Submitted:
19 July 2023
Posted:
21 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Cohort Sample
2.2. Data Collection
2.3. Blood Samples
2.4. Thrombin Generation Assay
2.5. Outcome Variables
2.6. Statistical Analysis
3. Results
3.1. Study Sample
3.2. Thrombin Generation Parameters in Study Samples
3.3. Incidence Rates of VTE Recurrence, Major Bleeding, and Mortality, and Thrombin Generation
3.4. Discriminative Power of Thrombin Generation Parameters for Outcomes
3.5. Association between Thrombin Generation Parameters and Outcomes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef] [PubMed]
- Cushman, M. Epidemiology and risk factors for venous thrombosis. Seminars in hematology 2007, 44, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Deitelzweig, S.B.; Johnson, B.H.; Lin, J.; Schulman, K.L. Prevalence of clinical venous thromboembolism in the USA: current trends and future projections. American journal of hematology 2011, 86, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Heit, J.A. Predicting the risk of venous thromboembolism recurrence. American journal of hematology 2012, 87 Suppl 1, S63–67. [Google Scholar] [CrossRef]
- Spencer, F.A.; Gore, J.M.; Lessard, D.; Emery, C.; Pacifico, L.; Reed, G.; Gurwitz, J.H.; Goldberg, R.J. Venous thromboembolism in the elderly. A community-based perspective. Thrombosis and haemostasis 2008, 100, 780–788. [Google Scholar]
- Spencer, F.A.; Gurwitz, J.H.; Schulman, S.; Linkins, L.A.; Crowther, M.A.; Ginsberg, J.S.; Lee, A.Y.; Saczynski, J.S.; Anand, S.; Lessard, D.; et al. Venous thromboembolism in older adults: A community-based study. The American journal of medicine 2014, 127, 530–537. [Google Scholar] [CrossRef]
- Silverstein, M.D.; Heit, J.A.; Mohr, D.N.; Petterson, T.M.; O'Fallon, W.M.; Melton, L.J. , 3rd. Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Archives of internal medicine 1998, 158, 585–593. [Google Scholar] [CrossRef]
- Ainle, F.N. , Kevane, B. Which patients are at high risk of recurrent venous thromboembolism (deep vein thrombosis and pulmonary embolism)? Blood Adv 2020, 4, 5595–5606. [Google Scholar] [CrossRef]
- Abdulla, A. , Davis, W.M.; Ratnaweera, N.; Szefer, E.; Ballantyne Scott, B.; Lee, A.Y.Y. A Meta-Analysis of Case Fatality Rates of Recurrent Venous Thromboembolism and Major Bleeding in Patients with Cancer. Thrombosis and haemostasis 2020, 120, 702–713. [Google Scholar]
- Iorio, A.; Kearon, C.; Filippucci, E.; Marcucci, M.; Macura, A.; Pengo, V.; Siragusa, S.; Palareti, G. Risk of recurrence after a first episode of symptomatic venous thromboembolism provoked by a transient risk factor: a systematic review. Archives of internal medicine 2010, 170, 1710–1716. [Google Scholar] [CrossRef]
- Khan, F.; Rahman, A.; Carrier, M.; Kearon, C.; Weitz, J.I.; Schulman, S.; Couturaud, F.; Eichinger, S.; Kyrle, P.A.; Becattini, C.; et al. Long term risk of symptomatic recurrent venous thromboembolism after discontinuation of anticoagulant treatment for first unprovoked venous thromboembolism event: systematic review and meta-analysis. Bmj 2019, 366, l4363. [Google Scholar] [CrossRef] [PubMed]
- Agnelli, G.; Prandoni, P.; Santamaria, M.G.; Bagatella, P.; Iorio, A.; Bazzan, M.; Moia, M.; Guazzaloca, G.; Bertoldi, A.; Tomasi, C.; et al. Three months versus one year of oral anticoagulant therapy for idiopathic deep venous thrombosis. Warfarin Optimal Duration Italian Trial Investigators. The New England journal of medicine 2001, 345, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Kearon, C.; Gent, M.; Hirsh, J.; Weitz, J.; Kovacs, M.J.; Anderson, D.R.; Turpie, A.G.; Green, D.; Ginsberg, J.S.; Wells, P.; et al. A comparison of three months of anticoagulation with extended anticoagulation for a first episode of idiopathic venous thromboembolism. The New England journal of medicine 1999, 340, 901–907. [Google Scholar] [CrossRef]
- Kearon, C.; Ginsberg, J.S.; Kovacs, M.J.; Anderson, D.R.; Wells, P.; Julian, J.A. , MacKinnon, B.; Weitz, J.I.; Crowther, M.A.; Dolan, S.; et al. Comparison of low-intensity warfarin therapy with conventional-intensity warfarin therapy for long-term prevention of recurrent venous thromboembolism. The New England journal of medicine 2003, 349, 631–639. [Google Scholar] [CrossRef]
- Palareti, G.; Leali, N.; Coccheri, S.; Poggi, M.; Manotti, C.; D'Angelo, A.; Pengo, V.; Erba, N.; Moia, M.; Ciavarella, N.; et al. Bleeding complications of oral anticoagulant treatment: an inception-cohort, prospective collaborative study (ISCOAT). Italian Study on Complications of Oral Anticoagulant Therapy. Lancet 1996, 348, 423–428. [Google Scholar] [CrossRef]
- Pinede, L.; Ninet, J.; Duhaut, P.; Chabaud, S.; Demolombe-Rague, S.; Durieu, I.; Nony, P.; Sanson, C.; Boissel, J.P.; Investigators of the "Duree Optimale du Traitement AntiVitamines KS. Comparison of 3 and 6 months of oral anticoagulant therapy after a first episode of proximal deep vein thrombosis or pulmonary embolism and comparison of 6 and 12 weeks of therapy after isolated calf deep vein thrombosis. Circulation 2001, 103, 2453–2460. [Google Scholar] [CrossRef]
- Schulman, S.; Rhedin, A.S.; Lindmarker, P.; Carlsson, A.; Larfars, G.; Nicol, P.; Loogna, E.; Svensson, E.; Ljungberg, B.; Walter, H. A comparison of six weeks with six months of oral anticoagulant therapy after a first episode of venous thromboembolism. Duration of Anticoagulation Trial Study Group. The New England journal of medicine 1995, 332, 1661–1665. [Google Scholar] [CrossRef]
- Cosmi, B.; Legnani, C.; Tosetto, A.; Pengo, V.; Ghirarduzzi, A.; Testa, S.; Prisco, D.; Poli, D.; Tripodi, A.; Marongiu, F.; et al. Usefulness of repeated D-dimer testing after stopping anticoagulation for a first episode of unprovoked venous thromboembolism: the PROLONG II prospective study. Blood 2010, 115, 481–488. [Google Scholar] [CrossRef]
- Douketis, J.; Tosetto, A.; Marcucci, M.; Baglin, T.; Cushman, M.; Eichinger, S.; Palareti, G.; Poli, D.; Tait, R.C.; Iorio, A. Patient-level meta-analysis: effect of measurement timing, threshold, and patient age on ability of D-dimer testing to assess recurrence risk after unprovoked venous thromboembolism. Annals of internal medicine 2010, 153, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Palareti, G.; Cosmi, B.; Legnani, C.; Tosetto, A.; Brusi, C.; Iorio, A.; Pengo, V.; Ghirarduzzi, A.; Pattacini, C. ; Testa S; et al: D-dimer testing to determine the duration of anticoagulation therapy. The New England journal of medicine 2006, 355, 1780–1789. [Google Scholar] [CrossRef]
- Kearon, C. Natural history of venous thromboembolism. Seminars in vascular medicine 2001, 1, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Kearon, C.; Parpia, S.; Spencer, F.A.; Schulman, S.; Stevens, S.M.; Shah, V.; Bauer, K.A.; Douketis, J.D.; Lentz, S.R.; Kessler, C.M.; et al. Long-term risk of recurrence in patients with a first unprovoked venous thromboembolism managed according to d-dimer results; A cohort study. Journal of thrombosis and haemostasis : JTH 2019, 17, 1144–1152. [Google Scholar] [CrossRef] [PubMed]
- Hemker, H.C.; Al Dieri, R.; De Smedt, E.; Beguin, S. Thrombin generation, a function test of the haemostatic-thrombotic system. Thrombosis and haemostasis 2006, 96, 553–561. [Google Scholar]
- Tripodi, A.; Legnani, C.; Chantarangkul, V.; Cosmi, B.; Palareti, G.; Mannucci, P.M. High thrombin generation measured in the presence of thrombomodulin is associated with an increased risk of recurrent venous thromboembolism. Journal of thrombosis and haemostasis : JTH 2008, 6, 1327–1333. [Google Scholar] [CrossRef]
- van Hylckama Vlieg, A.; Baglin, C.A.; Luddington, R.; MacDonald, S.; Rosendaal, F.R.; Baglin, T.P. The risk of a first and a recurrent venous thrombosis associated with an elevated D-dimer level and an elevated thrombin potential: results of the THE-VTE study. Journal of thrombosis and haemostasis : JTH 2015, 13, 1642–1652. [Google Scholar] [CrossRef] [PubMed]
- Eichinger, S.; Hron, G.; Kollars, M.; Kyrle, P.A. Prediction of recurrent venous thromboembolism by endogenous thrombin potential and D-dimer. Clinical chemistry 2008, 54, 2042–2048. [Google Scholar] [CrossRef]
- Hron, G.; Kollars, M.; Binder, B.R.; Eichinger, S.; Kyrle, P.A. Identification of patients at low risk for recurrent venous thromboembolism by measuring thrombin generation. Jama 2006, 296, 397–402. [Google Scholar] [CrossRef]
- Brummel-Ziedins, K.E.; Vossen, C.Y.; Butenas, S.; Mann, K.G.; Rosendaal, F.R. Thrombin generation profiles in deep venous thrombosis. Journal of thrombosis and haemostasis : JTH 2005, 3, 2497–2505. [Google Scholar] [CrossRef]
- Dielis, A.W.; Castoldi, E.; Spronk, H.M.; van Oerle, R.; Hamulyak, K.; Ten Cate, H.; Rosing, J. Coagulation factors and the protein C system as determinants of thrombin generation in a normal population. Journal of thrombosis and haemostasis : JTH 2008, 6, 125–131. [Google Scholar] [CrossRef]
- Haidl, H.; Cimenti, C.; Leschnik, B.; Zach, D.; Muntean, W. Age-dependency of thrombin generation measured by means of calibrated automated thrombography (CAT). Thrombosis and haemostasis 2006, 95, 772–775. [Google Scholar] [CrossRef]
- Wang, H.; Rosendaal, F.R.; Cushman, M.; van Hylckama Vlieg, A. D-dimer, thrombin generation, and risk of a first venous thrombosis in the elderly. Research and practice in thrombosis and haemostasis 2021, 5, e12536. [Google Scholar] [CrossRef]
- Mean, M.; Aujesky, D.; Lammle, B.; Gerschheimer, C.; Trelle, S.; Angelillo-Scherrer, A. Design and establishment of a biobank in a multicenter prospective cohort study of elderly patients with venous thromboembolism (SWITCO65+). Journal of thrombosis and thrombolysis 2013, 36, 484–491. [Google Scholar] [CrossRef]
- Mean, M.; Righini, M.; Jaeger, K.; Beer, H.J.; Frauchiger, B.; Osterwalder, J.; Kucher, N.; Lammle, B.; Cornuz, J.; Angelillo-Scherrer, A.; et al. The Swiss cohort of elderly patients with venous thromboembolism (SWITCO65+): rationale and methodology. Journal of thrombosis and thrombolysis 2013, 36, 475–483. [Google Scholar] [CrossRef]
- Calzavarini, S.; Brodard, J.; Quarroz, C.; Maire, L.; Nutzi, R.; Jankovic, J.; Rotondo, L.C.; Giabbani, E.; Fiedler, G.M.; Nagler, M.; et al. Thrombin generation measurement using the ST Genesia Thrombin Generation System in a cohort of healthy adults: Normal values and variability. Research and practice in thrombosis and haemostasis 2019, 3, 758–768. [Google Scholar] [CrossRef] [PubMed]
- Dargaud, Y.; Wolberg, A.S.; Gray, E.; Negrier, C.; Hemker, H.C.; Subcommittee on Factor Viii FIX, Rare Coagulation D. Proposal for standardized preanalytical and analytical conditions for measuring thrombin generation in hemophilia: communication from the SSC of the ISTH. Journal of thrombosis and haemostasis : JTH 2017, 15, 1704–1707. [Google Scholar] [CrossRef]
- Brodard, J.; Calzavarini, S.; Quarroz, C.; Berzigotti, A.; De Gottardi, A.; Angelillo-Scherrer, A. Resistance to thrombomodulin correlates with liver stiffness in chronic liver disease a prospective single-center cohort study. Thrombosis research 2021, 207, 40–49. [Google Scholar] [CrossRef]
- Tripodi, A. Detection of procoagulant imbalance. Modified endogenous thrombin potential with results expressed as ratio of values with-to-without thrombomodulin. Thrombosis and haemostasis 2017, 117, 830–836. [Google Scholar] [PubMed]
- Hemker, H.C.; Giesen, P.; Al Dieri, R.; Regnault, V.; de Smedt, E.; Wagenvoord, R.; Lecompte, T.; Beguin, S. Calibrated automated thrombin generation measurement in clotting plasma. Pathophysiology of haemostasis and thrombosis 2003, 33, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Prandoni, P.; Noventa, F.; Ghirarduzzi, A.; Pengo, V.; Bernardi, E.; Pesavento, R.; Iotti, M.; Tormene, D.; Simioni, P.; Pagnan, A. The risk of recurrent venous thromboembolism after discontinuing anticoagulation in patients with acute proximal deep vein thrombosis or pulmonary embolism. A prospective cohort study in 1,626 patients. Haematologica 2007, 92, 199–205. [Google Scholar] [CrossRef]
- Schulman, S.; Kearon, C.; Subcommittee on Control of Anticoagulation of the S, Standardization Committee of the International Society on T, Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. Journal of thrombosis and haemostasis : JTH 2005, 3, 692–694. [Google Scholar] [CrossRef]
- Fine, J.P.; Gray, R.J. A proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association 1999, 94, 496–509. [Google Scholar] [CrossRef]
- Agnelli, G.; Prandoni, P.; Becattini, C.; Silingardi; M. ; Taliani, M.R.; Miccio, M.; Imberti, D.; Poggio, R.; Ageno, W.; Pogliani, E; et al. Extended oral anticoagulant therapy after a first episode of pulmonary embolism. Annals of internal medicine 2003, 139, 19–25. [Google Scholar] [CrossRef]
- Boutitie, F.; Pinede, L.; Schulman, S.; Agnelli, G.; Raskob, G.; Julian, J.; Hirsh, J.; Kearon, C. Influence of preceding length of anticoagulant treatment and initial presentation of venous thromboembolism on risk of recurrence after stopping treatment: analysis of individual participants' data from seven trials. Bmj 2011, 342, d3036. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, S.C.; Lijfering, W.M.; Helmerhorst, F.M.; Rosendaal, F.R.; Cannegieter, S. C: Sex difference in risk of recurrent venous thrombosis and the risk profile for a second event. Journal of thrombosis and haemostasis : JTH 2010, 8, 2159–2168. [Google Scholar] [CrossRef]
- Hansson, P.O.; Sorbo, J.; Eriksson, H. Recurrent venous thromboembolism after deep vein thrombosis: incidence and risk factors. Archives of internal medicine 2000, 160, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Heit, J.A.; Mohr, D.N.; Silverstein, M.D.; Petterson, T.M.; O'Fallon, W.M.; Melton, L.J. , 3rd. Predictors of recurrence after deep vein thrombosis and pulmonary embolism: a population-based cohort study. Archives of internal medicine 2000, 160, 761–768. [Google Scholar] [CrossRef]
- Huang, W.; Goldberg, R.J.; Anderson, F.A.; Cohen, A.T.; Spencer, F.A. Occurrence and predictors of recurrence after a first episode of acute venous thromboembolism: population-based Worcester Venous Thromboembolism Study. Journal of thrombosis and thrombolysis 2016, 41, 525–538. [Google Scholar] [CrossRef] [PubMed]
- Insam, C.; Mean, M.; Limacher, A.; Angelillo-Scherrer, A.; Aschwanden, M.; Banyai, M.; Beer, J.H.; Bounameaux, H.; Egloff, M.; Frauchiger, B.; et al. Anticoagulation Management Practices and Outcomes in Elderly Patients with Acute Venous Thromboembolism: A Clinical Research Study. PloS one 2016, 11, e0148348. [Google Scholar] [CrossRef]
- Lopez-Jimenez, L.; Montero, M.; Gonzalez-Fajardo, J.A.; Arcelus, J.I.; Suarez, C.; Lobo, J.L.; Monreal, M.; RIETE Investigators. Venous thromboembolism in very elderly patients: findings from a prospective registry (RIETE). Haematologica 2006, 91, 1046–1051. [Google Scholar]
- Prandoni, P.; Lensing, A.W.; Piccioli, A.; Bernardi, E.; Simioni, P.; Girolami, B.; Marchiori, A.; Sabbion, P.; Prins, M.H.; Noventa, F.; et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood 2002, 100, 3484–3488. [Google Scholar] [CrossRef]
- Wattanakit, K.; Cushman, M.; Stehman-Breen, C.; Heckbert, S.R.; Folsom, A.R. Chronic kidney disease increases risk for venous thromboembolism. Journal of the American Society of Nephrology : JASN 2008, 19, 135–140. [Google Scholar] [CrossRef]
- Gussoni, G.; Frasson, S.; La Regina, M.; Di Micco, P.; Monreal, M.; RIETE Investigators. Three-month mortality rate and clinical predictors in patients with venous thromboembolism and cancer. Findings from the RIETE registry. Thrombosis research 2013, 131, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Beyth, R.J.; Quinn, L.M.; Landefeld, C.S. Prospective evaluation of an index for predicting the risk of major bleeding in outpatients treated with warfarin. The American journal of medicine 1998, 105, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.C.; Go, A.S.; Chang, Y.; Borowsky, L.H.; Pomernacki, N.K.; Udaltsova, N.; Singer, D.E. A new risk scheme to predict warfarin-associated hemorrhage: The ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) Study. Journal of the American College of Cardiology 2011, 58, 395–401. [Google Scholar] [CrossRef]
- Gage, B.F.; Yan, Y.; Milligan, P.E.; Waterman, A.D.; Culverhouse, R.; Rich, M.W.; Radford, M.J. Clinical classification schemes for predicting hemorrhage: results from the National Registry of Atrial Fibrillation (NRAF). American heart journal 2006, 151, 713–719. [Google Scholar] [CrossRef]
- Hutten, B.A.; Prins, M.H.; Gent, M.; Ginsberg, J.; Tijssen, J.G.; Buller, H.R. ; Incidence of recurrent thromboembolic and bleeding complications among patients with venous thromboembolism in relation to both malignancy and achieved international normalized ratio: a retrospective analysis. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2000, 18, 3078–3083. [Google Scholar] [CrossRef] [PubMed]
- Kuijer, P.M.; Hutten, B.A.; Prins, M.H.; Buller, H.R. Prediction of the risk of bleeding during anticoagulant treatment for venous thromboembolism. Archives of internal medicine 1999, 159, 457–460. [Google Scholar] [CrossRef]
- Landefeld, C.S.; Goldman, L. Major bleeding in outpatients treated with warfarin: incidence and prediction by factors known at the start of outpatient therapy. The American journal of medicine 1989, 87, 144–152. [Google Scholar] [CrossRef]
- Lip, G.Y.; Frison, L.; Halperin, J.L.; Lane, D.A. Comparative validation of a novel risk score for predicting bleeding risk in anticoagulated patients with atrial fibrillation: the HAS-BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly) score. Journal of the American College of Cardiology 2011, 57, 173–180. [Google Scholar]
- Nieto, J.A.; Bruscas, M.J.; Ruiz-Ribo, D.; Trujillo-Santos, J.; Valle, R.; Ruiz-Gimenez, N.; Monreal, M.; RIETE Investigators. Acute venous thromboembolism in patients with recent major bleeding. The influence of the site of bleeding and the time elapsed on outcome. Journal of thrombosis and haemostasis : JTH 2006, 4, 2367–2372. [Google Scholar] [CrossRef]
- Olesen, J.B.; Lip, G.Y.; Hansen, P.R.; Lindhardsen, J.; Ahlehoff, O.; Andersson, C.; Weeke, P.; Hansen, M.L.; Gislason, G.H.; Torp-Pedersen, C. Bleeding risk in 'real world' patients with atrial fibrillation: comparison of two established bleeding prediction schemes in a nationwide cohort. Journal of thrombosis and haemostasis : JTH 2011, 9, 1460–1467. [Google Scholar] [CrossRef]
- Pisters, R.; Lane, D.A.; Nieuwlaat, R.; de Vos, C.B.; Crijns, H.J.; Lip, G.Y. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest 2010, 138, 1093–1100. [Google Scholar] [CrossRef]
- Prandoni, P.; Lensing, A.W.; Cogo, A.; Cuppini, S.; Villalta, S.; Carta, M.; Cattelan, A.M.; Polistena, P.; Bernardi, E.; Prins, M.H. The long-term clinical course of acute deep venous thrombosis. Annals of internal medicine 1996, 125, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Gimenez, N.; Suarez, C.; Gonzalez, R.; Nieto, J.A.; Todoli, J.A.; Samperiz, A.L.; Monreal, M.; RIETE Investigators. Predictive variables for major bleeding events in patients presenting with documented acute venous thromboembolism. Findings from the RIETE Registry. Thrombosis and haemostasis 2008, 100, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Shireman, T.I.; Mahnken, J.D.; Howard, P.A.; Kresowik, T.F.; Hou, Q.; Ellerbeck, E.F. Development of a contemporary bleeding risk model for elderly warfarin recipients. Chest 2006, 130, 1390–1396. [Google Scholar] [CrossRef] [PubMed]
- Torn, M.; Bollen, W.L.; van der Meer, F.J.; van der Wall, E.E.; Rosendaal, F.R. Risks of oral anticoagulant therapy with increasing age. Archives of internal medicine 2005, 165, 1527–1532. [Google Scholar] [CrossRef]
- van der Meer, F.J.; Rosendaal, F.R.; Vandenbroucke, J.P.; Briet, E. Bleeding complications in oral anticoagulant therapy. An analysis of risk factors. Archives of internal medicine 1993, 153, 1557–1562. [Google Scholar] [CrossRef]
- White, R.H.; Beyth; R. J.; Zhou, H.; Romano, P.S. Major bleeding after hospitalization for deep-venous thrombosis. The American journal of medicine 1999, 107, 414–424. [Google Scholar] [CrossRef]
- Chaireti, R.; Jennersjo, C.; Lindahl, T.L. Is thrombin generation at the time of an acute thromboembolic episode a predictor of recurrence? The LInkoping Study on Thrombosis (LIST)--a 7-year follow-up. Thrombosis research 2013, 131, 135–139. [Google Scholar] [CrossRef]
- Loeffen, R.; van Oerle, R.; Leers, M.P.; Kragten, J.A.; Crijns, H.; Spronk, H.M.; Ten Cate, H. Factor XIa and Thrombin Generation Are Elevated in Patients with Acute Coronary Syndrome and Predict Recurrent Cardiovascular Events. PloS one 2016, 11, e0158355. [Google Scholar] [CrossRef]
- Attanasio, M.; Marcucci, R.; Gori, A.M.; Paniccia, R.; Valente, S.; Balzi, D.; Barchielli, A.; Carrabba, N.; Valenti, R.; Antoniucci, D.; et al. Residual thrombin potential predicts cardiovascular death in acute coronary syndrome patients undergoing percutaneous coronary intervention. Thrombosis research 2016, 147, 52–57. [Google Scholar] [CrossRef] [PubMed]
- van Paridon, P.C.S.; Panova-Noeva, M.; van Oerle, R.; Schultz, A.; Hermanns, I.M.; Prochaska, J.H.; Arnold, N.; Binder, H.; Schmidtmann, I.; Beutel, M.E.; et al. Thrombin generation in cardiovascular disease and mortality - results from the Gutenberg Health Study. Haematologica 2020, 105, 2327–2334. [Google Scholar] [CrossRef] [PubMed]
- Vambergue, A.; Rugeri, L.; Gaveriaux, V.; Devos, P.; Martin, A.; Fermon, C.; Fontaine, P.; Jude, B. Factor VII, tissue factor pathway inhibitor, and monocyte tissue factor in diabetes mellitus: influence of type of diabetes, obesity index, and age. Thrombosis research 2001, 101, 367–375. [Google Scholar] [CrossRef] [PubMed]
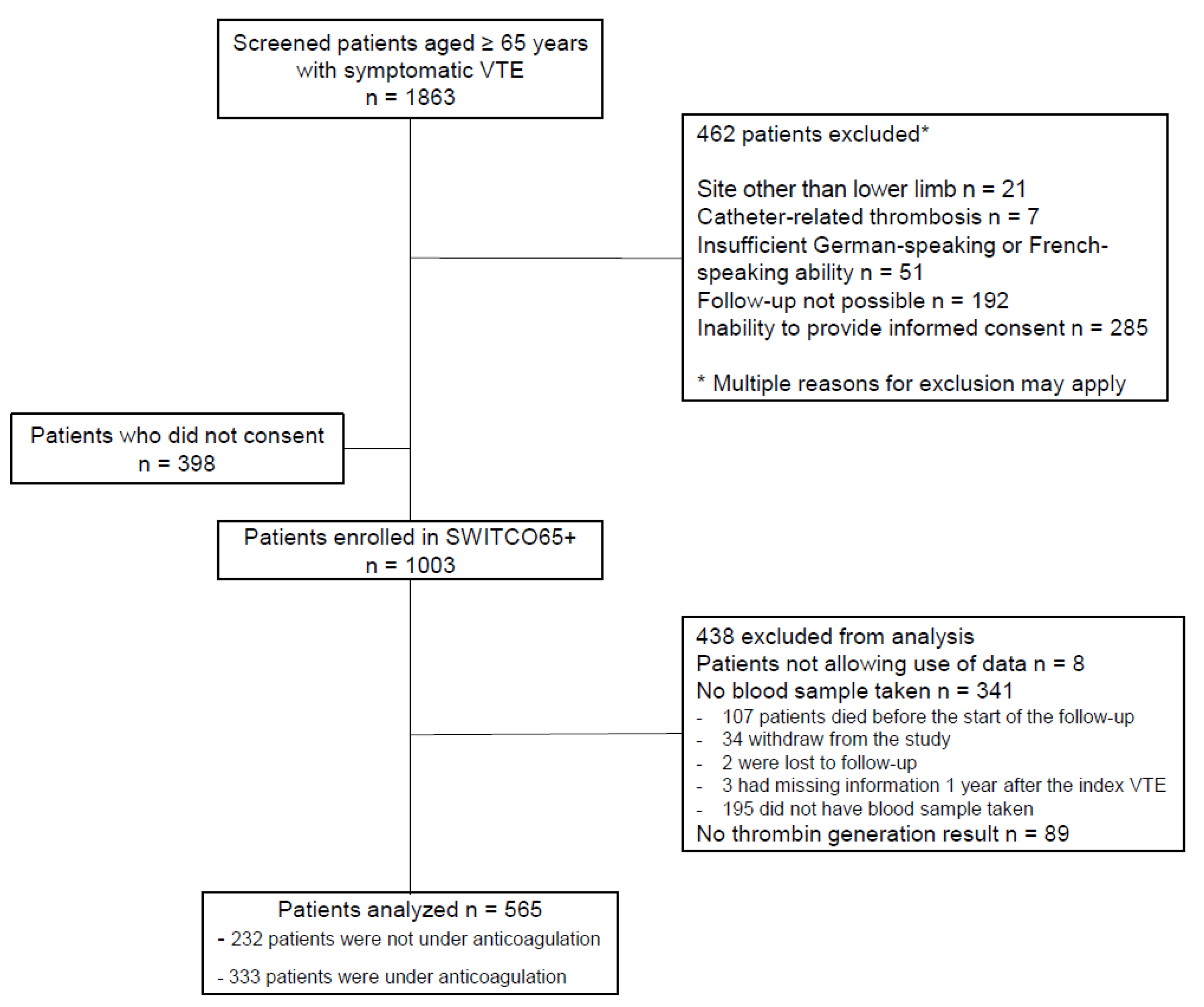
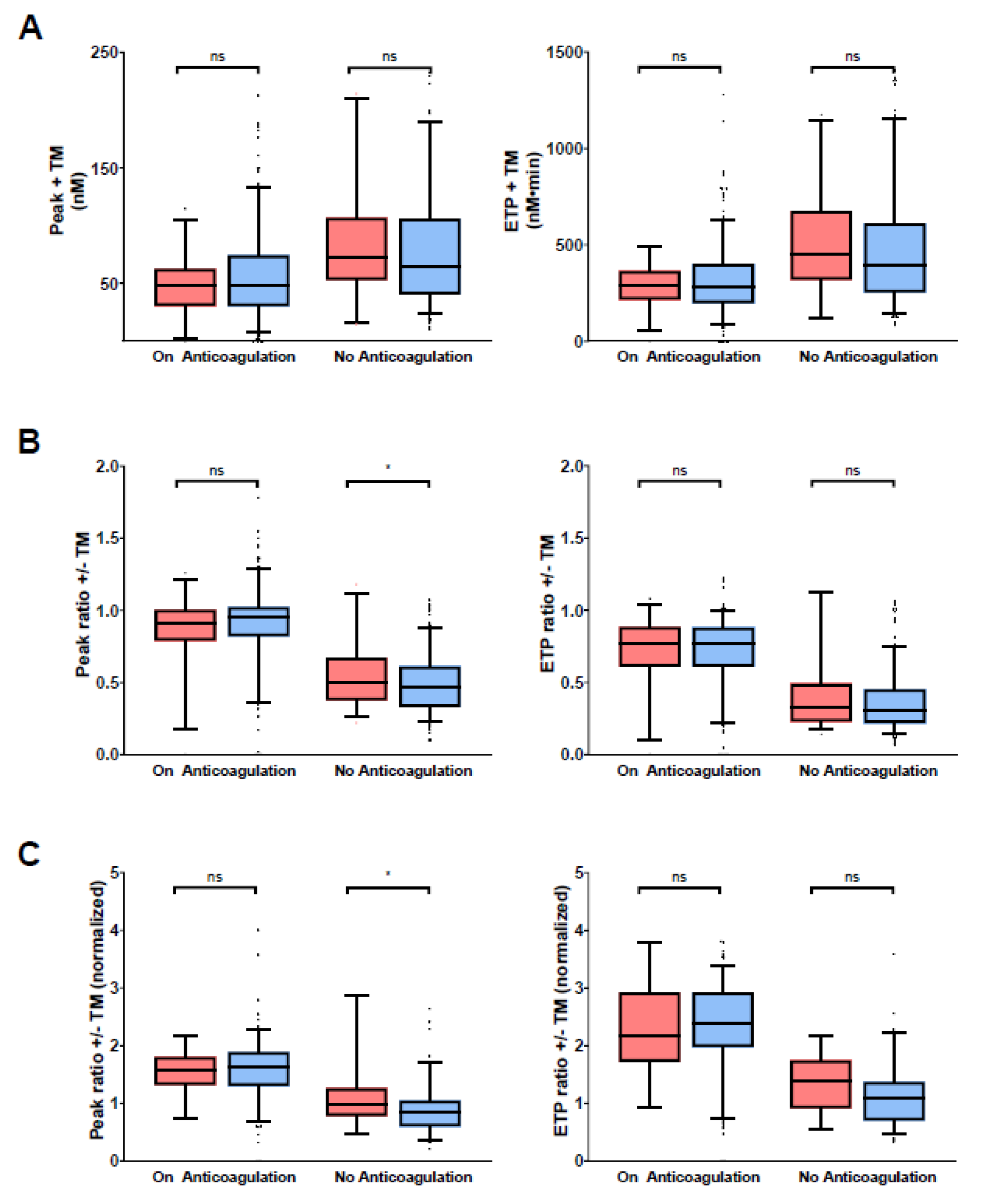
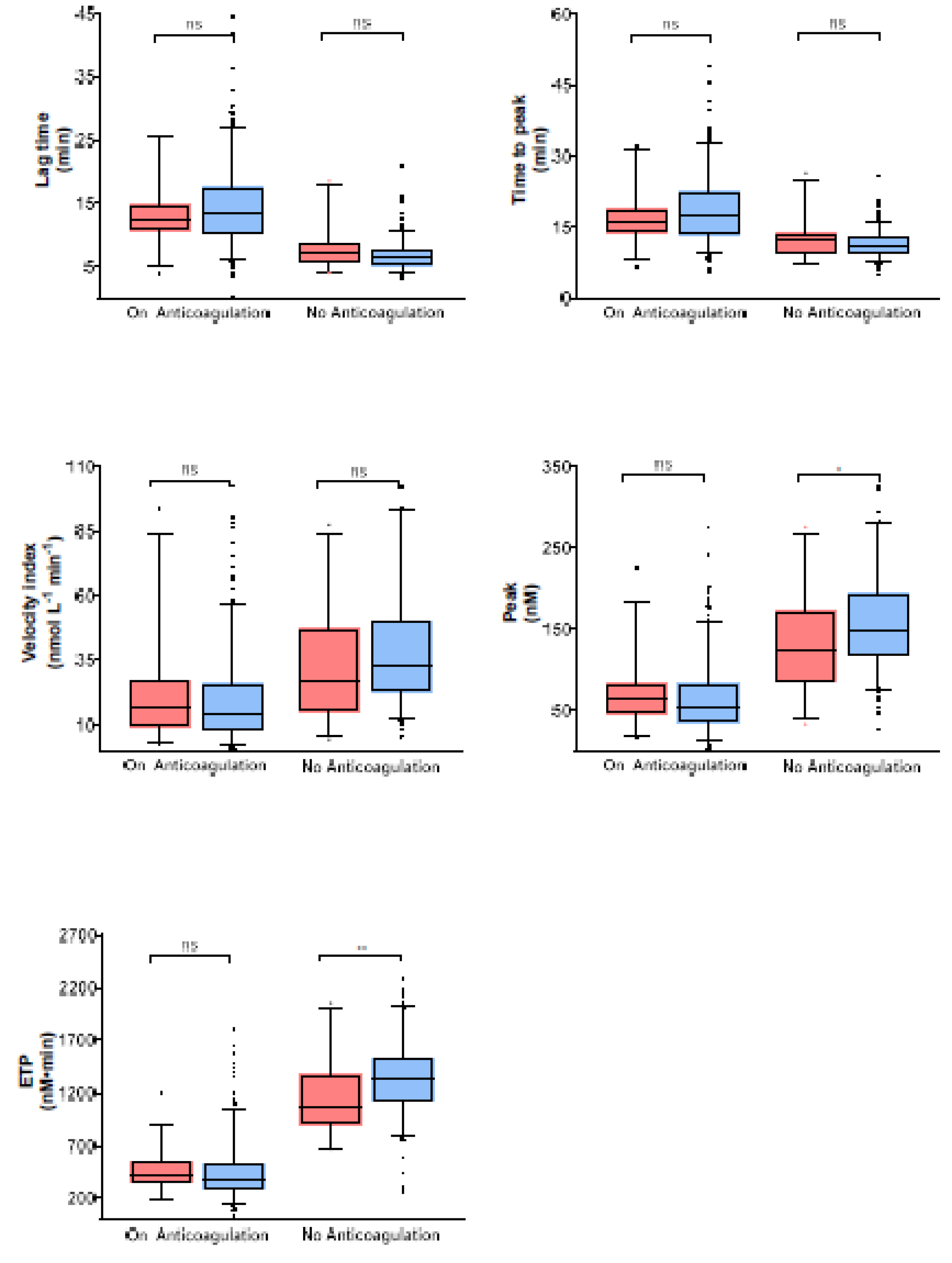
| Characteristic | Alln (%) or Median (IQ-Range) | Not under Anticoagulation One Year after Index VTE n (%) or Median (IQ-Range) |
Under Anticoagulation one Year after Index VTE n (%) or Median (IQ-Range) |
p-Value | |||
|---|---|---|---|---|---|---|---|
| Total number of patients | 565 | 232 | 333 | ||||
| Patient age (years) | 74.0 (69.0;79.0) | 74.0 (68.0;78.0) | 75.0 (69.0;80.0) | 0.262 | |||
| Female sex1 | 239 (42) | 107 (46) | 132 (40) | 0.141 | |||
| Patient race | |||||||
| Caucasian | 564 (100) | 231 (100) | 333 (100) | ||||
| African | 1 (0) | 1 (0) | 0 (0) | ||||
| Index VTE event | <0.001 | ||||||
| PE only | 317 (56) | 111 (48) | 206 (62) | ||||
| DVT only | 175 (31) | 98 (42) | 77 (23) | ||||
| PE and DVT | 73 (13) | 23 (10) | 50 (15) | ||||
| Index DVT type2 | <0.001 | ||||||
| proximal DVT only | 101 (18) | 47 (20) | 54 (16) | ||||
| distal DVT only | 58 (10) | 42 (18) | 16 (5) | ||||
| proximal and distal DVT | 89 (16) | 32 (14) | 57 (17) | ||||
| Type of index VTE | <0.001 | ||||||
| cancer-related VTE | 64 (11) | 27 (12) | 37 (11) | ||||
| provoked index VTE | 114 (20) | 69 (30) | 45 (14) | ||||
| unprovoked index VTE | 387 (68) | 136 (59) | 251 (75) | ||||
| Current oestrogen therapy during the last 3 months | 19 (3) | 9 (4) | 10 (3) | 0.638 | |||
| Immobilization during the last 3 months | 96 (17) | 60 (26) | 36 (11) | <0.001 | |||
| Major surgery during the last 3 months | 81 (14) | 53 (23) | 28 (8) | <0.001 | |||
| Prior VTE | 172 (30) | 29 (13) | 143 (43) | <0.001 | |||
| PTS2 | 295 (52) | 123 (53) | 172 (52) | 0.665 | |||
| History of major bleeding2 | 44 (8) | 18 (8) | 26 (8) | 1.000 | |||
| Chronic liver disease | 8 (1) | 4 (2) | 4 (1) | 0.722 | |||
| Chronic renal disease | 101 (18) | 34 (15) | 67 (20) | 0.118 | |||
| Chronic or acute heart failure | 67 (12) | 17 (7) | 50 (15) | 0.005 | |||
| Anemia2 | 189 (33) | 88 (38) | 101 (30) | 0.020 | |||
| Concomitant antiplatelet therapy | 177 (31) | 66 (28) | 111 (33) | 0.232 | |||
| Concomitant antiplatelet/NSAID therapy | 207 (37) | 77 (33) | 130 (39) | 0.183 | |||
| Heart rate of ≥ 110 beats min-1 2 | 47 (8) | 15 (6) | 32 (10) | 0.218 | |||
| Systolic BP of < 100 mmHg2 | 12 (2) | 3 (1) | 9 (3) | 0.376 | |||
| Arterial oxygen saturation of < 90%2 | 54 (10) | 16 (7) | 38 (11) | 0.233 | |||
| D-dimer at the time of the index VTE2 | 2482 (1599;3757) | 2407 (1687;3758) | 2513 (1554;3757) | 0.967 | |||
| D-dimer 1 year after the index VTE2 | 630 (393;1125) | 945 (567;1537) | 503 (327;807) | <0.001 | |||
| Overall anticoagulation duration (days) | 645 (213;976) | 192 (145;281) | 900 (709;1210) | <0.001 | |||
| Anticoagulation duration until 1 year after the index VTE (days) | 354 (194;365) | 186 (121;212) | 363 (357;371) | <0.001 | |||
| Anticoagulation duration from 1 year after the index VTE (days) | 338 (0;686) | 0 (0;0) | 535 (351; 865) | <0.001 | |||
| No of Patients | No of Events/Person-Years | Incidence Rate (95%-CI) | ||
|---|---|---|---|---|
| Peak ratio obtained in presence/absence of TM | ||||
| VTE recurrence | ||||
| All | 222 | 32 / 322.3 | 9.9 (7.0 to 14.0) | |
| ≤ median | 111 | 12 / 172.0 | 7.0 (4.0 to 12.3) | |
| > median | 111 | 20 / 150.3 | 13.3 (8.6 to 20.6) | |
| Major bleeding | ||||
| All | 222 | 11 / 342.2 | 3.2 (1.8 to 5.8) | |
| ≤ median | 111 | 2 / 182.0 | 1.1 (0.3 to 4.4) | |
| > median | 111 | 9 / 160.3 | 5.6 (2.9 to 10.8) | |
| Overall mortality | ||||
| All | 222 | 13 / 348.5 | 3.7 (2.2 to 6.4) | |
| ≤ median | 111 | 4 / 182.8 | 2.2 (0.8 to 5.8) | |
| > median | 111 | 9 / 165.8 | 5.4 (2.8 to 10.4) | |
| Normalized peak ratio in presence/absence of TM | ||||
| VTE recurrence | ||||
| All | 122 | 17 / 156.1 | 10.9 (6.8 to 17.5) | |
| ≤ median | 61 | 4 / 89.1 | 4.5 (1.7 to 12.0) | |
| > median | 61 | 13 / 67.0 | 19.4 (11.3 to 33.4) | |
| Major bleeding | ||||
| All | 122 | 3 / 165.6 | 1.8 (0.6 to 5.6) | |
| ≤ median | 61 | 0 / 91.2 | 0.0 (-) | |
| > median | 61 | 3 / 74.4 | 4.0 (1.3 to 12.5) | |
| Overall mortality | ||||
| All | 122 | 4 / 167.0 | 2.4 (0.9 to 6.4) | |
| ≤ median | 61 | 1 / 91.2 | 1.1 (0.2 to 7.8) | |
| > median | 61 | 3 / 75.8 | 4.0 (1.3 to 12.3) | |
| ETP ratio obtained in presence/absence of TM | ||||
| VTE recurrence | ||||
| All | 221 | 31 / 321.5 | 9.6 (6.8 to 13.7) | |
| ≤ median | 111 | 12 / 174.5 | 6.9 (3.9 to 12.1) | |
| > median | 110 | 19 / 147.0 | 12.9 (8.2 to 20.3) | |
| Major bleeding | ||||
| All | 221 | 11 / 340.2 | 3.2 (1.8 to 5.8) | |
| ≤ median | 111 | 4 / 182.9 | 2.2 (0.8 to 5.8) | |
| > median | 110 | 7 / 157.4 | 4.4 (2.1 to 9.3) | |
| Overall mortality | ||||
| All | 221 | 3.8 (2.2 to 6.5) | ||
| ≤ median | 111 | 2.7 (1.1 to 6.5) | ||
| > median | 110 | 8 / 161.0 | 5.0 (2.5 to 9.9) | |
| Normalized ETP ratio obtained in presence/absence of TM | ||||
| VTE recurrence | ||||
| All | 122 | 17 / 156.1 | 10.9 (6.8 to 17.5) | |
| ≤ median | 61 | 6 / 84.9 | 7.1 (3.2 to 15.7) | |
| > median | 61 | 11 / 71.2 | 15.5 (8.6 to 27.9) | |
| Major bleeding | ||||
| All | 122 | 3 / 165.6 | 1.8 (0.6 to 5.6) | |
| ≤ median | 61 | 1 / 87.8 | 1.1 (0.2 to 8.1) | |
| > median | 61 | 2 / 77.8 | 2.6 (0.6 to 10.3) | |
| Overall mortality | ||||
| All | 122 | 4 / 167.0 | 2.4 (0.9 to 6.4) | |
| ≤ median | 61 | 2 / 87.8 | 2.3 (0.6 to 9.1) | |
| > median | 61 | 2 / 79.1 | 2.5 (0.6 to 10.1) |
| Thrombin Generation Parameters Measured one Year after the Index VTE | No. of Events/no. of Patients |
C-Statistics (95% Confidence Interval) |
|---|---|---|
|
Peak ratio obtained in presence/absence of TM VTE recurrence Major bleeding Overall mortality |
32/222 11/222 13/222 |
0.60 (0.51 to 0.69) 0.65 (0.50 to 0.80) 0.59 (0.45 to 0.73) |
|
Normalized peak ratio obtained in presence/absence of TM VTE recurrence Major bleeding Overall mortality |
17/122 3/122 4/122 |
0.70 (0.59 to 0.81) 0.65 (0.55 to 0.75) 0.63 (0.36 to 0.89) |
|
ETP ratio obtained in presence/absence of TM VTE recurrence Major bleeding Overall mortality |
31/221 11/221 13/221 |
0.59 (0.50 to 0.69) 0.63 (0.48 to 0.77) 0.44 (0.32 to 0.56) |
|
Normalized ETP ratio obtained in presence/absence of TM VTE recurrence Major bleeding Overall mortality |
17/122 3/122 4/122 |
0.70 (0.60 to 0.80) 0.48 (0.37 to 0.58) 0.66 (0.46 to 0.87) |
| n/N (%) | Crude Subhazard Ratio (95% Confidence Interval) |
Adjusted Subhazard Ratio (95% Confidence Interval) |
|||
|---|---|---|---|---|---|
| Peak ratio obtained in presence/absence of TM | |||||
| VTE recurrence | 32/222 (14.4) | 3.94 (1.00 to 15.49) | 4.09 (1.12 to 14.92) | ||
| Major bleeding | 11/222 (5.0) | 5.01 (0.67 to 37.24) | 5.65 (0.83 to 38.71) | ||
| Overall mortality | 13/222 (5.9) | 1.89 (0.33 to 10.75) | 2.93 (0.39 to 21.71) | ||
| Normalized peak ratio obtained in presence/absence of TM | |||||
| VTE recurrence | 17/122 (13.9) | 2.21 (1.30 to 3.77) | 2.18 (1.28 to 3.73) | ||
| Major bleeding | 3/122 (2.5) | 1.35 (0.84 to 2.18) | - | ||
| Overall mortality | 4/122 (3.3) | 1.36 (0.50 to 3.67) | - | ||
| ETP ratio obtained in presence/absence of TM | |||||
| VTE recurrence | 31/221 (14.0) | 3.10 (0.86 to 11.24) | 2.88 (0.82 to 10.09) | ||
| Major bleeding | 11/221 (5.0) | 3.38 (0.40 to 28.79) | 3.02 (0.38 to 23.97) | ||
| Overall mortality | 13/221 (5.9) | 0.80 (0.16 to 3.97) | 0.80 (0.10 to 6.55) | ||
| Normalized ETP ratio obtained in presence/absence of APC | |||||
| VTE recurrence | 17/122 (13.9) | 1.82 (1.01 to 3.29) | 1.80 (0.99 to 3.27) | ||
| Major bleeding | 3/122 (2.5) | 0.81 (0.54 to 1.23) | - | ||
| Overall mortality | 4/122 (3.3) | 1.58 (0.71 to 3.50) | - | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





