Submitted:
20 July 2023
Posted:
21 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
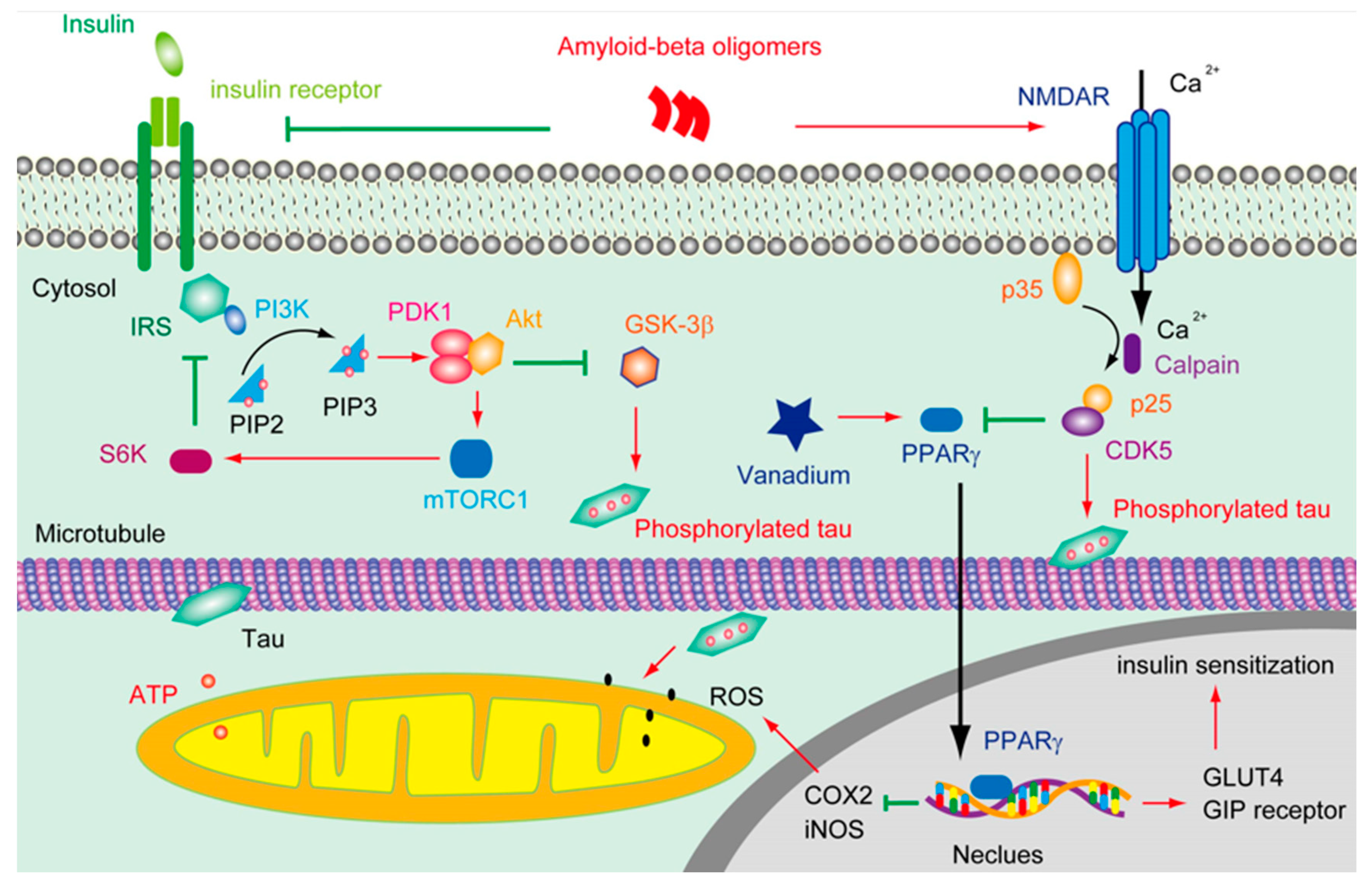
2. The Deficits of Insulin Signal in AD
2.1. The Role of APP in Glucose Metabolism
2.2. The Influence of Tau on Insulin Signal
2.3. The Impaired Insulin Signal in AD
3. The Effects of Vanadium on Curing AD
4. The Potential Mechanisms of Vanadium in Curing AD for Future Study
5. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Aβ | amyloid-beta |
| AD | Alzheimer’s disease |
| ADAM10 | a disintegrin and metalloproteinase 10 |
| AGD | argyrophilic grain disease |
| AICD | APP intracellular domain |
| APOE | apolipoprotein E |
| APP | amyloid precursor protein |
| BEOV | bis(2-ethyl-3-hydroxy-4-pyronato) oxovanadium (IV) |
| BMOV | bis(maltolato) oxovanadium(IV) |
| CAA | cerebral amyloid angiopathy |
| CBD | corticobasal degeneration |
| CDK5 | cyclin-dependent kinase 5 |
| COX2 | cyclooxygenase-2 |
| CTE | chronic traumatic encephalopathy |
| CTF-83 | C-terminal fragment |
| CNS | central nervous system |
| Drp1 | dynamin-related GTPase |
| FAD | familial AD |
| FTDP-17 | frontotemporal dementia with Parkinsonism linked to chromosome 17 |
| GIP | glucose-dependent insulinotropic polypeptide |
| GLUT4 | glucose transporter 4 |
| GSIS | glucose-stimulated insulin secretion |
| GSK-3β | 3glycogen synthase kinase -3 |
| IDE | insulin degrading enzyme |
| IGF | insulin-like growth factor |
| iNOS | inducible nitric oxide synthase |
| IR | insulin receptor |
| IRS2 | insulin receptor substrate |
| LTP | long-term potentiation |
| MFN | mitofusion |
| NFTs | neurofibrillary tangles |
| Nrf2 | nuclear factor-erythroid-2-related factor 2 |
| PGC-1α | proliferator activated receptor gamma coactivator 1 α |
| PI3K | phosphatidylinositol-3-kinase |
| PiD | Pick’s disease |
| PP2A | protein phosphatase 2A |
| PPARγ | proliferator-activated receptor gamma |
| PS1/2 | presenilin-1/2 |
| PSP | progressive supranuclear palsy |
| PTEN | phosphatase and tension homologue on chromosome 10 |
| PTPase | protein tyrosine phosphatase |
| SCS | Succinyl-CoA synthetase |
| SCOT/OXCT1 | Succinyl-CoA: 3-ketoacid-CoA transferase catalyzes |
| STZ | streptozotocin |
References
- Harland, B.F.; Harden-Williams, B.A. Is vanadium of human nutritional importance yet? J Am Diet Assoc 1994, 94, 891–894. [Google Scholar] [CrossRef] [PubMed]
- Zaporowska, H.; Wasilewski, W. Hematological Effects of Vanadium on Living Organisms. Comparative Biochemistry and Physiology C-Pharmacology Toxicology & Endocrinology 1992, 102, 223–231. [Google Scholar]
- Beauge, L.A.; Glynn, I.M. A modifier of (Na+ + k+) atpase in commercial ATP. Nature 1977, 268, 355–356. [Google Scholar] [CrossRef] [PubMed]
- Beauge, L.A.; Glynn, I.M. Commercial ATP containing traces of vanadate alters the response of (Na+ + K+) ATPase to external potassium. Nature 1978, 272, 551–552. [Google Scholar] [CrossRef] [PubMed]
- Shechter, Y.; Karlish, S.J. Insulin-like stimulation of glucose oxidation in rat adipocytes by vanadyl (IV) ions. Nature 1980, 284, 556–558. [Google Scholar] [CrossRef]
- Huyer, G.; et al. Mechanism of inhibition of protein-tyrosine phosphatases by vanadate and pervanadate. J Biol Chem 1997, 272, 843–851. [Google Scholar] [CrossRef]
- Liu, J.C.; et al. Bis(acetylacetonato)-oxovanadium(IV), bis(maltolato)-oxovanadium(IV) and sodium metavanadate induce antilipolytic effects by regulating hormone-sensitive lipase and perilipin via activation of Akt. Metallomics 2013, 5, 813–820. [Google Scholar] [CrossRef]
- Wu, J.X.; Hong, Y.H.; Yang, X.G. Bis(acetylacetonato)-oxidovanadium(IV) and sodium metavanadate inhibit cell proliferation via ROS-induced sustained MAPK/ERK activation but with elevated AKT activity in human pancreatic cancer AsPC-1 cells. J Biol Inorg Chem 2016, 21, 919–929. [Google Scholar] [CrossRef]
- Montaigne, D.; Butruille, L.; Staels, B. PPAR control of metabolism and cardiovascular functions. Nature Reviews Cardiology 2021, 18, 809–823. [Google Scholar] [CrossRef]
- Zhao, P.; Yang, X.D. Vanadium compounds modulate PPAR gamma activity primarily by increasing PPAR gamma protein levels in mouse insulinoma NIT-1 cells. Metallomics 2013, 5, 836–843. [Google Scholar] [CrossRef]
- Wu, Y.L.; et al. Vanadyl acetylacetonate upregulates PPAR gamma and adiponectin expression in differentiated rat adipocytes. Journal of Biological Inorganic Chemistry 2013, 18, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Ladagu, A.D.; et al. Novel NMDA-receptor antagonists ameliorate vanadium neurotoxicity. Naunyn Schmiedebergs Arch Pharmacol 2020, 393, 1729–1738. [Google Scholar] [CrossRef] [PubMed]
- Colin-Barenque, L.; et al. Neuroprotective effect of carnosine in the olfactory bulb after vanadium inhalation in a mouse model. Int J Exp Pathol 2018, 99, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Bishayee, A.; et al. Vanadium in the detection, prevention and treatment of cancer: the in vivo evidence. Cancer Lett 2010, 294, 1–12. [Google Scholar] [CrossRef]
- Llobet, J.M.; Domingo, J.L. Acute toxicity of vanadium compounds in rats and mice. Toxicol Lett 1984, 23, 227–231. [Google Scholar] [CrossRef]
- Yang, X.G.; Wang, K. Chemical, biochemical, and biological behaviors of vanadate and its oligomers. Prog Mol Subcell Biol 2013, 54, 1–18. [Google Scholar] [PubMed]
- Scibior, A.; et al. Vanadium: Risks and possible benefits in the light of a comprehensive overview of its pharmacotoxicological mechanisms and multi-applications with a summary of further research trends. J Trace Elem Med Biol 2020, 61, 126508. [Google Scholar] [CrossRef] [PubMed]
- Scibior, A.; Kurus, J. Vanadium and Oxidative Stress Markers - In Vivo Model: A Review. Curr Med Chem 2019, 26, 5456–5500. [Google Scholar] [CrossRef]
- Zhao, Y.B.; et al. Vanadium compounds induced mitochondria permeability transition pore (PTP) opening related to oxidative stress. Journal of Inorganic Biochemistry 2010, 104, 371–378. [Google Scholar] [CrossRef]
- Thompson, K.H.; Orvig, C. Vanadium compounds in the treatment of diabetes. Metal Ions in Biological Systems 2004, 41, 221–252. [Google Scholar]
- Mcneill, J.H.; et al. Bis(Maltolato)Oxovanadium(Iv) Is a Potent Insulin Mimic. Journal of Medicinal Chemistry 1992, 35, 1489–1491. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; et al. Synthesis, characterization and anti-diabetic therapeutic potential of novel aminophenol-derivatized nitrilotriacetic acid vanadyl complexes. J Inorg Biochem 2015, 152, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Yang, J.; Yang, X. Synthesis and anti-diabetic activity of new N,N-dimethylphenylenediamine-derivatized nitrilotriacetic acid vanadyl complexes. J Inorg Biochem 2017, 177, 291–299. [Google Scholar] [CrossRef]
- Du, J.; et al. Vanadium coordination compounds loaded on graphene quantum dots (GQDs) exhibit improved pharmaceutical properties and enhanced anti-diabetic effects. Nanoscale 2020, 12, 9219–9230. [Google Scholar] [CrossRef] [PubMed]
- Setyawati, I.A.; et al. Kinetic analysis and comparison of uptake, distribution, and excretion of 48V-labeled compounds in rats. J Appl Physiol (1985) 1998, 84, 569–575. [Google Scholar] [CrossRef]
- Gerhardsson, L.; et al. Metal concentrations in plasma and cerebrospinal fluid in patients with Alzheimer's disease. Dement Geriatr Cogn Disord 2008, 25, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Szabo, S.T.; et al. Comparison of Metal Levels between Postmortem Brain and Ventricular Fluid in Alzheimer's Disease and Nondemented Elderly Controls. Toxicological Sciences 2016, 150, 292–300. [Google Scholar] [CrossRef]
- Busche, M.A.; Hyman, B.T. Synergy between amyloid-beta and tau in Alzheimer's disease. Nature Neuroscience 2020, 23, 1183–1193. [Google Scholar] [CrossRef]
- Muller, U.C.; Deller, T.; Korte, M. Not just amyloid: physiological functions of the amyloid precursor protein family. Nat Rev Neurosci 2017, 18, 281–298. [Google Scholar] [CrossRef]
- Kim, M.; et al. Potential late-onset Alzheimer's disease-associated mutations in the ADAM10 gene attenuate alpha-secretase activity. Human Molecular Genetics 2009, 18, 3987–3996. [Google Scholar] [CrossRef]
- Aguero, P.; et al. alpha-Secretase nonsense mutation (ADAM10 Tyr167*) in familial Alzheimer's disease. Alzheimers Res Ther 2020, 12, 139. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.J.; Chen, Y.R. The coexistence of an equal amount of Alzheimer's amyloid-beta 40 and 42 forms structurally stable and toxic oligomers through a distinct pathway. Febs Journal 2014, 281, 2674–2687. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.; et al. Mechanisms for the Insertion of Toxic, Fibril-like beta-Amyloid Oligomers into the Membrane. Journal of Chemical Theory and Computation 2013, 9, 822–833. [Google Scholar] [CrossRef] [PubMed]
- Morkuniene, R.; et al. Small A beta(1-42) Oligomer-Induced Membrane Depolarization of Neuronal and Microglial Cells: Role of N-Methyl-D-Aspartate Receptors. Journal of Neuroscience Research 2015, 93, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Li, S.M.; et al. Soluble A beta Oligomers Inhibit Long-Term Potentiation through a Mechanism Involving Excessive Activation of Extrasynaptic NR2B-Containing NMDA Receptors. Journal of Neuroscience 2011, 31, 6627–6638. [Google Scholar] [CrossRef]
- Wang, X.L.; et al. Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins. Proceedings of the National Academy of Sciences of the United States of America 2008, 105, 19318–19323. [Google Scholar] [CrossRef]
- Du, H.; et al. Early deficits in synaptic mitochondria in an Alzheimer's disease mouse model. Proceedings of the National Academy of Sciences of the United States of America 2010, 107, 18670–18675. [Google Scholar] [CrossRef]
- He, Y.; et al. Soluble oligomers and fibrillar species of amyloid beta-peptide differentially affect cognitive functions and hippocampal inflammatory response. Biochemical and Biophysical Research Communications 2012, 429, 125–130. [Google Scholar] [CrossRef]
- Zhang, Y.L.; et al. The relationship between amyloid-beta and brain capillary endothelial cells in Alzheimer's disease. Neural Regen Res 2022, 17, 2355–2363. [Google Scholar]
- Jansen, W.J.; et al. Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA 2015, 313, 1924–1938. [Google Scholar] [CrossRef]
- Pallo, S.P.; Johnson, G.V.W. Tau facilitates A beta-induced loss of mitochondrial membrane potential independent of cytosolic calcium fluxes in mouse cortical neurons. Neuroscience Letters 2015, 597, 32–37. [Google Scholar] [CrossRef]
- Roberson, E.D.; et al. Reducing endogenous tau ameliorates amyloid beta-induced deficits in an Alzheimer's disease mouse model. Science 2007, 316, 750–754. [Google Scholar] [CrossRef] [PubMed]
- Vossel, K.A.; et al. Tau reduction prevents Abeta-induced defects in axonal transport. Science 2010, 330, 198. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, M.; et al. Tau is essential to beta-amyloid-induced neurotoxicity. Proceedings of the National Academy of Sciences of the United States of America 2002, 99, 6364–6369. [Google Scholar] [CrossRef] [PubMed]
- Marciniak, E.; et al. Tau deletion promotes brain insulin resistance. Journal of Experimental Medicine 2017, 214, 2257–2269. [Google Scholar] [CrossRef]
- Lei, P.; et al. Tau deficiency induces parkinsonism with dementia by impairing APP-mediated iron export. Nat Med 2012, 18, 291–295. [Google Scholar] [CrossRef]
- Abbondante, S.; et al. Genetic Ablation of Tau Mitigates Cognitive Impairment Induced by Type 1 Diabetes. American Journal of Pathology 2014, 184, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Trujillo-Estrada, L.; et al. Tau underlies synaptic and cognitive deficits for type 1, but not type 2 diabetes mouse models. Aging Cell 2019, 18. [Google Scholar] [CrossRef] [PubMed]
- Steen, E.; et al. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer's disease - is this type 3 diabetes? Journal of Alzheimers Disease 2005, 7, 63–80. [Google Scholar] [CrossRef]
- Frolich, L.; et al. Brain insulin and insulin receptors in aging and sporadic Alzheimer's disease. J Neural Transm (Vienna) 1998, 105, 423–438. [Google Scholar] [CrossRef]
- de la Monte, S.M.; et al. Early-Stage Alzheimer's Disease Is Associated with Simultaneous Systemic and Central Nervous System Dysregulation of Insulin-Linked Metabolic Pathways. J Alzheimers Dis 2019, 68, 657–668. [Google Scholar] [CrossRef]
- Confettura, A.D.; et al. Neddylation-dependent protein degradation is a nexus between synaptic insulin resistance, neuroinflammation and Alzheimer's disease. Translational Neurodegeneration 2022, 11. [Google Scholar] [CrossRef]
- Ma, Q.L.; et al. Beta-amyloid oligomers induce phosphorylation of tau and inactivation of insulin receptor substrate via c-Jun N-terminal kinase signaling: suppression by omega-3 fatty acids and curcumin. J Neurosci 2009, 29, 9078–9089. [Google Scholar] [CrossRef] [PubMed]
- Farris, W.; et al. Insulin-degrading enzyme regulates the levels of insulin, amyloid beta-protein, and the beta-amyloid precursor protein intracellular domain in vivo. Proc Natl Acad Sci U S A 2003, 100, 4162–4167. [Google Scholar] [CrossRef]
- Cook, D.G.; et al. Reduced hippocampal insulin-degrading enzyme in late-onset Alzheimer's disease is associated with the apolipoprotein E-epsilon 4 allele. American Journal of Pathology 2003, 162, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Morelli, L.; et al. Insulin-degrading enzyme in brain microvessels: proteolysis of amyloid {beta} vasculotropic variants and reduced activity in cerebral amyloid angiopathy. J Biol Chem 2004, 279, 56004–56013. [Google Scholar] [CrossRef] [PubMed]
- Schechter, R.; Beju, D.; Miller, K.E. The effect of insulin deficiency on tau and neurofilament in the insulin knockout mouse. Biochemical and Biophysical Research Communications 2005, 334, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Clodfelder-Miller, B.J.; et al. Tau is hyperphosphorylated at multiple sites in mouse brain in vivo after streptozotocin-induced insulin deficiency. Diabetes 2006, 55, 3320–3325. [Google Scholar] [CrossRef]
- Schubert, M.; et al. Insulin receptor substrate-2 deficiency impairs brain growth and promotes tau phosphorylation. Journal of Neuroscience 2003, 23, 7084–7092. [Google Scholar] [CrossRef]
- Duarte, A.I.; Moreira, P.I.; Oliveira, C.R. Insulin in central nervous system: more than just a peripheral hormone. J Aging Res 2012, 2012, 384017. [Google Scholar] [CrossRef]
- Banks, W.A. The source of cerebral insulin. European Journal of Pharmacology 2004, 490, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A.; Owen, J.B.; Erickson, M.A. Insulin in the brain: There and back again. Pharmacology & Therapeutics 2012, 136, 82–93. [Google Scholar]
- Grillo, C.A.; et al. Insulin-stimulated translocation of GLUT4 to the plasma membrane in rat hippocampus is PI3-kinase dependent. Brain Research 2009, 1296, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Pearson-Leary, J.; Mcnay, E.C. Novel Roles for the Insulin-Regulated Glucose Transporter-4 in Hippocampally Dependent Memory. Journal of Neuroscience 2016, 36, 11851–11864. [Google Scholar] [CrossRef]
- Radhakrishnan, Y.; et al. Insulin-like growth factor-I stimulates Shc-dependent phosphatidylinositol 3-kinase activation via Grb2-associated p85 in vascular smooth muscle cells. Journal of Biological Chemistry 2008, 283, 16320–16331. [Google Scholar] [CrossRef]
- Rodriguez-Rodriguez, P.; et al. Tau hyperphosphorylation induces oligomeric insulin accumulation and insulin resistance in neurons. Brain 2017, 140, 3269–3285. [Google Scholar] [CrossRef]
- Wijesekara, N.; et al. Tau ablation in mice leads to pancreatic beta cell dysfunction and glucose intolerance. Faseb Journal 2018, 32, 3166–3173. [Google Scholar] [CrossRef]
- Zhao, N.; et al. Apolipoprotein E4 Impairs Neuronal Insulin Signaling by Trapping Insulin Receptor in the Endosomes. Neuron 2017, 96, 115–+. [Google Scholar] [CrossRef]
- Craft, S.; et al. Effects of Regular and Long-Acting Insulin on Cognition and Alzheimer's Disease Biomarkers: A Pilot Clinical Trial. J Alzheimers Dis 2017, 57, 1325–1334. [Google Scholar] [CrossRef]
- Born, J.; et al. Sniffing neuropeptides: a transnasal approach to the human brain. Nature Neuroscience 2002, 5, 514–516. [Google Scholar] [CrossRef]
- Adzovic, L.; et al. Insulin improves memory and reduces chronic neuroinflammation in the hippocampus of young but not aged brains. J Neuroinflammation 2015, 12, 63. [Google Scholar] [CrossRef] [PubMed]
- Reger, M.A.; et al. Effects of intranasal insulin on cognition in memory-impaired older adults: modulation by APOE genotype. Neurobiol Aging 2006, 27, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Reger, M.A.; et al. Intranasal insulin improves cognition and modulates beta-amyloid in early AD. Neurology 2008, 70, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Claxton, A.; et al. Long Acting Intranasal Insulin Detemir Improves Cognition for Adults with Mild Cognitive Impairment or Early-Stage Alzheimer's Disease Dementia. J Alzheimers Dis 2015, 45, 1269–1270. [Google Scholar] [CrossRef]
- Craft, S.; et al. Safety, Efficacy, and Feasibility of Intranasal Insulin for the Treatment of Mild Cognitive Impairment and Alzheimer Disease Dementia: A Randomized Clinical Trial. JAMA Neurol 2020, 77, 1099–1109. [Google Scholar] [CrossRef]
- Sato, T.; et al. Efficacy of PPAR-gamma agonist pioglitazone in mild Alzheimer disease. Neurobiol Aging 2011, 32, 1626–1633. [Google Scholar] [CrossRef]
- Watson, G.S.; et al. Preserved cognition in patients with early Alzheimer disease and amnestic mild cognitive impairment during treatment with rosiglitazone: a preliminary study. Am J Geriatr Psychiatry 2005, 13, 950–958. [Google Scholar] [CrossRef]
- Liu, J.; Wang, L.N.; Jia, J.P. Peroxisome Proliferator-Activated Receptor-Gamma Agonists for Alzheimer's Disease and Amnestic Mild Cognitive Impairment: A Systematic Review and Meta-Analysis. Drugs & Aging 2015, 32, 57–65. [Google Scholar]
- Risner, M.E.; et al. Efficacy of rosiglitazone in a genetically defined population with mild-to-moderate Alzheimer's disease. Pharmacogenomics Journal 2006, 6, 246–254. [Google Scholar] [CrossRef]
- Harrington, C.; et al. Rosiglitazone Does Not Improve Cognition or Global Function when Used as Adjunctive Therapy to AChE Inhibitors in Mild-to-Moderate Alzheimer's Disease: Two Phase 3 Studies. Current Alzheimer Research 2011, 8, 592–606. [Google Scholar] [CrossRef]
- Lu, X.Y.; et al. Metformin Ameliorates A beta Pathology by Insulin-Degrading Enzyme in a Transgenic Mouse Model of Alzheimer's Disease. Oxidative Medicine and Cellular Longevity 2020. [Google Scholar]
- Ou, Z.R.; et al. Metformin treatment prevents amyloid plaque deposition and memory impairment in APP/PS1 mice. Brain Behavior and Immunity 2018, 69, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Kickstein, E.; et al. Biguanide metformin acts on tau phosphorylation via mTOR/protein phosphatase 2A (PP2A) signaling. Proceedings of the National Academy of Sciences of the United States of America 2010, 107, 21830–21835. [Google Scholar] [CrossRef] [PubMed]
- Nabizadeh, F.; et al. Metformin use and brain atrophy in nondemented elderly individuals with diabetes. Exp Gerontol 2022, 166, 111890. [Google Scholar] [CrossRef]
- Koenig, A.M.; et al. Effects of the Insulin Sensitizer Metformin in Alzheimer Disease: Pilot Data From a Randomized Placebo-controlled Crossover Study. Alzheimer Dis Assoc Disord 2017, 31, 107–113. [Google Scholar] [CrossRef]
- Han, F.; et al. The vanadium (IV) compound rescues septo-hippocampal cholinergic neurons from neurodegeneration in olfactory bulbectomized mice. Neuroscience 2008, 151, 671–679. [Google Scholar] [CrossRef]
- Dong, Y.; et al. Anti-diabetic vanadyl complexes reduced Alzheimer's disease pathology independent of amyloid plaque deposition. Sci China Life Sci 2019, 62, 126–139. [Google Scholar] [CrossRef]
- He, Z.; et al. Bis(ethylmaltolato)oxidovanadium (IV) attenuates amyloid-beta-mediated neuroinflammation by inhibiting NF-kappaB signaling pathway via a PPARgamma-dependent mechanism. Metallomics 2021, 13. [Google Scholar]
- He, Z.J.; et al. Bis(ethylmaltolato)oxidovanadium (IV) mitigates neuronal apoptosis resulted from amyloid-beta induced endoplasmic reticulum stress through activating peroxisome proliferator-activated receptor gamma. Journal of Inorganic Biochemistry 2020, 208. [Google Scholar] [CrossRef]
- He, Z.J.; et al. The Protective Effect of Vanadium on Cognitive Impairment and the Neuropathology of Alzheimer's Disease in APPSwe/PS1dE9 Mice. Frontiers in Molecular Neuroscience 2020, 13. [Google Scholar] [CrossRef]
- He, Z.J.; et al. Bis(ethylmaltolato)oxidovanadium (IV) alleviates neuronal apoptosis through regulating peroxisome proliferator-activated receptor gamma in a triple transgenic animal model of Alzheimer's disease. Journal of Biological Inorganic Chemistry 2021, 26, 551–568. [Google Scholar] [CrossRef] [PubMed]
- He, Z.J.; et al. Bis(ethylmaltolato)oxidovanadium(iv) inhibited the pathogenesis of Alzheimer's disease in triple transgenic model mice (vol 71, pg 521, 2020). Metallomics 2020, 12, 631–631. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; et al. Is the hypoglycemic action of vanadium compounds related to the suppression of feeding? Biol Trace Elem Res 2014, 157, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Spires-Jones, T.L.; et al. Tau pathophysiology in neurodegeneration: a tangled issue. Trends Neurosci 2009, 32, 150–159. [Google Scholar] [CrossRef]
- Prokopovich, D.V.; et al. Impact of Phosphorylation and Pseudophosphorylation on the Early Stages of Aggregation of the Microtubule-Associated Protein Tau. Journal of Physical Chemistry B 2017, 121, 2095–2103. [Google Scholar] [CrossRef]
- Chang, E.; et al. Pseudophosphorylation of tau protein directly modulates its aggregation kinetics. Biochimica Et Biophysica Acta-Proteins and Proteomics 2011, 1814, 388–395.
- SantaCruz, K.; et al. Tau suppression in a neurodegenerative mouse model improves memory function. Science 2005, 309, 476–481. [Google Scholar] [CrossRef]
- Cieri, D.; et al. Tau localises within mitochondrial sub-compartments and its caspase cleavage affects ER-mitochondria interactions and cellular Ca2+ handling. Biochimica Et Biophysica Acta-Molecular Basis of Disease 2018, 1864, 3247–3256. [Google Scholar] [CrossRef]
- David, D.C.; et al. Proteomic and functional analyses reveal a mitochondrial dysfunction in P301L Tau transgenic mice. Journal of Biological Chemistry 2005, 280, 23802–23814. [Google Scholar] [CrossRef]
- Esteras, N.; et al. Mitochondrial hyperpolarization in iPSC-derived neurons from patients of FTDP-17 with 10+16 MAPT mutation leads to oxidative stress and neurodegeneration. Redox Biology 2017, 12, 410–422. [Google Scholar] [CrossRef]
- Manczak, M.; Reddy, P.H. Abnormal interaction between the mitochondrial fission protein Drp1 and hyperphosphorylated tau in Alzheimers disease neurons: implications for mitochondrial dysfunction and neuronal damage. Human Molecular Genetics 2012, 21, 2538–2547. [Google Scholar] [CrossRef]
- Gamblin, T.C.; et al. Caspase cleavage of tau: Linking amyloid and neurofibrillary tangles in Alzheimer's disease. Proceedings of the National Academy of Sciences of the United States of America 2003, 100, 10032–10037. [Google Scholar] [CrossRef]
- de Calignon, A.; et al. Caspase activation precedes and leads to tangles. Nature 2010, 464, 1201–U123. [Google Scholar] [CrossRef] [PubMed]
- Quintanilla, R.A.; et al. Caspase-cleaved Tau Expression Induces Mitochondrial Dysfunction in Immortalized Cortical Neurons IMPLICATIONS FOR THE PATHOGENESIS OF ALZHEIMER DISEASE. Journal of Biological Chemistry 2009, 284, 18754–18766. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.J.; et al. Caspase-Cleaved Tau Impairs Mitochondrial Dynamics in Alzheimer's Disease. Molecular Neurobiology 2018, 55, 1004–1018. [Google Scholar] [CrossRef] [PubMed]
- Li, X.C.; et al. Human wild-type full-length tau accumulation disrupts mitochondrial dynamics and the functions via increasing mitofusins. Scientific Reports 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Kandimalla, R.; et al. Reduced dynamin-related protein 1 protects against phosphorylated Tau-induced mitochondrial dysfunction and synaptic damage in Alzheimer's disease. Human Molecular Genetics 2016, 25, 4881–4897. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; et al. The Protective Effect of Icariin on Mitochondrial Transport and Distribution in Primary Hippocampal Neurons from 3 x Tg-AD Mice. International Journal of Molecular Sciences 2016, 17. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; et al. Impaired Balance of Mitochondrial Fission and Fusion in Alzheimer's Disease. Journal of Neuroscience 2009, 29, 9090–9103. [Google Scholar] [CrossRef] [PubMed]
- Kandimalla, R.; et al. Hippocampal phosphorylated tau induced cognitive decline, dendritic spine loss and mitochondrial abnormalities in a mouse model of Alzheimer's disease. Human Molecular Genetics 2018, 27, 30–40. [Google Scholar] [CrossRef]
- Abtahi, S.L.; Masoudi, R.; Haddadi, M. The distinctive role of tau and amyloid beta in mitochondrial dysfunction through alteration in Mfn2 and Drp1 mRNA Levels: A comparative study in Drosophila melanogaster. Gene 2020, 754. [Google Scholar] [CrossRef]
- Yin, J.X.; et al. Effect of ApoE isoforms on mitochondria in Alzheimer disease. Neurology 2020, 94, E2404–E2411. [Google Scholar] [CrossRef]
- Song, J.Y.; et al. The association between MFN2 (mitofusin 2) gene polymorphism and late-onset Alzheimer's disease in Korean population. European Neuropsychopharmacology 2015, 25, S169–S169. [Google Scholar] [CrossRef]
- Kim, Y.J.; et al. Association between Mitofusin 2 Gene Polymorphisms and Late-Onset Alzheimer's Disease in the Korean Population. Psychiatry Investigation 2017, 14, 81–85. [Google Scholar] [CrossRef]
- Wang, L.W.; et al. Mitochondrial Fusion Suppresses Tau Pathology-Induced Neurodegeneration and Cognitive Decline. Journal of Alzheimers Disease 2021, 84, 1057–1069. [Google Scholar] [CrossRef]
- Jara, C.; et al. Genetic ablation of tau improves mitochondrial function and cognitive abilities in the hippocampus. Redox Biology 2018, 18, 279–294. [Google Scholar] [CrossRef]
- Li, Y.J.; et al. Structural insights of human mitofusin-2 into mitochondrial fusion and CMT2A onset. Nature Communications 2019, 10. [Google Scholar] [CrossRef]
- Sidarala, V.; et al. Mitofusin 1 and 2 regulation of mitochondrial DNA content is a critical determinant of glucose homeostasis. Nature Communications 2022, 13. [Google Scholar] [CrossRef]
- Barbosa, D.J.; et al. MDMA impairs mitochondrial neuronal trafficking in a Tau- and Mitofusin2/Drp1-dependent manner. Archives of Toxicology 2014, 88, 1561–1572. [Google Scholar] [CrossRef]
- Tracy, T.E.; et al. Tau interactome maps synaptic and mitochondrial processes associated with neurodegeneration. Cell 2022, 185, 712–+. [Google Scholar] [CrossRef]
- Drummond, E.; et al. Phosphorylated tau interactome in the human Alzheimer's disease brain. Brain 2020, 143, 2803–2817. [Google Scholar] [CrossRef]
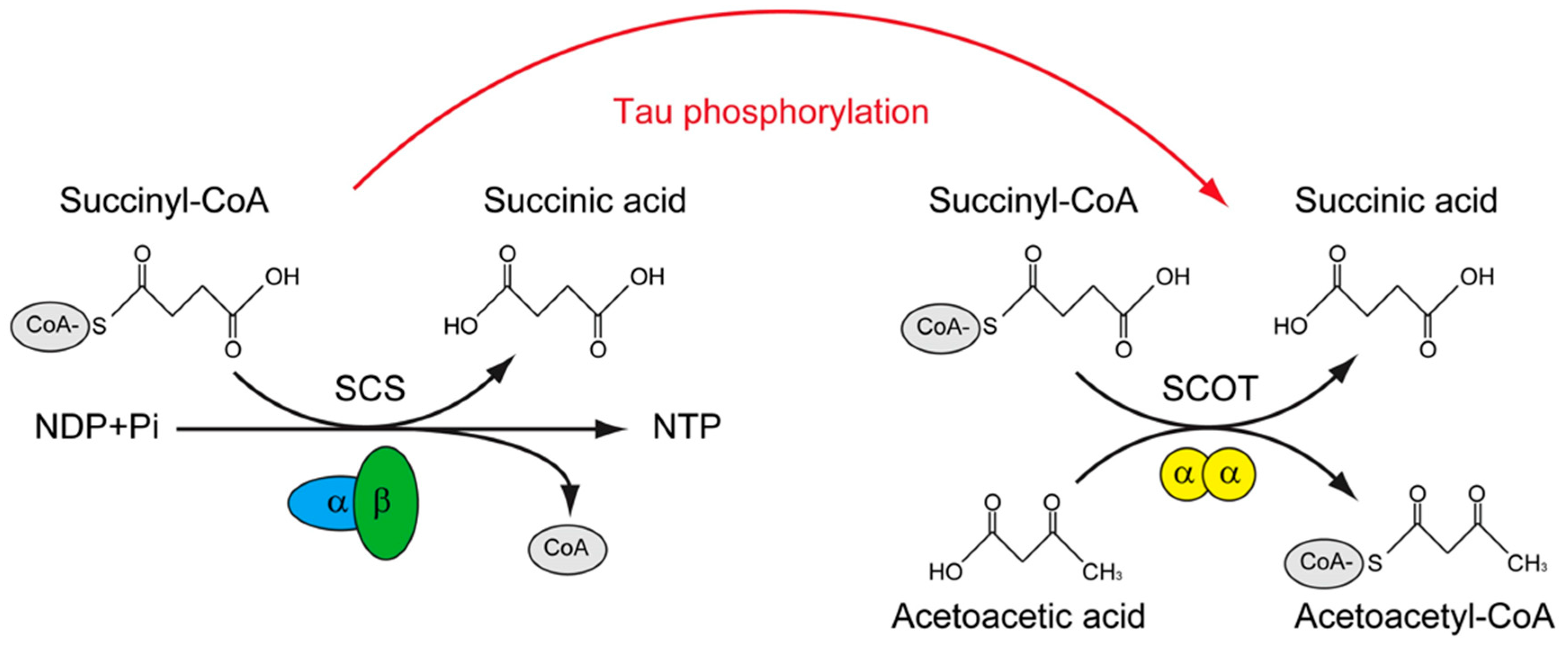
- Zhang, F.; et al. Identification of Potential Therapeutic Targets of Alzheimer's Disease By Weighted Gene Co-Expression Network Analysis. Chin Med Sci J 2020, 35, 330–341. [Google Scholar]
- Ramirez, A.; et al. SUCLG2 identified as both a determinator of CSF A beta(1-42) levels and an attenuator of cognitive decline in Alzheimer's disease. Human Molecular Genetics 2014, 23, 6644–6658. [Google Scholar] [CrossRef]
- Wang, H.; et al. Integrated analysis of ultra-deep proteomes in cortex, cerebrospinal fluid and serum reveals a mitochondrial signature in Alzheimer's disease. Molecular Neurodegeneration 2020, 15. [Google Scholar] [CrossRef]
- Huang, J.; Fraser, M.E. The structure of succinyl-CoA synthetase bound to the succinyl-phosphate intermediate clarifies the catalytic mechanism of ATP-citrate lyase. Acta Crystallogr F Struct Biol Commun 2022, 78 Pt 10 Pt 10, 363–370. [Google Scholar] [CrossRef]
- Alghamdi, M.A.; et al. Clinical variability and outcome of succinyl-CoA:3-ketoacid CoA transferase deficiency caused by a single OXCT1 mutation: Report of 17 cases. JIMD Rep 2021, 62, 91–96. [Google Scholar] [CrossRef]
- Meshkini, A.; Yazdanparast, R.; Nouri, K. Intracellular GTP level determines cell's fate toward differentiation and apoptosis. Toxicol Appl Pharmacol 2011, 253, 188–196. [Google Scholar] [CrossRef]
- Dagher, P.C. Apoptosis in ischemic renal injury: Roles of GTP depletion and p53. Kidney International 2004, 66, 506–509. [Google Scholar] [CrossRef]
- Cleland, M.M.; et al. Bcl-2 family interaction with the mitochondrial morphogenesis machinery. Cell Death Differ 2011, 18, 235–247. [Google Scholar] [CrossRef]
- Hoppins, S.; et al. The soluble form of Bax regulates mitochondrial fusion via MFN2 homotypic complexes. Mol Cell 2011, 41, 150–160. [Google Scholar] [CrossRef]
- Brooks, C.; et al. Bak regulates mitochondrial morphology and pathology during apoptosis by interacting with mitofusins. Proc Natl Acad Sci U S A 2007, 104, 11649–11654. [Google Scholar] [CrossRef]
- Pallo, S.P.; Johnson, G.V. Tau facilitates Abeta-induced loss of mitochondrial membrane potential independent of cytosolic calcium fluxes in mouse cortical neurons. Neurosci Lett 2015, 597, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Quintanilla, R.A.; et al. Phosphorylated tau potentiates Abeta-induced mitochondrial damage in mature neurons. Neurobiol Dis 2014, 71, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Lopes, S.; et al. Tau Deletion Prevents Stress-Induced Dendritic Atrophy in Prefrontal Cortex: Role of Synaptic Mitochondria. Cerebral Cortex 2017, 27, 2580–2591. [Google Scholar] [CrossRef]
- He, Z.; et al. An Adequate Supply of Bis(ethylmaltolato)oxidovanadium(IV) Remarkably Reversed the Pathological Hallmarks of Alzheimer's Disease in Triple-Transgenic Middle-Aged Mice. Biol Trace Elem Res 2022, 200, 3248–3264. [Google Scholar] [CrossRef]
- Fasulo, L.; Ugolini, G.; Cattaneo, A. Apoptotic effect of caspase-3 cleaved tau in hippocampal neurons and its potentiation by tau FTDP-mutation N279K. Journal of Alzheimers Disease 2005, 7, 3–13. [Google Scholar] [CrossRef]
- Ho, K.H.; et al. Glucose Regulates Microtubule Disassembly and the Dose of Insulin Secretion via Tau Phosphorylation. Diabetes 2020, 69, 1936–1947. [Google Scholar] [CrossRef]
- Benderradji, H.; et al. Impaired Glucose Homeostasis in a Tau Knock-In Mouse Model. Frontiers in Molecular Neuroscience 2022, 15. [Google Scholar]
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer's disease at 25 years. EMBO Mol Med 2016, 8, 595–608. [Google Scholar] [CrossRef]
- Swerdlow, R.H.; Burns, J.M.; Khan, S.M. The Alzheimer's disease mitochondrial cascade hypothesis. J Alzheimers Dis 2010, 20 (Suppl 2), S265–279. [Google Scholar] [CrossRef] [PubMed]
- Bartus, R.T.; et al. The cholinergic hypothesis of geriatric memory dysfunction. Science 1982, 217, 408–414. [Google Scholar] [CrossRef]
- McGeer, P.L.; Rogers, J.; McGeer, E.G. , Neuroimmune mechanisms in Alzheimer disease pathogenesis. Alzheimer Dis Assoc Disord 1994, 8, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; et al. Alzheimer's disease: the two-hit hypothesis. Lancet Neurol 2004, 3, 219–226. [Google Scholar] [CrossRef]
- de la Monte, S.M. Insulin resistance and Alzheimer's disease. BMB Rep 2009, 42, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer's Association Calcium Hypothesis, W. Calcium Hypothesis of Alzheimer's disease and brain aging: A framework for integrating new evidence into a comprehensive theory of pathogenesis. Alzheimers Dement 2017, 13, 178–182.e17. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




