1. Introduction
The development of cardiology has led to increasing numbers of interventional procedures year after year. In recent decades, interventional pediatric cardiology (IPC) has evolved from a mainly diagnostic tool to a therapeutic modality that has substantially improved the prognosis of congenital cardiac malformations [
1,
2].
However, it is known that these procedures may involve high doses of radiation, which increases the potential risk of stochastic effects, especially in pediatric patients; this, given the higher sensitivity of their tissues compared to adults [
3]. In addition, IPC procedures can lead to a high risk of developing cancer due to their longer life expectancy [
3,
4].
Therefore, radiation protection strategies exist to avoid deterministic effects and reduce, as much as possible, the probability of stochastic effects [
3,
5]. This objective is embodied in three principles: justification of the study by being clear that the exposure produces a net benefit versus the potential risks; limitation of the dose to avoid exceeding established values (only applicable to personnel and the general public, no dose limits are applied to patients); and optimization to achieve doses as low as reasonably achievable, called the "ALARA" concept [
5,
6].
To assist in the optimization process related with patient radiation exposure, one of the main tools available is the use of diagnostic reference levels (DRLs) [
7]. According to report 135 by the International Commission on Radiological Protection (ICRP), DRLs are a form of investigation level used for diagnostic and interventional procedures. Such are used in medical imaging with ionizing radiation to indicate whether, under routine conditions, the amount of radiation used for a specified procedure is unusually high or low for that procedure. This last report introduced the terms “DRL quantity”, “DRL value”, and “Typical values” and recommended using a facility’s median value (rather than mean value) for DRL quantity, given that this is recognized as more robust and representative of the patient population. Also, it is correct to use the third (3
rd) quartile values as DRLs for local, national, or regional DRLs [
8].
The ICRP has recommended using DRLs for fluoroscopy-guided procedures in all institutions, including IPC procedures [
3,
8]. Ideally, each institution should be able to establish its own values, each country should do the same and, finally, these values should be grouped for each region of the world to compare them with each other. This will make it possible to identify whether the values obtained in a specific institution are higher or lower than the existing DRL and, based on these results, to assess the need to take corrective measures.
Incipient efforts have been undertaken in our region to advance in the establishment of DRLs [
7,
9,
10,
11]; however, according to our knowledge, at the Colombian level, we only have the record by Mosquera
et al., 2014 [
12]. Therefore, this work sought to obtain the first local DRLs in our institution to perform a self-assessment and optimization of radiation protocols, in addition to contributing to the establishment of national DRLs for IPC.
This work was conducted as part of the program "Optimization of Protection in Pediatric Interventional Radiology in Latin America and the Caribbean" (OPRIPALC), established in 2018, as a joint response of the Pan-American Health Organization (PAHO) and the World Health Organization (WHO) in cooperation with the International Atomic Energy Agency (IAEA), to help its member states ensure that radiation exposure of pediatric patients is the minimum necessary during fluoroscopy-guided interventional procedures [
13,
14].
2. Materials and Methods
This was an observational, descriptive case series study with retrospective data collection [
15]. Data was collected from pediatric patients undergoing hemodynamic studies between April/2020 and July/2022 at the cardiac interventional area in a tertiary referral hospital in Latin America. The pediatric cardiologists working in our hospital are experienced specialists, with two senior interventional cardiologists, with 25 and 18 years of experience, respectively.
The equipment used was an Artis FLOOR (Siemens Healthcare GmbH) installed in 2020 and equipped with a zen30 HDR detector with a high-resolution crystalline silicon matrix with 160-μm pixel size and 16-bit digitization depth, in addition to a GIGALIX X-ray tube (40 – 125 kV). The equipment has three acquisition protocols for pediatric cardiology examinations selected according to patient weight (i.e., CARD PED < 40 kg; CARD PED < 20 kg, and CARD PED < 6 kg). Furthermore, different cine mode settings (Im Single; 7.5I/s; 10I/s; 15I/s, and 30I/s) and various fluoroscopy mode settings (0.5 - 30p/s). The field sizes used during the procedures are 32 and 42 cm, respectively.
All ionizing and non-ionizing radiation-emitting equipment is supervised by the Radiological Protection Officer, who ensures compliance with the quality control program for interventional fluoroscopy equipment. Quality control is performed annually by the Radiological Protection Officer or a Medical Physicist in charge (or when equipment modifications are made, such as X-ray tube replacement). All tests applied to the equipment are described in detail in the IAEA-TECDOC-1958 document, which include evaluation of environmental conditions and radiometric survey of the room, accuracy, and repeatability of the X-ray tube voltage, verification of the air kerma rate at the interventional reference point, and validation of the dose deployed by the system, as well as image quality tests [
16].
The OPRIPALC methodology and latest ICRP recommendations to collect patient dose data and calculate local DRLs were used [
8,
10]. The first local DRLs have been obtained as the 3rd quartile values from the database containing all the collected data, as one of the options suggested by the ICRP [
8]. Patient demographic data and the baseline diagnoses for which they were undergoing IPC procedures were collected. Moreover, data were collected in terms of air kerma-area product (Pka), cumulative dose magnitude (Ka,r) [
17,
18], fluoroscopy time (FT), and several cine frames. The Pka and Ka,r for each procedure were corrected by a calibration and mean attenuation factor of 0.85, derived from the table and mattress attenuation measured for the X-ray beam qualities used in this system for pediatric procedures [
7]. The data were divided into three groups, according to the type of procedure (non-complex diagnostic, complex diagnostic, and therapeutic), grouped into five age ranges (< 1 year, 1 to < 5 years, 5 to < 10 years, 10 to < 16 years, and > 16 years) and five weight groups (< 5 kg, 5 - 15 kg, 15 - 30 kg, 30 - 50 kg, and 50 - 80 kg), according to ICRP.
The Mann-Whitney test (95% CI) was used to compare the
Pka medians for the two procedure groups (diagnostic and therapeutic). This nonparametric comparison procedure tests hypotheses and is used to find differences between two independent samples that do not necessarily have a normal distribution. Values of p < 0.05 were considered statistically significant [
19], and STATA 16
® software was used [
20].
The study was approved by the Institutional Ethics Committee (No. 1795). Due to the study's observational, descriptive, and retrospective nature, an exception to informed consent was requested.
3. Results
During the evaluation time, 255 pediatric procedures were performed (38.4% diagnostic and 61.6% therapeutic).
Table 1 shows the anthropometric characteristics of the patients.
Table 2 and
Table 3 summarize the median and 3
rd quartile values for
Pka,
Ka,r, and FT magnitude for all pediatric procedures (diagnostic and therapeutic) by weight and age bands, respectively.
Table 4 shows the median
Pka values by age group reported in this work compared to that published by other groups in similar studies.
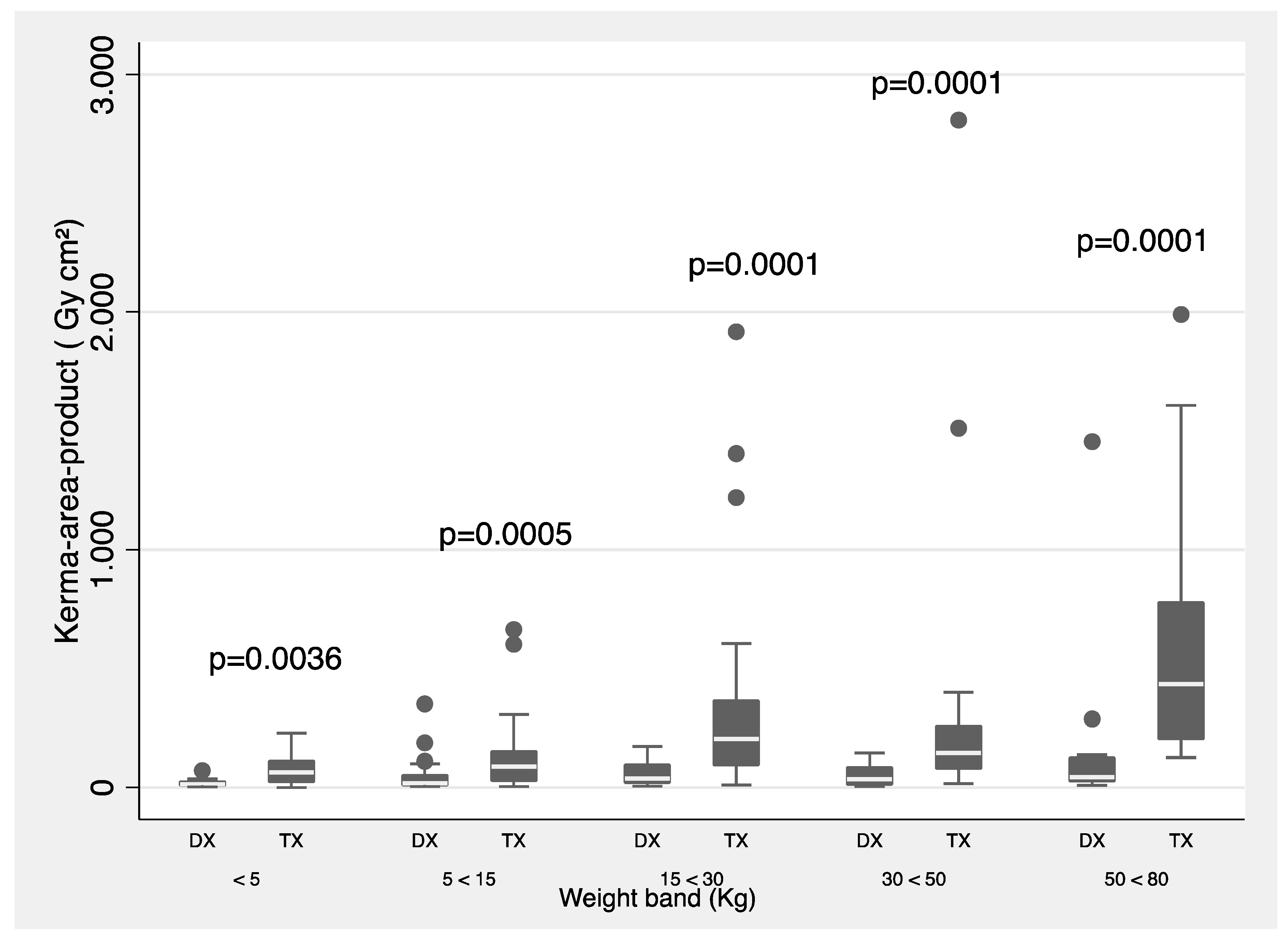
Figure 1 and
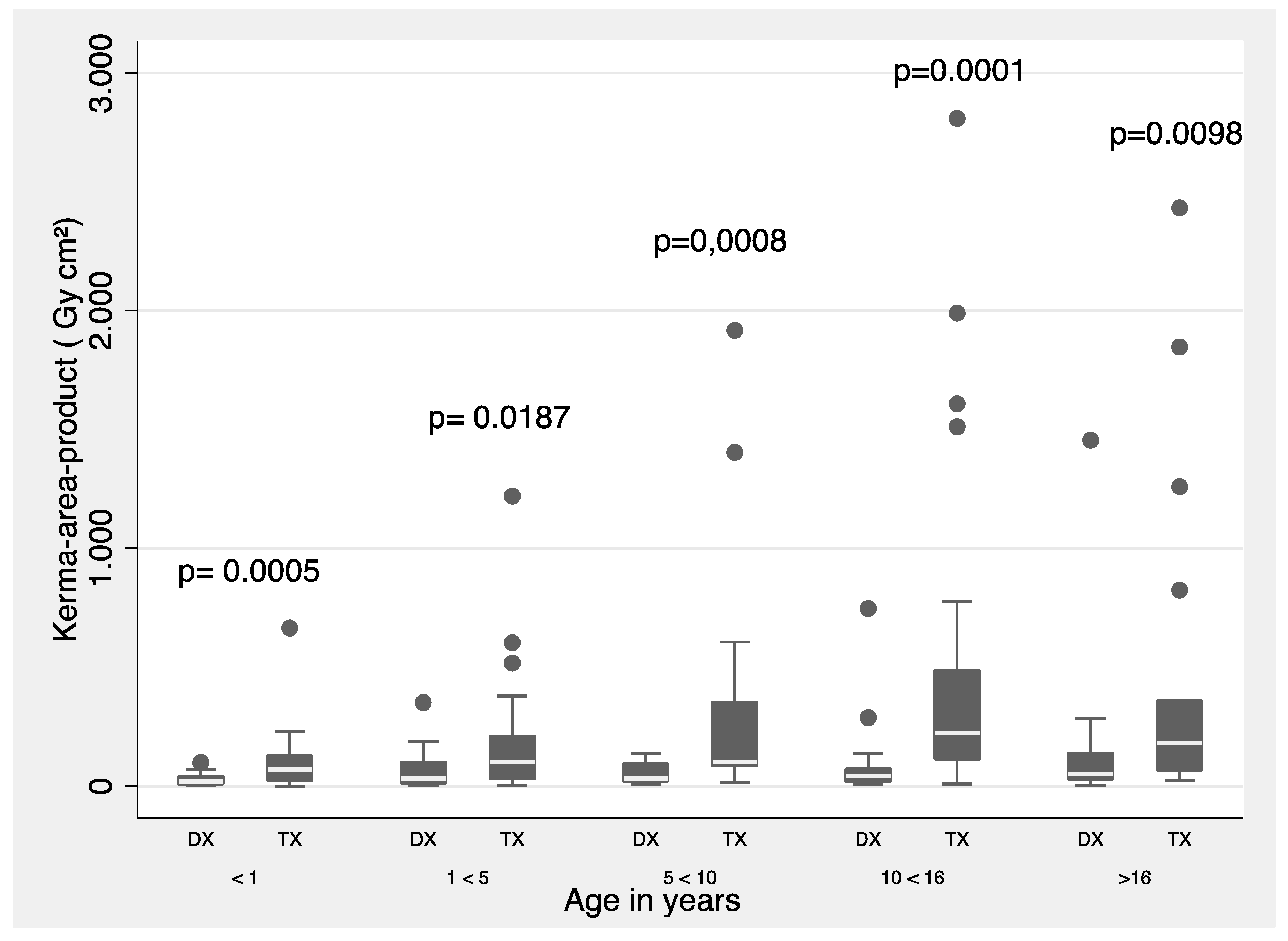
Figure 2 show a summary of 3
rd quartile
Pka values (proposed as local DRLs) separated by type of procedure (diagnostic and therapeutic) and for all procedures grouped by weight band. The Mann-Whitney test was used for weight and age groups.
Table 5 groups the procedures according to type: diagnostic (complex or non-complex) and therapeutic (atrial septal defect closure, ventricular septal defect closure, patent ductus arteriosus closure, aortopulmonary collateral embolization, aortic coarctation angioplasty, aortic or pulmonary valvotomy, and others).
4. Discussion
Pediatric cardiac catheterization is a tool of increasing importance in the diagnosis, treatment, and follow up of patients with congenital heart disease. However, the use of radiation provides inherent procedural risks and adverse side effects that could be avoided by considering controlled radiation exposure.
European DRL guidelines for pediatric imaging [
27] suggest that generic DRL levels for diagnostic and therapeutic procedures are generally inadequate. Particularly in therapeutic procedures, higher radiation levels have been evidenced with greater variation in each procedure. Therefore, current guideline recommendations propose creating specific DRLs for pediatric interventions.
Considering the foregoing, the OPRIPALC program, together with WHO, PAHO, and IAEA, conducted a study with several reference medical centers in Latin America and the Caribbean, evidencing that the difference in DRLs between diagnostic and therapeutic procedures was statistically significant between the age groups of children under 1 year and patients between 10 and 16 years of age [
10]. Hopefully, with this initiative and with the results of DRLs, these data can be used as DRLs in different health institutions, and if levels above the established regional values are found, they can be corrected and set up in countries without defined DRLs.
In this study, a 2-year follow up was performed in the pediatric cardiac catheterization laboratory to obtain DRLs, optimize radiation protocols, and provide local DRL values for IPC procedures in the country. All our age groups met the minimum number of 30 patients per group recommended for a DRL study [
8].
The results of this paper show that radiation doses have a wide range, as expected (
Table 2 and
Table 3). Local DRLs were 3.82 Gy/cm
2 (< 5 kg), 7.39 Gy/cm
2 (5 - < 15 kg), 19.72 Gy/cm
2 (15 - < 30 kg), 28.99 Gy/cm
2 (30 - < 50 kg), and 81.71 Gy/cm
2 (50-<80 kg), respectively. For age bands, the DRLs were 3.97 Gy/cm
2 (< 1 y), 9.94 Gy/cm
2 (1 - < 5 y), 20.82 Gy/cm
2 (5 - < 10 y), 58.00 Gy/cm
2 (10 - < 16 y), and 31.56 Gy/cm
2 (< 16 y), respectively. In addition, it is worth noting that all
Ka,r values are below 2,000 mGy, which complies with current recommendations and reduces the risk of skin lesions [
28].
In
Table 4, with the comparison of
Pka median values by age range reported in this paper and others reported in similar surveys, differences exist that can be explained in several ways, from differences in terms of the technology used, level of staff training, or procedure optimization. An example of the latter, are the results achieved by Calvo Mackenna Hospital that has been involved in several IAEA programs to optimize radiation dose management in IPC procedures since 2009. An optimization program has been applied for 8 to 10 years at this hospital, which has allowed it to maintain lower dose levels than those usually published elsewhere [
9]. Likewise, we consider that the results of this study allow us to continue with the process of optimization of radiological protection and continuous improvement of our institution.
A special analysis aims to compare our current results with those reported in a previous multicenter work by Kobayashi
et al., [
12] in which our institution had participated and a statistically significant difference (p < 0.05) was found when comparing DRLs between diagnostic and therapeutic procedures. Now, according to
Figure 1 and
Figure 2, statistically significant difference is shown in all weight and age ranges, except for the age group between 1 and 5 years, when comparing the
Pka between diagnostic and therapeutic procedures. Also, the 3
rd quartile values are proposed as local DRLs obtained in IPC procedures by weight an age bands.
Likewise, according to
Table 5, higher
Pka and
Ka,r values were found in the group of patients who underwent therapeutic procedures compared to those who underwent complex or non-complex diagnostic procedures. This could be related to the fact that during therapeutic procedures the complexity of the cases implies a longer time and higher radiation exposure dose [
29]. Note that Ventricular septal defect closure procedure showed the highest mean values for FT (30.3 min) and
Pka value of 69.3 Gy.cm
2, and the patent ductus arteriosus closure value was the lowest with 9.2 Gy.cm
2.
It is important to take into count that the process to set and update DRLs should be both flexible and dynamic. Flexibility is necessary for procedures where few data are available, as in interventional procedures in pediatric patients. A dynamic process is necessary to allow initial DRLs to be derived from these data while waiting for a wider survey to be conducted [
8].
Regarding the study’s limitations, it is a single-center study where, based on statistics, it was possible to establish a proposal of DRLs for diagnosis in diagnostic and therapeutic procedures for classification categories based on age and weight ranges, despite having a low number of procedures. Another variable to consider was the manual collection of data, which may add some error while typing the information. To minimize the possibility of typing error, we validated the data by looking at the DICOM dose structured report for each procedure.
In pursuit of providing state-of-the-art radiological protection, our institution has updated the fluoroscopic systems used in cardiac catheterization laboratories. Integration of bi-plane systems offers superior imaging capabilities, allowing for precise visualization during procedures. This advancement enhances diagnostic accuracy and aids in reducing radiation exposure to pediatric patients.
Efficient and accurate radiation data management is crucial to assess and optimize radiological protection measures. To this end, our institution has undertaken the implementation of an automated tool to manage radiation data. This tool is designed to gather and analyze radiation dosage data in real-time, allowing medical staff to make informed decisions during procedures; further ensuring the safety of pediatric patients.
A cornerstone of our institution's radiological protection strategy is an ongoing education program designed to equip healthcare professionals with the latest techniques in safeguarding children from ionizing radiation. This continuous education initiative covers various aspects, including radiation dose reduction techniques, proper use of shielding devices, and appropriate positioning during procedures.
Recognizing the varying sizes and anatomical considerations in pediatric patients, our institution places special attention on tailoring radiological protection measures to each age group. By refining the DAP/Weight ratio, we account for significant differences in children’s sizes, ensuring that radiation exposure is kept to a minimum while maintaining the required diagnostic quality. Future work includes complementing with DAP/Weight ratio and refining these values for the age bands, accounting for large differences in children’s sizes.
5. Conclusions
Within the framework of an international initiative (OPRIPALC) supported by WHO, PAHO, and IAEA, we obtained an initial set of institutional values of DRLs in IPC procedures for diagnostic and therapeutic procedures by weight and age groups. According to our results, radiation doses are within the ranges of other international initiatives.
It should be noted that strict radiological protection protocols have been implemented in our institution for several years, as well as using high-sensitivity angiographic systems that allow imaging at low doses.
By establishing a baseline of radiation data for cardiac procedures and meticulously implementing comprehensive quality control procedures, the institution ensures that its interventional fluoroscopy equipment operates at the highest level of performance and safety. Regular assessments and validations of equipment functionality not only enhance the accuracy and reliability of the imaging process but also contribute to the overall radiological protection of patients, particularly children undergoing cardiac catheterization procedures.
In conclusion, our institution is committed to continuously enhancing radiological protection for children undergoing cardiac catheterization procedures. By implementing advanced fluoroscopic technology, a comprehensive education program, and tailored protection measures, we ensure that pediatric patients receive the highest standard of care while minimizing their exposure to ionizing radiation. Through prioritizing research, collaboration, and data analysis, we strive to set new benchmarks for radiological protection in pediatric cardiology.
To our knowledge, this is one of the first proposals for local DRL in PIC procedures in Colombia, and we hope that it will serve as a starting point to continue with the efforts to have DRL at a national level, as well as to implement optimization actions in these procedures in pediatrics.
Author Contributions
Conceptualization, WM., JAG., CU and AMA.; methodology, VMQ., AMA.; software, VM., ER.; validation, VM., WM., CU., and AMA.; formal analysis, VM, AMA; investigation, AMA., WFM; data curation, VM.; writing—original draft preparation, AMA., WM., CU.; writing—review and editing, AMA., WM., CU.; project administration, AMA.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The Fundación Valle del Lili Review Board approves this study.
Informed Consent Statement
Patient consent was waived due to the study's observational, descriptive, and retrospective nature.
Acknowledgments
The authors thank Dina Maribel Mingan P. and Duván Gonzales for their technical support.
Conflicts of Interest
The authors declare having no conflict of interest.
References
- Kaang, S.-L.; Benson, L. Recent advances in cardiac catheterization for congenital heart disease. F1000Res 2018, 7, 370. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H. Recent advances in pediatric interventional cardiology. Korean J Pediatr 2017, 60, 237–244. [Google Scholar] [CrossRef] [PubMed]
- ICRP. International Commission on Radiological Protection. The recommendations of the international commission on radiological protection. Ann ICRP 2007, 37, 1–332. [Google Scholar] [CrossRef]
- ICRP. International Commission on Radiological Protection. Radiological protection in paediatric diagnostic and interventional radiology. Ann. ICRP 2013, 42, 1–63. [Google Scholar] [CrossRef] [PubMed]
- Connolly, B.; Racadio, J.; Towbin, R. Practice of ALARA in the pediatric interventional suite. Pediatr Radiol 2006, 36 (Suppl. S2), 163–167. [Google Scholar] [CrossRef]
- Ubeda, C. New Optimization Strategies on Radiation Protection in Fluoroscopy-Guided Interventional Procedures in Pediatrics. Children 2023, 10, 883. [Google Scholar] [CrossRef]
- Ubeda, C.; Miranda, P.; Vano, E. Local patient dose diagnostic reference levels in pediatric interventional cardiology in Chile using age bands and patient weight values. Med Phys 2015, 42, 615–622. [Google Scholar] [CrossRef]
- 8 Vañó, E.; Miller, D.L.; Martin, C.J.; Rehani, M.M.; Kang, K.; Rosenstein, M.; Ortiz-López, P.; Mattsson, S.; Padovani, R.; Rogers, A.; et al. ICRP Publication 135: Diagnostic Reference Levels in Medical Imaging. Ann ICRP 2017, 46, 1–144. [Google Scholar] [CrossRef]
- Ubeda, C.; Vano, E.; Miranda, P.; Leyton, F. Pilot program on patient dosimetry in pediatric interventional cardiology in Chile. Med Phys 2012, 39, 2424–2430. [Google Scholar] [CrossRef]
- Ubeda, C.; Vano, E.; Perez, M.D.; Jímenez, P.; Ramirez, R.; Nader, A.; Miranda, P.; Azcurra, P.; Damsky, J.; Capdevila, S.; et al. Setting up regional diagnostic reference levels for pediatric interventional cardiology in Latin America and the Caribbean countries: Preliminary results and identified challenges. J Radiol Prot 2022, 42. [Google Scholar] [CrossRef]
- Ubeda, C.; Salazar, L.; Retana, V.; Gutiérrez, R.; Santos, F.; Reyes, C. Niveles de referencia diagnósticos en procedimientos cardiológicos intervencionistas pediátricos en Costa Rica por bandas de edad. Journal of Health and Medical Sciences. 2018, 4, 203–206. [Google Scholar]
- Kobayashi, D.; Meadows, J.; Forbes, T.J.; Moore, P.; Javois, A.J.; Pedra, C.A.; Du, W.; Gruenstein, D.H.; Wax, D.F.; Hill, J.A.; et al. Standardizing radiation dose reporting in the pediatric cardiac catheterization laboratory-a multicenter study by the CCISC (Congenital Cardiovascular Interventional Study Consortium). Catheter Cardiovasc Interv 2014, 84, 785–793. [Google Scholar] [CrossRef]
- Carlos Ubeda, Eliseo Vaño, María del Rosario Pérez, Pablo Jiménez, Raúl Ramírez, Alejandro Nader, Patricia Miranda et al. Optimización de la protección en radiología y cardiología intervencionista pediatrica en América Latina y el Caribe (OPRIPALC). Journal of Health and Medical Sciences. 2021, 7, 215–221. [Google Scholar]
- Optimization of Protection in Pediatric Interventional Radiology in Latin America and the Caribbean (OPRIPALC). Available online: www.opripalc.org (accessed on 5 April 2023).
- Manterola, C.; Otzen, T. Checklist for reporting results using observational descriptive studies as research designs. The MInCir initiative. Int. J. Morphol. 2017, 35, 72–76. [Google Scholar] [CrossRef]
- Available online: https://www.pub.iaea.org/MTCD/Publications/PDF/TE-1958web.pdf; www.pub.iaea.org (accessed on 10 April 2023).
- ICRU 2005 Patient dosimetry for x-rays used in medical imaging ICRU Report 74 (Bethesda, MD: International Commission on Radiological Units and Measurements).
- IEC 2010 Medical Electrical Equipment—Part 2-43: Particular Requirements for the Basic Safety and Essential Performance of X-ray Equipment for Interventional Procedures 60601-2-43 2nd edn (Geneva, Switzerland: International Electrotechnical Commission).
- Andrade, C. The P Value and Statistical Significance: Misunderstandings, Explanations, Challenges, and Alternatives. Indian J Psychol Med 2019, 41, 210–215. [Google Scholar] [CrossRef]
- StataCorp. Stata Statistical Software: Release 16; StataCorp LLC: College Station, TX, USA, 2019. [Google Scholar]
- Martinez, L.; Vano, E.; Gutierrez, F.; Rodriguez, C.; Gilarranz, R.; Manzanas, M.J. Patient doses from fluoroscopically guided cardiac procedures in paediatrics. Phys. Med. Biol. 2007, 52, 4749–4759. [Google Scholar] [CrossRef] [PubMed]
- Verghese, G.; McElhinney, D.; Strauss, K.; Bergersen, L. Characterization of radiation monitoring policy in a large pediatric cardiac catheterization lab Catheter. Cardiovasc. Interv. 2012, 79, 294–301. [Google Scholar]
- Corredoira, E.; Vañó, E.; Ubeda, C.; Gutiérrez-Larraya, F. Patient doses in paediatric interventional cardiology: Impact of 3D rotational angiography. J. Radiol. Prot. 2015, 35, 179–195. [Google Scholar] [CrossRef]
- Kottou, S.; Kollaros, N.; Plemmenos, C.; Mastorakou, I.; Apostolopoulou, S.C.; Tsapaki, V. Towards the definition of Institutional diagnostic reference levels in paediatric interventional cardiology procedures in Greece. Phys. Med. 2018, 46, 52–58. [Google Scholar] [CrossRef]
- Ubeda, C.; Vano, E.; Riquelme, N.; Aguirre, D.; Vasquez, H.; Chavez, C.; Dalmazzo, D. Patient radiation doses in paediatric interventional cardiology and optimization actions Radiat. Phys. Chem. 2020, 168, 108539. [Google Scholar]
- Ishibashi, T.; et al. Pediatric diagnostic reference levels for diagnostic and therapeutic cardiac catheterization in Japan, PREPRINT (Version 1). Research Square 2021. [Google Scholar] [CrossRef]
- IAEA 2018 Radiation Protection and Safety in Medical Uses of Ionizing Radiation, (IAEA Safety Standards Series No. SSG-46) (Vienna: IAEA).
- ICRP. Avoidance of radiation injuries from medical interventional procedures. Ann. ICRP 2000, 30, 7–67. [Google Scholar] [CrossRef] [PubMed]
- Andreassi, M.G. Radiation risk from pediatric cardiac catheterization: Friendly fire on children with congenital heart disease. Circulation 2009, 120, 1847–1849. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).