1. Introduction
Our knowledge on non-visual eyestalk organs in mysidaceans is far from exhaustive. An Organ of Bellonci (OB) was indicated mostly without details as a circle or as an oval in drawings of eyes for an estimated fifty of the currently acknowledged 1224 species of Mysida (own census 2 July 2023). The OB is located near the base of each eyestalk and on or near a sensory papilla, if present. It was described as a bipartite vesicle connected to a proximal nerve; the completely internal OB is closely accompanied by sensory pores reaching the surface of the cuticle [
1]. OBs are found in the eyestalks of most podophthalmous crustaceans, whereas sensory pore organs (SPOs) have a more restricted distribution in this crustacean group [
2]. OBs and SPOs form separate organs developing independently in the shrimp
Palaemon serratus [
3].
To date, non-visual eyestalk organs were completely unknown in all 16 currently acknowledged species of Stygiomysida, most of which are anophthalmic, living in hypogean aquatic environments. Only three species show a few functional ommatidia disto-laterally on the eyestalks:
Spelaeomysis servata (gender of species name here corrected due to fixation of ‘
Mysis’ as feminine [
4] (p. 129)) from a dimly-lit dolina in Zanzibar and a tide-pool in Aldabra, W-Indian Ocean;
S. cardisomae from burrows of land crabs along the Caribbean coast of Columbia and from the coast of Peru, this species also occurring in caves; and
S. cochinensis from a prawn culture field fully exposed to sunlight in Cochin, India. A fourth species not fully restricted to darkness, the anophthalmic
S. bottazzii from the Salento Peninsula (S-Italy) inhabits deep brackish groundwater but also shallow wells and dolinas, where it exhibits micro-phytophagy in or near the margins of the photic zone during part of its life cycle [
5,
6]. With these four species occurring at least partly in photic habitats, the genus
Spelaeomysis appears less strongly tied to dark deep groundwater than generally assumed up to the 1960s. Future intensified studies in ‘small’ habitats such as dolinas and wells could reveal additional ties of Stygiomysida species to photic habitats.
A potential chemoreceptive function of sensory pore organs was considered to be speculative as long as it was based solely on ultrastructure [
2]. Based on the structure of the OB, a sensory function – either chemosensory or photoreceptive – rather than a neurosecretory function was hypothesized as most probable. Nonetheless, this did not exclude potentially differing functions between crustacean groups [
2]. In favor of this interpretation, a neurosecretory role was demonstrated mainly in the gut, where genes coding for neuropeptides were expressed, and less in the OB, optic nerve in the eyestalk or in other organs of a decapod shrimp [
7].
An ultrastructural study of the OB in the amphipod
Gammarus setosus led to the statement that [
8] “The large size, cephalic position, elaborate structure, and suspension of the organ suggest that it is of considerable importance in the sensory capability of aquatic Malacostraca”. Nonetheless, a potential sensory capability could hardly be generalized for all aquatic Malacostraca in view of recent findings pointing to the function of the OB in endocrine control [
7,
9]. The precise functions are unknown in Mysida and Stygiomysida. The large sized OB shared by mysidaceans with
Gammarus species would be strange if the OB were an exclusively endocrine organ. To shed light on this issue, the first ample multi-species investigation on potential ties between habitat and both qualitative and metric features of the eyestalk is here presented. The intention is to provide novel insights and stimuli for future research in this field.
4. Discussion
Stammer [
22] (p. 91) was the first to study the internal structure of the eye rudiments in
T. vjetrenicensis. Subapically, he noted a lamella separating a short, nearly transverse portion from the main body of the eyestalk. From inside of this portion he reported fibers and large nuclei of hypodermal cells. With some reservation he identified the separate portion of the eye as the former cornea with some nuclei, but did not identify rudimentary ommatidia. As discussed below, he erroneously claimed more proximal structures to represent main, proximally shifted parts of the cornea. The hunchbacked demarcated distalmost portion of the eye rudiments actually presents the only homolog of the cornea [
23]. The sub-spherical to oviform structures in the rugged area are here interpreted as strongly reduced ommatidia (
Figure 1C).
Stammer [
22] (p. 91) noted a cavity with complex content closely below the cuticle of the mesial margin of the eye rudiments and initially interpreted this as a rudiment of lenses. In the appendix [
22] (p. 93), clearly added in press, he discussed and left open whether or not the observed cavity may represent an endocrine structure possibly homologous to the X-Organ previously reported [
24] for decapods. In a study on the eye of another mysid species, Mayrat [
1] finally acknowledged the interpretation as an X-organ (here termed ‘Organ of Bellonci’) by attributing a typical aspect to the “
pars distalis” of this organ in
T. vjetrenicensis. The subunit structuring containing small cavities and sensory cells as in
Figure 1D fits well with the type of OBs known [
2] from many crustacean groups.
A neurosecretory function involved in controlling molting and reproduction was attributed to the OB of a mysid species [
20]. Evidence for a non-photoreceptive role was claimed for the OB of another decapod [
25]. Nonetheless, other authors [
2] assumed a chemosensory or photosensitive function as being most probable. Evidence was also provided that the crustacean hyperglycemic hormone is synthesized in the X-organ/sinus gland complex of the eyestalk, a hormone involved in osmoregulation of a freshwater decapod [
26]. This complex therefore appears as the major endocrine control center in most stalk-eyed crustaceans [
9]. Finally, the expression of genes coding for neuropeptides was demonstrated in the eyestalk organs of a decapod species [
7]. So far, however, there is no evidence in favor or against multiple functions.
Whatever else that organ may contribute, a photosensitivity appears ecologically plausible in subterranean mysids [
6,
21]: the anophthalmic hypogean
Spelaeomysis bottazzii (order Stygiomysida) and the eye-bearing semihypogean
Diamysis camassai (order Mysida), co-occurring in brackish dolinas, exhibit micro-phytophagy in or near the margins of the photic zone during part of their life cycle. Both species have mandibles capable of scratching epigrowth from hard substrate. A preceding replenishment of trophic reserves could be important prior to entering deep, nutrient-poor groundwater where the incubating females of the hypogean
Spelaeomysis may escape from predation by epigean animals. Only ‘fat’ specimens produce eggs [
6]. Nonetheless, females incubating eggs are on average fatter than those with larvae, which points to unfavorable nutritional conditions during the very long incubation, which ends with release of the young and a molt to a post-reproductive stage with reduced oostegites. This makes it plausible that the females could profit from detailed sensory feedback when transitioning between risky photic and nearby, safer aphotic habitats. The contribution of potential photic, olfactory and ‘auditory’ sensitivity to this feedback remains a challenging question.
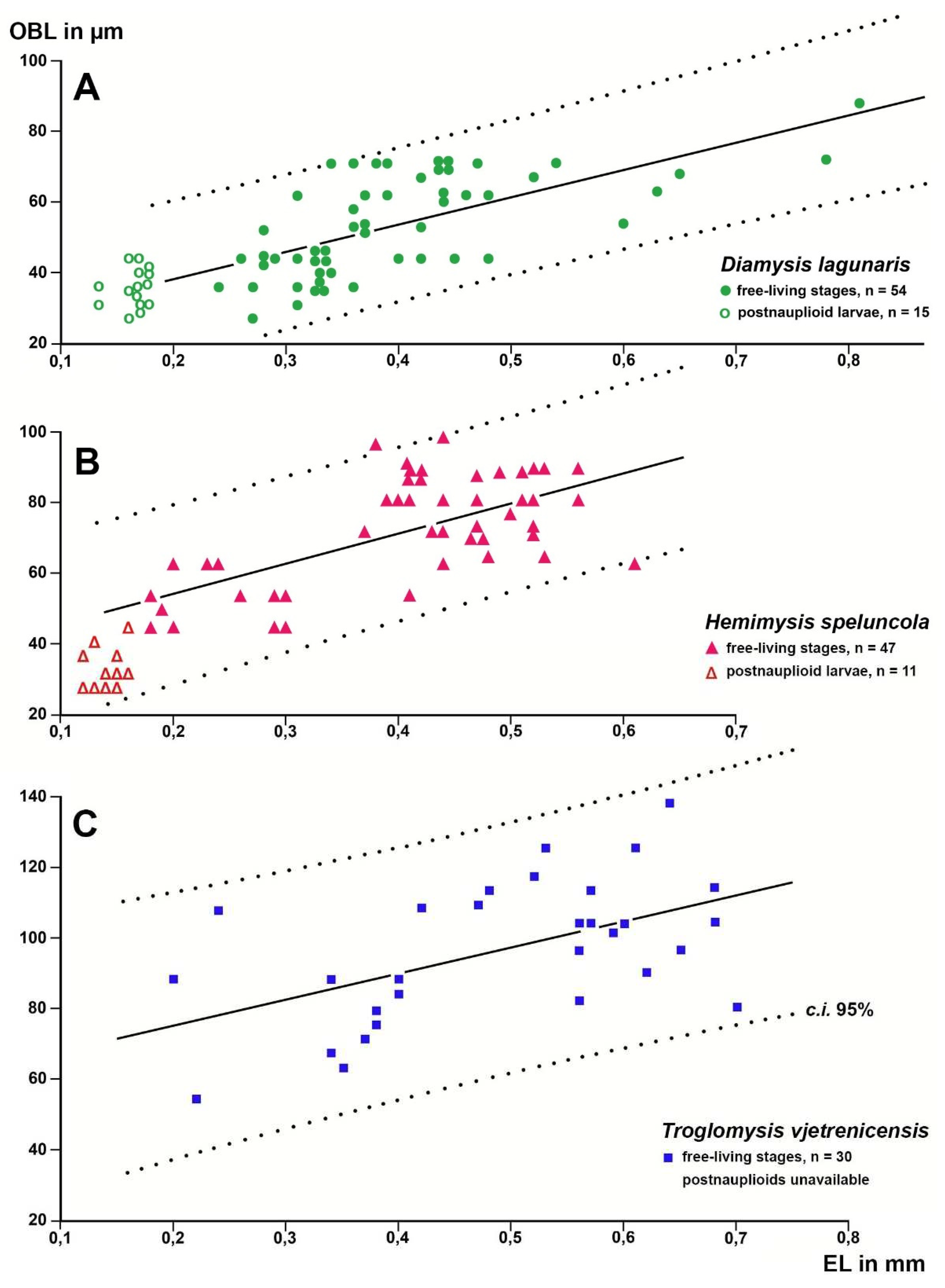
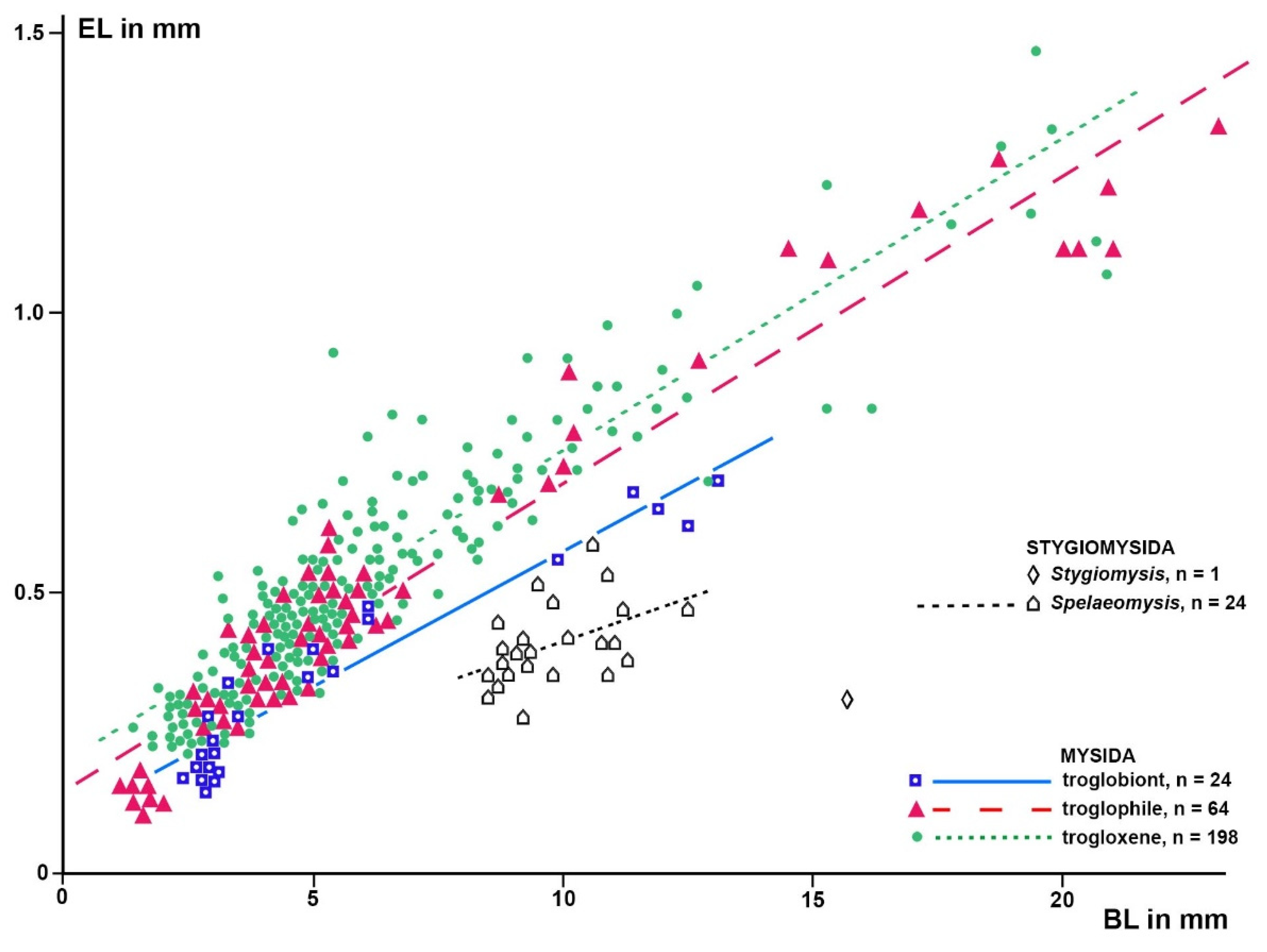
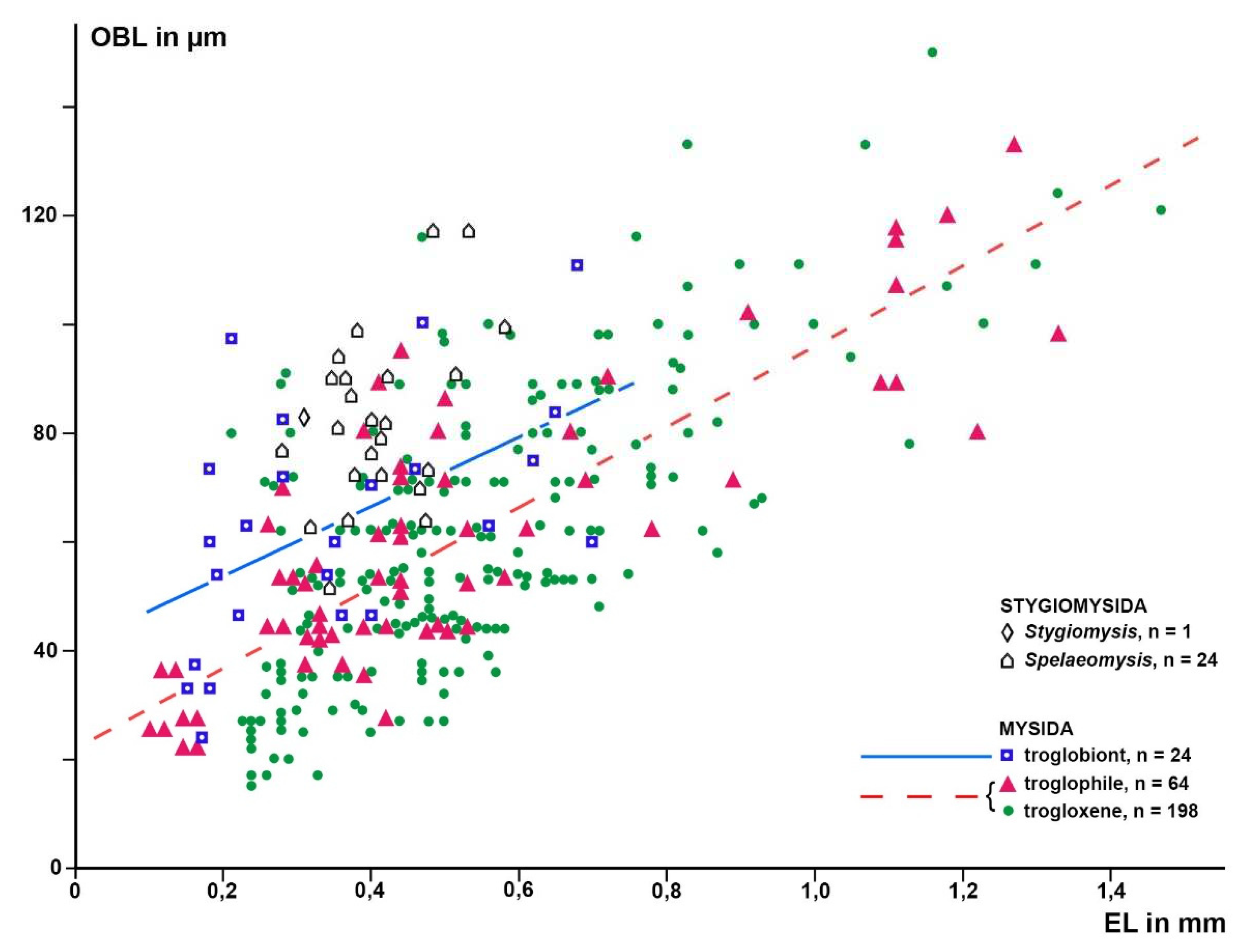
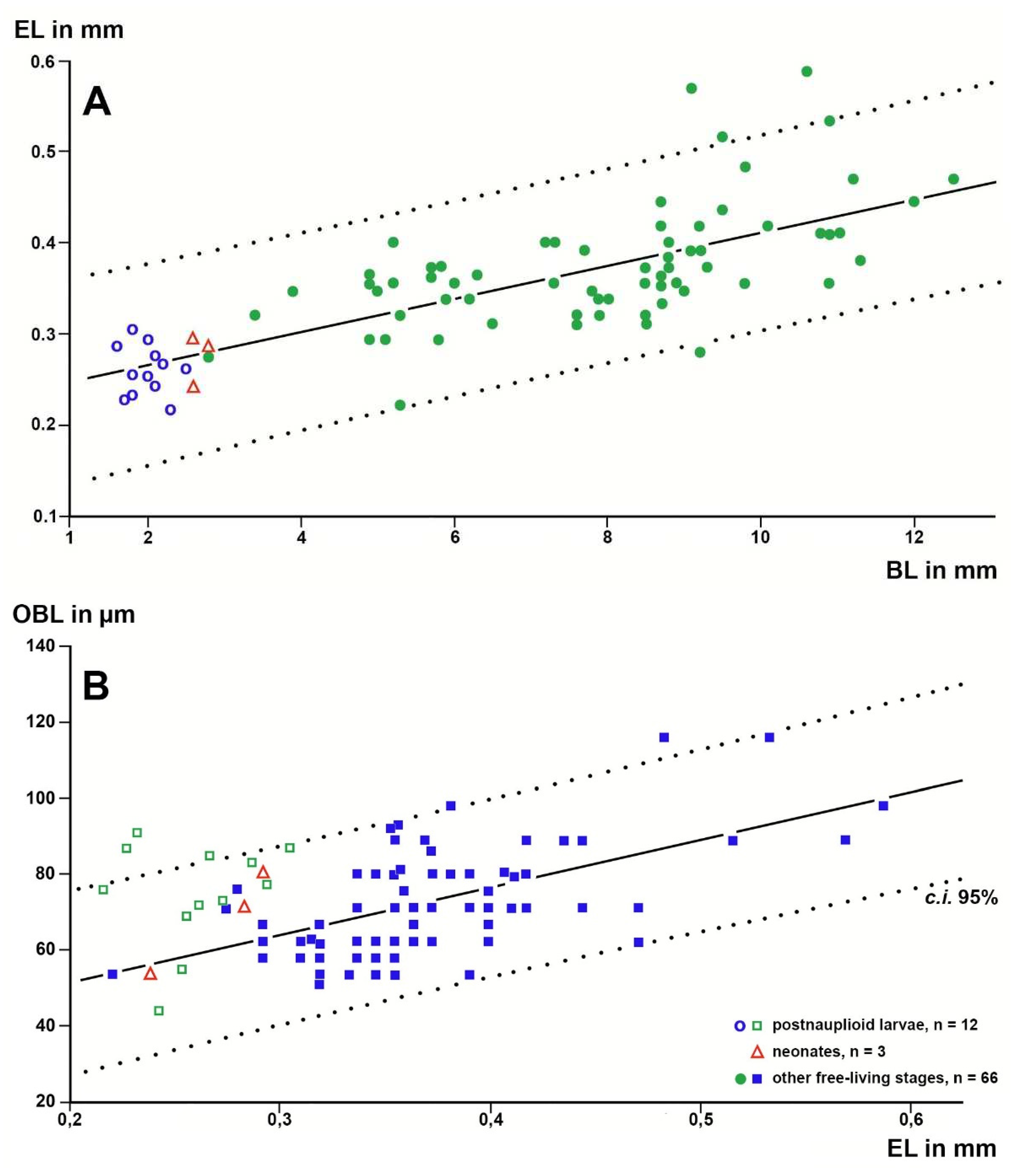
Based on a sample of 68 Mysida species,
Table 1 and
Figure 5,
Figure 6 show that adult eye length at a given body length is on average 26% (c.i. 95% based on specimen numbers: 19−33%) shorter in troglobiont versus trogloxene species, while the Organ of Bellonci is 54% longer (41−66%) at a given eye length and 40% (27−53%) longer at given body length, respectively, in troglobiont species. These findings clearly demonstrate that the OB proliferates whereas the eyes are reduced in troglobiont species. This points to several potential sensory functions (possibly in addition to other functions) of the OB in Mysida. The structure of antennal sensilla in a subterranean mysid species suggests that the loss of visual abilities could be counterbalanced by more effective tactile and chemosensory abilities [
27]. In that respect, the role of OBs remains pending and calls for additional physiological data from anophthalmic mysids before drawing further conclusions.
Using standard microscopy, no OBs were found in nauplioid larvae (the first larval stage in the marsupium of Mysida and Stygiomysida). The OBs became well visible at the subsequent postnauplioid stage in the marsupium. The first appearance of OBs varies in the range of larval stages I to V among species of decapods [
28]. The here-presented first finding for mysidacean larvae may be related to the incomplete eyes (
Figure 3A−D) of the almost immotile nauplioid larvae versus well-formed eyes (
Figure 3F and
Figure 8B) in postnauplioids, which are capable of small movements in the marsupium.
In the troglobiont
Spelaeomysis bottazzii the postnauplioid larvae still show almost spherical eyes (
Figure 8B) with pale cornea, whereas neonates feature large eyes (
Figure 8D,E) shaped as flattened ovoids with some differentiation in eyestalk and rudimentary cornea [
6]. During subsequent molt stages the eyes once again become more flattened and take on a quadrangular shape without clear differentiation into eyestalk and cornea. The eye rudiments remain separate up to the adult stage. This species shows a clear regressive ontogenetic eye development. Similarly, a strong ontogenetic regression occurs in
Spelaeomysis longipes, whose paired conical eyes show no trace of ommatidia in postnauplioids, the eye rudiments then fusing mesially to form a single eyeplate in the adult [
29]. Optical ganglia inside eyeplates have been reported for this species from freshwater wells in India, but neither OBs nor SPOs [
29]. If the large eyes of early stages (
Figure 8D,E) in
S. bottazzii have any sensorial functions, they may be related to life in near-surface habitats, whereby adult females are rare near the surface, probably mainly incubating in deep brackish groundwater [
5,
6].
Although a total of eight genus names were proposed in the past in the order Stygiomysida, only two genera,
Spelaeomysis with nine species and
Stygiomysis with seven species, are currently acknowledged. Toroidal structures with a central pore dorsally on the eyestalks (
Figure 10B), interpreted as SPOs, were detected here for the first time for this order, namely in one species for each of both genera, representing separate families (Stygiomysidae and Lepidomysidae). In analogy, toroids with a central pore were found at the tip of the sensory papilla on the eyestalks in the mysid
Mysidopsis gibbosa (
Figure 2E,F). In most mysids, however, the sensory pores are not on papillae but associated with the Organ of Bellonci (
Figure 1E and
Figure 3C,D). Sensory pores with putative chemoreceptive function on eyestalk papillae are also present in certain decapods [
2]. A chemosensory function appears plausible also for the pores on the eyestalks of Mysida and Stygiomysida, but experimental und ultrastructural data are still wanting.
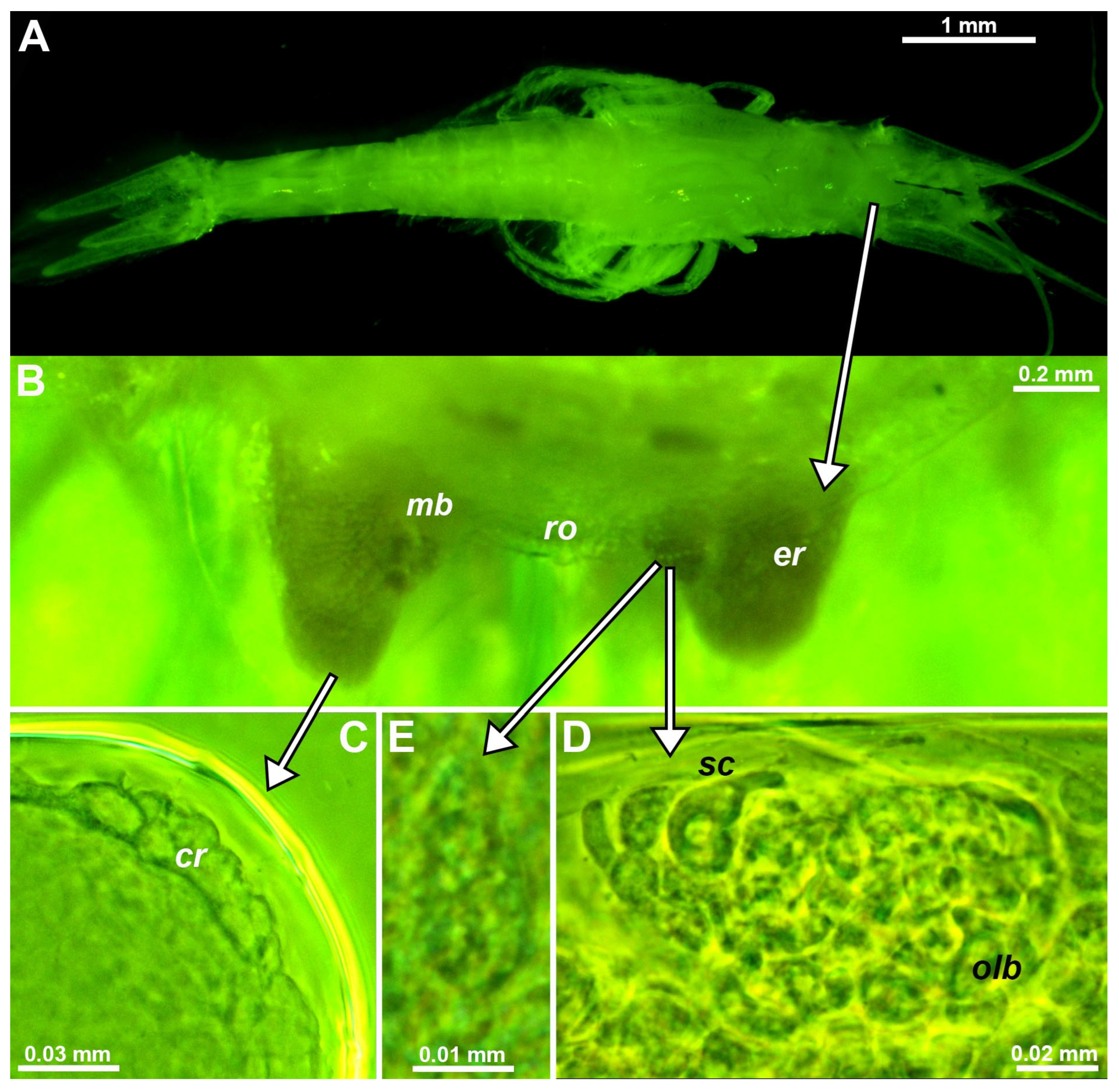
Figure 1.
Eye structure in the troglobiont Troglomysis vjetrenicensis. (A) habitus of adult male, dorsal. (B) anterior margin of carapace and eye rudiments in upside-down orientation, dorsal, details from other specimens showing corneal rudiment (C) at distal margin of the eye, Organ of Bellonci (D) close to mesial margin and pore field (E) at the dorsal face above the Organ of Bellonci. Lower case labels indicate corneal rudiment (cr), eye rudiment (er), onion-like bodies (olb), rostrum (ro) and sensory cells (sc). C−E, objects expanded on slide. Green coloring of objects artificial.
Figure 1.
Eye structure in the troglobiont Troglomysis vjetrenicensis. (A) habitus of adult male, dorsal. (B) anterior margin of carapace and eye rudiments in upside-down orientation, dorsal, details from other specimens showing corneal rudiment (C) at distal margin of the eye, Organ of Bellonci (D) close to mesial margin and pore field (E) at the dorsal face above the Organ of Bellonci. Lower case labels indicate corneal rudiment (cr), eye rudiment (er), onion-like bodies (olb), rostrum (ro) and sensory cells (sc). C−E, objects expanded on slide. Green coloring of objects artificial.
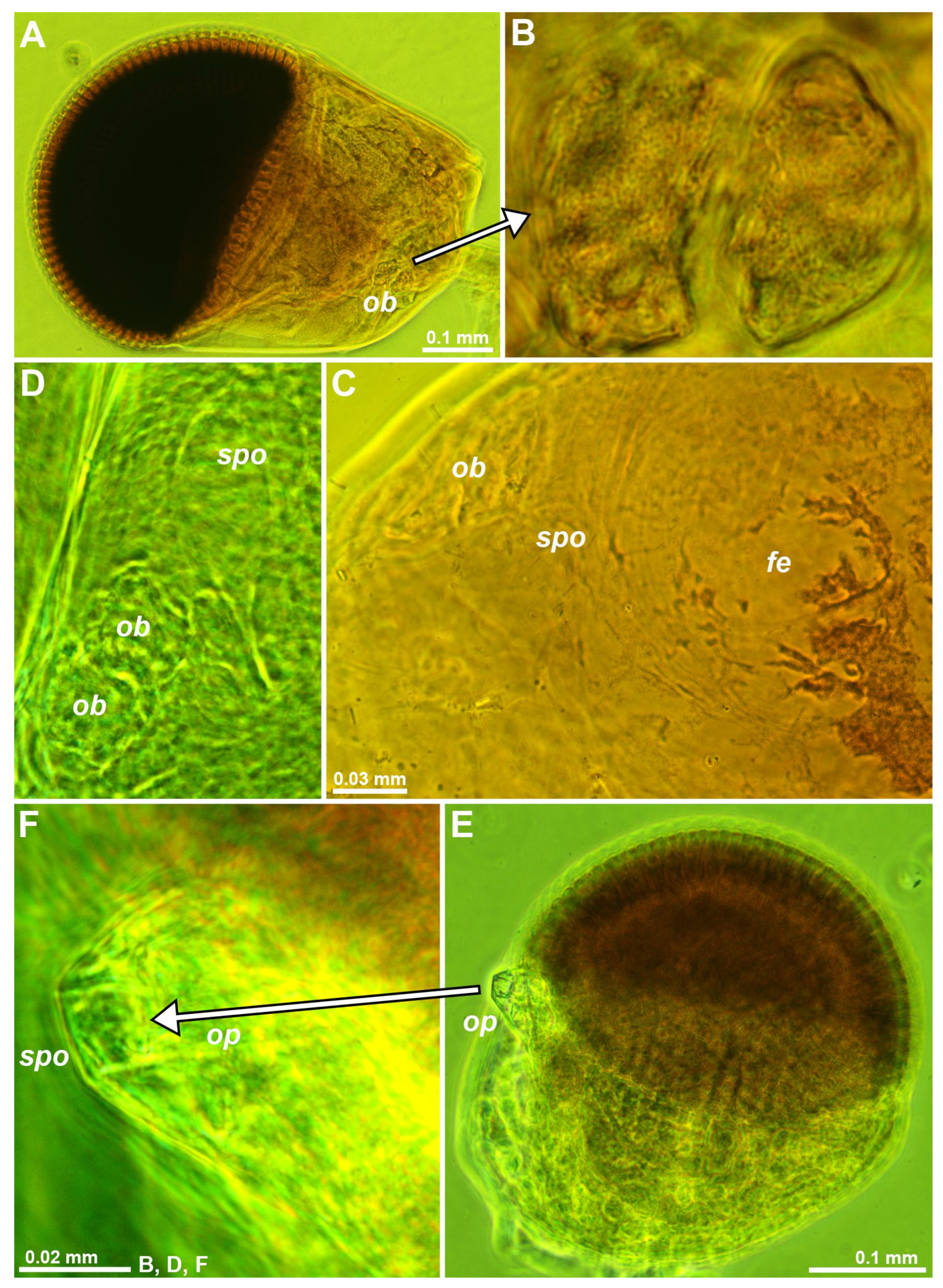
Figure 2.
Structure of eyestalks in the trogloxenes Diamysis fluviatilis (A, B), D. lagunaris (C, D) and Mysidopsis gibbosa (E, F). (A) left eye, dorsal, detail (B) showing vesicular form of the Organ of Bellonci (OB). (C) antero-mesial area of eyestalk, dorsal, focus on a ‘fenestra’. (D) OB and sensory pore organ (SPO) in another specimen. (E) left eye, dorsal, detail (F) showing tip of ocular papilla with SPO. Lower-case labels indicate fenestra (fe), OB (ob), ocular papilla (op) and SPO (spo). Objects expanded on slide. Green coloring of objects is artificial.
Figure 2.
Structure of eyestalks in the trogloxenes Diamysis fluviatilis (A, B), D. lagunaris (C, D) and Mysidopsis gibbosa (E, F). (A) left eye, dorsal, detail (B) showing vesicular form of the Organ of Bellonci (OB). (C) antero-mesial area of eyestalk, dorsal, focus on a ‘fenestra’. (D) OB and sensory pore organ (SPO) in another specimen. (E) left eye, dorsal, detail (F) showing tip of ocular papilla with SPO. Lower-case labels indicate fenestra (fe), OB (ob), ocular papilla (op) and SPO (spo). Objects expanded on slide. Green coloring of objects is artificial.
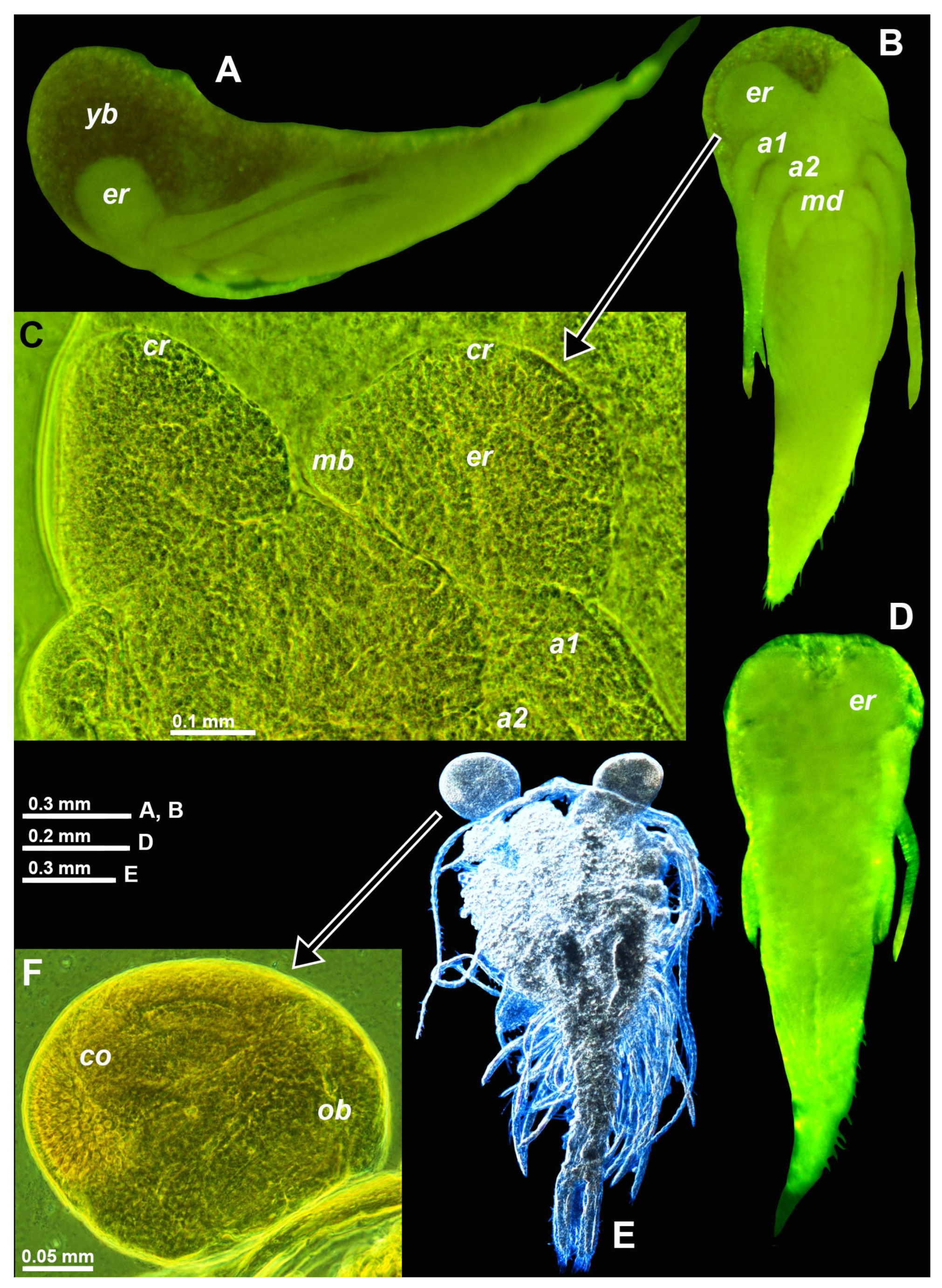
Figure 3.
Eye development of marsupial larvae in the troglobiont Troglomysis vjetrenicensis from the cave Donja Vjetrenica (A−C), in the trogloxene Diamysis fluviatilis from the Sile River (D) and Heteromysis microps from the marine sublittoral in Sardinia (E, F). (A, B, D) nauplioids in toto, lateral (A) and ventral (B, D). (C) cephalic region of nauplioid expanded on slide, ventral. (E) postnauplioid larva expanded on slide, dorsal, detail (F) showing left eye. Lower-case labels indicate antennula (a1), antenna (a2), cornea (co), corneal rudiment (cr), eye rudiment (er), mesial bulge (mb), mandible (md), Organ of Bellonci (ob) and yolk or fat bodies (yb). A, B, D, E, objects artificially separated from background. Green and blue coloring of objects artificial.
Figure 3.
Eye development of marsupial larvae in the troglobiont Troglomysis vjetrenicensis from the cave Donja Vjetrenica (A−C), in the trogloxene Diamysis fluviatilis from the Sile River (D) and Heteromysis microps from the marine sublittoral in Sardinia (E, F). (A, B, D) nauplioids in toto, lateral (A) and ventral (B, D). (C) cephalic region of nauplioid expanded on slide, ventral. (E) postnauplioid larva expanded on slide, dorsal, detail (F) showing left eye. Lower-case labels indicate antennula (a1), antenna (a2), cornea (co), corneal rudiment (cr), eye rudiment (er), mesial bulge (mb), mandible (md), Organ of Bellonci (ob) and yolk or fat bodies (yb). A, B, D, E, objects artificially separated from background. Green and blue coloring of objects artificial.
Figure 4.
Length of the Organ of Bellonci (OBL) as a response variable with eye length (EL) as a predictor variable in linear regressions of larval and free-living specimens of trogloxene (A), troglophile (B) and troglobiont (C) mysid species. Regressions calculated with data from free-living specimens only; data points for postnauplioid larvae added post hoc. Coinciding points slightly displaced.
Figure 4.
Length of the Organ of Bellonci (OBL) as a response variable with eye length (EL) as a predictor variable in linear regressions of larval and free-living specimens of trogloxene (A), troglophile (B) and troglobiont (C) mysid species. Regressions calculated with data from free-living specimens only; data points for postnauplioid larvae added post hoc. Coinciding points slightly displaced.
Figure 5.
Eye length (EL) as a response variable versus body length (BL) as a predictor variable in separate linear regressions for the stygiomysid Spelaeomysis bottazzii (24 adult specimens) and for troglobiont, troglophile and trogloxene mysids (286 specimens of 68 species). Coinciding points slightly displaced.
Figure 5.
Eye length (EL) as a response variable versus body length (BL) as a predictor variable in separate linear regressions for the stygiomysid Spelaeomysis bottazzii (24 adult specimens) and for troglobiont, troglophile and trogloxene mysids (286 specimens of 68 species). Coinciding points slightly displaced.
Figure 6.
Length of the Organ of Bellonci (OBL) as a response variable versus eye length (EL) as a predictor variable in separate linear regressions for troglobiont versus combined troglophile and trogloxene mysids (286 specimens of 68 species); data for stygiomysids give no significant regression. Coinciding points slightly displaced.
Figure 6.
Length of the Organ of Bellonci (OBL) as a response variable versus eye length (EL) as a predictor variable in separate linear regressions for troglobiont versus combined troglophile and trogloxene mysids (286 specimens of 68 species); data for stygiomysids give no significant regression. Coinciding points slightly displaced.
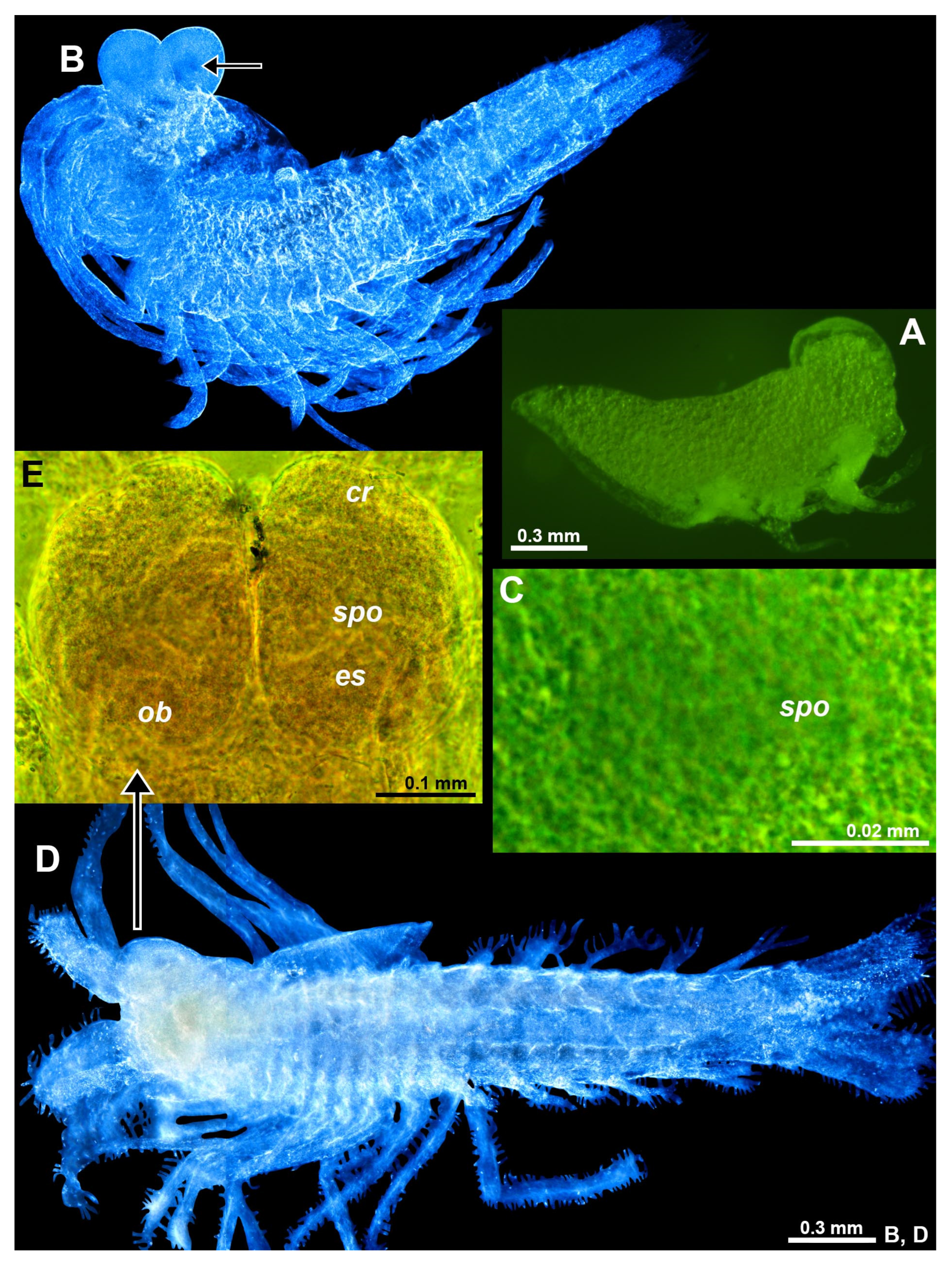
Figure 7.
Position and structure of eye rudiments in adult Spelaeomysis bottazzii. (A) habitus of adult female with nauplioid larvae, lateral (pigment spots are on thoracomeres of the female, not on nauplioids). (B) head of another adult female, dorsal, focus on eye rudiments (er), anterior margin of carapace out of focus. (C) detail of panel (B) showing sensory pore organ (spo), dorsal. (D) detail of panel (B) showing Organ of Bellonci (ob), dorsal, small arrow points to sensory cells with a neck. A, object artificially separated from background. Green coloring of objects artificial.
Figure 7.
Position and structure of eye rudiments in adult Spelaeomysis bottazzii. (A) habitus of adult female with nauplioid larvae, lateral (pigment spots are on thoracomeres of the female, not on nauplioids). (B) head of another adult female, dorsal, focus on eye rudiments (er), anterior margin of carapace out of focus. (C) detail of panel (B) showing sensory pore organ (spo), dorsal. (D) detail of panel (B) showing Organ of Bellonci (ob), dorsal, small arrow points to sensory cells with a neck. A, object artificially separated from background. Green coloring of objects artificial.
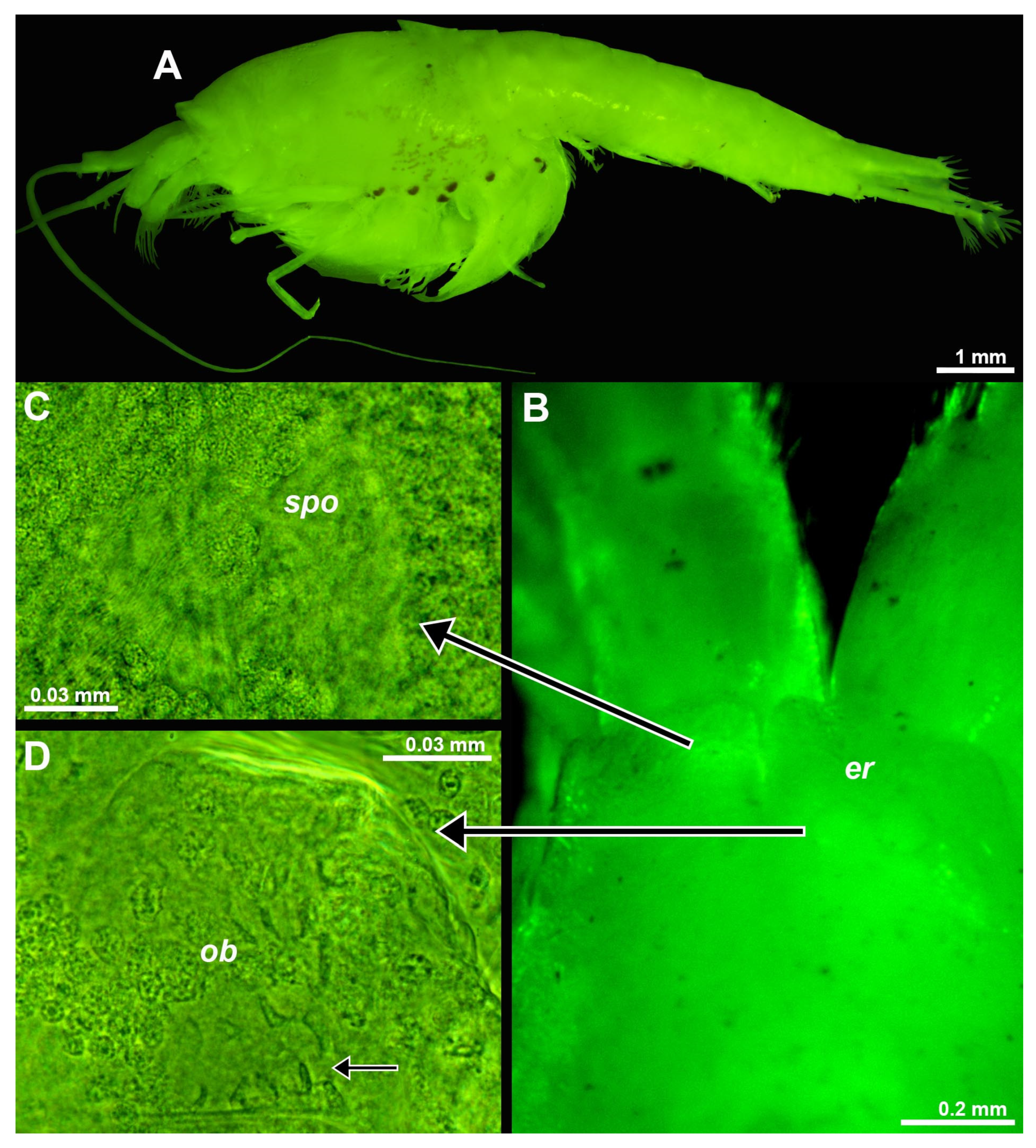
Figure 8.
Eye rudiments in larval and neonate Spelaeomysis bottazzii. (A) nauplioid larva, lateral. (B) postnauplioid larva, obliquely dorsal, arrow points to sensory pore organ. (C) detail of panel (B) showing sensory pore organ, dorsal. (D) neonate, ventral. (E) eyes of another neonate, dorsal. Lower-case labels indicate corneal rudiment (cr), eyestalk (es), Organ of Bellonci (ob) and sensory pore organ (spo). Green and blue coloring of objects artificial. B, D, objects artificially separated from background.
Figure 8.
Eye rudiments in larval and neonate Spelaeomysis bottazzii. (A) nauplioid larva, lateral. (B) postnauplioid larva, obliquely dorsal, arrow points to sensory pore organ. (C) detail of panel (B) showing sensory pore organ, dorsal. (D) neonate, ventral. (E) eyes of another neonate, dorsal. Lower-case labels indicate corneal rudiment (cr), eyestalk (es), Organ of Bellonci (ob) and sensory pore organ (spo). Green and blue coloring of objects artificial. B, D, objects artificially separated from background.
Figure 9.
Linear regression analysis of length of Organ of Bellonci (OBL), eye length (EL) and body length (BL) in postnauplioid larvae and free-living specimens of the troglobiont stygiomysid Spelaeomysis bottazzii. (A) eye length as a response variable and body length as a predictor variable. (B) length of the Organ of Bellonci as a response variable and eye length as a predictor variable. Regressions calculated with data from free-living specimens only; data points for postnauplioid larvae added post hoc. Coinciding points slightly displaced.
Figure 9.
Linear regression analysis of length of Organ of Bellonci (OBL), eye length (EL) and body length (BL) in postnauplioid larvae and free-living specimens of the troglobiont stygiomysid Spelaeomysis bottazzii. (A) eye length as a response variable and body length as a predictor variable. (B) length of the Organ of Bellonci as a response variable and eye length as a predictor variable. Regressions calculated with data from free-living specimens only; data points for postnauplioid larvae added post hoc. Coinciding points slightly displaced.
Figure 10.
Position and structure of the right eye rudiment in Stygiomysis major. (A) habitus of subadult female, dorsal. (B) right eye rudiment, dorsal. (C) cephalic region, lateral. Lower-case labels indicate carapace (ca), corneal rudiment (cr), eyestalk (es), longitudinal groove (lg), sensory pore organ (spo) and rostrum (ro). A, object artificially separated from background. A−C, green coloring of objects artificial.
Figure 10.
Position and structure of the right eye rudiment in Stygiomysis major. (A) habitus of subadult female, dorsal. (B) right eye rudiment, dorsal. (C) cephalic region, lateral. Lower-case labels indicate carapace (ca), corneal rudiment (cr), eyestalk (es), longitudinal groove (lg), sensory pore organ (spo) and rostrum (ro). A, object artificially separated from background. A−C, green coloring of objects artificial.
Table 1.
Effects of troglophilia on length of eyes (mm) and Organ of Bellonci (µm) in mysidaceans.
Table 1.
Effects of troglophilia on length of eyes (mm) and Organ of Bellonci (µm) in mysidaceans.
| |
|
Troglobiont vs. troglophile Mysida |
Troglobiont vs. trogloxene Mysida |
Troglophile vs. trogloxene Mysida |
Troglobiont Mysida vs. Stygiomysida |
| |
n species |
5 + 14 |
5 + 49 |
14 + 49 |
5 + 2 |
| |
n specimens |
24 + 64 |
24 + 198 |
64 + 198 |
24 + 25 |
Effects on EL
at given BL
|
mean ± s.e. |
−0.086 ± 0.019 |
−0.143 ± 0.020 |
−0.057 ± 0.013 |
−0.077 ± 0.025 |
| c.i. 95% |
−0.123 to −0.049 |
−0.182 to −0.103 |
−0.084 to −0.031 |
−0.127 to −0.028 |
| P (2-tailed) |
<0.0001 |
<0.0001 |
<0.0001 |
0.003 |
| % mean (c.i.) |
−17% (76−90%) |
−26% (67−81%) |
−10% (85−94%) |
−19% (69−93%) |
Eff. on OBL
at given BL
|
mean ± s.e. |
25.64 ± 3.70 |
24.53 ± 4.05 |
−2.63 ± 2.63 |
−5.17 ± 4.13 |
| c.i. 95% |
12.29 to 32.99 |
16.56 to 32.50 |
−7.80 to +2.54 |
−13.48 to +3.14 |
| P (2-tailed) |
<0.0001 |
<0.0001 |
0.32 (n.s.) |
0.22 (n.s.) |
| % mean (c.i.) |
42% (120−154%) |
40% (127−153%) |
−4% (83−104%) |
−6% (84−104%) |
Eff. on OBL
at given EL
|
mean ± s.e. |
29.81 ± 3.55 |
32.98 ± 4.00 |
1.67 ± 2.57 |
2.97 ± 3.88 |
| c.i. 95% |
22.76 to 36.86 |
25.11 to 40.85 |
−3.31 to +6.82 |
−4.83 to +10.78 |
| P (2-tailed) |
<0.0001 |
<0.0001 |
0.50 (n.s.) |
0.45 (n.s.) |
| % mean (c.i.) |
49% (137−160%) |
54% (141−166%) |
3% (95−111%) |
4% (94−113%) |