Submitted:
06 September 2023
Posted:
07 September 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Colony
2.2. Experimental procedures
2.3. BrdU treatment
2.4. Running test
2.5. Histological and immunohistochemistry procedures
2.6. Golgi staining
2.7. Image acquisition and measurements
2.7.1. Dendritic spine number and morphology
2.7.2. Cell Density
2.7.3. Morphometric microglial cell analysis
2.7.4. Intensity-based analysis of BDNF staining
2.8. Western Blotting
2.9. Behavioral Testing
2.9.1. Hind-Limb Clasping
2.9.2. Object exploration in an Open Field arena
2.9.3. Passive Avoidance Test
2.10. Statistical Analysis
3. Results
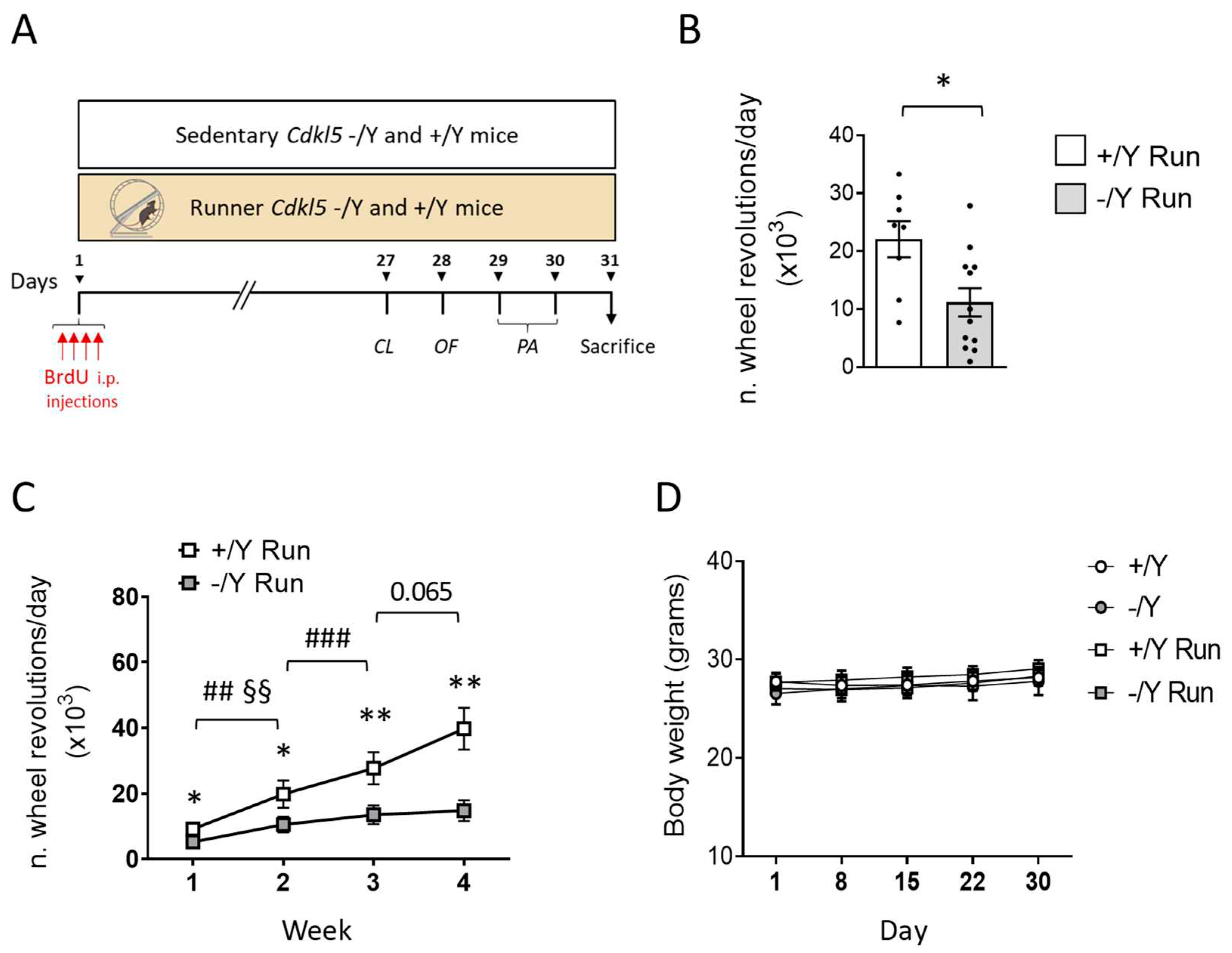
3.1. Running test and effect of voluntary wheel running on body weight
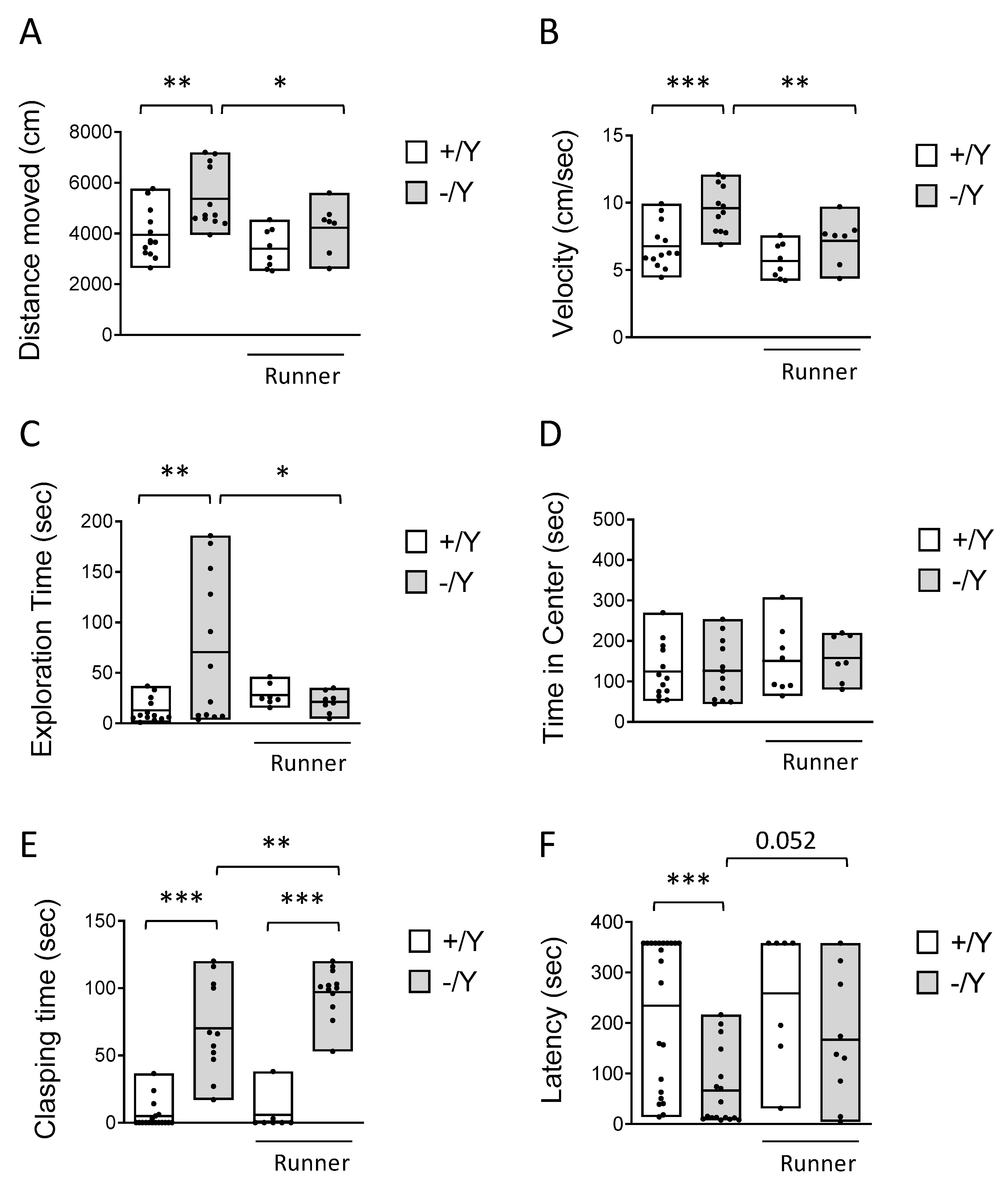
3.2. Effect of voluntary wheel running on hyperlocomotion, impulsivity, and cognitive behavior in Cdkl5 KO mice
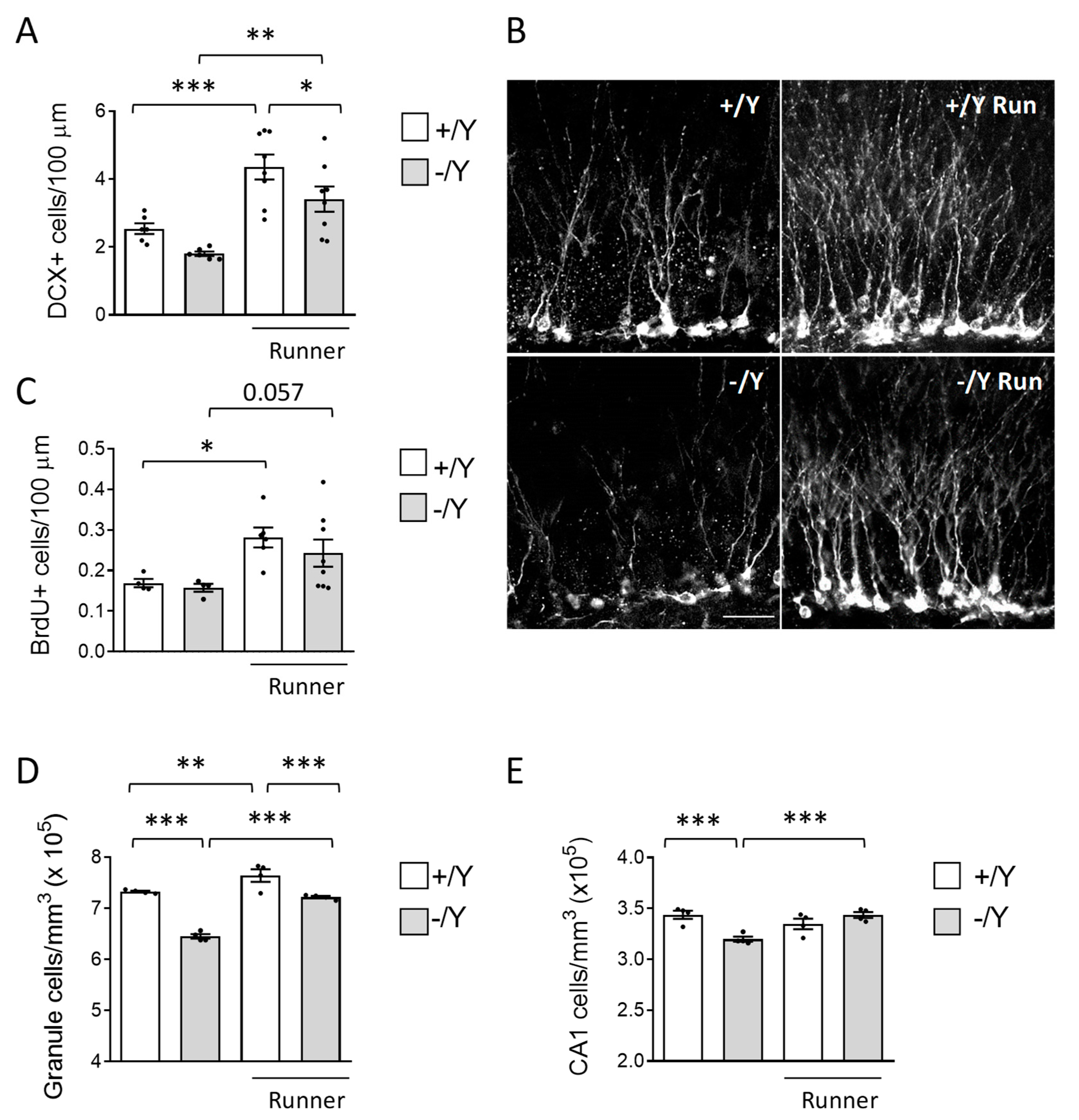
3.3. Effect of voluntary wheel running on hippocampal neurogenesis and neuronal survival in Cdkl5 KO mice
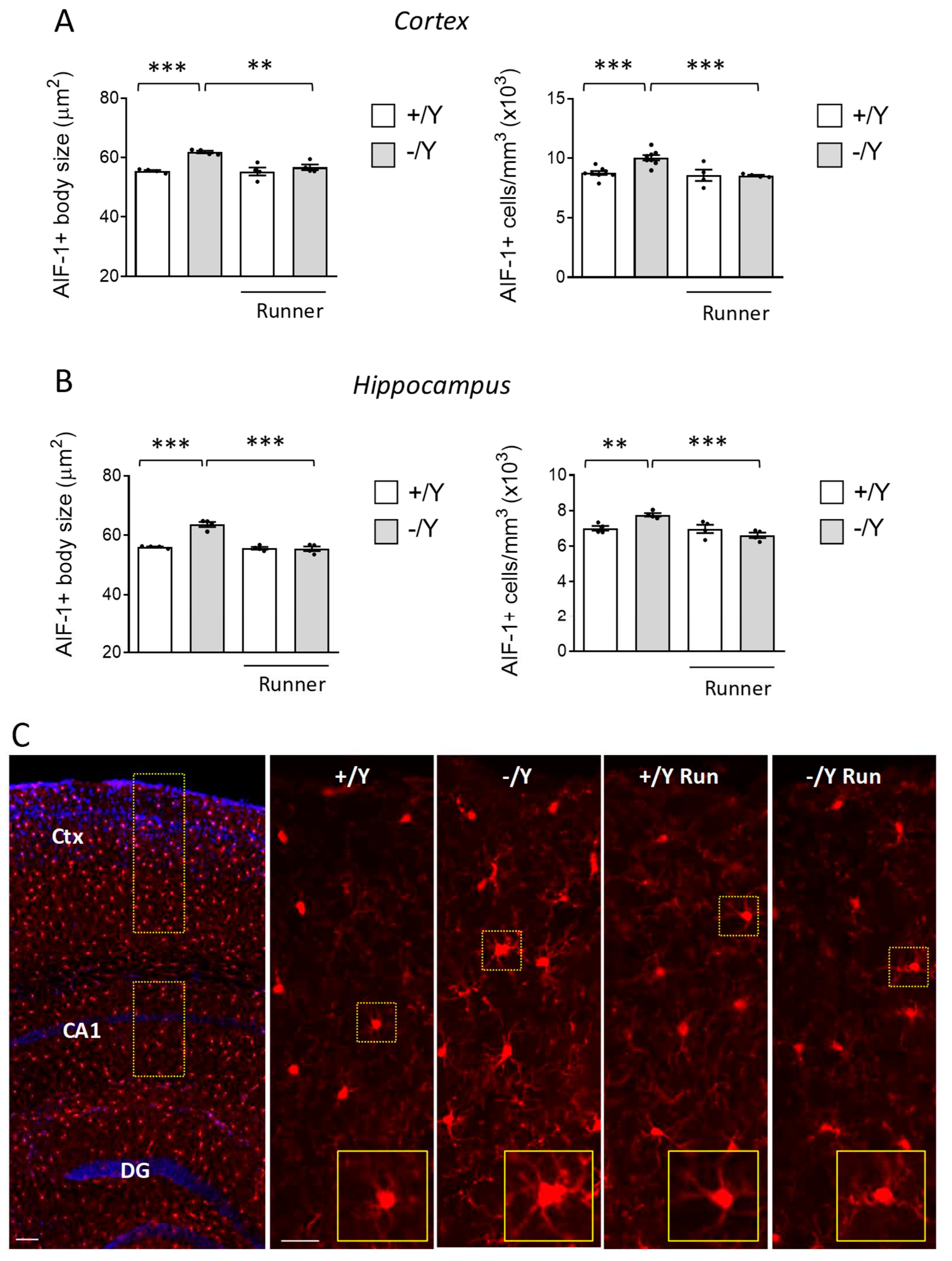
3.4. Effect of voluntary wheel running on microglia overactivation in Cdkl5 KO mice
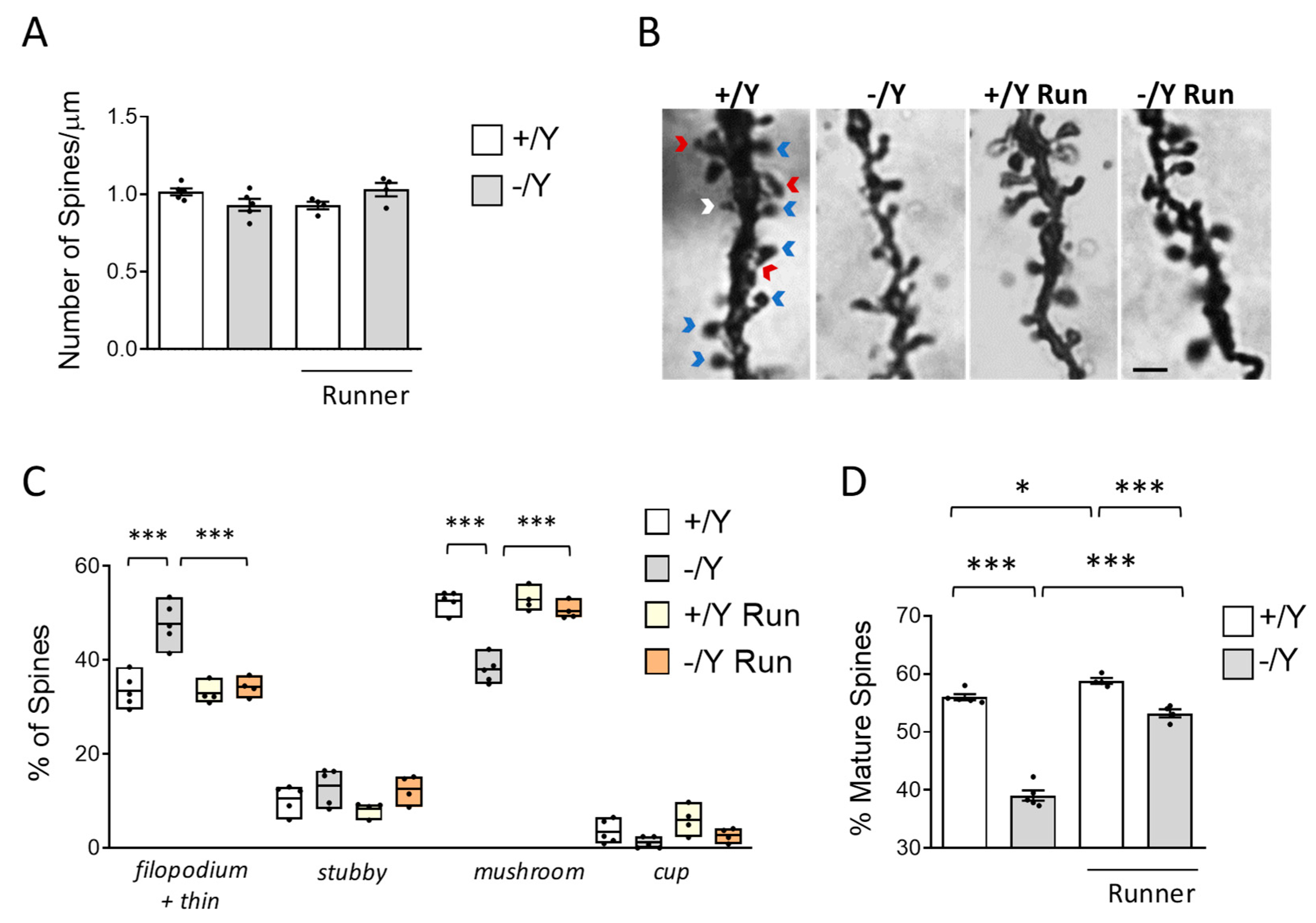
3.5. Effect of voluntary wheel running on spine development in Cdkl5 KO mice
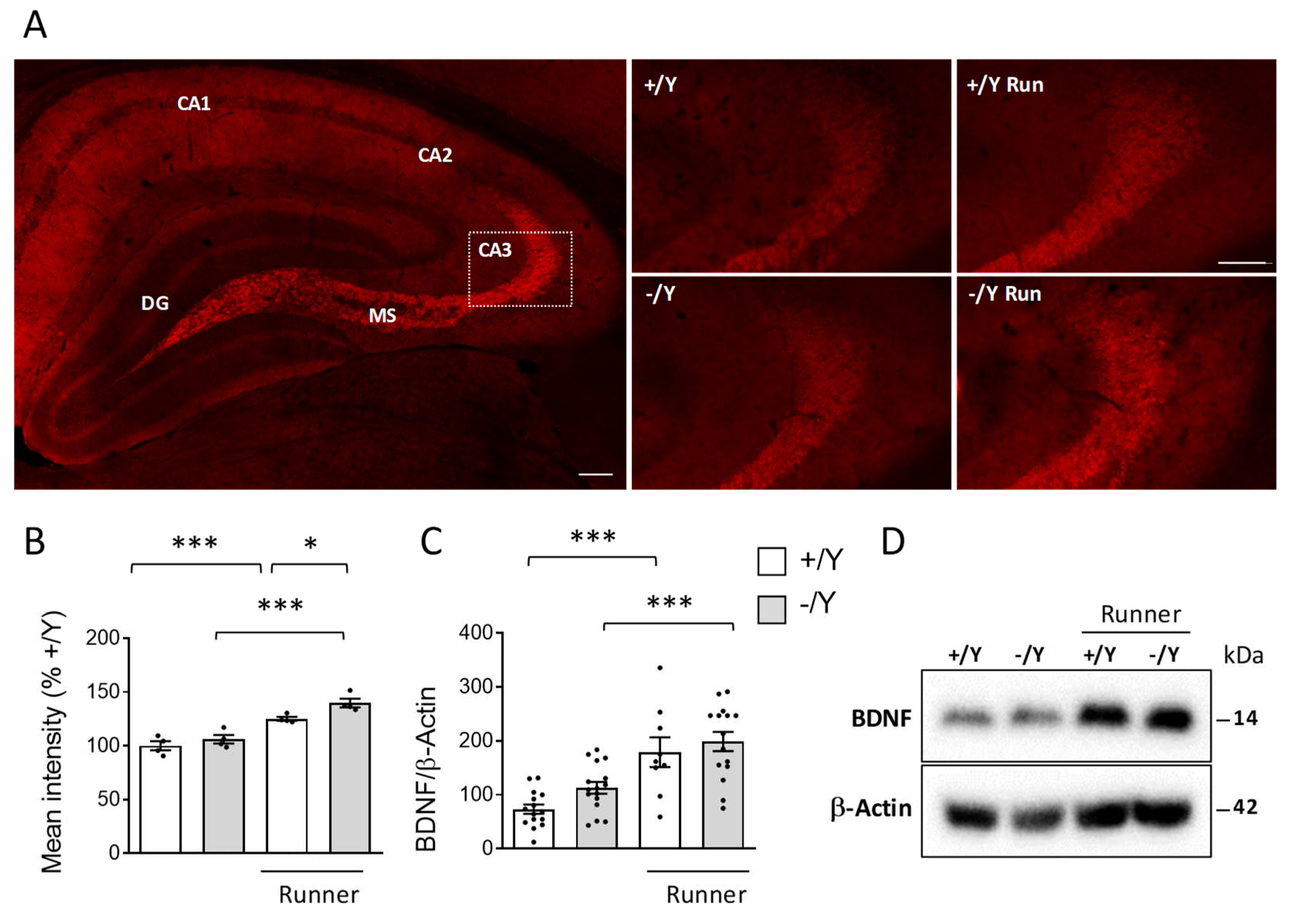
3.6. Effect of voluntary wheel running on BDNF expression in Cdkl5 KO mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kalscheuer, V.M.; Tao, J.; Donnelly, A.; Hollway, G.; Schwinger, E.; Kubart, S.; Menzel, C.; Hoeltzenbein, M.; Tommerup, N.; Eyre, H.; et al. Disruption of the serine/threonine kinase 9 gene causes severe X-linked infantile spasms and mental retardation. Am J Hum Genet 2003, 72, 1401–1411. [Google Scholar] [CrossRef]
- Rusconi, L.; Salvatoni, L.; Giudici, L.; Bertani, I.; Kilstrup-Nielsen, C.; Broccoli, V.; Landsberger, N. CDKL5 expression is modulated during neuronal development and its subcellular distribution is tightly regulated by the C-terminal tail. J Biol Chem 2008, 283, 30101–30111. [Google Scholar] [CrossRef]
- Bertani, I.; Rusconi, L.; Bolognese, F.; Forlani, G.; Conca, B.; De Monte, L.; Badaracco, G.; Landsberger, N.; Kilstrup-Nielsen, C. Functional consequences of mutations in CDKL5, an X-linked gene involved in infantile spasms and mental retardation. J Biol Chem 2006, 281, 32048–32056. [Google Scholar] [CrossRef] [PubMed]
- Olson, H.E.; Demarest, S.T.; Pestana-Knight, E.M.; Swanson, L.C.; Iqbal, S.; Lal, D.; Leonard, H.; Cross, J.H.; Devinsky, O.; Benke, T.A. Cyclin-Dependent Kinase-Like 5 Deficiency Disorder: Clinical Review. Pediatr Neurol 2019, 97, 18–25. [Google Scholar] [CrossRef]
- Fehr, S.; Wilson, M.; Downs, J.; Williams, S.; Murgia, A.; Sartori, S.; Vecchi, M.; Ho, G.; Polli, R.; Psoni, S.; et al. The CDKL5 disorder is an independent clinical entity associated with early-onset encephalopathy. Eur J Hum Genet 2013, 21, 266–273. [Google Scholar] [CrossRef]
- Wang, I.T.; Allen, M.; Goffin, D.; Zhu, X.; Fairless, A.H.; Brodkin, E.S.; Siegel, S.J.; Marsh, E.D.; Blendy, J.A.; Zhou, Z. Loss of CDKL5 disrupts kinome profile and event-related potentials leading to autistic-like phenotypes in mice. Proc Natl Acad Sci U S A 2012, 109, 21516–21521. [Google Scholar] [CrossRef]
- Amendola, E.; Zhan, Y.; Mattucci, C.; Castroflorio, E.; Calcagno, E.; Fuchs, C.; Lonetti, G.; Silingardi, D.; Vyssotski, A.L.; Farley, D.; et al. Mapping pathological phenotypes in a mouse model of CDKL5 disorder. PLoS One 2014, 9, e91613. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, C.; Rimondini, R.; Viggiano, R.; Trazzi, S.; De Franceschi, M.; Bartesaghi, R.; Ciani, E. Inhibition of GSK3beta rescues hippocampal development and learning in a mouse model of CDKL5 disorder. Neurobiol Dis 2015, 82, 298–310. [Google Scholar] [CrossRef]
- Fuchs, C.; Gennaccaro, L.; Trazzi, S.; Bastianini, S.; Bettini, S.; Lo Martire, V.; Ren, E.; Medici, G.; Zoccoli, G.; Rimondini, R.; et al. Heterozygous CDKL5 Knockout Female Mice Are a Valuable Animal Model for CDKL5 Disorder. Neural Plast 2018, 2018, 9726950. [Google Scholar] [CrossRef] [PubMed]
- Della Sala, G.; Putignano, E.; Chelini, G.; Melani, R.; Calcagno, E.; Michele Ratto, G.; Amendola, E.; Gross, C.T.; Giustetto, M.; Pizzorusso, T. Dendritic Spine Instability in a Mouse Model of CDKL5 Disorder Is Rescued by Insulin-like Growth Factor 1. Biol Psychiatry 2016, 80, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Wang, I.J.; Yue, C.; Takano, H.; Terzic, B.; Pance, K.; Lee, J.Y.; Cui, Y.; Coulter, D.A.; Zhou, Z. Loss of CDKL5 in Glutamatergic Neurons Disrupts Hippocampal Microcircuitry and Leads to Memory Impairment in Mice. J Neurosci 2017, 37, 7420–7437. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, C.; Trazzi, S.; Torricella, R.; Viggiano, R.; De Franceschi, M.; Amendola, E.; Gross, C.; Calza, L.; Bartesaghi, R.; Ciani, E. Loss of CDKL5 impairs survival and dendritic growth of newborn neurons by altering AKT/GSK-3beta signaling. Neurobiol Dis 2014, 70, 53–68. [Google Scholar] [CrossRef] [PubMed]
- Pizzo, R.; Gurgone, A.; Castroflorio, E.; Amendola, E.; Gross, C.; Sassoe-Pognetto, M.; Giustetto, M. Lack of Cdkl5 Disrupts the Organization of Excitatory and Inhibitory Synapses and Parvalbumin Interneurons in the Primary Visual Cortex. Front Cell Neurosci 2016, 10, 261. [Google Scholar] [CrossRef]
- Galvani, G.; Mottolese, N.; Gennaccaro, L.; Loi, M.; Medici, G.; Tassinari, M.; Fuchs, C.; Ciani, E.; Trazzi, S. Inhibition of microglia overactivation restores neuronal survival in a mouse model of CDKL5 deficiency disorder. J Neuroinflammation 2021, 18, 155. [Google Scholar] [CrossRef]
- Tassinari, M.; Mottolese, N.; Galvani, G.; Ferrara, D.; Gennaccaro, L.; Loi, M.; Medici, G.; Candini, G.; Rimondini, R.; Ciani, E.; et al. Luteolin Treatment Ameliorates Brain Development and Behavioral Performance in a Mouse Model of CDKL5 Deficiency Disorder. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef]
- Fuchs, C.; Medici, G.; Trazzi, S.; Gennaccaro, L.; Galvani, G.; Berteotti, C.; Ren, E.; Loi, M.; Ciani, E. CDKL5 deficiency predisposes neurons to cell death through the deregulation of SMAD3 signaling. Brain Pathol 2019, 29, 658–674. [Google Scholar] [CrossRef] [PubMed]
- Loi, M.; Trazzi, S.; Fuchs, C.; Galvani, G.; Medici, G.; Gennaccaro, L.; Tassinari, M.; Ciani, E. Increased DNA Damage and Apoptosis in CDKL5-Deficient Neurons. Mol Neurobiol 2020, 57, 2244–2262. [Google Scholar] [CrossRef]
- Rogers, R.L.; Meyer, J.S.; Mortel, K.F. After reaching retirement age physical activity sustains cerebral perfusion and cognition. J Am Geriatr Soc 1990, 38, 123–128. [Google Scholar] [CrossRef]
- Kramer, A.F.; Colcombe, S. Fitness Effects on the Cognitive Function of Older Adults: A Meta-Analytic Study-Revisited. Perspect Psychol Sci 2018, 13, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Colcombe, S.; Kramer, A.F. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci 2003, 14, 125–130. [Google Scholar] [CrossRef]
- Chapman, S.B.; Aslan, S.; Spence, J.S.; Defina, L.F.; Keebler, M.W.; Didehbani, N.; Lu, H. Shorter term aerobic exercise improves brain, cognition, and cardiovascular fitness in aging. Front Aging Neurosci 2013, 5, 75. [Google Scholar] [CrossRef]
- Kramer, A.F.; Erickson, K.I.; Colcombe, S.J. Exercise, cognition, and the aging brain. J Appl Physiol (1985) 2006, 101, 1237–1242. [Google Scholar] [CrossRef]
- van Praag, H.; Christie, B.R.; Sejnowski, T.J.; Gage, F.H. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci U S A 1999, 96, 13427–13431. [Google Scholar] [CrossRef]
- Anderson, B.J.; Rapp, D.N.; Baek, D.H.; McCloskey, D.P.; Coburn-Litvak, P.S.; Robinson, J.K. Exercise influences spatial learning in the radial arm maze. Physiol Behav 2000, 70, 425–429. [Google Scholar] [CrossRef]
- Vaynman, S.; Ying, Z.; Gomez-Pinilla, F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci 2004, 20, 2580–2590. [Google Scholar] [CrossRef]
- Kondo, M.A.; Gray, L.J.; Pelka, G.J.; Leang, S.K.; Christodoulou, J.; Tam, P.P.; Hannan, A.J. Affective dysfunction in a mouse model of Rett syndrome: Therapeutic effects of environmental stimulation and physical activity. Dev Neurobiol 2016, 76, 209–224. [Google Scholar] [CrossRef]
- Lee, T.H.; Devaki, M.; Formolo, D.A.; Rosa, J.M.; Cheng, A.S.K.; Yau, S.Y. Effects of Voluntary Wheel Running Exercise on Chemotherapy-Impaired Cognitive and Motor Performance in Mice. Int J Environ Res Public Health 2023, 20. [Google Scholar] [CrossRef]
- van Praag, H.; Kempermann, G.; Gage, F.H. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci 1999, 2, 266–270. [Google Scholar] [CrossRef]
- Naylor, A.S.; Persson, A.I.; Eriksson, P.S.; Jonsdottir, I.H.; Thorlin, T. Extended voluntary running inhibits exercise-induced adult hippocampal progenitor proliferation in the spontaneously hypertensive rat. J Neurophysiol 2005, 93, 2406–2414. [Google Scholar] [CrossRef]
- Holmes, M.M.; Galea, L.A.; Mistlberger, R.E.; Kempermann, G. Adult hippocampal neurogenesis and voluntary running activity: circadian and dose-dependent effects. J Neurosci Res 2004, 76, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.C.; Huddleston, D.E.; Brickman, A.M.; Sosunov, A.A.; Hen, R.; McKhann, G.M.; Sloan, R.; Gage, F.H.; Brown, T.R.; Small, S.A. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci U S A 2007, 104, 5638–5643. [Google Scholar] [CrossRef]
- Stranahan, A.M.; Khalil, D.; Gould, E. Running induces widespread structural alterations in the hippocampus and entorhinal cortex. Hippocampus 2007, 17, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Brockett, A.T.; LaMarca, E.A.; Gould, E. Physical exercise enhances cognitive flexibility as well as astrocytic and synaptic markers in the medial prefrontal cortex. PLoS One 2015, 10, e0124859. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Hong, E.J.; Cohen, S.; Zhao, W.N.; Ho, H.Y.; Schmidt, L.; Chen, W.G.; Lin, Y.; Savner, E.; Griffith, E.C.; et al. Brain-specific phosphorylation of MeCP2 regulates activity-dependent Bdnf transcription, dendritic growth, and spine maturation. Neuron 2006, 52, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Jou, J.; Wolff, L.J.; Sun, H.; Gage, F.H. Spine morphogenesis in newborn granule cells is differentially regulated in the outer and middle molecular layers. J Comp Neurol 2014, 522, 2756–2766. [Google Scholar] [CrossRef]
- Kohman, R.A.; DeYoung, E.K.; Bhattacharya, T.K.; Peterson, L.N.; Rhodes, J.S. Wheel running attenuates microglia proliferation and increases expression of a proneurogenic phenotype in the hippocampus of aged mice. Brain Behav Immun 2012, 26, 803–810. [Google Scholar] [CrossRef]
- Gebara, E.; Sultan, S.; Kocher-Braissant, J.; Toni, N. Adult hippocampal neurogenesis inversely correlates with microglia in conditions of voluntary running and aging. Front Neurosci 2013, 7, 145. [Google Scholar] [CrossRef]
- Barrientos, R.M.; Frank, M.G.; Crysdale, N.Y.; Chapman, T.R.; Ahrendsen, J.T.; Day, H.E.; Campeau, S.; Watkins, L.R.; Patterson, S.L.; Maier, S.F. Little exercise, big effects: reversing aging and infection-induced memory deficits, and underlying processes. J Neurosci 2011, 31, 11578–11586. [Google Scholar] [CrossRef]
- Vivar, C.; Potter, M.C.; van Praag, H. All about running: synaptic plasticity, growth factors and adult hippocampal neurogenesis. Curr Top Behav Neurosci 2013, 15, 189–210. [Google Scholar] [CrossRef]
- Brenner, D.R.; Ruan, Y.; Adams, S.C.; Courneya, K.S.; Friedenreich, C.M. The impact of exercise on growth factors (VEGF and FGF2): results from a 12-month randomized intervention trial. Eur Rev Aging Phys Act 2019, 16, 8. [Google Scholar] [CrossRef]
- Neeper, S.A.; Gomez-Pinilla, F.; Choi, J.; Cotman, C.W. Physical activity increases mRNA for brain-derived neurotrophic factor and nerve growth factor in rat brain. Brain Res 1996, 726, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Cotman, C.W.; Engesser-Cesar, C. Exercise enhances and protects brain function. Exerc Sport Sci Rev 2002, 30, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Griffin, E.W.; Bechara, R.G.; Birch, A.M.; Kelly, A.M. Exercise enhances hippocampal-dependent learning in the rat: evidence for a BDNF-related mechanism. Hippocampus 2009, 19, 973–980. [Google Scholar] [CrossRef]
- Voss, M.W.; Erickson, K.I.; Prakash, R.S.; Chaddock, L.; Kim, J.S.; Alves, H.; Szabo, A.; Phillips, S.M.; Wojcicki, T.R.; Mailey, E.L.; et al. Neurobiological markers of exercise-related brain plasticity in older adults. Brain Behav Immun 2013, 28, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.L.; Chen, F.C.; Pan, C.Y.; Wang, C.H.; Huang, T.H.; Chen, T.C. Impact of acute aerobic exercise and cardiorespiratory fitness on visuospatial attention performance and serum BDNF levels. Psychoneuroendocrinology 2014, 41, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Heijnen, S.; Hommel, B.; Kibele, A.; Colzato, L.S. Neuromodulation of Aerobic Exercise-A Review. Front Psychol 2015, 6, 1890. [Google Scholar] [CrossRef]
- Chroboczek, M.; Kujach, S.; Luszczyk, M.; Soya, H.; Laskowski, R. Exercise-Induced Elevated BDNF Concentration Seems to Prevent Cognitive Impairment after Acute Exposure to Moderate Normobaric Hypoxia among Young Men. Int J Environ Res Public Health 2023, 20. [Google Scholar] [CrossRef]
- Fuchs, C.; Cosentino, L.; Urbinati, C.; Talamo, M.C.; Medici, G.; Quattrini, M.C.; Mottolese, N.; Pietraforte, D.; Fuso, A.; Ciani, E.; et al. Treatment with FRAX486 rescues neurobehavioral and metabolic alterations in a female mouse model of CDKL5 deficiency disorder. CNS Neurosci Ther 2022, 28, 1718–1732. [Google Scholar] [CrossRef]
- Fuchs, C.; Gennaccaro, L.; Ren, E.; Galvani, G.; Trazzi, S.; Medici, G.; Loi, M.; Conway, E.; Devinsky, O.; Rimondini, R.; et al. Pharmacotherapy with sertraline rescues brain development and behavior in a mouse model of CDKL5 deficiency disorder. Neuropharmacology 2019, 107746. [Google Scholar] [CrossRef]
- Gennaccaro, L.; Fuchs, C.; Loi, M.; Roncace, V.; Trazzi, S.; Ait-Bali, Y.; Galvani, G.; Berardi, A.C.; Medici, G.; Tassinari, M.; et al. A GABA(B) receptor antagonist rescues functional and structural impairments in the perirhinal cortex of a mouse model of CDKL5 deficiency disorder. Neurobiol Dis 2021, 153, 105304. [Google Scholar] [CrossRef]
- Loi, M.; Gennaccaro, L.; Fuchs, C.; Trazzi, S.; Medici, G.; Galvani, G.; Mottolese, N.; Tassinari, M.; Giorgini, R.R.; Milelli, A.; et al. Treatment with a GSK-3beta/HDAC Dual Inhibitor Restores Neuronal Survival and Maturation in an In Vitro and In Vivo Model of CDKL5 Deficiency Disorder. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef]
- Medici, G.; Tassinari, M.; Galvani, G.; Bastianini, S.; Gennaccaro, L.; Loi, M.; Mottolese, N.; Alvente, S.; Berteotti, C.; Sagona, G.; et al. Expression of a Secretable, Cell-Penetrating CDKL5 Protein Enhances the Efficacy of Gene Therapy for CDKL5 Deficiency Disorder. Neurotherapeutics 2022, 19, 1886–1904. [Google Scholar] [CrossRef] [PubMed]
- Trazzi, S.; De Franceschi, M.; Fuchs, C.; Bastianini, S.; Viggiano, R.; Lupori, L.; Mazziotti, R.; Medici, G.; Lo Martire, V.; Ren, E.; et al. CDKL5 protein substitution therapy rescues neurological phenotypes of a mouse model of CDKL5 disorder. Hum Mol Genet 2018, 27, 1572–1592. [Google Scholar] [CrossRef]
- Trovo, L.; Fuchs, C.; De Rosa, R.; Barbiero, I.; Tramarin, M.; Ciani, E.; Rusconi, L.; Kilstrup-Nielsen, C. The green tea polyphenol epigallocatechin-3-gallate (EGCG) restores CDKL5-dependent synaptic defects in vitro and in vivo. Neurobiol Dis 2020, 138, 104791. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, C.; Gennaccaro, L.; Ren, E.; Galvani, G.; Trazzi, S.; Medici, G.; Loi, M.; Conway, E.; Devinsky, O.; Rimondini, R.; et al. Pharmacotherapy with sertraline rescues brain development and behavior in a mouse model of CDKL5 deficiency disorder. Neuropharmacology 2020, 167, 107746. [Google Scholar] [CrossRef] [PubMed]
- Ren, E.; Roncacé, V.; Trazzi, S.; Fuchs, C.; Medici, G.; Gennaccaro, L.; Loi, M.; Galvani, G.; Ye, K.; Rimondini, R.; et al. Functional and Structural Impairments in the Perirhinal Cortex of a Mouse Model of CDKL5 Deficiency Disorder Are Rescued by a TrkB Agonist. Frontiers in Cellular Neuroscience 2019, 13. [Google Scholar] [CrossRef]
- Pinar, C.; Yau, S.Y.; Sharp, Z.; Shamei, A.; Fontaine, C.J.; Meconi, A.L.; Lottenberg, C.P.; Christie, B.R. Effects of Voluntary Exercise on Cell Proliferation and Neurogenesis in the Dentate Gyrus of Adult FMR1 Knockout Mice. Brain Plast 2018, 4, 185–195. [Google Scholar] [CrossRef]
- Kida, E.; Rabe, A.; Walus, M.; Albertini, G.; Golabek, A.A. Long-term running alleviates some behavioral and molecular abnormalities in Down syndrome mouse model Ts65Dn. Exp Neurol 2013, 240, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Kida, E.; Walus, M.; Albertini, G.; Golabek, A.A. Long-term voluntary running modifies the levels of proteins of the excitatory/inhibitory system and reduces reactive astrogliosis in the brain of Ts65Dn mouse model for Down syndrome. Brain Res 2021, 1766, 147535. [Google Scholar] [CrossRef]
- Guidi, S.; Stagni, F.; Bianchi, P.; Ciani, E.; Ragazzi, E.; Trazzi, S.; Grossi, G.; Mangano, C.; Calza, L.; Bartesaghi, R. Early pharmacotherapy with fluoxetine rescues dendritic pathology in the Ts65Dn mouse model of down syndrome. Brain Pathol 2013, 23, 129–143. [Google Scholar] [CrossRef]
- Jhang, C.L.; Huang, T.N.; Hsueh, Y.P.; Liao, W. Mice lacking cyclin-dependent kinase-like 5 manifest autistic and ADHD-like behaviors. Hum Mol Genet 2017, 26, 3922–3934. [Google Scholar] [CrossRef]
- Jhang, C.L.; Lee, H.Y.; Chen, J.C.; Liao, W. Dopaminergic loss of cyclin-dependent kinase-like 5 recapitulates methylphenidate-remediable hyperlocomotion in mouse model of CDKL5 deficiency disorder. Hum Mol Genet 2020, 29, 2408–2419. [Google Scholar] [CrossRef]
- Adhikari, A.; Buchanan, F.K.B.; Fenton, T.A.; Cameron, D.L.; Halmai, J.; Copping, N.A.; Fink, K.D.; Silverman, J.L. Touchscreen cognitive deficits, hyperexcitability and hyperactivity in males and females using two models of Cdkl5 deficiency. Hum Mol Genet 2022, 31, 3032–3050. [Google Scholar] [CrossRef]
- Viglione, A.; Sagona, G.; Carrara, F.; Amato, G.; Totaro, V.; Lupori, L.; Putignano, E.; Pizzorusso, T.; Mazziotti, R. Behavioral impulsivity is associated with pupillary alterations and hyperactivity in CDKL5 mutant mice. Hum Mol Genet 2022, 31, 4107–4120. [Google Scholar] [CrossRef]
- Gennaccaro, L.; Fuchs, C.; Loi, M.; Pizzo, R.; Alvente, S.; Berteotti, C.; Lupori, L.; Sagona, G.; Galvani, G.; Gurgone, A.; et al. Age-Related Cognitive and Motor Decline in a Mouse Model of CDKL5 Deficiency Disorder is Associated with Increased Neuronal Senescence and Death. Aging Dis 2021, 12, 764–785. [Google Scholar] [CrossRef] [PubMed]
- Kohman, R.A.; Bhattacharya, T.K.; Wojcik, E.; Rhodes, J.S. Exercise reduces activation of microglia isolated from hippocampus and brain of aged mice. J Neuroinflammation 2013, 10, 114. [Google Scholar] [CrossRef] [PubMed]
- Mee-Inta, O.; Zhao, Z.W.; Kuo, Y.M. Physical Exercise Inhibits Inflammation and Microglial Activation. Cells 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Luo, Y.; Liang, X.; Tang, J.; Wang, J.; Xiao, Q.; Qi, Y.; Li, Y.; Zhu, P.; Yang, H.; et al. Beneficial effects of running exercise on hippocampal microglia and neuroinflammation in chronic unpredictable stress-induced depression model rats. Transl Psychiatry 2021, 11, 461. [Google Scholar] [CrossRef]
- Ricciardi, S.; Ungaro, F.; Hambrock, M.; Rademacher, N.; Stefanelli, G.; Brambilla, D.; Sessa, A.; Magagnotti, C.; Bachi, A.; Giarda, E.; et al. CDKL5 ensures excitatory synapse stability by reinforcing NGL-1-PSD95 interaction in the postsynaptic compartment and is impaired in patient iPSC-derived neurons. Nat Cell Biol 2012, 14, 911–923. [Google Scholar] [CrossRef]
- Trazzi, S.; Fuchs, C.; Viggiano, R.; De Franceschi, M.; Valli, E.; Jedynak, P.; Hansen, F.K.; Perini, G.; Rimondini, R.; Kurz, T.; et al. HDAC4: a key factor underlying brain developmental alterations in CDKL5 disorder. Hum Mol Genet 2016, 25, 3887–3907. [Google Scholar] [CrossRef]
- Conner, J.M.; Lauterborn, J.C.; Yan, Q.; Gall, C.M.; Varon, S. Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: evidence for anterograde axonal transport. J Neurosci 1997, 17, 2295–2313. [Google Scholar] [CrossRef]
- Dieni, S.; Matsumoto, T.; Dekkers, M.; Rauskolb, S.; Ionescu, M.S.; Deogracias, R.; Gundelfinger, E.D.; Kojima, M.; Nestel, S.; Frotscher, M.; et al. BDNF and its pro-peptide are stored in presynaptic dense core vesicles in brain neurons. J Cell Biol 2012, 196, 775–788. [Google Scholar] [CrossRef]
- van Praag, H.; Shubert, T.; Zhao, C.; Gage, F.H. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci 2005, 25, 8680–8685. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Q.; Spitzer, N.C. Exercise enhances motor skill learning by neurotransmitter switching in the adult midbrain. Nat Commun 2020, 11, 2195. [Google Scholar] [CrossRef] [PubMed]
- Willuhn, I.; Steiner, H. Motor-skill learning in a novel running-wheel task is dependent on D1 dopamine receptors in the striatum. Neuroscience 2008, 153, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Sivilia, S.; Mangano, C.; Beggiato, S.; Giuliani, A.; Torricella, R.; Baldassarro, V.A.; Fernandez, M.; Lorenzini, L.; Giardino, L.; Borelli, A.C.; et al. CDKL5 knockout leads to altered inhibitory transmission in the cerebellum of adult mice. Genes Brain Behav 2016, 15, 491–502. [Google Scholar] [CrossRef]
- Pang, T.Y.C.; Stam, N.C.; Nithianantharajah, J.; Howard, M.L.; Hannan, A.J. Differential effects of voluntary physical exercise on behavioral and brain-derived neurotrophic factor expression deficits in Huntington's disease transgenic mice. Neuroscience 2006, 141, 569–584. [Google Scholar] [CrossRef]
- Svensson, M.; Andersson, E.; Manouchehrian, O.; Yang, Y.; Deierborg, T. Voluntary running does not reduce neuroinflammation or improve non-cognitive behavior in the 5xFAD mouse model of Alzheimer's disease. Sci Rep 2020, 10, 1346. [Google Scholar] [CrossRef]
- Lupori, L.; Sagona, G.; Fuchs, C.; Mazziotti, R.; Stefanov, A.; Putignano, E.; Napoli, D.; Strettoi, E.; Ciani, E.; Pizzorusso, T. Site-specific abnormalities in the visual system of a mouse model of CDKL5 deficiency disorder. Hum Mol Genet 2019, 28, 2851–2861. [Google Scholar] [CrossRef]
- Atucha, E.; Roozendaal, B. The inhibitory avoidance discrimination task to investigate accuracy of memory. Front Behav Neurosci 2015, 9, 60. [Google Scholar] [CrossRef]
- Bolijn, S.; Lucassen, P.J. How the Body Talks to the Brain; Peripheral Mediators of Physical Activity-Induced Proliferation in the Adult Hippocampus. Brain Plast 2015, 1, 5–27. [Google Scholar] [CrossRef]
- Ko, Y.J.; Ko, I.G. Voluntary Wheel Running Improves Spatial Learning Memory by Suppressing Inflammation and Apoptosis via Inactivation of Nuclear Factor Kappa B in Brain Inflammation Rats. Int Neurourol J 2020, 24, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.H.; Lee, C.H.; Seo, M.K.; Cho, H.; Lee, J.G.; Lee, B.J.; Park, S.W.; Kim, Y.H. Effect of treadmill exercise on the BDNF-mediated pathway in the hippocampus of stressed rats. Neurosci Res 2013, 76, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Saucedo Marquez, C.M.; Vanaudenaerde, B.; Troosters, T.; Wenderoth, N. High-intensity interval training evokes larger serum BDNF levels compared with intense continuous exercise. J Appl Physiol (1985) 2015, 119, 1363–1373. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Bylykbashi, E.; Chatila, Z.K.; Lee, S.W.; Pulli, B.; Clemenson, G.D.; Kim, E.; Rompala, A.; Oram, M.K.; Asselin, C.; et al. Combined adult neurogenesis and BDNF mimic exercise effects on cognition in an Alzheimer's mouse model. Science 2018, 361. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, M.E.; Xu, B.; Lu, B.; Hempstead, B.L. New insights in the biology of BDNF synthesis and release: implications in CNS function. J Neurosci 2009, 29, 12764–12767. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wei, N.; Lu, T.; Zhu, J.; Xu, G.; Liu, X. Intranasal brain-derived neurotrophic factor protects brain from ischemic insult via modulating local inflammation in rats. Neuroscience 2011, 172, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wei, N.; Zhu, J.; Lu, T.; Chen, Z.; Xu, G.; Liu, X. Effects of brain-derived neurotrophic factor on local inflammation in experimental stroke of rat. Mediators Inflamm 2010, 2010, 372423. [Google Scholar] [CrossRef]
- Makar, T.K.; Trisler, D.; Sura, K.T.; Sultana, S.; Patel, N.; Bever, C.T. Brain derived neurotrophic factor treatment reduces inflammation and apoptosis in experimental allergic encephalomyelitis. J Neurol Sci 2008, 270, 70–76. [Google Scholar] [CrossRef]
- Bovolenta, R.; Zucchini, S.; Paradiso, B.; Rodi, D.; Merigo, F.; Navarro Mora, G.; Osculati, F.; Berto, E.; Marconi, P.; Marzola, A.; et al. Hippocampal FGF-2 and BDNF overexpression attenuates epileptogenesis-associated neuroinflammation and reduces spontaneous recurrent seizures. J Neuroinflammation 2010, 7, 81. [Google Scholar] [CrossRef]
- von Bohlen Und Halbach, O.; von Bohlen Und Halbach, V. BDNF effects on dendritic spine morphology and hippocampal function. Cell Tissue Res 2018, 373, 729–741. [Google Scholar] [CrossRef]
- Eadie, B.D.; Redila, V.A.; Christie, B.R. Voluntary exercise alters the cytoarchitecture of the adult dentate gyrus by increasing cellular proliferation, dendritic complexity, and spine density. J Comp Neurol 2005, 486, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.W.; Chen, S.J.; Huang, T.Y.; Chang, C.Y.; Chuang, J.I.; Wu, F.S.; Kuo, Y.M.; Jen, C.J. Different types of exercise induce differential effects on neuronal adaptations and memory performance. Neurobiol Learn Mem 2012, 97, 140–147. [Google Scholar] [CrossRef]
- Stranahan, A.M.; Lee, K.; Martin, B.; Maudsley, S.; Golden, E.; Cutler, R.G.; Mattson, M.P. Voluntary exercise and caloric restriction enhance hippocampal dendritic spine density and BDNF levels in diabetic mice. Hippocampus 2009, 19, 951–961. [Google Scholar] [CrossRef]
- Zhao, C.; Teng, E.M.; Summers, R.G., Jr.; Ming, G.L.; Gage, F.H. Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J Neurosci 2006, 26, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Zagrebelsky, M.; Tacke, C.; Korte, M. BDNF signaling during the lifetime of dendritic spines. Cell Tissue Res 2020, 382, 185–199. [Google Scholar] [CrossRef] [PubMed]
- An, J.J.; Gharami, K.; Liao, G.Y.; Woo, N.H.; Lau, A.G.; Vanevski, F.; Torre, E.R.; Jones, K.R.; Feng, Y.; Lu, B.; et al. Distinct role of long 3' UTR BDNF mRNA in spine morphology and synaptic plasticity in hippocampal neurons. Cell 2008, 134, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.C.; Novais, A.; Sousa, N.; Sousa, J.C.; Marques, F. Voluntary running rescues the defective hippocampal neurogenesis and behaviour observed in lipocalin 2-null mice. Sci Rep 2019, 9, 1649. [Google Scholar] [CrossRef]
- Lin, Y.; Lu, X.; Dong, J.; He, X.; Yan, T.; Liang, H.; Sui, M.; Zheng, X.; Liu, H.; Zhao, J.; et al. Involuntary, Forced and Voluntary Exercises Equally Attenuate Neurocognitive Deficits in Vascular Dementia by the BDNF-pCREB Mediated Pathway. Neurochem Res 2015, 40, 1839–1848. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




