1. Pre-clinical models for human prostate
The prostate is a sex-specific organ that shows significant differences in morphology and secreted proteins among mammals. The secreted proteins have specific functions that can vary between species, and in humans, only PSA (Prostate-Specific Antigen) and PSMA (Prostate-Specific Membrane Antigen) are expressed, serving as biomarkers for prostate inflammation and prostate cancer [
1]. The human prostate has a ball-shaped morphology with distinct zones [
2]. Dogs and pigs also exhibit a similar morphology, while rodents have four well-defined bilateral lobes [
3,
4]. Among these species, only dogs develop sporadic prostate cancer, but typically at a late age, which makes it challenging to conduct animal studies on prostate cancer (PCa) [
5]. The choice of an appropriate pre-clinical model for PCa has been a topic of extensive discussion. Most of the research in this field has been carried out in mice, which can be genetically modified to study PCa. Particularly, the dorsal and lateral lobes of the mouse prostate resemble the peripheral zone of the human prostate, where most cancers arise [
6]. Although no perfect pre-clinical model exists, the mouse is preferred due to its amenability to genetic modifications, allowing for the study of cancer initiation and progression within a relatively short time frame.
2. Evolution of mouse models for prostate cancer
2.1. Prostate specific promotors
Genetic mouse models have transformed cancer studies, but in the case of prostate cancer, which progresses slowly, mice with broad genetic alterations often develop cancer in organs other than the prostate. Therefore, to study prostate cancer, tissues specific gene alterations must be applied, and researchers have focused on finding prostate-specific promoters to drive transgene expression. In the 1990s, Greenberg and Zhang utilized the promoter from the rat secreted protein Probasin to drive tissue-specific expression in the mouse prostate [
7]. Through the delineation of the promoter, they identified a fragment, ARR2PB, that exhibited high specificity and strong expression when four elements were used [
8]. Using this modified promoter, they generated a Cre-expressing mouse line called PB4Cre, which is the most commonly used Cre line for studying prostate cancer [
9]. However, the expression is not uniform across the prostatic tissues, with the highest expression in the dorsal and lateral lobes, but only 10-20 percent of cells in the anterior and ventral lobes shows expression [
9]. Other prostate-specific Cre-expressing lines have been generated, including PSA-Cre and Nkx3.1 [
10,
11,
12]. The Nkx3.1-Cre line has further been developed as a tamoxifen-inducible Cre line, enabling researchers to control the timing of Cre activation or performed a label experiment with a pulse of Cre activation by tamoxifen treatment [
13]. These diverse prostate-specific Cre-expressing mouse lines are indispensable for genetic studies of prostate cancer and are commonly employed.
2.2. Prostate specific gene alteration
The development of a prostate-specific promoter allows researchers to generate mice with oncogene expression restricted to the mouse prostatic epithelium. One of the first mice established using this technique was the TRAMP mouse, in which the SV40 large and small antigens were expressed from the Probasin-modified promoter. This generated a model of prostate cancer that has been widely used. The TRAMP mouse model exhibits rapid tumor progression with high-grade PIN at 12 weeks and displays metastasis, reaching a humane endpoint around 30 weeks of age [
14]. This model has enabled researchers to study PCa progression and conduct pre-clinical studies to intervene cancer progression by targeting androgen and other pathways [
15]. However, it should be noted that the TRAMP model displays heterogenic cancer, with aggressiveness driven by neuroendocrine cells rather than the transformed prostatic epithelium [
16]. This limits the use of the TRAMP mouse, as only a minor part of human PCa is nuroendocrine-driven cancer even though this cancer form is on the raise [
17].
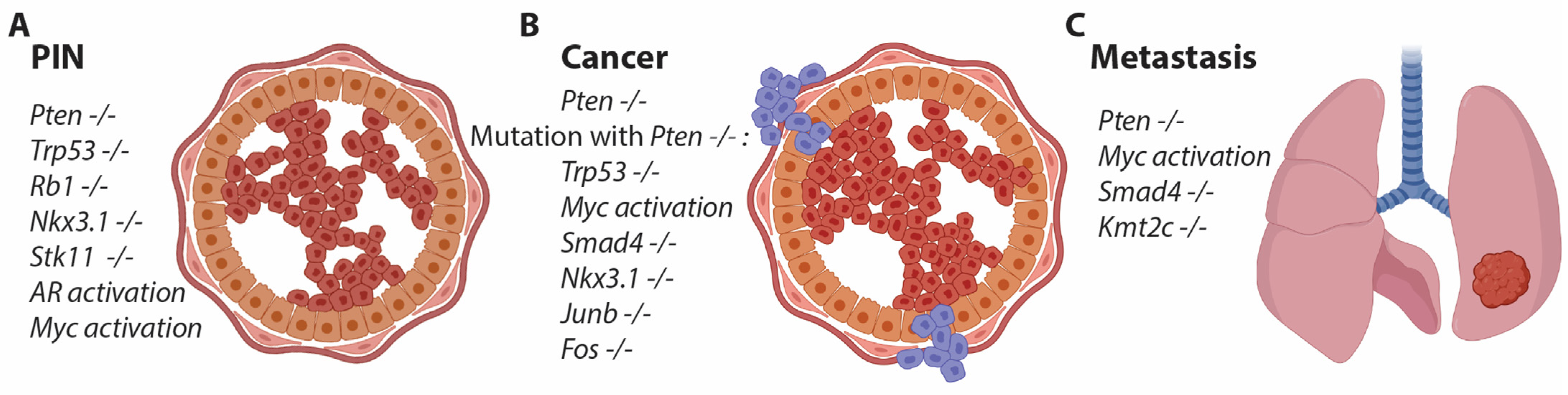
Only a few genetically modified mice exhibit alterations in the prostatic epithelium. Loss of Nkx3.1 results in prostatic intraepithelial neoplasia (PIN) development, but not until around 1 year of age, and this condition does not progress to cancer (
Figure 1) [
18]. Additionally, heterozygous loss of Pten induces PIN formation at 6 months of age [
19]. Since both Nkx3.1 and Pten are commonly mutated in human PCa, studying these gene alterations in the mouse prostate is biologically relevant as a model for human PCa (
Figure 1) [
20]. The group led by Hong Wu generated a mouse with a conditional allele for Pten and crossed it with the PB4Cre line to achieve prostate-specific depletion of Pten. This resulted in a robust mouse model for PCa with early PIN formation and progression to cancer, eventually leading to metastatic formation at a late stage [
21]. The background of the mouse strain interfered with the onset of cancer, but most studies are now conducted in C57bl/6 mice, which exhibit a delayed phenotype [
22]. Pten is a phosphatase that dephosphorylates PIP3, thereby antagonizing PI3K. Loss of Pten increases phosphorylation through the PI3K pathway, activating AKT and mTOR, which in turn leads to increased proliferation of the prostatic epithelium - a hallmark of cancer initiation [
23]. Therefore, loss of Pten or activation of the downstream pathway is considered an essential event in prostate cancer and is applied to most mouse models of PCa to ensure transformation and proliferation of the prostatic epithelium (
Figure 1).
2.3. Targeting multiple genes in the mouse prostate
The use of conditional alleles in combination with prostate-specific Cre lines has allowed researchers to explore specific gene functions in the prostate. However, depletion of many different genes has shown either no or very minor phenotypical changes in the prostate, often occurring at a late stage. This has led the scientific community to interbreed the strain with a conditional allele for Pten with the conditional strain of interest. One of the initial studies was conducted by Pandolfi's group, where the combination of Pten and Trp53 loss was investigated. These mice exhibited an accelerated phenotype, revealing a clear positive genetic alteration associated with the loss of these two tumor suppressor genes. The mice reached a humane endpoint at around 6 months of age, characterized by a significantly enlarged prostate [
24]. However, the humane endpoint is not directly related to cancer but rather to the obstruction of kidney function [
24]. Cancer progression can be further accelerated in this model by the additional deletion of Rb1, whereas the loss of either Trp53 or Rb1 alone results in an indolent phenotype at a later age (
Figure 1) [
25].
Another interesting mouse study was conducted by DePhino's group, where the conditional allele for Smad4 was combined with Pten loss [
26]. These mice exhibited rapid tumor progression with metastatic formation in various organs, including the bones [
27]. Metastasis and, in particular, bone metastasis are rare events in pre-clinical models for PCa, presumably due to the presence of a large primary tumor, which defines the humane endpoint. However, metastasis are important features of PCa and are highly relevant for studying PCa progression, as patients with metastatic PCa have a poor prognosis. Other genes have been studied in combination with Pten loss, contributing to the understanding of how gene alterations in PCa can accelerate or differentiate mutated cells [
28,
29,
30,
31,
32,
33,
34,
35,
36,
37]. A commonly mutated gene in human PCa is KMT2C, but mice with the loss of Kmt2c alone do not display a clear cancer-related phenotype. However, when combined with Pten loss, metastatic formation is often observed [
38], and we have unpublished data that confirm the role of Kmt2c in the dissemination of prostate cancer. Overall, the application of mouse models with the loss of Pten has generated essential knowledge about PCa and gene alterations, which do not manifest phenotypically when the genes are deficient alone (
Figure 1).
2.4. Gain-of-function studies for PCa
Preclinical mouse models with gain-of-function mutations have been generated in a similar fashion to the aforementioned TRAMP mouse. This includes a mouse model with androgen expressed under the Probasin promoter, where a point mutation has been introduced to the reading frame. This mutation is often found in human PCa with androgen-insensitive cancer. These mice developed aggressive cancer after 50 weeks, with the formation of metastasis, confirming the oncogenic properties of mutated AR [
39]. Genomic translocation is a common event in PCa, particularly the fusion between the promoter of the AR-regulated TMPRSS2 and ETV1 or EGF genes [
40]. Multiple mouse models have been generated with overexpression of either ETV1 or EGF under the modified Probasin promoter, resulting in PIN formation and possible invasive adenocarcinoma at late stages [
41,
42,
43].
Amplification of MYC is observed in numerous of human cancers and is a frequent occurrence in prostate cancer (PCa), emphasizing its oncogenic function in this particular cancer [
44]. MYC's role in PCa has been modeled using gain-of-function models with prostate-specific expression. Overexpression of MYC alone is sufficient to transform the prostatic epithelium, leading to the formation of PIN and adenocarcinoma [
45,
46]. However, when combined with the loss of Pten or both Pten and Trp53, MYC overexpression accelerates cancer progression and facilitates cancer dissemination [
47,
48]. These findings demonstrate that MYC amplification in human cancer is mirrored in mouse models of PCa, and RNAseq data from various mouse models of PCa confirms the upregulation of MYC target genes [
49].
In humans, point mutations in the E3 ligase SPOP are seen in around 10% of PCa cases and are an early event. A mouse model of SPOP point mutation has been generated by two independent groups by the expression of SPOPF133V from the Rosa26 locus controlled by lox-stop-lox. This mouse line has been interbred with the Probasin Cre line to generate prostate-specific expression. A minor phenotype was observed, but by intercrossing with the conditional allele for Pten, invasive adenocarcinoma is developed [
50,
51]. SPOPF133V increases the expression of AR-regulated genes and underpins the oncogenic property in PCa [
50]. Other gain-of-function studies have been conducted in mice to understand the principles of amplified genes in PCa initiation and progression. For most models, it requires transgenic mice and tissue-specific expression to study the function in the prostatic epithelium, which can be time consuming.
3. Orthotopic models for PCa
The mouse prostate differs from the human prostate by having four bilateral lobes. The anterior lobe contains a large lumen, which allows researchers to inject a small volume of approximately 25 µl for orthotopic delivery. This is done through a small operation involving an insertion in the abdominal skin, followed by lifting the seminal vesicles to expose the anterior lobes of the prostate [
52]. In a study by Leow et al., orthotopic delivery of adenovirus to the mouse prostate was employed to demonstrate tissue-specific delivery without the need for an additional transgenic mouse line such as Probasin Cre. The researchers conducted the experiment in a reporter mouse line to activate the expression of beta-galactosidase [
53]. We applied a similar method to deliver adenovirus expressing Cre, inducing conditional loss of Junb and Pten in the prostatic tissues to avoid use of a Cre-specific mouse line [
30].
The group led by Trotman also utilized orthotopic delivery to the mouse prostate to establish a novel mouse model of prostate cancer called RapidCaP [
47]. In their approach, they used lentivirus to express Cre protein and luciferase to track cells with integrated virus. Lentivirus was applied to mice with double conditional alleles for Trp53 and Pten, allowing them to monitor tumor progression and cancer dissemination through illumination from the luciferase. They demonstrated that tumor progression was driven by Myc activation, leading them to overexpress Myc through orthotopic lentivirus delivery to the mouse prostate as a proof of concept. This study highlighted the effectiveness of applying orthotopic lentivirus delivery for studying prostate cancer, as transgene expression can proceed in the transformed cells [
37,
47].
3.1. CRISPR generated PCa models
CRISPR technology has been applied to generate preclinical models in different species. For mice, CRISPR is used to genetically modify embryonic stem cells and is also applied to induce somatic mutations to study cancer in specific organs [
54]. For somatic mutations, Cas9 transgenic mice have been used to avoid the delivery of Cas9 and ensure biosafety without combined delivery of Cas9 and sgRNA [
54]. To study prostate cancer (PCa), we applied CRISPR through orthotopic delivery of adeno-associated virus (AAV) to the anterior mouse prostatic lobe [
31,
32]. The advantage of AAV is that it rarely integrates into the host cell's genome and has very little immunogenicity [
55]. However, the AAV genome is small and cannot exceed 5kb, which imposes limitations on the genomic cargo that can be delivered. Therefore, we took advantage of Cas9 transgenic mice with either conditional expression of Cas9 and EGFP for visualization or Cas9 expression from a ubiquitin promoter [
31,
56,
57]. Each line has advantages: with conditional Cas9-EGFP expression, only cells transduced with a virus expressing Cre will express Cas9 and EGFP. However, Cre expression will have to be delivered by the virus or, as an alternative, the conditional Cas9 mouse line needs to be interbred with a Cre line to obtain prostate-specific expression. The ubiquitin Cas9-expressing line does not have these limitations but lacks EGFP expression, and tumors can occur in other organs if the virus has spread outside the prostate [
54].
One advantage of CRISPR-generated tumors is the ability of multiplex sgRNAs to target multiple genes simultaneously in the same prostatic epithelial cell. This mimics the occurrence of multiple mutations over time in the same cell, allowing for the study of genetic interactions. Using this method, it is feasible to mutate 3-4 genes simultaneously, and we have successfully targeted up to eight genes (Cai et al., under revision), which would not be possible through traditional interbreeding of conditional mouse strains. However, there are limitations associated with CRISPR-generated tumors. First, the AAV virus only allows for a cargo of 5kb, which is equivalent to 10-12 sgRNAs or 6 sgRNAs and Cre expression cassette. Second, CRISPR-induced mutations are stochastic and can result in the insertion or deletion of bases, often leading to truncated proteins. However, they can also generate functional proteins with loss or gain of amino acids. Furthermore, the expression of sgRNAs does not always result in a double-strand break, which can lead to tumors with different mutation profiles among the animals. Therefore, it is necessary to sequence the mutation sites for each tumor to validate the appropriate gene mutations before analyzing the phenotype [
54]. In lung cancer studies, CRISPR technology has shown the generation of tumors with combined loss- and gain-of-function mutations [
57,
58]. Future studies in the prostate can take advantage of similar methodologies to model different gain-of-function mutations commonly observed in human PCa, in combination with the loss of tumor suppressor genes.
Orthotopic delivery of viral particles to induce PCa has advantages over traditional mouse models. Since the orthotopic delivery targets somatic cells, the virus can be titrated to transduce only a few cells. This allows for clonal expansion and the development of a defined tumor, whereas models with Cre expression in the majority of prostatic epithelium can result in hyperplastic lesions with limited progression to cancer before reaching the humane endpoint [
52]. This has been observed in mice with double mutations of Pten and Trp53, where mice developed kidney problems due to dysfunction in emptying the bladder as the entire prostate became significantly enlarged [
24]. The most commonly used Cre line is the PBCre4, where expression occurs from 1-3 weeks after birth, allowing for genetic modifications in the pre-adult organ [
9]. In contrast, orthotopic delivery is typically performed in adult mice, and researchers can mimic when in the life cycle the cancer is induced. Overall, orthotopic delivery has many benefits, and future preclinical models of PCa will rely more on this methodology.
4. Pre-clinical models by cell implantation
4.1. Classical cell-line derived xenografts
The most widely used preclinical models for studying PCa are xenografts of human PCa cells. In this model, previously established patient-derived monoclonal cell lines are grown in immunocompromised mice, offering several advantages. Commercially available cell lines are well-characterized, allowing for a direct comparison between in vitro determined molecular vulnerabilities, druggability, and their translation to potential treatments in complementary in vivo scenarios. Such research is invaluable and has played a central role in cancer research for several decades, and the importance of these models should not be dismissed.
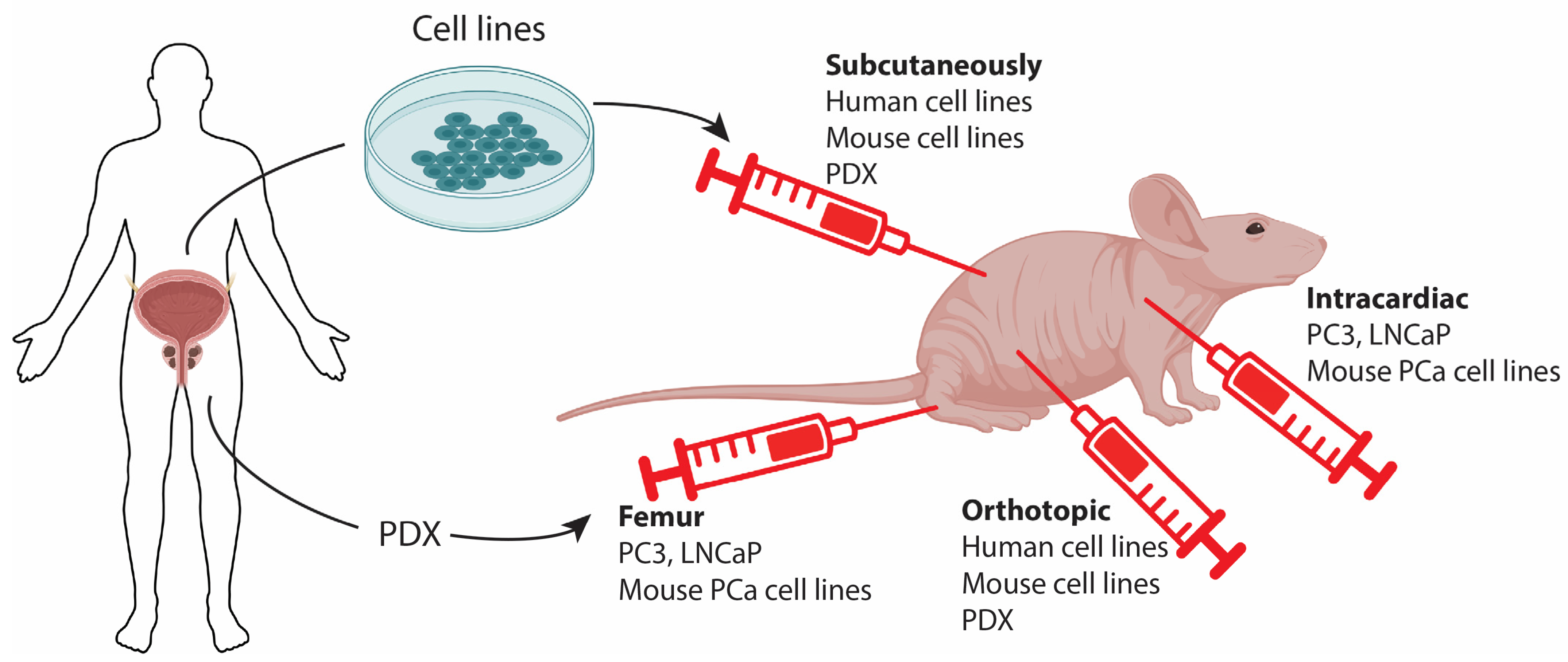
The most commonly used prostate cell lines are PC3, DU145, and LNCaP. However, the first two cell lines are not androgen-sensitive and do not secrete PSA. Typically, cells are inoculated subcutaneously (SC), often in the flank, which is a relatively simple procedure and enables easy caliper-based assessment of tumor growth (
Figure 2). This method is highly valuable for evaluating the efficacy of interventions. SC inoculations also generally result in higher take-rates compared to orthotopic inoculations since a larger number of cells can be administered. Additionally, SC tumors can grow to a considerable size without compromising animal welfare, which is crucial for certain applications such as the development of functional imaging in oncology (e.g., PET) [
59]. However, SC tumors may develop differently than patient tumors. Studies have shown that orthotopic prostate tumors exhibit better perfusion and less hypoxia compared to SC growing tumors [
60]. This difference in hypoxia levels is significant because hypoxia is associated with aggressive growth, metastasis formation, and reduced treatment sensitivity. Similar observations have been made in lung tumors [
61], and it may be an inherent characteristic of growth in the SC niche. Nevertheless, in certain contexts, maintaining similarity to the average patient tumor is not crucial or even desirable. For example, when developing hypoxia-targeting treatments, tumors that mimic the extent of hypoxia in the most hypoxic patient tumors, rather than the typical patient, are optimal. As an example, the development and testing of the radiosensitizer Nimorazole, which is now standard therapy for head and neck cancer patients in some countries, was based exclusively on mouse studies using a single SC tumor model [
62]. Additionally, for localized treatments such as radiotherapy or hyperthermia, a SC location in an extremity (foot/thigh) is extremely helpful to ensure accurate dosing and minimize side effects on crucial organs, which may limit treatment intensity and the testing of potentially curative treatments [
63].
4.2. Patient derived xenografts.
Although classical xenograft tumor models are established from cells originating from patients, the term "Patient-derived xenograft" (PDX) specifically refers to models that are directly established from patients by inoculating tumor material (e.g., a biopsy or a surgical specimen) into mice, typically through subcutaneous injection. This bypasses the step of establishing cell lines (
Figure 2). The major advantage of PDX models is that they may more faithfully recapitulate the biology of the original tumors, including cellular and microenvironmental heterogeneity. This, in turn, can provide valuable information on biology, microenvironment, biomarkers, treatment sensitivity, and more [
64].
PDX models have been successfully established from a variety of cancers, but the establishment of prostate PDX tumors has proven to be problematic compared to tumors from many other sites [
65]. One major obstacle is the significantly lower levels of androgens in male mice compared to men. One study showed that testosterone supplementation increased the take-rate, but it still remained low (10%) compared to other cancers [
66]. A low take-rate is problematic not only because it is labor-intensive and costly but also because more advanced prostate cancers tend to have a higher take-rate, leading to an inherent bias. It is important to consider this issue for all PDX models. Another concern with PDX models is the potential loss of crucial traits of the original patient tumor during passaging in mice. This can be tumor-type specific, but in general, it is recommended to minimize passaging and conduct a thorough histopathological examination of different passages [
67].
4.3. Allograft and Metastatic models for PCa.
Until now, immunotherapeutic strategies against prostate cancers have largely failed [
68], and further research is warranted. The application of xenograft models for immunotherapy research is clearly challenging when using human-derived cell lines, even though efforts are being made to humanize immunocompromised mice to establish a patient-like immune system [
69]. Instead, mouse-derived tumor cells can be allografted to syngeneic immunocompetent mice to study immune surveillance of the tumor. However, there haven't been many cell lines derived from mouse PCa, and this area generally requires further research. One cell line was derived from a transgenic Myc-driven PCa on a C57BL/6 background. This cell line showed clear expression of prostate epithelial markers and responded to castration. Furthermore, when injected intracardiacally, this cell line could form bone metastasis, which is highly relevant when studying PCa metastasis [
70]. The work by the Abate-Shen group also established cell lines from primary and secondary PCa, which exhibited loss of Pten and gain-of-function mutation of Kras. Their work revealed that increased Myc gene expression signature promoted metastasis formation in the bone, but also that inoculation of the cells by intracardiac injection favored metastasis in the bone (
Figure 2) [
49].
Intracardiac injections have also been performed with human PCa cell lines in immunodeficient mice. Specifically, PC3 cells have been injected intracardiacally, resulting in the formation of bone metastasis much more efficiently than orthotopic implantation [
71] [
72]. Some researchers have taken a step further and performed femur grafts to ensure the establishment of tumors in the bone. This model is useful for studying PCa cells in the bone microenvironment but cannot be used to investigate the homing of prostatic cells to the bone (
Figure 2) [
72].
5. Future perspectives
Preclinical models of PCa have proven crucial for elucidating the molecular mechanisms involved in PCa initiation and progression. Genetic mouse models and the interbreeding of different genetic modifications have shed light on key mutations and their implications for PCa. A tremendous effort has been made to generate prostate-specific genetic alterations, enabling researchers to ask specific questions focused on PCa. The genetic mouse models have been complemented by xenograft and patient-derived xenograft (PDX) models, which have brought human cancer into preclinical settings. Future work will be driven by the aim to replicate human PCa and develop effective treatments. In vivo models are essential for this purpose, as drug delivery poses a significant challenge in treatment, and the study of immune therapy necessitates appropriate models. Therefore, more refined models will be developed, including the orthotopic delivery of viral particles to generate clonal tumor expansion, which is valuable for understanding cancer evolution. These genetic mouse models should also be complemented by the generation of cancer cell lines, particularly derived from the mouse prostate at different stages of cancer. These cell lines are important for allograft studies, with a focus on the tumor microenvironment and the testing of cancer interventions.
6. Conclusions
Preclinical models have significantly contributed to our understanding of the molecular alterations that initiate and drive the progression of PCa. This is especially crucial since PCa can remain indolent for many years before progressing to an aggressive cancer. The mouse has emerged as an essential model for grafting human cells and utilizing genetically modified strains. Mutations frequently identified in human PCa have been studied in this model, providing valuable insights into the molecular pathways and implications for cancer progression. In future PCa research, preclinical models will continue to play an essential role in development of interventions targeting disease progression and the elimination of cancer as the primary objective.
Author Contributions
Conceptualization M.K.T.; Writing—original draft preparation M.K.T., M.B; Writing—review and editing, M.K.T., M.B; funding acquisition, M.K.T., M.B.
Funding
This research was funded by Danish Cancer Society (R204-A12490, R231-A13828), Dagmar Marshall Fond, P.A. Messerschmidt og Hustrus Fond and The Aarhus University Research Foundation, Denmark, to MKT. Danish Cancer Society: R231-A14066 to MB.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this article and supplementary material.
Acknowledgments
We would like to thank Cecilia Fominaya for reading the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Donin, N.M.; Reiter, R.E. Why Targeting PSMA Is a Game Changer in the Management of Prostate Cancer. J Nucl Med 2018, 59, 177–182. [Google Scholar] [CrossRef]
- McNeal, J.E. The zonal anatomy of the prostate. Prostate 1981, 2, 35–49. [Google Scholar] [CrossRef]
- Price, D. Comparative Aspects of Development and Structure in the Prostate. Natl Cancer Inst Monogr 1963, 12, 1–27. [Google Scholar]
- Russell, P.J.; Voeks, D.J. Animal models of prostate cancer. Methods Mol Med 2003, 81, 89–112. [Google Scholar] [CrossRef]
- Bryan, J.N.; Keeler, M.R.; Henry, C.J.; Bryan, M.E.; Hahn, A.W.; Caldwell, C.W. A population study of neutering status as a risk factor for canine prostate cancer. Prostate 2007, 67, 1174–1181. [Google Scholar] [CrossRef]
- McNeal, J.E. The anatomic heterogeneity of the prostate. Prog Clin Biol Res 1980, 37, 149–160. [Google Scholar]
- Greenberg, N.M.; DeMayo, F.J.; Sheppard, P.C.; Barrios, R.; Lebovitz, R.; Finegold, M.; Angelopoulou, R.; Dodd, J.G.; Duckworth, M.L.; Rosen, J.M.; et al. The rat probasin gene promoter directs hormonally and developmentally regulated expression of a heterologous gene specifically to the prostate in transgenic mice. Mol Endocrinol 1994, 8, 230–239. [Google Scholar] [CrossRef]
- Zhang, J.; Thomas, T.Z.; Kasper, S.; Matusik, R.J. A small composite probasin promoter confers high levels of prostate-specific gene expression through regulation by androgens and glucocorticoids in vitro and in vivo. Endocrinology 2000, 141, 4698–4710. [Google Scholar] [CrossRef]
- Wu, X.; Wu, J.; Huang, J.; Powell, W.C.; Zhang, J.; Matusik, R.J.; Sangiorgi, F.O.; Maxson, R.E.; Sucov, H.M.; Roy-Burman, P. Generation of a prostate epithelial cell-specific Cre transgenic mouse model for tissue-specific gene ablation. Mech Dev 2001, 101, 61–69. [Google Scholar] [CrossRef]
- Ma, X.; Ziel-van der Made, A.C.; Autar, B.; van der Korput, H.A.; Vermeij, M.; van Duijn, P.; Cleutjens, K.B.; de Krijger, R.; Krimpenfort, P.; Berns, A.; et al. Targeted biallelic inactivation of Pten in the mouse prostate leads to prostate cancer accompanied by increased epithelial cell proliferation but not by reduced apoptosis. Cancer Res 2005, 65, 5730–5739. [Google Scholar] [CrossRef]
- Abdulkadir, S.A.; Magee, J.A.; Peters, T.J.; Kaleem, Z.; Naughton, C.K.; Humphrey, P.A.; Milbrandt, J. Conditional loss of Nkx3.1 in adult mice induces prostatic intraepithelial neoplasia. Mol Cell Biol 2002, 22, 1495–1503. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, M.K.; Butler, C.M.; Shen, M.M.; Swain, A. Sox9 is required for prostate development. Dev Biol 2008, 316, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Kruithof-de Julio, M.; Economides, K.D.; Walker, D.; Yu, H.; Halili, M.V.; Hu, Y.P.; Price, S.M.; Abate-Shen, C.; Shen, M.M. A luminal epithelial stem cell that is a cell of origin for prostate cancer. Nature 2009, 461, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, N.M.; DeMayo, F.; Finegold, M.J.; Medina, D.; Tilley, W.D.; Aspinall, J.O.; Cunha, G.R.; Donjacour, A.A.; Matusik, R.J.; Rosen, J.M. Prostate cancer in a transgenic mouse. Proceedings of the National Academy of Sciences of the United States of America 1995, 92, 3439–3443. [Google Scholar] [CrossRef]
- Gelman, I.H. How the TRAMP Model Revolutionized the Study of Prostate Cancer Progression. Cancer Res 2016, 76, 6137–6139. [Google Scholar] [CrossRef]
- Chiaverotti, T.; Couto, S.S.; Donjacour, A.; Mao, J.H.; Nagase, H.; Cardiff, R.D.; Cunha, G.R.; Balmain, A. Dissociation of epithelial and neuroendocrine carcinoma lineages in the transgenic adenocarcinoma of mouse prostate model of prostate cancer. Am J Pathol 2008, 172, 236–246. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Ci, X.; Choi, S.Y.C.; Crea, F.; Lin, D.; Wang, Y. Molecular events in neuroendocrine prostate cancer development. Nat Rev Urol 2021, 18, 581–596. [Google Scholar] [CrossRef]
- Bhatia-Gaur, R.; Donjacour, A.A.; Sciavolino, P.J.; Kim, M.; Desai, N.; Young, P.; Norton, C.R.; Gridley, T.; Cardiff, R.D.; Cunha, G.R.; et al. Roles for Nkx3.1 in prostate development and cancer. Genes Dev 1999, 13, 966–977. [Google Scholar] [CrossRef]
- Trotman, L.C.; Niki, M.; Dotan, Z.A.; Koutcher, J.A.; Di Cristofano, A.; Xiao, A.; Khoo, A.S.; Roy-Burman, P.; Greenberg, N.M.; Van Dyke, T.; et al. Pten dose dictates cancer progression in the prostate. PLoS Biol 2003, 1, E59. [Google Scholar] [CrossRef]
- Abate-Shen, C.; Banach-Petrosky, W.A.; Sun, X.; Economides, K.D.; Desai, N.; Gregg, J.P.; Borowsky, A.D.; Cardiff, R.D.; Shen, M.M. Nkx3.1; Pten mutant mice develop invasive prostate adenocarcinoma and lymph node metastases. Cancer Res 2003, 63, 3886–3890. [Google Scholar]
- Wang, S.; Gao, J.; Lei, Q.; Rozengurt, N.; Pritchard, C.; Jiao, J.; Thomas, G.V.; Li, G.; Roy-Burman, P.; Nelson, P.S.; et al. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell 2003, 4, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Svensson, R.U.; Haverkamp, J.M.; Thedens, D.R.; Cohen, M.B.; Ratliff, T.L.; Henry, M.D. Slow disease progression in a C57BL/6 pten-deficient mouse model of prostate cancer. Am J Pathol 2011, 179, 502–512. [Google Scholar] [CrossRef]
- Lee, Y.R.; Chen, M.; Pandolfi, P.P. The functions and regulation of the PTEN tumour suppressor: new modes and prospects. Nat Rev Mol Cell Biol 2018, 19, 547–562. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Trotman, L.C.; Shaffer, D.; Lin, H.K.; Dotan, Z.A.; Niki, M.; Koutcher, J.A.; Scher, H.I.; Ludwig, T.; Gerald, W.; et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature 2005, 436, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Ku, S.Y.; Rosario, S.; Wang, Y.; Mu, P.; Seshadri, M.; Goodrich, Z.W.; Goodrich, M.M.; Labbe, D.P.; Gomez, E.C.; Wang, J.; et al. Rb1 and Trp53 cooperate to suppress prostate cancer lineage plasticity, metastasis, and antiandrogen resistance. Science 2017, 355, 78–83. [Google Scholar] [CrossRef]
- Ding, Z.; Wu, C.J.; Chu, G.C.; Xiao, Y.; Ho, D.; Zhang, J.; Perry, S.R.; Labrot, E.S.; Wu, X.; Lis, R.; et al. SMAD4-dependent barrier constrains prostate cancer growth and metastatic progression. Nature 2011, 470, 269–273. [Google Scholar] [CrossRef]
- Ding, Z.; Wu, C.J.; Jaskelioff, M.; Ivanova, E.; Kost-Alimova, M.; Protopopov, A.; Chu, G.C.; Wang, G.; Lu, X.; Labrot, E.S.; et al. Telomerase reactivation following telomere dysfunction yields murine prostate tumors with bone metastases. Cell 2012, 148, 896–907. [Google Scholar] [CrossRef]
- Francis, J.C.; McCarthy, A.; Thomsen, M.K.; Ashworth, A.; Swain, A. Brca2 and Trp53 deficiency cooperate in the progression of mouse prostate tumourigenesis. PLoS genetics 2010, 6, e1000995. [Google Scholar] [CrossRef]
- Thomsen, M.K.; Ambroisine, L.; Wynn, S.; Cheah, K.S.; Foster, C.S.; Fisher, G.; Berney, D.M.; Moller, H.; Reuter, V.E.; Scardino, P.; et al. SOX9 elevation in the prostate promotes proliferation and cooperates with PTEN loss to drive tumor formation. Cancer Res 2010, 70, 979–987. [Google Scholar] [CrossRef]
- Thomsen, M.K.; Bakiri, L.; Hasenfuss, S.C.; Wu, H.; Morente, M.; Wagner, E.F. Loss of JUNB/AP-1 promotes invasive prostate cancer. Cell Death Differ 2015, 22, 574–582. [Google Scholar] [CrossRef]
- Riedel, M.; Berthelsen, M.F.; Cai, H.; Haldrup, J.; Borre, M.; Paludan, S.R.; Hager, H.; Vendelbo, M.H.; Wagner, E.F.; Bakiri, L.; et al. In vivo CRISPR inactivation of Fos promotes prostate cancer progression by altering the associated AP-1 subunit Jun. Oncogene 2021, 40, 2437–2447. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Agersnap, S.N.; Sjøgren, A.; Simonsen, M.K.; Blaavand, M.S.; Jensen, U.V.; Thomsen, M.K. In Vivo Application of CRISPR/Cas9 Revealed Implication of Foxa1 and Foxp1 in Prostate Cancer Proliferation and Epithelial Plasticity. Cancers 2022, 14, 4381. [Google Scholar] [CrossRef] [PubMed]
- Hubner, A.; Mulholland, D.J.; Standen, C.L.; Karasarides, M.; Cavanagh-Kyros, J.; Barrett, T.; Chi, H.; Greiner, D.L.; Tournier, C.; Sawyers, C.L.; et al. JNK and PTEN cooperatively control the development of invasive adenocarcinoma of the prostate. Proceedings of the National Academy of Sciences of the United States of America 2012, 109, 12046–12051. [Google Scholar] [CrossRef] [PubMed]
- Pencik, J.; Schlederer, M.; Gruber, W.; Unger, C.; Walker, S.M.; Chalaris, A.; Marie, I.J.; Hassler, M.R.; Javaheri, T.; Aksoy, O.; et al. STAT3 regulated ARF expression suppresses prostate cancer metastasis. Nat Commun 2015, 6, 7736. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Lunardi, A.; Zhang, J.; Chen, Z.; Ala, U.; Webster, K.A.; Tay, Y.; Gonzalez-Billalabeitia, E.; Egia, A.; Shaffer, D.R.; et al. Zbtb7a suppresses prostate cancer through repression of a Sox9-dependent pathway for cellular senescence bypass and tumor invasion. Nat Genet 2013, 45, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Wu, S.P.; Creighton, C.J.; Dai, F.; Xie, X.; Cheng, C.M.; Frolov, A.; Ayala, G.; Lin, X.; Feng, X.H.; et al. COUP-TFII inhibits TGF-beta-induced growth barrier to promote prostate tumorigenesis. Nature 2013, 493, 236–240. [Google Scholar] [CrossRef]
- Nowak, D.G.; Cho, H.; Herzka, T.; Watrud, K.; Demarco, D.V.; Wang, V.M.Y.; Senturk, S.; Fellmann, C.; Ding, D.; Beinortas, T.; et al. MYC Drives Pten/Trp53 Deficient Proliferation and Metastasis due to IL6 Secretion and AKT Suppression via PHLPP2. Cancer Discovery 2015, 5, 636–651. [Google Scholar] [CrossRef]
- Limberger, T.; Schlederer, M.; Trachtova, K.; Garces de Los Fayos Alonso, I.; Yang, J.; Hogler, S.; Sternberg, C.; Bystry, V.; Oppelt, J.; Tichy, B.; et al. KMT2C methyltransferase domain regulated INK4A expression suppresses prostate cancer metastasis. Mol Cancer 2022, 21, 89. [Google Scholar] [CrossRef]
- Han, G.; Buchanan, G.; Ittmann, M.; Harris, J.M.; Yu, X.; Demayo, F.J.; Tilley, W.; Greenberg, N.M. Mutation of the androgen receptor causes oncogenic transformation of the prostate. Proceedings of the National Academy of Sciences of the United States of America 2005, 102, 1151–1156. [Google Scholar] [CrossRef]
- Tomlins, S.A.; Laxman, B.; Varambally, S.; Cao, X.; Yu, J.; Helgeson, B.E.; Cao, Q.; Prensner, J.R.; Rubin, M.A.; Shah, R.B.; et al. Role of the TMPRSS2-ERG gene fusion in prostate cancer. Neoplasia 2008, 10, 177–188. [Google Scholar] [CrossRef]
- Klezovitch, O.; Risk, M.; Coleman, I.; Lucas, J.M.; Null, M.; True, L.D.; Nelson, P.S.; Vasioukhin, V. A causal role for ERG in neoplastic transformation of prostate epithelium. Proceedings of the National Academy of Sciences of the United States of America 2008, 105, 2105–2110. [Google Scholar] [CrossRef]
- Graff, R.E.; Pettersson, A.; Lis, R.T.; DuPre, N.; Jordahl, K.M.; Nuttall, E.; Rider, J.R.; Fiorentino, M.; Sesso, H.D.; Kenfield, S.A.; et al. The TMPRSS2:ERG fusion and response to androgen deprivation therapy for prostate cancer. Prostate 2015, 75, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.T.; Tretiakova, M.S.; Silvis, M.R.; Lucas, J.; Klezovitch, O.; Coleman, I.; Bolouri, H.; Kutyavin, V.I.; Morrissey, C.; True, L.D.; et al. ERG Activates the YAP1 Transcriptional Program and Induces the Development of Age-Related Prostate Tumors. Cancer Cell 2015, 27, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Kalkat, M.; De Melo, J.; Hickman, K.A.; Lourenco, C.; Redel, C.; Resetca, D.; Tamachi, A.; Tu, W.B.; Penn, L.Z. MYC Deregulation in Primary Human Cancers. Genes (Basel) 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Ellwood-Yen, K.; Graeber, T.G.; Wongvipat, J.; Iruela-Arispe, M.L.; Zhang, J.; Matusik, R.; Thomas, G.V.; Sawyers, C.L. Myc-driven murine prostate cancer shares molecular features with human prostate tumors. Cancer Cell 2003, 4, 223–238. [Google Scholar] [CrossRef]
- Nandana, S.; Ellwood-Yen, K.; Sawyers, C.; Wills, M.; Weidow, B.; Case, T.; Vasioukhin, V.; Matusik, R. Hepsin cooperates with MYC in the progression of adenocarcinoma in a prostate cancer mouse model. Prostate 2010, 70, 591–600. [Google Scholar] [CrossRef]
- Cho, H.; Herzka, T.; Zheng, W.; Qi, J.; Wilkinson, J.E.; Bradner, J.E.; Robinson, B.D.; Castillo-Martin, M.; Cordon-Cardo, C.; Trotman, L.C. RapidCaP, a novel GEM model for metastatic prostate cancer analysis and therapy, reveals myc as a driver of Pten-mutant metastasis. Cancer Discov 2014, 4, 318–333. [Google Scholar] [CrossRef]
- Hubbard, G.K.; Mutton, L.N.; Khalili, M.; McMullin, R.P.; Hicks, J.L.; Bianchi-Frias, D.; Horn, L.A.; Kulac, I.; Moubarek, M.S.; Nelson, P.S.; et al. Combined MYC Activation and Pten Loss Are Sufficient to Create Genomic Instability and Lethal Metastatic Prostate Cancer. Cancer Res 2016, 76, 283–292. [Google Scholar] [CrossRef]
- Arriaga, J.M.; Panja, S.; Alshalalfa, M.; Zhao, J.; Zou, M.; Giacobbe, A.; Madubata, C.J.; Kim, J.Y.; Rodriguez, A.; Coleman, I.; et al. A MYC and RAS co-activation signature in localized prostate cancer drives bone metastasis and castration resistance. Nat Cancer 2020, 1, 1082–1096. [Google Scholar] [CrossRef]
- Blattner, M.; Liu, D.; Robinson, B.D.; Huang, D.; Poliakov, A.; Gao, D.; Nataraj, S.; Deonarine, L.D.; Augello, M.A.; Sailer, V.; et al. SPOP Mutation Drives Prostate Tumorigenesis In Vivo through Coordinate Regulation of PI3K/mTOR and AR Signaling. Cancer Cell 2017, 31, 436–451. [Google Scholar] [CrossRef]
- Shoag, J.; Liu, D.; Blattner, M.; Sboner, A.; Park, K.; Deonarine, L.; Robinson, B.D.; Mosquera, J.M.; Chen, Y.; Rubin, M.A.; et al. SPOP mutation drives prostate neoplasia without stabilizing oncogenic transcription factor ERG. J Clin Invest 2018, 128, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Riedel, M.; Berthelsen, M.F.; Bakiri, L.; Wagner, E.F.; Thomsen, M.K. Virus Delivery of CRISPR Guides to the Murine Prostate for Gene Alteration. J Vis Exp 2018. [Google Scholar] [CrossRef]
- Leow, C.C.; Wang, X.D.; Gao, W.Q. Novel method of generating prostate-specific Cre-LoxP gene switching via intraductal delivery of adenovirus. Prostate 2005, 65, 1–9. [Google Scholar] [CrossRef]
- Thomsen, M.K. Application of CRISPR for In Vivo Mouse Cancer Studies. Cancers (Basel) 2022, 14. [Google Scholar] [CrossRef]
- Daya, S.; Berns, K.I. Gene therapy using adeno-associated virus vectors. Clin Microbiol Rev 2008, 21, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Chiou, S.H.; Winters, I.P.; Wang, J.; Naranjo, S.; Dudgeon, C.; Tamburini, F.B.; Brady, J.J.; Yang, D.; Grüner, B.M.; Chuang, C.H.; et al. Pancreatic cancer modeling using retrograde viral vector delivery and in vivo CRISPR/Cas9-mediated somatic genome editing. Genes Dev 2015, 29, 1576–1585. [Google Scholar] [CrossRef]
- Platt, R.J.; Chen, S.; Zhou, Y.; Yim, M.J.; Swiech, L.; Kempton, H.R.; Dahlman, J.E.; Parnas, O.; Eisenhaure, T.M.; Jovanovic, M.; et al. CRISPR-Cas9 knockin mice for genome editing and cancer modeling. Cell 2014, 159, 440–455. [Google Scholar] [CrossRef]
- Berthelsen, M.F.; Leknes, S.L.; Riedel, M.; Pedersen, M.A.; Joseph, J.V.; Hager, H.; Vendelbo, M.H.; Thomsen, M.K. Comparative Analysis of Stk11/Lkb1 versus Pten Deficiency in Lung Adenocarcinoma Induced by CRISPR/Cas9. Cancers (Basel) 2021, 13. [Google Scholar] [CrossRef]
- Busk, M.; Horsman, M.R.; Overgaard, J. Resolution in PET hypoxia imaging: voxel size matters. Acta Oncol 2008, 47, 1201–1210. [Google Scholar] [CrossRef]
- Zhang, W.; Fan, W.; Rachagani, S.; Zhou, Z.; Lele, S.M.; Batra, S.K.; Garrison, J.C. Comparative Study of Subcutaneous and Orthotopic Mouse Models of Prostate Cancer: Vascular Perfusion, Vasculature Density, Hypoxic Burden and BB2r-Targeting Efficacy. Sci Rep 2019, 9, 11117. [Google Scholar] [CrossRef]
- Graves, E.E.; Vilalta, M.; Cecic, I.K.; Erler, J.T.; Tran, P.T.; Felsher, D.; Sayles, L.; Sweet-Cordero, A.; Le, Q.T.; Giaccia, A.J. Hypoxia in models of lung cancer: implications for targeted therapeutics. Clin Cancer Res 2010, 16, 4843–4852. [Google Scholar] [CrossRef]
- Overgaard, J.; Overgaard, M.; Nielsen, O.S.; Pedersen, A.K.; Timothy, A.R. A comparative investigation of nimorazole and misonidazole as hypoxic radiosensitizers in a C3H mammary carcinoma in vivo. Br J Cancer 1982, 46, 904–911. [Google Scholar] [CrossRef]
- Mortensen, L.S.; Busk, M.; Nordsmark, M.; Jakobsen, S.; Theil, J.; Overgaard, J.; Horsman, M.R. Accessing radiation response using hypoxia PET imaging and oxygen sensitive electrodes: a preclinical study. Radiother Oncol 2011, 99, 418–423. [Google Scholar] [CrossRef]
- Lilja-Fischer, J.K.; Ulhoi, B.P.; Alsner, J.; Stougaard, M.; Thomsen, M.S.; Busk, M.; Lassen, P.; Steiniche, T.; Nielsen, V.E.; Overgaard, J. Characterization and radiosensitivity of HPV-related oropharyngeal squamous cell carcinoma patient-derived xenografts. Acta Oncol 2019, 58, 1489–1494. [Google Scholar] [CrossRef]
- Shi, C.; Chen, X.; Tan, D. Development of patient-derived xenograft models of prostate cancer for maintaining tumor heterogeneity. Transl Androl Urol 2019, 8, 519–528. [Google Scholar] [CrossRef]
- Russell, P.J.; Russell, P.; Rudduck, C.; Tse, B.W.; Williams, E.D.; Raghavan, D. Establishing prostate cancer patient derived xenografts: lessons learned from older studies. Prostate 2015, 75, 628–636. [Google Scholar] [CrossRef]
- Abdolahi, S.; Ghazvinian, Z.; Muhammadnejad, S.; Saleh, M.; Asadzadeh Aghdaei, H.; Baghaei, K. Patient-derived xenograft (PDX) models, applications and challenges in cancer research. J Transl Med 2022, 20, 206. [Google Scholar] [CrossRef]
- Slovin, S.F. Immunotherapy combinations for metastatic castration-resistant prostate cancer - failed trials and future aspects. Curr Opin Urol 2023. [Google Scholar] [CrossRef]
- Jin, K.T.; Du, W.L.; Lan, H.R.; Liu, Y.Y.; Mao, C.S.; Du, J.L.; Mou, X.Z. Development of humanized mouse with patient-derived xenografts for cancer immunotherapy studies: A comprehensive review. Cancer Sci 2021, 112, 2592–2606. [Google Scholar] [CrossRef]
- Simons, B.W.; Kothari, V.; Benzon, B.; Ghabili, K.; Hughes, R.; Zarif, J.C.; Ross, A.E.; Hurley, P.J.; Schaeffer, E.M. A mouse model of prostate cancer bone metastasis in a syngeneic immunocompetent host. Oncotarget 2019, 10, 6845–6854. [Google Scholar] [CrossRef]
- Park, S.H.; Eber, M.R.; Shiozawa, Y. Models of Prostate Cancer Bone Metastasis. Methods Mol Biol 2019, 1914, 295–308. [Google Scholar] [CrossRef]
- Wu, T.T.; Sikes, R.A.; Cui, Q.; Thalmann, G.N.; Kao, C.; Murphy, C.F.; Yang, H.; Zhau, H.E.; Balian, G.; Chung, L.W. Establishing human prostate cancer cell xenografts in bone: induction of osteoblastic reaction by prostate-specific antigen-producing tumors in athymic and SCID/bg mice using LNCaP and lineage-derived metastatic sublines. Int J Cancer 1998, 77, 887–894. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).