1. Introduction
Interstitial lung disease (ILD) is a heterogeneous group of diffuse parenchymal processes in the lung characterized by varying degrees of inflammation and fibrosis. Idiopathic pulmonary fibrosis (IPF) is one of the most common forms of ILD (accounting for 20-30% of all ILD cases) and is characterized by progressive parenchymal fibrosis with a poor prognosis [
1,
2]. Currently, the incidence and prevalence of IPF are increasing worldwide [
3,
4]. The median survival of patients with IPF is 3-5 years [
5]. Fibrogenesis in IPF is known to be mainly mediated by abnormal release of profibrogenic factors such as tumor necrosis factor-α (TNF-α) and interleukin 1β (IL-1β), which promote myofibroblast differentiation/activation, transforming growth factor-beta (TGF-β) [
2] and extracellular matrix (ECM) deposition [
6]. Levels of the profibrotic cytokine TGF-β directly correlate with the severity of IPF disease [
7,
8]. TGF-β is believed to induce epithelial-mesenchymal transition and differentiation of fibroblasts into myofibroblasts, which contributes to the excessive synthesis and deposition of ECM proteins in the lung [
9,
10]. In addition, the concentration of hyaluronic acid (HA), a key component of the ECM, increases in the lung in IPF [
11,
12]. In IPF, there is an imbalance between the synthesis and degradation of HA [
13]. The main enzyme that regulates HA metabolism is hyaluronidase (HD), which remodels the extracellular matrix by cleaving HA into fragments (glucosamines and glucuronic acid) [
13,
14]. Given the role of HA in the organization of fibrin, fibronectin and collagen, it is considered one of the biomarkers of fibrosis and a potential target for antifibrotic therapy, in particular for drugs based on HD [
15]. The pathogenesis of pulmonary fibrosis is complex and not fully understood, which hinders the development of specific therapies. Current treatment options for IPF remain limited [
8,
10]. Pirfenidone axunio and Nintedanib esilat, known on the pharmaceutical market for the treatment of IPF, have not shown the expected high antifibrotic activity, they can only slow down the progression of the disease, and due to the risk of serious side effects, their use is limited [
16].
Several animal models of pulmonary fibrosis are used to study and understand the biological mechanisms of fibrogenesis in IPF and to test the efficacy of therapeutic compounds. In particular, systemic or intratracheal administration of bleomycin, radiation, intratracheal administration of silica or asbestos are used to induce fibrosis [
17]. At the same time, histologic staining and morphometric analysis of lung tissue, quantitative determination of hydroxyproline and collagen are used to quantify lung fibrosis in animals [
17]. Bleomycin injection into the respiratory tract of small rodents is the most commonly used experimental and preclinical model to study the pathophysiology of IPF and to test potential therapeutic compounds [
17,
18,
19]. The histologic picture of lung tissue in animals after bleomycin administration is similar in some histologic features to that observed in patients with IPF [
17,
20]. Thus, the drugs Pirfenidone axunio and Nintedanib esilat first showed therapeutic efficacy in a model of bleomycin-induced pulmonary fibrosis. As pulmonary fibrosis is also an inflammatory disease [
21] it is important to explore the role of immune cells in the pathogenesis of the disease.
In 2011, Bitencourt C.S. and colleagues showed that intranasal administration of HD reduced the fibrotic response induced by bleomycin in the lungs of laboratory animals, reducing TGF-β production and collagen deposition [
22]. Thus, HD may represent a new and promising approach/agent for the treatment of pulmonary fibrosis. However, despite the demonstrated efficacy of the drug, the use of native HD is largely limited due to the short plasma half-life when administered systemically [
23]. Previously, we investigated the effects of poloxamergaluronidase (pHD - a conjugate of Pluronic L31 and hyaluronidase) compared to native HD in a model of bleomycin-induced pulmonary fibrosis in C57BL/6 mice. It was shown that the administration of pHD reduced the content of connective tissue in the lung by more than twofold, decreased the concentration of TGF-β, hydroxyproline, type 1 collagen and total collagen in the lung homogenate [
15].
The present study aims to investigate the potential anti-inflammatory and antifibrotic effects of Longidaza (OOO NPO Petrovax Pharma, Moscow, Russia) containing HD on a bleomycin model of pulmonary fibrosis in mice. Longidaza is able to influence the fibrotic process and is already used for various diseases in urology, gynecology, dermatology, etc [
24]. In addition, the efficacy and safety of Longidaza was evaluated in patients with post-COVID-19 syndrome with lung abnormalities. Patients with post-COVID-19 syndrome and lung abnormalities may benefit from treatment with longidaza, as evidenced by patients showing improvement in forced vital capacity (FVC), pulse oximetry (SpO
(2)), functional capacity and dyspnea mMRC score [
25]. The World Health Organization (WHO) assigned an international non-proprietary name (INN) to the Russian product Longidaza in 2015.
2. Materials and Methods
2.1. Animals
8-week-old male C57BL/6 mice (Surgical Bio-modelling Department of the Goldberg ED Research Institute of Pharmacology and Regenerative Medicine, Russia) were used in all experiments. Animals were randomly assigned into the experimental groups. All experimental protocols were approved by the animal care and use committee of the Goldberg ED Research Institute of Pharmacology and Regenerative Medicine, Tomsk NRMC (IACUC Protocol No. 204092022). Within this study, 200 mice were used.
2.2. Modeling of Experimental Pulmonary Fibrosis
Experimental pulmonary fibrosis was induced by a single intratracheal bleomycin (BLM, Nippon Kayaku Co., Ltd., Tokyo, Japan) administration at a dose 80 μg/mouse in 0.03 mL of 0.9% NaCl, which was slowly instilled in the tracheal lumen [
26]. All procedures were performed under anesthesia induced by inhalation of isoflurane using an apparatus for inhalation anesthesia UGO BASILE model 21050 (UGO BASILE, Comerio, Italy). The introduction of BLM was taken for the d0. All mice were euthanized on d7 and d21 by CO
2 overdose.
Mice were co-housed (5–6 mice per cage) and entrained to a reverse 12 h light/12 h dark cycle. During the 21 days, mice had ad libitum access to standard rodent chow.
2.3. Study Design and Experimental Groups
Mice were divided into 10 groups (n=20 in a group): group 1 – intact control (Intact); group 2 – mice treated with bleomycin (pulmonary fibrosis) + NaCl intramuscularly (Vehicle i.m.); group 3 – mice treated with bleomycin (pulmonary fibrosis) + NaCl intranasally (Vehicle i.n.); group 4 – pulmonary fibrosis + Pirfenidone (Pirfenidone 300 mg/kg p.o.); group 5 – pulmonary fibrosis + Longidaza intramuscularly at a dose of 60 U/kg (LG 60 U/kg i.m.); group 6 – pulmonary fibrosis + Longidaza intramuscularly at a dose of 120 U/kg (LG 120 U/kg i.m.); group 7 – pulmonary fibrosis + Longidaza intramuscularly at a dose of 1200 U/kg (LG 1200 U/kg i.m.); group 8 – pulmonary fibrosis + Longidaza intranasally at a dose of 10 U/kg (LG 10 U/kg i.n.); group 9 – pulmonary fibrosis + Longidaza intranasally at a dose of 30 U/kg (LG 30 U/kg i.n.); group 10 – pulmonary fibrosis + Longidaza intranasally at a dose of 120 U/kg (LG 120 U/kg i.n.).
Control animals received 0.03 ml of 0.9% NaCl intramuscularly (group 2) and 0.1 ml or intratracheally (group 3). The animals from groups 5-7 received 0.03 ml of Longidaza intramuscularly and animals from groups 8-10 received 0.1 ml of Longidaza intratracheally.
On the d7 hematological blood parameters were studied, inflammation in the lungs was histologically evaluated, levels of IL-6, TNF-α and hyaluronic acid were assessed in the lung homogenate in mice from groups 1-10 (n=10/group). On the d21 hematological blood parameters were examined, inflammation and connective tissue area in the lungs were histologically evaluated, ELISA levels of TGF-β, type I collagen, hydroxyproline and hyaluronic acid were assessed in a lung homogenate of mice from groups 1–10 (n=10/group).
2.4. Reagents
2.4.1. Longidaza
Longidaza® (NPO Petrovax Pharm LLC, Moscow, Russia) contains hyaluronidase enzyme stabilized on a carrier as an active ingredient. The enzyme is stabilized by conjugation of hyaluronidase with a polymeric carrier, a copolymer derivative of N-oxide 1,4-ethylenpiperazine and (N-carboxymethyl)-1,4-ethylenpiperazine bromide, an analog of Azoximer bromide with a molecular mass of about 40 kilodalton, which has its own pharmacological activity: immunomodulator, antioxidant, detoxifier. International nonproprietary name of the substance of Longidaza® preparation is Bovhyaluronidaze azoximer. Bovhyaluronidase azoxymer has the ability to regulate the concentration of hyaluronic acid and retains the pharmacological properties of azoxymer bromide with chelating, antioxidant, anti-inflammatory and immunomodulatory activity [
25].
Longidaza® was administered intramuscularly at doses of 60, 120 and 1200 U/kg and intranasally at doses of 10, 30 and 120 U/kg in a course according to the following scheme: d2-d6, d8, d10, d12, d14, d16, d18, and d20.
2.4.2. Pirfenidone
Pirfenidone («TOKYO CHEMICAL INDUSTRY CO., LTD», Japan) was used as the reference drug. Pirfenidone was dissolved in 0.5% carboxymethylcellulose solution (vehicle) and was administered intragastrically daily at a dose of 300 mg/kg/day on d2-d6, d8, d10, d12, d14, d16, d18, d20. The volume of administration was determined according to body weight. The Pirfenidone dose was selected according to a report published elsewhere [
27].
2.5. The Study of Blood Parameters
To determine hematological parameters, blood was taken from the tail vein of the tip of the tail (in vivo) into a microvette with K3EDTA spraying for a hematological analyzer in a total volume of 0.2 ml. The analysis was performed on the day of sampling on a Mythic 18 Vet hematology analyzer (EU).
2.6. ELISA
2.6.1. IL-6, TGF-β, TNF-α measurements
The concentrations of IL-1β, TNF-α and TGF-β in the lung homogenate of the right lung were determined by ELISA according to the manufacturer’s instructions (Cusabio Biotech CO., Ltd., Wuhan, China). Sensitivity was >7.8 pg/ml for IL-6, >4.7 pg/ml for TNF-α and >0.2 ng/ml for TGF-β. Results are expressed in ng/ml for TGF-β and pg/ml for TNF-α and IL-6.
2.6.2. Measurements of hyaluronic acid, hydroxyproline, type I collagen
Hyaluronic acid, hydroxyproline and type I collagen levels were determined by ELISA according to the manufacturer’s instructions (Cusabio Biotech CO., Ltd, Wuhan, China). Sensitivity was >15.6 pg/ml for hyaluronic acid, >1.95 ng/ml for hydroxyproline, >0.039 ng/ml for type I collagen. The results are expressed in ng/ml.
2.7. Histological Examination of Lung Tissue
2.7.1. Analysis of lung tissue
For histological examination, the left lung was fixed in 10% neutral formalin solution, embedded in paraffin, and sections 5 µm thick were prepared. Sections were stained with hematoxylin and eosin. The structure of the lungs, edema, infiltration by inflammatory cells, venous hyperemia, thickening of the walls of blood vessels and bronchi were assessed. Micropreparations from each animal were analyzed under an Axio Lab.A1 light microscope (Carl Zeiss, MicroImaging GmbH; Göttingen, Germany) at 100- and 400-fold magnification.
2.7.2. Analysis of pulmonary inflammation
The degrees of peribronchial and perivascular infiltrates were assessed by the scale of inflammation and quantitative assessment of peribronchial and perivascular mononuclear cells [
28]. The drugs were coded and peribronchial inflammation was assessed in a blinded manner using a reproducible scoring system. Each tissue slice was assigned a value from 0 to 3. A value of 0 was taken when no inflammation was detected, a value of 1 was when there was a rare encounter with inflammatory cells, a value of 2 was when most of the bronchi or vessels were surrounded by a thin layer (from one to five cells) of inflammatory cells, and a value of 3 was when most of the bronchi and blood vessels were surrounded by a thick layer (more than five cells) of inflammatory cells. Since there were five to seven randomly selected tissue sections per mouse, inflammation scores could be expressed as an average per animal and compared between groups. In addition, cells in the peribronchial and perivascular segments were counted relative to the length of the basement membrane. The total index of bronchial and vascular inflammation was expressed as the number of inflammatory cells/m of the basement membrane.
2.7.3. The quantification of the area of connective tissue in the lungs
To quantify area of connective tissue, the histological slides were stained using Van Gieson method. Micrographs of the histological surface section of the lung tissue at 4x magnification were taken for each experimental animal using a Cytation5 multi-mode reader (BioTek Instruments, Inc., Winooski, VT, USA). The resulting images were processed using Gen5 software (Bad Friedrichshall, Germany). The area of connective tissue in the lung was determined using a function for counting the area of the object in the image. Bronchovascular strands were carefully removed from the analyzed areas. The area of connective tissue (X) was calculated by the formula:
where ∑ a is the sum of pixels occupied by fibrosis tissue on 10 images of one slide, S is the number of pixels corresponding to the total area of the image (when using this camera and program - 4423680), b is the sum of pixels occupied by the empty part of the slide, on 10 images one slide. The content of connective tissue in the lungs was expressed as a percentage of the lung tissue area.
2.8. Statistical Analysis
Statistical analysis was performed using SPSS (version 15.0, SPSS Inc., Chicago, IL, USA). Data were analyzed and presented as means ± standard error of the mean. Statistical significance was evaluated by Student’s t-test (for parametric data), or Mann–Whitney test (for nonparametric data) when appropriate. A p-value of less than 0.05 (by two-tailed testing) was considered an indicator of statistical significance. Statistical analysis of hematology and ELISA data was performed using the Kruskal-Wallis test, post-hoc Wilcoxon test for pairwise comparisons with the bleomycin control group (with appropriate route of administration), Bonferroni correction (ns - p>0.05, * p<0.05, **p <0.01, *** p<0.001, **** p< 0.0001).
3. Results
3.1. Mortality Assessment
The condition of mice in all experimental groups was satisfactory, no deaths were observed.
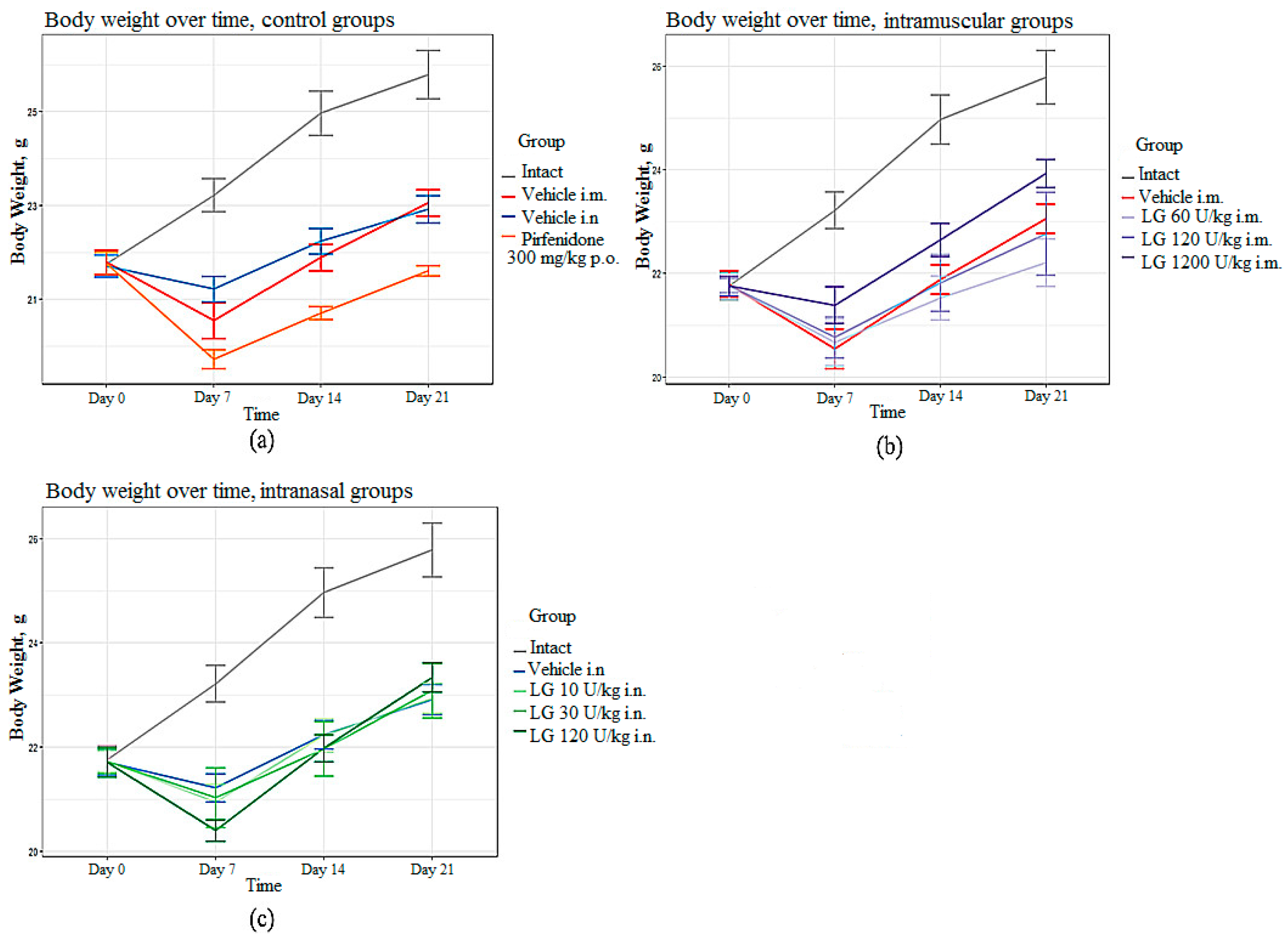
3.2. Body Weight of Mice in Formation of Pulmonary Fibrosis
In the groups of mice with pulmonary fibrosis (groups 2 and 3) without treatment, the body weight of the animals was significantly reduced from day 7 to day 21 of the experiment compared with the intact group (
Figure 1a). In the group of mice treated with the reference drug pirfenidone (group 4), a significant decrease in body weight was observed from day 7 to day 21 of the experiment compared to untreated animals (
Figure 1a). Body weight loss is common with Pirfenidone as it has a metabolic side effect. In mice treated with Longidaza, body weight was significantly higher compared to animals treated with the Pirfenidone (
Figure 1b,c). The most pronounced effect of Longidaza was seen at a dose of 1200 U/kg when administered intramuscularly and at a dose of 120 U/kg when administered intranasally (
Figure 1).
Figure 1.
Body weight in C57BL/6 mice from group of intact control, from group with pulmonary fibrosis after Pirfenidone administration per os (a), after intramuscular (b) and intranasal (c) administration of various doses of Longidaza compared to mice without treatment.
Figure 1.
Body weight in C57BL/6 mice from group of intact control, from group with pulmonary fibrosis after Pirfenidone administration per os (a), after intramuscular (b) and intranasal (c) administration of various doses of Longidaza compared to mice without treatment.
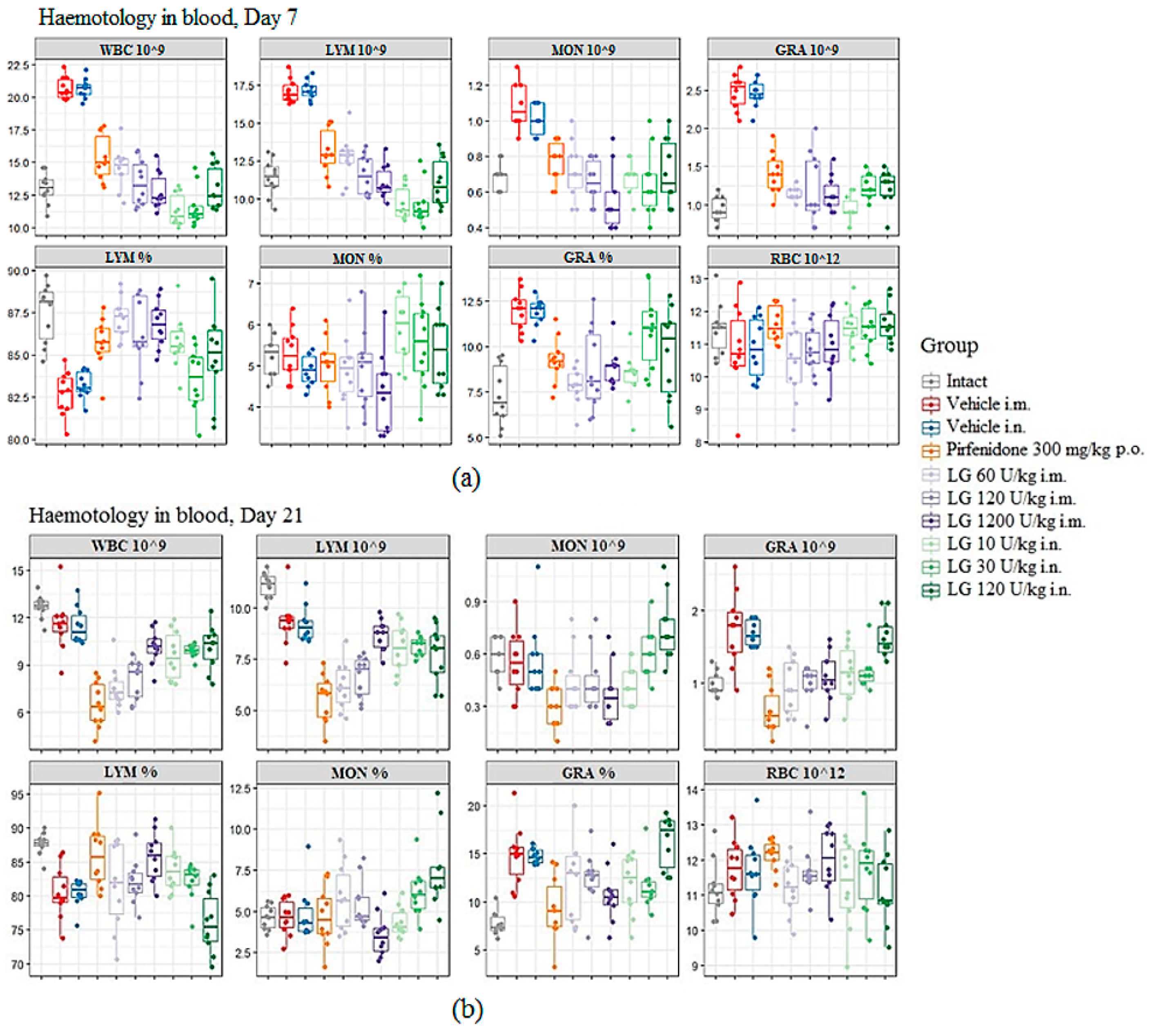
3.3. Influence of Longidaza on Hematologic Parameters of Blood
During the inflammatory phase of fibrosis development, on d7 after bleomycin administration, an increase in the white blood cells (WBC) in the peripheral blood of mice was observed in both pathological control groups (groups 2 and 3) compared to the group of intact animals (
Figure 2a). A shift in the relative content of blood cells towards neutrophils was observed in these groups. Against the background of Longidaza administration at all doses, regardless of the route of administration, a decrease in WBC was observed compared to groups 2 and 3, with a shift in the relative content of cells towards lymphocytes. With the intramuscular injection of Longidaza, an increase in the dose of the drug induces an increase in the effect (
Figure 2a). The most pronounced effect of the drug was observed at a dose of 1200 U/kg administered intramuscularly (group 7). With the inhalation administration of Longidaza, the greatest activity during these periods of the experiment was observed at doses of 10 U/kg (group 8) and 30 U/kg (group 9) (
Figure 2a). Administration of Pirfenidone prevented the increase in blood WBC, but the effect of Longidaza was more pronounced.
On the d21 the inflammatory response was still present in the peripheral blood of mice in the pathological control groups, as evidenced by the increased content of neutrophils and the continued shift in the relative content of blood cells toward neutrophils compared with the intact control (
Figure 2b). At the same time, a decrease in WBC and lymphocyte content was observed in the blood of animals from these groups compared to the intact control. Administration of the Pirfenidone to mice with pulmonary fibrosis (group 4) resulted in the development of an even more pronounced leukopenia in the peripheral blood of the animals by the d21 of the experiment (
Figure 2b). During these study periods, administration of Longidaza at a dose of 1200 U/kg (intramuscular administration, group 7) and 120 U/kg (inhalation administration, group 10) normalized the levels of neutrophils and monocytes, although the levels of lymphocytes remained reduced compared to the intact control (
Figure 2b). The positive effect of the drug Longidaza compared to the reference drug was remarkable: Longidaza prevented the leukopenia that develops after the bleomycin administration and increases after the Pirfenidone administration.
Figure 2.
The effect of Longidaza on the hematological parameters of C57BL/6 mice at the modeling of bleomycin-induced pulmonary fibrosis on d7 (a) and d21 (b). WBC – white blood cells (109/L blood); GRA 10^9, LYM 10^9, MON 10^9 (absolute number), GRA %, LYM %, MON % (relative number) - the number of neutrophils, lymphocytes, monocytes; RBC 10^12 – red blood cells (1012/L blood). Statistical analysis of data was performed using the Kruskal-Wallis test, post-hoc Wilcoxon test for pairwise comparisons with the bleomycin control group (with appropriate route of administration), Bonferroni correction (ns - p>0.05, * p<0.05, **p <0.01, *** p<0.001, **** p< 0.0001).
Figure 2.
The effect of Longidaza on the hematological parameters of C57BL/6 mice at the modeling of bleomycin-induced pulmonary fibrosis on d7 (a) and d21 (b). WBC – white blood cells (109/L blood); GRA 10^9, LYM 10^9, MON 10^9 (absolute number), GRA %, LYM %, MON % (relative number) - the number of neutrophils, lymphocytes, monocytes; RBC 10^12 – red blood cells (1012/L blood). Statistical analysis of data was performed using the Kruskal-Wallis test, post-hoc Wilcoxon test for pairwise comparisons with the bleomycin control group (with appropriate route of administration), Bonferroni correction (ns - p>0.05, * p<0.05, **p <0.01, *** p<0.001, **** p< 0.0001).
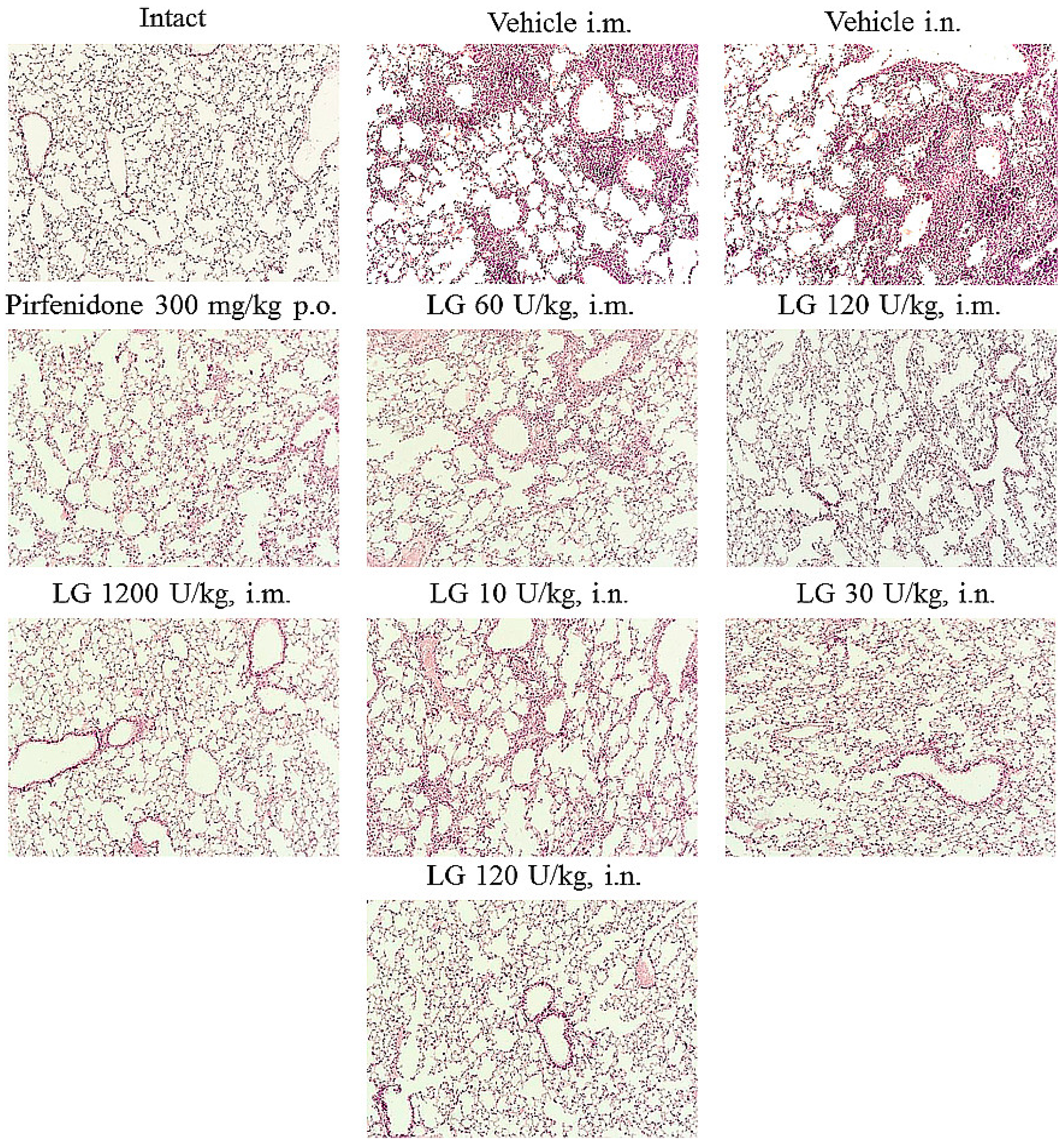
3.4. Effects of Longidaza on Bleomycin-damaged Lungs
Histologic examination showed that bleomycin induced lesions similar to acute fibrosing alveolitis in the lungs of mice. On d7, inflammatory infiltrates of lymphocytes and macrophages in the parenchyma, hyperemia of large vessels and vessels of the microvasculature were observed (
Figure 3). Small hemorrhages were present in the lung parenchyma. The inflammatory response was predominantly perivascular and peribronchial. There was infiltration of the alveolar septa with lymphocytes, plasmocytes, and multiple accumulations of macrophages in the alveoli.
Figure 3.
Micrographs of lung sections obtained from male C57BL/6 mice (Intact) mice of intact control (group 1); (Vehicle i.m. and Vehicle i.n.) mice treated by bleomycin (groups 2 and 3); (Pirfenidone 300 mg/kg p.o.) pulmonary fibrosis+Pirfenidone (group 4); (LG 60 U/kg i.m.) pulmonary fibrosis+Longidaza intramuscularly at a dose of 60 U/kg (group 5); (LG 120 U/kg i.m.) pulmonary fibrosis+Longidaza intramuscularly at a dose of 120 U/kg (group 6); (LG 1200 U/kg i.m.) pulmonary fibrosis+Longidaza intramuscularly at a dose of 1200 U/kg (group 7); (LG 10 U/kg i.n.) pulmonary fibrosis+Longidaza intranasally at a dose of 10 U/kg (group 8); (LG 30 U/kg i.n.) pulmonary fibrosis+Longidaza intranasally at a dose of 30 U/kg (group 9); (LG 120 U/kg i.n.) pulmonary fibrosis+Longidaza intranasally at a dose of 120 U/kg (group 10) on d7. Tissues were stained with hematoxylin–eosin. x100.
Figure 3.
Micrographs of lung sections obtained from male C57BL/6 mice (Intact) mice of intact control (group 1); (Vehicle i.m. and Vehicle i.n.) mice treated by bleomycin (groups 2 and 3); (Pirfenidone 300 mg/kg p.o.) pulmonary fibrosis+Pirfenidone (group 4); (LG 60 U/kg i.m.) pulmonary fibrosis+Longidaza intramuscularly at a dose of 60 U/kg (group 5); (LG 120 U/kg i.m.) pulmonary fibrosis+Longidaza intramuscularly at a dose of 120 U/kg (group 6); (LG 1200 U/kg i.m.) pulmonary fibrosis+Longidaza intramuscularly at a dose of 1200 U/kg (group 7); (LG 10 U/kg i.n.) pulmonary fibrosis+Longidaza intranasally at a dose of 10 U/kg (group 8); (LG 30 U/kg i.n.) pulmonary fibrosis+Longidaza intranasally at a dose of 30 U/kg (group 9); (LG 120 U/kg i.n.) pulmonary fibrosis+Longidaza intranasally at a dose of 120 U/kg (group 10) on d7. Tissues were stained with hematoxylin–eosin. x100.
Injection of Longidaza and the reference drug pirfenidone reduced the intensity of the inflammatory reaction in the lung parenchyma. On d7 intramuscular injection of Longidaza in doses of 60, 120 U/kg (groups 5 and 6) and intranasal application of this drug in doses of 30, 120 U/kg (groups 9 and 10) showed a moderate anti-inflammatory effect similar to that of Pirfenidone. The severity of hyperemia of the microvascular bed decreased, the prevalence of inflammatory infiltration of the lung parenchyma, the number of macrophages in the alveoli decreased, inflammatory infiltrates remained mainly peribronchial and perivascular (
Figure 3). Intranasal administration of Longidaza at a minimum dose of 10 U/kg (group 8) did not affect the intensity of the inflammatory response. The most pronounced anti-inflammatory effect was observed with the intramuscular administration of Longidaza at a dose of 1200 U/kg on d7 (group 7). In this group of animals, a significant decrease in the intensity of inflammatory infiltration of the lung parenchyma by lymphocytes, plasmocytes and macrophages and a significant decrease in the cellularity of peribronchial and perivascular infiltrates were observed. Isolated macrophages were observed in the alveolar lumen. There was local hyperemia, small vessels were filled with blood corresponding to intact animals.
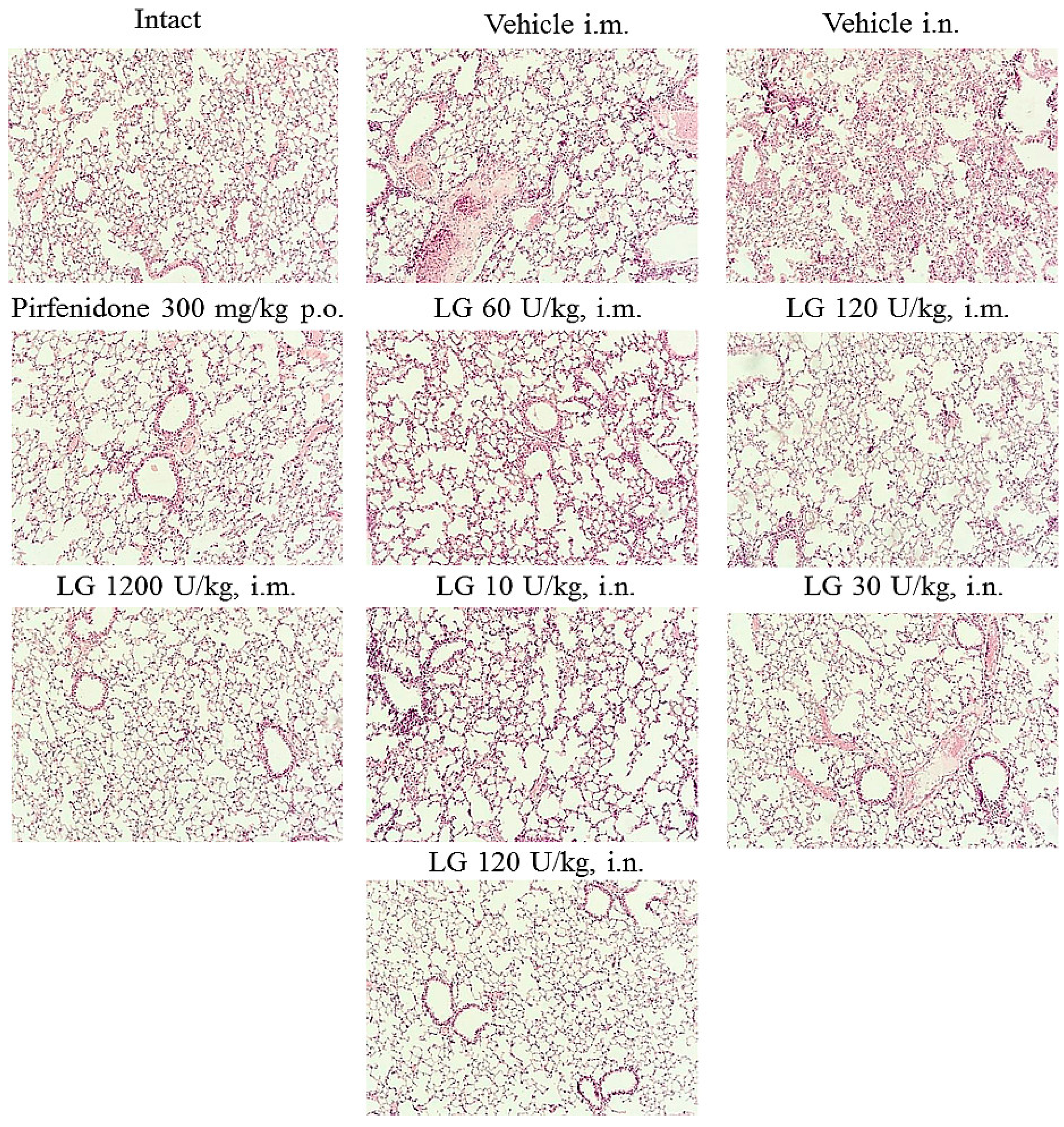
On d21 after bleomycin instillation, there was an increase in inflammatory infiltration, mainly by lymphocytes and fibroblasts, resulting in thickening of the alveolar septa (
Figure 4). Most of the alveoli were filled with macrophages. There were areas of parenchyma where the lung pattern was completely absent. An overgrowth of fibrous tissue was observed in the lungs of the mice. Fibrotic foci were localized peribronchially and perivascularly. Extensive collagen deposition was found in the lung parenchyma, with a significant decrease in the airiness of the organ and the formation of a "honeycomb lung". There were areas of emphysematous enlarged alveoli, atelectasis. Morphological changes in the lungs of animals in groups 2 and 3 were of the same type.
Figure 4.
Micrographs of lung sections obtained from male C57BL/6 mice (Intact) mice of intact control (group 1); (Vehicle i.m. and Vehicle i.n.) mice treated by bleomycin (groups 2 and 3); (Pirfenidone 300 mg/kg p.o.) pulmonary fibrosis+Pirfenidone (group 4); (LG 60 U/kg i.m.) pulmonary fibrosis+Longidaza intramuscularly at a dose of 60 U/kg (group 5); (LG 120 U/kg i.m.) pulmonary fibrosis+Longidaza intramuscularly at a dose of 120 U/kg (group 6); (LG 1200 U/kg i.m.) pulmonary fibrosis+Longidaza intramuscularly at a dose of 1200 U/kg (group 7); (LG 10 U/kg i.n.) pulmonary fibrosis+Longidaza intranasally at a dose of 10 U/kg (group 8); (LG 30 U/kg i.n.) pulmonary fibrosis+Longidaza intranasally at a dose of 30 U/kg (group 9); (LG 120 U/kg i.n.) pulmonary fibrosis+Longidaza intranasally at a dose of 120 U/kg (group 10) on d21. Tissues were stained with hematoxylin–eosin. x100.
Figure 4.
Micrographs of lung sections obtained from male C57BL/6 mice (Intact) mice of intact control (group 1); (Vehicle i.m. and Vehicle i.n.) mice treated by bleomycin (groups 2 and 3); (Pirfenidone 300 mg/kg p.o.) pulmonary fibrosis+Pirfenidone (group 4); (LG 60 U/kg i.m.) pulmonary fibrosis+Longidaza intramuscularly at a dose of 60 U/kg (group 5); (LG 120 U/kg i.m.) pulmonary fibrosis+Longidaza intramuscularly at a dose of 120 U/kg (group 6); (LG 1200 U/kg i.m.) pulmonary fibrosis+Longidaza intramuscularly at a dose of 1200 U/kg (group 7); (LG 10 U/kg i.n.) pulmonary fibrosis+Longidaza intranasally at a dose of 10 U/kg (group 8); (LG 30 U/kg i.n.) pulmonary fibrosis+Longidaza intranasally at a dose of 30 U/kg (group 9); (LG 120 U/kg i.n.) pulmonary fibrosis+Longidaza intranasally at a dose of 120 U/kg (group 10) on d21. Tissues were stained with hematoxylin–eosin. x100.
On d21 the most pronounced anti-inflammatory effect was observed in the group of mice treated intramuscularly with Longidaza at a dose of 1200 U/kg (group 7) (
Figure 4). A significant decrease in the number of macrophages, lymphocytes, plasmocytes and fibroblasts was observed in the lung parenchyma, peribronchial and perivascular infiltrates, and there were no inflammatory cells in the alveoli. Lung tissue aeration was significantly higher than in pathological control animals (groups 2 and 3). The anti-inflammatory effect of Longidaza was somewhat weaker in the groups of animals that received the drug intramuscularly and intranasally at the dose of 120 U/kg (groups 6 and 10); the intensity of the inflammatory infiltrates decreased in these groups individual macrophages were found in the alveoli. The nature of the inflammatory response of the lung parenchyma in other groups of mice treated with Longidaza did not differ from the similar parameter in the model groups (
Figure 4).
The most effective antifibrotic effect of Longidaza was demonstrated at a dose of 1200 U/kg (group 7) administered intramuscularly. In this group of mice, the area of fibrous tissue approached that of intact animals. A pronounced antifibrotic effect of Longidaza was observed at a dose of 120 U/kg (group 6) administered intramuscularly. The area of fibrous tissue in the lungs of mice in this group decreased compared to the model animals, and focal collagen deposition was noted, mainly around the large bronchi. In groups of mice receiving Longidaza intramuscularly at a dose of 60 U/kg (group 5) and intranasally at doses of 10, 30 and 120 U/kg (groups 8-10), the antifibrotic effect of the corrector was less pronounced and close to that of the reference drug pirfenidone (group 4).
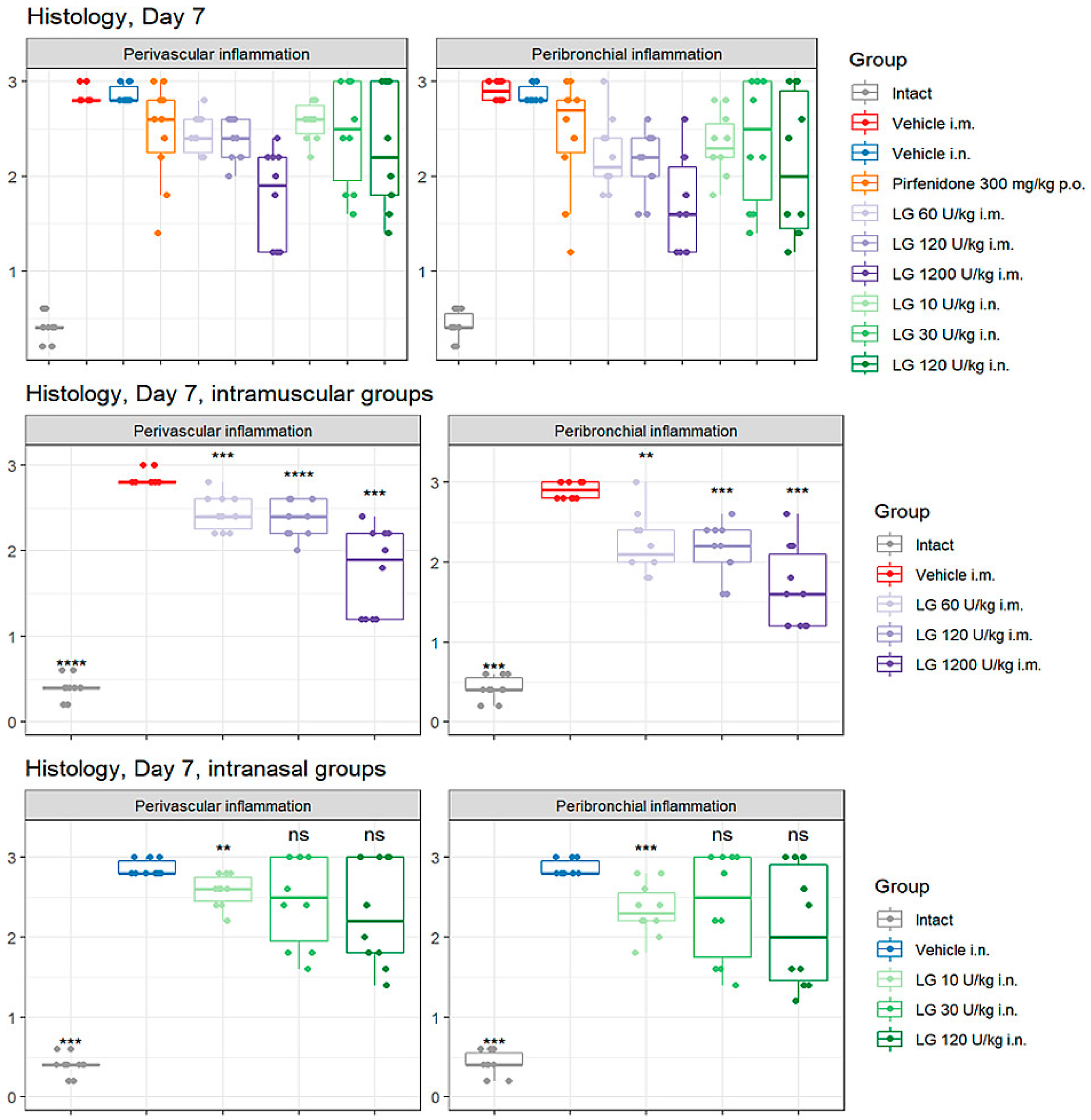
3.5. Effects of Longidaza on Perivascular and Peribronchial Inflammation in the Lungs
On the d7 after bleomycin instillation, an accumulation of inflammatory cells was observed in the peribronchial and perivascular areas of the lung tissue in animals of groups 2 and 3 (
Figure 5). Pirfenidone (group 4) had little effect on the severity of the inflammatory response in the lungs of mice during these experimental periods. Intramuscular administration of Longidaza (groups 5-7) caused a decrease in the recruitment of inflammatory cells to the lung tissue compared to group 2, with the effect becoming greater as the dose of the drug increased. The most pronounced effect was observed with intramuscular injection of Longidaza at a dose of 1200 U/kg (group 7) (
Figure 5). The effect of the drug Longidaza with intranasal administration (groups 8-10) was weakly expressed during these periods of the experiment.
On the d21 after bleomycin instillation in the lungs of mice of groups 2 and 3, a pronounced inflammatory reaction was still observed in the peribronchial and perivascular spaces of the respiratory tract. With the injection of the reference drug pirfenidone (group 4) on the d21, the level of perivascular and peribronchial inflammation decreased compared to groups 2 and 3 and was significantly higher than in intact animals. The effect of intramuscularly administered Longidaza at a dose of 120 U/kg (group 6) was at the level of the reference drug, and at a dose of 1200 U/kg (group 7) was significantly higher than the effect of pirfenidone. Intranasal administration of Longidaza contributed to a decrease in the accumulation of inflammatory cells in the peribronchial and perivascular space of the respiratory tract only at a dose of 120 U/kg (group 10), while the efficacy was comparable to that of the reference drug pirfenidone.
Figure 5.
Effects of Longidaza on Perivascular and Peribronchial Inflammation in the Lungs of C57BL/6 mice on d7. Groups: (Intact) mice of intact control (group 1); (Vehicle i.m. and Vehicle i.n.) mice treated by bleomycin (groups 2 and 3); (Pirfenidone 300 mg/kg p.o.) mice with pulmonary fibrosis+Pirfenidone (group 4); (LG 60 U/kg i.m.) mice with pulmonary fibrosis+Longidaza intramuscularly at a dose of 60 U/kg (group 5); (LG 120 U/kg i.m.) mice with pulmonary fibrosis+Longidaza intramuscularly at a dose of 120 U/kg (group 6); (LG 1200 U/kg i.m.) mice with pulmonary fibrosis+Longidaza intramuscularly at a dose of 1200 U/kg (group 7); (LG 10 U/kg i.n.) mice with pulmonary fibrosis+Longidaza intranasally at a dose of 10 U/kg (group 8); (LG 30 U/kg i.n.) mice with pulmonary fibrosis+Longidaza intranasally at a dose of 30 U/kg (group 9); (LG 120 U/kg i.n.) mice with pulmonary fibrosis+Longidaza intranasally at a dose of 120 U/kg (group 10). Statistical analysis of data was performed using the Kruskal-Wallis test, post-hoc Wilcoxon test for pairwise comparisons with the bleomycin control group (with appropriate route of administration), Bonferroni correction (ns - p>0.05, * p<0.05, **p <0.01, *** p<0.001, **** p< 0.0001).
Figure 5.
Effects of Longidaza on Perivascular and Peribronchial Inflammation in the Lungs of C57BL/6 mice on d7. Groups: (Intact) mice of intact control (group 1); (Vehicle i.m. and Vehicle i.n.) mice treated by bleomycin (groups 2 and 3); (Pirfenidone 300 mg/kg p.o.) mice with pulmonary fibrosis+Pirfenidone (group 4); (LG 60 U/kg i.m.) mice with pulmonary fibrosis+Longidaza intramuscularly at a dose of 60 U/kg (group 5); (LG 120 U/kg i.m.) mice with pulmonary fibrosis+Longidaza intramuscularly at a dose of 120 U/kg (group 6); (LG 1200 U/kg i.m.) mice with pulmonary fibrosis+Longidaza intramuscularly at a dose of 1200 U/kg (group 7); (LG 10 U/kg i.n.) mice with pulmonary fibrosis+Longidaza intranasally at a dose of 10 U/kg (group 8); (LG 30 U/kg i.n.) mice with pulmonary fibrosis+Longidaza intranasally at a dose of 30 U/kg (group 9); (LG 120 U/kg i.n.) mice with pulmonary fibrosis+Longidaza intranasally at a dose of 120 U/kg (group 10). Statistical analysis of data was performed using the Kruskal-Wallis test, post-hoc Wilcoxon test for pairwise comparisons with the bleomycin control group (with appropriate route of administration), Bonferroni correction (ns - p>0.05, * p<0.05, **p <0.01, *** p<0.001, **** p< 0.0001).
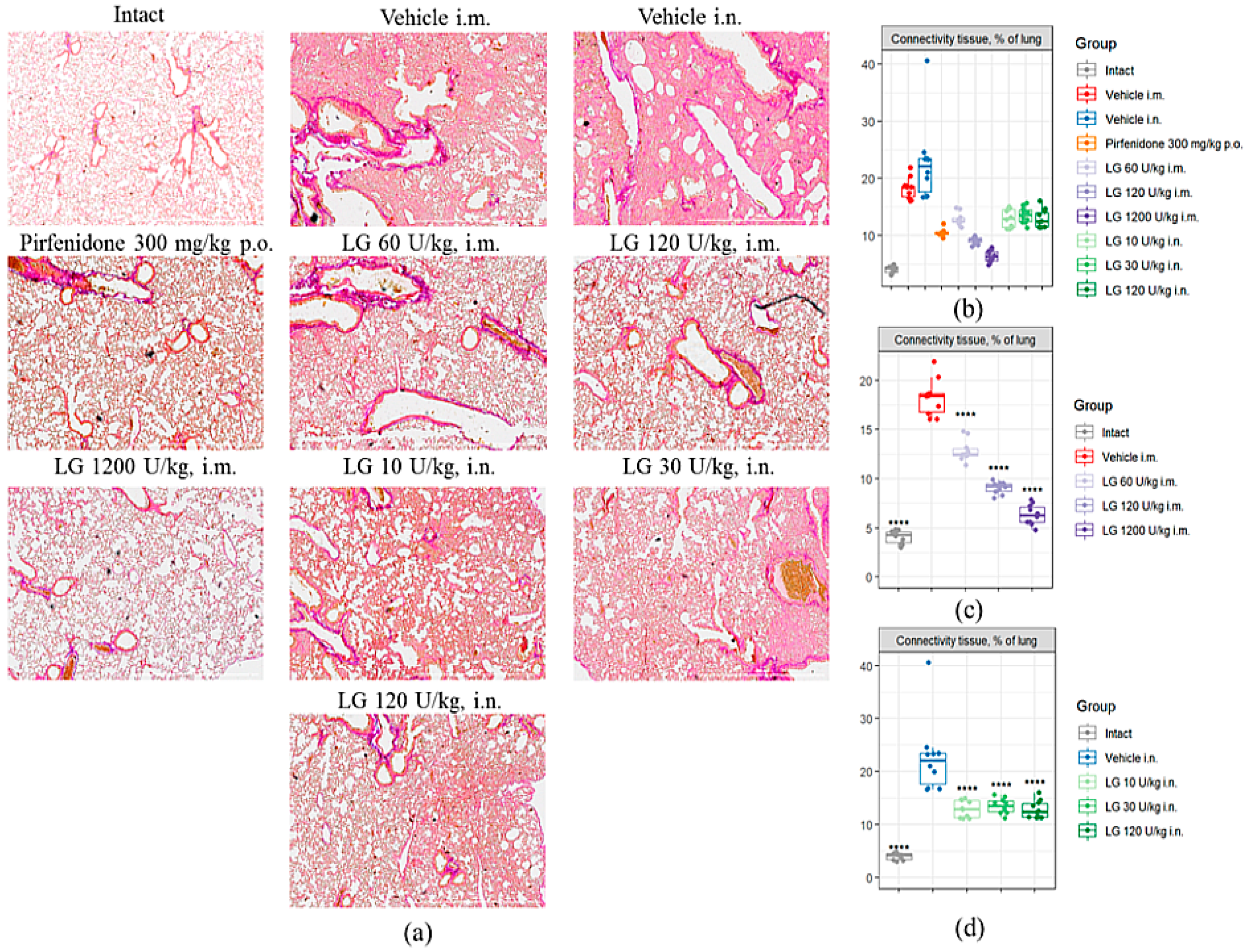
3.6. Effects of Longidaza on Pulmonary Connective Tissue Content
Fibrosis modeling caused a significant increase in the area of connective tissue in the lungs of mice in the pathological control groups by d21 (
Figure 6a,b). Injection of Pirfenidone (group 4) resulted in a twofold decrease in the area of connective tissue in the lungs compared to groups 2 and 3. Treatment of pulmonary fibrosis with Longidaza at doses of 10 U/kg, 30 U/kg and 120 U/kg (groups 8-10) by intranasal and intramuscular administration at a dose of 60 U/kg (group 5) produced an effect comparable to Pirfenidone: the connective tissue area was significantly lower than the corresponding pathological control, while the indicator remained 23-29% higher than in group 4 (
Figure 6bd). Longidaza at doses of 120 U/kg (group 6) and 1200 U/kg (group 7) showed the most pronounced antifibrotic effect when administered intramuscularly. The area of connective tissue in the lungs of animals treated with Longidaza at a dose of 120 U/kg was 50% lower than the corresponding values in group 2 and 13% lower than the reference drug group (group 4) (
Figure 6b). In the treatment of pulmonary fibrosis with Longidaza at a dose of 1200 U/kg (Group 7), the area of connective tissue in the lungs decreased by 65% compared to Group 2 and by 39% compared to the group of animals treated with the reference drug Pirfenidone (
Figure 6c).
The highest therapeutic efficacy was observed with intramuscular administration of Longidaza at doses of 120 and 1200 U/kg (groups 6 and 7). Intranasally administered Longidaza showed anti-inflammatory and antifibrotic properties at a dose of 120 U/kg (group 10).
Figure 6.
Effects of Longidaza on the Content of Connective Tissue in the Lungs of C57BL/6 Mice on d21. (a) Photomicrographs of left lung sections (middle pulmonary field) obtained from male C57BL/6 mice on d21. Tissues stained by Van Gieson, scale bar 100μm. (b) Content of the connective tissue in the lungs of C57BL/6 mice from all groups; (c) Content of the connective tissue in the lungs of C57BL/6 mice from groups after intramuscularly administration; (d) Content of the connective tissue in the lungs of C57BL/6 mice from groups after intranasal administration. Groups: (Intact) mice of intact control (group 1); (Vehicle i.m. and Vehicle i.n.) mice treated by bleomycin (groups 2 and 3); (Pirfenidone 300 mg/kg p.o.) mice with pulmonary fibrosis+Pirfenidone (group 4); (LG 60 U/kg i.m.) mice with pulmonary fibrosis+Longidaza intramuscularly at a dose of 60 U/kg (group 5); (LG 120 U/kg i.m.) mice with pulmonary fibrosis+Longidaza intramuscularly at a dose of 120 U/kg (group 6); (LG 1200 U/kg i.m.) mice with pulmonary fibrosis+Longidaza intramuscularly at a dose of 1200 U/kg (group 7); (LG 10 U/kg i.n.) mice with pulmonary fibrosis+Longidaza intranasally at a dose of 10 U/kg (group 8); (LG 30 U/kg i.n.) mice with pulmonary fibrosis+Longidaza intranasally at a dose of 30 U/kg (group 9); (LG 120 U/kg i.n.) mice with pulmonary fibrosis+Longidaza intranasally at a dose of 120 U/kg (group 10). Statistical analysis of data was performed using the Kruskal-Wallis test, post-hoc Wilcoxon test for pairwise comparisons with the bleomycin control group (with appropriate route of administration), Bonferroni correction (ns - p>0.05, * p<0.05, **p <0.01, *** p<0.001, **** p< 0.0001).
Figure 6.
Effects of Longidaza on the Content of Connective Tissue in the Lungs of C57BL/6 Mice on d21. (a) Photomicrographs of left lung sections (middle pulmonary field) obtained from male C57BL/6 mice on d21. Tissues stained by Van Gieson, scale bar 100μm. (b) Content of the connective tissue in the lungs of C57BL/6 mice from all groups; (c) Content of the connective tissue in the lungs of C57BL/6 mice from groups after intramuscularly administration; (d) Content of the connective tissue in the lungs of C57BL/6 mice from groups after intranasal administration. Groups: (Intact) mice of intact control (group 1); (Vehicle i.m. and Vehicle i.n.) mice treated by bleomycin (groups 2 and 3); (Pirfenidone 300 mg/kg p.o.) mice with pulmonary fibrosis+Pirfenidone (group 4); (LG 60 U/kg i.m.) mice with pulmonary fibrosis+Longidaza intramuscularly at a dose of 60 U/kg (group 5); (LG 120 U/kg i.m.) mice with pulmonary fibrosis+Longidaza intramuscularly at a dose of 120 U/kg (group 6); (LG 1200 U/kg i.m.) mice with pulmonary fibrosis+Longidaza intramuscularly at a dose of 1200 U/kg (group 7); (LG 10 U/kg i.n.) mice with pulmonary fibrosis+Longidaza intranasally at a dose of 10 U/kg (group 8); (LG 30 U/kg i.n.) mice with pulmonary fibrosis+Longidaza intranasally at a dose of 30 U/kg (group 9); (LG 120 U/kg i.n.) mice with pulmonary fibrosis+Longidaza intranasally at a dose of 120 U/kg (group 10). Statistical analysis of data was performed using the Kruskal-Wallis test, post-hoc Wilcoxon test for pairwise comparisons with the bleomycin control group (with appropriate route of administration), Bonferroni correction (ns - p>0.05, * p<0.05, **p <0.01, *** p<0.001, **** p< 0.0001).
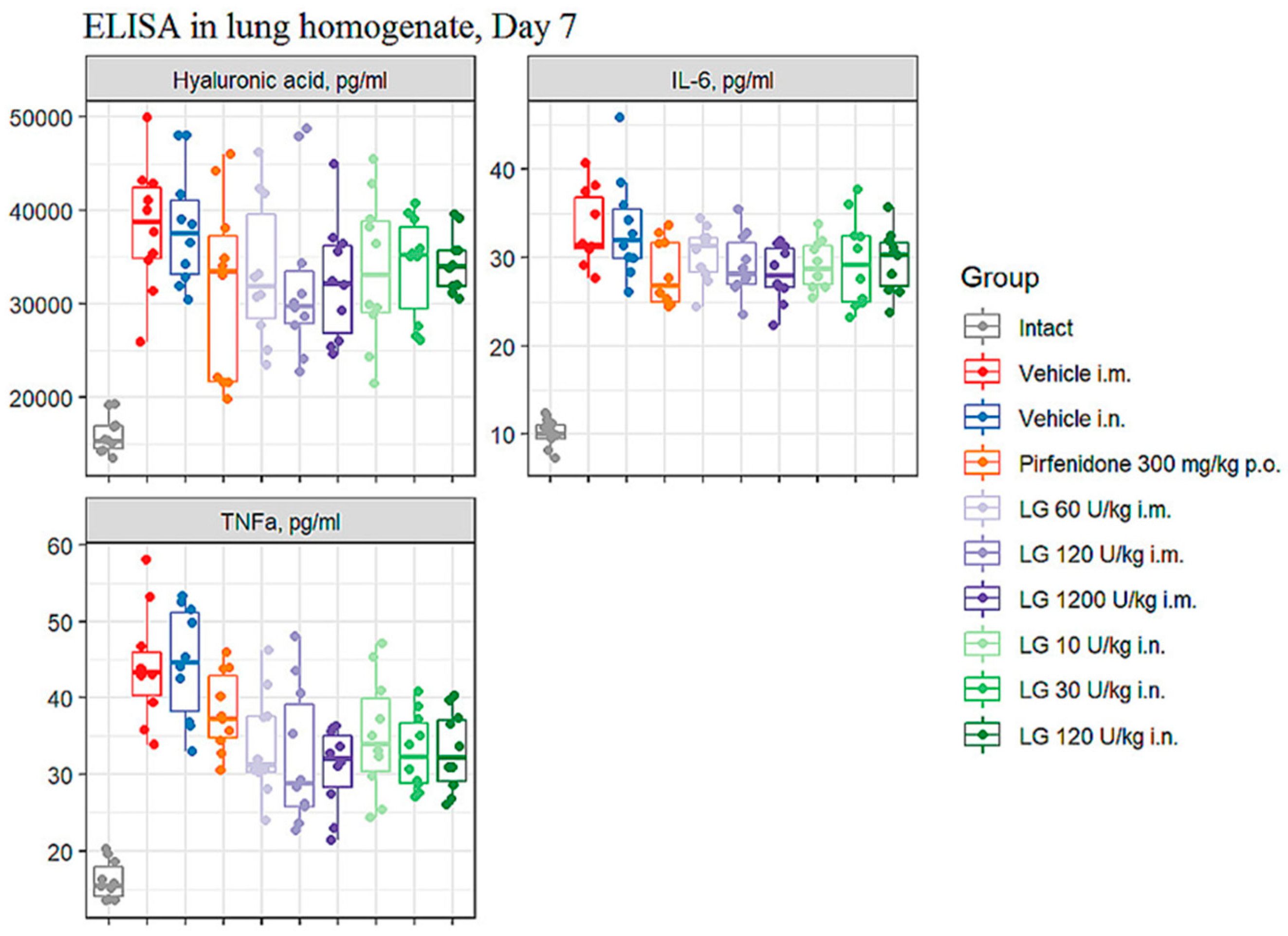
3.7. Enzyme-linked Immunosorbent Assay
3.7.1. Levels of IL-6, TNF-α, and hyaluronic acid in bleomycin-induced lung injury
On the d7 after bleomycin instillation in the lungs of mice of both groups of pathological control (groups 2 and 3), an increase in the level of IL-6, TNF-α and hyaluronic acid was observed in comparison with intact mice (
Figure 7). Intramuscular administration of Longidaza at doses of 120 U/kg (group 6) and 1200 U/kg (group 7) contributed to a decrease in the level of IL-6, TNF-α and HA in the lung tissue of mice on the d7 compared to animals from group 2 (
Figure 7). It should be noted that the effect of these doses of Longidaza on the level of IL-6 and HA was comparable to the effect of the reference drug pirfenidone, while Pirfenidone did not affect the level of TNF-α in the lung tissue on the d7. The level of TNF-α in the lung tissue on the d7 was positively influenced by the drug Longidaza at an inhalation dose of 30 U/kg (group 9). Notably, the inhalation use of Longidaza at all doses studied (groups 8-10) decreased the level of IL-6 and practically did not change the HA level (
Figure 7).
Figure 7.
Effects of Longidaza treatment on the IL-6, TNF-α, and hyaluronic acid levels in homogenate of right lung lobes received from male C57BL/6 mice (d7). Groups: (Intact) mice of intact control (group 1); (Vehicle i.m. and Vehicle i.n.) mice treated by bleomycin (groups 2 and 3); (Pirfenidone 300 mg/kg p.o.) mice with pulmonary fibrosis+Pirfenidone (group 4); (LG 60 U/kg i.m.) mice with pulmonary fibrosis+Longidaza intramuscularly at a dose of 60 U/kg (group 5); (LG 120 U/kg i.m.) mice with pulmonary fibrosis+Longidaza intramuscularly at a dose of 120 U/kg (group 6); (LG 1200 U/kg i.m.) mice with pulmonary fibrosis+Longidaza intramuscularly at a dose of 1200 U/kg (group 7); (LG 10 U/kg i.n.) mice with pulmonary fibrosis+Longidaza intranasally at a dose of 10 U/kg (group 8); (LG 30 U/kg i.n.) mice with pulmonary fibrosis+Longidaza intranasally at a dose of 30 U/kg (group 9); (LG 120 U/kg i.n.) mice with pulmonary fibrosis+Longidaza intranasally at a dose of 120 U/kg (group 10). Statistical analysis of data was performed using the Kruskal-Wallis test, post-hoc Wilcoxon test for pairwise comparisons with the bleomycin control group (with appropriate route of administration), Bonferroni correction (ns - p>0.05, * p<0.05, **p <0.01, *** p<0.001, **** p< 0.0001).
Figure 7.
Effects of Longidaza treatment on the IL-6, TNF-α, and hyaluronic acid levels in homogenate of right lung lobes received from male C57BL/6 mice (d7). Groups: (Intact) mice of intact control (group 1); (Vehicle i.m. and Vehicle i.n.) mice treated by bleomycin (groups 2 and 3); (Pirfenidone 300 mg/kg p.o.) mice with pulmonary fibrosis+Pirfenidone (group 4); (LG 60 U/kg i.m.) mice with pulmonary fibrosis+Longidaza intramuscularly at a dose of 60 U/kg (group 5); (LG 120 U/kg i.m.) mice with pulmonary fibrosis+Longidaza intramuscularly at a dose of 120 U/kg (group 6); (LG 1200 U/kg i.m.) mice with pulmonary fibrosis+Longidaza intramuscularly at a dose of 1200 U/kg (group 7); (LG 10 U/kg i.n.) mice with pulmonary fibrosis+Longidaza intranasally at a dose of 10 U/kg (group 8); (LG 30 U/kg i.n.) mice with pulmonary fibrosis+Longidaza intranasally at a dose of 30 U/kg (group 9); (LG 120 U/kg i.n.) mice with pulmonary fibrosis+Longidaza intranasally at a dose of 120 U/kg (group 10). Statistical analysis of data was performed using the Kruskal-Wallis test, post-hoc Wilcoxon test for pairwise comparisons with the bleomycin control group (with appropriate route of administration), Bonferroni correction (ns - p>0.05, * p<0.05, **p <0.01, *** p<0.001, **** p< 0.0001).
3.7.2. The concentration of TGF-β1 and hyaluronic acid in bleomycin-induced lung damage on d21
It was found that the modeling of pulmonary fibrosis on d21 led to a significant increase in the levels of HA and TGF-β1 in the lung homogenate obtained from mice of both pathological control groups (
Figure 8). Treatment of animals with Pirfenidone resulted in a decrease in the levels of HA and TGF-β1 in lung homogenate compared to those in groups 2 and 3 (
Figure 8). Treatment with Longidaza at all doses and routes of administration decreased HA and TGF-β1 levels to varying degrees. The most pronounced decrease was observed with intramuscular injection of Longidaza at a dose of 1200 U/kg (Group 7) (
Figure 8).
Figure 8.
Effects of Longidaza treatment on the hyaluronic acid, Collagen I, hydroxyproline, and TGF-β1 levels in homogenate of right lung lobes received from male C57BL/6 mice (d21). Groups: (Intact) mice of intact control (group 1); (Vehicle i.m. and Vehicle i.n.) mice treated by bleomycin (groups 2 and 3); (Pirfenidone 300 mg/kg p.o.) mice with pulmonary fibrosis+Pirfenidone (group 4); (LG 60 U/kg i.m.) mice with pulmonary fibrosis+Longidaza intramuscularly at a dose of 60 U/kg (group 5); (LG 120 U/kg i.m.) mice with pulmonary fibrosis+Longidaza intramuscularly at a dose of 120 U/kg (group 6); (LG 1200 U/kg i.m.) mice with pulmonary fibrosis+Longidaza intramuscularly at a dose of 1200 U/kg (group 7); (LG 10 U/kg i.n.) mice with pulmonary fibrosis+Longidaza intranasally at a dose of 10 U/kg (group 8); (LG 30 U/kg i.n.) mice with pulmonary fibrosis+Longidaza intranasally at a dose of 30 U/kg (group 9); (LG 120 U/kg i.n.) mice with pulmonary fibrosis+Longidaza intranasally at a dose of 120 U/kg (group 10). Statistical analysis of data was performed using the Kruskal-Wallis test, post-hoc Wilcoxon test for pairwise comparisons with the bleomycin control group (with appropriate route of administration), Bonferroni correction (ns - p>0.05, * p<0.05, **p <0.01, *** p<0.001, **** p< 0.0001).
Figure 8.
Effects of Longidaza treatment on the hyaluronic acid, Collagen I, hydroxyproline, and TGF-β1 levels in homogenate of right lung lobes received from male C57BL/6 mice (d21). Groups: (Intact) mice of intact control (group 1); (Vehicle i.m. and Vehicle i.n.) mice treated by bleomycin (groups 2 and 3); (Pirfenidone 300 mg/kg p.o.) mice with pulmonary fibrosis+Pirfenidone (group 4); (LG 60 U/kg i.m.) mice with pulmonary fibrosis+Longidaza intramuscularly at a dose of 60 U/kg (group 5); (LG 120 U/kg i.m.) mice with pulmonary fibrosis+Longidaza intramuscularly at a dose of 120 U/kg (group 6); (LG 1200 U/kg i.m.) mice with pulmonary fibrosis+Longidaza intramuscularly at a dose of 1200 U/kg (group 7); (LG 10 U/kg i.n.) mice with pulmonary fibrosis+Longidaza intranasally at a dose of 10 U/kg (group 8); (LG 30 U/kg i.n.) mice with pulmonary fibrosis+Longidaza intranasally at a dose of 30 U/kg (group 9); (LG 120 U/kg i.n.) mice with pulmonary fibrosis+Longidaza intranasally at a dose of 120 U/kg (group 10). Statistical analysis of data was performed using the Kruskal-Wallis test, post-hoc Wilcoxon test for pairwise comparisons with the bleomycin control group (with appropriate route of administration), Bonferroni correction (ns - p>0.05, * p<0.05, **p <0.01, *** p<0.001, **** p< 0.0001).
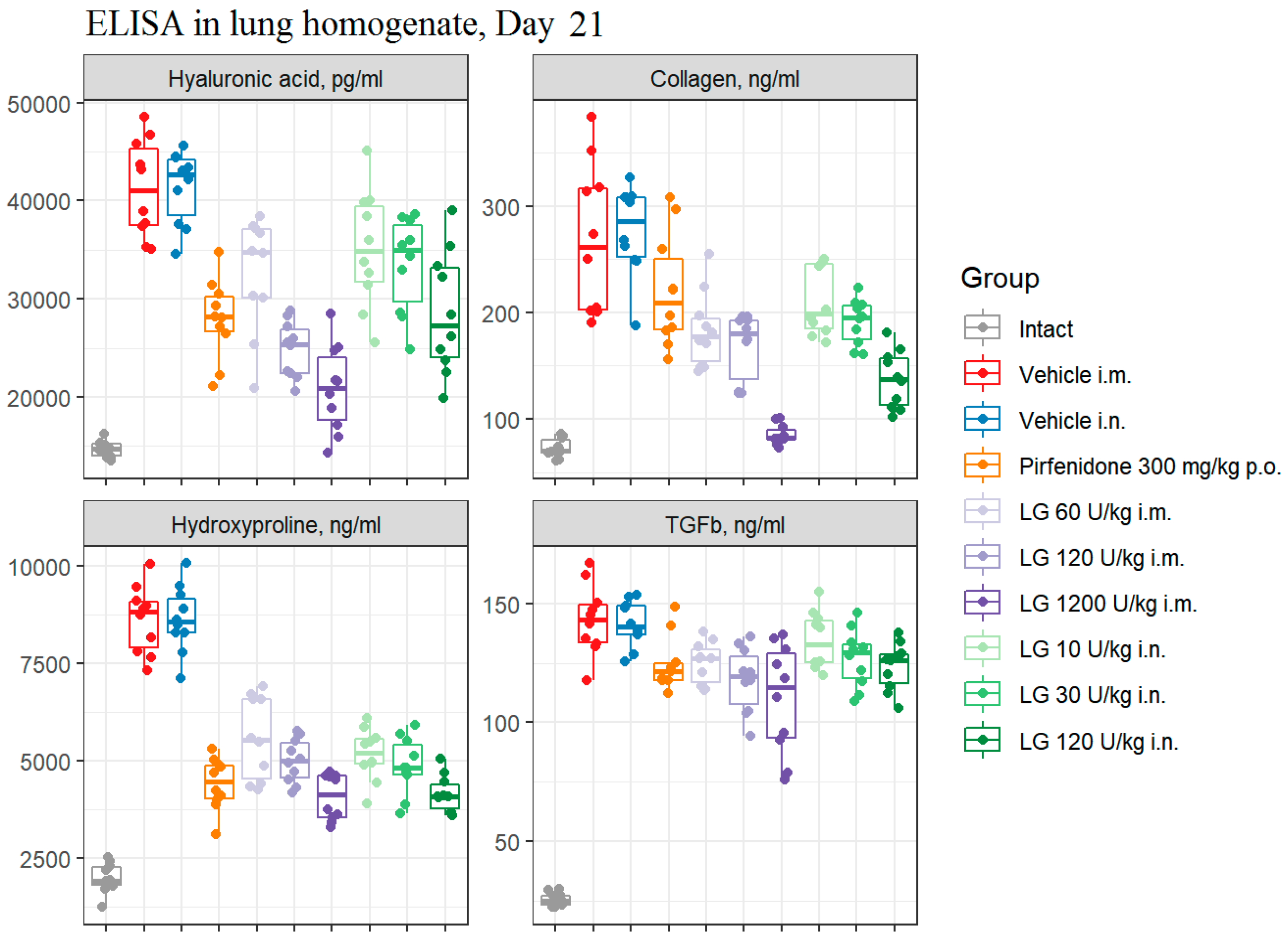
3.7.3. The collagen I and hydroxyproline concentration in bleomycin-induced lung damage on d21
Modeling pulmonary fibrosis caused a significant increase in the levels of fibrosis markers - collagen I (3.7-3.8 fold) and hydroxyproline (4.3 fold) in the lung homogenate on d21 in animals of groups 2 and 3 (
Figure 8). The use of the Pirfenidone (group 4) resulted in a 2-fold and 1.2-fold decrease in the concentration of hydroxyproline and collagen I, respectively, in the lung homogenate of mice with pulmonary fibrosis (
Figure 8). The concentration of hydroxyproline (2-fold) and collagen I (3-fold) in lung homogenate was most effectively reduced by Longidaza administered intramuscularly at a dose of 1200 U/kg (group 7) (
Figure 8). The effect was weaker at a dose of 120 U/kg with intranasal (group 10) and intramuscular (group 6) administration, but the efficacy of Longidaza even at this dose exceeded that of Pirfenidone (
Figure 8). Thus, the use of Longidaza at all doses and routes of administration studied reduced the levels of hydroxyproline and collagen I in lung homogenate compared to untreated mice with pulmonary fibrosis (
Figure 8). The most pronounced effect, exceeding that of the reference drug pirfenidone, was observed in groups of mice treated with Longidaza intramuscularly at a dose of 1200 U/kg (group 7) and intranasally at a dose of 120 U/kg (group 10) (
Figure 8).
4. Discussion
IPF is a chronic progressive disease with the development of interstitial fibrosis and progressive respiratory failure [
2,
29]. In most cases, the prognosis of IPF is unfavorable, with an average life expectancy of about five years from diagnosis [
5]. Current therapies, nintedanib and pirfenidone, slow progression by preserving lung function. However, an adequate drug treatment that would stop the progression of the disease has not been discovered, which requires the development of new effective antifibrotic agents.
Previously in preclinical studies on rabbits, it was shown that maximum concentration of Longidaza in blood serum after intramuscular injection is reached after 15 minutes (
Figure S1). It was found that
3H-Longidaza after intravenously and intramuscularly administration to rabbits is well distributed in the body, the steady-state volume of distribution was 3.43 and 3.58 L/kg, respectively. The semi-elimination period of 3H-Longidaza after intravenously and intramuscularly administration was 32.6 and 32.7 hours respectively. Bioavailability of Longidaza was about 96% after intramuscularly administration. In the study of metabolism it was found that Longidaza at a concentration up to 10 µM does not inhibit the isoenzymes 1A2, 2C9, 2C19, 2D6, 2C8 and 3A4 of human cytochrome P-450. The data obtained give grounds for prescribing Longidaza together with other drugs without fear of drug interaction.
In this study, the effect of various doses and routes of administration of Longidaza on the development of bleomycin-induced pulmonary fibrosis in mice was evaluated in comparison to the well-known drug Pirfenidone. However, Pirfenidone has a number of side effects in addition to its antifibrotic activity. Gastrointestinal and skin reactions are the most common adverse events caused by Pirfenidone treatment [
30]. In contrast to Pirfenidone, administration of Longidaza at all doses tested did not adversely affect body weight in mice with pulmonary fibrosis. At the highest intramuscular dose of 1200 U/kg, the drug even contributed to an increase in body weight of mice with pulmonary fibrosis compared to untreated animals.
The early stage of bleomycin-induced lung injury is manifested by acute inflammation, including alveolar epithelial damage, inflammatory cell infiltration, release of inflammatory mediators, and peripheral blood leukocytosis [
31]. On the d7 of the study, Longidaza had anti-inflammatory activity, there was a decrease in the total number of leukocytes in the blood, the level of pro-inflammatory cytokines IL-6 and TNFα, as well as the component of extracellular matrix HA, decreased in the lungs, and peribronchial and perivascular inflammation decreased compared to groups of mice with pulmonary fibrosis without treatment. It should be noted that the effect on the level of various leukocyte populations in the blood was dose dependent after intramuscular administration of Longidaza, no clear dose dependence was observed after intranasal administration. The reference drug pirfenidone also contributed to the reduction of leukocytosis in the peripheral blood of the animals but had little effect on the severity of the inflammatory response in the lungs of the mice on d7 of the experiment.
Summarizing the results obtained on the d7 after bleomycin administration, it can be concluded that the highest anti-inflammatory activity of the drug Longidaza is recorded with intramuscular injection at the dose of 1200 U/kg and the effect is superior to that of Pirfenidone. At the same time, Longidaza does not have the side effects associated with Pirfenidone. The anti-inflammatory effect of Longidaza at low and medium doses (both routes of administration) is comparable to that of the reference drug.
Importantly, uncontrolled lung injury is a hallmark of the initiation and progression of IPF, resulting in the release of proinflammatory and profibrotic cytokines, leading to further fibrosis-associated immune cell influx and ECM remodeling. Our data showed that Longidaza had anti-inflammatory and antifibrotic activity in the study on d21 after bleomycin administration. It prevented the infiltration of inflammatory cells into peribronchial and perivascular spaces, reduced the expression of pro-inflammatory mediators (IL-6, TNF-α, hyaluronic acid) in lung tissue, and also reduced the activity of synthesis and deposition of connective tissue. The most pronounced positive effect on the d21 after bleomycin administration was observed with Longidaza at a dose of 1200 U/kg intramuscularly and 120 U/kg intranasally.
TGF-β is recognized as an important regulator of tissue fibrosis in general, and numerous studies have convincingly confirmed its role in pulmonary fibrosis in particular [
32]. It has been shown that TGF-β is required for the development of bleomycin-induced pulmonary fibrosis, which is a potent stimulator of HA production and promotes the transition of resident fibroblasts to myofibroblasts
in vitro [
33]. The antifibrotic effect of Longidaza in the pulmonary fibrosis model was manifested by a decrease in the area of connective tissue in the lungs of mice and a decrease in the levels of profibrotic mediators (TGF-β1, type 1 collagen, hydroxyproline) in the lung tissue. At the same time, the effect of intramuscular application of Longidaza at doses of 120 U/kg and 1200 U/kg is most pronounced and exceeds that of the reference drug pirfenidone. Thus, the investigated drug Longidaza showed significant anti-inflammatory and antifibrotic activity in the bleomycin-induced model of pulmonary fibrosis. The highest therapeutic efficacy is observed with the use of Longidaza at doses of 120 and 1200 U/kg intramuscularly, which is superior to that of the reference drug pirfenidone. It should be noted that Longidaza does not have the side effects associated with Pirfenidone.
5. Conclusions
The data presented in this study indicate that Longidaza is a new promising drug for the treatment of IPF.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Figure S1: Graph of the Longidaza concentration (ng/ml) versus time after intramuscular injection.
Author Contributions
“Conceptualization, S.A. and A.P.; methodology, A.P. and O.P.; software, E.P.; validation, A.P. and O.P.; formal analysis, S.A.; investigation, A.P., O.P., N.E., E.P. and M.Z.; data curation, A.P. and O.P.; writing—original draft preparation, A.P., O.P. and L.K.; writing—review and editing, A.P., O.P., P.B., W-D.G. and S.A.; visualization, N.E., L.S. and E.P.; supervision, S.A.; project administration, Y.D. All authors have read and agreed to the published version of the manuscript.”.
Funding
“This research received no external funding”.
Institutional Review Board Statement
“The animal study protocol was approved by the Institutional Ethics Committee of GOLDBERG ED RESEARCH INSTITUTE OF PHARMACOLOGY AND REGENERATIVE MEDICINE (protocol code 204092022, 27.09.2022).”.
Conflicts of Interest
“The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.”.
References
- Bowman, W.S.; Echt, G.A.; Oldham, J.M. Biomarkers in Progressive Fibrosing Interstitial Lung Disease: Optimizing Diagnosis, Prognosis, and Treatment Response. Front Med (Lausanne). 2021, 10, p.8:680997. https://doi.org/10.3389/fmed.2021.680997. [CrossRef]
- Abdelhady, R.; Cavalu, S.; Saber, S.; Elmowafy, R.; Morsy, N.E.; Ibrahim, S.; Abdeldaiem, M.S.I.; Samy, M.; Abd-Eldayem, M.A.; Shata, A.; Elgharabawy, R.M. Mirtazapine, an atypical antidepressant, mitigates lung fibrosis by suppressing NLPR3 inflammasome and fibrosis-related mediators in endotracheal bleomycin rat model. Biomed Pharmacother. 2023,161, 114553. https://doi.org/10.1016/j.biopha.2023.114553. [CrossRef]
- Shukla, A.K.; Misra, S. An overview of post COVID sequelae. J Basic Clin Physiol Pharmacol. 2022, 15, 33(6), 715-726. https://doi.org/10.1515/jbcpp-2022-0057. [CrossRef]
- Zohny, M.H.; Cavalu, S.; Youssef, M.E.; Kaddah, M.M.Y.; Mourad, A.A.E. et al. Coomassie brilliant blue G-250 dye attenuates bleomycin-induced lung fibrosis by regulating the NF-κB and NLRP3 crosstalk: A novel approach for filling an unmet medical need. Biomed. Pharmacother. 2022, 148, 112723. https://doi.org/10.1016/j.biopha.2022.112723. [CrossRef]
- Liu, J.; Gao, D.; Ding, Q.; Zhang, B.; Zhu, W.; Shi, Y. Sparganii Rhizoma alleviates pulmonary fibrosis by inhibiting fibroblasts differentiation and epithelial-mesenchymal transition mediated by TGF-β1/ Smad2/3 pathway. Journal of Ethnopharmacology. 2023, 309, 116305. https://doi.org/10.1016/j.jep.2023.116305. [CrossRef]
- Xia, J.; Xiong, Z.; Guo, J.; Wang, Y.; Luo, Y. et al. Study of paraquat-induced pulmonary fibrosis using biomimetic micro-lung chips. Biofabrication 2022, 7,15(1). https://doi.org/10.1088/1758-5090/ac999e. [CrossRef]
- Wynn, T.A. Integrating mechanisms of pulmonary fibrosis. J. Exp. Med. 2011, 208 (7), 1339-1350.
- Ye, Z.; Hu, Y. TGF-β1: gentlemanly orchestrator in idiopathic pulmonary fibrosis (Review). Int. J. Mol. Med. 2021, 48 (1), 132. https://doi.org/10.3892/ijmm.2021.4965. [CrossRef]
- Gamad, N.; Malik, S.; Suchal. K.; Vasisht, S.; Tomar, A.; Arava, S.; Arya, D.S.; Bhatia, J. Metformin alleviates bleomycin-induced pulmonary fibrosis in rats: Pharmacological effects and molecular mechanisms. Biomed Pharmacother. 2018, 97, 1544-1553. https://doi.org/10.1016/j.biopha.2017.11.101. [CrossRef]
- Qi, F.; Lv. Z.D.; Huang, W.D.; Wei, S.C.; Liu, X.M.; Song, W.D. LncRNA TUG1 promotes pulmonary fibrosis progression via up-regulating CDC27 and activating PI3K/Akt/mTOR pathway. Epigenetics 2023, 18(1), 2195305. https://doi.org/10.1080/15592294.2023.2195305. [CrossRef]
- Pandolfi, L.; Frangipane, V.; Bocca, C.; Marengo, A.; Tarro Genta, E.; Bozzini, S. et al. Hyaluronic Acid-Decorated Liposomes as Innovative Targeted Delivery System for Lung Fibrotic Cells. Molecules 2019, 10, 24(18), 3291. https://doi.org/10.3390/molecules24183291. [CrossRef]
- Spataro, S.; Guerra, C.; Cavalli, A.; Sgrignani, J.; Sleeman, J. et al. CEMIP (HYBID, KIAA1199): structure, function and expression in health and disease. FEBS J. 2022, 23. https://doi.org/10.1111/febs.16600. [CrossRef]
- Noble, P.W.; Jiang D. Matrix Regulation of Lung Injury, Inflammation, and Repair. The Role of Innate Immunity. Proc Am Thorac Soc. 2006, 3, pp. 401–404.
- Kemparaju, K.; Girish, K.S. Snake venom hyaluronidase: a therapeutic target. Cell Biochem. Funct. 2006, 24, pp. 7-12.
- Skurikhin, E.; Madonov, P.; Pershina, O.; Ermakova, N.; Pakhomova, A. at al. Micellar Hyaluronidase and Spiperone as a Potential Treatment for Pulmonary Fibrosis. Int J Mol Sci. 2021, 25,22(11):5599. https://doi.org/10.3390/ijms22115599. [CrossRef]
- Sgalla, G.; Franciosa, C.; Simonetti, J.; Richeldi, L. Pamrevlumab for the treatment of idiopathic pulmonary fibrosis. Expert Opin Investig Drugs. 2020, 29(8), pp.771-777. https://doi.org/10.1080/13543784.2020.1773790. [CrossRef]
- Kolb, P.; Upagupta, C.; Vierhout, M.; Ayaub, E.; Bellaye, P.S. et al. The importance of interventional timing in the bleomycin model of pulmonary fibrosis. Eur Respir J. 2020, 11;55(6), 1901105. https://doi.org/10.1183/13993003.01105-2019. [CrossRef]
- Abidi, A.; Robbe, A.; Kourda, N.; Ben Khamsa, S.; Legrand, A. Nigella sativa, a traditional Tunisian herbal medicine, attenuates bleomycin-induced pulmonary fibrosis in a rat model. Biomed Pharmacother. 2017, 90, pp.626-637. https://doi.org/10.1016/j.biopha.2017.04.009. [CrossRef]
- Jenkins, R.G.; Moore, B.B.; Chambers, R.C., et al. An official American Thoracic Society workshop report: use of animal models for the preclinical assessment of potential therapies for pulmonary fibrosis. Am J Respir Cell Mol Biol. 2017, 56, pp. 667–679.
- Degryse, A.L.; Tanjore, H.; Xu, X.C., et al. Repetitive intratracheal bleomycin models several features of idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2010, 299, L442–L452. https://doi.org/10.1152/ajplung.00026.2010. [CrossRef]
- Shenderov, K.; Collins, S.L.; Powell, J.D.; Horton, M.R. Immune dysregulation as a driver of idiopathic pulmonary fibrosis. J Clin Invest. 2021, 131(2),e143226. https://doi.org/10.1172/JCI143226. [CrossRef]
- Bitencourt, C.S.; Pereira, P.A.; Ramos, S.G.; Sampaio, S.V.; Arantes, E.C.; Aronoff, D.M.; Faccioli L.H. Hyaluronidase recruits mesenchymal-like cells to the lung and ameliorates fibrosis. Fibrogenesis Tissue Repair. 2011, 4, 3. https://doi.org/10.1186/1755-1536-4-3. [CrossRef]
- Thompson C.B., Shepard M.H., O’Connor P.M. et al. Enzymatic Depletion of Tumor Hyaluronan Induces Antitumor Responses in Preclinical Animal Models. Mol Cancer Ther. 2010, 9, pp. 3052-3064.
- Kulchavenya, E.V.; Shevchenko, S.Y.; Cherednichenko, A.G.; Breusov, A.A.; Vinitskiy AA. [New opportunities of using gialuronidase in chronic prostatitis]. Urologiia. 2020, Jun(3), pp.56-62.
- Chuchalin, A.G.; Yablonskiy, P.K.; Rubanik, T.V.; Chernyavskaya, O.A.; Naumov, V.V. et al. Efficacy and safety of bovhyaluronidase azoximer (Longidase) in patients with post-COVID syndrome: results of an open, prospective, controlled, comparative, multicenter clinical trial. Medicine, Pulmonologiya, 2023, 10 February. https://doi.org/10.18093/0869-0189-2023-33-1-52-63. [CrossRef]
- Ortiz, L.A.; Gambelli, F.; McBride, C.; Gaupp, D.; Baddoo, M.; Kaminski, N.; Phinney, D.G. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proc. Nat.l Acad. Sci. USA, 2003, 100, 8407–8411. https://doi.org/10.1073/pnas.1432929100. [CrossRef]
- Liu, J.; Shi, G. Pirfenidone activates cannabinoid receptor 2 in a mouse model of bleomycin-induced pulmonary fibrosis. Exp Ther Med. 2019, 18(6), pp. 4241-4248. https://doi.org/10.3892/etm.2019.8045. [CrossRef]
- Gueders, M.M.; Bertholet, P.; Perin, F.; Rocks, N.; Maree, R.; Botta, V.; Louis, R.; Foidart, J.M.; Noel, A.; Evrard, B.; et al. A novel formulation of inhaled doxycycline reduces allergen-induced inflammation, hyperrespon: siveness and remodeling by matrix metalloproteinases and cytokines modulation in a mouse model of asthma. Biochem. Pharmacol. 2008, 75, pp. 514–526.
- Costabel, U.; Albera, C.; Lancaster, L.H.; Lin C.Y., Hormel P., Hulter H.N., Noble P.W. An Open-Label Study of the Long-Term Safety of Pirfenidone in Patients with Idiopathic Pulmonary Fibrosis (RECAP). Respiration.2017, 94(5). pp. 408-415.
- Lancaster, L.H.; de Andrade, J.A.; Zibrak, J.D.; Padilla, M.L.; Albera, C. et al. Pirfenidone safety and adverse event management in idiopathic pulmonary fibrosis. European respiratory review: an official journal of the European Respiratory Society, 2017, 26(146), 170057. https://doi.org/10.1183/16000617.0057-2017. [CrossRef]
- Gul, A.; Yang, F.; Xie, C.; Du, W.; Mohammadtursun, N.; Wang, B.; Le, J.; Dong, J. Pulmonary fibrosis model of mice induced by different administration methods of bleomycin. BMC pulmonary medicine 2023, 23(1), 91. https://doi.org/10.1186/s12890-023-02349-z. [CrossRef]
- Tanner, L.; Single, A.B.; Bhongir, R.K.V.; Heusel, M.; Mohanty, T. et al. Small-molecule-mediated OGG1 inhibition attenuates pulmonary inflammation and lung fibrosis in a murine lung fibrosis model. Nat Commun. 2023, 14(1), 643. https://doi.org/10.1038/s41467-023-36314-5. [CrossRef]
- Webber, J.; Meran, S.; Steadman, R.; Phillips A. Hyaluronan orchestrates transforming growth factor-beta1-dependent maintenance of myofibroblast phenotype. J. Biol. Chem. 2009, 284, pp.9083–9092. https://doi.org/10.1074/jbc.M806989200. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).