Submitted:
18 July 2023
Posted:
19 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
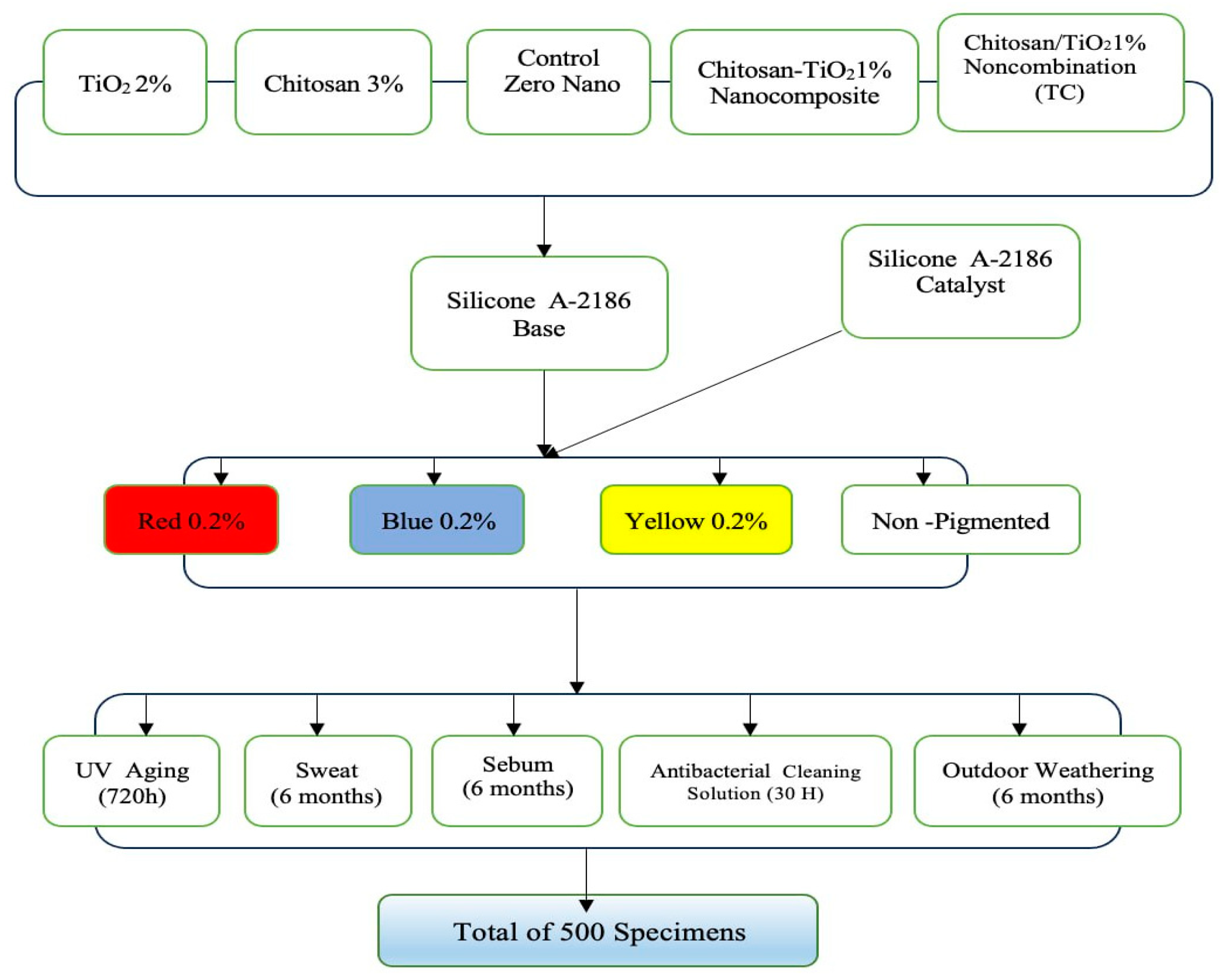
2.1. Materials
2.2. Methods
2.2.1. pilot study
2.2.2. Preparation of Nanocomposite
2.2.3. Preparation of control group specimens
2.2.4. Preparation of experimental group specimens
2.2.5. Preparation of experimental group specimens reinforced with Chitosan-TiO2 synthesized nanocomposite
2.2.6. Color stability test
2.2.7. Conditioning modes
2.3. Statistical Analysis
3. Results
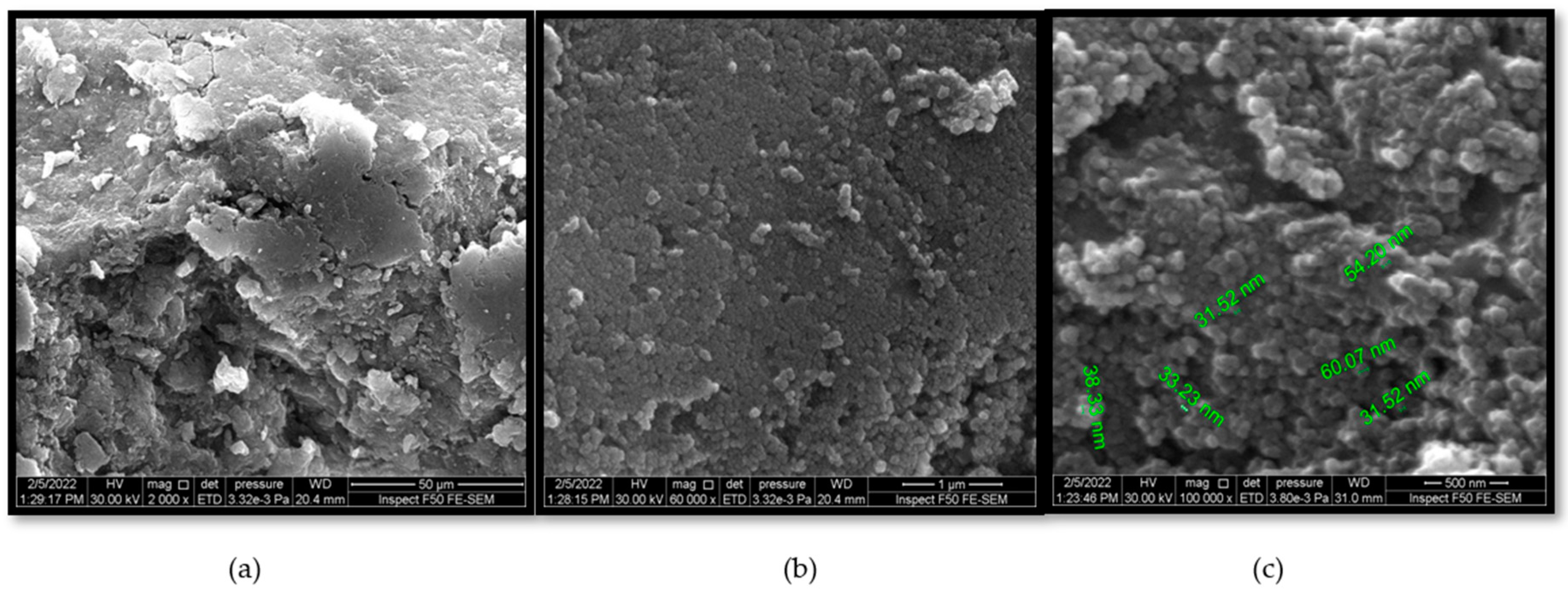
3.1. Morphological Characteristics
3.1.1. Scanning Electron Microscope (SEM)
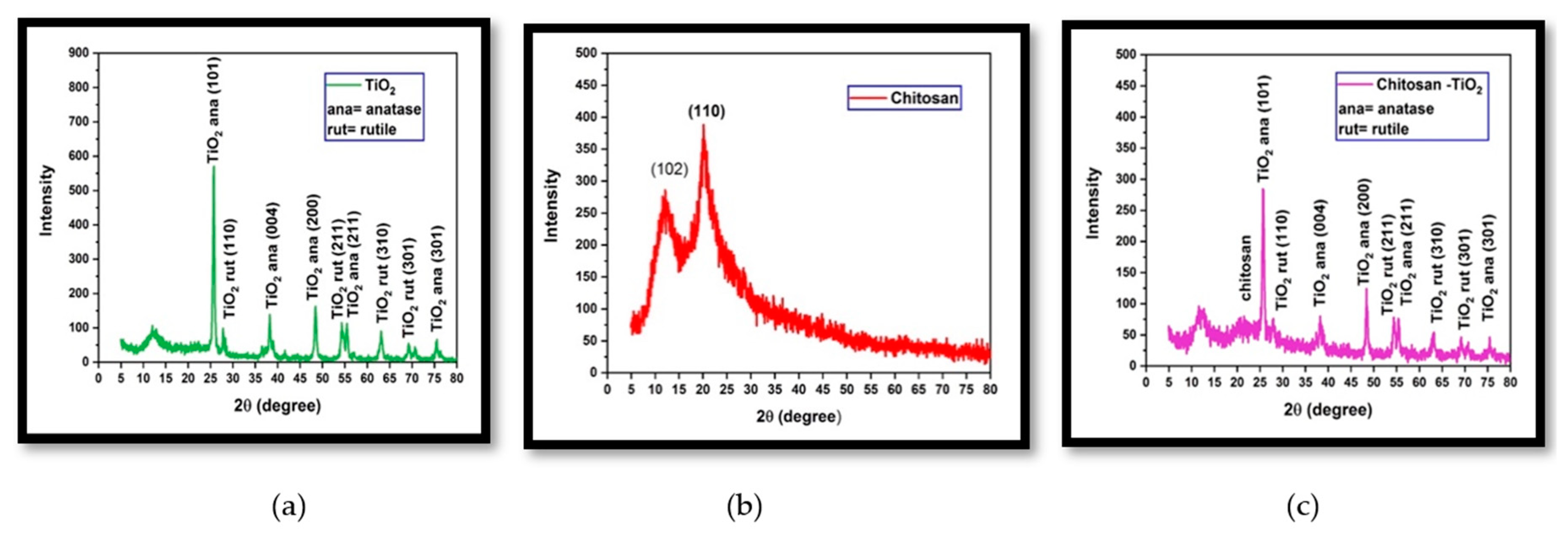
3.1.2. X-ray diffraction (XRD)
3.1.3. Fourier Transform Infrared Spectroscopy (FTIR)
3.2. Pilot study
3.3. Color stability results
3.3.1. The red color
| Groups | Brilliant Red ΔE | |||||
|---|---|---|---|---|---|---|
| Chitosan- TiO2 1% | TC 1% | TiO2 2% | Chitosan 3% | Control (Zero Nano ) |
P-value | |
| Sweat (6 months) | 6.66±3.60 | 6.14 ± 0.38 | 5.45 ± 0.58 | 5.01 ± 2.02 | 5.15 ± 1.43 | 0.561 |
| Antibacterial Cleaning Solution (30 h) | 3.31 ± 1.67 | 1.35 ± 0.49c,d | 1.03 ± 0.98c,d | 4.62 ± 2.39 | 4.66 ± 1.11 | 0.000 |
| Outdoor weather (6 months) |
16.61 ± 6.32 | 21.19 ±1.70 | 19.74±2.95 | 20.87±1.03 | 18.91 ± 9.90 | 0.695 |
| UV Weather 1 month (720h) |
27.93 ± 1.24a,c | 31.38 ± 0.6b,c | 27.36±0.71c | 41.19 ± 2.6 | 40.84±7.15 | 0.000 |
| Sebum (6 months) | 6.24 ± 1.66d | 7.48 ± 1.66d | 6.25±2.34d | 9.26 ± 1.85d | 14.44±2.36 | 0.000 |
| P-value | 0.000* | 0.000* | 0.000* | 0.000* | 0.000* | |
3.3.2. The blue color
| Groups | Blue ΔE | |||||
|---|---|---|---|---|---|---|
| Chitosan-TiO21% | TC 1% | TiO2 2% | Chitosan 3% | Control (Zero Nano) |
P-value | |
| Sweat (6 months) | 3.49±2.57 | 1.18±0.82c | 0.96±0.26c | 3.46±1.02d | 1.50±0.55 | 0.001 |
| Antibacterial Cleaning Solution (30 h) | 1.21±0.67 | 1.37±0.93 | 0.74±0.43c | 2.94±1.39 | 1.32±0.31 | 0.001 |
| Outdoor weather (6 months) | 9.08±1.44d | 8.92±0.59d | 10.65±0.25d,c | 7.73±0.66d | 5.71±1.57 | 0.000 |
| UV Weather 1 month (720h) | 3.73±0.89d | 2.67±0.58 | 2.67±0.37 | 3.63±0.93d | 1.88±0.86 | 0.005 |
| Sebum (6 months) | 4.21±1.79 | 2.84±1.34c | 3.09±1.36c | 5.76±2.19 | 4.85±1.26 | 0.018 |
| P-value | 0.000* | 0.000* | 0.000* | 0.000* | 0.000* | |
3.3.3. The yellow color
| Groups | Yellow ΔE | |||||
|---|---|---|---|---|---|---|
| Chitosan-TiO21% | TC 1% | TiO2 2% | Chitosan 3% | Control (Zero Nano ) |
P-value | |
| Sweat (6 months) | 1.34 ± 0.48b | 1.69 ± 0.85b | 0.48 ± 0.23c,d | 1.5 ± 0.60 | 2.21 ± 1.24 | 0.006 |
| Antibacterial Cleaning Solution (30 h) | 1.84 ± 0.50 | 1.14 ± 0.35 | 0.86 ± 0.38 | 1.41 ± 0.74 | 1.45 ± 0.78 | 0.103 |
| Outdoor weather (6 months) | 2.61 ± 0.56 | 2.64 ± 0.63 | 2.72 ± 0.74 | 2.72 ± 0.40 | 3.04 ± 0.61 | 0.803 |
| UV Weather 1 month (720h) | 0.95 ± 0.26 | 0.7 ± 0.26 | 1.02 ± 0.16 | 0.89 ± 0.32 | 1.13 ± 0.43 | 0.267 |
| Sebum (6 months) |
1.07 ± 0.36 | 1.49 ± 0.87 | 1.38 ± 0.50 | 1.32 ± 0.40 | 1.6 ± 1.29 | 0.788 |
| P-value | 0.000* | 0.002* | 0.000* | 0.000* | 0.035* | |
3.3.4. The non-pigmented
| Groups | Non-Pigmented ΔE | |||||
|---|---|---|---|---|---|---|
| Chitosan-TiO2 1% | TC 1% | TiO2 2% | Chitosan 3% | Control (Zero Nano) |
P-value | |
| Sweat (6 months) | 1.12 ± 0.41c | 1.70 ± 0.99c | 1.26 ± 0.59c | 7.63 ± 3.5d | 1.39 ± 0.46 | 0.000 |
| Antibacterial Cleaning Solution (30 h) | 1.24 ± 0.48 | 1.10 ± 0.37 | 0.76 ± 0.37 | 1.17 ± 0.32 | 0.95 ± 0.39 | 0.294 |
| Outdoor weather (6 months) | 7.77 ± 0.36a,b,c,d | 3.42 ± 0.45 | 4.11 ± 0.93 | 3.13 ± 1.61 | 4.67 ± 2.54 | 0.000 |
| UV Weather 1 month (720h) | 3.81 ± 0.35a,b,d | 2.00 ± 0.51c | 1.8 ± 0.34c | 5.04 ± 1.21d | 1.88 ± 0.99 | 0.000 |
| Sebum (6 months) | 4.81 ± 0.92b | 4.84 ± 0.92b | 2.55 ± 0.52d | 5.5 ± 4.28 | 9.02 ± 3.96 | 0.009 |
| P-value | 0.000* | 0.000* | 0.000* | 0.014* | 0.000* | |
4. Discussion
5. Conclusion
Author Contributions
Funding
Conflicts of Interest
References
- Rangel Goulart, D.; Sigua-Rodriguez, E.A.; Alvarez-Pinzón, N.; Rocha Fernandes, A.Ú.; Queiroz, E. Quality of life of patients with facial prosthesis. Revista Facultad de Odontología 2017, 29, 131–147. [Google Scholar] [CrossRef]
- Montgomery, P.C.; Kiat-Amnuay, S. Survey of currently used materials for fabrication of extraoral maxillofacial prostheses in North America, Europe, Asia, and Australia. J Prosthodont 2010, 19, 482–490. [Google Scholar] [CrossRef]
- Kotra, R.; Malhotra, P.; Yadav, B.; Bhardwaj, H.; Yadav, T. Color in Maxillofacial Prosthetics. Indian Journal of Health Sciences and Care 2016, 3. [Google Scholar] [CrossRef]
- P. N. Eleni, I.K., M. K. Krodkida, G. L. polyzois,. color stability of facial silicon prosthetic elastomer after artificial weathering. Dental Reearch journal 2008, 5, 71–79. [Google Scholar]
- Keith, M. Industrial applications of colour science. Physics in Technology 1988, 19, 51. [Google Scholar] [CrossRef]
- Rashid, F.; Barman, A.; Farook, TH; Jamayet, N. B.; Yhaya, M.F.B.; Alam, M.K. Factors affecting color stability of maxillofacial prosthetic silicone elastomer: A systematic review and meta-analysis. Journal of Elastomers & Plastics 2020, 53, 698–754. [Google Scholar] [CrossRef]
- Beatty, M.W.; Mahanna, G.K.; Dick, K.; Jia, W. Color changes in dry-pigmented maxillofacial elastomer resulting from ultraviolet light exposure. J Prosthet Dent 1995, 74, 493–498. [Google Scholar] [CrossRef]
- Kiat-amnuay, S. ; Lemon, JC; Powers, JM Effect of opacifiers on color stability of pigmented maxillofacial silicone A-2186 subjected to artificial aging. Journal of Prosthodontics 2002, 11, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Akash, R.N.; Guttal, S.S. Effect of Incorporation of Nano-Oxides on Color Stability of Maxillofacial Silicone Elastomer Subjected to Outdoor Weathering. J Prosthodont 2015, 24, 569–575. [Google Scholar] [CrossRef]
- Han, Y.; Kiat-amnuay, S.; Powers, JM; Zhao, Y. Effect of nano-oxide concentration on the mechanical properties of a maxillofacial silicone elastomer. J Prosthet Dent 2008, 100, 465–473. [Google Scholar] [CrossRef]
- Han, Y.; Zhao, Y.; Xie, C. ; Powers, JM; Kiat-amnuay, S. Color stability of pigmented maxillofacial silicone elastomer: effects of nano-oxides as opacifiers. J Dent, 2. [CrossRef]
- Prameshwari, F.; Karlina, E.; Hasratiningsih, Z. Synthesis of Ca-Psz nanoparticles using sol-gel technique with Chitosan as a dispersant for raw materials restoration and dental rehabilitation equipment. Padjadjaran Journal of Dentistry 2013, 25. [Google Scholar] [CrossRef]
- Jaiswal, T.; Pisulkar, S.; Kambala, S. Evaluation of antifungal effect of maxillofacial silicone after incorporation of chitosan nanoparticles: Evidence in pharmaceutical therapeutics. Journal of Datta Meghe Institute of Medical Sciences University 2020, 15. [Google Scholar] [CrossRef]
- Anaya-Esparza, L.M.; Ruvalcaba-Gómez, J.M.; Maytorena-Verdugo, C.I.; González-Silva, N.; Romero-Toledo, R.; Aguilera-Aguirre, S.; Pérez-Larios, A.; Montalvo-González, E. Chitosan-TiO2: A Versatile Hybrid Composite. Materials 2020, 13, 811. [Google Scholar] [CrossRef]
- Castellano, M.; Dodero, A.; Scarfi, S.; Mirata, S.; Pozzolini, M.; Tassara, E.; Sionkowska, A.; Adamiak, K.; Alloisio, M.; Vicini, S. Chitosan–Collagen Electrospun Nanofibers Loaded with Curcumin as Wound-Healing Patches. Polymers 2023, 15, 2931. [Google Scholar] [CrossRef]
- Sonnahalli, N.K.; Chowdhary, R. Effect of nanoparticles on color stability and mechanical and biological properties of maxillofacial silicone elastomer: A systematic review. J Indian Prosthodont Soc 2020, 20, 244–254. [Google Scholar] [CrossRef]
- Al-Hakam J Ibrahim, H.J.A.-J. Mechanical properties of Chitosan incorporated in maxillofacial silicone and its anti candidal activity in vitro. J Res Med Dent Sci 2018, 6, 101–107. [Google Scholar]
- Gandhi, D.S.; Sethuraman, R. Comparative evaluation of tensile strength, tear strength, color stability and hardness of conventional and 1% trisnorbornenylisobutyl polyhedralsilsesquioxane modified room temperature vulcanizing maxillofacial silicone after a six month artificial aging period. J Indian Prosthodont Soc 2022, 22, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Hatamleh, M.M.; Polyzois, G.L.; Nuseir, A.; Hatamleh, K.; Alnazzawi, A. Mechanical Properties and Simulated Aging of Silicone Maxillofacial Elastomers: Advancements in the Past 45 Years. J Prosthodont 2016, 25, 418–426. [Google Scholar] [CrossRef]
- Nair, A.; Saratchandran, S. Comparative Evaluation of Color Stability of Maxillofacial Silicones Following Accelerated Aging Conditions. Face 2022, 3, 362–368. [Google Scholar] [CrossRef]
- Hatamleh, M.M.; Polyzois, G.L.; Silikas, N.; Watts, D.C. Effect of extraoral aging conditions on mechanical properties of maxillofacial silicone elastomer. J Prosthodont 2011, 20, 439–446. [Google Scholar] [CrossRef]
- dos Santos, D.M.; Goiato, M.C.; Moreno, A.; Pesqueira, A.A.; Haddad, M.F. Influence of pigments and opacifiers on color stability of an artificially aged facial silicone. J Prosthodont 2011, 20, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Sethi, T.; Kheur, M.; Coward, T.; Patel, N. Change in color of a maxillofacial prosthetic silicone elastomer, following investment in molds of different materials. J Indian Prosthodont Soc 2015, 15, 153–157. [Google Scholar] [CrossRef]
- Polyzois, G.L. Color stability of facial silicone prosthetic polymers after outdoor weathering. J Prosthet Dent 1999, 82, 447–450. [Google Scholar] [CrossRef]
- Mancuso, D.; Goiato, M.; Dekon, S.; Gennari-Filho, H. Visual evaluation of color stability after accelerated aging of pigmented and nonpigmented silicones to be used in facial prostheses. Indian Journal of Dental Research 2009, 20, 77–80. [Google Scholar] [CrossRef]
- Kiat-amnuay, S.; Johnston, D.A.; Powers, JM; Jacob, R. F. Color stability of dry earth pigmented maxillofacial silicone A-2186 subjected to microwave energy exposure. J Prosthodont 2005, 14, 91–96. [Google Scholar] [CrossRef]
- Chamaria, A.; Aras, MA; Chitre, V. ; Rajagopal, P. Effect of Chemical Disinfectants on the Color Stability of Maxillofacial Silicones: An In Vitro Study. J Prosthodont 2019, 28, e869–e872. [Google Scholar] [CrossRef] [PubMed]
- Eltayyar NH, A.A. , Abushelib MN. Evaluation of intrinsic color stability of Facial silicone elastomer reinforced with Different nanoparticles. Alexandria Dental Journal 2016, 41, 50–54. [Google Scholar]
- Abdalqadir, M.; Faraj, S.; Azhdar, B. An evaluation of a technique to improve the mechanical properties of maxillofacial silicone elastomers with zinc oxide nanoparticles. J Prosthet Dent 2022, 128, 531–538. [Google Scholar] [CrossRef]
- Beatty, M.W.; Mahanna, G.K.; Jia, W. Ultraviolet radiation-induced color shifts occurring in oil-pigmented maxillofacial elastomers. J Prosthet Dent 1999, 82, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Kiat-Amnuay, S.; Mekayarajjananonth, T.; Powers, JM; Chambers, M. S.; Lemon, JC Interactions of pigments and opacifiers on color stability of MDX4-4210/type A maxillofacial elastomers subjected to artificial aging. J Prosthet Dent 2006, 95, 249–257. [Google Scholar] [CrossRef]
- Kiat-amnuay, S.; Beerbower, M.; Powers, JM; Paravina, R. D. Influence of pigments and opacifiers on color stability of silicone maxillofacial elastomer. J Dent 2009, 37 Suppl 1, e45–50. [Google Scholar] [CrossRef]
- Kantola, R.; Lassila, L.V.; Tolvanen, M.; Valittu, P.K. Color stability of thermochromic pigment in maxillofacial silicone. J Adv Prosthodont 2013, 5, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Kheur, M.G.; Kakade, D.; Trevor, C.J.; Lakha, T.A.; Sethi, T. Effect of newly developed pigments and ultraviolet absorbers on the color change of pigmented silicone elastomer. J Indian Prosthodont Soc 2017, 17, 395–400. [Google Scholar] [CrossRef] [PubMed]
- Cevik, P.; Yildirim-Bicer, A.Z. Effect of different types of disinfection solution and aging on the hardness and colour stability of maxillofacial silicone elastomers. Int J Artif Organs 2017, 0. [Google Scholar] [CrossRef]
- Haldorai, Y.; Shim, J.-J. Novel chitosan-TiO2 nanohybrid: Preparation, characterization, antibacterial, and photocatalytic properties. Polymer Composites 2014, 35, 327–333. [Google Scholar] [CrossRef]
- Farah, A.; Sherriff, M.; Coward, T. Color stability of nonpigmented and pigmented maxillofacial silicone elastomer exposed to 3 different environments. J Prosthet Dent 2018, 120, 476–482. [Google Scholar] [CrossRef]
- Hatamleh, M.M.; Watts, D.C. Effect of extraoral aging conditions on color stability of maxillofacial silicone elastomer. J Prosthodont 2010, 19, 536–543. [Google Scholar] [CrossRef]
- Polyzois, G.L.; Tarantili, P.A.; Frangou, M.J.; Andreopoulos, A.G. Physical properties of a silicone prosthetic elastomer stored in simulated skin secretions. J Prosthet Dent 2000, 83, 572–577. [Google Scholar] [CrossRef]
- Stefaniak, A.B.; Harvey, C.J. Dissolution of materials in artificial skin surface film liquids. Toxicol In Vitro 2006, 20, 1265–1283. [Google Scholar] [CrossRef] [PubMed]
- Al-Harbi, F.A.; Ayad, NM; Saber, M. A.; ArRejaie, A.S.; Morgano, S.M. Mechanical behavior and color change of facial prosthetic elastomers after outdoor weathering in a hot and humid climate. J Prosthet Dent 2015, 113, 146–151. [Google Scholar] [CrossRef]
- Salloum, M.G.; Ganji, KK; Aldajani, A. M.; Sonune, S. Colour Stability of Two Commercially Available Maxillofacial Prosthetic Elastomers after Outdoor Weathering in Al Jouf Province. Materials (Basel) 2023, 16. [Google Scholar] [CrossRef]
- Chotprasert, N.; Shrestha, B.; Sipiyaruk, K. Effects of Disinfection Methods on the Color Stability of Precolored and Hand-Colored Maxillofacial Silicone: An In Vitro Study. Int J Biomater 2022, 2022, 7744744. [Google Scholar] [CrossRef]
- Anaya-Esparza, L.M.; Ruvalcaba-Gomez, J.M.; Maytorena-Verdugo, C.I.; Gonzalez-Silva, N.; Romero-Toledo, R.; Aguilera-Aguirre, S.; Perez-Larios, A.; Montalvo-Gonzalez, A.E. Chitosan-TiO(2): A Versatile Hybrid Composite. Materials (Basel) 2020, 13. [Google Scholar] [CrossRef] [PubMed]
- Hood, M.A.; Mari, M.; Munoz-Espi, R. Synthetic Strategies in the Preparation of Polymer/Inorganic Hybrid Nanoparticles. Materials (Basel) 2014, 7, 4057–4087. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, D.N.; Goiato, M.C.; Dekon, S.F.; Gennari-Filho, H. Visual evaluation of color stability after accelerated aging of pigmented and nonpigmented silicones to be used in facial prostheses. Indian J Dent Res 2009, 20, 77–80. [Google Scholar] [CrossRef]
- dos Santos, D.M.; Goiato, M.C.; Sinhoreti, M.A.; Fernandes, A.U.; Ribeiro Pdo, P.; Dekon, S.F. Color stability of polymers for facial prosthesis. J Craniofac Surg 2010, 21, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Bishal, A.K.; Wee, AG; Barao, VAR; Yuan, J. C.; Landers, R.; Sukotjo, C.; Takoudis, C.G. Color stability of maxillofacial prosthetic silicone functionalized with oxide nanocoating. J Prosthet Dent 2019, 121, 538–543. [Google Scholar] [CrossRef]
- Bangera, B.S.; Guttal, S.S. Evaluation of varying concentrations of nano-oxides as ultraviolet protective agents when incorporated in maxillofacial silicones: an in vitro study. J Prosthet Dent 2014, 112, 1567–1572. [Google Scholar] [CrossRef]
- Marrega Malavazi, E.; Dos Santos, D.M.; de Moraes Melo Neto, C.L.; Pereira de Caxias, F.; Freitas da Silva, E.V.; Bannwart, L.C.; Pesqueira, A.A.; de Melo Moreno, A.L.; de Magalhaes Bertoz, A.P.; Goiato, M.C. Influence of Different Pigmentations and Accelerated Aging on the Hardness and Tear Strength of the A-2186 and MDX4-4210 Silicones. Int J Dent 2020, 2020, 8492091. [Google Scholar] [CrossRef] [PubMed]
- Haug, S.P.; Moore, B.K.; Andres, C.J. Color stability and colorant effect on maxillofacial elastomers. Part II: Weathering effect on physical properties. The Journal of Prosthetic Dentistry 1999, 81, 423–430. [Google Scholar] [CrossRef]

| Date (2022) |
Temperature Co | Average Humidity % | Pressure (mbar) | ||
|---|---|---|---|---|---|
| Max | Min | Average | |||
| February | 15.2 | 5.4 | 10.3 | 64.0 | 917.4 |
| March | 15.3 | 6.4 | 10.8 | 59.6 | 915.9 |
| April | 26.2 | 14.6 | 20.4 | 43.5 | 914.5 |
| May | 28.2 | 17.3 | 22.8 | 41.8 | 913.2 |
| June | 37.4 | 24.6 | 31.0 | 28.3 | 910.1 |
| July | 40.7 | 26.8 | 33.7 | 24.2 | 906.4 |
| August | 42.1 | 28.0 | 35.0 | 23.9 | 908.5 |
| Groups | Brilliant Red ΔE (Pilot Study) | |||
|---|---|---|---|---|
| Chitosan-TiO2 1% | Chitosan-TiO2 1.5% | Chitosan-TiO2 0.5% | p-value | |
| Sweat (6 months) | 6.66 ± 3.60 | 5.20± 0.36 | 4.12± 0.87 | 0.305 |
| Antibacterial Cleaning Solution (30 h) | 3.31 ± 1.67 | 2.99 ± 2.02 | 1.28 ± 0.64 | 0.179 |
| Outdoor weather (6 months) | 16.62 ± 6.32b | 17.59 ± 1.68b | 24.64 ± 0.68 | 0.035 |
| UV Weather 1 month (720h) | 27.92 ± 1.24 | 26.45 ± 1.11 | 25.61 ± 2.64 | 0.162 |
| Sebum (6 months) | 6.24 ± 1.66a,b | 10.09 ± 2.96 | 11.14 ± 0.92 | 0.01 |
| p-value | 0.000* | 0.000* | 0.000* | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





