Submitted:
20 July 2023
Posted:
25 July 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
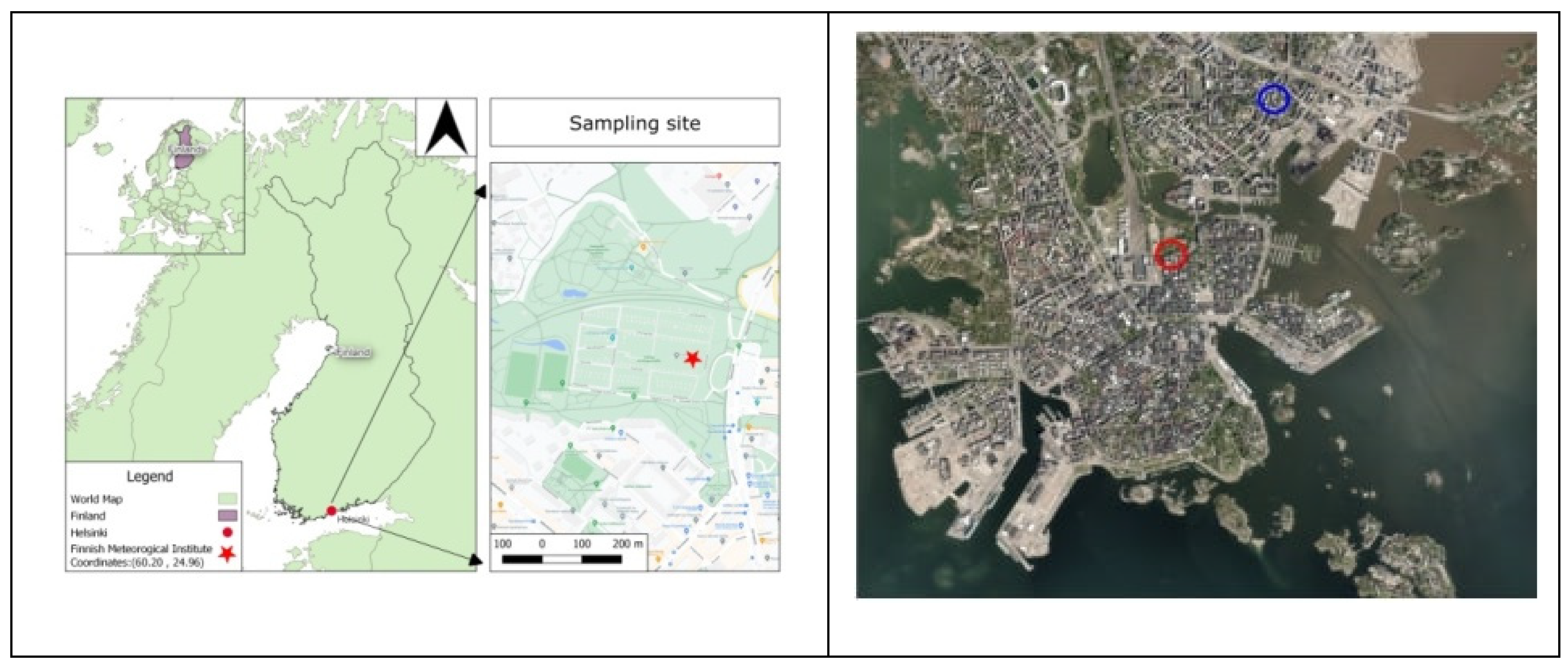
2.1. Study area, sampling and measurements
2.2. Source apportionment methodology
3. Results
3.1. Atmospheric concentration levels
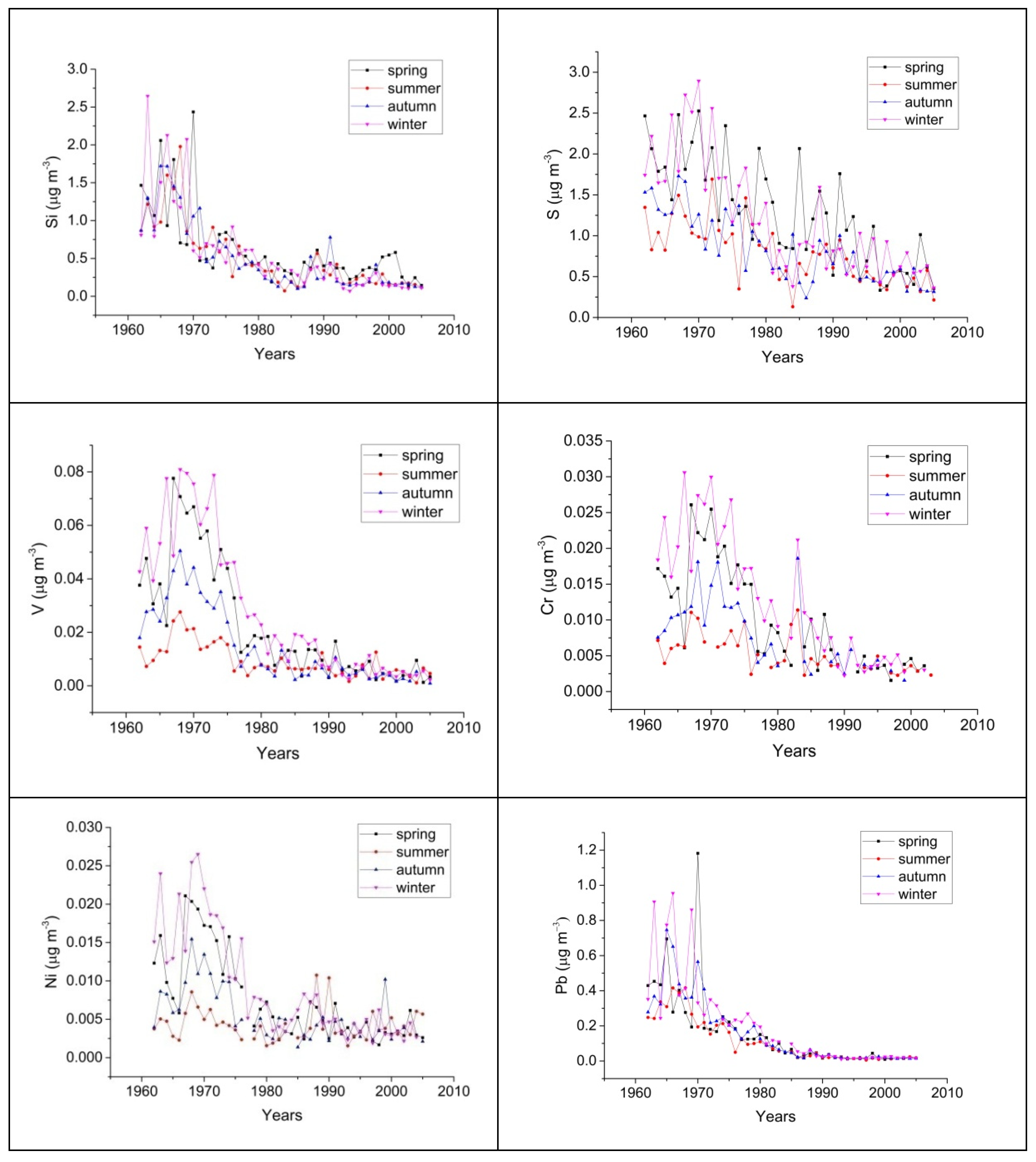
3.2. Seasonal variation
3.3. Source apportionment
2Pb + O2-> 2PbO
3.4. An overview in the literature
4. Conclusions
Acknowledgments
References
- Miller, A.J. , Raduma, D.M., George, L.A., Fry, J.L. Source apportionment of trace elements and black carbon in an urban industrial area (Portland, Oregon). Atmospheric Pollution Research, 2019, 10, 784–794. [Google Scholar] [CrossRef]
- WHO. WHO global air quality guidelines: particulate matter (PM2.5 and PM10), ozone, nitrogen dioxide, sulfur dioxide and carbon monoxide: executive summary. 2021. Available online: https://www.who.int/publications/i/item/9789240034433.
- Taghvaee, S. , Sowlat, M.H., Diapouli, E., Manousakas, M.I., Vasilatou, V., Eleftheriadis, K., Sioutas, C. Source apportionment of the oxidative potential of fine ambient particulate matter (PM2.5) in Athens, Greece. Science of the Total Environment, 2019, 653, 1407–1416. [Google Scholar] [CrossRef]
- Kyllönen, K. , Vestemius, M., Anttila, P., Makkonen, U., Aurela, M., Wängberg, I., Mastromonaco, M.N., Hakola, H. Trends and source apportionment of atmospheric heavy metals at a subarctic site during 1996-2018. Atmospheric Environment, 2020, 236, 117644. [Google Scholar] [CrossRef]
- Lieu, W. , Wei, J., Cai, M., Qian, Z., Long, Z., Wang, L., Vaughn, M.G., Aaron, H.E., Tong, X., Li, Y., Yin, P., Lin, H., Zhou, M. Particulate matter pollution and asthma mortality in China: A nationwide time-stratified case cross-over study from 2015-2020. Chemosphere, 2022, 308, 136316. [Google Scholar] [CrossRef] [PubMed]
- Manousakas, M. , Diapouli, E., Papaefthymiou, H., Migliori, A., Karydas, A.G., Padilla-Alvarez, R., Bogovac, M., Kaiser, R.B., Jaksic M., Bogdanovic-Radovic, I., Eleftheriadis, K. Source apportionment by PMF on elemental concentrations obtained by PIXE analysis of PM10 samples collected at the vicinity of lignite power plants and mines in Megalopolis, Greece. Nuclear Instruments and Methods in Physics Research B, 2015, 349, 114–124. [Google Scholar] [CrossRef]
- Kim, K.H. , Kabir, E., Kabir, S. A review on the human health impact of airborne particulate matter. Environment International, 2015, 74, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Almeida, S.M. , Faria, T., Martins, V., Canha, N., Diapouli, E., Eleftheriadis, K., Manousakas, M.I. Source apportionment of children daily exposure to particulate matter. SSRN Electronic Journal 2022. [Google Scholar] [CrossRef]
- Gu, X. Yu, Chu, X., Zeng, X.L., Bao, H.R., Liu, X.J. Effects of PM2.5 exposure on the Notch signaling pathway and immune imbalance in chronic obstructive pulmonary disease. Environmental Pollution, 2017, 226, 163–173. [Google Scholar] [CrossRef]
- Buoli, M. , Grassi, S., Caldiroli, A., Camevali, G.S., Mucci, F., Iodice, S., Cantone, L., Pergoli, L., Bollati, V. Is there a link between air pollution and mental disorders? Environmental International, 2018, 118, 154–168. [Google Scholar] [CrossRef]
- Lee, S. , Kang, J.E. Impact of particulate matter and urban spatial characteristics on urban vitality using spatiotemporal big data. Cities, 2022, 131, 104030. [Google Scholar] [CrossRef]
- Zhang, C. , Hu, Y., Adams, M.D., Liu, M., Li, B., Shi, T., Li, C. Natural and human factors influencing urban particulate matter concentrations in central heating areas with long-term wearable monitoring devices. Environmental Research, 2022, 215, 114393. [Google Scholar] [CrossRef]
- Seinfeld, J.H. , Pandis, S.N. Atmospheric Chemistry and Physics: from Air Pollution to Climate Change. Wiley, 1998, New York.
- Wagner, A. , Boman, J., Gatari, M.J. Elemental analysis of size-fractionated particulate matter sampled in Göteborg, Sweden. Spectrochimica Acta Part B: Atomic Spectroscopy 2008, 63, 1426–1431. [Google Scholar] [CrossRef]
- Boman, J. , Wagner, A., Gatari, M.J. Trace elements in PM2.5 in Gothenburg, Sweden. Spectrochimica Acta Part B: Atomic Spectroscopy, 2010, 65, 478–482. [Google Scholar] [CrossRef]
- Pacyna, J.M. , Pacyna, E.G. An assessment of global and regional emissions of trace metals to the atmosphere from anthropogenic sources worldwide. Environmental Reviews, 2001, 9, 269–298. [Google Scholar] [CrossRef]
- Christian, T.J. , Yokelson, R.J., Cárdenas, B., Molina, L.T., Engling, G., Hsu, S.C. Trace gas and particle emissions from domestic and industrial biofuel use and garbage burning in central Mexico. Atmospheric Chemistry and Physics, 2010, 10, 565–584. [Google Scholar] [CrossRef]
- Dung, T.T.T. , Vassilieva, E., Swennen, R., Cappuyns, V. Release of Trace Elements from Bottom Ash from Hazardous Waste Incinerators. Recycling 2018, 3, 36. [Google Scholar] [CrossRef]
- EEA, Heavy Metal Emissions. European Environmental Agency, 2020. Available online: https://www.eea.europa.eu/data-and-maps/indicators/eea32-heavy -metal-hm-emissions-1/assessment-10.
- Diapouli, E. , Manousakas, M., Vratolis, S., Vasilatou, V., Maggos, T., Saraga, D., Grigoratos, T., Argyropoulos, G., Voutsa, D., Samara, C., Eleftheriadis, K. Evolution of air pollution source contributions over one decade, derived by PM10 and PM2.5 source apportionment in two metropolitan urban areas in Greece. Atmos. Environ. 2017, 164, 416–430. [Google Scholar] [CrossRef]
- AMAP. AMAP Assessment 2002: Heavy Metals in the Arctic. Arctic Monitoring and Assessment Programme (AMAP), Oslo, Norway. 2005, Xvi+265, ISBN 82-7971-018-3 (first published as electronic document in 2004).
- Anttila, P. , Salmi, T. Characterizing temporal and spatial patterns of urban PM10 using six years of Finnish monitoring data. Boreal Environmental Research, 2006, 11, 463–479. [Google Scholar]
- Infante, R. , Acosta, I.L. Size distribution of trace metals in Ponce, Puerto Rico air particulate matter. Atmospheric Environment, Part B, Urban Atmosphere, 1991, 25, 1–121. [Google Scholar] [CrossRef]
- Pacyna, J.M. , Pacyna, E.G., Aas, W. Changes of emissions and atmospheric deposition of mercury, lead and cadmium. Atmospheric Environment 2009, 43, 117–127. [Google Scholar] [CrossRef]
- EU. Council Directive 1999/30/EC of 22 April 1999 relating to limit values for sulfur dioxide, nitrogen dioxide and oxides of nitrogen, particulate matter and lead in ambient air. Off J 1999, L 163, 41-60. EN:HTML (Jan, 2020). Available online: http://eurlex.europa.eu/LexUriServ.do?uri=CELEX:31999L0030.
- EU. Directive 2004/107/EC of the European Parliament and of the Council of 15 December 2004 relating to arsenic, cadmium, mercury, nickel and polycyclic aromatic hydrocarbons in ambient air. Off J 2004, L 23, 3-16. EN:PDF (Jan, 2020). Available online: http://eurlex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2005:023:0003:0016.
- EU. Directive 2008/50/EC of the European Parliament and of the Council of 21 May 2008 on ambient air quality and cleaner air for Europe. Off J 2008, L 152, 1-44. Available online: http://eurlex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32008L0050from=EN.
- EU. Commission Directive (EU) 2015/1480 of 28 August 2015 amending several annexes to Directives 2004/107/EC and 2008/50/EC of the European Parliament and of the Council laying down the rules concerning reference methods, data validation and location of sampling points for the assessment of ambient air quality. Off J 2015, L 226, 4-11. Available online: https://eurlex.europa.eu/eli/dir/2015/1480/oj.
- Wiklund, J.A. , Kirk, J.L., Muir, D.C.G., Gleason, A., Carrier, J., Yang, F. Atmospheric trace metal deposition to remote Northwest Ontario, Canada: Anthropogenic fluxes and inventories from 1860 to 2010. Science of the Total Environment, 2020, 749, 142276. [Google Scholar] [CrossRef] [PubMed]
- Voukantsis, D. , Karatzas, K., Kukkonen, J., Räsänen, T., Karppinen, A., Kolehmainen, M. Intercomparison of air quality data using principal component analysis, and forecasting of PM10 and PM2.5 concentrations using artificial neural networks, in Thessaloniki and Helsinki. Science of the Total Environment, 2011, 409, 1266–1276. [Google Scholar] [CrossRef] [PubMed]
- Paatero, J. , Hatakka, J., Mattsson, R., Lehtinen, I. A Comprehensive Station for Monitoring Atmospheric Radioactivity. Radiation Protection Dosimetry, 1994, 54, 33–39. [Google Scholar] [CrossRef]
- Mattsson, R. , Paatero, J., Hatakka, J. Automatic Alpha/Beta Analyser for Air Filter Samples-Absolute Determination of Radon Progeny by Pseudo-coincidence Techniques. Radiation Protection Dosimetry, 1996, 63, 133–139. [Google Scholar] [CrossRef]
- Manousakas, M. , Diapouli, E., Papaefthymiou, H., Kentarelou, V., Zarkadas, C., Kalogridis, A.C., Karydas A.C., Eleftheriadis, K. XRF characterization and source apportionment of PM10 samples collected in a coastal city. X Ray Spectrom., 2017, 47, 190–200. [Google Scholar] [CrossRef]
- Canonaco, F. , Crippa, M., Slowik, J.G., Baltensperger, U., Prévôt, A.S.H. SoFi, an IGOR-based interface for the efficient use of the generalized multilinear engine (ME-2) for the source apportionment: ME-2 application to aerosol mass spectrometer data. Atmospheric Measurement Techniques 2013, 6, 3649–3661. [Google Scholar] [CrossRef]
- Polissar, A.V. , Hopke, P.K., Poirot, R.L. Atmospheric aerosol over Vermont: chemical composition and sources. Environmental Science & amp; Technology 2001, 35, 4604–4621. [Google Scholar] [CrossRef]
- Reff, A. , Eberly, S.I., Bhave, P.V. Receptor Modeling of Ambient Particulate Matter Data Using Positive Matrix Factorization: Review of Existing Methods. Journal of the Air & Waste Management Association 2007, 57, 146–154. [Google Scholar] [CrossRef]
- Canonaco, F. , Tobler, A., Chen, G., Sosedova,., Slowik, J.G., Bozzetti, C., Daellenbach, K.R., El Haddad, I., Crippa, M., Huang, R.J., Furger, M., Baltensperger, U., Prévôt, A.S.H. A new method for long-term source apportionment with time-dependent factor profiles and uncertainty assessment using SoFi Pro: application to 1 year of organic aerosol data. Atmospheric Measurement Techniques 2021, 14, 923–943. [Google Scholar] [CrossRef]
- Nriagu, J.O. A global assessment of natural sources of atmospheric trace metals. Nature, 1989, 338, 47–49. [Google Scholar] [CrossRef]
- Athanasopoulou, E. , Tombrou, M., Russell, A.G., Karanasiou, A., Eleftheriadis, K., Dandou, A. Implementation of road and soil dust emission parameterizations in the aerosol model CAMx: applications over the greater Athens urban area affected by natural sources. Journal of Geophysical Research, 2010, 115, D17301. [Google Scholar] [CrossRef]
- Chapman, S. Notes of Atmospheric Sodium. Astrophysical Journal, 1939, 90, 309. [Google Scholar] [CrossRef]
- Zhuang, H. , Chan, C.K., Fang, M., Wexler, A.S. Formation of nitrate and non-sea-salt sulfate on coarse particles. Atmospheric Environment 1999, 33, 4223–4233. [Google Scholar] [CrossRef]
- Eleftheriadis, K. , Ochsenkuhn, K.M., Lymperopoulou, Th., Karanasiou, A., Razos, P., Ochsenkuhn-Petropoulou, M. Influence of local and regional sources on the observed spatial and temporal variability of size resolved atmospheric aerosol mass concentrations and water-soluble species in the Athens metropolitan area. Atmospheric Environment, 2014, 97, 252–261. [Google Scholar] [CrossRef]
- Lucarelli, F. , Mandò, P.A., Nava, S., Prati, P., Zucchiatti, A. One-year study of the elemental composition and source apportionment of PM10 aerosols in Florence, Italy. Journal of the Air & Waste Management Association, 2014, 54, 1372–1382. [Google Scholar] [CrossRef]
- Viana, M. , Kuhlbusch, T.A.J., Querol, X., Alastuey, A., Harrison, R.M., Hopke, P.K., Winiwarter, W., Vallius, M., Szidat, S., Prevôt, A.S.H., Hueglin, C., Bloemen, H., Wahlin, P., Vecchi, R., Miranda, A.I., Kasper-Giebl, A., Maenhaut, W., Hitzenberger, R. Source apportionment of particulate matter in Europe: a review of methods and results. Journal of Aerosol Science, 2008, 39, 827–849. [Google Scholar] [CrossRef]
- Santoso, M. , Hopke, P.K., Hidayat, A., Dwiana, D.L. Sources identification of the atmospheric aerosol at urban and suburban sites in Indonesia by positive matrix factorization. Science of the Total Environment, 2008, 397, 229–237. [Google Scholar] [CrossRef]
- Pacyna, J. Pacyna, J. Workpackage 02-D01b source-sector analysis and evaluation report. Estimation of willingness-to-pay to reduce risks of exposure to heavy metals and cost-benefit analysis for reducing heavy metals occurrence in Europe. 2007. Available online: http://espreme.ier.uni-stuttgart.de/.
- 47. EEA. European Union Emission Inventory Report 1990-2017 under the UNECE Convection on Long-Range Transboundary Air Pollution (LRTAP). In EEA Report No 08/2019; 2019; ISBN 978-92-9480-078-7. [CrossRef]
- Virkkula, A. Teinilä, K., Hillamo, R., Stohl, A. A decade of trace gas measurements using DOAS in Finnish Lapland. Boreal Environ. Res., 2003, 8, 351–363. [Google Scholar]
- Jokiniemi, J. , Raunemaa, T., Mattsson, R., Hautojärvi, A. Analysis of aerosol samples from radioactivity registration stations. Nuclear Instruments and Methods in Physics, Research Section B: Beam Interactions with Materials and Atoms, 1984, 3, 1–3. [Google Scholar] [CrossRef]
- Kummer, U. , Pacyna, J., Pacyna, E., Friedrich, R. Assessment of heavy metal releases from the use phase of road transport in Europe. Atmospheric Environment 2009, 43, 640–647. [Google Scholar] [CrossRef]
- Mattsson, R. , Jaakkola, T. An Analysis of Helsinki Air 1962 to 1977 Based on Trace Metals and Radionuclides. Geophysica, 1979, 16, 1–42. [Google Scholar]
- ECHA, Inclusion of Substances of Very High Concern in the Candidate List – Decision of the European Chemicals Agency ED/169/2012, 2012. Available online: https://echa.europa.eu/documents/10.162/0b417b76-b533-42a1-9bd2-519fldc1990d.
- NESTE, Bensiiniopas, Neste Oyj, Engineering Services Company, 2022.
- Kloprogge, J.T. , Ponce, C.P., Loomis, T.A. The Periodic Table: Nature’s Building Blocks (J. Theo Kloprogge, C.P. Ponce, T.A. Loomis (eds.); First). Elsevier, 2021. [Google Scholar]
- Manousakas, M. , Diapouli, E., Belis, C.A., Vasilatou, V., Gini, M., Lucarelli, F., Querol, X., Eleftheriadis, K. Quantitative Assessment of the variability in chemical profiles from source apportionment analysis of PM10 and PM2.5 at different sites within a large Metropolitan area. Environmental Research, 2021, 192, 110257. [Google Scholar] [CrossRef]
- Kim, E. , Hopke, P.K. Source characterization of ambient fine particles at multiple sites in the Seattle area. Atmospheric Environment 2008, 42, 6047–6056. [Google Scholar] [CrossRef]
- Lopes, D. , Rafael, S., Ferreira, J., Relvas, H., Almeida, S.M., Faria, T., Martins, V., Diapouli, E., Manousakas, M., Vasilatou, V., Fetfatzis, P., Miranda, A.I. Assessing the levels of regulated metals in an urban area: A modeling and experimental approach. Atmospheric Environment, 2022, 290, 119366. [Google Scholar] [CrossRef]
- 58. Traficom. Open Data for Vehicles. The Finnish Transport and Communications Agency Traficom.
- Gini, M. , Manousakas, M.I., Karydas, A.G., Eleftheriadis, K. Mass size distributions composition and dose estimates of particulate matter in Saharan dust outbreaks. Environmental Pollution, 2022, 298, 118768. [Google Scholar] [CrossRef]
- WHO. Air quality guide lines global update 2005. Particulate matter, ozone, nitrogen dioxide and sulfur dioxide. 2005. Available online: http://www.euro.who.int/en/health-topics/environment-and-health/air-quality/publications/pre2009/air-quality-guidelines.-global-update-2005.-particulate-matter,-ozone,-nitrogen-dioxide-and-sulfur-dioxide.
- WHO. Review of Evidence on Health Aspects of Air Pollution-REVIHAAP Project Technical Report. The WHO European Centre for Environment and Health, WHO Regional Office for Europe, Bonn, Germany, 2013. Available online: http://www.euro.who.int/_data/assets/pdf_file/004/193108/REVIHAAP-Final-technicalreport-final-version.pdf?ua=1.
- Maas, R., Grennfelt, P. Towards cleaner air. Scientific assessment report 2016. EMEP Steering Body and Working Group on Effects of the Convention on Long-Range Transboundary Air Pollution: Oslo, Norway, 2016, p. 50. Available online: https://unece.org/DAM/env/lrtap/ExecutiveBody/35th_session/CLRTAP_Scientific_Assessment_Report_-_Final_20-5-2016.pdf.
- Brimblecombe, P. The Effects of Air Pollution on the Built Environment: Air Pollution Reviews. Imperial College Press, 2003, 2, London. [Google Scholar]
- Watt, J. , Tidblad, J., Kucera, V., Hamilton, R. The Effects of Air Pollution on Cultural Heritage. Springer, New York, 2009, 105-127. Available online: https://link.springer.com/book/10.1007/978-0-387-84893-8.
- Grøntoft, T. Recent trends in maintenance costs for facades due to air pollution in the Oslo quadrature, Norway. Atmosphere 2019, 10, 529. [Google Scholar] [CrossRef]
- EMEP. The co-operative programme for monitoring and evaluation of the long-range transmission of air pollutants in Europe. 2021. Available online: https://www.emep.int/.
- Kyllönen, K. , Karlsson, V., Ruoho-Airola, T. Trace elements deposition and trends during a ten year period in Finland. Science of the Total Environment, 2009, 407, 2260–2269. [Google Scholar] [CrossRef]
- Laing, J.R. , Hopke, P.K., Kopke, E.F., Husian, L., Dutkiewicz, V.A., Paatero, J., Viisanen, Y. Long-term particle measurements in Finnish Arctic: Part I – Chemical composition and trace metal solubility. Atmospheric Environment, 2014, 88, 275–284. [Google Scholar] [CrossRef]
- Laing, J.R. , Hopke, P.K., Hopke, E.F., Husian, L., Dutkiewicz, V.A., Paatero, J., Viisanen, Y. Long-term particle measurements in Finnish Arctic: Part II – Trend analysis and source location identification. Atmospheric Environment, 2014, 88, 285–296. [Google Scholar] [CrossRef]
- Jalkanen, L. Mäkinen, A., Häsänen, E., Juhanoja, J. The effect of large anthropogenic particulate emissions on atmospheric aerosols, deposition and bioindicators in the eastern Gulf of Finland region. Science of the Total Environment, 2000, 262, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Hosiokangas, J. , Ruuskanen, J., Pekkanen, J. Effects of soil dust episodes and mixed fuel source on source apportionment of PM10 particles in Kuopio, Finland. Atmospheric Environment, 1999, 33, 3821–3829. [Google Scholar] [CrossRef]
- Vallius, M. , Ruuskanen, J., Mirme, A., Pekkanen, J. Concentrations and estimated soot content of PM1, PM2.5 and PM10 in subarctic urban atmosphere. Environ. Sci. Technol., 2000, 34, 10–1919. [Google Scholar] [CrossRef]
- Pakkanen, T. , Loukkola, K., Korhonen, C., Aurela, M., Mäkelä, T., Hillamo, R., Aamio, P., Koskentalo, T., Kousa, A., Maenhaut, W. Sources and chemical composition of atmospheric fine and coarse particles in the Helsinki area. Atmospheric Environment 2001, 35, 5381–5391. [Google Scholar] [CrossRef]
- Pohjola, M. , Kousa, A., Kukkonen, J., Härkönen, J., Karppinen, A., Aamio, P., Koskentalo, T. The spatial and temporal variation of measured urban PM10 and PM2.5 in the Helsinki metropolitan area. Urban Air quality – Recent Advances 2002. [Google Scholar] [CrossRef]
- Laakso, L. , Hussein, T., Aamio, P., Komppula, M., Hiltunen, V., Viisanen, Y., Kulmala, M. Diurnal and annual characteristics of particle mass and number concentrations in urban, rural and arctic environments in Finland. Atmospheric Environment, 2003, 37, 19–2629. [Google Scholar] [CrossRef]
- Hosiokangas, J. , Vallius, M., Ruuskanen, J., Mirme, A., Pekkanen, J. Resuspended dust episodes as an urban air-quality problem in subarctic regions. Scandinavian Journal of Work, Environment and Health 2004, 30, 28–35. [Google Scholar]
- Kukkonen, J. , Pohjola, M., Sokhi, R.S., Luhana, L., Kitwiroon, N., Fragkou, L., Rantamäki, M., Berge, E., Ødegaard, V., Slørdal, L.H., Denby, B. & Finardi, S. Analysis and evaluation of selected local-scale PM10 air pollution episodes in four European cities: Helsinki, London, Milan and Oslo. Atmospheric Environment, 2005, 39, 15–2759. [Google Scholar] [CrossRef]
- Sivertsen, B. , Makarova, T., Hagen, L.O. Baklanov, A.A. Air Pollution in the Border Areas of Norway and Russia. 1992. NILU-Norwegian Institute for Air Reasearch, Lillestrom, pp. 1-14.
- Berg, T. , Aas, W., Pacyna, J., Uggerud, H., Vdste, M. Atmospheric trace metal concentrations at Norwegian background sites during 25 years and its relation to European emissions. Atmospheric Environment, 2008, 42, 7494–7501. [Google Scholar] [CrossRef]
- Pacyna, J.M., Pacyna, E, G., Panasiuk, D., Fudala,J., Strzelecka-Jastrzab,E., Hlawiczka S. Heavy metals emissions in Europe: first results from the EU ESPREME project. Paper presented at Joint Workshop of the UN ECE Task Force on Emission Inventories and Projections at the EU ESPREME Project, 2005, Rovaniemi, Finland 18-19 October.
- Grøntoft, T. Historical dry deposition of air pollution in the urban background in Oslo, Norway, compared to Western European data. Atmospheric Environment, 2021, 267, 118777. [Google Scholar] [CrossRef]
- Victorin, K. Health effects of urban air pollutants. Guideline values and conditions in Sweden. Chemosphere, 1993, 27, 9–1691. [Google Scholar] [CrossRef]
- Isakson, J. , Persson, T.A., Selin Lindgren, E. Identification and assessment of ship emissions and their effects in the harbor of Göteborg, Sweden. Atmospheric Environment, 2001, 35, 21–3659. [Google Scholar] [CrossRef]
- Mukai, H. , Machida, T., Tanaka, A., Vera, Y.P., Uematsu, M. Lead isotope ratios in the urban air of eastern and central Russia. Atmospheric Environment, 2001, 35, 15–2783. [Google Scholar] [CrossRef]
- Shevchenko, V. , Lisitzin, A., Vinogradova, A., Stein, R. Heavy metals in aerosols over the seas of the Russian Arctic. Science of the Total Environment, 2003, 306, 11–25. [Google Scholar] [CrossRef]
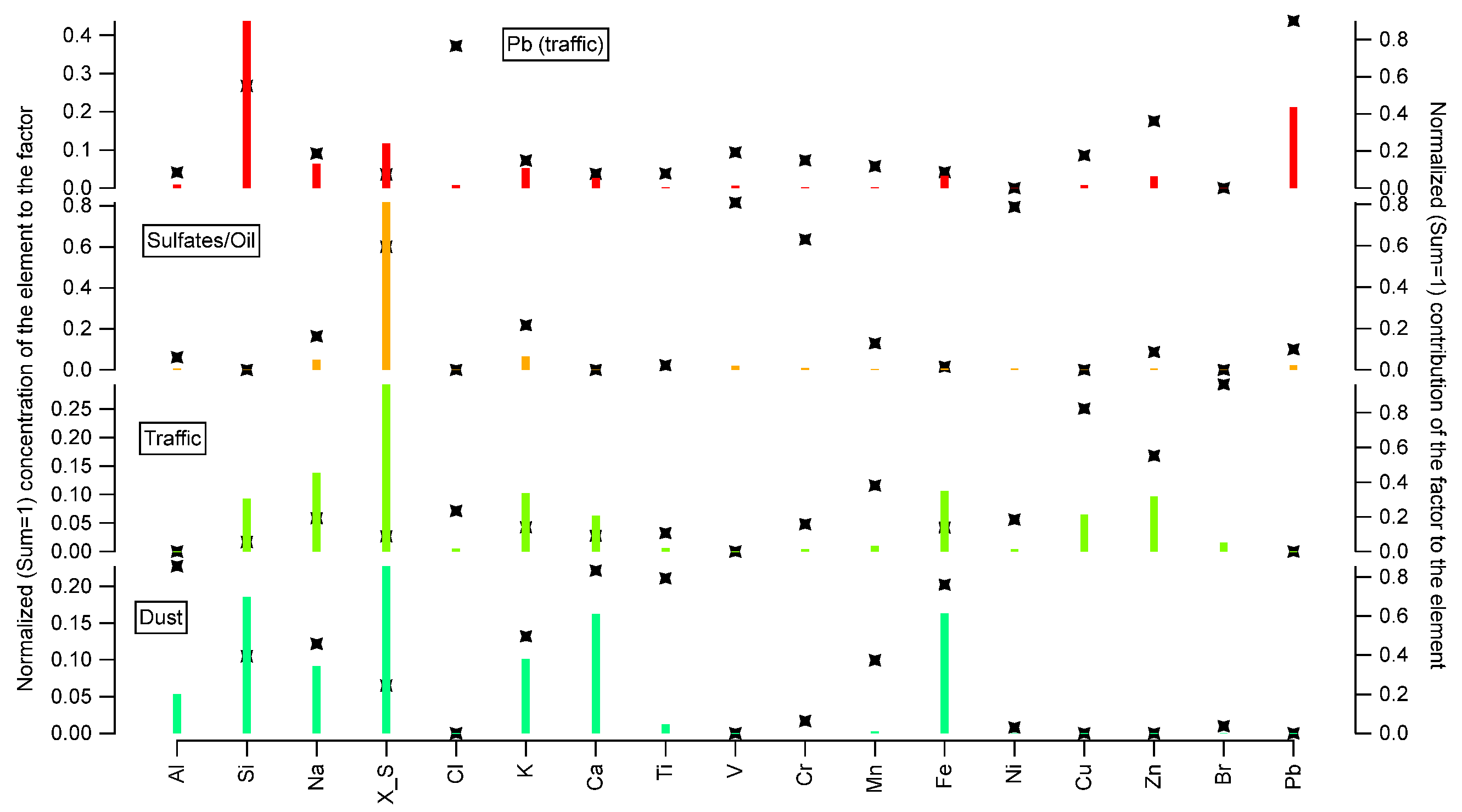
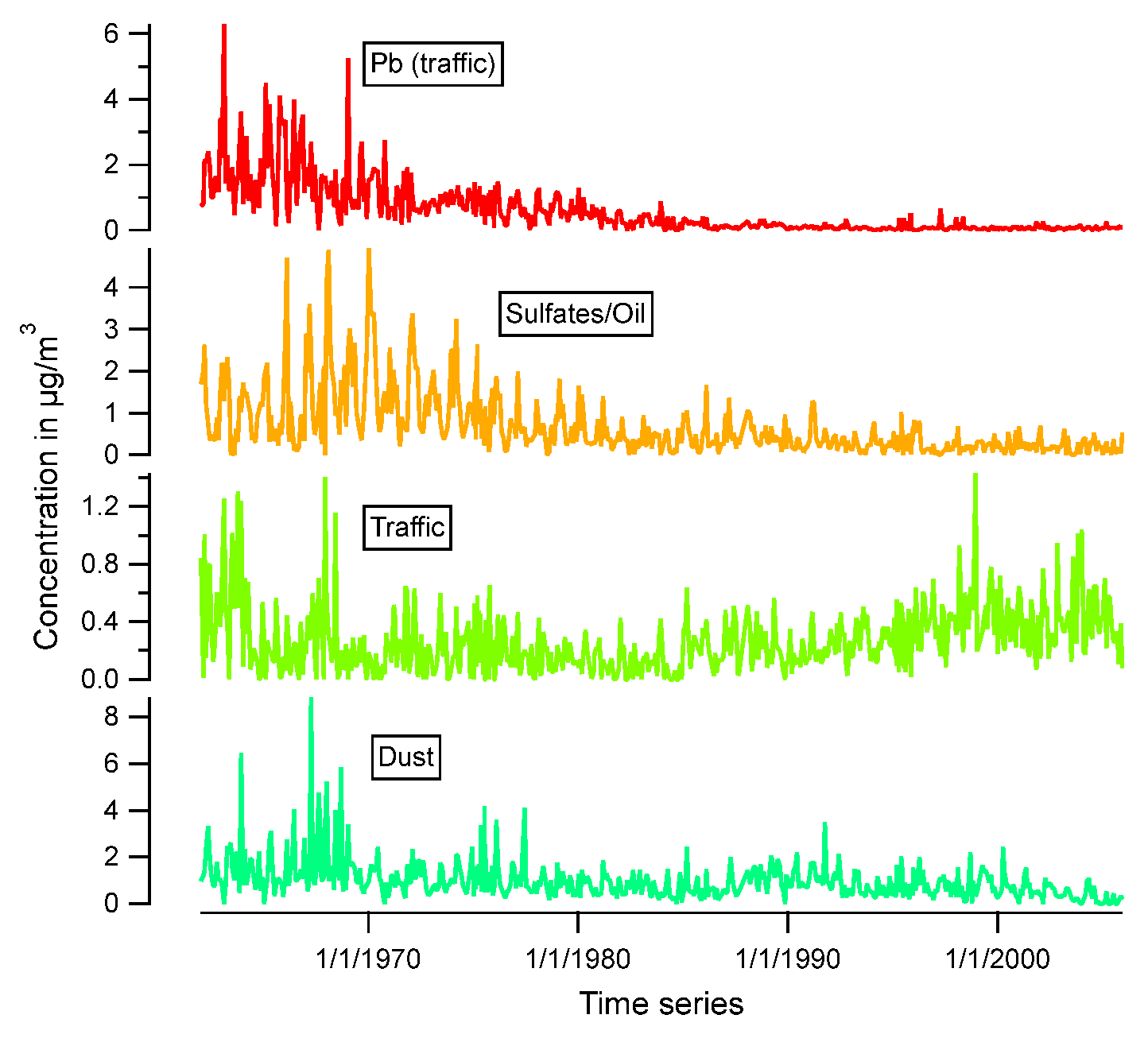
| Element | (MU) (%) |
Number of samples |
LOD (μg m-3) |
| Na | 30.2 | 599 | 0.0307 |
| Al | 16.0 | 599 | 0.0098 |
| Si | 15.8 | 599 | 0.0028 |
| S | 9.1 | 599 | 0.0009 |
| Cl | 15.8 | 599 | 0.0009 |
| K | 9.1 | 599 | 0.0009 |
| Ca | 10.4 | 599 | 0.0068 |
| Ti | 10.9 | 599 | 0.0025 |
| V | 13.2 | 599 | 0.0015 |
| Cr | 21.2 | 599 | 0.0025 |
| Mn | 16.8 | 599 | 0.0012 |
| Fe | 10.4 | 599 | 0.0012 |
| Ni | 17.8 | 599 | 0.0009 |
| Cu | 11.2 | 599 | 0.0012 |
| Zn | 12.1 | 599 | 0.0015 |
| Br | 18.0 | 599 | 0.0012 |
| Pb | 13.5 | 599 | 0.0022 |
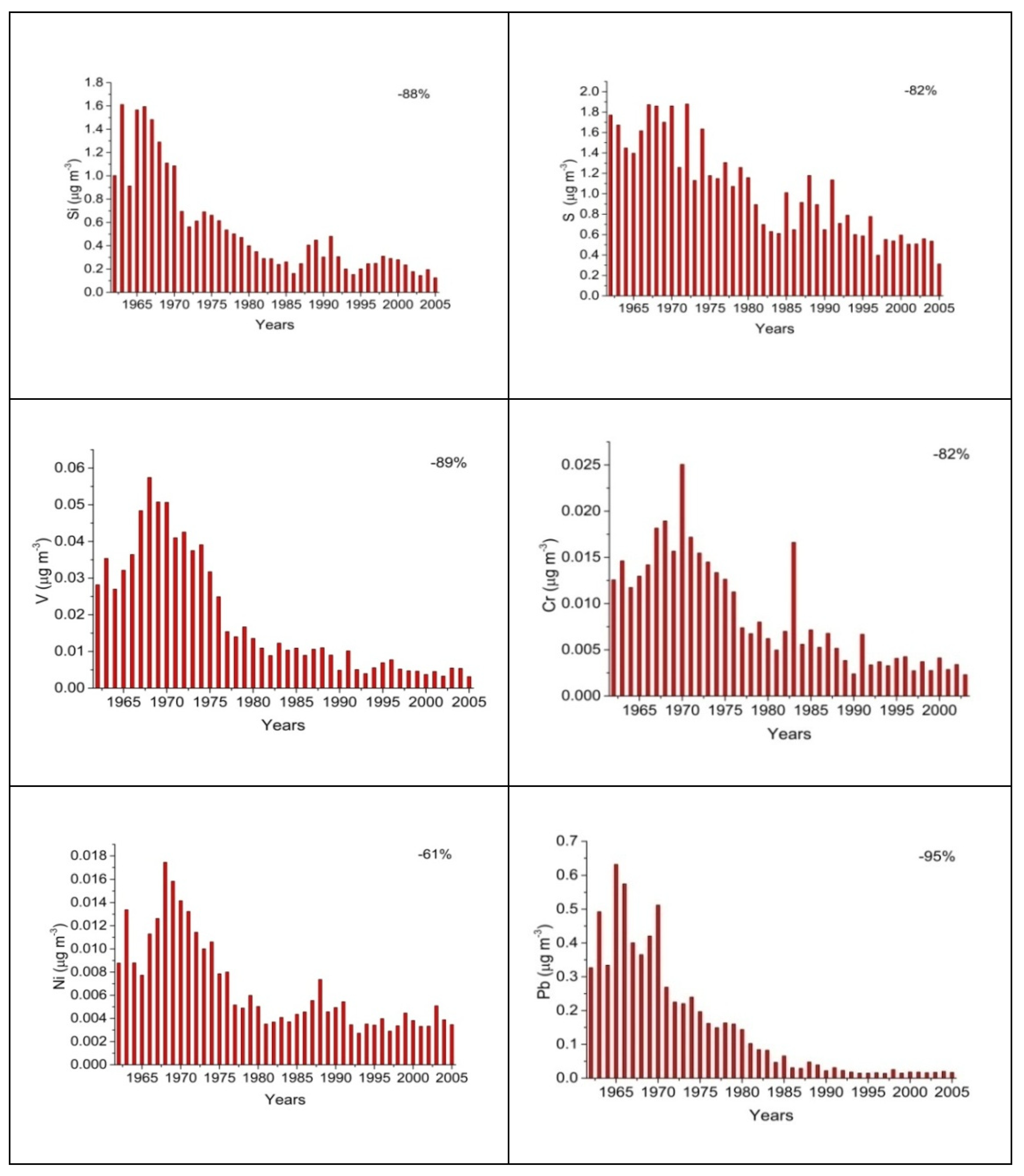
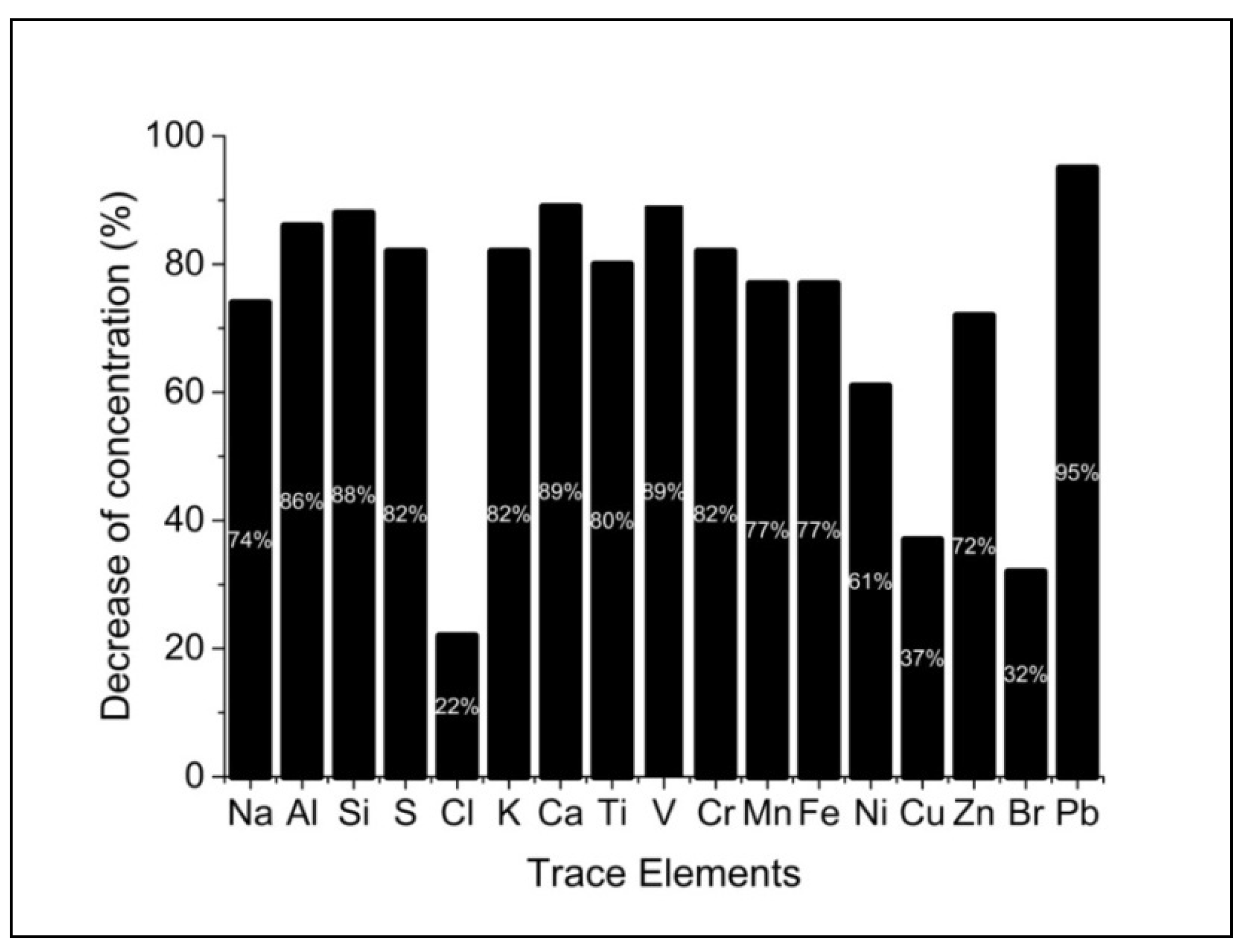
| Element | Min | Max | Median | Mean | St. Dev. | Total decrease |
| (μg m-3) | (%) | |||||
| Na | 0.07 | 1.37 | 0.26 | 0.30 | 0.19 | -74.0 |
| Al | <0.01 | 1.04 | 0.09 | 0.13 | 0.13 | -86.0 |
| Si | 0.03 | 3.84 | 0.38 | 0.55 | 0.58 | -88.0 |
| S | 0.09 | 5.12 | 0.84 | 1.02 | 0.71 | -82.0 |
| Cl | <0.01 | 0.49 | 0.02 | 0.03 | 0.06 | -22.0 |
| K | <0.01 | 1.21 | 0.17 | 0.22 | 0.15 | -82.0 |
| Ca | 0.01 | 1.76 | 0.17 | 0.21 | 0.19 | -89.0 |
| Ti | <0.01 | 0.15 | 0.01 | 0.02 | 0.01 | -80.0 |
| V | <0.01 | 0.14 | 0.01 | 0.02 | 0.02 | -89.0 |
| Cr | <0.01 | 0.05 | 0.01 | 0.01 | 0.01 | -82.0 |
| Mn | <0.01 | 0.18 | 0.01 | 0.01 | 0.01 | -77.0 |
| Fe | 0.01 | 1.56 | 0.18 | 0.21 | 0.15 | -77.0 |
| Ni | <0.01 | 0.04 | 0.00 | 0.01 | 0.01 | -61.0 |
| Cu | <0.01 | 0.44 | 0.02 | 0.03 | 0.04 | -37.0 |
| Zn | <0.01 | 0.44 | 0.04 | 0.06 | 0.05 | -72.0 |
| Br | <0.01 | 0.11 | 0.01 | 0.01 | 0.02 | -32.0 |
| Pb | <0.01 | 1.96 | 0.10 | 0.17 | 0.23 | -95.0 |
| Na | Al | Si | S | Cl | K | Ca | Ti | V | Cr | Mn | Fe | Ni | Cu | Zn | Br | Pb | |
| Na | 1 | ||||||||||||||||
| Al | 0.87 | 1 | |||||||||||||||
| Si | 0.89 | 0.86 | 1 | ||||||||||||||
| S | 0.81 | 0.83 | 0.84 | 1 | |||||||||||||
| Cl | -0.24 | -0.32 | -0.21 | -0.30 | 1 | ||||||||||||
| K | 0.91 | 0.81 | 0.93 | 0.85 | -0.13 | 1 | |||||||||||
| Ca | 0.85 | 0.83 | 0.90 | 0.79 | -0.18 | 0.92 | 1 | ||||||||||
| Ti | 0.88 | 0.89 | 0.81 | 0.82 | -0.21 | 0.86 | 0.84 | 1 | |||||||||
| V | 0.80 | 0.83 | 0.83 | 0.90 | -0.31 | 0.78 | 0.69 | 0.80 | 1 | ||||||||
| Cr | 0.76 | 0.72 | 0.76 | 0.83 | -0.34 | 0.68 | 0.59 | 0.67 | 0.93 | 1 | |||||||
| Mn | 0.34 | 0.61 | 0.45 | 0.31 | -0.13 | 0.42 | 0.44 | 0.44 | 0.26 | 0.18 | 1 | ||||||
| Fe | 0.87 | 0.89 | 0.88 | 0.81 | -0.15 | 0.89 | 0.94 | 0.91 | 0.77 | 0.67 | 0.42 | 1 | |||||
| Ni | 0.82 | 0.83 | 0.82 | 0.87 | -0.28 | 0.78 | 0.70 | 0.83 | 0.96 | 0.87 | 0.26 | 0.79 | 1 | ||||
| Cu | 0.51 | 0.43 | 0.38 | 0.29 | -0.11 | 0.43 | 0.40 | 0.48 | 0.34 | 0.40 | 0.08 | 0.50 | 0.38 | 1 | |||
| Zn | 0.86 | 0.70 | 0.84 | 0.68 | -0.12 | 0.89 | 0.81 | 0.76 | 0.66 | 0.60 | 0.29 | 0.81 | 0.72 | 0.62 | 1 | ||
| Br | 0.50 | 0.54 | 0.33 | 0.43 | -0.02 | 0.36 | 0.35 | 0.40 | 0.42 | 0.32 | 0.19 | 0.40 | 0.40 | 0.21 | 0.22 | 1 | |
| Pb | 0.81 | 0.77 | 0.96 | 0.84 | -0.26 | 0.87 | 0.78 | 0.69 | 0.86 | 0.82 | 0.37 | 0.75 | 0.82 | 0.28 | 0.77 | 0.29 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





