Submitted:
20 July 2023
Posted:
21 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Published study search and selection criteria
2.2. Data extraction
2.3. Quality assessment
2.4. Statistical analysis
3. Results
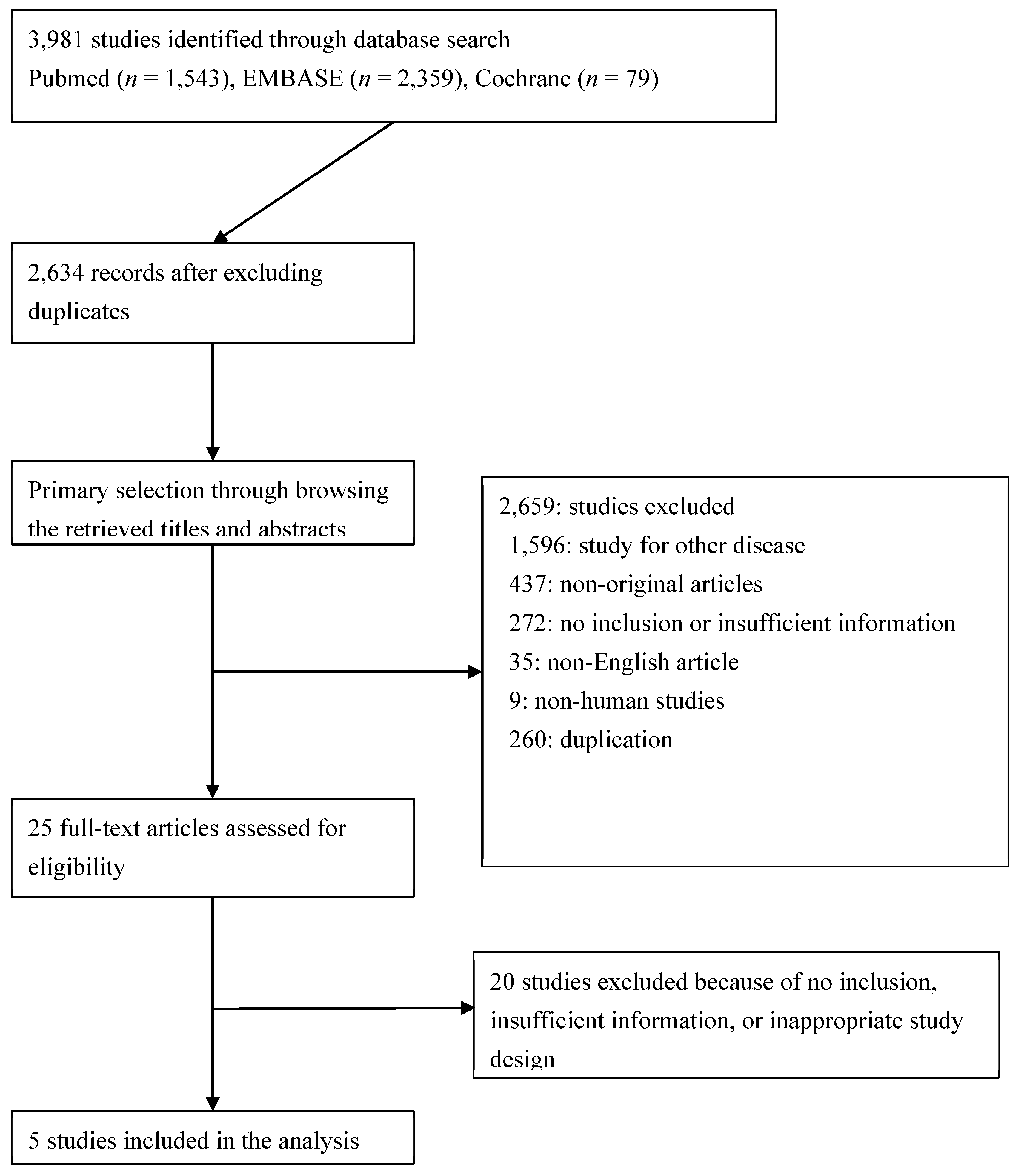
3.1. Selection and characteristics
3.2. Included and excluded studies
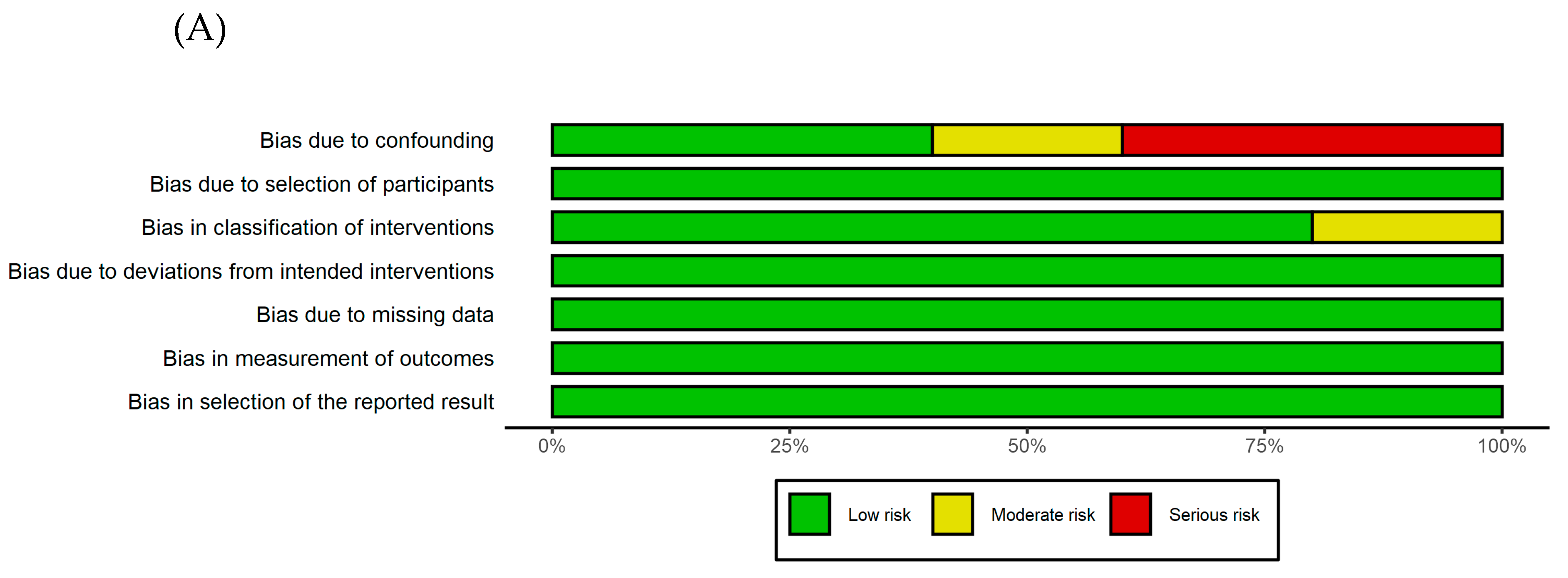
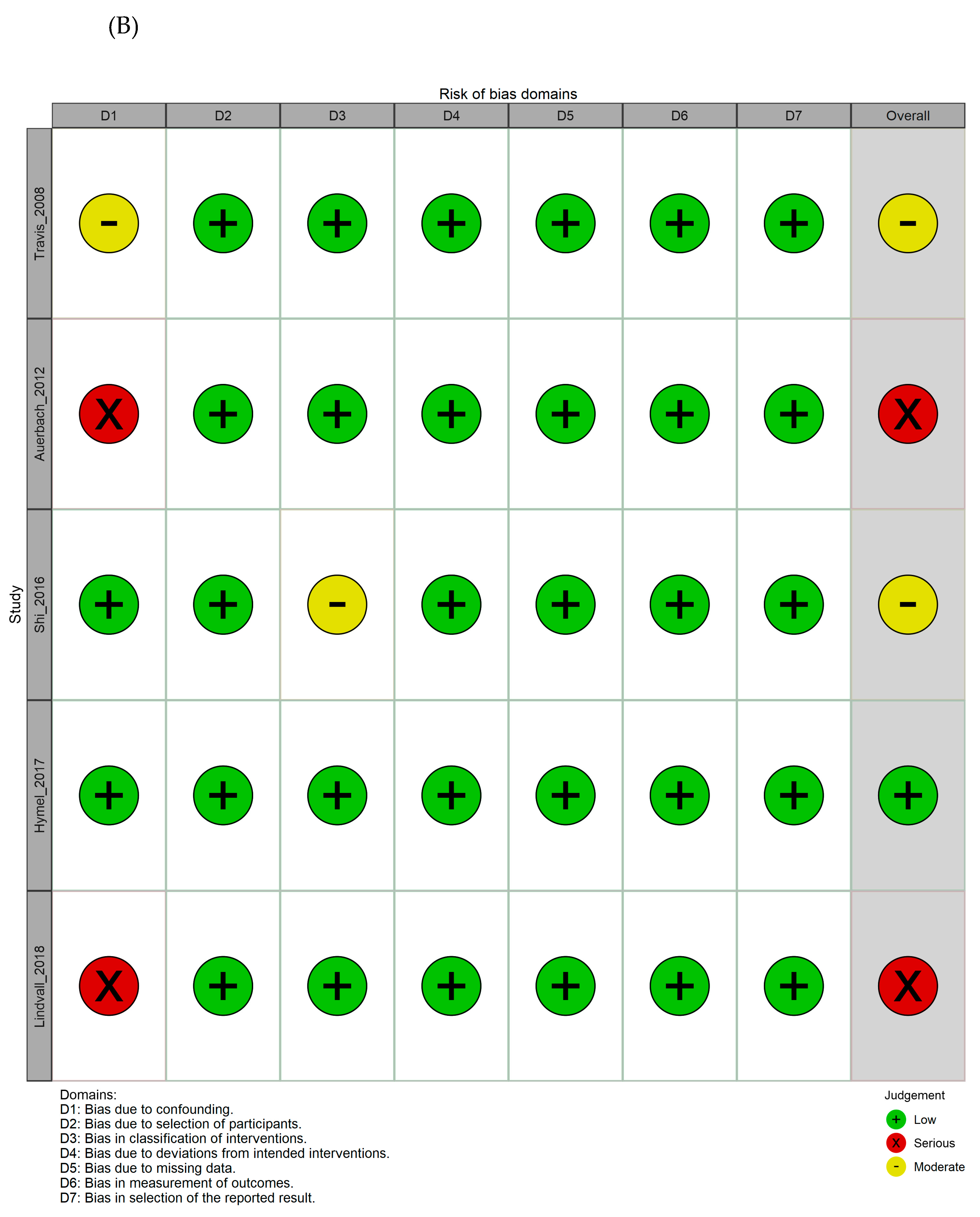
3.3. Quality assessment
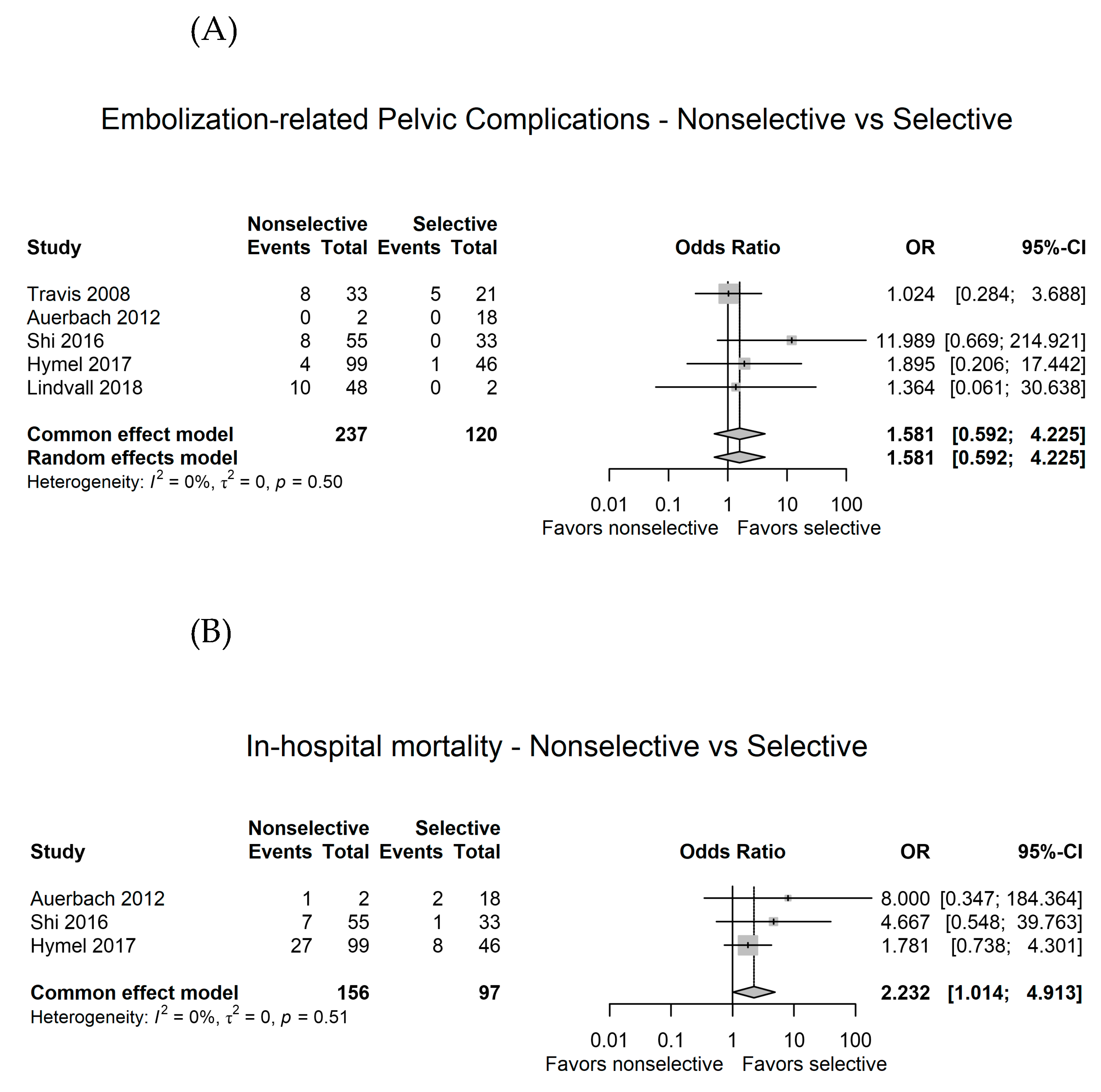
3.4. Comparison between nonselective and selective angioembolization for pelvic injury with hemorrhage
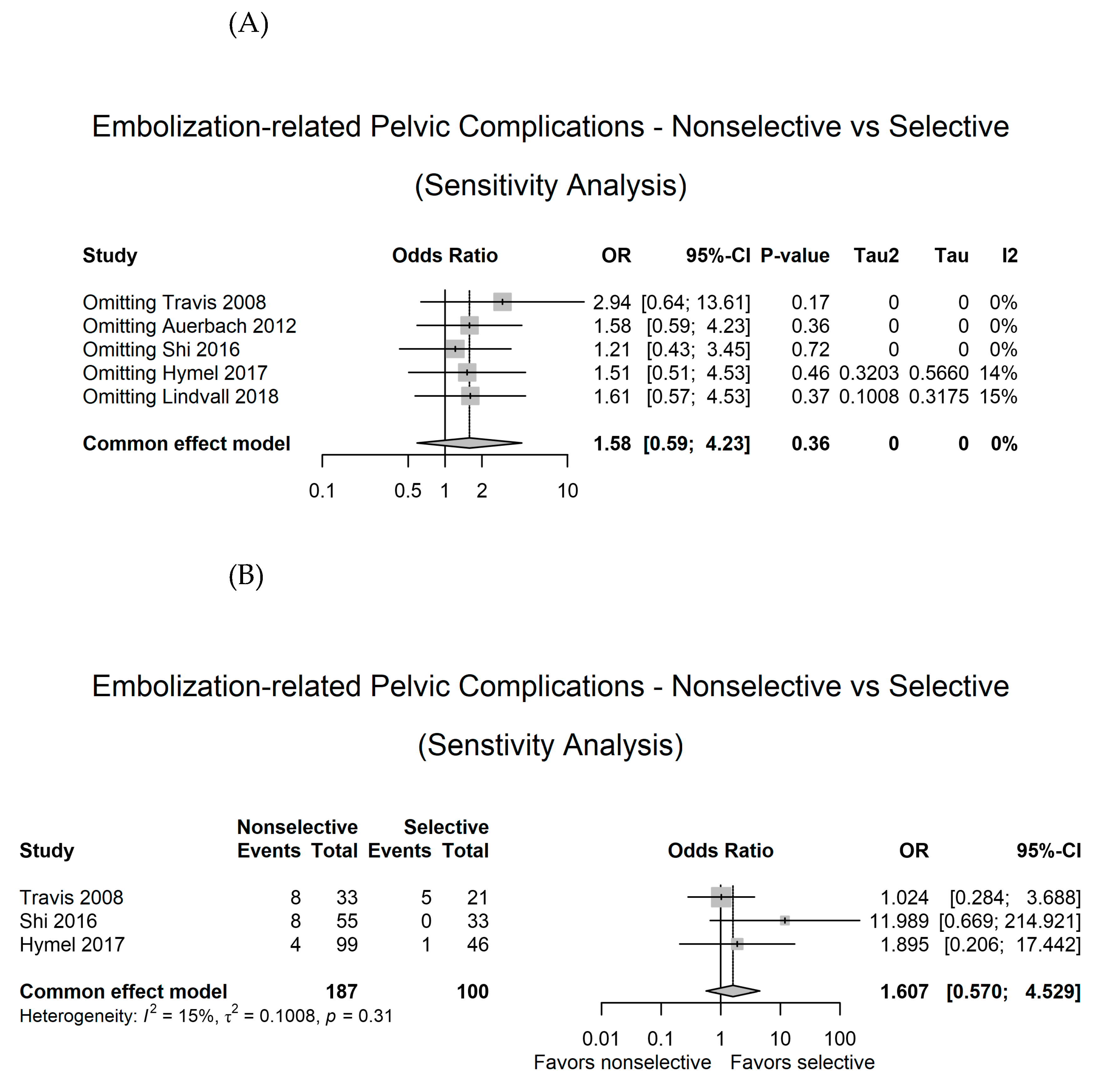
3.5. Sensitivity analysis
4. Discussion
5. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tran, T.L.N.; Brasel, K.J.; Karmy-Jones, R.; Rowell, S.; Schreiber, M.A.; Shatz, D.V.; Albrecht, R.M.; Cohen, M.J.; DeMoya, M.A.; Biffl, W.L.; et al. Western Trauma Association Critical Decisions in Trauma: Management of Pelvic Fracture with Hemodynamic Instability-2016 Updates. J. Trauma Acute Care Surg. 2016, 81, 1171–1174. [Google Scholar] [CrossRef]
- Moon, S.N.; Pyo, J.-S.; Kang, W.S. Accuracy of Contrast Extravasation on Computed Tomography for Diagnosing Severe Pelvic Hemorrhage in Pelvic Trauma Patients: A Meta-Analysis. Med. Kaunas Lith. 2021, 57, 63. [Google Scholar] [CrossRef]
- Papakostidis, C.; Kanakaris, N.; Dimitriou, R.; Giannoudis, P.V. The Role of Arterial Embolization in Controlling Pelvic Fracture Haemorrhage: A Systematic Review of the Literature. Eur. J. Radiol. 2012, 81, 897–904. [Google Scholar] [CrossRef]
- Choi, H.; Kim, J.; Yeo, K.; Kwon, O.; Kim, K.; Kang, W.S. Quality Monitoring of Resuscitative Endovascular Balloon Occlusion of the Aorta Using Cumulative Sum Analysis in Korea: A Case Series. J. Trauma Inj. 2022, 36, 78–86. [Google Scholar] [CrossRef]
- Jung, P.Y.; Yu, B.; Park, C.-Y.; Chang, S.W.; Kim, O.H.; Kim, M.; Kwon, J.; Lee, G.J. Clinical Practice Guideline for the Treatment of Traumatic Shock Patients from the Korean Society of Traumatology. J. Trauma Inj. 2020, 33, 1–12. [Google Scholar] [CrossRef]
- Anand, T.; El-Qawaqzeh, K.; Nelson, A.; Hosseinpour, H.; Ditillo, M.; Gries, L.; Castanon, L.; Joseph, B. Association Between Hemorrhage Control Interventions and Mortality in US Trauma Patients With Hemodynamically Unstable Pelvic Fractures. JAMA Surg. 2023, 158, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Lopera, J.E. Embolization in Trauma: Review of Basic Principles and Techniques. Semin. Interv. Radiol. 2021, 38, 18–33. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. PRISMA Group Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A Tool for Assessing Risk of Bias in Non-Randomised Studies of Interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. Introduction to Meta-Analysis; John Wiley & Sons, 2021; ISBN 978-1-119-55838-5. [Google Scholar]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring Inconsistency in Meta-Analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Lau, J.; Ioannidis, J.P.A.; Terrin, N.; Schmid, C.H.; Olkin, I. The Case of the Misleading Funnel Plot. BMJ 2006, 333, 597–600. [Google Scholar] [CrossRef] [PubMed]
- Travis, T.; Monsky, W.L.; London, J.; Danielson, M.; Brock, J.; Wegelin, J.; Link, D.P. Evaluation of Short-Term and Long-Term Complications after Emergent Internal Iliac Artery Embolization in Patients with Pelvic Trauma. J. Vasc. Interv. Radiol. JVIR 2008, 19, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Auerbach, A.D.; Rehman, S.; Kleiner, M.T. Selective Transcatheter Arterial Embolization of the Internal Iliac Artery Does Not Cause Gluteal Necrosis in Pelvic Trauma Patients. J. Orthop. Trauma 2012, 26, 290–295. [Google Scholar] [CrossRef]
- Shi, J.; Gomes, A.; Lee, E.; Kee, S.; Moriarty, J.; Cryer, H.; McWilliams, J. Complications after Transcatheter Arterial Embolization for Pelvic Trauma: Relationship to Level and Laterality of Embolization. Eur. J. Orthop. Surg. Traumatol. Orthop. Traumatol. 2016, 26, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Hymel, A.; Asturias, S.; Zhao, F.; Bliss, R.; Moran, T.; Marshall, R.H.; Benjamin, E.; Phelan, H.A.; Krause, P.C.; Marecek, G.S.; et al. Selective versus Nonselective Embolization versus No Embolization in Pelvic Trauma: A Multicenter Retrospective Cohort Study. J. Trauma Acute Care Surg. 2017, 83, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Lindvall, E.; Davis, J.; Martirosian, A.; Garcia, G.; Husak, L. Bilateral Internal Iliac Artery Embolization Results in an Unacceptably High Rate of Complications in Patients Requiring Pelvic/Acetabular Surgery. J. Orthop. Trauma 2018, 32, 445–451. [Google Scholar] [CrossRef]
- Velmahos, G.C.; Chahwan, S.; Hanks, S.E.; Murray, J.A.; Berne, T.V.; Asensio, J.; Demetriades, D. Angiographic Embolization of Bilateral Internal Iliac Arteries to Control Life-Threatening Hemorrhage after Blunt Trauma to the Pelvis. Am. Surg. 2000, 66, 858–862. [Google Scholar] [CrossRef]
- Takahira, N.; Shindo, M.; Tanaka, K.; Nishimaki, H.; Ohwada, T.; Itoman, M. Gluteal Muscle Necrosis Following Transcatheter Angiographic Embolisation for Retroperitoneal Haemorrhage Associated with Pelvic Fracture. Injury 2001, 32, 27–32. [Google Scholar] [CrossRef]
- Suzuki, T.; Shindo, M.; Kataoka, Y.; Kobayashi, I.; Nishimaki, H.; Yamamoto, S.; Uchino, M.; Takahira, N.; Yokoyama, K.; Soma, K. Clinical Characteristics of Pelvic Fracture Patients with Gluteal Necrosis Resulting from Transcatheter Arterial Embolization. Arch. Orthop. Trauma Surg. 2005, 125, 448–452. [Google Scholar] [CrossRef]
- Fu, C.-Y.; Hsieh, C.-H.; Wu, S.-C.; Chen, R.-J.; Wang, Y.-C.; Shih, C.-H.; Huang, H.-C.; Huang, J.-C.; Tsuo, H.-C.; Tung, H.-J. Anterior-Posterior Compression Pelvic Fracture Increases the Probability of Requirement of Bilateral Embolization. Am. J. Emerg. Med. 2013, 31, 42–49. [Google Scholar] [CrossRef]
- Bonde, A.; Velmahos, A.; Kalva, S.P.; Mendoza, A.E.; Kaafarani, H.M.A.; Nederpelt, C.J. Bilateral Internal Iliac Artery Embolization for Pelvic Trauma: Effectiveness and Safety. Am. J. Surg. 2020, 220, 454–458. [Google Scholar] [CrossRef]
- Lai, C.-Y.; Tseng, I.-C.; Su, C.-Y.; Hsu, Y.-H.; Chou, Y.-C.; Chen, H.-W.; Yu, Y.-H. High Incidence of Surgical Site Infection May Be Related to Suboptimal Case Selection for Non-Selective Arterial Embolization during Resuscitation of Patients with Pelvic Fractures: A Retrospective Study. BMC Musculoskelet. Disord. 2020, 21, 335. [Google Scholar] [CrossRef] [PubMed]
- Maruhashi, T.; Kashimi, F.; Kotoh, R.; Kasahara, S.; Minehara, H.; Kataoka, Y.; Nishimaki, H.; Asari, Y. Novel Transcatheter Arterial Embolization Method for Hemodynamically Unstable Pelvic Fractures to Prevent Complications of Gluteal Necrosis. Eur. J. Trauma Emerg. Surg. Off. Publ. Eur. Trauma Soc. 2020, 46, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.-F.; Shih, L.-Y.; Wong, Y.-C.; Lin, B.-C.; Hsu, Y.-P. Repeat Transcatheter Arterial Embolization for the Management of Pelvic Arterial Hemorrhage. J. Trauma 2009, 66, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Zhu, R.; Ghaffarian, A.; Wu, W.; Weng, C.; Chen, X.; Shalhub, S.; Starnes, B.W.; Zhang, W.W. A Systematic Review and Meta-Analysis of the Clinical Effectiveness and Safety of Unilateral versus Bilateral Iliac Branch Devices for Aortoiliac and Iliac Artery Aneurysms. J. Vasc. Surg. 2022, 76, 1089–1098.e8. [Google Scholar] [CrossRef]
- Tanizaki, S.; Maeda, S.; Matano, H.; Sera, M.; Nagai, H.; Ishida, H. Time to Pelvic Embolization for Hemodynamically Unstable Pelvic Fractures May Affect the Survival for Delays up to 60 Min. Injury 2014, 45, 738–741. [Google Scholar] [CrossRef]
| Author | Year | Location | Study period | Study design | Inclusion and exclusion | Hemodynamic status | Injury severity | Modality (embolic agent) | Indication of non-selective embolization | Primary and secondary outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| Travis [13] | 2008 | USA | 1994-2006 | obs, single center, comparative |
Inclusion: Patients who underwent pelvic angiography after blunt trauma Exclusion: NR |
NR |
ISS: NR Pelvic AIS: NR |
Nonselective (BIIA or UIIA) (33 patients) vs. selective embolization (21 patients) (gelfoam) | NR | Shor-term outcome (<30 day) – pelvic or perineal infeciton, nerve damage, skin necrosis; Long-term outcome (> 30 days) – pain, paresthesia, ulceration |
| Auerbach [14] | 2012 | USA | 2004-2009 | obs, single center, comparative |
Inclusion: Patients who underwent TAE after blunt trauma Exclusion: Death within 48 hours, No evidence of pelvic fracture |
NR |
ISS: Nonselective (13-41) vs. selective (9-29) Pelvic AIS: NR |
Nonselective (UIIA) (2 patients) vs. selective embolization (18 patients) (coil [14 procedure] or gelfoam) | NR | Ischemic complications (gluteal necrosis, infection) |
| Shi [15] | 2016 | USA | 2003-2013 | obs, single center, comparative |
Inclusion: Patients who underwent pelvic angiography Exclusion: < 18 years old, death within 48 hours, patients transffered from external hospitals, those who underwent angiography without embolization, those who had indications other than pelvic trauma, those who died from immediate complications related to their trauma (e.g., traumatic brain injury) |
Lowest SBP: Nonselective, 87.9 (±21.1) vs. selective, 90.2 (±28.4); p<0.20 |
ISS: Nonselective, 30.2 (±11.0) vs. selective, 22.7 (±7.4); p<0.01 Pelvic AIS: NR |
Nonselective (BIIA or UIIA) (55 patients) vs. selective embolization (33 patients) (gelfoam [70.1%] or coil) | NR | Major pelvic complications (gluteal necrosis, bladder necrosis, rectal necrosis, skin necrosis, surgical wound breakdown, superficial wound infection, deep soft tissue infection, abscess formation) during hospitalization or within 1 year |
| Hymel [16] | 2017 | USA | 2002-2014 | obs, multicenter (3 level 1 trauma center), comparative |
Inclusion: Blunt trauma with pelvic fracture undergoing angiography Exclusion: < 18 years old |
Initial SBP: Nonselective, 120.7 (±27.7) vs. selective, 118.9 (±29.7); p=0.727 |
ISS: Nonselective, 25.9 (±11.2) vs. selective, 27.4 (±13.9); p=0.483 Pelvic AIS: Nonselective, 2.8 (±0.9) vs. selective, 2.6 (±0.8); p=0.128 |
Nonselective (BIIA or UIIA) (99 patients) vs. selective embolization (46 patients) (no information of embolic agent) | NR | Mortality; Hemorrhagic control; Emblization-related complication (short-term: wound infection or breakdown, gluteal or skin nerosis, and osteomyelitis; long-term: claudicationm sexual dysfucntion, paresthesia, pain, urinary dysfuction, wound infection or breakdown, fracture nununion or ostermyelitis); Thromboembolic complications |
| Lindvall [17] | 2018 | USA | 2007-2014 | obs, single center, comparative |
Inclusion: Patients presenting with trauma activation with an associated pelvic and/or acetabular fracture who underwent pelvic angiography All patients underwent open reduction internal fixation of their pelvic/acetabular fractures Exclusion: Death during initial hospital stay, Loss to follow up |
NR |
ISS: NR Pelvic AIS: NR |
Nonselective (BIIA) (48 patients) vs. selective (2 patients) (gelfoam or coil) | NR | Surgical complications in patients undergoing surgical fixation of a pelvic and/or acetabular fracture after TAE |
| Author | Year | Location | Study period | Study design | Inclusion | Modality (embolic agent) | Indication of nonselective embolization | Complications | Mortality |
|---|---|---|---|---|---|---|---|---|---|
| Velmahos [18] | 2000 | USA | 1991 - 1998 | obs, single center |
Inclusion: blunt pelvic trauma with BIIA Exclusion: patients with UIIA |
Nonselective BIIA (30 patients) (all gelfoam) | 1. Patients continued to require fluid and blood transfusions despite apparently successful sub selective embolization of different branches 2. Mutiple bleeding sites bilaterally 3. hemodynamic lability precluded technically challenging and time-consuming maneuvers to occlude small branches selectively |
2 patients with hematoma at arterial access site, No severe complication, No ischmic complications, 2 patients with repeated embolization |
10 patients (only one related uncontroled bleeding) |
| Takahira [19] | 2001 | Japan | 1979 - 1999 | obs, single center | Inclusion: pelvic fracture with gluteal necrosis after BIIA | Nonselective BIIA (5) (gelfoam or coil) | Hemorrhagic shock | 5 patients with gluteal necrosis | 3 patients with gluteal necrosis (60%) |
| Suzuki [20] | 2005 | Japan | 1995 - 2003 | obs, single center |
Inclusion: pelvic fracture managed with BIIA Exclusion: death within 48 hours |
Nonselective BIIA (132 patients) (all gelfoam) | Pelvic fracture with retroperitoneal bleeding | 12 patients with gluteal muscle necrosis and skin necrosis | 4/12 deaths among those with gluteal necrosis |
| Fu [21] | 2013 | Taiwan | 2005 - 2011 | obs, multi-center (2 hostpials) | Inclusion: patients with pelvic fracture undergoing computed tomography | Nonselective BIIA (27 patients), selective UIIA (43 patients) (gelfoam or coil) | Bilateral contast extravasation on CT scan | Repeated TAE: 1/27 in nonselective BIIA, 7/43 in selective UIIA no long-term complication in nonselective BIIA, 1 skin ulcer in BIIA |
NR |
| Bonde [22] | 2020 | USA | 1998 - 2018 | obs, single center |
Inclusion: pelvic fracture managed with BIIA Exclusion: angiography without embolization, unilateral embolization |
Nonselective BIIA (61 patients) (gelfoam [all] or coil [additional]) | Pelvic fracture with significant bleeding and labile hemodynamics | 10 patients with ongoing bleeding, 4 patiens with re-angiography, No pelvic/gluteal/perineal necrosis |
6 patients died pelvic bleeding, A total of 18 patients died (30%) |
| Lai [23] | 2020 | Taiwan | 2014 - 2017 | obs, single center |
Inclusion: pelvic fracture managed with AE Exclusion: dead on arrival, isolated acetabular fracture, diagnosed with pelvic fracture without imaging, AE as a hemostatic procedure targeting non-pelvic regions |
Nonselective BIIA (97 patients) (gelfoam [generally] or coil) | Discretion of the inverventional radiologist | 9/11 SSIs related to BIIA | 18/129 (13.7%) among patients with AE |
| Maruhashi [24] | 2020 | Japan | 2005 - 2015 | obs, single center |
Inclusion: pelvic fracture managed with BIIA Exclusion: selective embolization, embolic agent other than gelatine sponge |
Nonselective BIIA (70 patients) (only gelfoam) | Hemodynamic instability or extravasation on CT | No gluteal necrosis | Overall 12/70 (17.1%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





