. M.; and Pasut, G. PEGylation, successful approach to drug delivery. Drug Discovery Today. 2005, 10, 1451–1458. [Google Scholar] [CrossRef] [PubMed]
- Yamaoka, T.; Tabata, Y.; and Ikada, Y. Fate of water-soluble polymers administered via different routes. J. Pharm. Sci. 1995, 84, 349–354. [Google Scholar] [CrossRef]
- Final report on the safety assessment of Triethylene Glycol and PEG-4. Int. J. Toxicol. 2006, 25, 121–138. [CrossRef]
- Sarks, SH. Aging and degeneration in the macular region: a clinico-pathological study. Br. J. Ophthalmol. 1976, 60, 324–341. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, M.; Raisier, B.J.; Sakurai, E.; Sarma, J.V.; Barnum, S.R.; Lambris, J.D.; Chen, Y.; Zhang, K.; Ambati, B.K.; Baffi, J.Z.; Ambati, J. Drusen complement component C3a and C5a promote choroidal neovascularization. Proc. Natl. Acad. Sci. USA. 2006, 103, 2328–2333. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.J.; Zeiss, C.; Chew, E.Y.; Tsai, J.Y.; Sackler, R.S.; Haynes, C.; Henning, A.K.; sanGiovanni, J.P.; Mane, S.M.; Mayne, S.T.; Bracken, M.B.; Ferris, F.L.; Ott, J.; Barnstable, C.; Hoh, J. Complement factor H polymorphism in age-related macular degeneration. Science. 2005, 308, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Lyzogubov, V.V.; Tytarenko, R.G.; Liu, J.; Bora, N.S.; Bora, P.S. ; Polyethylene glycol (PEG)-induced model of choroidal neovascularization. J. Biol. Chem. 2011, 286, 16229–16237. [Google Scholar] [CrossRef] [PubMed]
- Lyzogubov, V.V.; Bora, N.S.; Tytarenko, R.G.; Bora, P.S. Polyethylene glycol induced mouse model of retinal degeneration. Exp. Eye Res. 2014, 27, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Shen, W. Y.; and Rakoczy, P. E. In vivo use of oligonucleotides to inhibit choroidal neovascularization in the eye. Antisense Nucleic Acid Drug Dev. 2001, 11, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Bora, N. S.; Jha, P.; Lyzogubov, V. V.; Kaliappan, S.; Liu, J.; Tytarenko, R. G.; Fraser, D. A.; Morgan, B. P.; and Bora, P. S. Recombinant membrane-targeted form of CD59 inhibits the growth of choroidal neovascular complex in mice. J. Biol. Chem. 2010, 285, 33826–33833. [Google Scholar] [CrossRef] [PubMed]
- Chader, GJ. Animal models in research on retinal degenerations: past progress and future hope. Vision Res. 2002, 42, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Ramkumar, H. L.; Zhang, J.; Chan, C. C. Retinal ultrastructure of murine models of dry age-related macular degeneration (AMD). Prog. Ret. Eye Res. 2010, 29, 169–190. [Google Scholar] [CrossRef] [PubMed]
- Keeler, C.E. The inheritance of a retinal abnormality in white mice. Proc. Natl. Acad. Sci. 1924, 10, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Hafezi, F.; Grimm, C.; Simmen, B.C.; Wenzel, A.; Remé, C.E. Molecular ophthalmology: an update on animal models for retinal degenerations and dystrophies. Br. J. Ophthalmol. 2000, 84, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.; Hawes, N.L.; Hurd, R.E.; Wang, J.; Howell, D.; Davisson, M.T.; Roderick, T.H.; Nusinowitz, S.; Heckenlively, J.R. Mouse models of ocular diseases. Vis. Neurosci. 2005, 22, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Killingsworth, M. C. Angiogenesis in early choroidal neovascularization secondary to age-related macular degeneration. Graefes Arch. Clin. Exp. Ophthalmol. 1995, 233, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Baba, T.; Bhutto, I. A.; Merges, C.; Grebe, R.; Emmert, D.; McLeod, D. S.; Armstrong, D.; and Lutty, G. A. A rat model for choroidal neovascularization using subretinal lipid hydroperoxide injection. Am. J. Pathol. 2010, 176, 3085–3097. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, Z.; Liu, Y.; Song, Y.; Li, Y.; Laties, A. M.; Wen, R. Translocation of the retinal pigment epithelium and formation of sub-retinal pigment epithelium deposit induced by subretinal deposit. Mol. Vis. 2007, 14, 873–880. [Google Scholar]
- Cao, J.; Zhao, L.; Li, Y.; Liu, Y.; Xiao, W.; Song, Y.; Luo, L.; Huang. D.; Yancopoulos, G. D.; Wiegand, S. J.; and Wen, R. A subretinal Matrigel rat choroidal neovascularization (CNV) model and inhibition of CNV and associated inflammation and fibrosis by VEGF trap. Invest. Ophthalmol. Vis. Sci. 2010, 51, 6009–6017. [Google Scholar] [CrossRef]
- Palarasah. Y.; Skjodt, K.; Brandt, J.; Teisner, B.; Koch, C.; Vitved, L.; and Skjoedt M. O. Generation of a C3c specific monoclonal antibody and assessment of a C3c as a putative inflammatory marker divided from complement factor C3. J. Immunol. Methods. 2010, 362, 142–150. [Google Scholar] [CrossRef]
- Haddad, S.; Chen, C. A.; Santangelo, S. L.; and Seddon, J. M. The genetics of age-related macular degeneration: a review of progress to date. Surv. Ophthalmol. 2006, 51, 316–363. [Google Scholar] [CrossRef]
- Donoso, L. A.; Kim, D.; Frost, A.; Callahan, A.; and Hageman, G. The role of inflammation in the pathogenesis of age-related macular degeneration. Surv. Ophthalmol. 2006, 51, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Thurman, J. M.; Renner, B.; Kunchithapautham, K.; Ferreira, V. P.; Pangburn, M. K.; Ablonczy, Z.; Tomlinson, S.; Holers, V. M.; and Rohrer, B. Oxidative stress renders retinal pigment epithelial cells susceptible to complement-mediated injury. J. Biol. Chem. 2009, 284, 16939–16947. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y. J.; Huang, L. Z.; Xu, X. L.; Du, W.; Zhou, A. Y.; Yu, W. Z.; and Li, X. X. Polyethylene glycol-modified pigment epithelial-derived factor: new prospects for treatment of retinal neovascularization. J. Pharm. Exp. Ther. 2012, 342, 131–139. [Google Scholar] [CrossRef]
- Fernandez-Bueno, I.; Alonso-Alonso, M. L.; Garcia-Gutierrez, M. T.; and Diebold, Y. Reliability and reproducibility of a rodent model of choroidal neovascularization based on the subretinal injection of polyethylene glycol. Mol. Vision. 2019, 25, 194–203. [Google Scholar]
- Grossniklaus, H.E.; Kang, S.J.; Berglin, L. Animal models of choroidal and retinal neovascularization. Prog. Retin. Eye Res. 2010, 29, 500–519. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




